- 1Department of Midwifery, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
- 2Department of Pediatrics and Child Health, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
- 3Department of Comprehensive Nursing, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
- 4Department of Emergency and Critical Care Nursing, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
- 5Department of Midwifery, College of Medicine and Health Science, Woldia University, Woldia, Ethiopia
- 6Department of Psychiatry, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
Background: The term of gestational trophoblastic disease (GTD) is used to describe a group of tumors that are characterized by abnormal trophoblastic proliferation. Histologically, GTD includes the pre-malignant partial hydatidiform mole, complete hydatidiform mole, and malignant invasive moles that are choriocarcinoma, placental site trophoblastic tumors, and epithelioid trophoblastic tumors.
Method and material: The protocol for this review was registered on PROSPERO, accessible at CRD42024560408. We used the “PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews and Meta–analysis. All original and published cross-sectional, cohort, and case-control types of studies were reported during the study period. Studies conducted in both community and institutional settings were considered. Two reviewers independently assessed the risk of bias in the included studies using tools developed by the Joanna Briggs Institute, which comprise eight criteria.
Result: Data was systematically searched by Google Scholar (n = 214), HINARI (n = 46), Scopus (n = 40), and PubMed (n = 146) on 1 May 2024. A total of 13 studies from five countries in East Africa have been included. The random effects model showed that the prevalence of GTD among pregnant women was 22% (95% CI: 10%–33%). Women who are aged between 30 and 39 years (AOR = 0.11, 95% CI: 0.05–0.16), women who have a previous history of GTD (AOR = 0.24, 95% CI: 0.00–0.47), women who have a previous complication of the reproductive organ system (AOR = 0.23, 95% CI: 0.10–0.35), and women who have more than two histories of pregnancy (AOR = 0.9, 95% CI: 0.06–0.11) were significantly associated with the outcome variable.
Conclusion: This finding revealed that the pooled prevalence of GTD among pregnant women was high. Women who are aged between 30 and 39 years, have a previous history of GTD, and women who have had previous complications during pregnancy, and women who have more than two histories of pregnancy were significantly associated with the outcome variable.
Introduction
Gestational trophoblastic disease (GTD) is defined as a spectrum of interconnected conditions but histologically distinct disease entities originating from the placenta (1). It commonly occurs during pregnancy and changes the process and outcome of pregnancy by developing abnormal fertilization and placenta (1).
GTD is used to describe a group of tumors that are characterized by abnormal trophoblastic proliferation. Trophoblastic produces human chorionic gonadotropin, which is why it is important to quantify this peptide for the diagnosis, treatment, and follow-up of this disease (2).
Some evidence shows that GTD most commonly develops after a molar pregnancy; however, it may follow any gestation, including term pregnancy. Histologically, GTD includes the pre-malignant partial hydatidiform mole, complete hydatidiform mole, and malignant invasive moles that are choriocarcinoma, placental site trophoblastic tumors (PSTT), epithelioid trophoblastic tumors (ETT), gestational trophoblastic neoplasm, and invasive mole. choriocarcinoma, PSTT, and ETT are malignant invasive moles that can arise after any type of pregnancy, and all are known as gestational tropgoblastic neoplasia. Therefore, by virtue of their origin, they are able to produce significant amounts of human chorionic gonadotropin (HCG), which is a reliable tumor marker for diagnosis and monitoring of response (3, 4).
Gestational trophoblastic neoplasia is a complication of pregnancy; it may also follow other pregnancy problems like miscarriages, ectopic pregnancy, or term pregnancy. Therefore, by virtue of their origin, they are able to produce a significant amount of HCG, which is a reliable tumor marker for diagnosis and monitoring of response (5–8).
Method and material
Registration and protocol
This review was registered by using the protocol on PROSPERO at CRD42024560408. We applied the PRISMA 2020 Statement for Reporting Systematic Reviews and Meta–analysis as a framework (9).
Eligibility criteria
All original and published cross-sectional and cohort types of study design reporting the magnitude and its determinant factors of GTD in East African countries, systematic review and meta-analysis influencing them were deemed eligible for the systematic review and meta-analysis. Studies conducted in both community and institutional settings were considered. The selection of studies was based on several parameters, including outcome variables, study population, year of the study, regional context, sample size, and response rate. Those studies that do not meet the eligibility criteria are excluded during the search for proper studies. In this research, we included studies that were published from 2015 to 2024.
Information sources and search strategy
We used Scopus, HINARI, PubMed, and Google Scholar on 1 May 2024 to searching for significant articles for this study, and used searching terms such as “Gestational” AND “trophoblastic” OR “Molar” OR “Hydatidiform” OR “Mole” OR “partial” OR “Complete” “AND “Disease” OR “problem” OR “Infection” OR “Syndrome” AND “Magnitude” AND “Determinant” “AND” Prevalence “OR” associated “AND “Factors” AND “pregnancy” OR “mother” AND “East” AND “Africa.” Manual searching was performed on PubMed, HINARI, Scopus, and Google Scholar by using the “Publish or Perish” database searching tool, version 8, to determine the published and unpublished articles (10).
Selection process
The remaining studies had been separately screened by NA and MA after duplicate studies were removed with EndNote 20. These researchers performed the selection process accurately. Articles were initially improved on the basis of their abstract and title; full-text modifications followed, first alternately and then collectively until a consensus had been reached. Third reviewer, GB, was consulted for an agreement in circumstances wherever there was disagreement.
Data collection process and data items
For data extraction, a Microsoft Excel 2019 spreadsheet was used. This spreadsheet was utilized to extract the following outcome variables: population (study units), year of study, context, sample size, response rate, and proportions. Following the extraction of the data and comparing their findings, the two independent reviewers, NA and MA, came to an agreement. While there was unwilling to reach agreement to a consensus, GB, a third reviewer, was requested to provide assistance. Associated factors of GTD in pregnant women in East Africa were the main outcomes of this comprehensive review and meta-analysis. Other factors influencing the primary result variables were incorporated in the additional outcomes.
Study risk of bias assessment
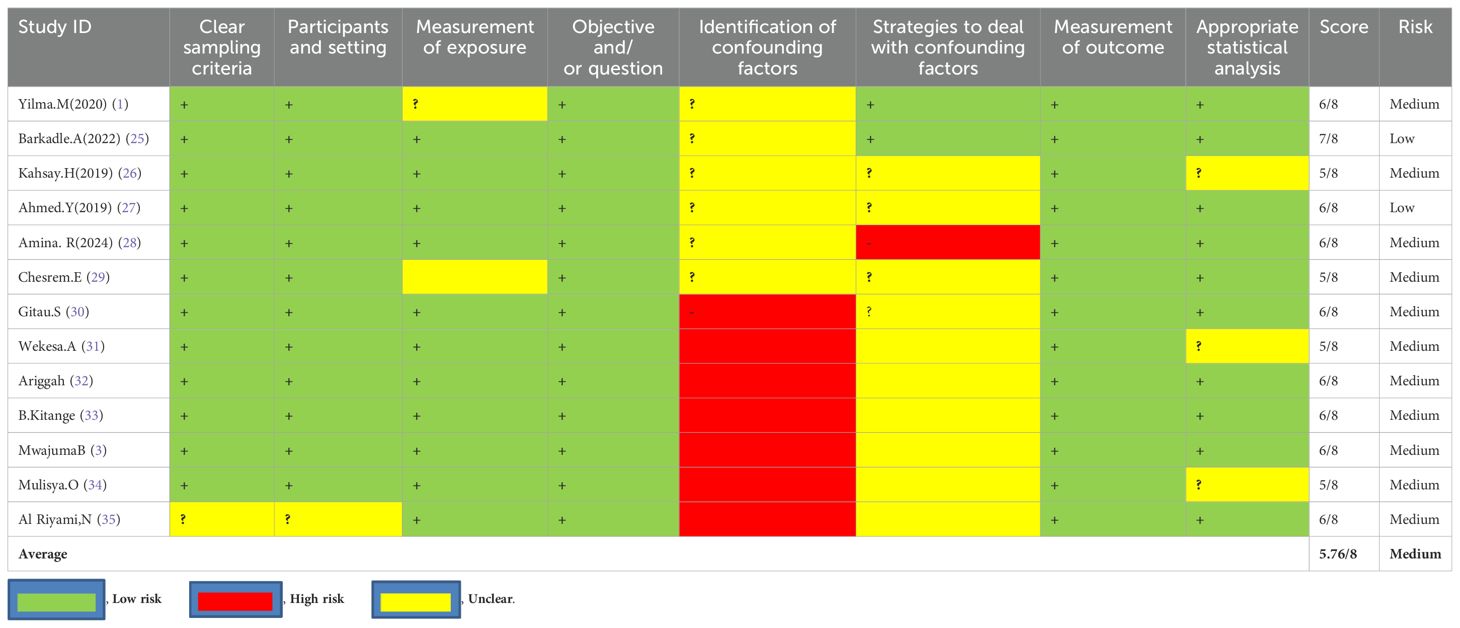
Two reviewers, NA and MA, independently assessed the risk of bias in the included studies using tools developed by the Joanna Briggs Institute, which comprise eight criteria. The assessment focused on several aspects: inclusion in the sample, descriptions of study subjects and settings, validity and reliability of measurements, confounding factors and strategies to address them, and appropriateness of the outcome measures. Scores of 7 or higher were classified as low risk, 5–6 as medium risk, and 4 or lower as high risk. Studies identified as low- and medium-risk were then included in the review. Depicts that the average risk of bias across the studies was 5.76, representing 72% (Table 1).
Effect measures and synthesis methods
The outcome variables were conceptually categorized for the qualitative synthesis using thematic approaches. Using a Microsoft Excel 2019 spreadsheet, preliminary effect measurements for the quantitative synthesis have been determined based on the qualitative synthesis. The effect estimates (proportions and odds ratios, or ORs) of the determinants of GTD in pregnant women were calculated by using STATA 17. Regardless of their significance levels, we included the associated variables, which were classified. These effect estimates were then compared among studies that targeted the outcome variables using subgroup statistical analysis. A 95% confidence interval (CI) and a p-value of less than 0.05 were taken to be the cutoff point for overall statistical significance.
Reporting bias and certainty assessment
The I2 statistic was used to evaluate study heterogeneity. By comparing the effect estimates (proportions and ORs) of the determinants of gestational trophoblastic illness between countries and based on research design, percentages of weights and subgroup analysis were used to gauge each study’s contribution to the overall meta-analysis. The outlier studies were also identified through a sensitivity analysis. Additionally, any inter-study bias was investigated using Doi plots.
Result
Study selection
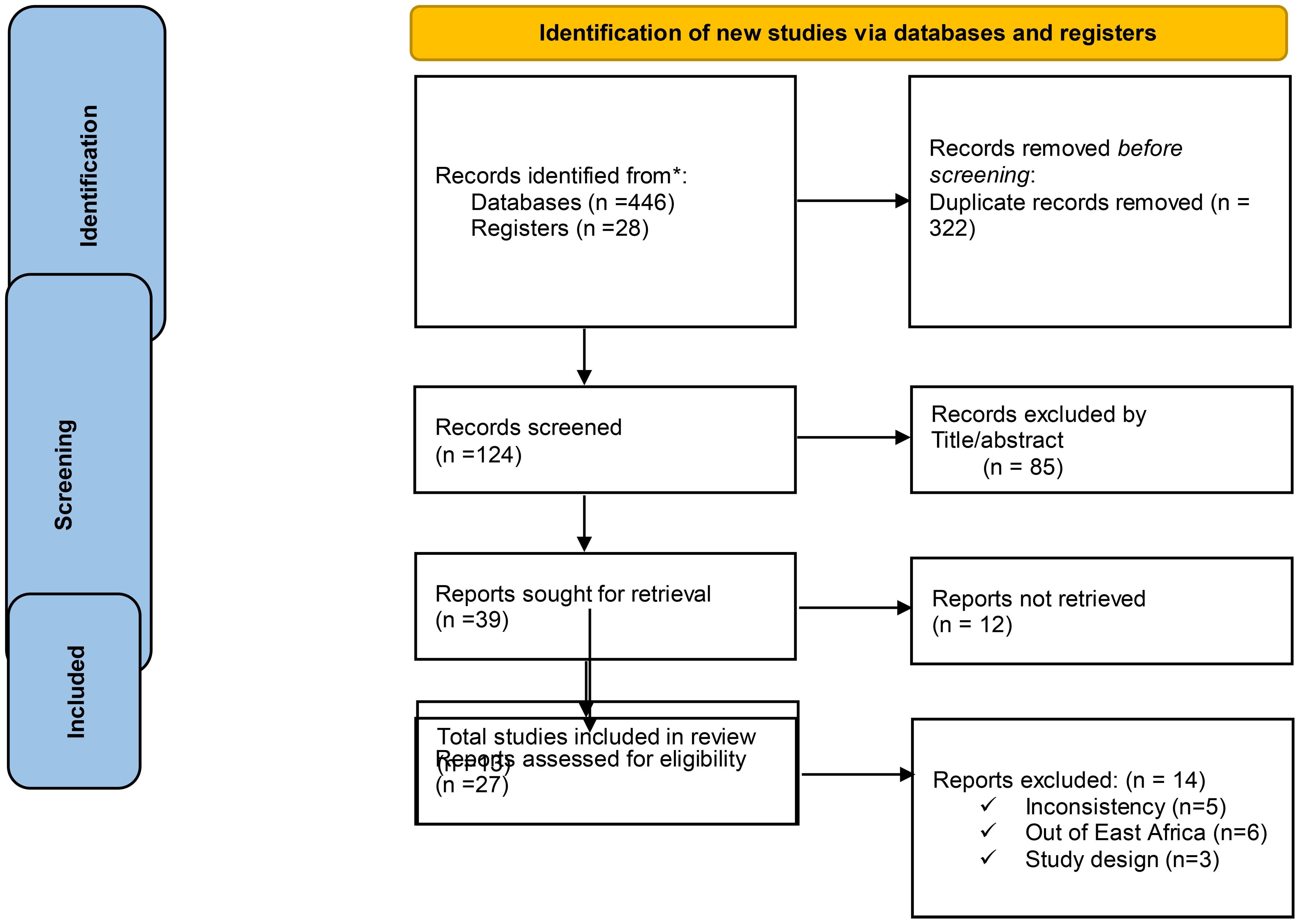
We systematically searched Google Scholar (n = 214), HINARI (n = 46), Scopus (n = 40), and PubMed (n = 146) on 1 May 2024. Additionally, 28 records were found through other sources, resulting in a total of 474 resources being searched. Following the removal of duplicates, 322 articles remained. Then, after excluding 124 resources through relevance, 85 articles were screened for title and abstract evaluation, which resulted in the exclusion of 39 resources by resource sought for retrieval (27 researches not fully filling the eligibility criteria and 12 researches by reports which were not retrieved) by full text review of each article. After the full text review of each article, 13 resources were identified for inclusion, all of that were considered suitable for the quantitative meta-analysis (Figure 1).
Qualitative synthesis
Characteristics of individual studies and participants pertain to GTD among pregnant women
A total of 13 studies from five countries in East Africa have been included [from Ethiopia (n = 4), Kenya (n = 5), Tanzania (n = 2), Uganda (n = 1), and Somalia (n = 1)]. Data were synthesized regarding the magnitude and its associated factors of GTD among pregnant women. Among the 13 studies, the majority (n = 9) were conducted by a cross-sectional study design, while the remainder (n = 4) were carried out by cohort types of study design (Table 2).

Table 2. Characteristics of individual Studies and participants pertain for gestational trophoblastic disease among pregnant women.
Prevalence of GTD among pregnant women
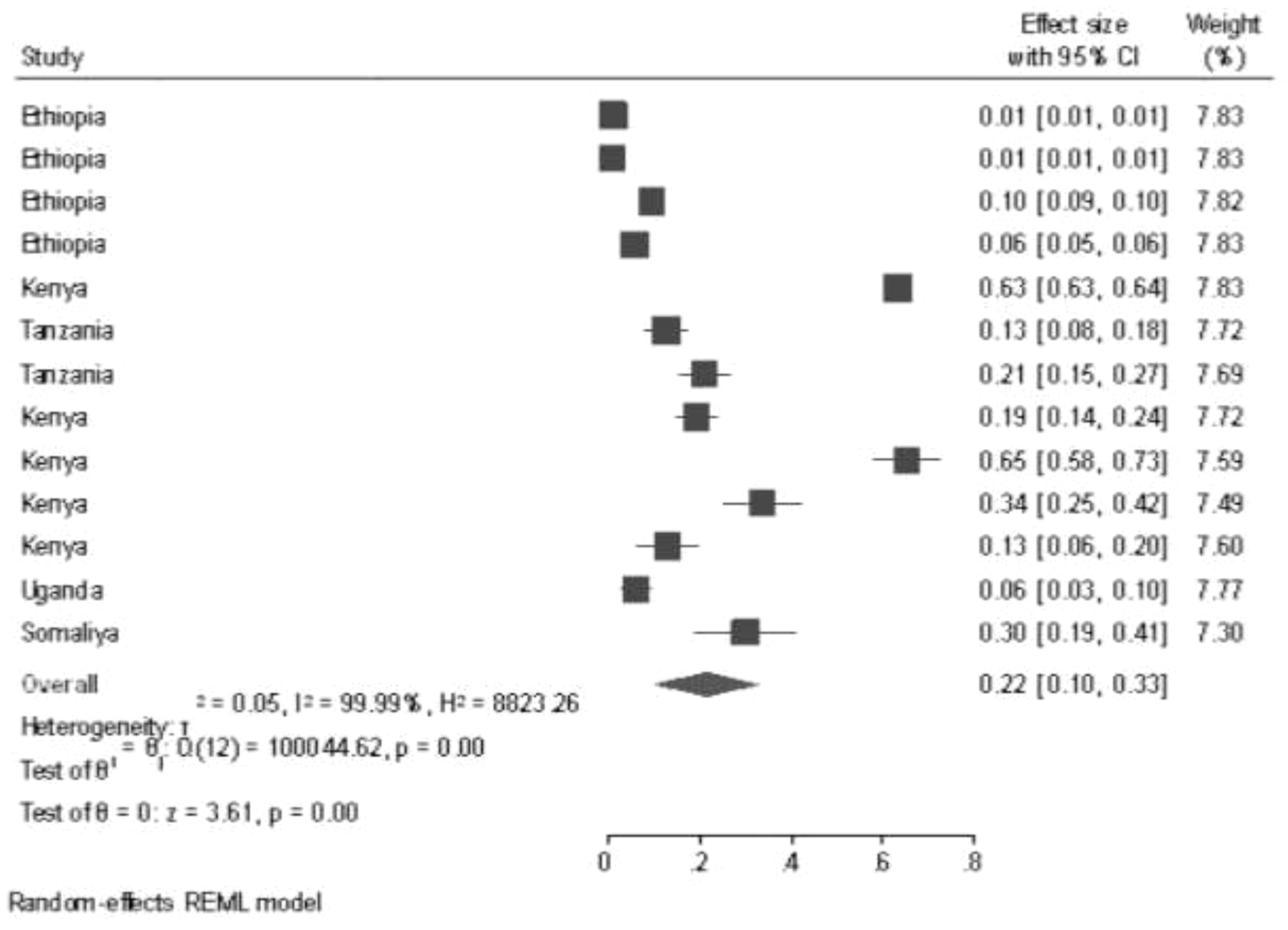
The estimated prevalence and ranges of GTD among pregnant women was reported by using a random effects model in East African countries. Double arcsine transformation was used to normalize the distribution of the effect size. This review revealed that there is a high prevalence of GTD among pregnant women in studies performed in Kenya, 65% (5% CI: 58%–73%), and this prevalence has declined in studies performed in Ethiopia, 0.1% (95% CI: 0.01–0.01).
The random effects model showed that the pooled prevalence of GTD among pregnant women was 22% (95% CI: 10%–33%), and there was significant heterogeneity among the studies (I2 = 99.99%, p < 0.001) (Figure 2).
Subgroup analysis and investigation of heterogeneity
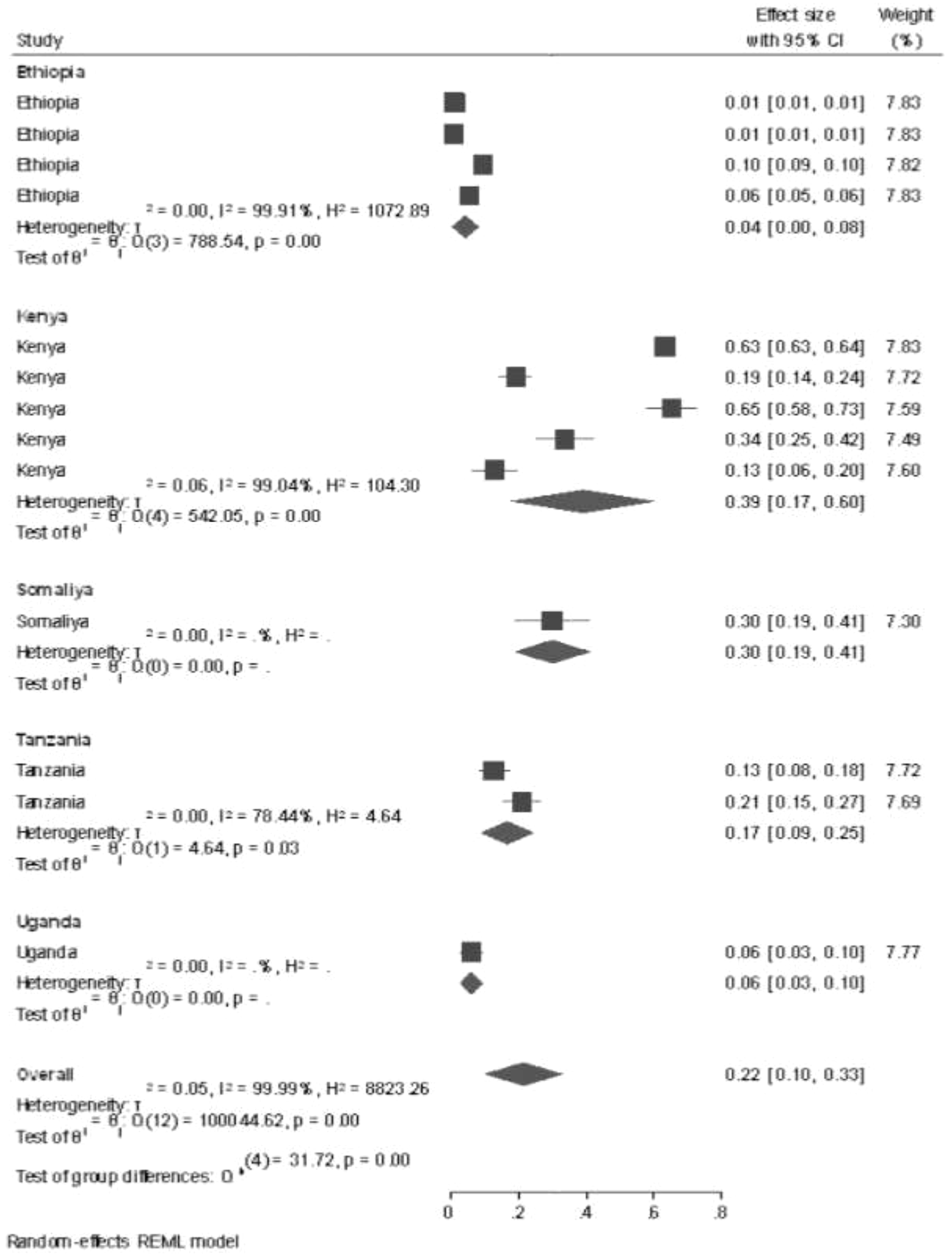
Subgroup analysis by country for all studies performed in East Africa was conducted by using a systematic review and meta-analysis method, and the prevalence of GTD among pregnant women was highest in the study conducted in Kenya (39%, 95% CI: 17%–60%), followed by Somalia (30%, 95% CI: 19%–41%), and the lowest prevalence of GTD among pregnant women was shown in the study conducted in Ethiopia (0.4%, 95% CI: 0.00%–0.8%) and 2015 (41%, 95% CI: 38%–45%). In the sub-country analysis, potential heterogeneity was detected between each study in the prevalence estimates of GTD among pregnant women (I2 = 99.99%; all p < 0.001). This potential heterogeneity took place using different sample sizes, sampling techniques, and different study populations (Figure 3).
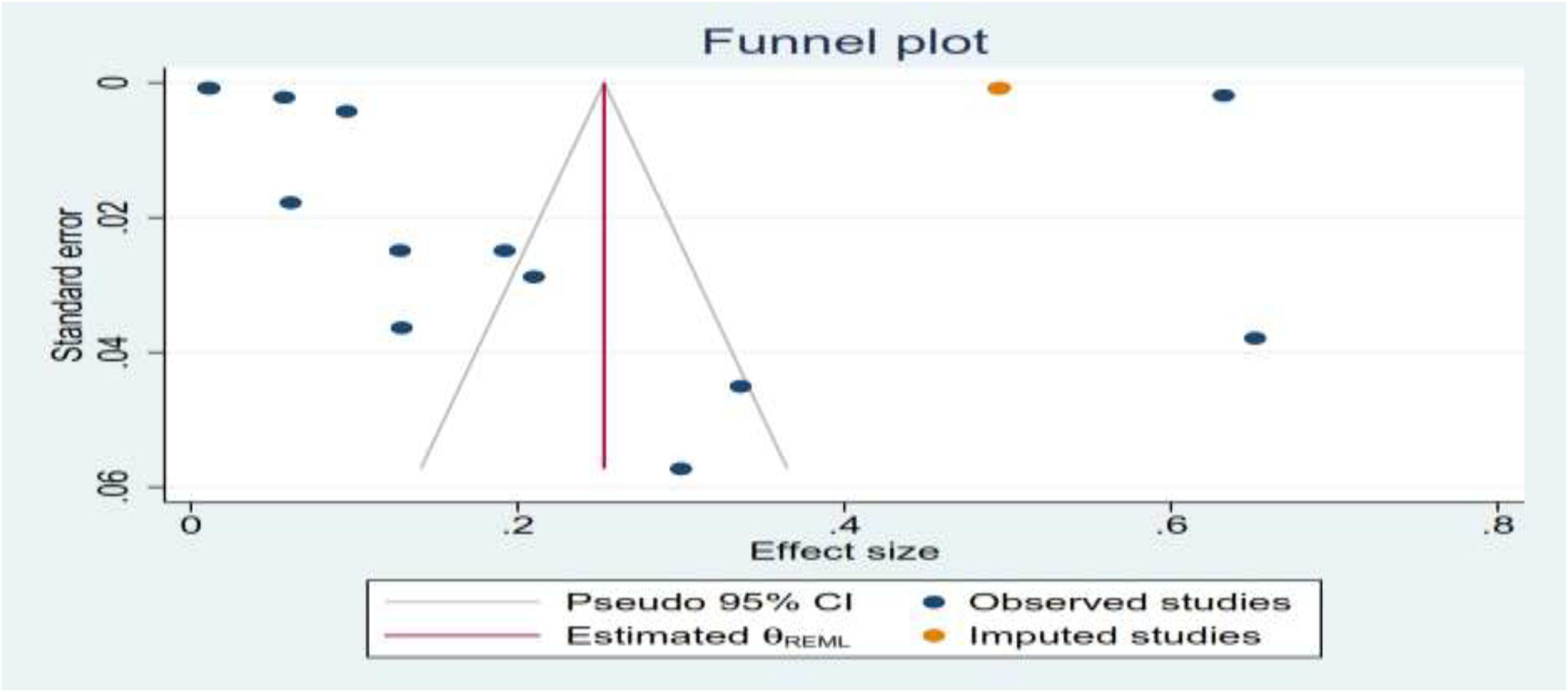
Publication bias of the study
Publication bias was assessed via visual inspection of funnel plots. Funnel plot analysis revealed that there were asymmetrical studies, which revealed publication bias between each study, but the publication bias analysis showed the test provides that there is evidence for the presence of small-study effects of the funnel plot; this effect takes place due to using of different studies done by different sample sizes, study populations, and different sampling techniques (Figure 4).
Sensitivity analysis
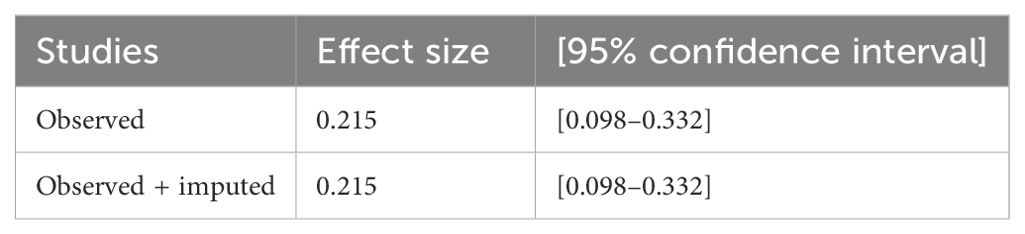
After assessing the variability effects between each study by considering the subgroup analysis and publication bias (I2 = 99.99%; all p < 0.001), there was detection of heterogeneity between each studies; therefore, a sensitivity analysis was done by using a trim and fill analysis to detect the studies that affect the variability effect between each variable. The nonparametric trim-and-fill analysis of publication bias showed that the heterogeneity effects were detected by omitting one study, which was done in Kenya, from the total findings (Table 3).
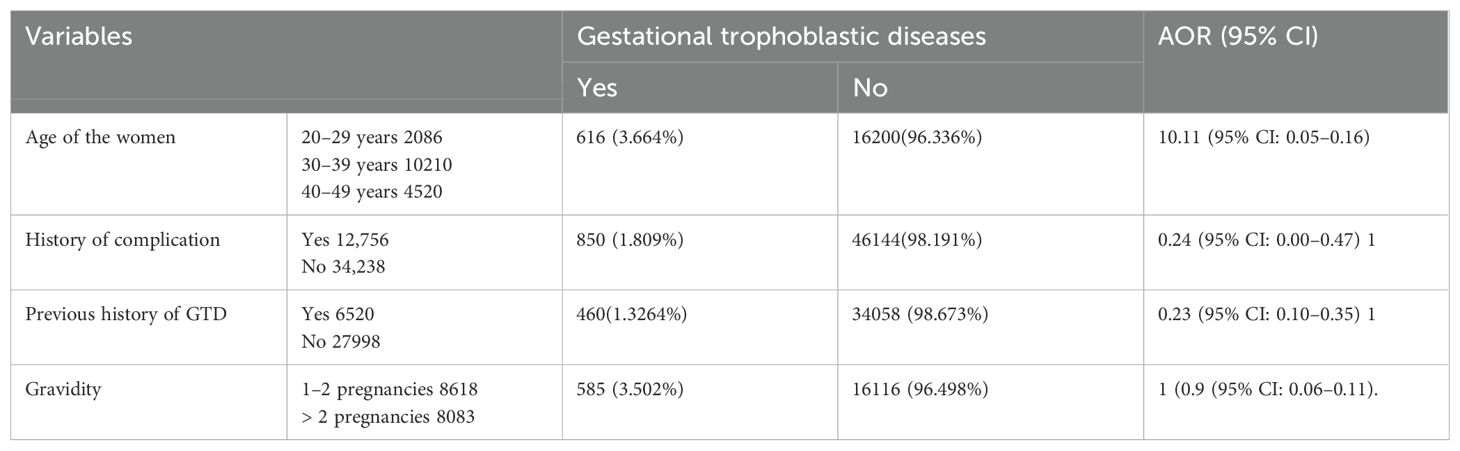
Associated factors of GTD among pregnant women
In this study, women who are aged between 30 and 39 years, the occurrence of complications during pregnancy, women who have more than two pregnancies, and women who have a previous history of GTD were significantly associated with GTDs during the pregnancy period.
In this study, women who were aged between 30 and 39 years were 11% less likely to develop GTD during pregnancy than those aged less than 30 years (AOR = 0.11, 95% CI: 0.05–0.16). Similarly, women who have a previous history of GTD were 24% less likely to develop GTD than those with no history of GTD during the pregnancy period (AOR = 0.24, 95% CI: 0.00–0.47).
Women who have previous complications of the reproductive organ system were 77% less likely to develop GTD during pregnancy (AOR= 0.23, 95% CI: 0.10–0.35). Women who have more than two histories of pregnancy were 91% less likely to develop GTD than those with a history of less than two pregnancies (AOR = 0.9, 95% CI: 0.06–0.11) (Table 4).
Discussion
In this study, the finding revealed that the pooled prevalence of GTD among pregnant women was 22% (95% CI: 10%–33%). This finding was similar to the study conducted in Egypt (11). But this study was higher than the study conducted in Tunisia (12), Egypt, Sindh (13), Enugu, Zaria (15), Kuanz-natal (16), Parma (17), Madhya (18), Odisha (19), and Mysore (20). But this finding was lower than the study conducted in Rajasthan (21) and Hyderabad (22). These differences might be due to study setting, healthcare access, diagnostic practices, and socio-demographic factors of each study.
In this finding age of the women between 30 and 39 years, complication history during the pregnancy period, previous history of GTD, and women who had more than two pregnancies were significantly associated with GTD during their pregnancy period.
In this finding, the ages of women between 30 and 39 years are highly risk factors for GTD; this finding was in line with studies conducted in Enugu, Nigeria (14), Tunisia (12), and Lower Egypt (11).
This study showed that women who have a previous history of GTD were significantly associated with GTD during her pregnancy period; this finding was in line with a study conducted in Qazvin (Iran) (23).
This finding showed that a history of complications was significantly associated with the GTD among women during the pregnant period; this finding was in line with a study conducted in Tehran, Iran (24).
This finding showed that gravity greater than 2 was significantly associated with the occurrence of GTD among pregnant women. This finding was congruent with a study conducted in Qazvin (Iran) (23); it is different from a study conducted in Indore, Madhya (18). This might be due to the socio-demographic status of the participants, types of study methodology, and study area of the participants, and this difference could be that the current study included pooled data from different East African countries.
Subgroup analysis by country for all studies performed in East Africa was conducted by using a systematic review and meta-analysis method, and the prevalence of GTD among pregnant women was highest in the study conducted in Kenya (39%, 95% CI: 17%–60%), followed by Somalia (30%, 95% CI: 19%–41%), and the lowest prevalence of GTD among pregnant women was shown in the study conducted in Ethiopia (0.4%, 95% CI: 0.00%–0.8%) and 2015 (41%, 95% CI: 38%–45%). The possible explanation of the difference might be due to the number of studies, types of study design, and sample size determinations; these are some factors affecting the sub-group results.
Limitation of the study
This study used only studies performed in East African countries. This study lacks comparator reviews done by systematic review and meta-analysis; we discussed the results of our systematic and meta-analysis against original studies conducted in various other countries out of the context area.
Conclusion
In this study, the finding revealed that the pooled prevalence of GTD among pregnant women was high compared with other African regions.
Women who are aged between 30 and 39 years, women with a previous history of GTD, women who have a previous complication of the reproductive organ system, and women who have more than two histories of pregnancy were significantly associated with the outcome variable. Therefore, all women should have preconception care before pregnancy and proper ANC follow-up during the pregnancy period, and also all concerned bodies should put their own efforts to eliminate the complication of the problem during the pregnancy period.
Author contributions
NA: Writing – original draft, Writing – review & editing. GB: Writing – original draft, Writing – review & editing. NY: Writing – original draft, Writing – review & editing. HG: Writing – original draft, Writing – review & editing. MW: Writing – original draft, Writing – review & editing. CM: Writing – original draft, Writing – review & editing. TE: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. ZB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yilma M, Fantu S, Hailu D, and Wassihun B. Magnitude of gestational trophoblastic disease at Hawassa university comprehensive specialized hospital, Ethiopia: A five-year retrospective analysis. Ethiopian J Reprod Health. (2020) 12:21–7. doi: 10.69614/ejrh.v12i02.370
2. UN E. FACTORS ASSOCIATED WITH GESTATIONAL TROFOBLASTIC DISEASE IN A PERUVIAN REFERENCE HOSPITAL. Rev Fac Med Hum. (2020) 20:64–9. doi: 10.25176/RFMH.v20i1.2547
3. Mdoe MB, Mwakigonja AR, and Mwampagatwa I. Gestational trophoblastic disease and associated factors among women experiencing first trimester pregnancy loss at a regional referral hospital in central Tanzania: a cross-sectional study. Int Health. (2023) 15:250–7. doi: 10.1093/inthealth/ihac015
4. Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecology Obstetrics. (2018) 143:79–85. doi: 10.1002/ijgo.2018.143.issue-S2
5. Omonua K, Isah A, and Adewole N. A review of gestational trophoblastic diseases in a tertiary hospital. Nigerian J Medicine. (2018) 27:342–8. doi: 10.4103/1115-2613.278800
6. Berkowitz RS and Goldstein DP. Current management of gestational trophoblastic diseases. Gynecologic oncology. (2009) 112:654–62. doi: 10.1016/j.ygyno.2008.09.005
7. Berkowitz RS and Goldstein DP. Gestational trophoblastic disease. Cancer. (1995) 76:2079–85. doi: 10.1002/1097-0142(19951115)76:10+<2079::AIDCNCR2820761329>3.0.CO;2-O
8. Gerulath A, Ehlen T, Bessette P, Jolicoeur L, and Savoie R. Gestational trophoblastic disease. J Obstetrics Gynaecology Canada: JOGC= J D’obstetrique Gynecologie du Canada: JOGC. (2002) 24:434–46.
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
10. Adams D. Publish or perish on microsoft windows st albans. United Kingdom: Tarma Software Research Ltd (2016). Available at: https://harzing.com/resources/publish-or-perish/windows.
11. Zakaria A, Hemida R, Elrefaie W, and Refaie E. Incidence and outcome of gestational trophoblastic disease in lower Egypt. Afr Health Sci. (2020) 20:73–82. doi: 10.4314/ahs.v20i1.12
12. Riadh BT, Abdellatif C, Wissal H, Leila A, Taher M, and Abdelhamid K. Clinical analysis and management of gestational trophoblastic diseases: a 90 cases study. Int J Biomed Science: IJBS. (2009) 5:321. doi: 10.59566/IJBS.2009.5321
13. Madhudas C, Khursheed F, and Srichand P. Analysis of patients with recurrent molar pregnancy. Eur J Biol Sci. (2011) 3:102–4. doi: 10.21608/EBWHJ.2024.44412.1113
14. Egwuatu V and Ozumba B. Observations on molar pregnancy in Enugu, Nigeria. Int J Gynecology Obstetrics. (1989) 29:219–25. doi: 10.1016/0020-7292(89)90258-0
15. Kolawole AO, Nwajagu JK, Oguntayo AO, Zayyan MS, and Adewuyi S. Gestational trophoblastic disease in Abuth Zaria, Nigeria: A 5−year review. Trop J Obstetrics Gynaecology. (2016) 33:209–15. doi: 10.4103/0189-5117.192228
16. Moodley M and Marishane T. Demographic variables of gestational trophoblastic disease in KwaZulu-Natal, South Africa. J Obstetrics Gynaecology. (2005) 25:482–5. doi: 10.1080/01443610500171102
17. Capozzi VA, Butera D, Armano G, Monfardini L, Gaiano M, Gambino G, et al. Obstetrics outcomes after complete and partial molar pregnancy: Review of the literature and meta-analysis. Eur J Obstetrics Gynecology Reprod Biol. (2021) 259:18–25. doi: 10.1016/j.ejogrb.2021.01.051
18. Singh T, Patel A, and Singh K. TO DETERMINE THE INCIDENCE, RISK FACTORS, CLINICAL PRESENTATIONS, DIAGNOSIS, TREATMENT OPTIONS, AND OUTCOMES OF MOLAR PREGNANCY. Obstetrics Gynaecology Forum. (2024).
19. Nayak AK, Hota S, Padhi M, and Jain MK. A prospective study on clinic oepidemiological PROFILE of molar pregnancy in a tertiary care HOspital. Int J Med Biomed Stud. (2019) 3:125. doi: 10.32553/ijmbs.v3i9.552
20. Neelakanthi A. A prospective clinical study of molar pregnancies in a tertiary care centre at Cheluvamba hospital. Mysore: Rajiv Gandhi University of Health Sciences (India (2014).
21. Kumari S, Bansal A, Poonia M, and Simlot A. A cross-sectional study of clinical analysis and management of molar pregnancy. J Med Sci Res. (2012) 3:13.
22. Aziz N, Yousfani S, Soomro I, and Mumtaz F. Gestational trophoblastic disease. J Ayub Med Coll Abbottabad. (2012) 24:7–9.
23. Alimohammadi N, Pakniat H, Mirzadeh M, Emami A, and Vasheghani Farahani A. Molar pregnancy and its associated risk factors: A case-control study in qazvin, Iran. J Advanced Biomed Sci. (2021) 11:4100–6. doi: 10.18502/jabs.v11i4.8635
24. Milani HS, Abdollahi M, Torbati S, Asbaghi T, and Azargashb E. Risk factors for hydatidiform mole: is husband’s job a major risk factor? Asian Pacific J Cancer prevention: APJCP. (2017) 18:2657. doi: 10.22034/APJCP.2017.18.10.2657
25. Barkadle A and Asebe T. MAGNITUDE, ASSOCIATED FACTORS AND POST-EVACUATION FOLLOW-UP OF GESTATIONAL TROPHOBLASTIC DISEASE AMONG DELIVERIES IN HIWOT FANA SPECIALISED UNIVERSITY HOSPITAL, HARAR, EASTERN ETHIOPIA. Haramaya University (2022).
26. Kahsay H. Histopathologic patterns of gestational trophoblastic disease in jimma university medical center, jimma, southwest Ethiopia : A three year retrospective study. (2019).
27. Ahmed Y. Incidence and clinical profiles of gestational trophoblastic diseases in south west Ethiopia. EC Gynaecology. (2019), 40–9.
28. Hassan AR, Itsura PM, Rosen BP, Covens AL, Shaffi AF, Odongo EB, et al. Mortality factors in high and ultra-high-risk gestational trophoblastic neoplasia at moi teaching & referral hospital: A decade-long observation in Kenya. Gynecologic Oncol Rep. (2024) 53. doi: 10.1016/j.gore.2024.101392
29. Cheserem EJ. Clinical-pathological characteristics and factorsassociated with mortality in patients managed forgestational trophoblastic neoplasia in Kenyatta nationalhospital, 2012–2020. A cross-sectionalstudy. University of Nairobi (2021).
30. Gitau SM. Predictors of gestational trophoblastic neoplasms chemotherapy outcomes at Kenyatta National Hospital: a retrospective cohort design study. University of Nairobi (2017).
31. Wekesa AN. Pregnancy outcomes after single agent versus multiagent chemotherapy for gestational trophoblastic neoplasia at Kenyatta national hospital. UON (2021).
32. Mwariggah RH. Management outcomes of gestational trophoblastic disease at Moi Teaching and Referral Hospital: An 8 year review. Moi University (2020).
33. Kitange B, Matovelo D, Konje E, Massinde A, and Rambau P. Hydatidiform moles among patients with incomplete abortion in Mwanza City, North western Tanzania. Afr Health Sci. (2015) 15:1081–6. doi: 10.4314/ahs.v15i4.5
34. Mulisya O, Roberts DJ, Sengupta ES, Agaba E, Laffita D, Tobias T, et al. Prevalence and factors associated with hydatidiform mole among patients undergoing uterine evacuation at mbarara regional referral hospital. Obstetrics gynecology Int. (2018) 2018:9561413. doi: 10.1155/2018/9561413
35. Al Riyami N, Al Riyami M, Al Saidi S, Salman B, and Al Kalbani M. Gestational trophoblastic disease at sultan qaboos university hospital: prevalence, risk factors, histological features, sonographic findings, and outcome. Oman Med J. (2018) 33:89–90. doi: 10.5001/omj.2019.39
Keywords: gestational trophoblastic disease, pregnant women, east africa, prevalence, associated factor
Citation: Abebaw N, Biset G, Yimer N, Gezie H, Workneh M, Mulugeta C, Emagneneh T, Alamrew A and Birhan Z (2025) Magnitude and its associated factors of gestational trophoblastic disease in East African countries, systematic review and meta-analysis. Front. Oncol. 15:1515246. doi: 10.3389/fonc.2025.1515246
Received: 22 October 2024; Accepted: 30 April 2025;
Published: 29 May 2025.
Edited by:
Payam Behzadi, Islamic Azad University, IranReviewed by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyQudus Olajide Lawal, Irrua Specialist Teaching Hospital, Nigeria
Montacer Hafsi, Tunis El Manar University, Tunisia
Copyright © 2025 Abebaw, Biset, Yimer, Gezie, Workneh, Mulugeta, Emagneneh, Alamrew and Birhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nigusie Abebaw, bmlndXNpZWFiZWJhd0BnbWFpbC5jb20=
 Nigusie Abebaw
Nigusie Abebaw Gebeyaw Biset
Gebeyaw Biset Nega Yimer
Nega Yimer Hailemariyam Gezie
Hailemariyam Gezie Moges Workneh2
Moges Workneh2 Chalie Mulugeta
Chalie Mulugeta Abebaw Alamrew
Abebaw Alamrew Zelalem Birhan
Zelalem Birhan