- 1Department of Medical Oncology, Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran
- 2Department of Medical Education, Education Development Center, Kerman University of Medical Sciences, Kerman, Iran
- 3Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran
- 4Institute of Neuropharmacology, Kerman Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran
- 5Department of Radiation Oncology, Kerman University of Medical Sciences, Kerman, Iran
- 6Department of Biostatistics and Epidemiology, Kerman University of Medical Sciences, Kerman, Iran
- 7Modeling in Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
- 8Department of Pathology, Pathology and Stem Cell Research Center, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
- 9Department of Medical Genetics, Kerman University of Medical Sciences, Kerman, Iran
Background: Apelin, a peptide implicated in various physiological processes, has been shown to be involved in cancer development and progression. However, its role in breast cancer remains unclear. This study aimed to investigate serum apelin levels in breast cancer patients and explore potential associations with clinicopathological features.
Methods: This study involved 137 histopathologically-confirmed female breast cancer patients and 71 healthy controls. Serum apelin levels were measured using enzyme-linked immunosorbent assay (ELISA) and patients’ clinicopathological data was collected retrospectively. Serum apelin levels were compared between the patients and control groups. Moreover, Youden’s J index and the receiver operating characteristic (ROC) curve analysis were utilized to select the optimal cut-off point to differentiate patients and healthy controls. A generalized linear model (GLM) was used to investigate the association of each histopathological variable with serum apelin levels.
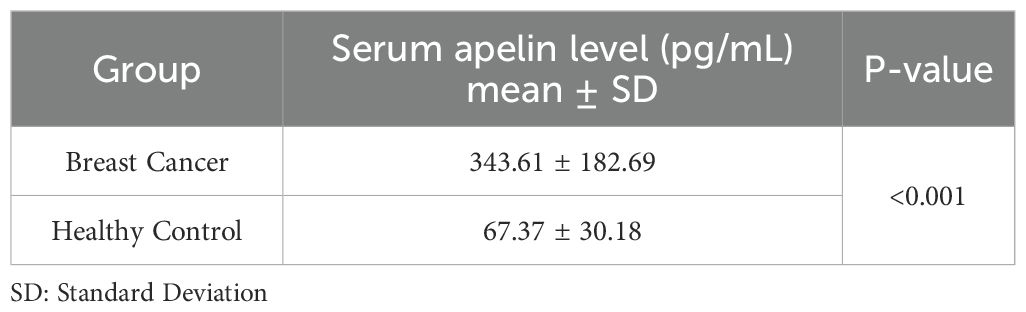
Results: Serum apelin levels were significantly higher in breast cancer patients (343.61 ± 182.69 pg/mL) compared to healthy controls (67.37 ± 30.18 pg/mL, p<0.001). ROC curve analysis revealed excellent discriminative ability of serum apelin in distinguishing breast cancer patients from healthy controls (AUC = 0.94, 95% CI: 0.91-0.98). The optimal cut-off value for serum apelin was determined to be 122.48 pg/mL, yielding 89% sensitivity and 97% specificity. However, GLM analysis found no statistically significant associations between serum apelin levels and clinicopathological features, including age, tumor size, histologic grade, lymphovascular invasion, lymph node involvement, microcalcification, in situ components, necrosis, Ki67 expression, molecular subtypes, and clinical stage.
Conclusions: Our findings suggest a potential role for serum apelin in breast cancer pathogenesis or progression. The high discriminative ability of serum apelin indicates its promise as a biomarker for breast cancer detection. However, the lack of association between serum apelin levels and specific clinicopathological features suggests a limited prognostic value and a complex role in breast cancer biology that warrants further investigation.
1 Introduction
In 2020, for the first time, female breast cancer emerged as the most prevalent diagnosed malignancy worldwide with an estimated 2.26 million new cases reported (1). Projections for the year 2040 indicate an escalation in the global burden of breast cancer, with an expected annual increase to over 3 million new cases (2). On the other hand, the latest global statistics reveal that breast cancer stood as the predominant cause of cancer-related mortality among women, ranking fifth in overall cancer deaths (3). Based on all this alarming evidence, breast cancer continues to constitute a significant global health concern, notwithstanding the rapid advancements within this domain.
The shortage of sensitive markers for early diagnosis and monitoring of breast cancer progression presents a notable challenge in the provision of effective treatment, resulting in suboptimal outcomes and decreased survival. Identifying objective and trustworthy biomarkers to improve cancer screening and treatment is essential, which is vital for advancing personalized and precision medicine. Initiatives focused on uncovering novel proteins and various molecules implicated in the development and progression of breast cancer have demonstrated promising findings (4–6).
One of these biomolecules, with an approved effect on breast cancer pathology, is apelin, which has received attention in the scientific community since 1998 (7, 8). This protein, found at elevated levels in the serum of patients diagnosed with breast cancer (6), is an endogenous ligand for the G-protein coupled receptor known as Apelin receptor (APJ) and was initially extracted from bovine stomach tissue (9). Subsequent research has revealed apelin expression in heart muscles, brain, kidneys, liver, lungs, spleen, mammary glands, placenta, and gastric mucosa (10). This small peptide plays a crucial role in numerous essential physiological functions, including angiogenesis, cell migration, cellular permeability, energy metabolism, neuroendocrine regulation, fluid homeostasis, and glucose metabolism (11, 12). It is particularly recognized for its impact on the cardiovascular system, exhibiting both inotropic effects and functioning as a vasopressor and vasodilator (13).
Recent investigations have demonstrated that the APJ is significantly overexpressed in tumor tissues, especially in those that have undergone metastasis (14–16). On the other side, increased levels of apelin expression have been observed in several cancer forms, particularly in breast cancer, where such elevations correlate with diminished survival durations and a higher incidence of cancer recurrence (17–19). Actually, apelin induces the maturation of tumor blood capillaries and prompts tumor vascularization (20). Additionally, it exhibits lymphangiogenic properties that influence tumor progression and lymph node metastasis, thereby mediating cancerous cells’ survival, proliferation, invasion, and metastasis (8, 11, 13, 21). It exerts the mentioned effects by stimulating the proliferation of lymphatic endothelial cells through the engagement of the ERK, STAT3, and PI3K signaling pathways (14, 21).
Research into tissue apelin levels has been comprehensive, revealing some discrepancies in findings while suggesting a link to clinicopathological traits. Since tumor tissue samples are often difficult to obtain, serum samples provide a less invasive method that may prove more effective for measuring apelin concentrations in various situations, including advanced cancers (17, 18, 22–24). Also, for clinical use, serum apelin holds the potential for enhancing cancer screening and providing personalized care for patients. In contrast, measures of tissue apelin are often only applicable after identifying the tumor (6). Thus, it is crucial to determine the optimal cutoff points of serum apelin concentration that indicate the presence of disease and its related processes. This can help identify breast cancer patients earlier, improving health outcomes and overall survival.
On the other side, breast cancer is a complex and heterogeneous disease, and its prognosis may vary depending on the combined characteristics of the tumor and clinicopathologic features (25). Hence, investigating the correlation between apelin and clinicopathologic characteristics is imperative in evaluating whether these relations possess sufficient robustness to anticipate outcomes such as the probability of lymphatic system involvement as a primary sign of metastasis and to figure out the specific associations that represent shorter survival or worse prognoses.
The aims of the present study included investigating the correlations of apelin in breast cancer patients with different clinicopathological features. These features comprise age, tumor size, histologic grade, lymphovascular invasion, lymph node involvement, microcalcification, in situ components, necrosis, Ki67 expression, and molecular subtypes. The study also aimed to determine the optimal cut-off value for serum apelin that indicates the presence of the disease.
2 Methods
2.1 Study design and participants
This study comprised female breast cancer patients who were histopathologically confirmed as invasive ductal carcinoma by core needle biopsy with immunohistochemistry staining. The participants were referred to the outpatient clinic of Kerman University of Medical Sciences, Kerman, Iran, between March 2021 and June 2023. We excluded individuals with a past medical history of other malignancies and any other inflammatory and severe metabolic diseases. We also recruited healthy individuals (in an approximately 1:2 ratio relative to patients) as controls from clinic attendants for routine check-ups. These individuals were not diagnosed with any prior malignancies and metabolic disease and were sex- and aged-matched as the breast cancer patients. All patients and control members provided written informed consent before proceeding with the study. This study was conducted under the approval of the Ethics Committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.AH.REC.1402.080).
2.2 Serum sampling
We took 5cc blood samples from the peripheral venous vessels of breast cancer patients and control-group participants, which were then kept in tubes containing clot activators allowed to clot at room temperature for 10–15 min before centrifugation. We prepared serum samples by centrifuging the clotted whole blood for 10 min at 2000 RPM. The serum was then transferred by sampler to sterile cryotubes and stored in a -60°C refrigerator until analysis.
2.3 Enzyme-linked immunosorbent assay
Apelin levels in human serum were measured using enzyme-linked immunosorbent assay (ELISA) methodology based on the biotin double-antibody sandwich technique. The assay followed the manufacturer’s instructions (Human Apelin (APLN) ELISA Kit, ZellBio GmbH, Ulm, Germany). The sensitivity of the apelin assay was 2.63 pg/mL. The inter and intra-assay coefficients of variation were <12% and <10%, respectively. To perform the assay, an antibody specific to apelin was pre-coated onto a 96-well plate. A competitive inhibition reaction was initiated between biotin-labeled and unlabeled apelin with the pre-coated antibody specific to apelin. The unbound conjugates were washed away, and Streptavidin-HRP conjugated to horseradish peroxidase was added to each microplate well and incubated. Then, the substrate solution was added, and the color changed to yellowish, appearing only in wells containing apelin by adding the acidic stop solution. The Optical Density (O.D) value was measured spectrophotometrically at 450 nm wavelength in a microplate reader Synergy™ Multi-purpose Microplate Reader (Nano Mabna; Iran) equipped. Apelin concentration was calculated based on the standard curve by Gen5 Software (BioTek; the USA).
2.4 Pathological assessments
Pathological data from breast cancer patients were collected retrospectively. The pathological assessment in our center involved breast mass sampling through core-needle biopsy (CNB) guided by ultrasound. A little incision (approximately ¼ inch) was occasionally made in the breast, allowing the insertion of a biopsy needle to extract tissue samples. In CNB, a thin needle was used (following local anesthesia) within the biopsy site, with ultrasound guidance ensuring the right spot. Afterward, tissue samples were fixed in 50% ethanol for 4–12 hours, then in 10% neutral buffered formalin for 6 hours. After formalin fixation, immunohistochemistry (IHC) was carried out using monoclonal mouse antibodies for estrogen receptor (ER, xbio, Framingham, Massachusett, USA), progesterone receptor (PR, xbio, Framingham, Massachusett, USA), and herceptin (xbio, Framingham, Massachusett, USA). Tissue sections were deparaffinized, rehydrated, and exposed to heat-induced epitope retrieval in citrate buffer (pH 6.0) using an electric pressure cooker at 12–15 PSI and 120°C for 3 minutes, followed by a 10-minute cooling period before immunostaining. All slides underwent hydrogen peroxide treatment for 5 minutes and incubation with primary antibodies for 30 minutes at room temperature, with TBS washes between incubations.
Herceptin staining involved immersion and incubation of slides in 10 mmol/L citrate buffer in a water bath at 95–99 °C for 40 minutes. Post-decanting, sections were washed and pre-soaked before staining, then treated with peroxidase blocker, incubated with anti-HER2 protein or negative control, stained with chromogen substrate (DAB), counterstained with hematoxylin, and coverslipped. Finally, the pathologist’s evaluation of the samples included scoring and grading based on ER, PR, and HER2 staining patterns and intensity.
In the process of Ki67 staining for immunohistochemistry, tissue samples were fixed in 10% neutral buffered formalin for 6 to 10 hours, followed by embedding in paraffin. After the deparaffinization and rehydration, antigen retrieval was performed using citrate buffer. The samples were stained with a monoclonal antibody against Ki-67 (clone MIB-1,xbio, Framingham, Massachusett, USA) and incubated accordingly. Finally, the samples were counterstained with hematoxylin and coverslipped for examination. A 14% cut-off value was used to categorize Ki-67 expression as low and high expression (26).
Tumor staging and other histopathological features were revised by the same expert pathologist using the 8th version of the Union for International Cancer Control (UICC) TNM classification of malignant tumors. Clinical staging was performed according to the classification by American College of Surgeons and American Cancer Society.
2.5 Statistical analysis
Statistical analysis was performed using SPSS version 22 (IBM, SPSS Inc., USA) and R 4.1.1 software. An independent sample t-test was employed to compare the serum apelin level between the patients and control group after checking and verifying the normality and homogeneity of variances. To select the optimal cut-off point with the highest sensitivity and specificity to differentiate patients and healthy controls, the Youden’s J index was employed through the “cutpointr” package in R software. Additionally, in order to evaluate the model’s performance, the receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated. To investigate the association of each histopathological variable with serum apelin levels, considering the structure of the study data, a generalized linear model (GLM) was utilized based on the Inverse Gaussian distribution for the response variable (serum apelin level). The significance level was set at 0.05 for all statistical tests.
3 Results
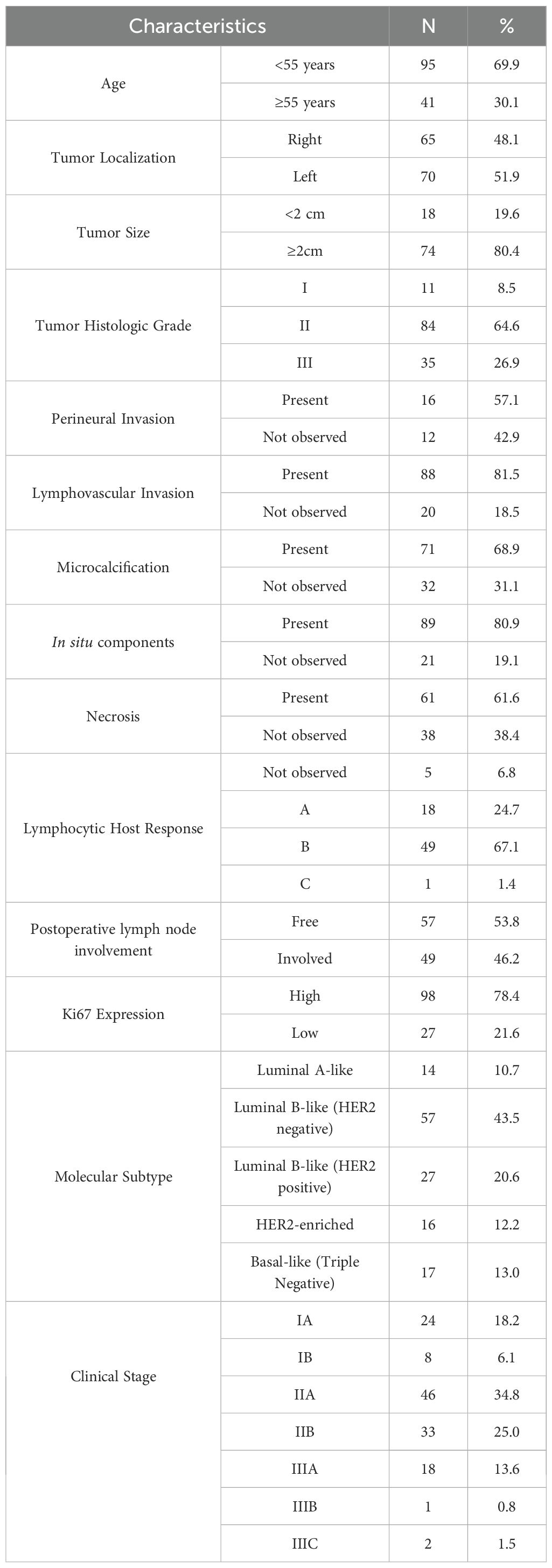
Data from a total of 137 breast cancer patients were investigated in this study. The mean (± SD) age of the patients was 49.48 (± 10.45) years. The majority of patients had a tumor histologic grade II (84.6%), followed by grades III (26.9%) and I (8.5%). The most prevalent molecular subtypes were Luminal B-like (HER2 negative), Luminal B-like (HER2 positive), and Basal-like (Triple Negative) in 43.5%, 20.6%, and 13.0% of cases, respectively. The majority of patients had a clinical stage of IIA (34.8%) and IIB (25.0%). High Ki67 expression was reported in 78.4% of the patients. The detailed clinicopathological characteristics of the patients are demonstrated in Table 1.
Our analysis revealed a significant difference in serum apelin levels between breast cancer patients and healthy controls. The mean (± SD) serum apelin level in breast cancer patients was 343.61 (± 182.69) pg/mL, which was significantly higher than that of healthy controls at 67.37 (± 30.18) pg/mL (p<0.001) (Table 2).
To evaluate the utility of serum apelin as a potential biomarker for breast cancer, we performed a ROC curve analysis (Figure 1). The area under the ROC curve (AUC) was 0.94 (95% CI: 0.91-0.98), indicating excellent discriminative ability between breast cancer patients and healthy individuals. Using Youden’s J index, we determined the optimal cut-off value of 122.48 pg/mL for serum apelin level to differentiate between breast cancer patients and healthy controls, which yielded a sensitivity of 0.89 (95% C.I: 0.84-0.94) and a specificity of (95% C.I: 0.94-1.00) (Table 3 and Figure 1).
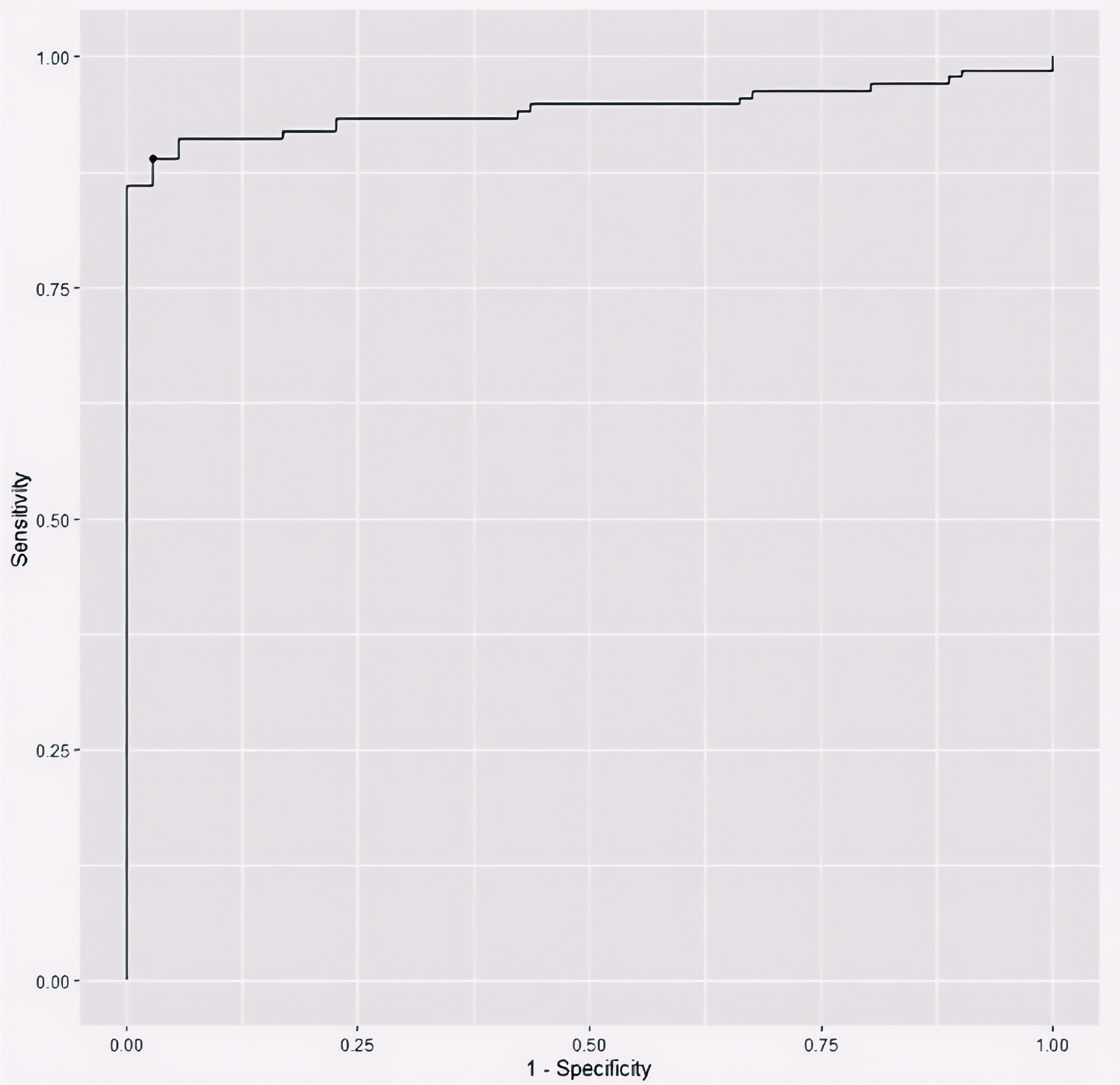
Figure 1. The receiver operating characteristic (ROC) curve plotting the discriminative utility of serum apelin level between breast cancer patients and healthy controls.
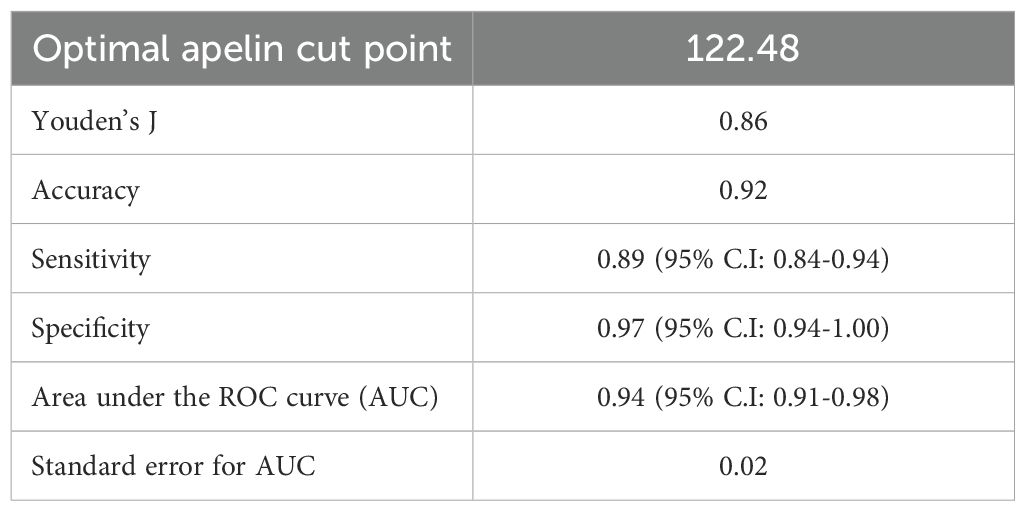
Table 3. Youden’s J index and ROC curve criteria for serum apelin level utility in differentiating between breast cancer patients and healthy controls.
The GLM analysis did not reveal any statistically significant associations between serum apelin levels and the clinicopathological variables examined, including age, tumor size, histologic grade, lymphovascular invasion, lymph node involvement, microcalcification, in situ components, necrosis, Ki67 expression, molecular subtypes, and clinical stage (all p-values > 0.05) (Table 4).
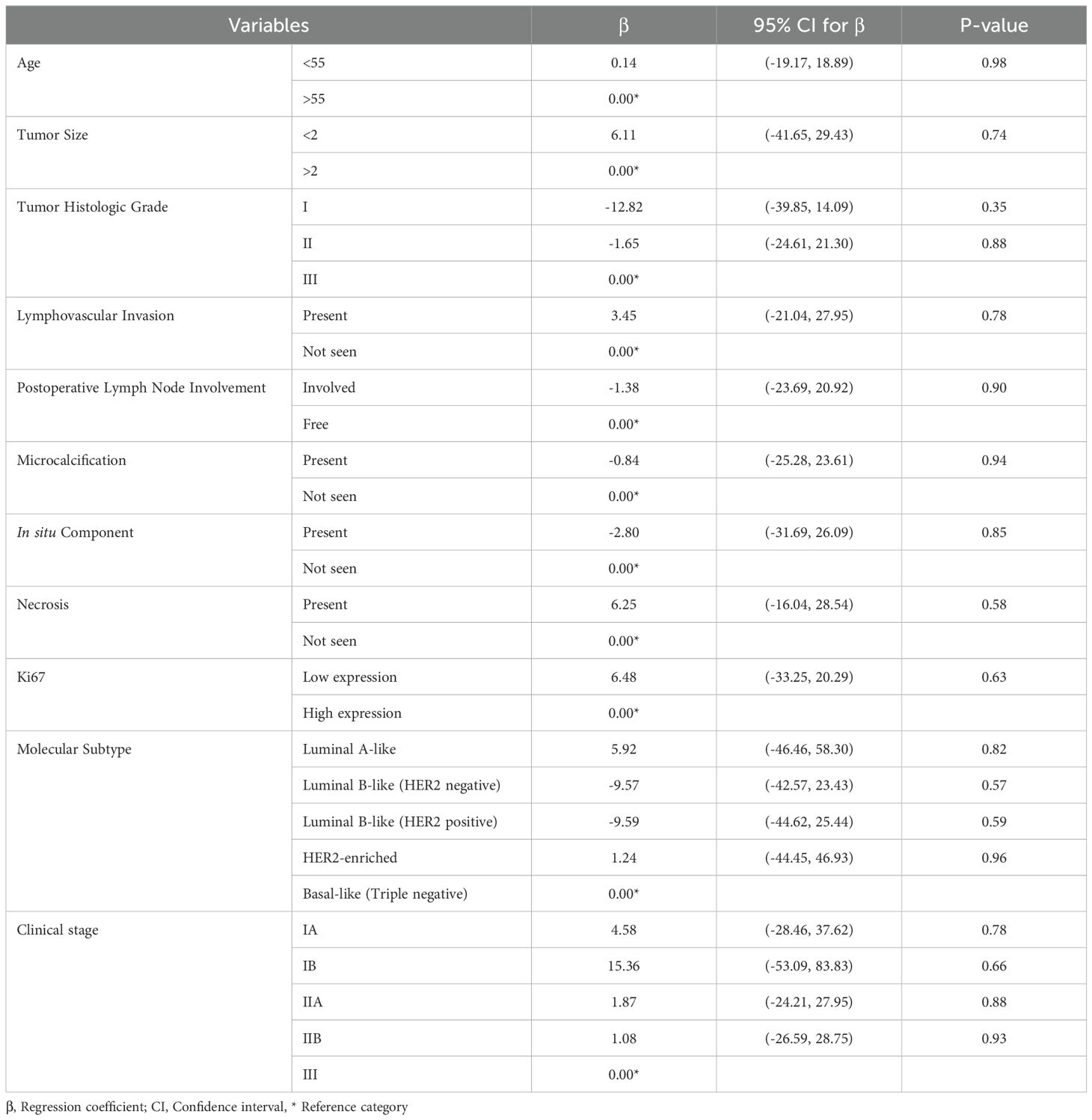
Table 4. Generalized linear model based on inverse Gaussian distribution exploring the clinicopathological predictive factors of serum apelin level in breast cancer patients.
4 Discussion
This study investigated serum apelin levels in breast cancer patients and their potential association with various clinicopathological features. Overall, we observed significantly elevated serum apelin levels in breast cancer patients compared to healthy controls, but no associations with clinicopathological features of the patients.
Several previous studies have reported altered tumoral apelin expression in breast cancer patients. In a study by Hu et al., the protein and mRNA expression of apelin was higher in breast cancer tissues compared to normal breast tissue (27). However, another study by Baran and colleagues showed that apelin expression was lower in the breast tissue of patients with invasive breast carcinoma compared to the normal breast tissue (28). On the other hand, studies on the serum apelin level in breast cancer patients have been limited. A recent systematic review of studies assessing serum or plasma apelin levels in all cancer types in the past decade only detected two studies assessing the circulating apelin levels in breast cancer patients (6), from which only in one study, the serum apelin level was compared with a control group, showing that the serum apelin levels were higher in early-stage postmenopausal breast cancer patients compared to healthy controls (29). The marked difference in serum apelin levels between patients and controls in our study suggests that apelin may play a role in breast cancer pathogenesis or progression. Furthermore, the ROC curve analysis in this study demonstrated excellent discriminative ability of serum apelin in distinguishing breast cancer patients from healthy controls, with an AUC of 0.94. This high AUC value, coupled with the optimal cut-off point of 122.48 (yielding 89% sensitivity and 97% specificity), suggests that serum apelin could potentially serve as a valuable biomarker for breast cancer detection.
To better contextualize our findings, it is important to note that previous experimental studies have identified several mechanisms through which apelin may influence cancer development and progression. So far, several mechanisms have been proposed for the potential role of apelin in cancer, including its involvement in angiogenesis, cell proliferation, and metastasis (19, 30). Studies have shown that apelin can activate the PI3K/Akt and MAPK/ERK signaling pathways, which are crucial for cell survival, growth, and migration (31, 32). Accordingly, it has been suggested that apelin contributes to tumor growth, angiogenesis, and metastasis (30, 33). Apelin has been shown to stimulate angiogenesis by promoting the proliferation and migration of endothelial cells (34), which could support tumor vascularization and growth. Recent findings have also shown that blocking the apelin/APJ axis could inhibit tumor growth and that APJ antagonists can also boost dendritic cell vaccine efficacy in controlling apoptotic, angiogenic, and metastatic properties of breast tumors (35). Our current clinical findings of elevated serum apelin in breast cancer patients are consistent with these previously reported biological activities, although the precise mechanisms underlying the elevation in our patient cohort remain to be elucidated in future studies.
Importantly, our analysis did not reveal any significant associations between serum apelin levels and the clinicopathological features examined, including tumor size, grade, lymphovascular invasion, lymph node involvement, molecular subtypes, and clinical stage. Similar to our findings, in a study by Grupińska et al., baseline apelin serum levels (1.30 ± 0.40 ng/mL) had no correlations with tumor size, histopathological grade, and regional lymph node metastasis of breast cancer patients (7). However, apelin level was associated with positive HER2/neu status (7). Nevertheless, this lack of association between serum apelin level and clinicopathological features contrasts with some previous studies that have reported correlations between tumoral apelin expression and certain prognostic factors in breast cancer. In a study by Hu and colleagues, apelin expression in breast cancer tissue had significant correlations with tumor size, stage, histological type, microvessel density, lymphatic vessel density, and lymph node metastasis (27). The findings on increased lymphatic vessel density and lymph node metastasis suggest that apelin may play an essential role in lymphangiogenesis as a route for the metastasis of cancer. Moreover, higher tumoral apelin expression has been associated with both worse disease-free survival and overall survival (27), indicating its potential as a prognostic factor. Interestingly, high tumoral apelin expression has been found to be associated with reduced response to neoadjuvant chemotherapy in breast cancer patients (36).
Overall, the absence of significant associations with clinicopathological features suggests that while serum apelin levels are elevated in breast cancer patients, they may not be prognostic factors indicative of specific tumor characteristics or disease severity. This study has several strengths, including a relatively large sample size of breast cancer patients and a comprehensive analysis of potential clinicopathological features. However, several limitations must be noted. The cross-sectional nature of the study prevents us from drawing conclusions about the causal relationship between apelin and breast cancer development or progression. Moreover, while the discriminative performance of serum apelin for present cancer is promising, our study design does not allow us to make definitive claims about apelin’s performance specifically in early-stage disease detection. Future prospective studies with stage-stratified analysis and longitudinal follow-up of at-risk individuals would be necessary to determine if apelin elevations can be detected at the earliest, most treatable stages of breast cancer, and whether screening for elevated apelin levels could improve patient outcomes. The high sensitivity and specificity observed in our study provide a strong rationale for such investigations. Additionally, we did not assess apelin expression in tumor tissues, which could provide complementary information to serum levels. We encourage research directions to include longitudinal studies to evaluate changes in serum apelin levels during cancer progression and treatment, as well as mechanistic studies to elucidate the specific roles of apelin in breast cancer biology. Moreover, investigating the potential of apelin as a therapeutic target in breast cancer could be a promising avenue for future research.
5 Conclusions
In conclusion, our study demonstrates significantly elevated serum apelin levels in breast cancer patients, with high discriminative ability between patients and healthy controls. While serum apelin shows promise as a potential biomarker for breast cancer detection, its lack of association with specific clinicopathological features suggests a limited prognostic role and a complex role in breast cancer biology that warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.AH.REC.1402.080). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft. AT: Conceptualization, Investigation, Methodology, Writing – original draft. YZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. MI: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. SA: Writing – original draft. AK: Formal Analysis, Software, Visualization, Writing – review & editing. MS: Conceptualization, Methodology, Validation, Writing – review & editing. EJ: Conceptualization, Methodology, Supervision, Writing – review & editing. AM: Formal analysis, Writing – review & editing. VM: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this study was provided by Kerman University of Medical Sciences (grant number: 402000122) for the supply of kits and other lab resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HER2, Human Epidermal Growth Factor Receptor 2; ROC, Receiver Operating Characteristic; GLM, Generalized Linear Model; AUC, Area Under the Curve; SD, Standard Deviation; CI, Confidence Interval.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. (2022) 66:15–23. doi: 10.1016/j.breast.2022.08.010
3. Xu H and Xu B. Breast cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. (2023) 35:565–83. doi: 10.21147/j.issn.1000-9604.2023.06.02
4. Hristova VA and Chan DW. Cancer biomarker discovery and translation: proteomics and beyond. Expert Rev Proteomics. (2019) 16:93–103. doi: 10.1080/14789450.2019.1559062
5. Umelo IA, Costanza B, and Castronovo V. Innovative methods for biomarker discovery in the evaluation and development of cancer precision therapies. Cancer Metastasis Rev. (2018) 37:125–45. doi: 10.1007/s10555-017-9710-0
6. Grinstead C and Yoon S. Apelin, a circulating biomarker in cancer evaluation: A systematic review. Cancers (Basel). (2022) 14:1–16.
7. Grupińska J, Budzyń M, Brzeziński JJ, Gryszczyńska B, Kasprzak MP, Kycler W, et al. Association between clinicopathological features of breast cancer with adipocytokine levels and oxidative stress markers before and after chemotherapy. BioMed Rep. (2021) 14:1–12. doi: 10.3892/br.2021.1406
8. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. (1998) 251:471–6. doi: 10.1006/bbrc.1998.9489
9. Ilaghi M, Soltanizadeh A, Amiri S, Kohlmeier KA, and Shabani M. The apelin/APJ signaling system and cytoprotection: Role of its cross-talk with kappa opioid receptor. Eur J Pharmacol. (2022) 936:175353. doi: 10.1016/j.ejphar.2022.175353
10. Kleinz MJ and Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. (2005) 107:198–211. doi: 10.1016/j.pharmthera.2005.04.001
11. Gandham R, Sumathi M, Dayanand C, Sheela S, and Kiranmayee P. Apelin and its receptor: an overview. J Clin Diagn Res. (2019). doi: 10.7860/JCDR/2019/41074.12930
12. Mughal A and O’Rourke ST. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol Ther. (2018) 190:139–47. doi: 10.1016/j.pharmthera.2018.05.013
13. Japp AG, Cruden NL, Barnes G, Van Gemeren N, Mathews J, Adamson J, et al. Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure. Circulation. (2010) 121:1818–27. doi: 10.1161/CIRCULATIONAHA.109.911339
14. Neelakantan D, Dogra S, Devapatla B, Jaiprasart P, Mukashyaka MC, Janknecht R, et al. Multifunctional APJ pathway promotes ovarian cancer progression and metastasis. Mol Cancer Res. (2019) 17:1378–90. doi: 10.1158/1541-7786.MCR-18-0989
15. Berta J, Török S, Tárnoki-Zách J, Drozdovszky O, Tóvári J, Paku S, et al. Apelin promotes blood and lymph vessel formation and the growth of melanoma lung metastasis. Sci Rep [Internet]. (2021) 11:1–12. doi: 10.1038/s41598-021-85162-0
16. Liu L, Yi X, Lu C, Wang Y, Xiao Q, Zhang L, et al. Study progression of apelin/APJ signaling and apela in different types of cancer. Front Oncol. (2021) 11: 1–9. doi: 10.3389/fonc.2021.658253
17. Lacquaniti A, Altavilla G, Picone A, Donato V, Chirico V, Mondello P, et al. Apelin beyond kidney failure and hyponatremia: a useful biomarker for cancer disease progression evaluation. Clin Exp Med. (2015) 15:97–105. doi: 10.1007/s10238-014-0272-y
18. Feng M, Yao G, Yu H, Qing Y, and Wang K. Tumor apelin, not serum apelin, is associated with the clinical features and prognosis of gastric cancer. BMC Cancer. (2016) 16:1–8. doi: 10.1186/s12885-016-2815-y
19. Yang Y, Lv SY, Ye W, and Zhang L. Apelin/APJ system and cancer. Clin Chim Acta. (2016) 457:112–6. doi: 10.1016/j.cca.2016.04.001
20. Muto J, Shirabe K, Yoshizumi T, Ikegami T, Aishima S, Ishigami K, et al. The apelin-APJ system induces tumor arteriogenesis in hepatocellular carcinoma. Anticancer Res. (2014) 34:5313–20.
21. Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget. (2014) 5:4426–37.
22. Altinkaya SO, Nergiz S, Küçük M, and Yüksel H. Apelin levels are higher in obese patients with endometrial cancer. J Obstet Gynaecol Res. (2015) 41:294–300. doi: 10.1111/jog.12503
23. Al-harithy RN and Al-otaibi WA. Apelin-12 levels in obese patients with colon cancer. Cancer Immunol Immunother. (2015) 1:1–5.
24. Tulubas F, Mete R, Oznur M, and Topcu B. The role of adipocytokines in colon cancer and adenomas. J Med Biochem. (2013) 33:135–42. doi: 10.2478/jomb-2013-0001
25. Januškevičienė I and Petrikaitė V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell populations. Life Sci. (2019) 239:117009. doi: 10.1016/j.lfs.2019.117009
26. Rewcastle E, Skaland I, Gudlaugsson E, Fykse SK, Baak JPA, and Janssen EAM. The Ki67 dilemma: investigating prognostic cut-offs and reproducibility for automated Ki67 scoring in breast cancer. Breast Cancer Res Treat. (2024), 1–12. doi: 10.1007/s10549-024-07352-4
27. Hu D, Cui Z, Peng W, Wang X, Chen Y, and Wu X. Apelin is associated with clinicopathological parameters and prognosis in breast cancer patients. Arch Gynecol Obstet. (2022) 306:1185–95.
28. Baran M, Ozturk F, Canoz O, Onder GO, and Yay A. The effects of apoptosis and apelin on lymph node metastasis in invasive breast carcinomas. Clin Exp Med. (2020) 20:507–14. doi: 10.1007/s10238-020-00635-2
29. Salman T, Demir L, Varol U, Akyol M, Oflazoglu U, Yildiz Y, et al. Serum apelin levels and body composition changes in breast cancer patients treated with an aromatase inhibitor. J BUON. (2016) 21:1419–24.
30. Masoumi J, Jafarzadeh A, Khorramdelazad H, Abbasloui M, Abdolalizadeh J, and Jamali N. Role of Apelin/APJ axis in cancer development and progression. Adv Med Sci. (2020) 65:202–13. doi: 10.1016/j.advms.2020.02.002
31. Yang Y, Zhang X, Cui H, Zhang C, Zhu C, and Li L. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci Lett. (2014) 568:44–9. doi: 10.1016/j.neulet.2014.03.037
32. Li Y, Bai Y-J, and Jiang Y-R. Apelin induces the proliferation, migration and expression of cytoskeleton and tight junction proteins in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways. Int J Clin Exp Pathol. (2017) 10:10711.
33. Sorli SC, Le Gonidec S, Knibiehler B, and Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. (2007) 26:7692–9.
34. Wu L, Chen L, and Li L. Apelin/APJ system: a novel promising therapy target for pathological angiogenesis. Clin Chim Acta. (2017) 466:78–84. doi: 10.1016/j.cca.2016.12.023
35. Masoumi J, Zainodini N, Basirjafar P, Tavakoli T, Zandvakili R, Nemati M, et al. Apelin receptor antagonist boosts dendritic cell vaccine efficacy in controlling angiogenic, metastatic and apoptotic-related factors in 4T1 breast tumor-bearing mice. Med Oncol. (2023) 40:179. doi: 10.1007/s12032-023-02030-9
Keywords: apelin, breast cancer, clinicopathological parameters, serum marker, histopathological
Citation: Soltanizadeh A, Tirgar A, Zamanian Y, Ilaghi M, Aflatoonian S, Karamoozian A, Shabani M, Jafari E, Mahmoudabadi A and Moazed V (2025) Elevated serum apelin levels in breast cancer patients: A potential biomarker with limited histopathological correlations. Front. Oncol. 15:1513693. doi: 10.3389/fonc.2025.1513693
Received: 18 October 2024; Accepted: 04 September 2025;
Published: 22 September 2025.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Shoko Kure, Dana–Farber Cancer Institute, United StatesJale Yuzugulen, Eastern Mediterranean University, Türkiye
Copyright © 2025 Soltanizadeh, Tirgar, Zamanian, Ilaghi, Aflatoonian, Karamoozian, Shabani, Jafari, Mahmoudabadi and Moazed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vahid Moazed, bW9hemVkLnZhaGlkQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Adel Soltanizadeh
Adel Soltanizadeh Aliasghar Tirgar
Aliasghar Tirgar Yasaman Zamanian3
Yasaman Zamanian3 Mehran Ilaghi
Mehran Ilaghi Mohammad Shabani
Mohammad Shabani