- 1Department of International Radiology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of International Radiology, Zhongshan People’s Hospital, Zhongshan, China
- 3Department of International Radiology, The First People’s Hosiptal of Kunshan, Suzhou, China
Purpose: To evaluate the efficacy and safety of a multimodal therapeutic approach involving transarterial chemoembolization (TACE) in conjunction with helical iodine-125 (I-125) seed implant, lenvatinib, and programmed cell death-1(PD-1) inhibitors for hepatocellular carcinoma (HCC) complicated by main portal vein tumor thrombus (MPVTT).
Material and methods: HCC patients with MPVTT treated with TACE coupled with helical I-125 implant, lenvatinib, PD-1 inhibitors between September 2019 and August 2022 were retrospectively analyzed, and constituted as study group. Those treated with TACE, helical I-125 seed implant, and sorafenib between December 2016 and August 2020 served as the historical control group. All patients received sorafenib or lenvatinib combined with PD-1 inhibitors within 3–7 days after TACE and helical I-125 seed implantation. The longest follow-up period for all patients in both groups was 36 months from the date of helical I-125 seed implantation. Primary outcome was overall survival time (OS), and secondary outcomes were progression free survival time (PFS), objective response rate (ORR), and disease control rate (DCR). The Cox proportional hazards regression model was employed to identify independent prognostic factors influencing OS and PFS. The value P < 0.05 was deemed statistically significant.
Results: A total of 53 patients were enrolled, with 22 assigned to the study group and 31 to the control group. The study group exhibited superior overall ORR(54.5% vs. 25.8%, P = 0.033) and overall DCR (77.3% vs. 64.5%, P = 0.319). Notably, the ORR and DCR of MPVTT were higher in the study group (86.4% vs. 51.6%, P = 0.008; and 95.5% vs. 83.9%, P = 0.382, respectively). Median OS (16.1 ± 6.1 months vs. 10.2 ± 0.8 months, P = 0.008) and PFS (13.6 ± 3.0 months vs. 6.1 ± 0.6 months, P = 0.014) were prolonged in the study group. The maximal tumor size, alpha fetoprotein level, and treatment modality were independent predictors for OS, while the maximal tumor size and treatment modality were independent determinants for PFS. Study group showed frequent hypothyroidism and reactive cutaneouscapillary (P < 0.01), with comparable grade 3/4 adverse events between groups.
Conclusions: The integration of the helical I-125 seed implant with TACE, lenvatinib, and PD-1 inhibitors is the safe and efficacious approach in the management of HCC complicated by MPVTT.
Introduction
Hepatocellular carcinoma (HCC) complicated by portal vein tumor thrombus (PVTT) is classified as Barcelona Clinic Liver Cancer (BCLC) stage C disease, where systemic therapies are recommended as the first-line treatment (1). Recent advancements in systemic therapies have yielded promising median overall survival (OS) of almost 2 years for HCC in BCLC stage C (2, 3). However, for patients with HCC complicated by main PVTT(MPVTT), the prognosis remains dismal. A post hoc analysis of the IMbrave150 study revealed that the median OS for such patients was merely 7.6 months and 5.5 months, respectively, treated with atezolizumab plus bevacizumab group(T+A) and the sorafenib (4).
The combined approach of endovascular brachytherapy with iodine-125 (I-125) seeds including I-125 seed strand plus stent (I-125 seed-stent), irradiation stent with I-125 seed and helical I-125 seed implant, and transarterial chemoembolization (TACE) is routinely utilized for the management of HCC complicated by MPVTT in China, with a favorable median OS ranging from 8.4 to 12.5 months (5–7). Furthermore, the incorporation of systemic therapies into endovascular brachytherapy with I-125 seeds and TACE has been shown to further enhance the prognostic outlook. Zhang et al. reported that a comprehensive treatment strategy incorporating I-125 seed strand-stent, TACE, molecular targeted therapies, and immune checkpoint inhibitors (ICIs) has remarkably extended the median OS of HCC with PVTT to 17.7 months (8).
Our previous researches demonstrated the safety and efficacy of integrating helical I-125 seed implant with TACE in the management of HCC with MPVTT (9). Juxtaposed against implantation of I-125 seed strand-stent or irradiation stent with I-125 seed, the helical I-125 seed implantation emerges as a less invasive approach, entailing reduced technical complexity. Whereas, the efficacy of combining helical I-125 seed implant with TACE and systemic therapies remains elusive. Therefore, the present study aims to elucidate a more efficacious treatment paradigm by assessing the therapeutic outcomes and safety profiles of treating HCC with MPVTT using helical I-125 seed implant in conjunction with TACE, lenvatinib, and programmed cell death-1 (PD-1) inhibitors, against a historical control of helical I-125 seed implant combined with TACE and sorafenib.
Materials and methods
Study design and patients
The clinical data of patients with HCC complicated by MPVTT who underwent TACE in combination with helical I-125 seed implant, lenvatinib, and PD-1 inhibitors in the first affiliated hospital of Soochow university between September 2019 and August 2022 were retrospectively collected. This cohort constituted the study group. Given the scarcity of cases where TACE, helical I-125 seed implant, and sorafenib were administered concurrently during the same period as the study group, records of HCC with MPVTT that underwent this combined therapy between December 2016 and August 2020 were gathered to form the historical control group. All patients underwent a standardized protocol involving synchronized helical I-125 seed implant and initial TACE treatment, followed by the introduction of a combination therapy with sorafenib, or lenvatinib plus PD-1 inhibitors, within 3 to 7 days. The inclusion criteria were as follows: (1) histological or radiological diagnosis of HCC based on the American Association for the Study of Liver Disease guideline; (2) HCC with Cheng’s type III PVTT; (3) at least one measurable tumor lesion in liver; (4) Child-Pugh class A/B with Eastern Cooperative Oncology Group performance status(ECOG-PS) of 0-2; (5) no prior history of systemic therapies; and (6) age range of 18–75 years, with life expectancy exceeding 3 months. Patients were excluded if they had: (1) tumor volume occupying 50% or more of the liver; (2) severe ascites, hepatic encephalopathy, or gastric fundal variceal bleeding; (3) high-flow intrahepatic arterioportal fistula; (5) coexistence of other malignancies; (6) brain metastasis; and (7) concurrent severe diseases affecting vital organs such as the heart, lungs, or kidneys. Informed consent was obtained from all patients participating in this retrospective study. This retrospective study conformed to the rules of the Declaration of Helsinki and was approved by the institutional review board. The requirement to obtain informed consent was waived.
Treatment protocols
The helical I-125 seed implant was composed of helical sleeves and I-125 seed. Production of the helical I-125 seed implant and the formula for calculating seed number were previously described in detail (7). An ultrasound-guided 22-gauge Chiba needle was used to puncture the left or right portal vein. A micro-guidewire was then introduced into the portal vein, a procedure which was confirmed by the injection of a contrast agent. Subsequently, a stiff guidewire was substituted. Thereafter, a 55-cm 4F sheath (Cook, US) was delivered along the guidewire to the portal vein, and the distal end of the main tumor thrombus was reached by the tip of the sheath. The helical I-125 seed implant, was loaded into the sheath and deployed in the portal vein at the site of MPVTT by pulling back the sheath to provide comprehensive coverage of the entire tumor thrombus. Following the deployment of the implant, the intrahepatic puncture tract was embolized with coils (Figures 1A-D). TACE was instantaneously performed following helical I-125 seed implantation. Following angiography, a solution consisting of 5–20 ml of lipiodol combined with pirarubicin was injected into the tumor feeding arteries via microcatheter. Subsequently, gelatin sponge particles with a diameter of 350-560μm were injected for embolization until the flow stasis of the tumor-feeding arterials was achieved under fluoroscopic guidance (Figure 1E). TACE was performed on demand in the event of intrahepatic tumor recurrence, the emergency of new tumors or the residual tumor lesions, following the rigorous exclusion of any contraindications. All of the treatment were performed by two interventionalists with more than 15-year experience.
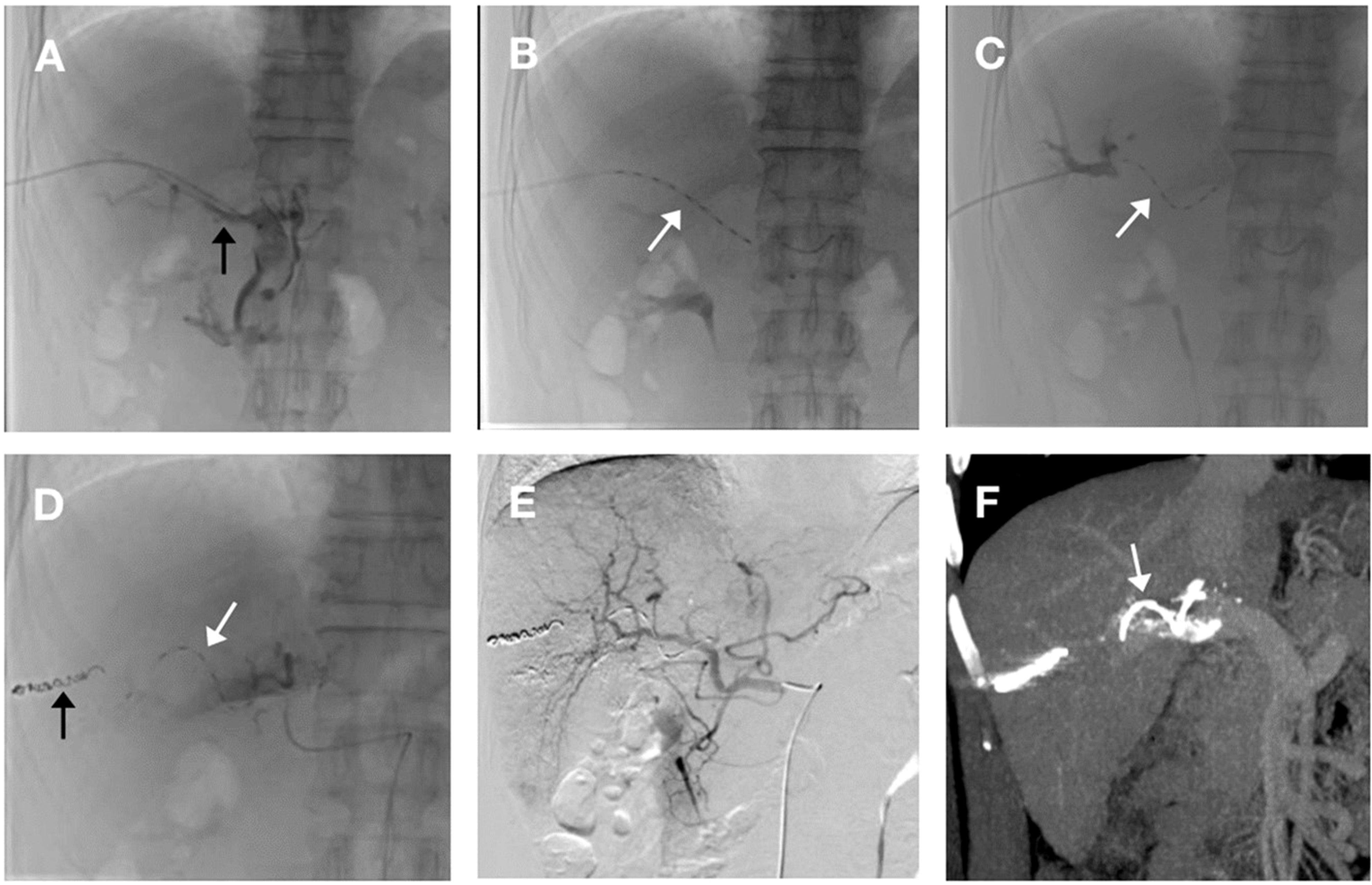
Figure 1. The procedure for helical Iodine-125 (I-125) seed implant and transarterial chemoembolization (TACE). (A) A 4-F catheter sheath was percutaneously inserted via transhepatic portal vein puncture into the right branch, subsequently advancing into the main portal vein. Portal venography reveals a filling defect in the main trunk, indicative of a main portal vein tumor thrombus (MPVTT) (black arrow). (B) Deployment of the helical I-125 seed was initiated through the catheter sheath. Prior to release, the seed was fully extended within the sheath(white arrow). (C) Following complete deployment, portal venography of the right branch demonstrated the helical I-125 seed self-anchoring in a spiral configuration within the main portal vein(white arrow). (D) After implantation of helical I-125 seed (white arrow), sealing of puncture tract using coil (black arrow). (E) Hepatic artery angiography after TACE treatment showed disappearance of staining in left liver tumor. (F) Enhanced computed tomography with portal vein phase reconstruction, performed after helical I-125 seed placement, clearly depicts the seed (white arrow) residing within the main portal vein, spanning across both proximal and distal extremities of the MPVTT.
The postoperative dose verification utilizing the Treatment Planning System (TPS) was rigorously executed via the 3D radiation treatment planning software, FtzyPlan version 1.3.118(Beijing FTT Technology Ltd., China), to precisely assess the radiation dose imparted to the brachytherapy target region following the helical I-125 seed implantation. Within the TPS framework, dedicated tools were employed to delineate the tumor thrombus contours and determine optimal particle implant locations and quantities, facilitating the generation of a concise dose-volume histogram, alongside an accurate calculation of the 90% target volume dose.
All patients adhered to the prescribed dosage of either lenvatinib (8mg/day for patients ≤60kg, 12mg/day for >60kg) or sorafenib (400mg administered twice daily) as molecular targeted therapies after and helical I-125 seed implantation and initial TACE. The dosage reduction or drug discontinuation was made in a timely manner based on the patient toleration. In addition, the two kinds of PD-1 inhibitors, namely sindilizumab and carelizumab, were administered in patients receiving lenvatinib after the first interventional therapy and repeated injection every 3 weeks. The drug dosage reduction, or, if necessary, complete drug discontinuation was made in a timely manner based on the onset of intolerance to adverse events.
Follow-up and outcomes
The patients were subjected to contrast-enhanced computed tomography or magnetic resonance imaging scans every 6–8 weeks following interventional therapy, complicated by assessments of liver and kidney function, as well as routine blood tests. The primary endpoint was OS, measured from the initiation of interventional therapy to death or the final follow-up on December 31, 2022. To balance the follow-up duration between the two groups, the maximum follow-up duration for all patients in both groups was capped at 36 months after the helical I-125 seed implantation and initial TACE. The secondary endpoints included progression-free survival (PFS), the tumor response rate, and the treatment safety. Tumor response was evaluated adhering to modified Response Evaluation Criteria in Solid Tumors (RECIST), with a particular focus on MPVTT response, which was based on alterations in the maximum width of MPVTT. Specifically, complete response was defined as the absence of arterial phase enhancement or the complete disappearance of MPVTT; partial response (PR) entailed ≥ 30% reduction in MPVTT width from baseline; stable disease excluded both progressive disease (PD) and PR criteria; and PD was defined as ≥ 20% increase in MPVTT width or the extension of the thrombus to additional branches. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and Student’s t-test or Mann-Whitney U test were employed for between-group comparisons. The data of frequencies were analyzed by the chi-square test or Fisher’s exact test. OS and PFS was conducted using the Kaplan-Meier method and the log-rank test. Cox proportional hazards regression model was applied for univariate and multivariate analysis to ascertain the independent factors influencing the survival prognosis of HCC patients with MPVTT. Variables with P < 0.1 in the univariate analysis were included in the multivariate analysis. P < 0.05 was deemed statistically significant. All statistical analyses were conducted using SPSS 22.0 software (IBM, USA).
Results
Patients
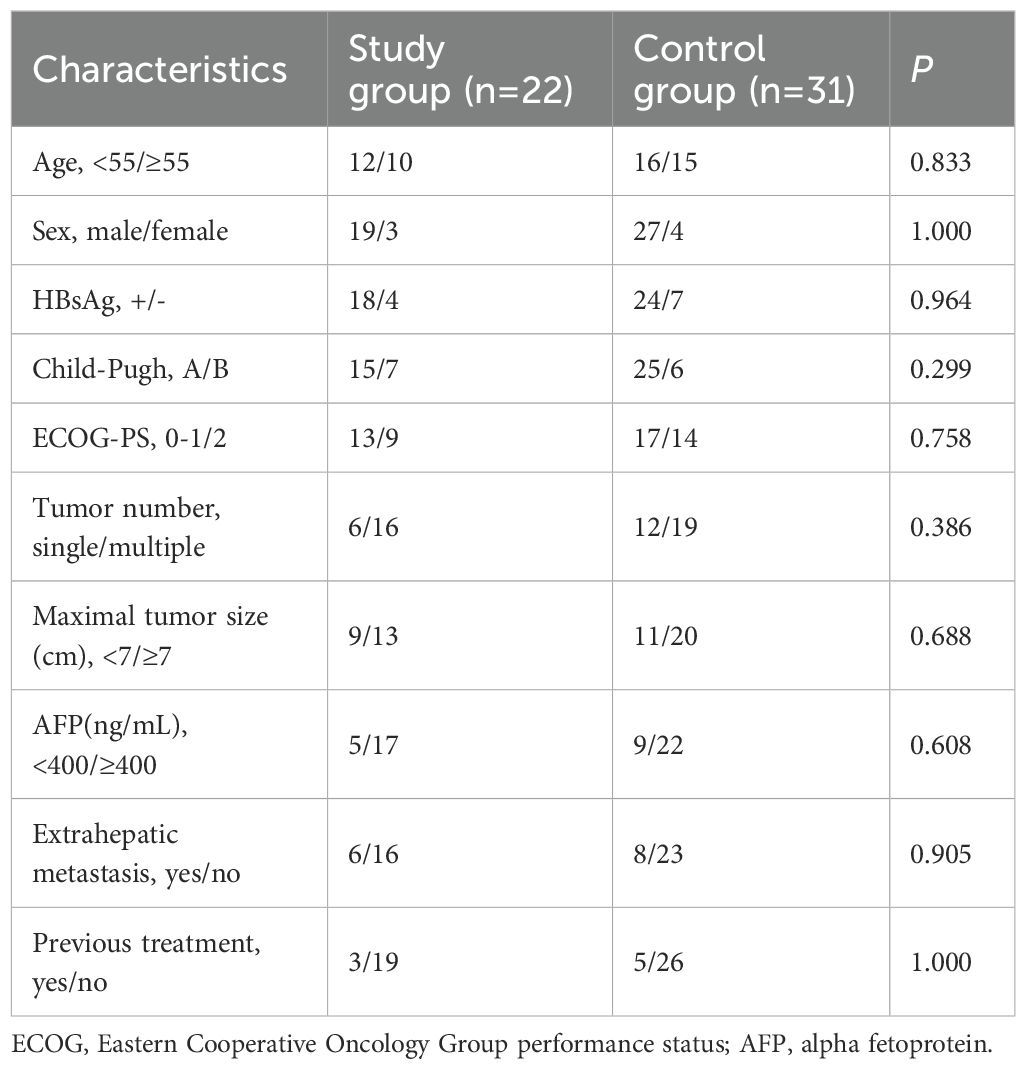
A total of 53 patients were enrolled in the study, with 22 and 31 patients, respectively, in the study group and control group. The majority of patients(42/53) with HCC had a hepatitis B surface antigen-positive status. Most patients exhibited well-preserved liver function and a favorable ECOG-PS. Notably, 62.2%(33/53) of patients suffered from a maximal tumor size of 7 cm or greater, and approximately a quarter (14/53) complicated with extrahepatic metastasis. The baseline characteristics were comparable between the two groups (Table 1). Each patient successfully underwent a helical I-125 seed implant and result in self-fixation of the particles in a spiral configuration within the main portal vein. No displacement was observed in either group (Figure 1F). The study group and control group received radiation doses of 51.2 ± 5.4Gy and 53.9 ± 4.5Gy, respectively, for MPVTT, with no significant difference (P = 0.367). The average number of TACE procedures performed was 4.1 ± 1.8 in the study group and 3.6 ± 2.3 in control group (P = 0.446).
Efficacy
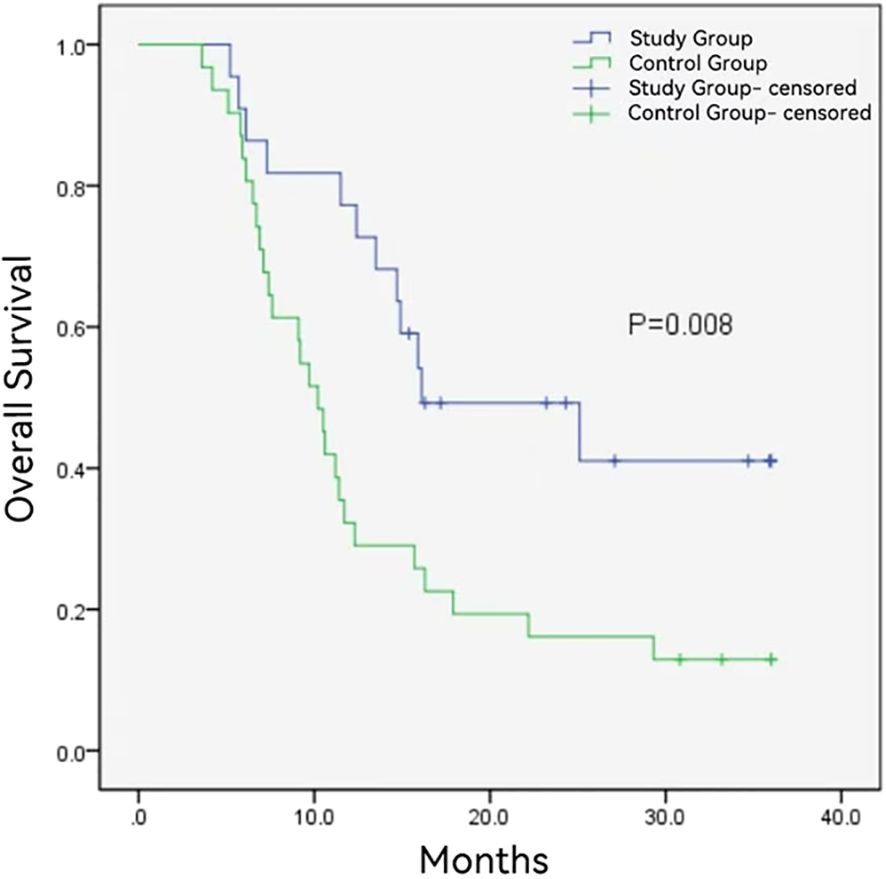
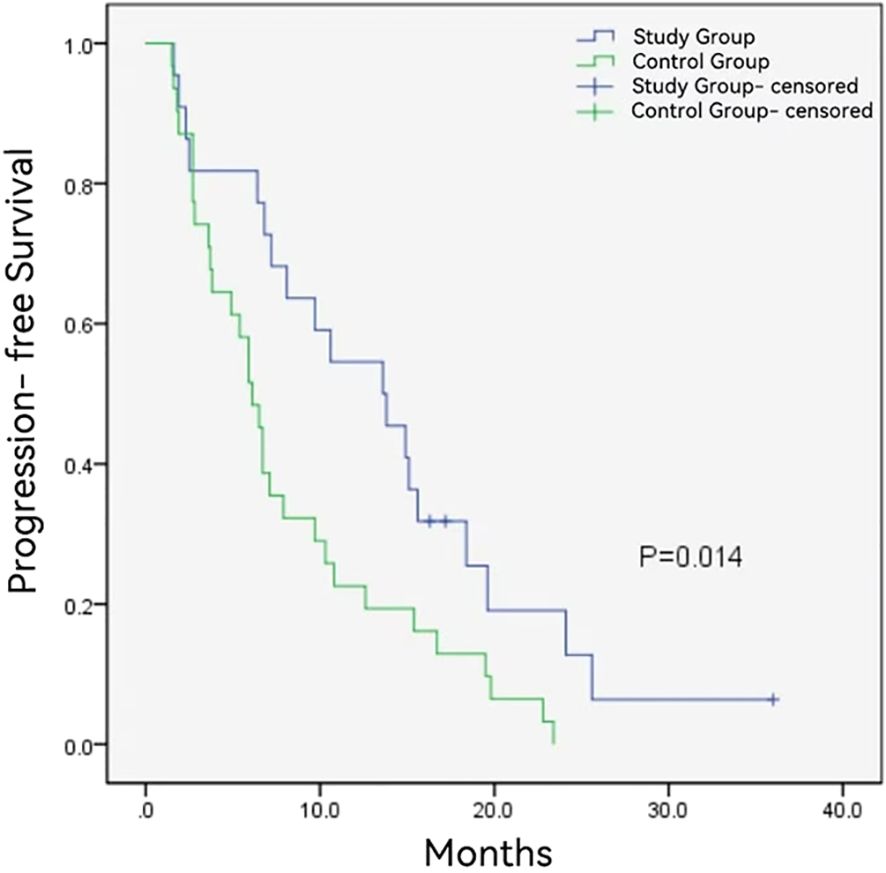
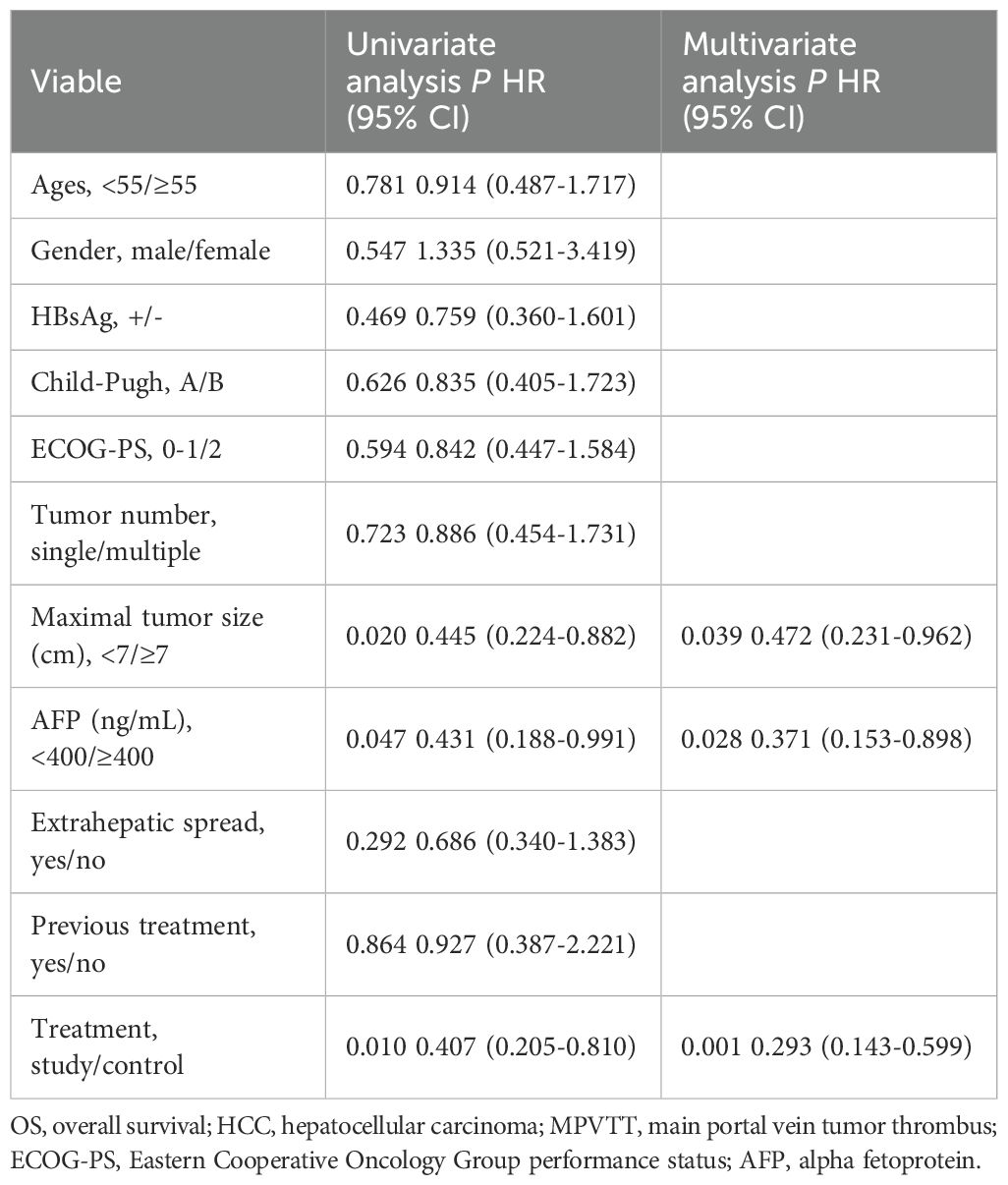
The follow-up duration in the control group and study group ranged from 3.6 to 36 months and 5.2 to 36 months, with a median follow-up time of 10.2 months and 16.0 months, respectively. During the follow-up period, 12 (54.5%) and 27 (87.1%) patients died in the study group and control group. The median OS was longer in the study group (16.1 ± 6.1months vs. 10.2 ± 0.8 months, P = 0.008) (Figure 2). Additionally, the study group demonstrated a favorable median PFS (13.6 ± 6.0 months vs. 6.1 ± 0.6, P = 0.014) (Figure 3), and a superior objective response rate (ORR) and disease control rate (DCR) for MPVTT compared to the control group(86.4% vs. 51.6%, P = 0.008; 95.5% vs. 83.9%, P = 0.382; respectively). Likewise, study group exhibited improved overall tumor control rates, with a favorable overall ORR (54.5% vs. 25.8%, P = 0.033) and overall DCR (77.3% vs. 64.5%, P = 0.319)(Figure 4). It is noteworthy that the two kinds PD-1 inhibitor employed in the study group (camrelizumab vs. sintilimab) exhibited comparable prognoses profiles, with no statistically significant differences observed in either median OS (16.9 ± 7.2 months vs. NA, P = 0.837) or median PFS (14.7 ± 4.9 vs. 13.9 ± 6.6 months, P = 0.756). In multivariate analysis, the maximal tumor size, alpha fetoprotein level, and treatment modality emerged as significant prognostic factors for OS (P all < 0.05)(Table 2). Furthermore, the maximal tumor size and treatment modality were independent prognosis for PFS (P all < 0.05) (Table 3).
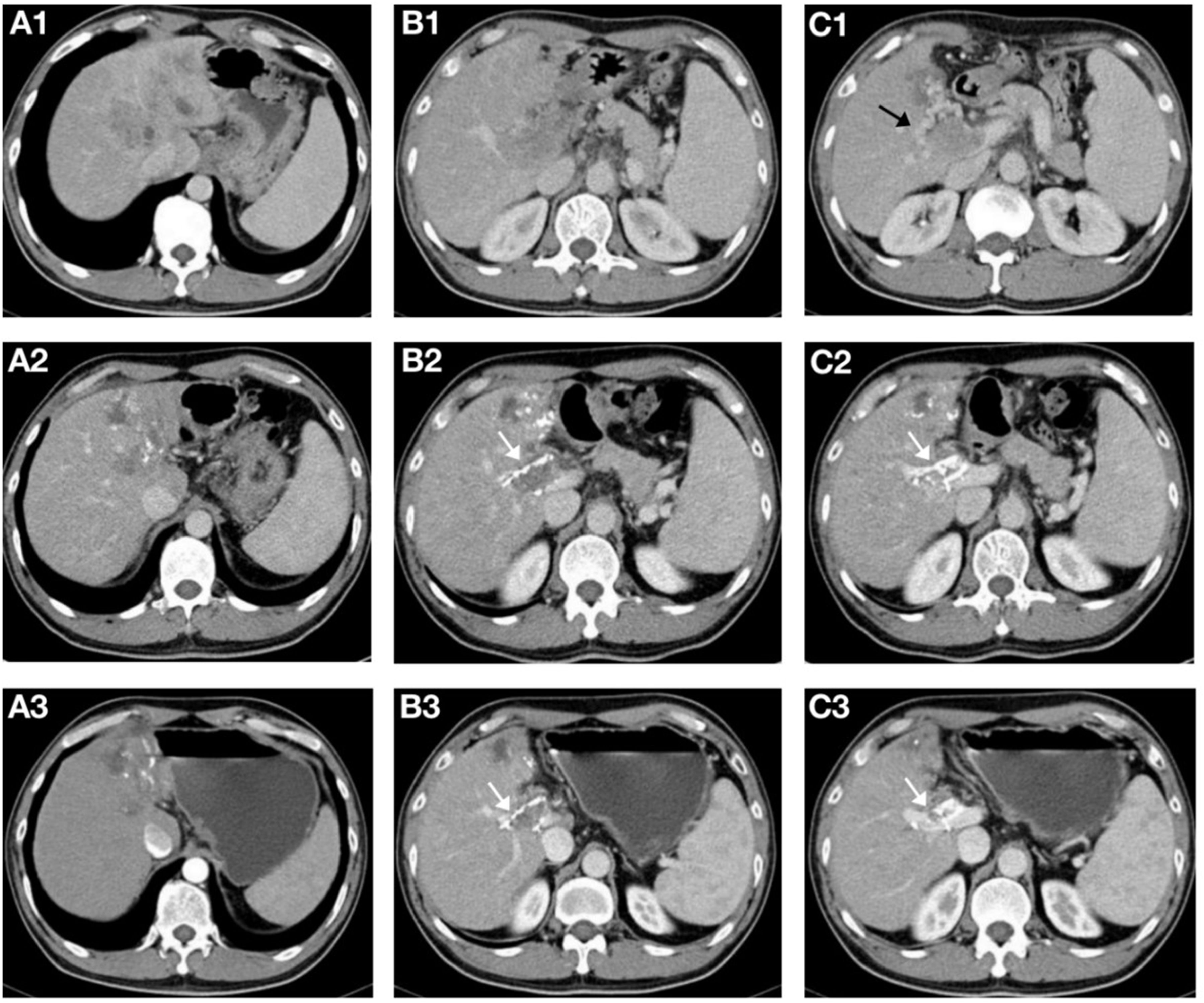
Figure 4. A 56-year-old male patient with hepatocellular carcinoma complicated with main portal vein tumor thrombus(MPVTT) treated with helical Iodine-125(I-125) seed implant, transarterial chemoembolization, lenvatinib, and PD-1 inhibitor. (A1-C1) Prior to the insertion of helical I-125 seed implant, the main trunk of the portal vein was occluded, forming peripheral collateral circulation(black arrows). (A2-C2) Two months after the insertion of the helical I-125 seed implant, the MPVTT and intrahepatic lesions shrank, and the peripheral collateral circulation of the main trunk of the portal vein disappeared; the helical I-125 seed implant can be seen beside the PVTT(white arrow). (A3-C3) After four months, the MPVTT and intrahepatic lesions further shrank, both achieving partial response, partial recanalization of the main trunk of the portal vein can be seen, and the helical I-125 seed implant did not shift (white arrow).
Safety
In the study group, the predominant adverse events(AEs) encompassed fever, elevated aminotransferases, abdominal pain, and reactive cutaneouscapillary(RCCEP). Conversely, the control group exhibited a high frequency of hand-foot skin reaction, fever, elevated aminotransferases, and diarrhea as AEs. Notably, the hypothyroidism and RCCEP were frequently witnessed in patients in study group(P all < 0.01). This may be associated with PD-1 inhibitor. In the study group, all patients who developed hypothyroidism and RCCEP were classified as Grade 1/2 AEs. These patients continued to receive the current PD-1 therapy while being managed with symptomatic supportive care. Any incidence of AEs in grade 3/4 were comparable between the two groups(P all >0.05)(Table 4), and no fatality attributed to AEs.
Discussion
This study utilized helical I-125 seed implantation, TACE and sorafenib as the historical control group to comparatively analyze the efficacy and safety of helical I-125 seed implantation combined with TACE, lenvatinib, and PD-1 inhibitors in the treatment of HCC with MPVTT. The results demonstrate that compared to the control group, the study group exhibits a better survival prognosis of HCC patients with MPVTT, with acceptable safety profiles for both treatment modalities. Notably, the discrepancy in follow-up duration between the two groups can be ascribed to inferior treatment outcomes, shorted survival, and a higher incidence of lost-to-follow-up cases in patients in control group.
Though BCLC stage system recommends systemic therapy for advanced HCC, systemic therapy has shown limited efficacy in patients with PVTT, particularly in cases involving the MPVTT (1, 4). I-125 seed-stent, irradiation stent with I-125 seed, and helical I-125 seed implantation have been reported as treatment modalities of endovascular brachytherapy with I-125 seeds to the management of HCC with MPVTT (5–7). The fundamental premise behind employing I-125 seed strand-stent or irradiation stent with I-125 seed for MPVTT is to facilitate recanalization the main portal vein, rejuvenating hepatic blood flow, and thereby mitigating liver dysfunction stemming from insufficient compensatory perfusion via an obstructed or stenotic portal vein after TACE-induced hepatic artery occlusion (6, 15). Whereas, many recent investigations have conclusively demonstrated the safety and efficacy of super-selective TACE or TACE-based combination therapies in the management of HCC with MPVTT, without the necessity for portal vein stenting (10–13). Consequently, the recanalization of the stenotic or obstructed portal vein is not mandatory when combining I-125 seed endovascular brachytherapy with TACE for the treatment of HCC in the presence of MPVTT. Our prior research underscores that, irrespective of portal vein stenosis severity, the helical I-125 seed implant can autonomously adhere adjacent to MPVTT, inhibiting its progression, without reliance on stent compression (7). This approach, coupled with TACE, exhibits favorable safety and efficacy profiles in the management of HCC with MPVTT. In comparison with the use of I-125 seed stent that is introduced through a 10-French sheath and necessitate anticoagulation therapy, helical I-125 seed implantation has been shown to be a less invasive procedure, less technically complex, and does not require anticoagulation therapy (14). Moreover, in comparison with I-125 seed strand, which has a risk of displacement, helical I-125 seed implantation has been demonstrated to be self-retaining (7).
Zhang et al. documented that I-125 seed strand-stent combined with TACE and sorafenib significantly elongated median OS (10.3 months vs. 6.0 months, P < 0.001), time to progression (9.0 months vs. 3.4 months, P < 0.001), ORR (45.9% vs. 16.1%, P = 0.009) and DCR (67.6% vs. 29.0%, P = 0.002) in HCC with MPVTT, as compared to TACE plus sorafenib. Furthermore, the safety of minimally invasive implantation of I-125 seeds strand-stent through a 6-F sheath was demonstrated (16). Conversely, an enlarged sheath is associated with the potential for increased complications, though these may be manageable. In a multicenter randomized controlled trial conducted by Lu et al., the introduction of an irradiation stent with I-125 through a 10-F sheath has been shown to induce more frequent abdominal pain(29% vs. 12%) though the combination of an irradiation stent with I-125 seeds with TACE has yielded a superior median OS (9.9 months vs. 6.3 months, P = 0.01), favorable tumor complete rate(21.6% vs. 5.9%, P = 0.109) and DCR(86.0% vs. 67.0%, P = 0.022) compared to sorafenib plus TACE in patients with HCC complicated by MPVTT (14). Notably, the historical control arm in this study achieved a commendable OS of 10.2 months, complicated by a satisfactory ORR(51.6%) and DCR(83.9%) for MPVTT. Despite the BCLC continuing to advocate T+A therapy as the preferred treatment for advanced HCC, the effectiveness of this approach for Vp4 HCC remains restricted (OS, 7 months; PFS, 5.4 months; and ORR, 23%) (4). Concurrently, patients undergoing T+A therapy exhibited an elevated risk of bleeding complication. The aforementioned treatment strategies that combined I-125 seeds endovascular brachytherapy with TACE or sorafenib have demonstrated a better prognosis profile (4, 6, 16). This may be attributed to the effective inhibition of multipolar vascular tumor thrombus by endovascular brachytherapy using I-125 seeds, thereby broadening the therapeutic spectrum and optimizing the therapeutic window for both TACE and systemic therapies. Nonetheless, T+A currently represents the highest level of evidence-based therapy for advanced HCC. The combination of TACE, helical I-125 seed implant, and T+A may offer enhanced therapeutic potential. This multimodal approach has the potential to leverage the ability of I-125 irradiation to recanalize portal vein thrombosis and mitigate bleeding risk.
In recent years, there has been an increasing focus on the field of tumor immunoregulation and therapy. It is imperative to acknowledge the intricate interplay between local and systemic therapy, particularly in the context of immune regulation. The immunosuppressive state of HCC itself is the primary factor hindering the efficacy of ICIs monotherapy, which is unable to significantly improve tumor response (17, 18). Radiotherapy and locoregional therapies, such as TACE, are postulated to induce immunogenic tumor necrosis, transforming the tumor from a ‘cold’ to a ‘hot’ state. Consequently, this transformation is anticipated to ultimately improve the tumor immune microenvironment(TIME) and present an avenue for the administration of ICIs (19). It has been demonstrated that TACE can upregulate PD-L1 expression, thus creating a favorable environment for subsequent anti-PD-1 or anti-PD-L1 monoclonal antibody therapy (20). However, it has also been reported that embolization may lead to a reduction in CD8+T cell infiltration, which might necessitate molecular targeted agents to recruit CD8+T cells (21). Lenvatinib, an effective anti-angiogenic agent, has the potential to improve the TIME in HCC by inhibiting the differentiation and stability of Tregs by targeting FGFR4 (22, 23). It is hypothesized that the administration of lenvatinib prior to and following TACE results in a reduction in tumor interstitial osmotic pressure by normalizing tumor blood vessels and reducing microvascular density. This, in turn, has been believed to increase drug delivery to maximize the efficacy of TACE (24). Furthermore, the use of anti-angiogenic agents has been shown to enhance the efficacy of ICIs. In HCC murine models, the combination of lenvatinib and PD-1 inhibitors demonstrated a superior anti-tumor efficacy over monotherapy. Additionally, lenvatinib reduces monocyte and macrophage populations while, in conjunction with PD-1 inhibitors, enhances the proportion of activated CD8+T cells (25). The Phase Ib studies of lenvatinib combined with pembrolizumab yielded promising anti-tumor responses, and the subsequent LEAP-012 trial further confirmed the therapeutic benefits of TACE in combination with lenvatinib and pembrolizumab (26, 27). Recently, Zhang et al. reported the safety and efficacy of augmenting PD-1 inhibitors to I-125 seed strand-stent, TACE, and lenvatinib in HCC with MPVTT, achieving a significantly prolonged median OS(17.7 months vs. 12.0 months, P = 0.01) (8). This finding underlines the synergistic anti-tumor effects of combining lenvatinib and PD-1 inhibitors with locoregional treatments, thereby enhancing survival prognosis. In the present study, compared with helical I-125 seed implant with TACE and sorafenib, the utilization of helical I-125 seed implant in conjunction with TACE, lenvatinib, and PD-1 inhibitors for HCC with MPVTT extend median OS to 16.1 months, mirroring the OS of 17.7 months by Zhang et al. and surpassing previous systemic treatment outcomes (8). The combination of radiotherapy with anti-angiogenic agents and PD-L1 monoclonal antibodies has been shown to activate exhausted T lymphocytes (28). In addition, the impact of radiotherapy and brachytherapy on the TIME, including changes in immune cells and cytokines, is also under investigation (29, 30). In a Phase II study, patients with HCC and macrovascular invasion experienced a 38.0% improvement in ORR after radiotherapy with a PD-1 monoclonal antibody compared to radiotherapy alone (31). From these perspectives, the combination of TACE, I-125 seeds brachytherapy, and lenvatinib may induce more profound improvements or alterations in the TIME. However, further in-depth basic research is required to ascertain whether this combination exerts a cascading amplification effect on immune activation.
This study is inherently constrained by several limitations. Firstly, its retrospective nature predisposes it to various confounding biases, which may compromise the robustness of the findings. Furthermore, owing to the absence of contemporaneous control cases involving TACE in conjunction with helical I-125 seed implant and sorafenib, a historical control group is devised as an alternative. Lastly, the modest sample sizes within both the study and the historical control cohorts pose challenges, and the employment of two distinct PD-1 inhibitors(camrelizumab and sintilimab) introduces a potential variable that may influence the precision of the outcomes. Multicenter prospective study is wanted to born out the present findings.
Conclusion
The integration of the helical I-125 seed implant with TACE, lenvatinib, and PD-1 inhibitors in the management of HCC concurrent with MPVTT demonstrates a safe and efficacious therapeutic approach.
Data availability statement
The datasets generated and/or analyzed during the current study are contained within the Office of Department of Interventional Radiology, the First Affiliated Hospital of Soochow University but are not publicly available because confidentiality, security and ownership matters. They may be available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because retrospective nature of the study.
Author contributions
J-WL: Methodology, Writing – original draft, Data curation, Formal Analysis, Investigation, Software. SZ: Investigation, Methodology, Supervision, Writing – original draft, Validation, Writing – review & editing. JS: Methodology, Conceptualization, Resources, Visualization, Writing – review & editing. YY: Data curation, Formal Analysis, Funding acquisition, Project administration, Software, Writing – original draft. JY: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review & editing. C-FN: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. W-SW: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Jiangsu Provincial Health Commission Scientific Research Project(M2022089).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
2. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
3. Abdelrahim M, Esmail A, Kim RD, Arora SP, Arshad J, Kournoutas I, et al. Real-world (RW) experience with atezolizumab + bevacizumab (A+B) for the treatment of unresectable HCC (uHCC): A multicenter study. J Clin Oncol. (2025) 43:537–7. doi: 10.1200/JCO.2025.43.4_suppl.537
4. Breder VV, Vogel A, Merle P, Finn RS, Galle PR, Zhu AX, et al. IMbrave150: Exploratory efficacy and safety results of hepatocellular carcinoma (HCC) patients (pts) with main trunk and/or contralateral portal vein invasion (Vp4) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in a global Ph III study. J Clin Oncol. (2021) 39:4073–3. doi: 10.1200/JCO.2021.39.15_suppl.4073
5. Luo J, Yan Z, Liu Q, Qu X, Wang J. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol. (2011) 22:479–89. doi: 10.1016/j.jvir.2010.11.029
6. Lu J, Guo JH, Zhu HD, Zhu GY, Chen L, Teng GJ. Safety and efficacy of irradiation stent placement for Malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: A single-center experience. J Vasc Interv Radiol. (2017) 28:786–794 e3. doi: 10.1016/j.jvir.2017.02.014
7. Wang W, Shen J, Wang C, Ren B, Zhu X, Ni C. Safety and feasibility of helical I-125 seed implants combined with transcatheter arterial chemoembolization in hepatocellular carcinomas with main portal vein tumor thrombus. Cardiovasc Intervent Radiol. (2019) 42:1420–8. doi: 10.1007/s00270-019-02256-z
8. Zhang ZH, Hou SN, Yu JZ, Zhang W, Ma JQ, Yang MJ, et al. Combined iodine-125 seed strand, portal vein stent, transarterial chemoembolization, lenvatinib and anti-PD-1 antibodies therapy for hepatocellular carcinoma and Vp4 portal vein tumor thrombus: A propensity-score analysis. Front Oncol. (2022) 12:1086095. doi: 10.3389/fonc.2022.1086095
9. Wang W, Wang C, Shen J, Ren B, Yin Y, Yang J, et al. Integrated I-125 seed implantation combined with transarterial chemoembolization for treatment of hepatocellular carcinoma with main portal vein tumor thrombus. Cardiovasc Intervent Radiol. (2021) 44:1570–8. doi: 10.1007/s00270-021-02887-1
10. Yang M, Fang Z, Yan Z, Luo J, Liu L, Zhang W, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. (2014) 140:211–9. doi: 10.1007/s00432-013-1568-0
11. Zhang YF, Guo RP, Zou RH, Shen JX, Wei W, Li SH, et al. Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study. Eur Radiol. (2016) 26:2078–88. doi: 10.1007/s00330-015-4021-8
12. Huang M, Lin Q, Wang H, Chen J, Bai M, Wang L, et al. Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol. (2016) 26:3428–36. doi: 10.1007/s00330-015-4198-x
13. Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. (2007) 18:1517–26; quiz 1527. doi: 10.1016/j.jvir.2007.07.035
14. Lu J, Guo J-H, Ji J-S, Li Y-L, Lv W-F, Zhu H-D, et al. Irradiation stent with 125I plus TACE versus sorafenib plus TACE for hepatocellular carcinoma with major portal vein tumor thrombosis: a multicenter randomized trial. Int J Surg. (2023) 109:1188–98. doi: 10.1097/JS9.0000000000000295
15. Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. (2016) 10:185–95. doi: 10.1007/s12072-015-9663-8
16. Zhang ZH, Liu QX, Zhang W, Ma JQ, Wang JH, Luo JJ, et al. Combined endovascular brachytherapy, sorafenib, and transarterial chemobolization therapy for hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol. (2017) 23:7735–45. doi: 10.3748/wjg.v23.i43.7735
17. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2015) 16:859–70. doi: 10.1016/S1470-2045(15)00050-9
18. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
19. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: A review. JAMA Oncol. (2021) 7:113–23. doi: 10.1001/jamaoncol.2020.3381
20. Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. (2021) 79:36–46. doi: 10.1111/his.14317
21. Zhang S, Xu L, Li JQ, Du MZ, Yin Y, Zhong BY, et al. Transarterial embolization enhances programmed cell death ligand 1 expression and influences CD8(+)T lymphocytes cytotoxicity in an orthotopic hepatocellular carcinoma rat model. Cardiovasc Intervent Radiol. (2024) 47:1372–81. doi: 10.1007/s00270-024-03813-x
22. Kudo M. Combination immunotherapy with anti-PD-1/PD-L1 antibody plus anti-VEGF antibody may promote cytotoxic T lymphocyte infiltration in hepatocellular carcinoma, including in the noninflamed subclass. Liver Cancer. (2022) 11:185–91. doi: 10.1159/000524977
23. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. (2021) 74:2544–60. doi: 10.1002/hep.31921
24. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117–27. doi: 10.1200/JCO.22.00392
25. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1–6 hepatocellular carcinoma model. Cancer Sci. (2018) 109:3993–4002. doi: 10.1111/cas.2018.109.issue-12
26. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
27. Kudo M, Ren Z, Guo Y, Han G, Lin H, Zheng J, et al. Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): a multicentre, randomised, double-blind, phase 3 study. Lancet. (2025) 405:203–15. doi: 10.1016/S0140-6736(24)02575-3
28. Li S, Li K, Wang K, Yu H, Wang X, Shi M, et al. Low-dose radiotherapy combined with dual PD-L1 and VEGFA blockade elicits antitumor response in hepatocellular carcinoma mediated by activated intratumoral CD8(+) exhausted-like T cells. Nat Commun. (2023) 14:7709. doi: 10.1038/s41467-023-43462-1
29. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: A randomized, open-label, multicenter controlled study. J Clin Oncol. (2019) 37:2141–51. doi: 10.1200/JCO.18.02184
30. Tang C, He Q, Feng J, Liao Z, Peng Y, Gao J. Portal vein tumour thrombosis radiotherapy improves the treatment outcomes of immunotherapy plus bevacizumab in hepatocellular carcinoma: a multicentre real-world analysis with propensity score matching. Front Immunol. (2023) 14:1254158. doi: 10.3389/fimmu.2023.1254158
Keywords: hepatocellular carcinoma, transarterial chemoembolization, helical iodine-125 seed implant, lenvatinib, programmed cell death ligand-1
Citation: Lin J-W, Zhang S, Shen J, Yin Y, Yang J, Ni C-F and Wang W-S (2025) The efficacy of transarterial chemoembolization combined with helical iodine-125 seed implant, lenvatinib and PD-1 inhibitors in patients with hepatocellular carcinoma complicated by main portal vein tumor thrombus: a retrospective study. Front. Oncol. 15:1514375. doi: 10.3389/fonc.2025.1514375
Received: 20 October 2024; Accepted: 09 April 2025;
Published: 08 May 2025.
Edited by:
Abdullah Esmail, Houston Methodist Hospital, United StatesReviewed by:
Xu-Hua Duan, First Affiliated Hospital of Zhengzhou University, ChinaJingsong Mao, Affiliated Hospital of Guilin Medical University, China
Copyright © 2025 Lin, Zhang, Shen, Yin, Yang, Ni and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-Sheng Wang, d3dzZGoyMDAyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jia-Wen Lin
Jia-Wen Lin Shen Zhang
Shen Zhang Jian Shen
Jian Shen Yu Yin
Yu Yin Jun Yang
Jun Yang Cai-Fang Ni
Cai-Fang Ni Wan-Sheng Wang
Wan-Sheng Wang