- 1Department of Breast Surgery, The Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming, Yunnan, China
- 2Department of General Surgery , No. 926 Hospital of the Joint Logistic Support Force of the Chinese People's Liberation Army, Kaiyuan, Yunnan, China
- 3Department of Thyroid and Breast Surgery, The Third People’s Hospital of Yunnan Province, Kunming, Yunnan, China
- 4Department of Breast Surgery, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 5Department of Gastroenterology and Oncology, The Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming, Yunnan, China
Background: This study aimed to investigate the risk factors associated with grade ≥3 diarrhea and neutropenia, which are the most common adverse events (AEs) leading to discontinuation and dose reduction in patients with hormone receptor-positive (HR+)/human epidermal growth factor 2-negative (HER2-) breast cancer treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitor abemaciclib combined with endocrine therapy (ET). Subsequently, two prediction nomograms were developed to serve as a foundation for enhancing the management of patients’ side effects and improving treatment quality.
Methods: A retrospective cohort analysis was conducted to explore the clinical characteristics and treatment variables of breast cancer patients treated with abemaciclib combined with ET in Yunnan Cancer Hospital from December 2021 to December 2022. Logistic regression was used to determine the risk factors for the occurrence of grade ≥3 diarrhea and neutropenia, and two kinds of nomograms were established. An external validation group of patients from three additional centers was used to validate the constructed nomograms. The area under the receiver operating characteristic (ROC) curve (AUC), calibration curve, and decision curve analysis (DCA) were used to assess the predictive performance and clinical applicability of the two nomograms.
Results: A total of 497 patients were included, including 403 in the modeling group and 94 in the external validation group. The results of the multifactorial analysis revealed that age ≥70 years, Eastern Cooperative Oncology Group (ECOG) score ≥1, and underlying gastrointestinal diseases were independent risk factors for grade ≥3 diarrhea. ECOG score ≥1, radiotherapy in the same period/within 1 month, and neutrophils ≤2.0×109/L before treatment were independent risk factors for grade ≥3 neutropenia. Two nomogram models were used to predict risk based on the above independent factors. The AUCs for the developmental and external validation groups were 0.747(95%CI:0.687-0.806) and 0.803(95%CI:0.702-0.918) for the diarrhea prediction nomogram and 0.765(95%CI:0.711-0.818) and 0.783(95%CI:0.691-0.892) for the neutropenia prediction nomogram, respectively. Calibration curves and DCA of both models also showed good predictive performance and clinical applicability.
Conclusion: We identified risk factors for grade ≥3 diarrhea and neutropenia in patients treated with abemaciclib combined with ET, and established a risk prediction nomogram, providing a scientific basis for safety assessment.
Introduction
Breast cancer is the number one health threat to women worldwide. Hormone receptor-positive (HR+)/human epidermal growth factor 2 negative (HER2–) breast cancer constitutes 60–70% of all breast cancer cases (1). For patients with HR+/HER2 metastatic or locally advanced breast cancer, as well as for patients with early breast cancer with specific risk factors, the use of CDK4/6 inhibitors combined with endocrine therapy (ET) has become the primary recommended regimen to significantly reduce the risk of disease progression or mortality (2–4). However, studies have shown that approximately 14.5–25.1% of patients discontinue treatment due to intolerance to severe adverse events (AEs) (5), which has a significant impact on patients’ ability to enjoy their quality of life and adhere to treatment. Nevertheless, the occurrence of AEs associated with drug therapy and the incidence and severity of these effects vary considerably (6, 7). Consequently, it is of paramount importance to accurately identify the risk factors for AEs following the use of CDK4/6 inhibitors and implement effective coping strategies in advance, with the objective of reducing the risk of AEs and optimizing the treatment effect and quality of life of patients.
Abemaciclib is a widely used oral CDK4/6 inhibitor in clinical practice. Current research shows that the most common AEs caused by dosage reduction or cessation in patients treated with abemaciclib are diarrhea and neutropenia (8, 9). Clinical studies have also shown that advanced age, menopause, and gastrointestinal diseases may increase the risk of diarrhea in patients with abemaciclib (10–12), and that neutropenia may be related to race, Eastern Cooperative Oncology Group (ECOG) score, and white blood cell level before treatment (13). Nevertheless, the enrollment of patients in large-scale clinical trials is strictly limited, precluding the ability to fully reflect the real-world occurrence of AEs. Currently, there is a lack of detailed studies on the risk factors related to the occurrence of AEs following the use of abemaciclib, a lack of systematic evaluation of the risk factors related to the occurrence of AEs in high-risk populations, and a lack of effective predictive models for intuitive risk assessment.
This study aimed to identify the risk factors associated with grade ≥3 diarrhea and neutropenia in patients with HR+/HER2- breast cancer treated with abemaciclib in combination with ET in a real-world setting. Furthermore, this study aimed to construct two risk prediction nomogram models to graphically represent the regression equation. The efficacy of the models was assessed through internal and external validations with the aim of developing a validated predictive tool for the individualized management of AEs.
Materials and methods
Study population
The clinical characteristics and therapeutic variables of patients with breast cancer treated with abemaciclib and ET were retrospectively collected at Yunnan Cancer Hospital, First Affiliated Hospital of Kunming Medical University, First People’s Hospital of Kunming and Third People’s Hospital of Yunnan Province from December 2021 to December 2022. Following the application of rigorous inclusion and exclusion criteria, 269 patients from the Yunnan Cancer Hospital and 94 patients from three additional hospitals were selected as the development and external validation groups, respectively. The following criteria were included (1): females aged ≥18 years (2); pathologically confirmed HR+/HER2– breast cancer (3); at least one cycle of standard-dose CDK4/6 inhibitor combined with ET; and (4) patient and family consent. The following were excluded (1): visceral crisis, other serious diseases, or other malignant tumors (2); inflammatory breast cancer (3); no assessment of adverse drug reactions and patients lost to follow-up; and (4) lack of baseline clinicopathological and hematological data. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital (approval number: KYLX2023-011).
Data collection
The following clinical data was obtained: age, body mass index (BMI), ECOG score, menopausal status, pathological information (clinical stage, molecular typing), types of combined ET (aromatase inhibitors (AI), fulvestrant), combined with other drugs (anti-bone metastasis therapy, chronic disease drugs, etc.), body surface area (BSA), metastasis status (quantity and position), previous antineoplastic therapy (chemotherapy, radiotherapy, ET), antibiotic use during treatment, combined underlying diseases, and hematological parameters before treatment. Underlying diseases included cardiovascular diseases (coronary heart disease, heart disease and hypertension), metabolic diseases (diabetes, hyperlipidemia, hyperuricemia, hyperthyroidism, and hypothyroidism), gastrointestinal diseases (colitis, irritable bowel syndrome, inflammatory bowel disease, constipation, chronic diarrhea, food intolerance, and pelvic radiotherapy), and a history of liver diseases (hepatitis, cirrhosis, and fatty liver). Hematological parameters before treatment included neutrophil (NE) count, white blood cell (WBC) count, albumin (propagated), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and serum creatinine (Cr) levels. The hematological data were obtained from the most recent hematological examination conducted 21 days before abemaciclib administration.
Drug use methods
The patients in this study received abemaciclib at an initial dose of 150 mg bis in die (BID), in combination with AI, such as anastrozole, letrozole, and exemestane, or in combination with selective estrogen receptor downregulatory (SERD) fulvestrant. Dose adjustments were made during patient administration based on safety and tolerability. The first recommended dose adjustment was 100 mg BID.
Follow-up
Patients were followed-up by telephone and case review for 12 months following drug administration or until they developed intolerable AEs and discontinuation was caused by disease progression. During the follow-up period, all AEs occurring in patients were recorded; if multiple AEs occurred, the highest-grade AE was recorded. The seriousness of the AEs was graded according to the Criteria for the Evaluation of Therapeutic Adverse Events (CTCAE version v5.0) (14).
Statistical analysis and model construction
Statistical analysis was conducted using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize measures, including averages with standard deviations, counts, or scores based on clinical cutoffs. Frequencies and percentages were used to describe nominal variables. Group comparisons were made using independent samples t-tests for measures and Pearson chi-squared or Fisher’s exact tests for counts. Univariate analyses evaluated all clinical characteristics and treatment variables associated with AEs. To ascertain the independent associations between the variables and AEs, those with P < 0.1 in the univariate analysis were entered into a multivariate logistic regression. Independent risk factors identified from the multifactorial analysis were used to construct a nomogram model using R software version 4.3.1 (Foundation for Statistical Computing, Vienna, Austria). Model discriminative ability was assessed using C-index and area under the receiver operating characteristic (ROC) curve (AUC), with internal validation using 1000 bootstrap resampling. Calibration curves assessed model accuracy, and clinical utility was assessed using decision curve analysis (DCA). External validation was performed using comparable differentiation, calibration, and clinical utility assessments. Statistical significance was set at α = 0.05. Figure 1 illustrates the study methodology flowchart.
Results
Real-world occurrence of AEs
In total, 363 patients treated with abemaciclib combined with ET were included. During the follow-up period, approximately 98% of patients developed AEs of varying severity. A total of 27 AEs were monitored, and a total of 23 AEs with an incidence ≥10% were observed (Supplementary Table S1). The three most prevalent grade ≥3 AEs were neutropenia (27.4%), diarrhea (19.9%), and fatigue (10.3%). AEs that resulted in treatment discontinuation were observed in 115 patients (23.4%), which included 38 patients (7.6%) with diarrhea and 28 patients (5.6%) with neutropenia. AEs resulting in dose reduction were observed in 91 patients (18.3%), which included 31 patients (6.2%) with diarrhea and 15 patients (3.0%) with neutropenia.
In the therapeutic regimen of abemaciclib combined with Endocrine Therapy (ET), the specific ET agents administered were as follows: fulvestrant in 210 cases (42.3%), exemestane in 47 cases (9.5%), anastrozole in 182 cases (36.6%), and letrozole in 58 cases (11.7%). Among the AEs leading to the highest incidence of drug withdrawal and dose reduction, the incidence of diarrhea and neutropenia of any grade and grade ≥3 did not differ significantly between the groups combined with different ET treatments (Supplementary Table S2).
Baseline characteristics for development and external validation groups
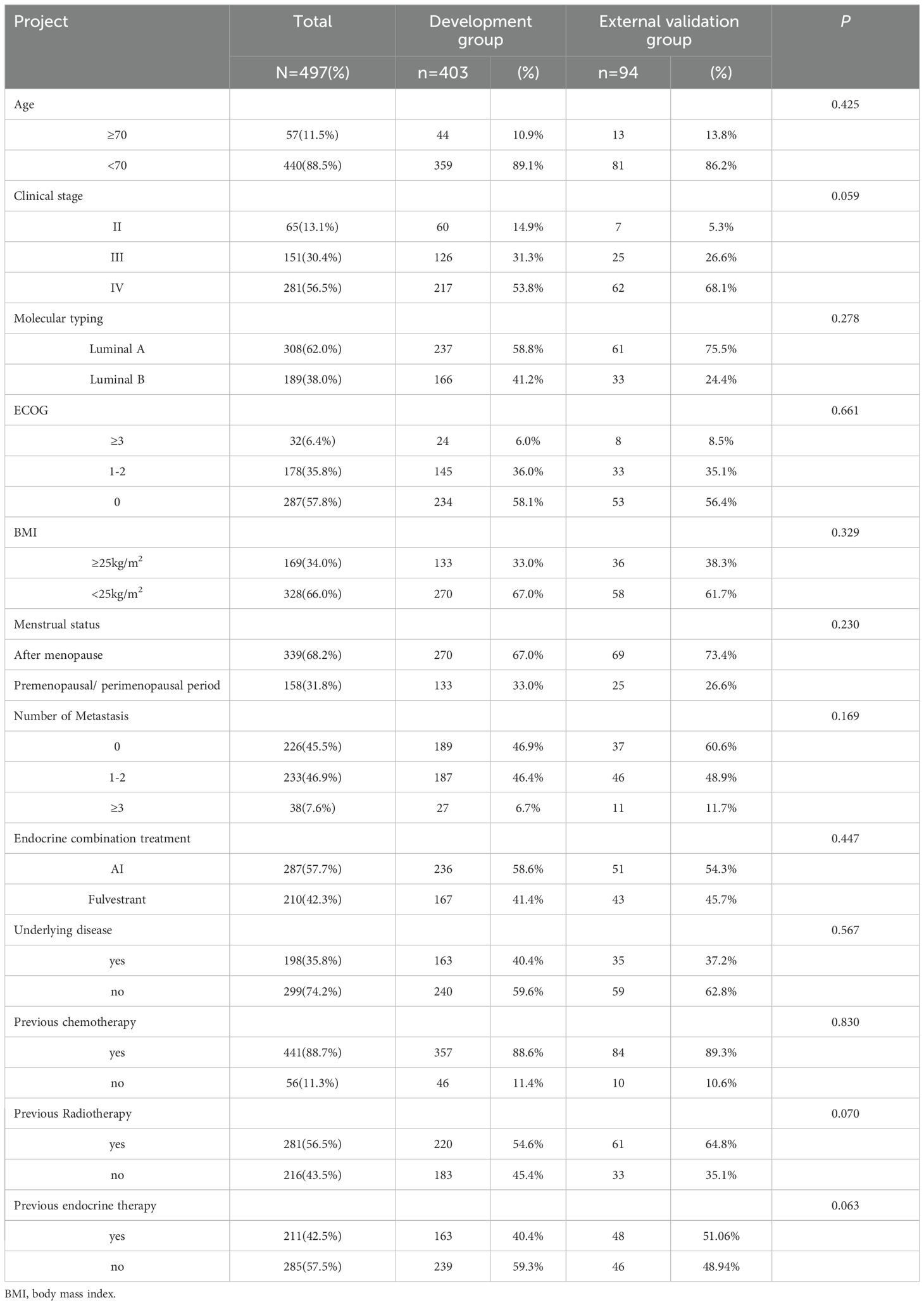
The study included 363 patients treated with abemaciclib plus ET, with 403 in the developmental group and 94 in the external validation group. The average age of the development group was 53.05 ± 11.14 years, and that of the external validation group was 51.12 ± 12.74 years. After all patients were followed up, it was found that 79 patients had ≥ grade 3 diarrhea in the modeling group, with an incidence of 19.6%, and 107 patients had ≥ grade 3 neutropenia, with an incidence of 26.6%. In the external validation group, 20 patients had grade ≥3 diarrhea, with an incidence of 21.3%, and 29 patients had grade ≥3 neutropenia, with an incidence of 30.9%. Baseline characteristics were not significantly different between the two groups, and general clinical information is shown in Table 1.
Risk factor analysis and nomogram construction for grade ≥3 diarrhea
Univariate and multivariate analysis of grade ≥3 diarrhea
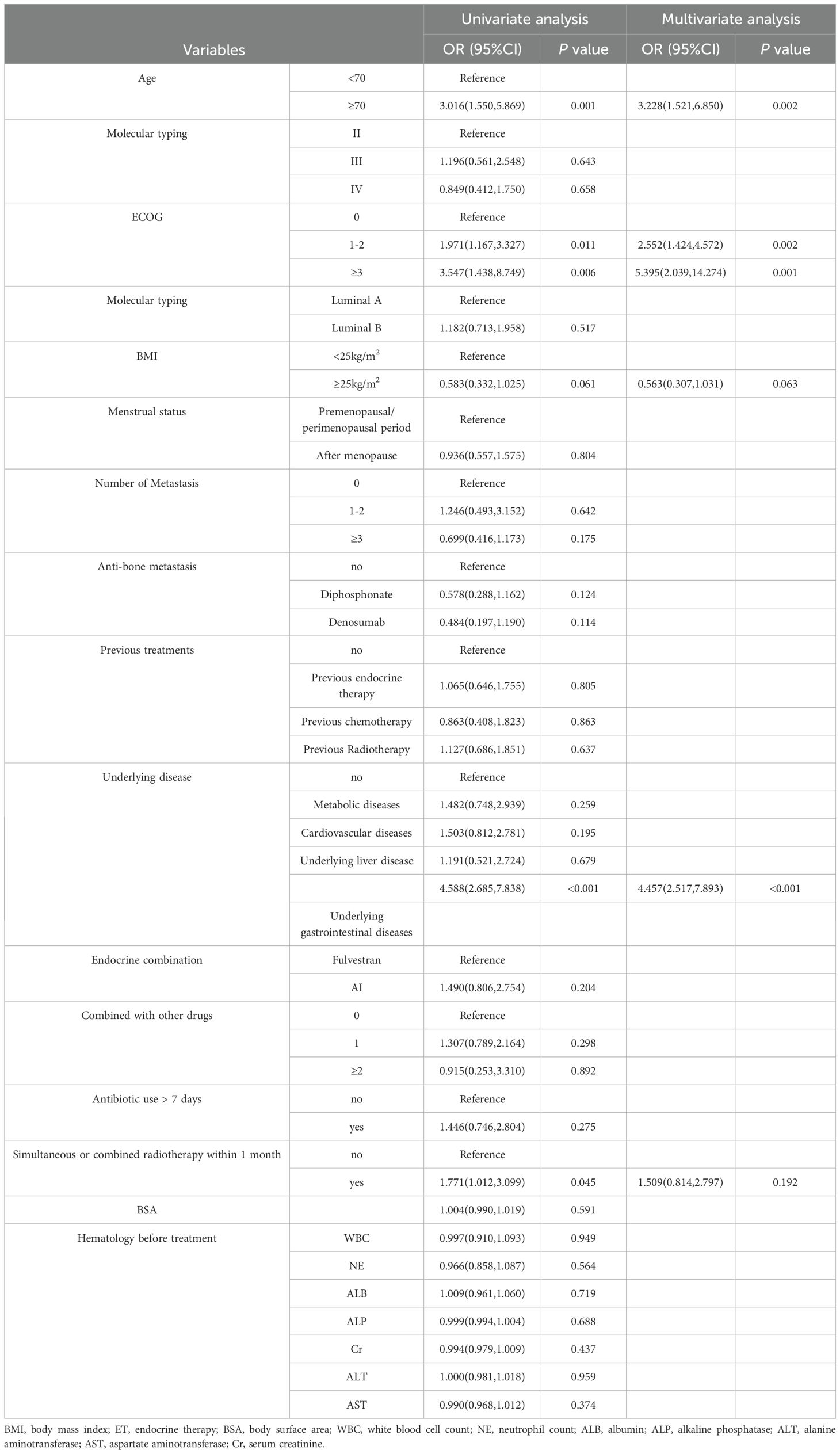
To determine the independent risk factors for the occurrence of grade ≥3 diarrhea with abemaciclib and ET, risk factors were analyzed using univariate and multivariate logistic regression, as shown in Table 2. From the results of univariate analysis, grade ≥3 diarrhea was associated with age, ECOG, combined with underlying gastrointestinal disease, and radiotherapy in the same period/within 1 month(p < 0.05). To mitigate the effects of confounding factors, a multivariate logistic regression analysis was conducted, and it incorporated variables with p < 0.1 in the univariate analysis. The results showed that age ≥70 years, ECOG score ≥1, and combined with underlying gastrointestinal diseases were independent risk factors for grade ≥3 diarrhea following abemaciclib combined with ET.
Development and validation of a nomogram for grade ≥3 diarrhea
According to the multifactorial logistic regression analysis of independent risk factors for grade ≥3 diarrhea, a nomogram model was constructed using the “rms” package in R software, as shown in Figure 2. The factors in the nomogram were assigned points according to their effect on the dependent variable, and the point value line represents the estimated point value for each of the risk factors. The scores for each factor were summed to obtain the total score, and the frequency of grade ≥3 diarrhea was estimated from the total score. A higher score corresponded to a higher likelihood.
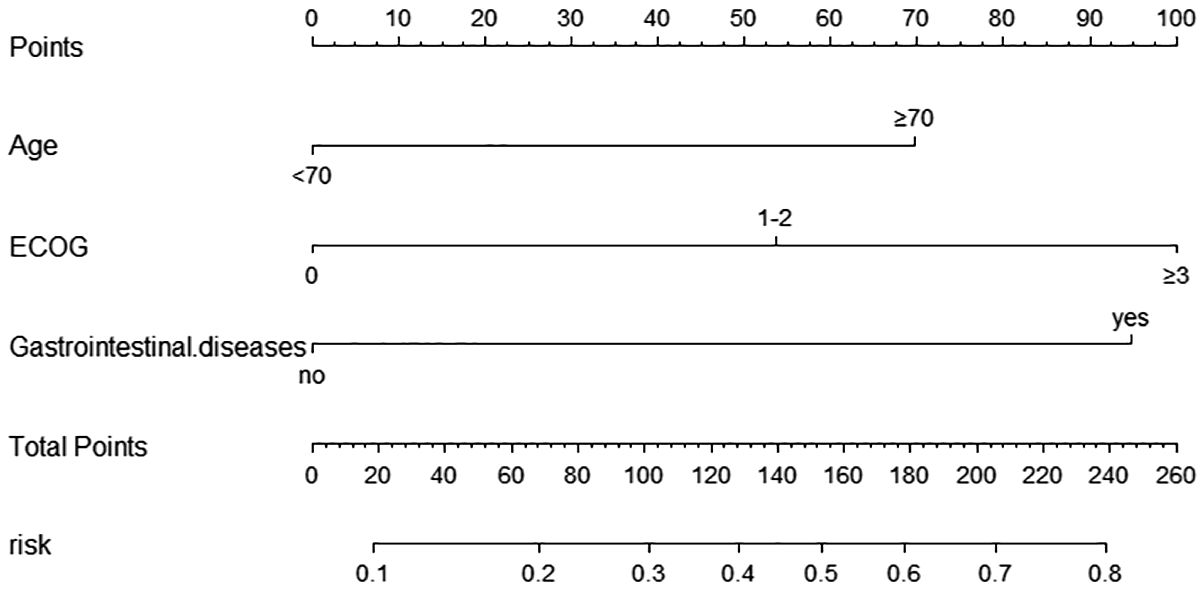
Figure 2. Nomogram for predicting the occurrence of ≥ grade 3 diarrhea in the modeling Group. Scores of risk factors: “Age” = Age, <70 = 0 points, ≥70 = 70 points; “ECOG” = ECOG score, 0 = 0points, 1-2 = 54 points, ≥3 = 100 points; “Gastrointestinal.disease” = complicated with underlying Gastrointestinal diseases, no=0 points, yes=95 points.
Nomogram model performance was evaluated by C-index calculation and plotting ROC, calibration, and DCA curves as shown in Figure 3. Internal validation of the model showed a C-index of 0.747, and the ROC curve results showed an AUC of 0.747(95% CI: 0.681–0.829), a sensitivity of 0.750, and a specificity of 0.646, which indicates that the model has a good value of prediction. The model predictions were in good correspondence with the actual observations, as shown by the calibration curve. The DCA results demonstrated the net clinical benefit of the nomogram model in identifying the risk of developing grade ≥3 diarrhea. The model was then externally validated. External validation ROC curve analysis showed an AUC of 0.803 (95% CI: 0.702-0.918), sensitivity of 0.784 and specificity of 0.750. The values predicted in the calibration curve agreed well with the measured values. The DCA curve showed a good net clinical benefit in the nomogram.
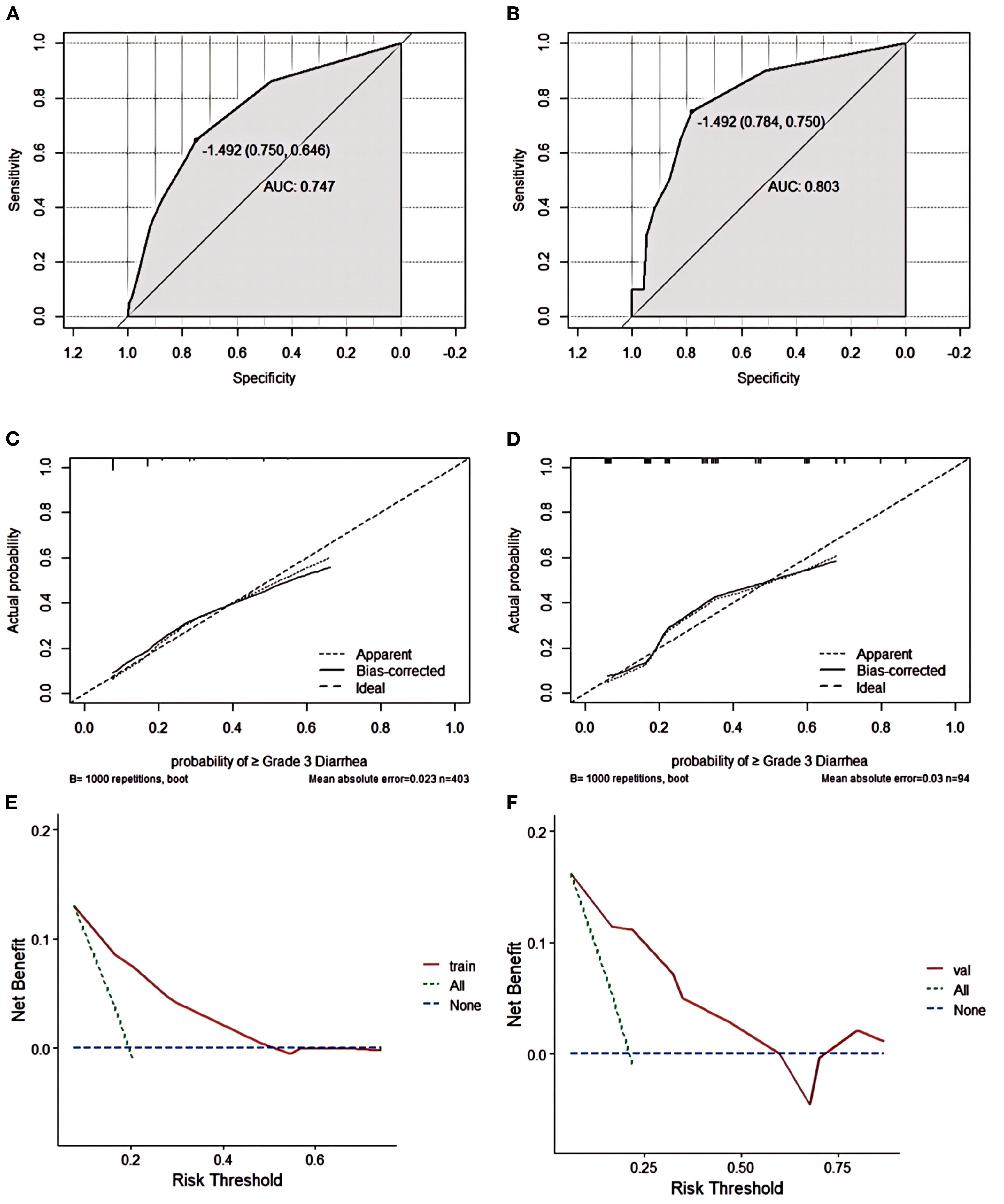
Figure 3. Grade ≥3 diarrhea nomogram evaluation (A–F). ROC curves of the nomogram for the training set (A) and the external validation set (B). The AUC values of the training cohort and the external validation cohort were 0.747 and 0.803, respectively.Nomogram calibration curves for training set (C) and validation set (D). DCA curves for the training set (E) and validation set (F).
Risk factor analysis and nomogram construction of grade ≥3 neutropenia
Univariate and multivariate analysis of grade ≥3 neutropenia
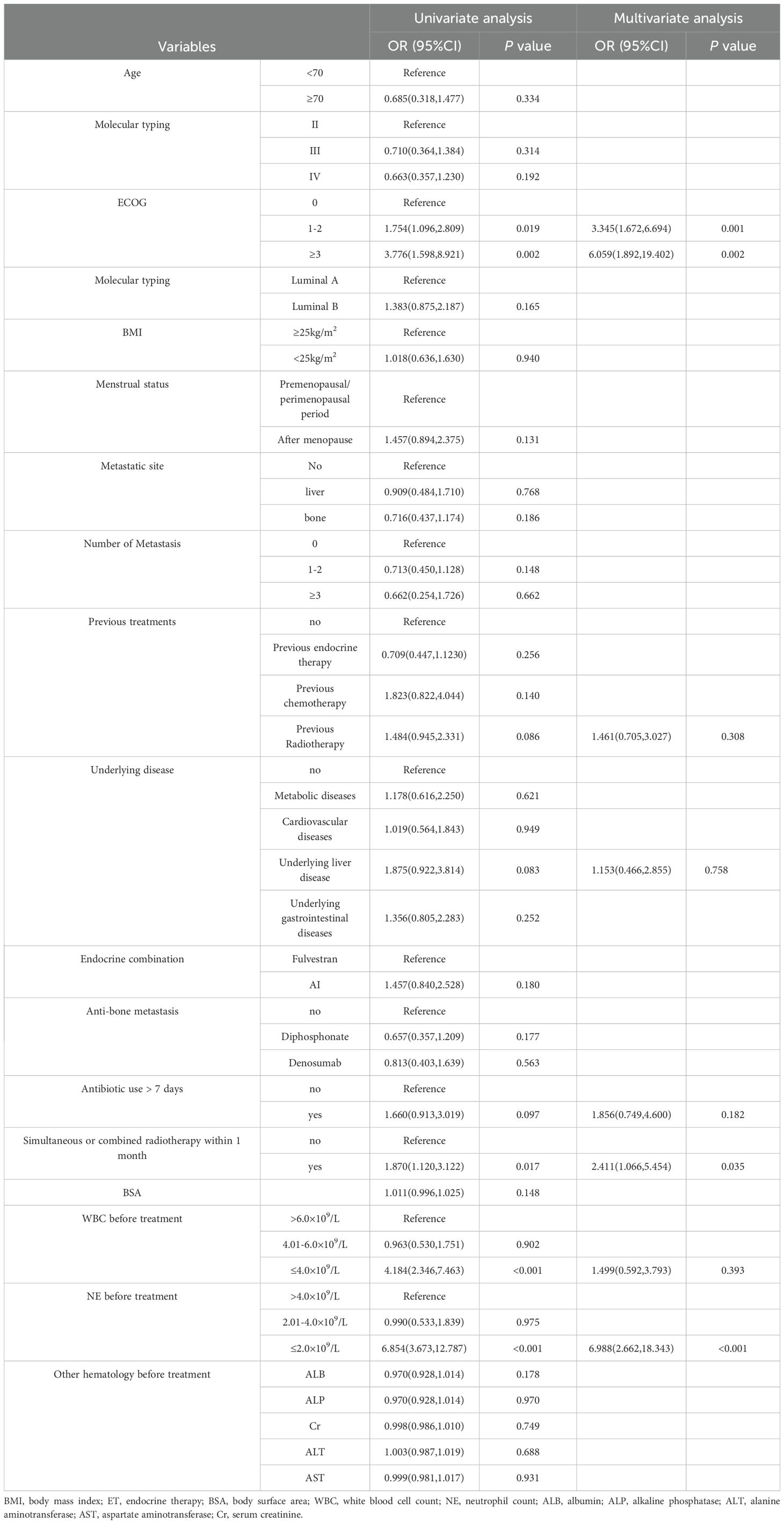
To determine the independent risk factors for the occurrence of grade ≥3 neutropenia with abemaciclib and ET, risk factors were analyzed using univariate and multivariate logistic regression as shown in Table 3. Univariate analysis revealed that grade ≥3 neutropenia was related to ECOG, radiotherapy in the same period/within 1-month, WBC before treatment, and NE before treatment (p < 0.05). To control for confounding factors, multivariate logistic regression analysis included variables with p<0.1 in the univariate analysis. The results showed that ECOG score ≥1, radiotherapy in the same period/within 1 month, and NE count ≤ 2.0 × 109/L before treatment were independent risk factors for grade ≥3 neutropenia following treatment with abemaciclib and ET.
Development and validation of a nomogram for grade ≥3 neutropenia
According to the multifactorial logistic regression analysis of independent risk factors for grade ≥3 neutropenia, a nomogram model was constructed using the “rms” package in R software as shown in Figure 4. Scores were assigned according to the influence of the factors on the dependent variable in the nomogram, with the score line representing the estimated score for each risk factor. The incidence of grade ≥3 neutropenia was estimated based on the sum of the scores for each factor and the total score.
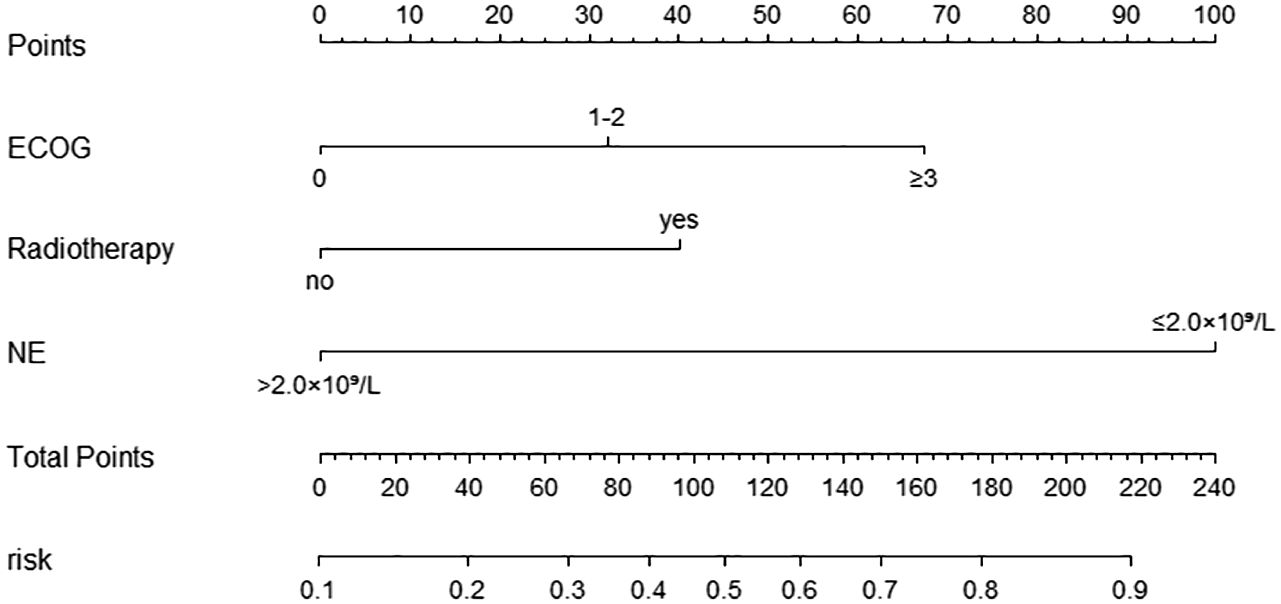
Figure 4. Nomogram for predicting the occurrence of ≥ grade 3 neutropenia in the modeling Group. Scores of risk factors: “ECOG” = ECOG score, 0 = 0 points, 1-2 = 32 points, ≥3 = 67 points; “Radiotherapy”= simultaneous or combined radiotherapy within 1 month, no=0 points, yes=40 points, “NE”= neutrophil level before treatment, > 2.0×109/L=0 points, ≤2.0×109/L=100 points.
Nomogram model performance was evaluated by C-index calculation and plotting ROC, calibration, and DCA curves as shown in Figure 5. The model was validated both internally and externally. In the development group, the C-index was 0.765, and the ROC curve showed that the AUC was 0.765 (95% CI: 0.711-0.818), sensitivity was 0.769, and specificity was 0.714. The ROC curve analysis of the external validation group showed that the AUC was 0.783(95% CI: 0.691-0.892), sensitivity was 0.785, and specificity was 0.690, indicating that the predictive value of the model was good. In both the development and external validation groups, the predicted values on the calibration curve were in good agreement with the measured values. The DCA results showed that both groups were clinically effective in identifying the risk of grade ≥3 neutropenia with a nomogram model within the threshold range.
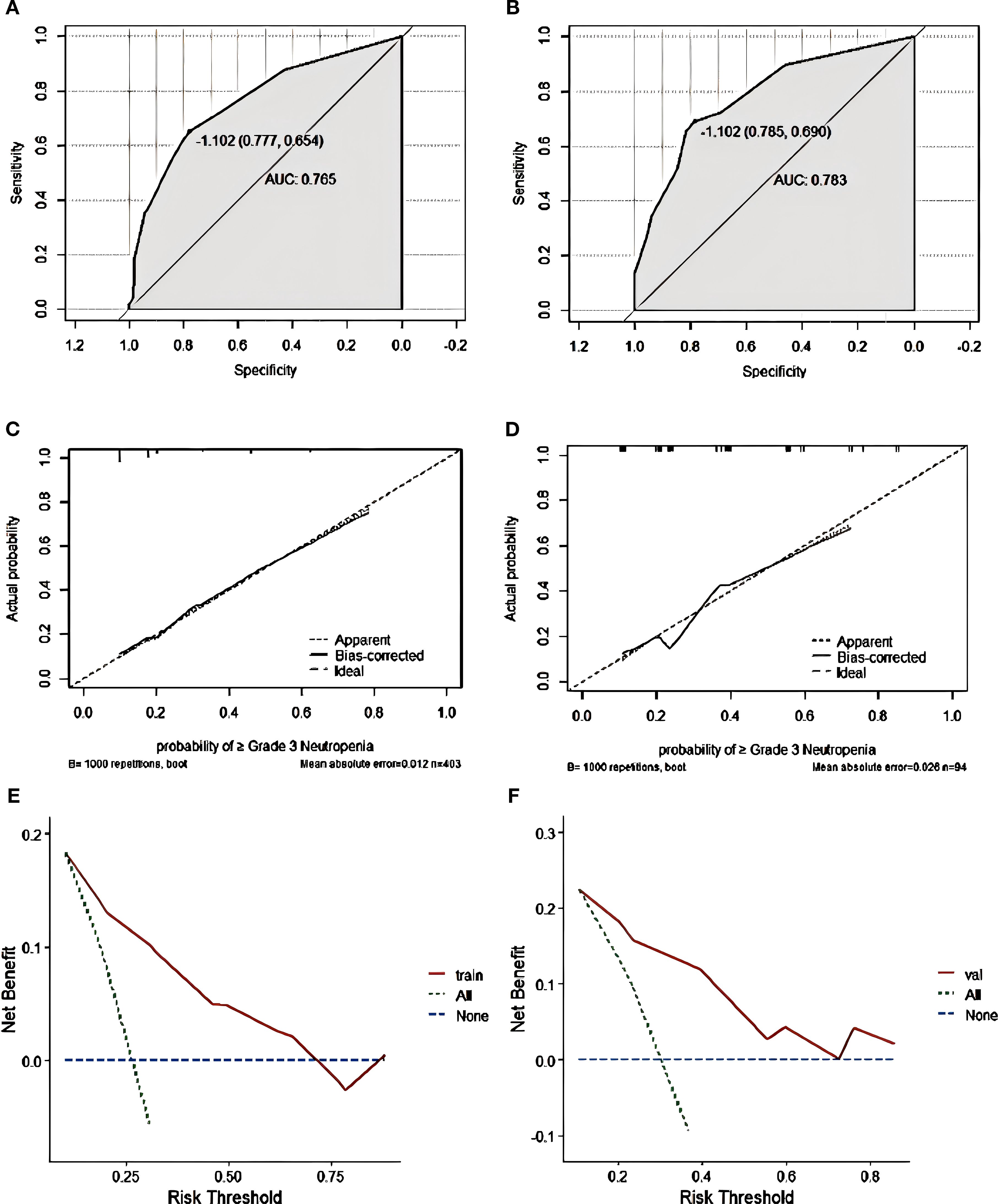
Figure 5. Grade ≥3 neutropenia nomogram evaluation (A–F). ROC curves of the nomogram for the training set (A) and the external validation set (B). The AUC values of the training cohort and the external validation cohort were 0.765 and 0.783, respectively. Nomogram calibration curves for training set (C) and validation set (D). DCA curves for the training set (E) and validation set (F).
Discussion
CDK4/6 inhibitors combined with ET have become the primary recommended regimen for salvage treatment of HR+/HER2- advanced breast cancer and adjuvant intensive treatment of locally advanced breast cancer with high-risk factors. Although the focus is on anticancer efficacy in clinical practice, monitoring and managing AEs is equally important. Notably, both single severe AEs and multiple superimposed AEs place a serious burden on the physical and mental health of patients and may even necessitate treatment interruption, thereby changing the survival outcome of patients. Both the clinical trial data reported in the current study and our real-world follow-up data show that diarrhea and neutropenia are the most common adverse events leading to treatment discontinuation and dose reduction of the CDK4/6 inhibitor abemaciclib plus endocrine therapy. Our study also revealed that approximately 54.7% (272 cases) of patients had grade 3 ≥ AEs, approximately 23.1% (115 cases) of patients discontinued treatment due to intolerable AEs, and approximately 18.3% (91 cases) of patients reduced the dose. These findings were slightly higher than the statistical data from the MONARCH 2 and MONARCH 3 clinical trials. This may also reflect the differences between clinical practice and clinical trials in the population and the standardized management of the treatment process. In the present study, we compared the occurrence of diarrhea and neutropenia following the use of the combination of abemaciclib with several ET agents and found no significant differences. Therefore, this study explored the risk factors for diarrhea and neutropenia after the use of CDK4/6 inhibitor abemaciclib combined with ET based on real-world evidence and integrated the risk factors in the regression model through R language to form a nomogram prediction model, which provided a convenient and individualized prediction tool for clinicians.
In addition to inhibiting CDK4 and CDK6, abemaciclib also has a strong inhibitory effect on CDK9 (15), which is mainly distributed in the gastrointestinal tract; therefore, abemaciclib-related AEs show a high incidence of diarrhea and fatigue. However, the precise mechanism underlying the induction of diarrhea by abemaciclib remains to be elucidated. Studies have shown that abemaciclib inhibits glycogen synthase kinase-3β (GSK3β) activity in patients with diarrhea, leading to impaired epithelial cell differentiation. Furthermore, abemaciclib-induced diarrhea also significantly downregulates the Ca2+/calmodulin-dependent protein kinase CAMKII (16), involved in intestinal motility, which may be associated with defecation. Previous studies have indicated that the incidence of diarrhea grade 2–3 diarrhea is significantly elevated in patients aged ≥70 years, those receiving multiple drugs concurrently, and those with pre-existing gastrointestinal diseases (13, 17). Our study results also showed that age ≥70 years and underlying gastrointestinal disease were independent risk factors for grade ≥3 diarrhea. The risk of multiple AEs increases with the use of CDK4/6 inhibitors in elderly patients, and a slightly higher rate of dose adjustment and discontinuation is observed in this population (12). Toxicity and efficacy analyses have shown that older women receiving CDK4/6 inhibitors experience more severe toxicity than younger women (18, 19). The underlying causes may include organ dysfunction, comorbidities, prolonged multidrug co-administration, drug distribution, and pharmacokinetic alterations in elderly patients (11). Patients with underlying gastrointestinal diseases have diminished gastrointestinal function, rendering them susceptible to severe gastrointestinal toxicity when treated with CDK4/6 inhibitors. Therefore, other CDK4/6 inhibitors are recommended for patients with poor gastrointestinal function. In addition, we considered the influence of concurrent use of other chronic drugs on the toxicity of CDK4/6 inhibitors. A statistical analysis revealed that approximately 30–50% of prescription drugs for chronic diseases in our country are not taken as prescribed, and that drugs taken in combination by each patient are inconsistent. Our study counted the number of drugs regularly taken by patients while taking abemaciclib and found no correlation with diarrhea. However, this issue may be regarded as a limitation of our study because our numerical definitions of multiple medications did not consider the role of the respective medications, making it difficult to assess treatment safety and applicability.
Unlike chemotherapy, which inhibits the bone marrow by directly damaging naive hematopoietic cells, CDK4/6 inhibitors induce cell cycle arrest by reducing hematopoietic stem cell proliferation, allowing proliferation to be restored after dose reduction or CDK4/6 blockade (20). The incidence of neutropenia was lower with abemaciclib than with other CDK4/6 inhibitors; however, it was still one of the most frequently reported serious (grade ≥3) AEs with abemaciclib (21). In our study, ECOG score >1, radiotherapy in the same period/within 1 month, and pretreatment neutrophils ≤2.0 × 109/L were identified as independent risk factors for grade ≥3 neutropenia with abemaciclib. Baseline myelosuppression with multiple CDK4/6 inhibitors has been shown in several previous studies to be an independent predictor of grade 3 or 4 neutropenia (22–24). In addition, race, ECOG score, and drug concentration have been reported to be associated with abemaciclib-induced neutropenia (13, 25). In our study, we explored the effect of baseline WBC and NE levels on the occurrence of neutropenia during the 21 days prior to treatment and analyzed them by numerical stratification (WBC: >6.0 vs. 4.01–6.0 vs. ≤4.0 × 109/L; NE: >4.0 vs. 2.01–4.0 vs. ≤2.0 × 109/L). The univariate results revealed an association with neutropenia only when the WBC and NE levels were below normal. After the multivariate analysis, only NE levels of ≤2.0 × 109/L before treatment were a risk factor for neutropenia, which may be due to the collinearity of WBCs and NE. However, patients with low neutrophil counts before treatment were more likely to experience severe neutropenia and were at a higher risk of infection-related complications (26, 27). However, overall, careful evaluation and correction of neutropenia before treatment, in addition to close monitoring during treatment, are essential.
The ECOG score is used to assess treatment tolerance and plays an important role in aspects such as prognosis. Patients with a high ECOG score tend to have poor tolerability to antitumor therapy and a higher incidence of AEs (28). ECOG performance status (PS) was a common risk factor for diarrhea and neutropenia in our study; a higher ECOG score predicted a higher risk. Therefore, in clinical practice, the potential benefits of CDK4/6 inhibitors in patients with relatively poor physical function cannot be completely ruled out. For these patients, clinicians should fully assess the feasibility of the treatment and choose a low initial treatment dose according to the situation. Good therapeutic effects can be achieved by implementing active and effective preventive measures. In addition, our study found that prior radiotherapy was not associated with diarrhea or neutrophilic development, but radiotherapy within the same period/within 1 month has been found to be an independent risk factor for grade ≥3 neutropenia. Evidence for combination therapy with CDK4/6 inhibitors and radiotherapy is currently mostly derived from limited retrospective studies, and there are no consensus guidelines to guide practice. Given their influence on the cell cycle, these two factors may have a synergistic effect, enhancing the efficacy and toxicity of radiotherapy (29, 30). Most retrospective studies have concluded that simultaneous radiotherapy may increase the incidence and severity of hematological and gastrointestinal AEs, but does not affect the delivery of systemic therapies (31–33). However, more efficacy and safety data need to be validated in prospective studies over a longer period.
Many factors may increase the risk of AEs associated with CDK4/6 inhibitors, as suggested by previous studies, including a history of radiotherapy for bone metastases, use of antibiotics, low body weight, and drug concentrations, which may increase the risk of neutropenia (20, 24, 34, 35). Furthermore, the risk of diarrhea is higher in postmenopausal patients, untreated patients, and patients who develop metastases (7), among others. These factors were included in our study; however, the results did not show a clear correlation. Although the influence of relevant independent risk factors has been reported, there is a lack of consensus on the influencing factors and predictive models that can include these factors in the analysis. In our study, two nomogram models were created for the prediction of the risk of grade ≥3 diarrhea and neutropenia. By calculation of the C-index and drawing of ROC, calibration, and DCA curves, the models were validated internally and externally. The prediction models constructed in this study included various clinical characteristics and treatment variables with strong predictive ability. Data were obtained from different hospitals for the development and external validation groups. Both the internal and external validation data demonstrated good predictive power, discrimination, and clinical utility of the predictive column line plots, which suggests that the models can be applied to different patient populations and medical centers.
This study had several limitations. First, it was a retrospective study, which is inevitably subject to bias; therefore, the findings need to be validated by multicenter, large prospective studies in the future. In addition, owing to drug accessibility problems, we examined only one type of CDK4/6 inhibitor. Future studies could build on this study by further increasing the number of samples and investigating the risk factors associated with the use of other CDK4/6 inhibitors to improve model stability and extrapolation.
Conclusion
This study evaluated the risk factors for grade ≥3 diarrhea and neutropenia in patients with HR+/HER2- breast cancer receiving abemaciclib in combination with ET in a real-world clinical setting. Two nomograms were generated and internally and externally validated, demonstrating that the models had good prediction performance and could be applied to various populations and medical centers. We expect this study to provide a scientific basis for the safety assessment of abemaciclib combined with ET in patients with HR+/HER2- breast cancer. Further prospective validation is required.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Yunnan Cancer Hospital, the Third Affiliated Hospital of Kunming Medical University (approval number: KYLX2023-011). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SY: Conceptualization, Methodology, Writing – original draft. JZ: Methodology, Supervision, Writing – original draft. HW: Data curation, Writing – original draft. YZ: Data curation, Writing – original draft. XW: Data curation, Investigation, Writing – original draft. MS: Data curation, Investigation, Writing – original draft. CY: Data curation, Investigation, Writing – review & editing. TD: Data curation, Formal Analysis, Writing – original draft. YY: Writing – review & editing, Data curation, Formal Analysis. YL: Conceptualization, Methodology, Supervision, Writing – review & editing. JN: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant numbers 82360555 and 81960479), Major Science and Technology Special Programme Projects in Yunnan Province (Grant numbers 202302AA310046-04) and Collaborative innovation center in Yunnan province colleges and universities (Grant numbers K1322147).
Acknowledgments
We thank all those involved in the research from the hospitals and research members and medical staff for their contribution to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1515420/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:1485–505. doi: 10.1200/JCO.20.03399
3. Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol Off J Eur Soc Med Oncol. (2021) 32:1571–81. doi: 10.1016/j.annonc.2021.09.015
4. Slamon DJ, Fasching PA, Patel R, Verma S, Hurvitz SA, Chia SKL, et al. NATALEE: Phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) early breast cancer (EBC). J Clin Oncol. (2019) 37:TPS597–7. doi: 10.1200/JCO.2019.37.15_suppl.TPS597
5. Desnoyers A, Nadler MB, Kumar V, Saleh R, and Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat Rev. (2020) 90:102086. doi: 10.1016/j.ctrv.2020.102086
6. Rugo HS, Huober J, García-Sáenz JA, Masuda N, Sohn JH, Andre VAM, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. (2021) 26:e53–65. doi: 10.1002/onco.13531
7. Onesti CE and Jerusalem G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev Anticancer Ther. (2021) 21:283–98. doi: 10.1080/14737140.2021.1852934
8. Ettl J. Management of adverse events due to cyclin-dependent kinase 4/6 inhibitors. Breast Care Basel Switz. (2019) 14:86–92. doi: 10.1159/000499534
9. Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol. (2020) 6:116–24. doi: 10.1001/jamaoncol.2019.4782
10. Shohdy KS, Lasheen S, Kassem L, and Abdel-Rahman O. Gastrointestinal adverse effects of cyclin-dependent kinase 4 and 6 inhibitors in breast cancer patients: a systematic review and meta-analysis. Ther Adv Drug Saf. (2017) 8:337–47. doi: 10.1177/2042098617722516
11. Roncato R, Angelini J, Pani A, Cecchin E, Sartore-Bianchi A, Siena S, et al. CDK4/6 inhibitors in breast cancer treatment: potential interactions with drug, gene, and pathophysiological conditions. Int J Mol Sci. (2020) 21:6350. doi: 10.3390/ijms21176350
12. Goetz MP, Okera M, Wildiers H, Campone M, Grischke E-M, Manso L, et al. Safety and efficacy of abemaciclib plus endocrine therapy in older patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: an age-specific subgroup analysis of MONARCH 2 and 3 trials. Breast Cancer Res Treat. (2021) 186:417–28. doi: 10.1007/s10549-020-06029-y
13. Modi ND, Abuhelwa AY, Badaoui S, Shaw E, Shankaran K, McKinnon RA, et al. Prediction of severe neutropenia and diarrhoea in breast cancer patients treated with abemaciclib. Breast Edinb Scotl. (2021) 58:57–62. doi: 10.1016/j.breast.2021.04.003
14. Freites-Martinez A, Santana N, Arias-Santiago S, and Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
15. Xie X, Zhang W, Zhou X, Ye Z, Wang H, Qiu Y, et al. Abemaciclib drives the therapeutic differentiation of acute myeloid leukaemia stem cells. Br J Haematol. (2023) 201:940–53. doi: 10.1111/bjh.18735
16. Hafner M, Mills CE, Subramanian K, Chen C, Chung M, Boswell SA, et al. Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem Biol. (2019) 26:1067–1080.e8. doi: 10.1016/j.chembiol.2019.05.005
17. Gebbia V, Martorana F, Sanò MV, Valerio MR, Giotta F, Spada M, et al. Abemaciclib-associated diarrhea: an exploratory analysis of real-life data. Anticancer Res. (2023) 43:1291–9. doi: 10.21873/anticanres.16276
18. Rugo HS, Turner NC, Finn RS, Joy AA, Verma S, Harbeck N, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer Oxf Engl. (2018) 1990:101. doi: 10.1016/j.ejca.2018.05.017
19. Di Cosimo S, Pérez-García JM, Bellet M, Dalenc F, Gil Gil MJ, Ruiz Borrego M, et al. Palbociclib with fulvestrant or letrozole in endocrine-sensitive patients with HR-positive/HER2-negative advanced breast cancer: A detailed safety analysis of the randomized PARSIFAL trial. Oncologist. (2023) 28:23–32. doi: 10.1093/oncolo/oyac205
20. Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res Off J Am Assoc Cancer Res. (2016) 22:2000–8. doi: 10.1158/1078-0432.CCR-15-1421
21. Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. (2023) 24:77–90. doi: 10.1016/S1470-2045(22)00694-5
22. Kimura M, Usami E, and Yoshimura T. Association between severe neutropenia and progression-free survival in patients with advanced or recurrent breast cancer treated with palbociclib. Pharm. (2020) 75:662–5. doi: 10.1691/ph.2020.0761
23. McAndrew NP, Dickson MA, Clark AS, Troxel AB, O’Hara MH, Colameco C, et al. Early treatment-related neutropenia predicts response to palbociclib. Br J Cancer. (2020) 123:912–8. doi: 10.1038/s41416-020-0967-7
24. Lavery L, DiSogra K, Lea J, Trufan SJ, Symanowski JT, Roberts A, et al. Risk factors associated with palbociclib-induced neutropenia in patients with metastatic breast cancer. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. (2022) 30:9803–9. doi: 10.1007/s00520-022-07400-z
25. Maeda A, Irie K, Hashimoto N, Fukushima S, Ando H, Okada A, et al. Serum concentration of the CKD4/6 inhibitor abemaciclib, but not of creatinine, strongly predicts hematological adverse events in patients with breast cancer: a preliminary report. Invest New Drugs. (2021) 39:272–7. doi: 10.1007/s10637-020-00994-3
26. Clarke RT, Jenyon T, van Hamel Parsons V, and King AJ. Neutropenic sepsis: management and complications. Clin Med Lond Engl. (2013) 13:185–7. doi: 10.7861/clinmedicine.13-2-185
27. Zimmer AJ and Freifeld AG. Optimal management of neutropenic fever in patients with cancer. J Oncol Pract. (2019) 15:19–24. doi: 10.1200/JOP.18.00269
28. Datta SS, Ghosal N, Daruvala R, Chakraborty S, Shrimali RK, van Zanten C, et al. How do clinicians rate patient’s performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience. (2019) 13:913. doi: 10.3332/ecancer.2019.913
29. Beddok A, Mouren V, Cottu P, Laki F, Fourquet A, and Kirova Y. Outcomes and toxicity of concurrent CDK4/6 inhibitor and locoregional radiotherapy for patients with de novo metastatic breast cancer. Int J Cancer. (2023) 153:1386–96. doi: 10.1002/ijc.34562
30. Kubeczko M, Jarząb M, Gabryś D, Krzywon A, Cortez AJ, and Xu AJ. Safety and feasibility of CDK4/6 inhibitors treatment combined with radiotherapy in patients with HR-positive/HER2-negative breast cancer. A systematic review and meta-analysis. Radiother Oncol J Eur Soc Ther Radiol Oncol. (2023) 187:109839. doi: 10.1016/j.radonc.2023.109839
31. Becherini C, Visani L, Caini S, Bhattacharya IS, Kirby AM, Nader Marta G, et al. Safety profile of cyclin-dependent kinase (CDK) 4/6 inhibitors with concurrent radiation therapy: A systematic review and meta-analysis. Cancer Treat Rev. (2023) 119:102586. doi: 10.1016/j.ctrv.2023.102586
32. Visani L, Livi L, Ratosa I, Orazem M, Ribnikar D, Saieva C, et al. Safety of CDK4/6 inhibitors and concomitant radiation therapy in patients affected by metastatic breast cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. (2022) 177:40–5. doi: 10.1016/j.radonc.2022.10.023
33. Kubeczko M, Gabryś D, Gawkowska M, Polakiewicz-Gilowska A, Cortez AJ, Krzywon A, et al. Safety and feasibility of radiation therapy combined with CDK 4/6 inhibitors in the management of advanced breast cancer. Cancers. (2023) 15:690. doi: 10.3390/cancers15030690
34. Iwata H, Umeyama Y, Liu Y, Zhang Z, Schnell P, Mori Y, et al. Evaluation of the association of polymorphisms with palbociclib-induced neutropenia: pharmacogenetic analysis of PALOMA-2/-3. Oncologist. (2021) 26:e1143–55. doi: 10.1002/onco.13811
Keywords: breast cancer, CDK4/6 inhibitors, abemaciclib, adverse events, risk factor, real-world
Citation: Wang L, Yang S, Zhang J, Wang H, Zhang Y, Wang X, Shen M, Ye C, Deng T, Ying Y, Li Y and Nie J (2025) Development and validation of nomograms for predicting grade ≥3 diarrhea and neutropenia after abemaciclib combined with endocrine therapy for breast cancer: a multicenter retrospective real-world study. Front. Oncol. 15:1515420. doi: 10.3389/fonc.2025.1515420
Received: 22 October 2024; Accepted: 18 September 2025;
Published: 06 October 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
J. Guilherme Gonçalves - Nobre, University of Lisbon, PortugalJulia Radosa, Saarland University Hospital, Germany
Copyright © 2025 Wang, Yang, Zhang, Wang, Zhang, Wang, Shen, Ye, Deng, Ying, Li and Nie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Li, bGl5YW5nMjMxNEAxNjMuY29t; Jianyun Nie, bmp5dmlwQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Lei Wang
Lei Wang Siyuan Yang
Siyuan Yang Ji Zhang1†
Ji Zhang1† Hairui Wang
Hairui Wang Xin Wang
Xin Wang Jianyun Nie
Jianyun Nie