- Department of Gastrointestinal Surgery, Cancer Hospital of Shantou University, Medical College, Shantou, Guangdong, China
Background and objectives: Gastric cancer is a common malignant tumor primarily treated through surgery. Concurrently, radiation therapy has gained attention as an important local treatment modality. However, its application in gastric cancer remains limited, with ongoing debates on radiation standards. Given that bibliometrics serves as a potent tool to unveil scientific literature, we conducted a bibliometric analysis of literature on radiation therapy for gastric cancer. We explored emerging trends, common patterns in research, tracked collaborations and networks, and anticipated future directions in this clinical context.
Materials and methods: We searched the electronic Web of Science (WOS) database using keywords “gastric cancer” and “radiation therapy” for manuscripts published in English from 2014 to 2023. Data analysis was conducted using R-Studio software, employing bibliometric methods based on the bib liometrix R package. Quantification involved assessing the most relevant authors based on document production and citation metrics. Author productivity was analyzed using Lotka’s law. Main thematic areas included isolated (niche) topics, emerging topics, hot (motor) topics, and necessary (basic) topics.
Results: A total of 2405 documents were initially retrieved, from which 484 articles closely related to gastric cancer radiation therapy were selected, showing an annual growth rate of -2.05%. Overall, publications were found in 186 different journals, with “FRONTIERS IN ONCOLOGY” being the most relevant journal. The most prolific authors were from South Korea. Clinical trials (survival, phase III clinical trials) and treatment strategies (surgery, chemotherapy, radiation therapy, perioperative treatment) represented the fundamental topics. Emerging topics included radiation dose, therapeutic response and immunotherapy.
Conclusion: Radiation therapy for gastric cancer has evolved in terms of timing, modes, target sites, and emerging combination therapies. It benefits patients with potentially resectable, unresectable, or isolated distant metastases. Immunotherapy combined with radiation shows significant potential and could become a new breakthrough in treatment strategies.
1 Introduction
Gastric cancer (GC) is one of the most significant contributors to the global cancer burden. According to the “Global Cancer Data Report 2022,” gastric cancer had a high number of new cases in 2022, ranking as the fifth most common cancer (4.9%) after lung cancer (2.48 million, 12.4%), female breast cancer (2.31 million, 11.6%), colorectal cancer (1.93 million, 9.6%), and prostate cancer (1.47 million, 7.3%). Due to the often late-stage diagnosis of gastric cancer, the mortality rate is alarmingly high. It is estimated that approximately 660,000 people worldwide died from gastric cancer in 2022, making it the fourth leading cause of cancer-related deaths globally (1).
Currently, radical surgery is still considered the only potentially curative treatment for gastric cancer. Over the years, survival rates and incidence have improved due to the adoption of radical surgical approaches. However, after surgical resection for gastric cancer, both local and distant recurrence rates remain high (2).The high recurrence rate makes gastric cancer a challenging disease to cure solely through surgery. Moreover, lacking typical clinical precursors for gastric cancer, over 75% of patients are diagnosed at an advanced stage. The survival rate for these patients with locally advanced gastric cancer ranges only from 20% to 50%. Approximately 50% of patients with locally advanced gastric cancer lose their opportunity for surgical treatment. Therefore, researchers have been exploring adjuvant therapies for gastric cancer following surgical resection, such as radiation therapy (RT), chemotherapy, and chemoradiotherapy. Consequently, comprehensive treatment focusing on radiation therapy (RT) and chemotherapy has gained significant attention in recent years.
Numerous scientists have dedicated their efforts to postoperative radiotherapy research, with the most famous being the Intergroup 0116 (INT-0116) study (3), renowned for its well-designed and large sample content. It has been demonstrated that postoperative chemoradiotherapy (fluorouracil and leucovorin followed by radiation therapy) improves the 5-year overall survival rate and reduces the rate of local recurrence compared to surgery alone. Since then, an increasing number of studies have sought to confirm the survival benefits of adjuvant chemoradiotherapy. However, the results of these trials have either been contradictory or inconclusive due to their relatively limited patient recruitment and varying inclusion criteria. As a result, the role of radiation therapy in the adjuvant treatment of gastric cancer following surgery remains controversial.
Preoperative radiotherapy is primarily used to reduce the tumor burden in patients with advanced gastric cancer. This process can render previously inoperable patients eligible for surgery. Additionally, preoperative radiotherapy may play a unique role in controlling micrometastases, and the pathological response observed post-radiotherapy can provide significant prognostic information. Several major clinical trials have demonstrated that cancer of the gastroesophageal junction (GEJ) has yielded better therapeutic outcomes from preoperative radiotherapy compared to gastric cancer. Stahl et al. found that preoperative radiotherapy significantly increased the pathological complete response rate for GEJ adenocarcinoma (15.6% vs. 2.0%) and improved the 3-year overall survival (OS) rate (47.4% vs. 27.7%, P=0.07) (4). Similarly, Hagen and colleagues investigated 366 cases of gastric or GEJ cancer and found that patients receiving preoperative chemoradiotherapy (carboplatin + paclitaxel, 5 weeks; 41.4 Gy/23 fractions, 5 days/week) had a significantly higher resection rate (92% vs. 69%, P < 0.001) and OS (49.4 months vs. 24 months, median survival) compared to those receiving surgery alone (5). Furthermore, preoperative chemoradiotherapy was associated with reduced local recurrence rates (LRR, 14% vs. 34%, P < 0.001) and distant metastasis rates (29% vs. 35%, P = 0.025) when compared to surgery alone. This regimen has become the recommended treatment for GEJ adenocarcinoma in the United States.
Common local symptoms in patients with gastric cancer include obstruction, bleeding, or pain. Interventions to alleviate these symptoms encompass palliative radiotherapy (RT), palliative chemotherapy, gastric bypass surgery, palliative gastrectomy, and endoscopic stent placement. Radiotherapy serves as a non-invasive treatment option for these local symptoms. Literature suggests that two-thirds of patients receiving radiotherapy will experience clinical benefits, with the highest response rate observed in hemorrhage control (6).Low Biologically Effective Dose (BED) regimens appear sufficient to alleviate symptoms. However, the optimal dose-fractionation scheme for symptom relief remains unclear.
Given that bibliometrics is a powerful tool for uncovering scientific literature on specific topics over defined time spans, we decided to conduct a bibliometric analysis of the radiotherapy literature for gastric cancer published over the past decade. Through this current analysis, we aim to explore emerging trends and common patterns in research, track collaboration and networks, and predict future directions for clinical research in radiation oncology as applied to gastric cancer.
2 Materials and methods
2.1 Literature search strategy
Web of Science (WoS) is recognized as one of the most comprehensive, systematic, and authoritative databases, encompassing a vast array of literature metrics and over 12,000 high-quality journals from around the world. It is widely utilized for bibliometric analysis and visualization of scientific literature.
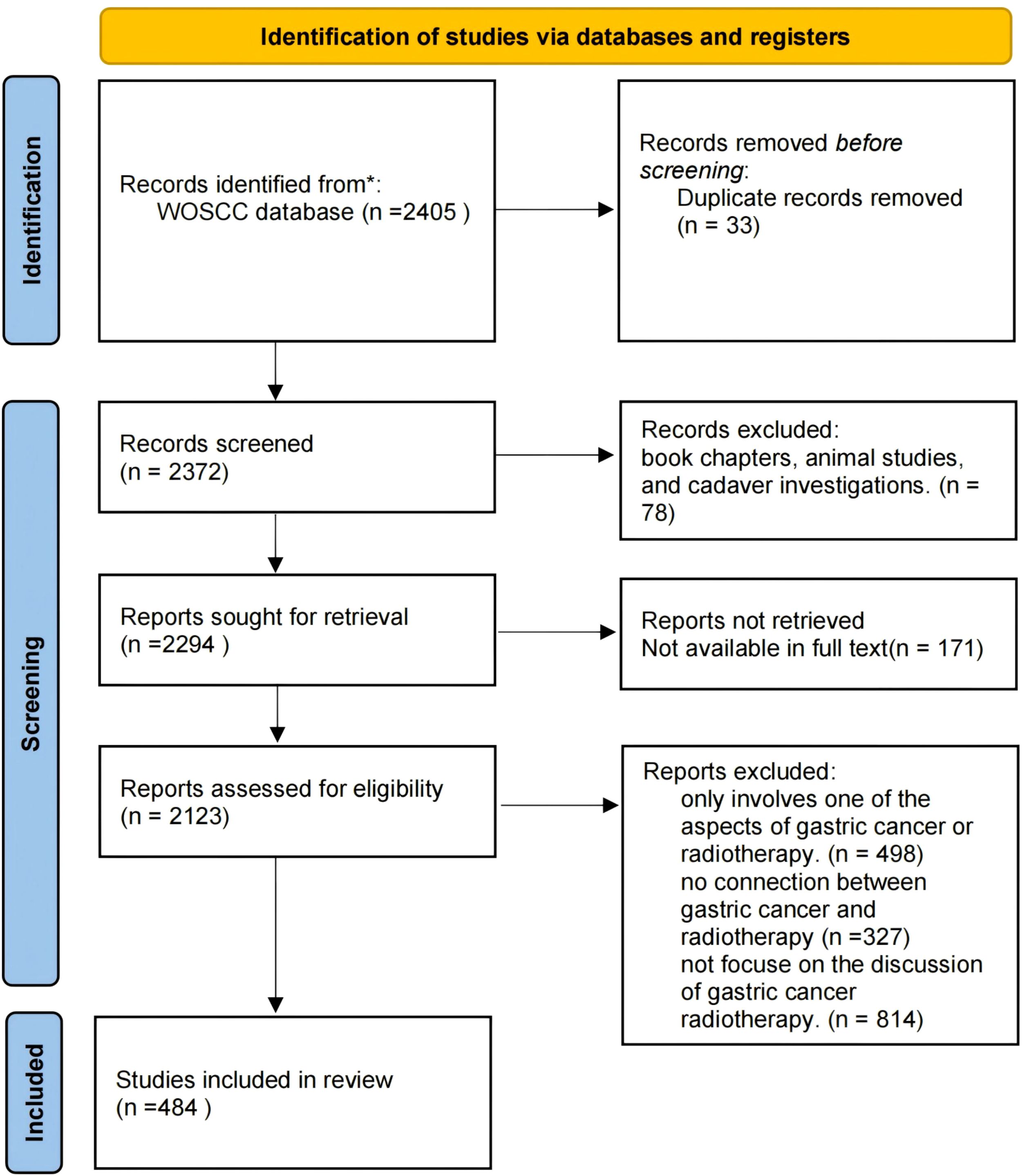
The literature screening process included in this study follows the PRISMA guidelines, as depicted in Figure 1. Publications were retrieved from the Science Citation Index Expanded (SCI-Expanded) of WoSCC for the period spanning from January 1, 2014 to October 25, 2023. Data were downloaded on October 25, 2023, within a single day to mitigate biases arising from daily database updates. Search terms used were: ((((((((((((((((((((ALL=(Neoplasm, Stomach)) OR ALL=(Stomach Neoplasm)) OR ALL=(Neoplasms, Stomach)) OR ALL=(Gastric Neoplasms)) OR ALL=(Gastric Neoplasm)) OR ALL=(Neoplasm, Gastric)) OR ALL=(Neoplasms, Gastric)) OR ALL=(Cancer of Stomach)) OR ALL=(Stomach Cancers)) OR ALL=(Gastric Cancer)) OR ALL=(Cancer, Gastric)) OR ALL=(Cancers, Gastric)) OR ALL=(Gastric Cancers)) OR ALL=(Stomach Cancer)) OR ALL=(Cancer, Stomach)) OR ALL=(Cancers, Stomach)) OR ALL=(Cancer of the Stomach)) OR ALL=(Gastric Cancer, Familial Diffuse) AND (((((((((((((((((TS=(Radiotherapy)) OR ALL=(Radiotherapies)) OR ALL=(Radiation Therapy)) OR ALL=(Radiation Therapies)) OR ALL=(Therapies, Radiation)) OR ALL=(Radiation Treatment)) OR ALL=(Radiation Treatments)) OR ALL=(Treatment, Radiation)) OR ALL=(Radiotherapy, Targeted)) OR ALL=(Radiotherapies, Targeted)) OR ALL=(Targeted Radiotherapies)) OR ALL=(Targeted Radiotherapy)) OR ALL=(Targeted Radiation Therapy)) OR ALL=(Radiation Therapies, Targeted)) OR ALL=(Targeted Radiation Therapies)) OR ALL=(Therapies, Targeted Radiation)) OR ALL=(Therapy, Targeted Radiation)) OR ALL=(Radiation Therapy, Targeted).
2.2 Inclusion and exclusion criteria
Inclusion criteria encompassed: (1)English-language articles, reviews and meta-analyses pertaining to radiotherapy for gastric cancer; and (2) only published data were used.
Exclusion criteria included conference abstract, editorial material, book chapters, animal studies, and cadaver investigations.
2.3 Data extraction and visualization methods
Data collection: All relevant details were retrieved from the Web of Science in text format, encompassing author names, article categories, citation counts, countries, digital object identifiers (DOIs), impact factors, journal names, institutions, keywords, sample sizes, study designs, titles, and publication years.
Data analysis: Data were analyzed using R-studio for summarizing and visualizing scientific literature. R-studio provides a rich set of literature analysis packages and user-friendly syntax for efficiently processing large-scale literature data, extracting valuable insights, and uncovering developmental trends and key influencing factors in academic fields. The main steps are as follows: (1) importing the dataset into R software, (2) selecting the type of analysis (i.e., “co-occurrence network,” “thematic evolution,” and “main information”), and (3) setting analysis parameters.
3 Results
3.1 Overview
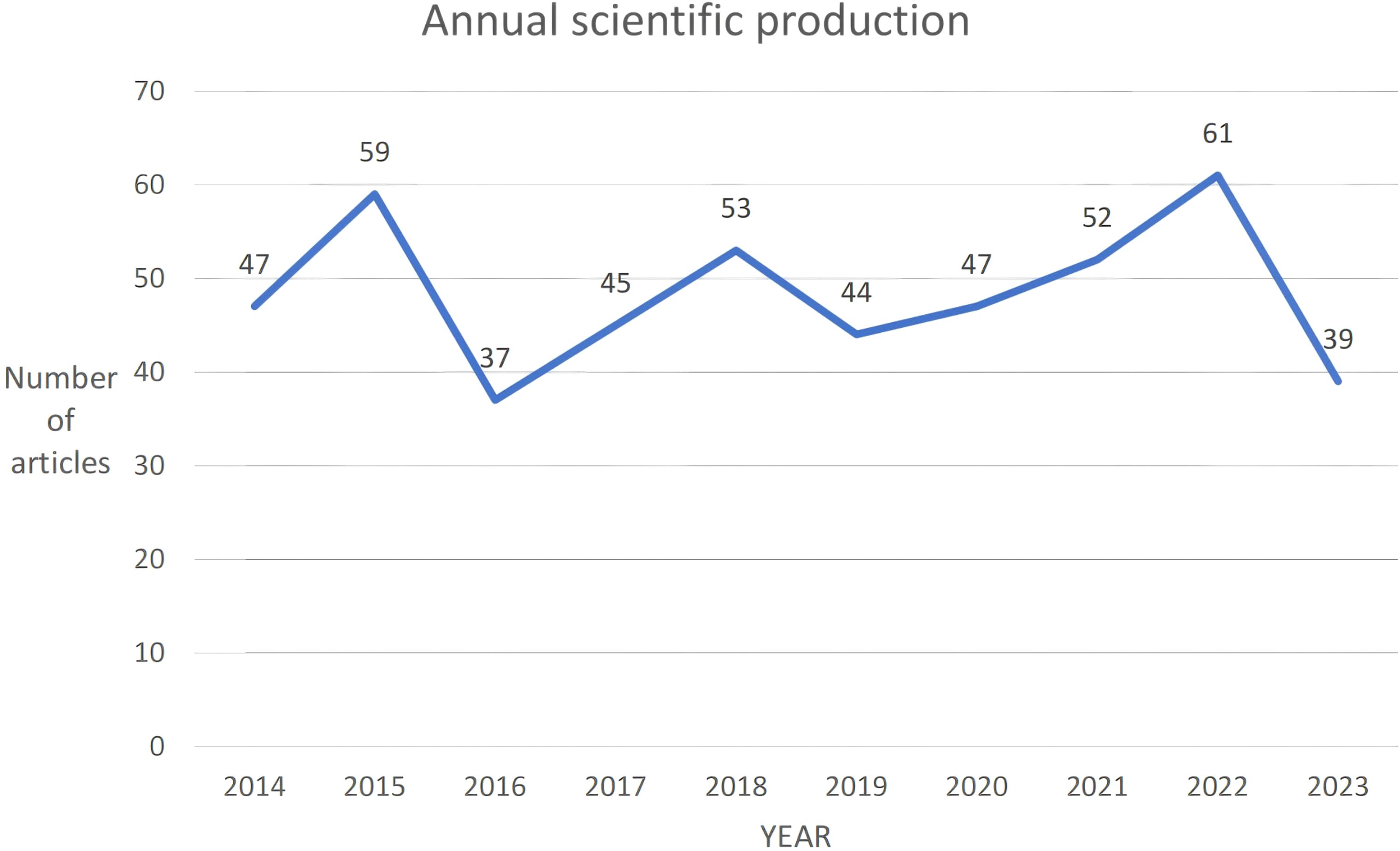
From 2014 to 2023, a total of 484 articles were collected. Overall, these articles involved 2940 authors, averaging 8 authors per publication. There was a negative annual growth rate of -2.05%, resulting in a decrease in scientific output from 47 articles in 2014 to 39 articles in 2023 (Figure 2). The average annual citations per article remained stable between 2 and 3 citations, with peaks in 2015 (2.46 citations per article) and 2019 (2.98 citations per article). The highest average citation per article was observed in 2015 at 24.63 citations, while the lowest was in 2023 at 0.54 citations, indicating a shorter duration of citable relevance.
3.2 Journals
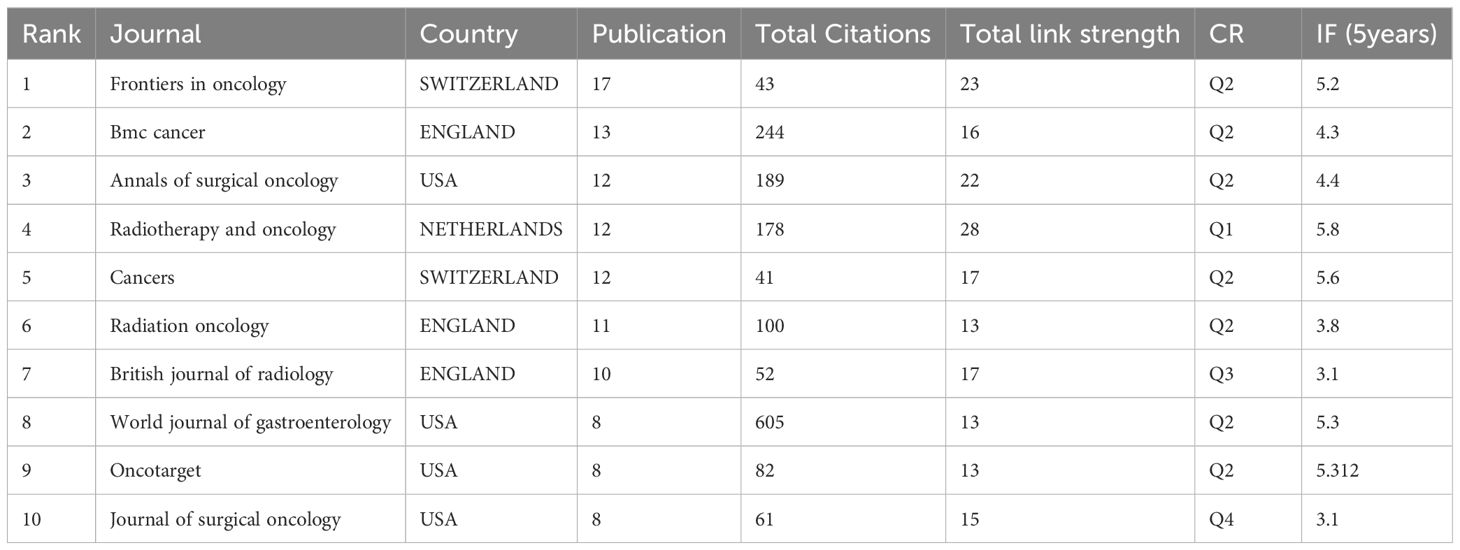
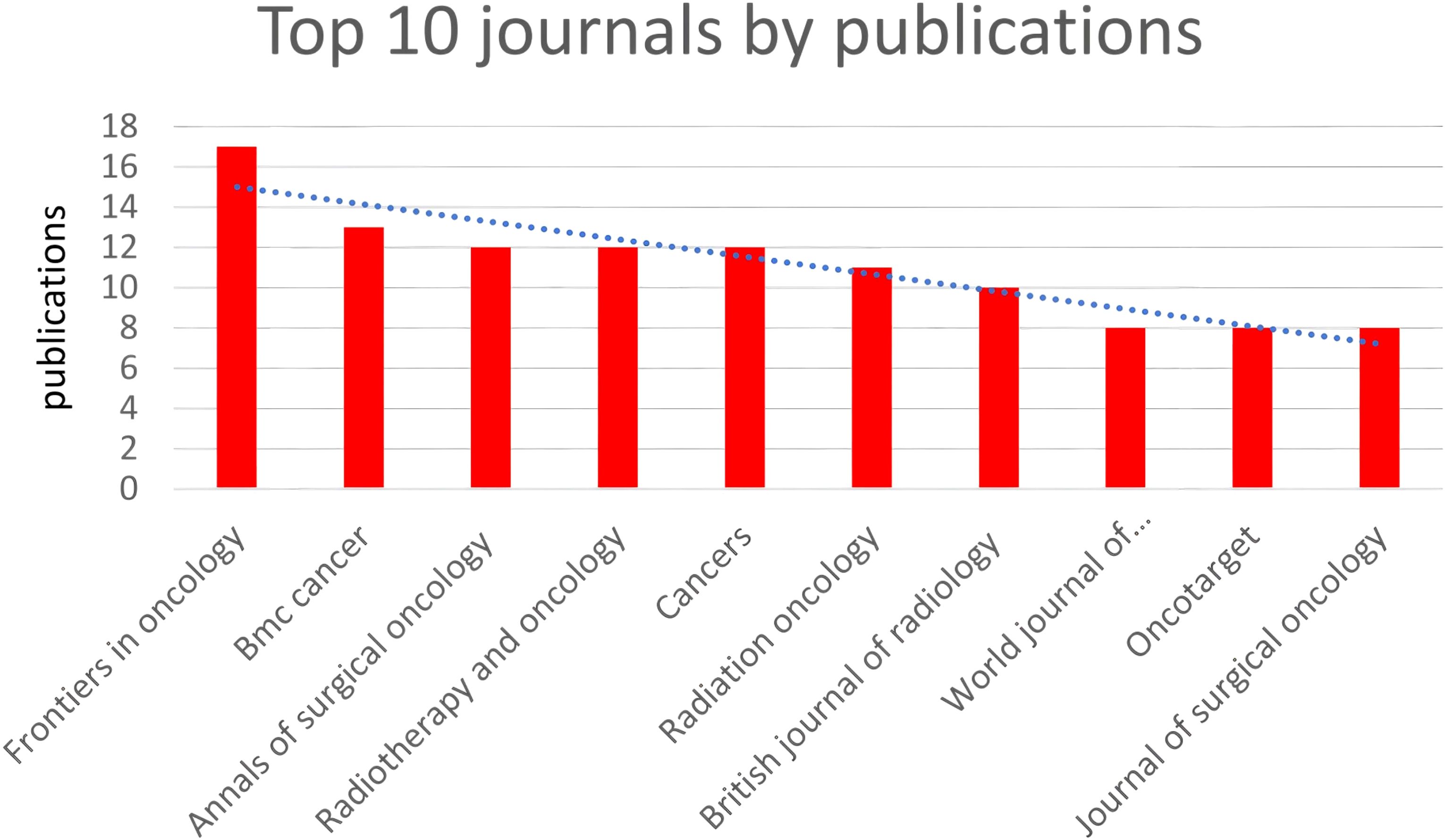
In total, 186 journals published one or more of these articles. Table 1 and Figure 3 summarize the top 10 journals by publication volume and their basic information. Impact Factor (IF), often used to measure a journal’s importance within its field, represents the 5-year average number of citations. 17 journals (core sources) published approximately one-third of the retrieved documents. The most relevant journal was “FRONTIERS IN ONCOLOGY,” which published 17 articles from 2014 to 2023, followed by “BMC CANCER” (n = 13), “ANNALS OF SURGICAL ONCOLOGY” (n = 12), “CANCERS” (n = 12), and “RADIOTHERAPY AND ONCOLOGY” (n = 12). These journals predominantly fall within Q2, with impact factors around 5. Similar to most research fields, articles in the field of gastric cancer radiotherapy are mostly exploratory, with few achieving significant breakthroughs or providing high-level evidence in evidence-based medicine. Overall, the quality of articles in the field of gastric cancer radiotherapy remains good, with most articles located in the Q2 JCR zone.
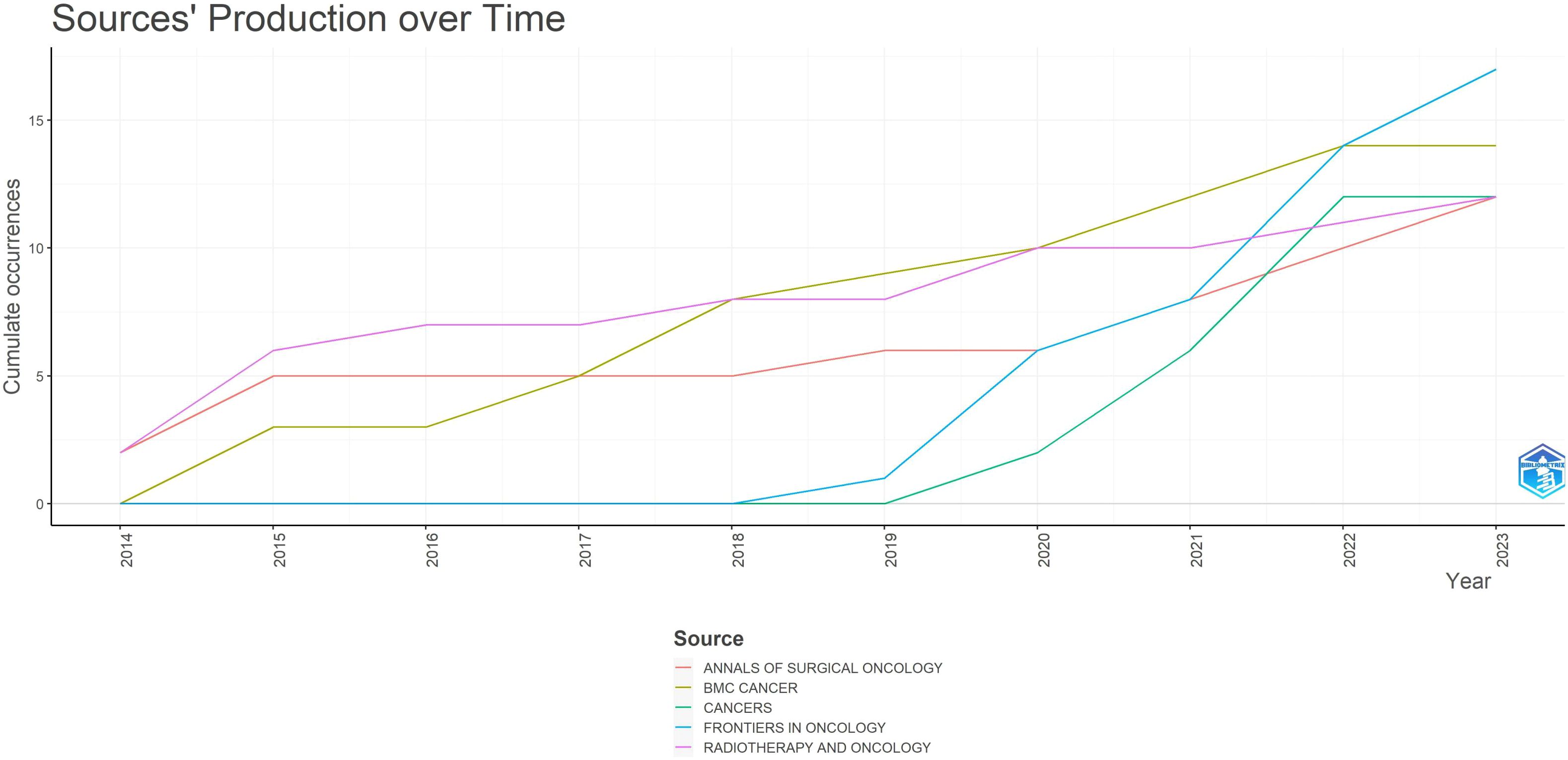
Figure 4 illustrates the dynamics of the top 5 relevant journals. Among the top 5 journals, “FRONTIERS IN ONCOLOGY” and “CANCER” saw a rapid increase in published articles starting from 2018 and 2019 respectively, both originating from Switzerland. In contrast, the publication rate of the other three journals remained relatively stable. This highlights how “FRONTIERS IN ONCOLOGY” and “CANCER” have gathered excellent articles discussing gastric cancer radiotherapy in recent years.
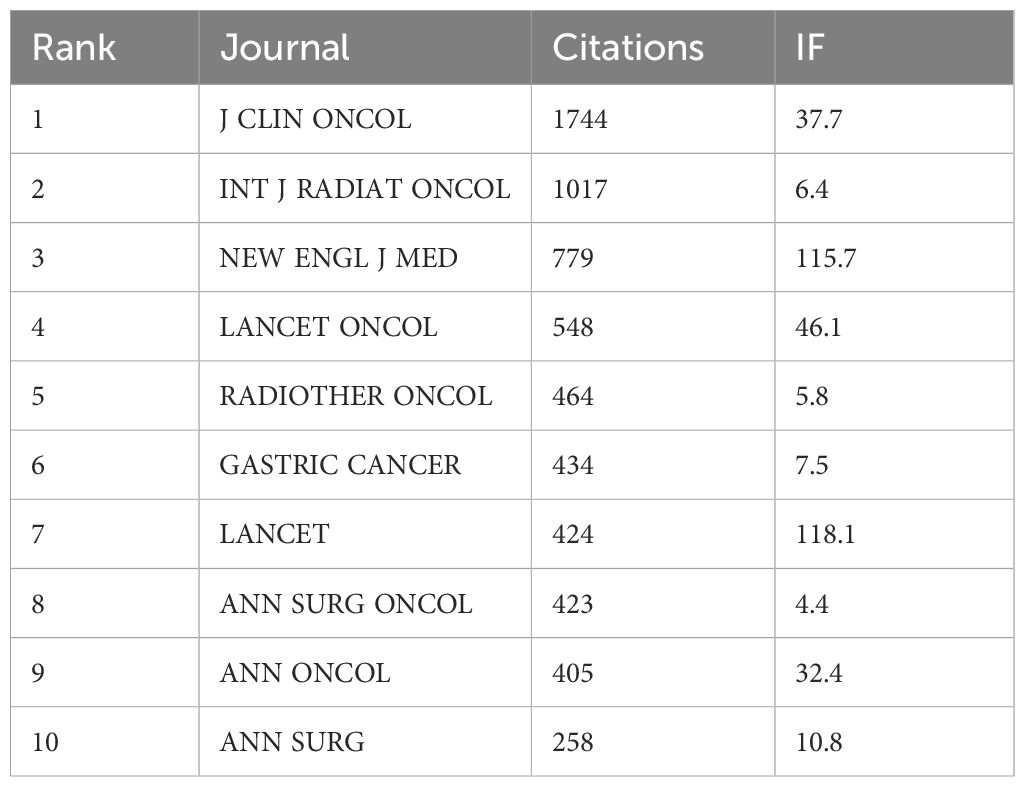
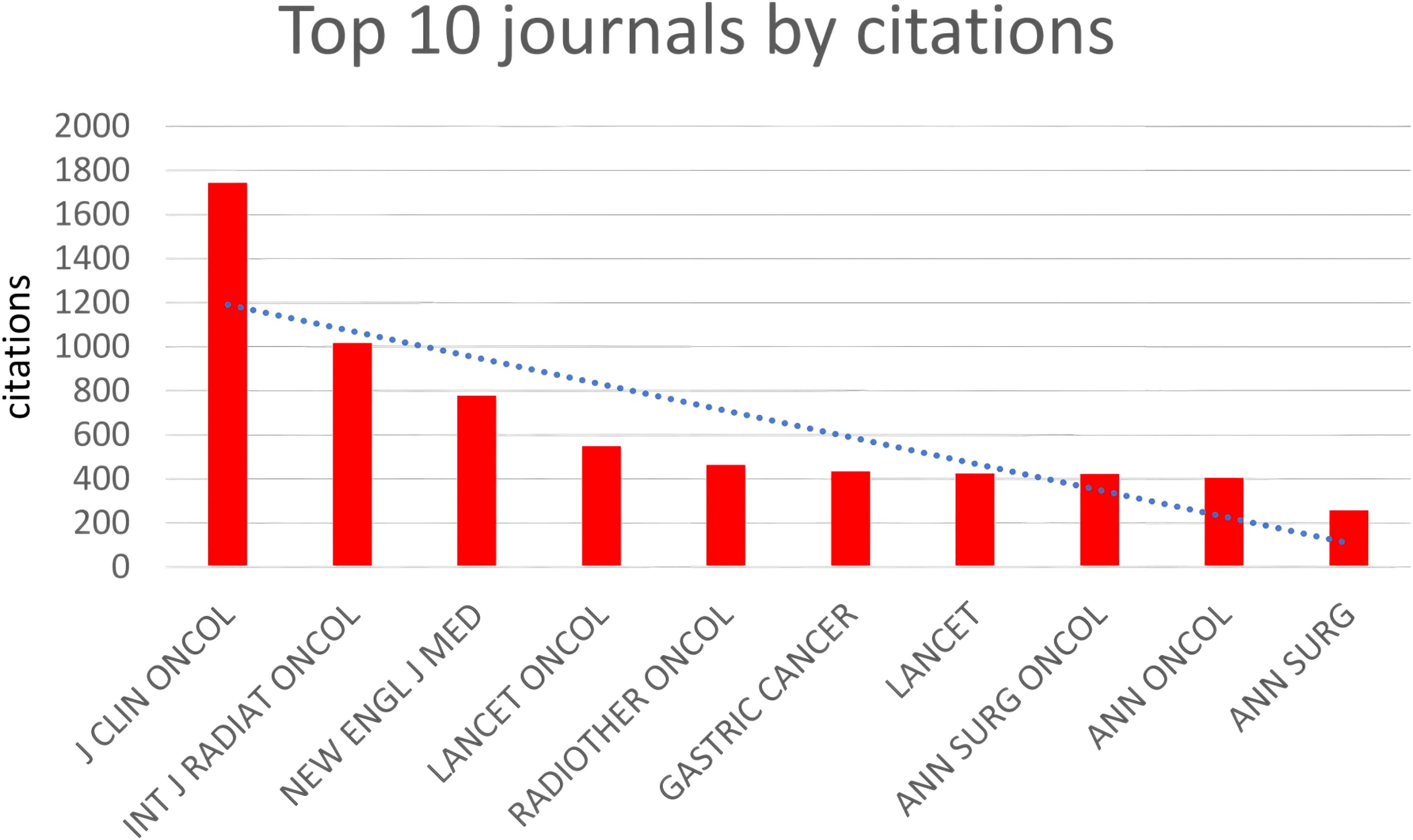
(Table 2, Figure 5) The most cited journals among all 484 articles (from the reference list) were “J CLIN ONCOL” with 1744 citations, followed by “INT J RADIAT ONCOL” with 1017 citations, “NEW ENGL J MED” with 779 citations, “LANCET ONCOL” with 548 citations, and “RADIOTHER ONCOL” with 464 citations. These journals are globally recognized top-tier publications that collect the highest quality articles on gastric cancer radiotherapy to date, many of which are considered landmark articles worthy of citation and reference by researchers worldwide. “J CLIN ONCOL” focuses on clinical oncology, while “INT J RADIAT ONCOL” specializes in radiation oncology, ranking first and second respectively among cited journals, underscoring their profound influence on the application of radiotherapy in gastric cancer.
3.3 Authors, affiliations, countries
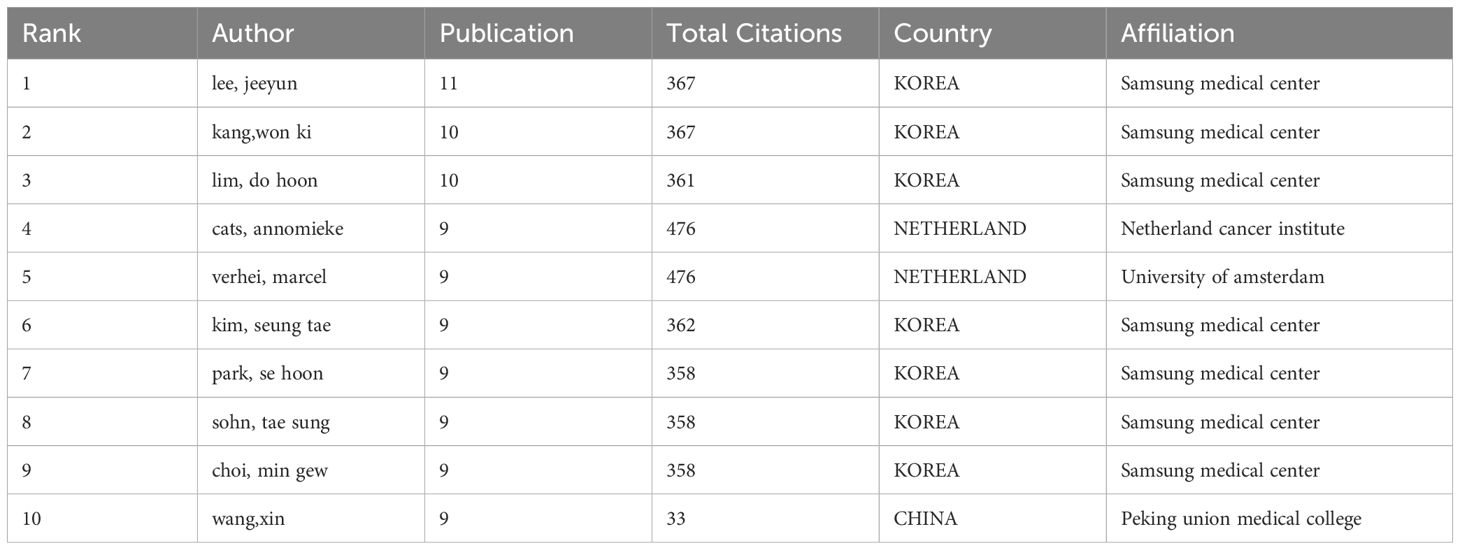
The most prolific authors in terms of publications are Lee Jeeyun, Kang Won Ki, and Lim Do Hoon, with 11, 10, and 10 articles respectively. Among the top 10 authors by publication volume, they hail from South Korea, the Netherlands, and China. South Korea is the most productive in this field, primarily represented by Samsung Medical Center (Table 3).
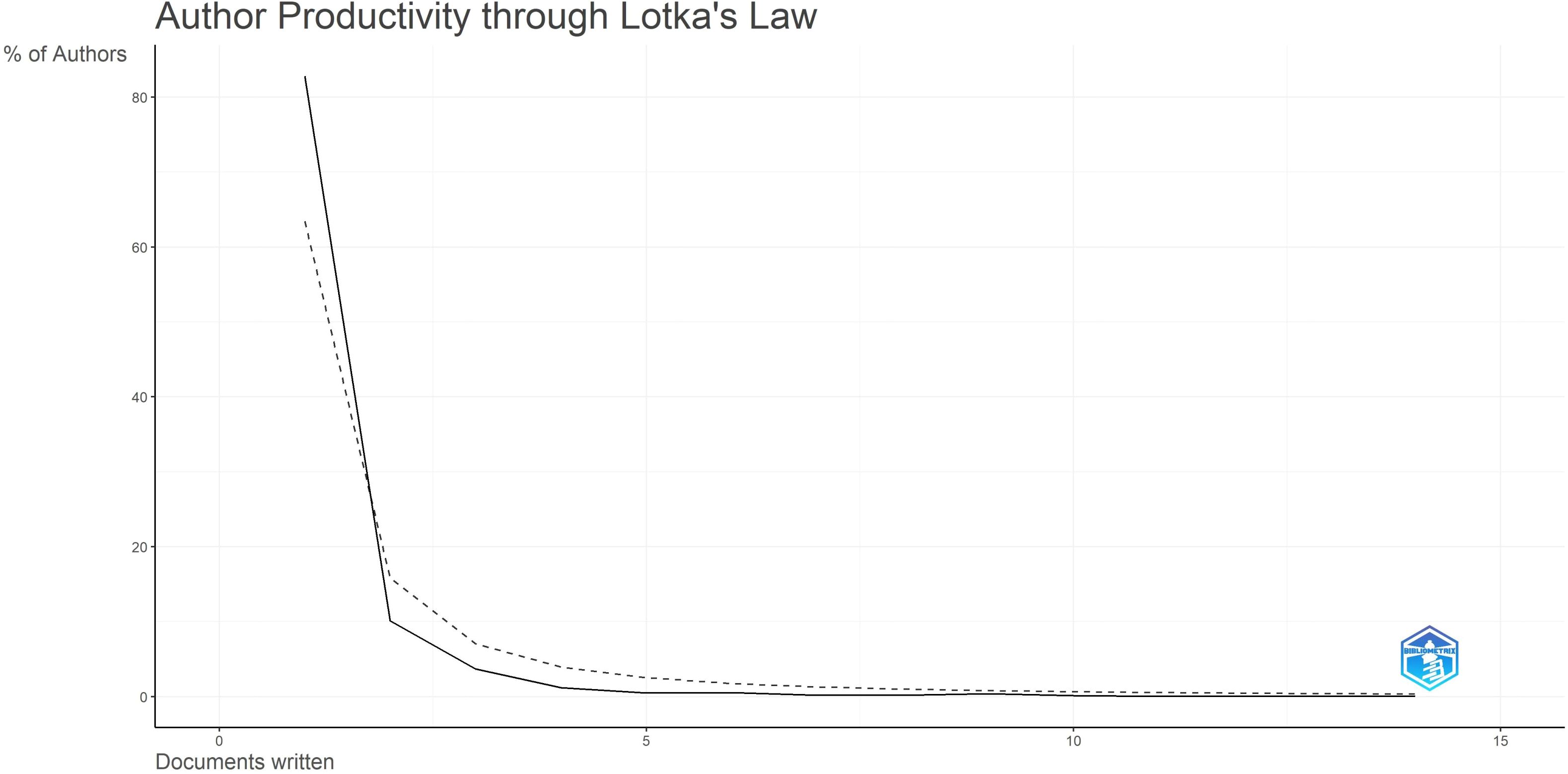
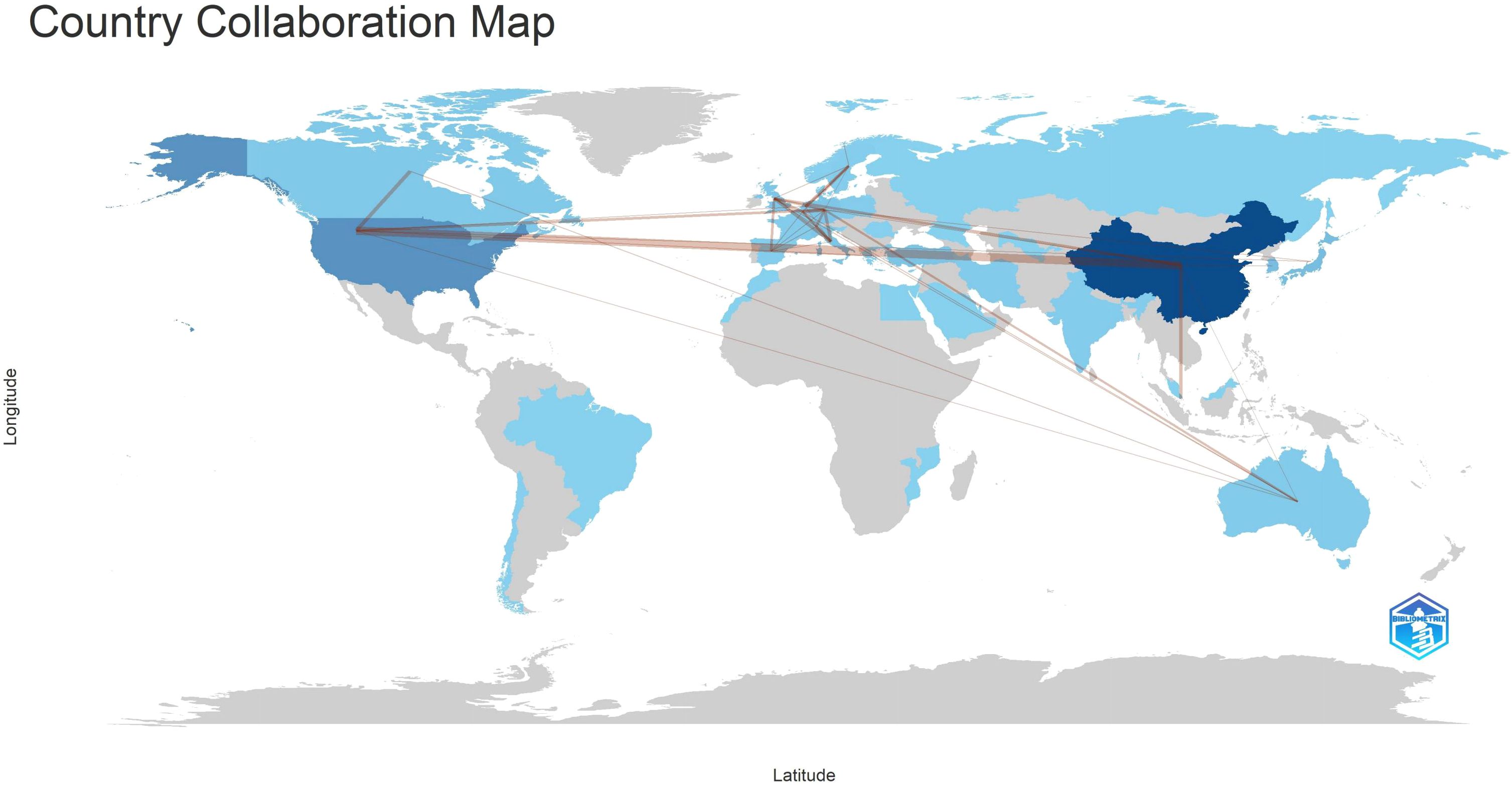
According to Lotka’s Law (7, 8)(Figure 6), the frequency distribution of scientific productivity identified 114 “core” authors who contributed to at least 3 articles (2.48%) and 2435 “occasional” authors who published only one paper (82.8%). Table 4 calculates the number of articles published by country based on the corresponding authors, with China topping the list. SCP (Single Country Publications) denotes articles where all authors are from the same country; MCP (Multiple Country Publications) indicates articles with authors from multiple countries, reflecting international collaboration. MCP Ratio = MCP/Articles denotes the proportion of collaborative articles in a country’s total publications. A higher value indicates more frequent international collaboration. According to MCP ratios, Turkey and major East Asian countries (China, Japan, South Korea) show relatively low rates of international collaboration, whereas North America (USA, Canada) and Europe (Netherlands, Germany, Italy) demonstrate higher rates. The national collaboration network based on publications shows the collaboration patterns between countries, with line thickness indicating the degree of collaboration closeness. Close collaborations are observed between the USA and Canada, USA and China, China and Singapore, and among European countries.
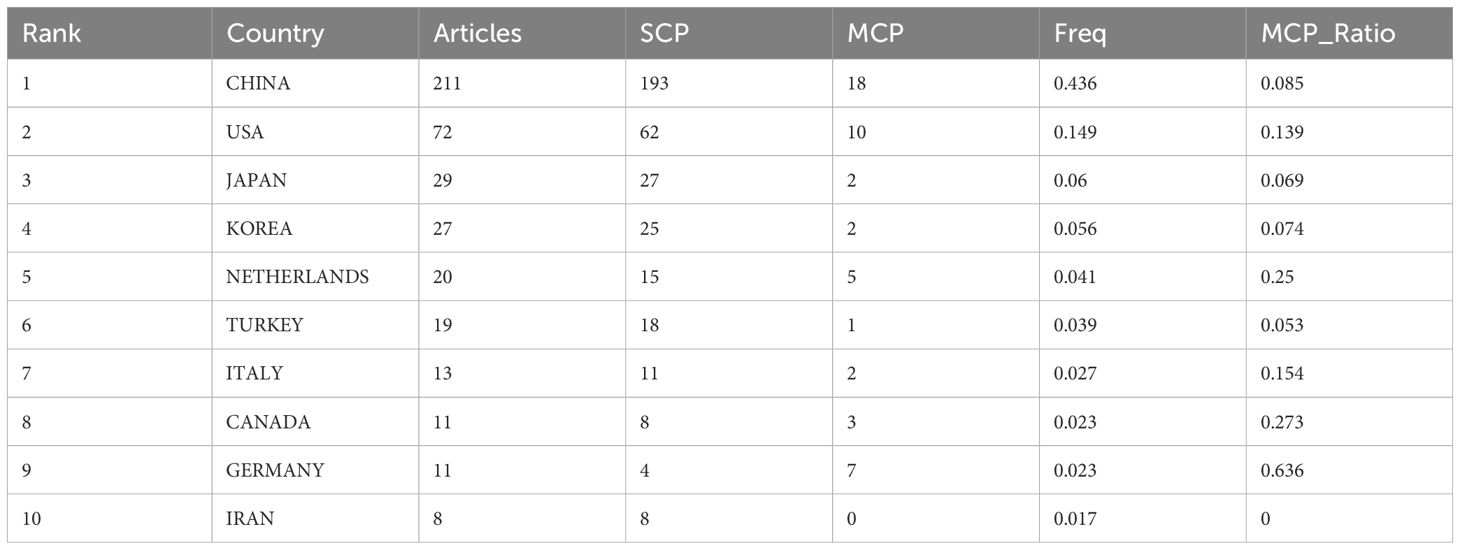
Table 4. Summarizes the top 10 countries with the highest volume of publications (Calculated by corresponding author).
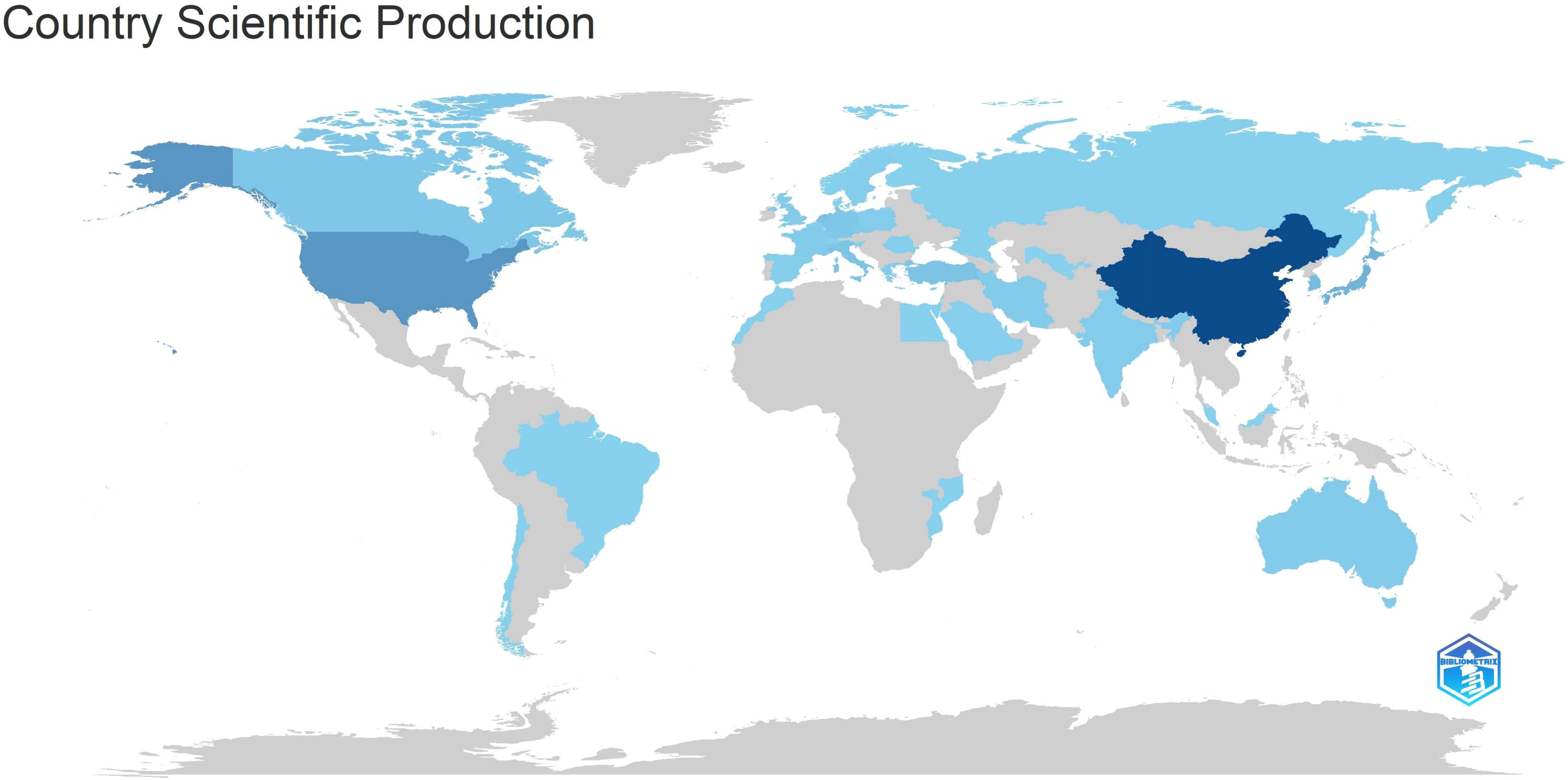
Table 5 and Figure 7 illustrate the production outputs by specific countries, with China leading with a total of 712 articles. In Figure 7, darker colors indicate that the country has a higher publication volume. Significant affiliated institutions include China (Fudan University, Peking Union Medical College, Chinese Academy of Medical Sciences - Peking Union Medical College), South Korea (Samsung Medical Center, Sungkyunkwan University (SKKU)), followed by the USA (University of Texas System, UTMD Anderson Cancer Center, Harvard University), and the Netherlands (Netherlands Cancer Institute) (Table 6).
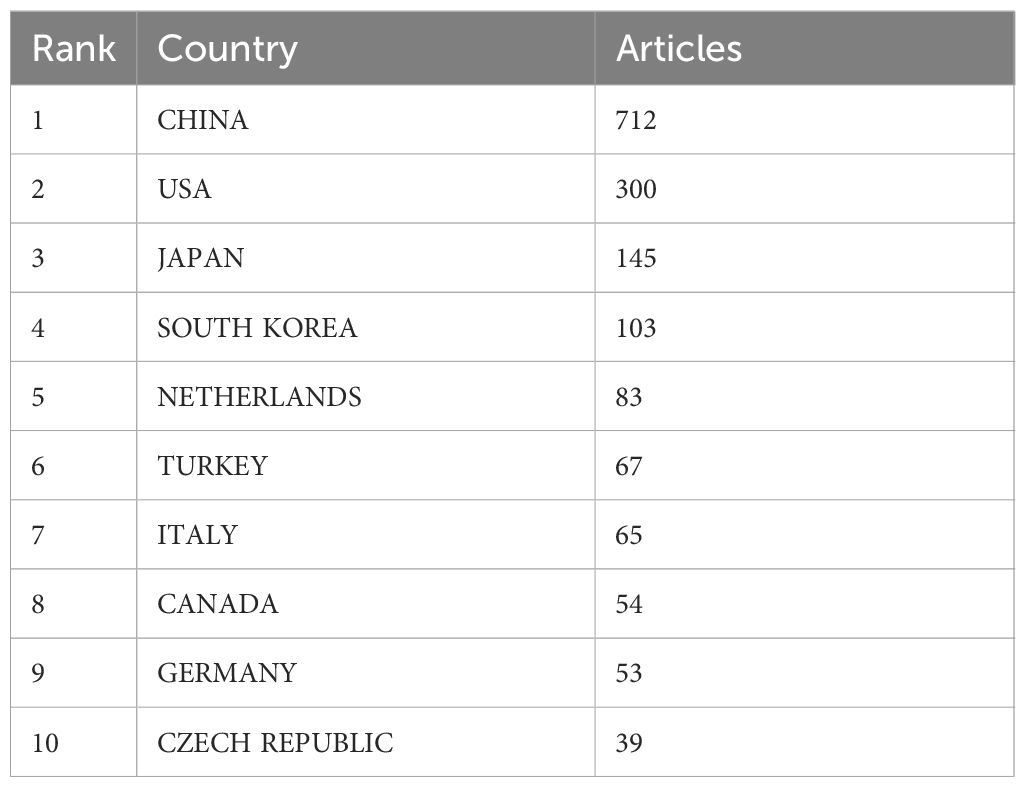
Table 5. Summarizes the top 10 countries with the highest volume of publications (Calculated by all authors).
3.4 Articles
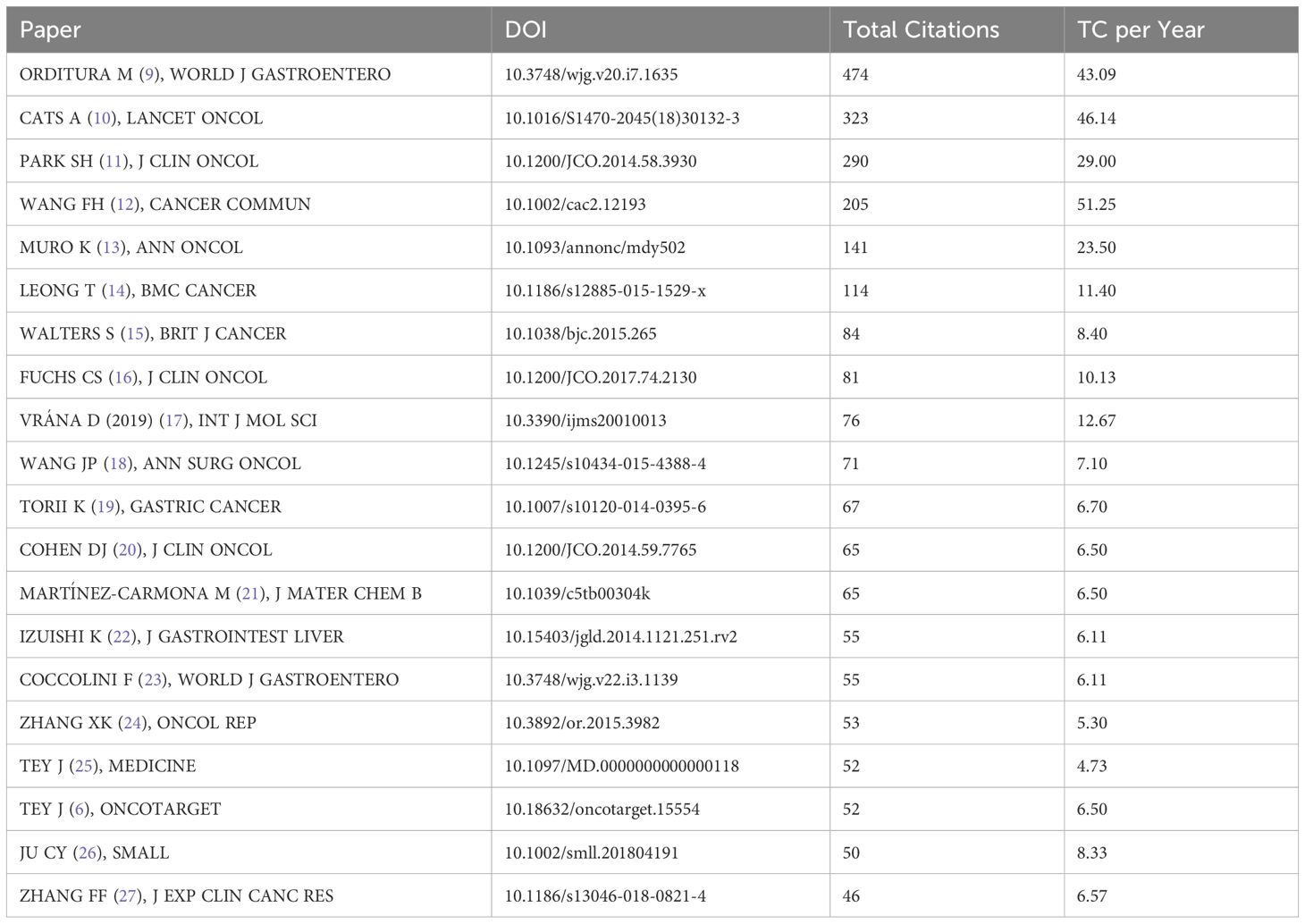
The Table 7 lists the top 20 most cited articles in the field of gastric cancer (6, 9–27). Among these twenty papers, 13 are original articles, with 4 being clinical trials; 6 are systematic reviews/commentary articles, and 1 is a clinical practice guideline/consensus. The top-ranked article is “Treatment of gastric cancer,” cited globally 474 times. Most of these articles (n = 16) were published between 2014 and 2018, with limited recent publications (2019-2023), possibly due to a shorter citation window.
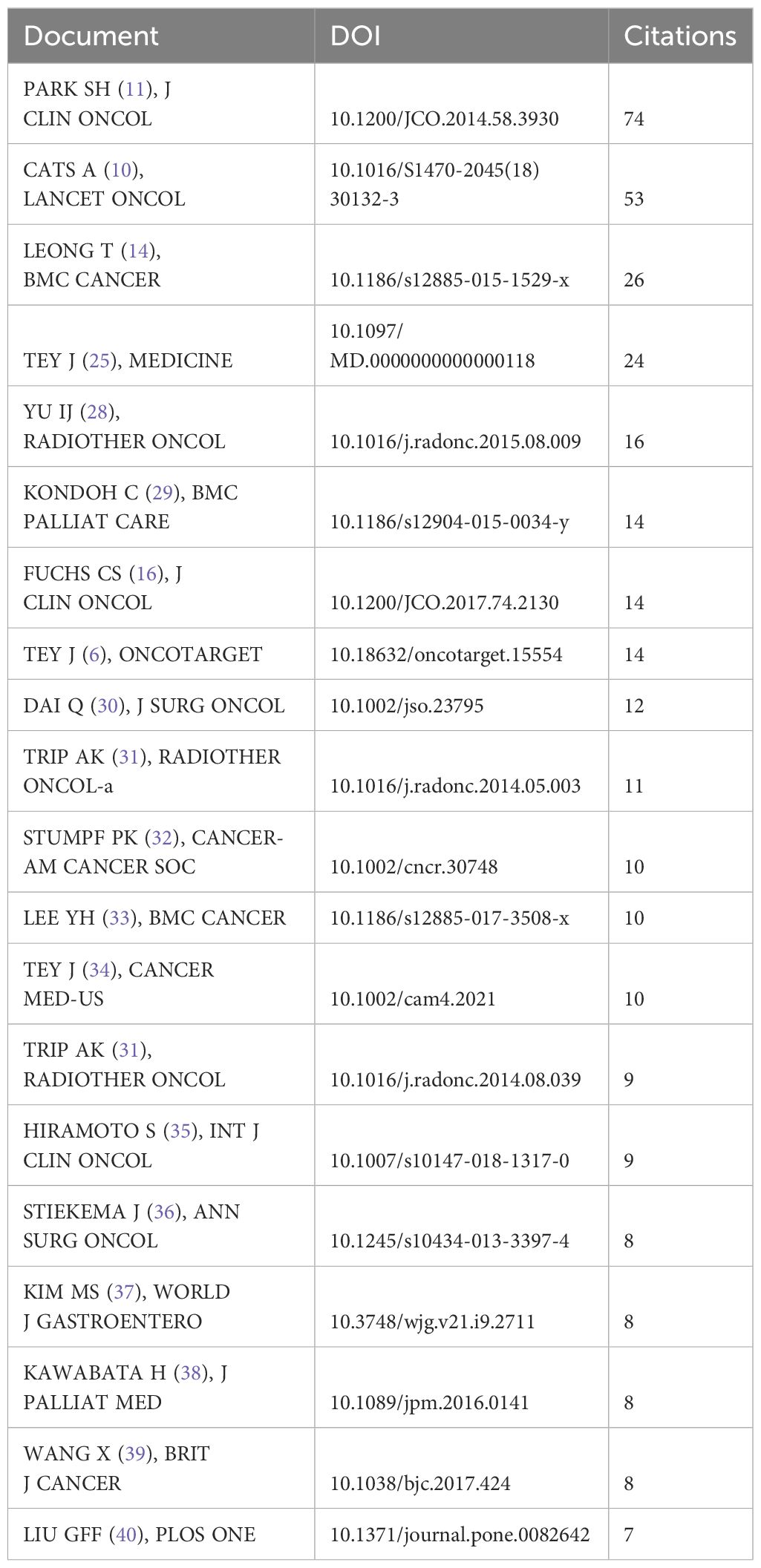
The Table 8 also lists the top 20 most cited articles in the field of gastric cancer radiotherapy (6, 10, 11, 14, 16, 25, 28–41).These articles better reflect the research advancements in radiotherapy for gastric cancer treatment.
3.5 Keywords
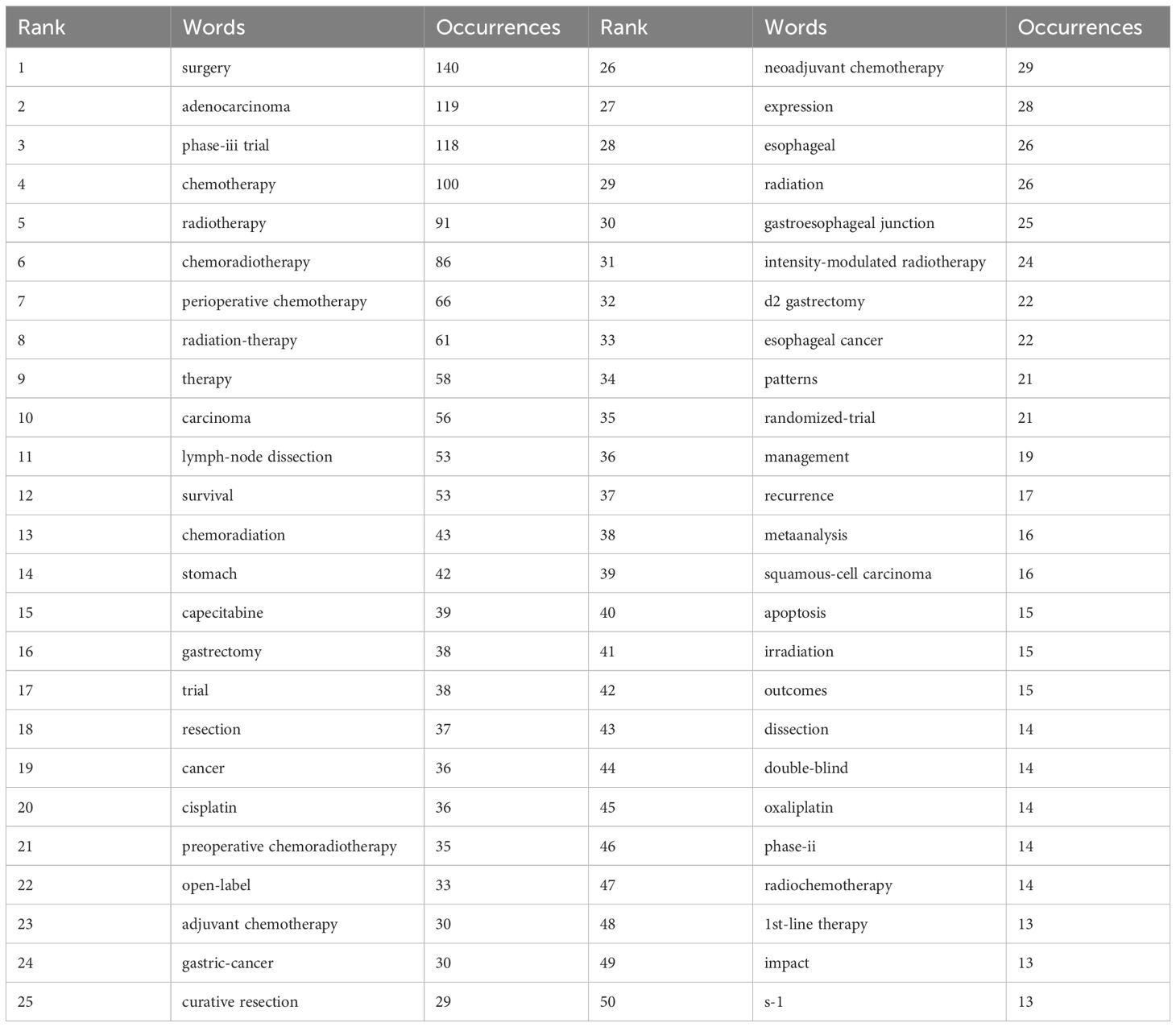
Keywords are crystallizations of the content of an article, possessing high generality and reflective power within a particular research field, directly pointing to the essence of the text. Therefore, commonly used high-frequency keywords can present the focal issues of a research field, macroscopically reflecting the research hotspots within a certain period. To depict the current research status and hotspots in the field of gastric cancer radiotherapy, frequencies of keywords and the emergence of hot topics in different time periods were analyzed using R Studio (Table 9, Figure 8).
After searching for the terms “gastric cancer” and “radiotherapy,” analysis of the top 50 high-frequency keywords reveals that “surgery,” “adenocarcinoma,” “phase-III trial,” and “chemotherapy” are the most frequently appearing terms, with 140, 119, 118, and 100 occurrences respectively. This indicates that adenocarcinoma is the predominant pathological type of gastric cancer, with surgery and chemotherapy currently being the mainstream treatments. Radiotherapy continues to play a supportive role in gastric cancer treatment, as complete pathological remission is challenging to achieve solely with radiotherapy. Phase-III clinical trials remain the most evidence-based approach among all articles. The top 25 keywords mainly focus on gastric cancer treatments involving radiotherapy, chemotherapy, and surgery. Specific hotspots related to gastric cancer radiotherapy are reflected in keywords ranked 26-50, such as “neoadjuvant radiochemotherapy,” “gastroesophageal,” and “intensity-modulated radiotherapy.”
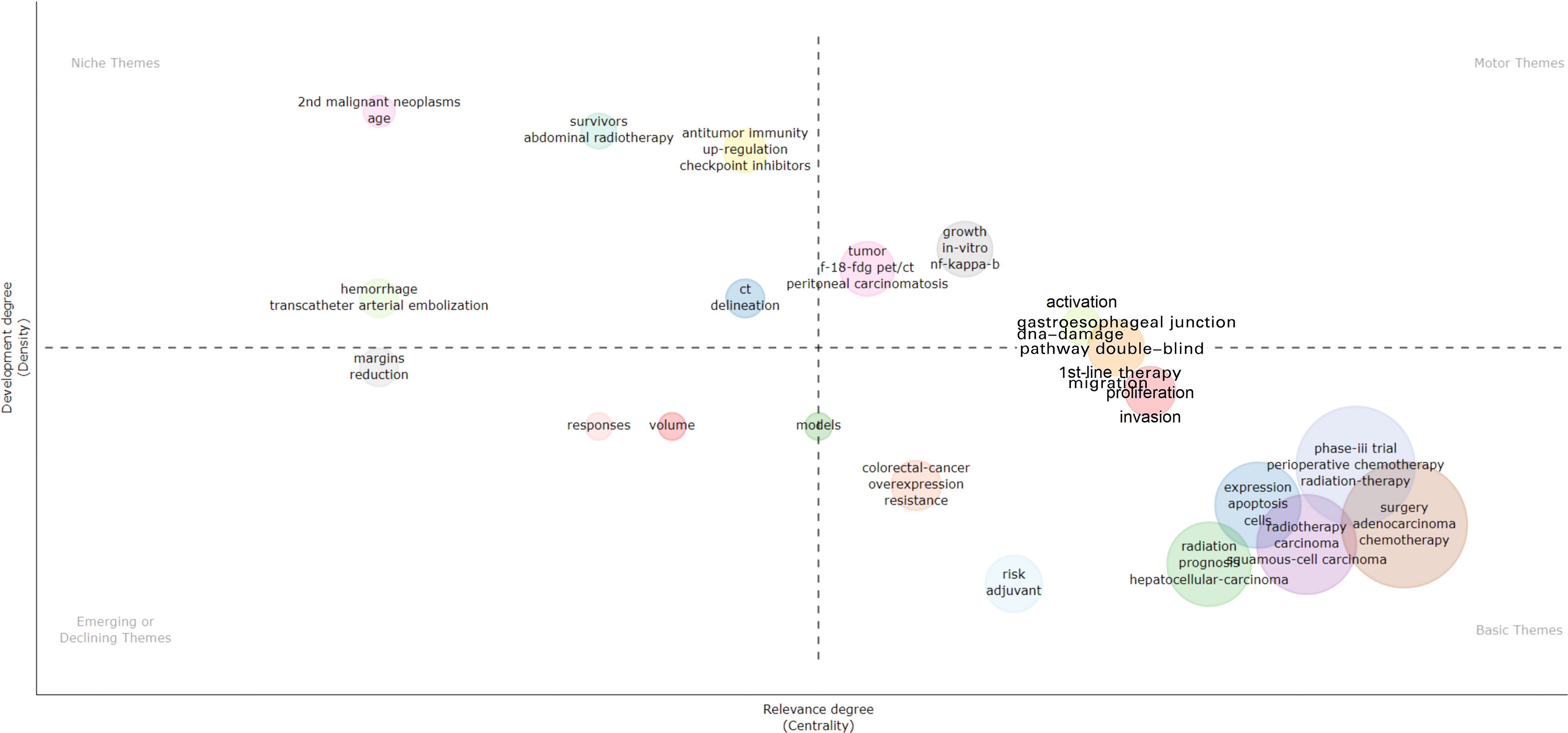
The main themes and trends are depicted in the Figure 9. In the thematic map, the x-axis represents centrality, which indicates the importance of the field, while the y-axis represents density, which indicates the potential for development. Based on this, four quadrants can be drawn:•First quadrant (top-right corner): motor-themes, which are both important and well-developed. Second quadrant (top-left corner): very specialized/niche themes, which are well-developed but not important for the current field. Third quadrant (bottom-left corner): emerging or disappearing themes, which are marginal and underdeveloped, possibly newly emerging or about to disappear. Fourth quadrant (bottom-right corner): basic themes, which are important for the field but have not yet been well-developed. These generally refer to fundamental concepts.
The thematic map indicates emerging topics such as radiotherapy dose and treatment response, while clinical trials (survival, phase III trials) and treatment strategies (surgery, chemotherapy, radiotherapy, perioperative treatment) represent fundamental and cross-sectional themes. Checkpoint inhibitors and bleeding are isolated topics.
3.6 Collaboration
The national collaboration network (Figure 10) shows country-based collaborations based on publications. The thickness of lines represents the proximity of collaboration. Close collaborations are observed between the United States and Canada, United States and China, China and Singapore, as well as Germany and Australia. There is a clear tendency for collaboration among European countries.
4 Discussion
In our bibliometric analysis of scientific literature on gastric cancer radiotherapy from 2014 to 2023, we observed an average annual growth rate of approximately -2.05%, indicating challenges in radiotherapy for gastric cancer treatment. The anatomical characteristics of the stomach, partially obscured by the liver and its frequent peristalsis, all limit the efficacy of radiotherapy. Additionally, since gastric cancer is predominantly adenocarcinoma, its sensitivity to radiotherapy is lower compared to squamous cell carcinoma. So, there were generally fewer articles discussing gastric cancer radiotherapy. With the results of several large-scale clinical trials on gastric cancer radiotherapy (such as the CRITICS and the ARTIST) over the past decade yielding less-than-satisfactory outcomes, the research interest in gastric cancer radiotherapy has declined.
4.1 Countries and institutions
Analyzing national and institutional distributions allows us to identify major contributors in this field. China leads in publication quantity, benefiting from East Asia’s highest gastric cancer incidence and its large population of patients. Moreover, China’s numerous doctors and several top institutions involved in gastric cancer radiotherapy research contribute significantly to its high productivity. However, the institution with the most publications is Samsung Medical Center in South Korea, highlighting Korean institutions’ deep exploration in this medical domain. Two of the top five highly cited articles in gastric cancer radiotherapy originate from South Korea, demonstrating its significant influence in this field. Despite China’s relatively late start in this field compared to South Korea, its development pace has been remarkable, with both quantity and quality of publications steadily increasing in recent years. These achievements stem from comprehensive advancements in Chinese healthcare, although their impact still requires further enhancement.
The geographical distribution of scientific publications spans continents, reflecting gastric cancer’s high incidence globally. Primary scientific production centers are in Asia (China, South Korea), North America (United States, Canada), and Europe (Netherlands, Germany, Italy). South Korea and China exhibit high quantitative scientific output, showcasing concentrated rather than dispersed scientific capabilities. North American and European countries maintain high scientific productivity, often engaging in regional scientific collaborations. The geographic distribution of scientific outputs highlights top academic institutions in gastric cancer radiotherapy across different regions. One limitation of the current analysis is that collaboration is measured simplistically using shared authors, which may not fully capture active scientific networks or the scientific value of published works.
4.2 Journals
By analyzing journal sources, researchers can efficiently identify suitable outlets for their papers. Journals like “Frontiers in Oncology” and “Cancer” have shown the highest output in recent years, indicating significant influence in gastric cancer radiotherapy and suitability for submission. The impact factors of these journals partly reflect the importance and priority of radiotherapy. Such high-quality journals underscore the significance of radiotherapy in gastric cancer as a vital research direction.
4.3 Authors
Authors frequently cited are considered more influential than less cited ones. In terms of contributions and citations, the most influential authors in this field are Jeeyun Lee from South Korea and Annomieke Cats from the Netherlands, both leading large-scale Phase III clinical trials in adjuvant radiotherapy and chemoradiotherapy for gastric cancer. Their teams represent excellent potential collaborators for researchers in gastric cancer radiotherapy studies.
4.4 Keywords
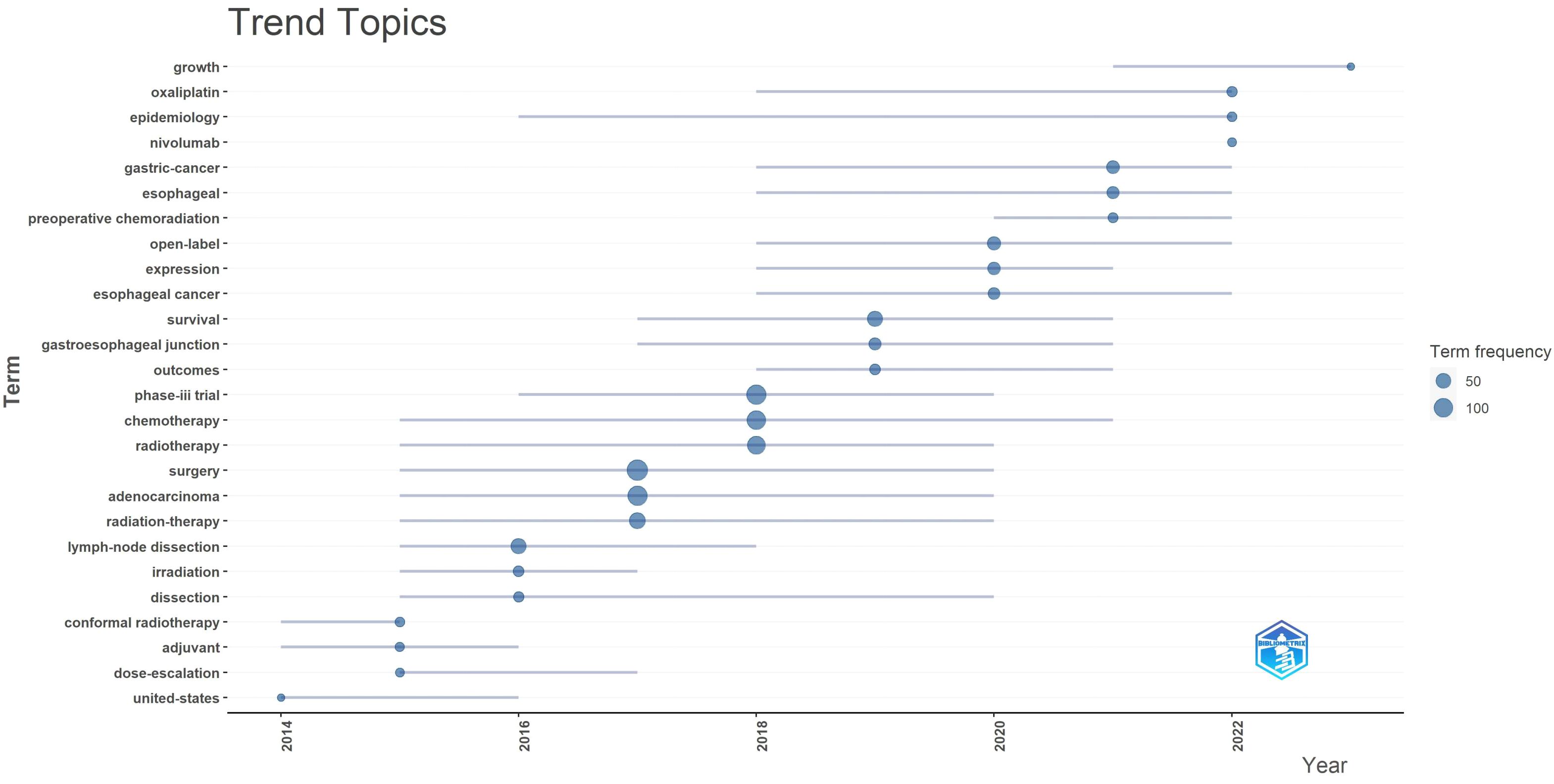
High-frequency keywords are pivotal in presenting hot topics within a research field, reflecting its evolving themes over different periods. From 2014 to 2016, gastric cancer radiotherapy primarily focused on “adjuvant therapy,” transitioning to “neoadjuvant chemoradiotherapy” from 2020 to 2022. Initially, studies emphasized radiotherapy forms (e.g., conformal radiotherapy) and dosages. Subsequently, due to the fixed location and high sensitivity of esophagogastric junction cancer, extensive research occurred from 2017 to 2019. In 2022, the advent of “nivolumab” marked the era of immunotherapy, resulting in a surge of articles on this topic. These developments illustrate discussions on treatment modalities, radiation patterns, treatment sites, and emerging combined therapies in gastric cancer radiotherapy.
4.5 Analysis of f highly cited articles
4.5.1 Postoperative adjuvant therapies
Since 2014, research on radiotherapy for gastric cancer has evolved, starting with postoperative adjuvant therapies, prominently represented by the Korean ARTIST study (11). This large-scale prospective, randomized, multicenter phase III clinical trial led by Se Hoon Park et al. explored whether adding radiotherapy to adjuvant chemotherapy could enhance disease-free survival (DFS) for D2-resected gastric cancer (GC) patients. The results indicated significant DFS improvement in lymph node-positive and intestinal-type GC patients with combined radiochemotherapy. Similar trends were observed for DFS and overall survival (OS) stratified by disease stage. Subsequently, the ARTIST 2 trial was initiated to evaluate adjuvant chemotherapy and radiotherapy specifically for lymph node-positive, D2-resected GC patients.
The CRITICS study (10), An international, multicenter, open-label, randomized controlled, phase III clinical trial led by the Netherlands in 2018, employed a research design similar to that of the ARTIST study. It found that compared to postoperative chemotherapy alone, postoperative radiochemotherapy did not improve overall survival for resectable gastric cancer patients who had undergone adequate preoperative chemotherapy and surgery. Given poor patient compliance in both treatment groups, future research should focus on optimizing preoperative treatment strategies. Postoperative adjuvant radiotherapy for gastric cancer had once again encountered challenges. Some scholars argued that the role of radiotherapy was not fully realized in this study. The controversy surrounding the delineation of the radiation target area, along with a treatment completion rate of less than 50% among enrolled patients, may have undermined the survival benefits of postoperative radiochemotherapy. This study is an open-label trial, meaning both the patients and the researchers were aware of the treatment regimen administered. This design may introduce potential biases, representing another limitation of the study, which could affect the accuracy and generalizability of the results.
Subsequently, the results of the ARTIST 2 study (42) published in 2020 indicated that for patients with stage II and III gastric cancer with positive lymph nodes, surgery plus SOX or SOXRT prolonged disease-free survival (DFS) compared to surgery plus S1. However, there was no significant reduction in local recurrence when comparing postoperative SOXRT with the postoperative SOX regimen, with both exhibiting similar DFS outcomes. This suggests that for stage II and III gastric cancer following D2 radical surgery, the SOX regimen or other combinations of fluoropyrimidine and oxaliplatin may serve as standard treatment. Postoperative chemoradiotherapy has still not been included as a standard treatment.
4.5.2 Neoadjuvant therapies
Due to suboptimal outcomes with postoperative radiotherapy, investigations have shifted towards preoperative radiochemotherapy models, exemplified by studies such as RTOG 9904 (43), CROSS (44), POET (45), and TOP_GEAR (14), aiming to provide high-level evidence for preoperative radiotherapy in gastric cancer treatment. RTOG 9904, an early-phase study, included 49 localized gastric cancer patients who received induction chemotherapy followed by synchronous fluoropyrimidine-based radiotherapy (45 Gy in 25 fractions). It reported pathologic complete response (pCR) and R0 resection rates of 26% and 77%, respectively. The CROSS study compared surgery alone with neoadjuvant radiochemotherapy plus surgery in 366 patients with esophageal or esophagogastric junction tumors. Results showed improved survival outcomes in the radiochemotherapy group (48.6 months vs. 24.0 months, P=0.003), with a higher R0 resection rate (92% vs. 69%). Long-term results from this study consistently suggested that radiochemotherapy provides better survival benefits. However, the study’s limitation lies in significant heterogeneity among enrolled patients, including various histological types such as adenocarcinoma, squamous cell carcinoma, and unspecified types, influencing treatment response and prognosis.
The POET study included 119 locally advanced esophagogastric junction tumor patients, comparing the efficacy of preoperative chemotherapy versus preoperative radiochemotherapy. Results showed significantly higher pCR rates (15.6% vs. 2.0%, P=0.03) and tumor-free lymph node rates (64.4% vs. 36.7%, P=0.001) in the radiochemotherapy group. Long-term outcomes demonstrated superior 3-year and 5-year survival rates in the radiochemotherapy group. Current research primarily focuses on esophagogastric junction tumors regarding preoperative radiotherapy, which notably enhances pCR and R0 resection rates. However, whether the increased pCR in initially resectable patients translates into survival benefits requires further confirmation.
4.5.3 Palliative therapies
Palliative radiotherapy for gastric cancer remains a research hotspot. Jeremy Tey et al. (6, 25, 34) highlighted the efficacy and good tolerability of 3D conformal external beam radiotherapy for local palliative treatment, which can extend patient survival. Short-term (39 Gy BED) radiotherapy plans are effective in symptom relief for these patients.
Overall, advancements in radiotherapy for locally advanced gastric cancer benefit potentially resectable, unresectable, and isolated distant metastatic patients alike. With the advent of immunotherapy, combined radiotherapy and immunotherapy may usher in new breakthroughs, as radiotherapy could enhance tumor cell sensitivity to immunotherapy. Some studies have reported promising pCR rates as high as 38.2% (46), indicating significant potential for immunotherapy-radiotherapy combinations.
5 Conclusion
The evolving objectives of research in radiotherapy reflect the advancements in global gastric cancer oncology over time. Current management of gastric cancer is based on multidisciplinary integration of treatments, including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. Radiotherapy offers benefits to gastric cancer patients with potentially resectable, unresectable, or isolated distant metastases. The combination of immunotherapy with radiotherapy holds significant potential and may represent a new breakthrough in treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
H-KH: Supervision, Validation, Visualization, Writing – review & editing. Z-HW: Data curation, Writing – original draft. J-RL: Supervision, Writing – review & editing. B-BC: Formal Analysis, Investigation, Writing – original draft. M-MX: Methodology, Project administration, Writing – review & editing. J-PY: Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63.
2. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. (2012) 379:315–21.
3. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. (2001) 345:725–30.
4. Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. (2009) 27:851–6.
5. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. (2014) 32:385–91.
6. Tey J, Soon YY, Koh WY, Leong CN, Choo BA, Ho F, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget. (2017) 8:25797–805.
7. Kawamura M, Thomas CD, Kawaguchi Y, and Sasahara H. Lotka’s law and the pattern of scientific productivity in the dental science literature. Med Inf Internet Med. (1999) 24:309–15.
8. Kawamura M, Thomas CD, Tsurumoto A, Sasahara H, and Kawaguchi Y. Lotka’s law and productivity index of authors in a scientific journal. J Sci. (2000) 42:75–8.
9. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. (2014) 20:1635–49.
10. Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. (2018) 19:616–28.
11. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. (2015) 33:3130–6.
12. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cance. Cancer Commun (London England). (2021) 41:747–95.
13. Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. (2019) 30:19–33.
14. Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC cancer. (2015) 15:532.
15. Walters S, Benitez-Majano S, Muller P, Coleman MP, Allemani C, Butler J, et al. Is England closing the international gap in cancer survival? Br J Cancer. (2015) 113:848–60.
16. Fuchs CS, Niedzwiecki D, Mamon HJ, Tepper JE, Ye X, Swanson RS, et al. Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (Alliance). J Clin Oncol. (2017) 35:3671–7.
17. Vrána D, Matzenauer M, Neoral Č, Aujeský R, Vrba R, Melichar B, et al. From tumor immunology to immunotherapy in gastric and esophageal cancer. Int J Mol Sci. (2018) 20(1):13. doi: 10.3390/ijms20010013
18. Wang J, Sun Y, and Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. (2015) 22:2965–71.
19. Torii K, Yamada S, Nakamura K, Tanaka H, Kajiyama H, Tanahashi K, et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric Cancer. (2015) 18:635–43.
20. Cohen DJ and Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. (2015) 33:1754–9.
21. Martínez-Carmona M, Baeza A, Rodriguez-Milla MA, García-Castro J, and Vallet-Regí M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J Mater Chem B. (2015) 3:5746–52.
22. Izuishi K and Mori H. Recent strategies for treating stage IV gastric cancer: roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointest Liver dis. (2016) 25:87–94.
23. Coccolini F, Montori G, Ceresoli M, Cima S, Valli MC, Nita GE, et al. Advanced gastric cancer: What we know and what we still have to learn. World J Gastroenterol. (2016) 22:1139–59.
24. Zhang X, Zheng L, Sun Y, Wang T, and Wang B. Tangeretin enhances radiosensitivity and inhibits the radiation-induced epithelial-mesenchymal transition of gastric cancer cells. Oncol Rep. (2015) 34:302–10.
25. Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine. (2014) 93:e118.
26. Ju C, Wen Y, Zhang L, Wang Q, Xue L, Shen J, et al. Neoadjuvant chemotherapy based on abraxane/human neutrophils cytopharmaceuticals with radiotherapy for gastric cancer. Small (Weinheim an der Bergstrasse Germany). (2019) 15:e1804191.
27. Zhang F, Li K, Pan M, Li W, Wu J, Li M, et al. miR-589 promotes gastric cancer aggressiveness by a LIFR-PI3K/AKT-c-Jun regulatory feedback loop. J Exp Clin Cancer Res. (2018) 37:152.
28. Yu JI, Lim DH, Ahn YC, Lee J, Kang WK, Park SH, et al. Effects of adjuvant radiotherapy on completely resected gastric cancer: A radiation oncologist’s view of the ARTIST randomized phase III trial. Radiother Oncol. (2015) 117:171–7.
29. Kondoh C, Shitara K, Nomura M, Takahari D, Ura T, Tachibana H, et al. Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: a retrospective cohort study. BMC Palliative Care. (2015) 14:37.
30. Dai Q, Jiang L, Lin RJ, Wei KK, Gan LL, Deng CH, et al. Adjuvant chemoradiotherapy versus chemotherapy for gastric cancer: a meta-analysis of randomized controlled trials. J Surg Oncol. (2015) 111:277–84.
31. Trip AK, Poppema BJ, van Berge Henegouwen MI, Siemerink E, Beukema JC, Verheij M, et al. Preoperative chemoradiotherapy in locally advanced gastric cancer, a phase I/II feasibility and efficacy study. Radiotherapy Oncol. (2014) 112:284–8.
32. Stumpf PK, Amini A, Jones BL, Koshy M, Sher DJ, Lieu CH, et al. Adjuvant radiotherapy improves overall survival in patients with resected gastric adenocarcinoma: A National Cancer Data Base analysis. Cancer. (2017) 123:3402–9.
33. Lee YH, Lee JW, and Jang HS. Palliative external beam radiotherapy for the treatment of tumor bleeding in inoperable advanced gastric cancer. BMC cancer. (2017) 17:541.
34. Tey J, Zheng H, Soon YY, Leong CN, Koh WY, Lim K, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med. (2019) 8:1447–58.
35. Hiramoto S, Kikuchi A, Tetsuso H, Yoshioka A, Kohigashi Y, and Maeda I. Efficacy of palliative radiotherapy and chemo-radiotherapy for unresectable gastric cancer demonstrating bleeding and obstruction. Int J Clin Oncol. (2018) 23:1090–4.
36. Stiekema J, Trip AK, Jansen EP, Boot H, Cats A, Ponz OB, et al. The prognostic significance of an R1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol. (2014) 21:1107–14.
37. Kim MS, Lim JS, Hyung WJ, Lee YC, Rha SY, Keum KC, et al. Neoadjuvant chemoradiotherapy followed by D2 gastrectomy in locally advanced gastric cancer. World J Gastroenterol. (2015) 21:2711–8.
38. Kawabata H, Uno K, Yasuda K, and Yamashita M. Experience of low-dose, short-course palliative radiotherapy for bleeding from unresectable gastric cancer. J Palliative Med. (2017) 20:177–80.
39. Wang X, Zhao DB, Yang L, Chi Y, Tang Y, Li N, et al. S-1 chemotherapy and intensity-modulated radiotherapy after D1/D2 lymph node dissection in patients with node-positive gastric cancer: a phase I/II study. Br J Cancer. (2018) 118:338–43.
40. Liu GF, Bair RJ, Bair E, Liauw SL, and Koshy M. Clinical outcomes for gastric cancer following adjuvant chemoradiation utilizing intensity modulated versus three-dimensional conformal radiotherapy. PLoS One. (2014) 9:e82642.
41. Trip AK, Nijkamp J, van Tinteren H, Cats A, Boot H, Jansen EP, et al. IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiotherapy Oncol. (2014) 112:289–94.
42. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. (2021) 32:368–74.
43. Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. (2006) 24:3953–8.
44. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84.
45. Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer (Oxford England: 1990). (2017) 81:183–90.
Keywords: gastric cancer, radiotherapy, bibliometrics, R studio, WoSCC
Citation: Weng Z-H, Chen B-B, Lin J-R, Xu M-M, Yuan J-P and Hu H-K (2025) Gastric cancer radiation therapy: a bibliometric analysis of the scientific literature. Front. Oncol. 15:1513255. doi: 10.3389/fonc.2025.1513255
Received: 18 October 2024; Accepted: 24 April 2025;
Published: 16 May 2025.
Edited by:
Stefano Forte, Mediterranean Institute of Oncology (IOM), ItalyReviewed by:
Zehua Zhang, School of Medicine, Tongji University, ChinaJie Shen, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2025 Weng, Chen, Lin, Xu, Yuan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-Kai Hu, MTM2NzYxMTUyMzZAMTYzLmNvbQ==
†These authors share first authorship
‡These authors contributed equally to this work
 Zhen-Hong Weng
Zhen-Hong Weng Bin-Bin Chen†
Bin-Bin Chen† Hao-Kai Hu
Hao-Kai Hu