- 1Department of PET/CT Diagnostic, Tianjin Key Lab of Functional Imaging & Tianjin Institute of Radiology, Tianjin Medical University General Hospital, Tianjin, China
- 2The Clinical Research and Translational Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China
- 3Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
Purpose: Delayed PET/CT imaging with 68Ga-PSMA-11 is valuable in the detection of primary prostate (PCa) lesions and the differentiation of suspicious lesions. However, 18F-FDG PET/CT has been overlooked due to its low sensitivity to PCa during routine examination. This study aimed to compare the clinical impact of PSMA and FDG in delayed PET/CT imaging in PCa diagnosis.
Methods: Between 2019 and 2024, 65 PCa patients who underwent early (1 h post-injection) and delayed (3 h p.i.) PSMA and FDG scans were retrospectively analyzed. The delayed scans were conducted to clarify unclear findings in early scans or to increase the tumor lesions uptake in negative early scans. All patients were asked to drink 1 L of water between early and delayed scans. The number of primary and metastatic lesions, sensitivity, specificity, diagnostic accuracy, lesions changes in SUVmax of early and delayed scans were evaluated. Correlation between SUVmax and Gleason score as well as SUVmax and PSA for PCa primary lesions diagnosis were analyzed.
Results: Overall, 83 and 84 lesions characteristic for PCa in 65 patients clearly presented at 1 h and 3 h p.i. in PSMA scans, respectively. 30 and 45 lesions characteristic for PCa in 65 patients clearly presented at 1 h and 3 h p.i. in FDG scans. The 3-hour delayed imaging of FDG found more primary foci than 1-hour imaging but was much less able to detect metastatic foci than PSMA. PSMA was more sensitive than FDG in delayed imaging (96.15% vs. 84.21%), and the diagnostic accuracy for primary foci was higher for PSMA than FDG in delayed imaging (83.87% vs. 73.91%). However, FDG delayed imaging greatly improved the diagnostic accuracy for primary PCa compared to early imaging (73.91% vs.53.33%). PSMA SUVmax of both 1 h and 3 h p.i. were correlated with the Gleason score PSA, but FDG SUVmax only showed a correlation with PSA at 3 h p.i.
Conclusion: PSMA PET/CT at 3 h p.i. detected the most lesions characteristic of primary PCa, and it showed higher uptake and contrast than FDG. However, to some extent, FDG delayed PET/CT imaging is still important in primary PCa diagnosis, particularly in hospitals without PSMA.
1 Introduction
Prostate cancer (PCa) is one of the most common malignant tumors in men, and early detection and accurate diagnosis have great significance in improving the treatment effect and survival rate of patients (1, 2).However, conventional imaging techniques, including MRI and CT (3), have limited sensitivity. In recent years, 68Ga-PSMA-11 PET/CT, an emerging non-invasive imaging method, has shown potential application in the diagnosis, staging, and restaging of PCa (4–7). Many studies have reported that the detection efficiency of PSMA PET is higher than that of conventional imaging methods (4, 8). In particular, PSMA delayed imaging 3 h postinjection (p.i.) has proven to be a valuable method to clarify unclear findings on routine scans at 1 h p.i. or to find new PCa lesions (9).
68Ga-PSMA-11 demonstrates clear advantages in the diagnosis of prostate cancer, especially in the precise detection and staging of metastatic and localized disease. 68Ga-PSMA-11 excels in detecting lymph node and distant metastases (e.g., bone, visceral metastases), providing more accurate staging information (10, 11). For localized prostate cancer, 68Ga-PSMA-11 helps determine the precise location and extent of the tumor, guiding surgical or radiotherapy planning (12). While FDG has limited utility in prostate cancer.
However, PSMA PET is owned only in some large hospitals, much more hospitals only have 18F-FDG for routine clinical diagnosis (13). Most studies reported that FDG has low sensitivity in detecting primary PCa (14). However, recent studies showed that FDG PET/CT were positive in patients with negative PSMA PET/CT findings (15, 16). In addition, a study confirmed increased sensitivity in FDG delayed (3h p. i.) PET/CT imaging of PCa (17). Therefore, a comparison of FDG and PSMA in delayed PET/CT imaging in PCa were needed.
At our institute, additional delayed scans have been subsequently used to increase the detection rate of PCa with FDG and PSMA in men with unclear findings 1 h p.i. In this study, we had performed a direct head-to-head comparison of FDG and PSMA delayed PET/CT imaging in PCa diagnosis.
2 Materials and methods
2.1 Patient selection
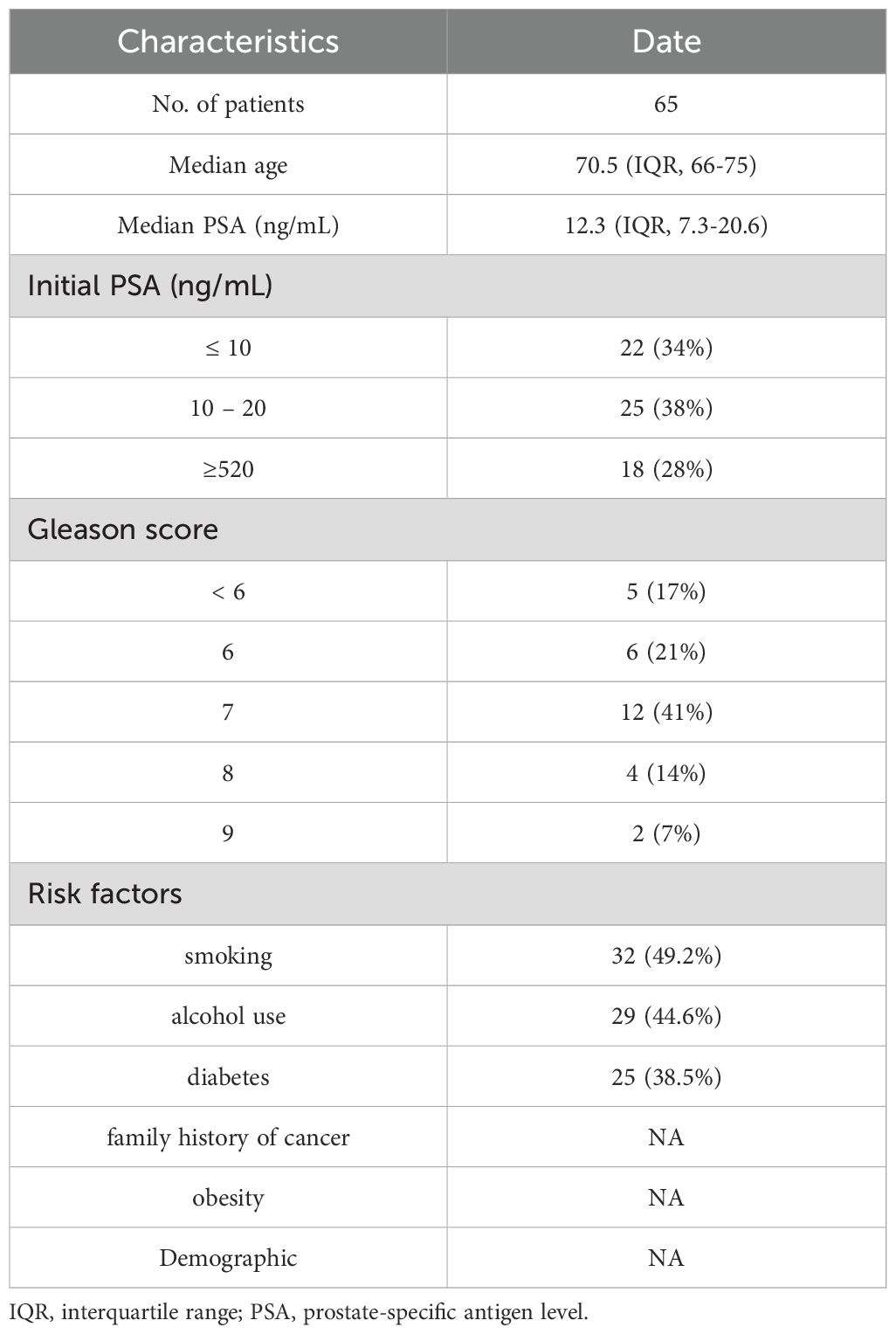
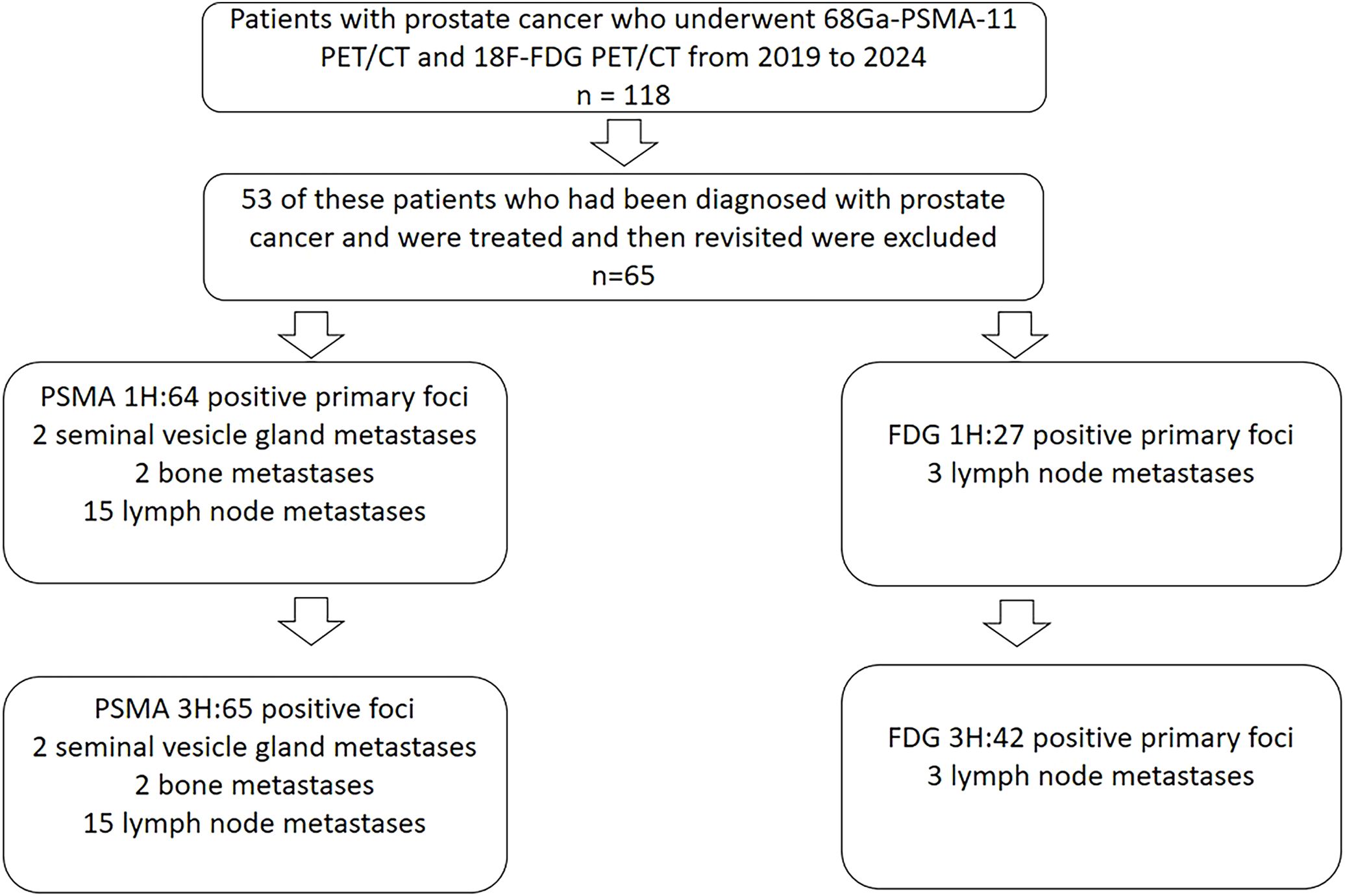
From October 2019 to April 2024, 118 patients received both 18F-FDG PET/CT and 68Ga-PSMA-11 PET/CT at Tianjin Medical University General Hospital. 53 of these patients who had been diagnosed with PCa and were treated and then revisited were excluded, and 65 patients with suspected PCa at first diagnosis were finally enrolled in our study. The following were the inclusion criteria: (a) patient suspected of possible PCa on other imaging tests such as MR or ultrasound; (b) patients had PSA laboratory results within the last two weeks; (c) FDG and PSMA-11 PET/CT examinations were performed at intervals of < 48 hours; and (d) there was no previous treatment related to PCa. The Ethics Committee of Tianjin Medical University General Hospital approved this retrospective study, and each patient signed an informed consent form. The patients’ characteristics are presented in Table 1. 65 patients in this study received both 1 h and 3 h p.i. delayed imaging with PSMA. 64 received 1 h imaging with FDG and 55 received 3 h p.i. delayed imaging with FDG. 29 of these patients were followed up with pathology of the prostate.
2.2 18F-FDG and 68Ga-PSMA-11 PET/CT
Patients were fasted for 6 h before receiving FDG injection, at a dose of 5.55 MBq/kg, and blood glucose should be controlled below 11.1 mmol/L. PET/CT (GE Discovery 710, GE Healthcare, USA) scanning was performed 1 h p.i., and CT images (3.75 mm slice thickness, automatic mA current:120kV, Scan Type: Helical, Rotation time:0.8, Rotation Length: Full, Pitch & Speed: 1.375:1&55.00) were scanned from the upper thigh to the skull. PET scanning time for each bed was 2 min in 3-dimensional mode, and the images were reconstructed in a 192×192 matrix with a pixel size of 3.27 mm. The reconstruction method was VUE Point FX (GE Healthcare), which uses time-of-flight information and includes a fully 3-dimensional ordered-subsets expectation maximization algorithm with 2 iterations, 24 subsets, and a filter cutoff of 6.4 mm. The quantitation method was SharpIR. A standard Z-filter was applied to smooth between transaxial slices. The Patients undergoing 68Ga-PSMA PET/CT were not required to fast. PSMA was injected at a dosage of 1.85 MBq/kg, and PET/CT was performed 1 h p.i. CT images (CT parameters are consistent with those used for FDG) were scanned from the upper thigh to the skull. PET scanning time at each bed was 3 min. The same reconstruction parameters were used for PSMA. All patients were instructed to drink 1 L of pure water after the first examination and were encouraged to urinate several times. Delayed scanning was performed at 3 h p.i., and the scanning range of delayed imaging for FDG PET/CT was the pelvis, with a scanning time of 3 min per bed. The range of delayed imaging for PSMA PET/CT was generally the pelvis. If suspected metastatic foci were detected during the first imaging, delayed scanning could be performed with a scanning time of 5 min per bed. In order to better visualize the lesion, we used higher CT parameters in delayed imaging, the details of which are as follows:1.25mm slice thichness;300mA current;120Kv; The Reconstruction Algorithm was Q.AC. All patients were examined on the same scanner.
2.3 Image evaluation
Two nuclear physicians with more than 10 y of PET/CT experience, on a computer-assisted reading application (AW Server, version 4.6; GE Healthcare), read all datasets independently and resolved any disagreements by consensus. Lesions suggestive of PCa observed with the naked eye were counted and analyzed for localization (primary foci, lymph nodes [LN], bone and soft tissue metastases) according to interpretive guidelines (18–21). Volumes of interest (VOI) were plotted around positive lesions and the maximum standardized uptake value (SUVmax) of each spherical VOI was measured. For calculation of the SUVmax, positive PSMA and FDG were defined as focal avidity greater than the background in the mediastinal blood pool after exclusion of other important pitfalls or physiological uptake of the two radiotracers. Circular regions of interest were drawn around areas with focally increased uptake in transaxial slices and automatically adapted to a 3-dimensional volume of interest at a 70% isocontour as previously described. For patients with negative test results, we measured the SUVmax of the entire prostate to be counted in the statistics. PSA measurements, imaging examination (including CT, MRI, whole-body bone scan, or PET/CT re-examination), and biopsies were used for follow-up. We used composite validation including histopathology, PSA decreases after PET-directed radiotherapy, and follow-up imaging to verify these positive results.
2.4 Statistical analysis
Results are shown as mean ± standard deviation or frequency (%). The Wilcoxon signed-rank tests were used to compare values of baseline and delayed imaging (SUVmax of FDG and PSMA lesions). Statistical significance of the association between positive/negative findings on FDG PET/CT and Gleason score was assessed by using the chi-squared test, where applicable. For PSMA we used the same method of data analysis. Unpaired two-tailed t-test was used to compare patients with FDG and PSMA SUVmax in terms of PSA level or Gleason score. PET uptake and PSA level or Gleason score were compared by use of the Spearman correlation. A P value of < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software (version 26.0; SPSS Inc.) or GraphPad Prism (version 9.0.0; GraphPad Software).
3 Result
3.1 Characteristics of enrolled patients with PCa
A total of 118 patients were retrospectively analyzed between October 2019 and April 2024, of which 53 patients were diagnosed after surgery as well as after radiotherapy and were excluded from the study. 65 patients, diagnosed by puncture or suspected to have probable PCa by other imaging methods and were not treated in any way, were recruited in this retrospective analysis. The clinical characteristics of patients were shown in Table 1. The median PSA was 12.3 ng/mL (interquartile range [IQR] 7.3-20.6), 65 (100%) of the patients underwent PSMA 1 h p.i. conventional imaging and 3 h delayed imaging. 64 (98%) patients underwent FDG 1 h p.i. conventional imaging and 55 (85%) underwent 3 h p.i. delayed imaging. 65 (100%) patients had PSA results, and 29 (45%) patients had pathologic puncture results, of which 24 (83%) had Gleason scores ≥ 6 and 5 (17%) were negative.
3.2 Detection of lesions by FDG and PSMA PET/CT
A total of 64 PSMA positive primary foci were found on 1 h p.i. conventional imaging and 65 positive foci were found on 3 h p.i. delayed imaging. There were 2 cases of seminal vesicle gland metastases, 2 bone metastases, and 15 lymph node metastases. All metastases were visible on conventional imaging at 1 h p.i. imaging and 3 h p.i. delayed imaging in PSMA. A total of 27 positive primary foci were found in 1 h p.i. routine FDG, 42 positive primary foci were found in 3 h p.i. delayed FDG, 3 lymph node metastases were found in 1 h and 3 h p.i. (Figure 1), and 31 foci had pathologic results, of which 26 were diagnosed with prostate cancer, and 5 were negative. 2 patients with PSMA-positive foci did not correspond to the location of FDG-positive foci. 1 patient with PSMA-negative results had positive pathology and FDG delayed results. 2 patients had positive PSMA results but the pathology and FDG results were negative.
3.3 The sensitivity, specificity, diagnostic accuracy of FDG and PSMA for prostate cancer primary foci
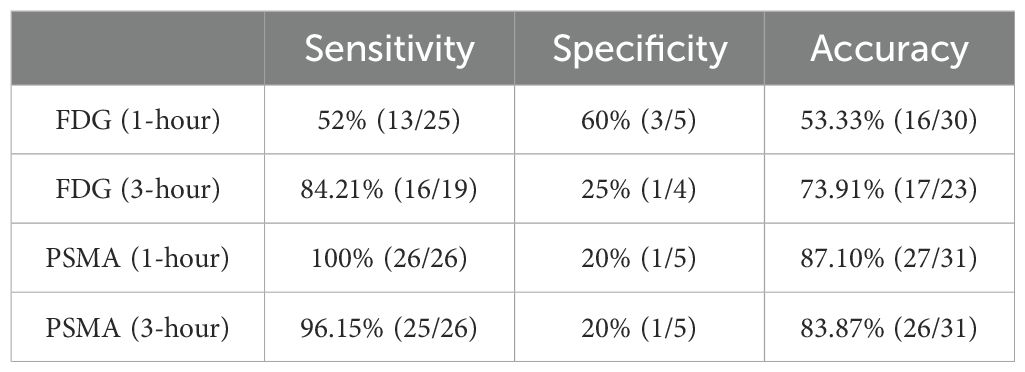
We compared the sensitivity specificity and accuracy of 1 h conventional and 3 h delayed imaging of FDG and PSMA in the diagnosis of primary foci of PCa. The sensitivity of PSMA is higher than that of FDG both in the 1 h conventional and 3 h delayed imaging, but there is no advantage of PSMA in specificity (Table 2). In terms of accuracy, 1 h image of PSMA was slightly higher than the 3 h delayed imaging (87.10% vs. 83.87%). However, delayed imaging of FDG had a significant improvement in diagnostic accuracy as compared to conventional imaging (73.91% vs. 53.55%).
3.4 Changes in SUVmax at two time point of FDG and PSMA
We measured SUVmax values of positive prostate foci in all patients (if the patient was negative, we measured SUVmax values of the whole prostate) both at 1 h and 3 h. On comparative analysis, the mean of PSMA SUVmax at 3 h p.i. was 18.16 (95% CI 14.73 – 21.60), which was significantly higher than the mean of SUVmax at 1 h p.i. 13.3 (95% CI 10.55 – 16.05), P < 0.0001. The same result was seen in FDG imaging, the mean of SUVmax at 3 hours was 6.13 (95% CI 5.00 – 7.25), which was significantly higher than the mean of SUVmax at 1 h p.i. 4.49 (95% CI 3.79 – 5.19), P < 0.0001. Delayed imaging improved the visibility of the lesions both in PSMA and FDG imaging (Table 3).
61 of the 64 positive primary foci identified in the 1 h image of PSMA had an increased SUVmax in the 3 h delayed image, and 3 positive foci SUVmax decreased. Only 27 positive foci were observed in the 1 h image of FDG, 26 positive foci had an increased SUVmax in the 3 h delayed image and an additional 16 positive foci with increased SUVmax were identified. After comparing the pathological results, we concluded that the 3 h delayed imaging significantly improves the diagnostic efficiency of FDG in the primary foci (Figure 2).
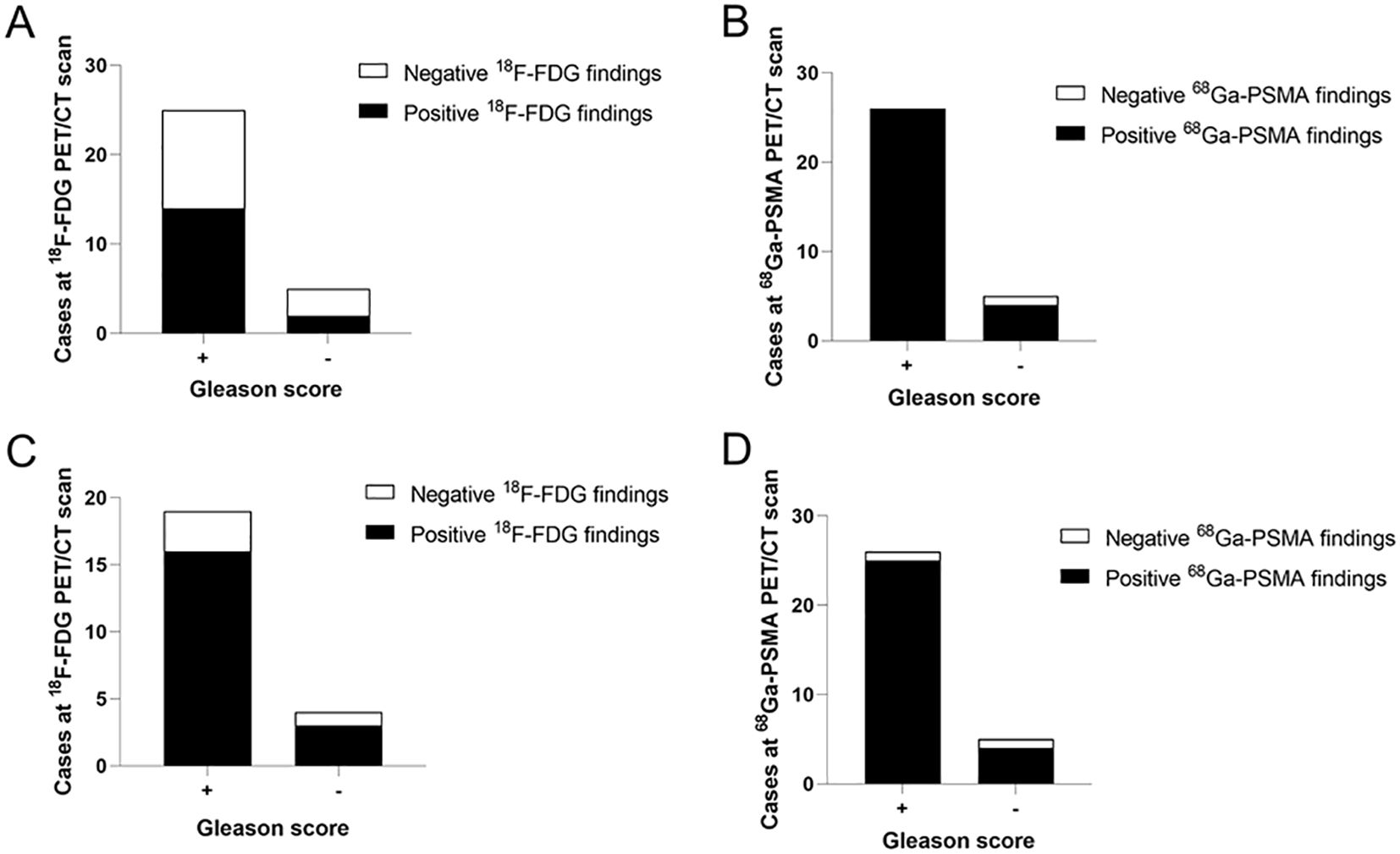
Figure 2. Analysis the relationship between cases at two types of imaging and pathological results. (A) FDG detection rate of PCa at 1 h was lower in the “Gleason score +” than in the “Gleason score -” group (56% vs. 60%). (B) The “Gleason score +” group had a higher PSMA detection rate than the “Gleason score -” group at 1 h p.i. (100% vs. 20%). (C) FDG detection rate of PCa at 3 h p.i. was higher in the “Gleason score +” than in the “Gleason score -” group (84.21% vs. 25%). (D) The “Gleason score +” group had a higher PSMA detection rate than the “Gleason score -” group at 3 h p.i. (96.15% vs. 20%). “+” mean Gleason score >= 6, “-” mean Gleason score < 6.
3.5 Correlation analysis of primary lesions SUVmax with PSA and Gleason score
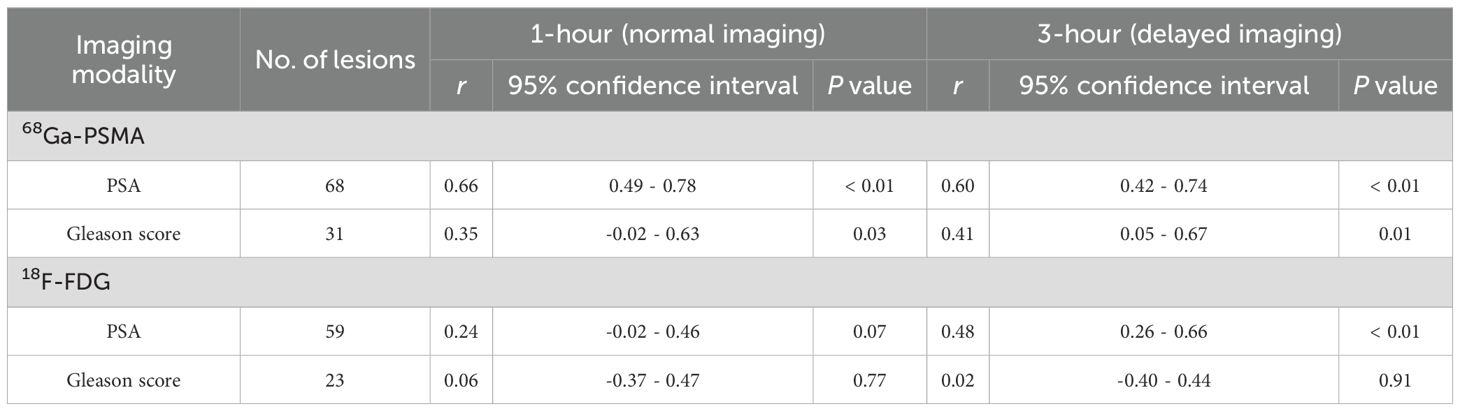
The relationship between PSMA uptake intensity and PSA expression and the comparison of PSMA uptake values with histopathological Gleason scores are shown in Figure 3. There was a significant correlation between SUVmax and PSA in samples obtained by PSMA 1 h p.i. (n = 68) (r, 0.66; P < 0.001). And there was also a significant correlation between SUVmax and PSA for samples (n = 68) obtained by PSMA 3 h p.i. (r =, 0.60; P < 0.01). A moderate correlation was detected between SUVmax and Gleason score for PSMA (1 h, r = 0.35; 3 h, r = 0.41) (Table 4).
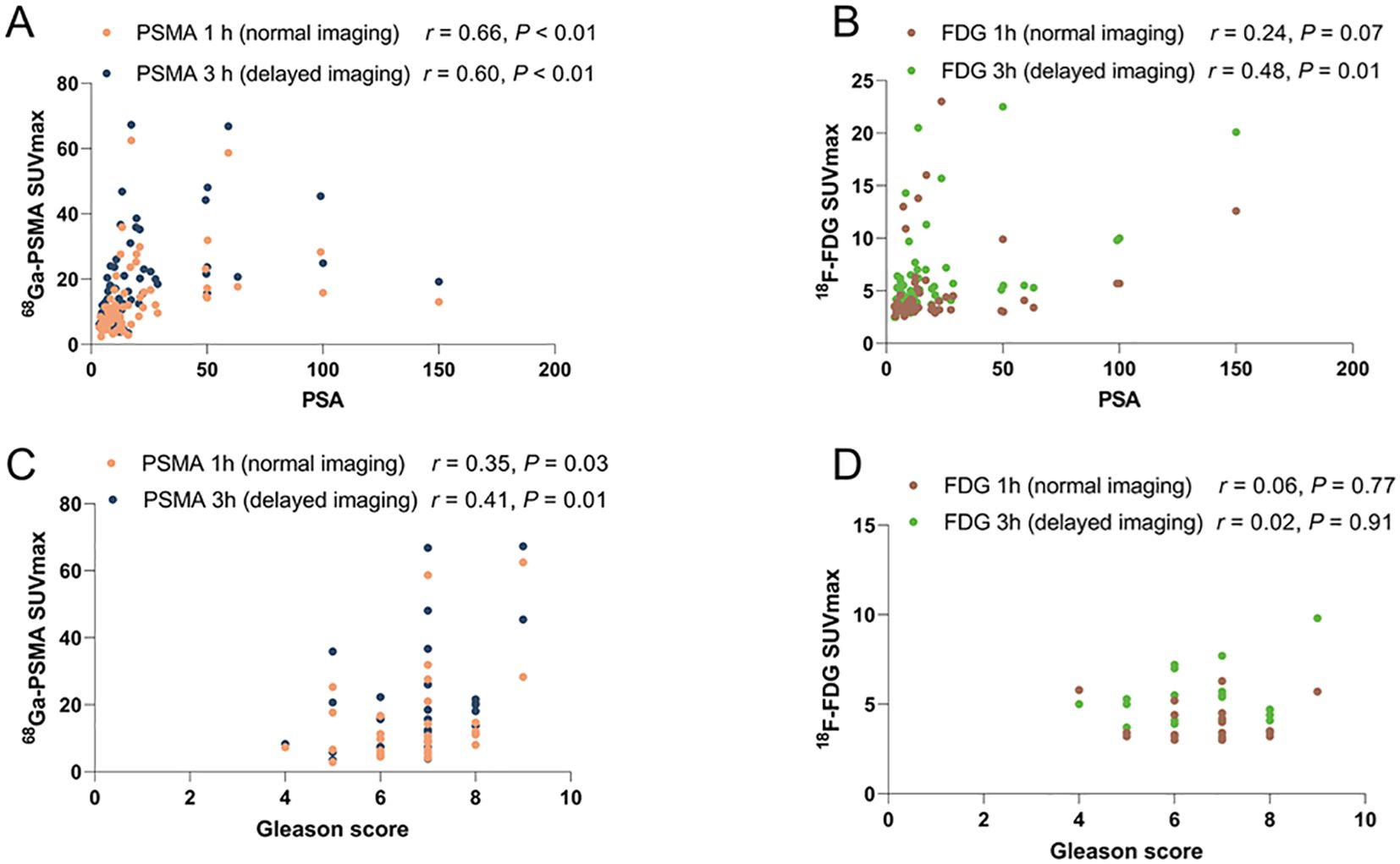
Figure 3. The association between PET uptake intensity and PSA or Gleason score. (A) Spearman r for PSMA 1 h SUVmax and PSMA 3 h SUVmax by PSA. Significant Correlation with PSMA 1 h p.i. (n = 68) (r, 0.66; P < 0.001); PSMA 3 h p.i. (r =, 0.60; P < 0.01). (B) Moderate correlation between SUVmax and PSA for FDG (n = 59; 1 h, r = 0.24; P = 0.07 3 h, r = 0.48; P=0.01). (C) Moderate correlation between SUVmax and Gleason score in PSMA 1 h p.i. (n = 68) (r = 0.35; P = 0.03); PSMA 3 h p.i. ( r = 0.41; P = 0.01). (D) No correlation between SUVmax and Gleason score for FDG (n = 59; 1 h, r = 0.06; 3 h, r = 0.02).
As shown in Figure 3, a moderate correlation between SUVmax and PSA was observed in FDG 1 h p.i. (n = 59; r = 0.24; P = 0.07). A moderate correlation between SUVmax and PSA was observed in FDG 3 h p.i. (n = 59; r = 0.48; P < 0.01). Strong correlation between SUVmax and PSA was detected for the samples obtained (n = 59; r = 0.48; P < 0.01). However, no correlation was observed between SUVmax and Gleason score for FDG (1 h, r = 0.06; 3 h, r = 0.02) (Table 4).
4 Discussion
The limitations of FDG in the diagnosis of prostate cancer can be confirmed from our study, especially in detecting prostate cancer lesions, specifically addressing the low sensitivity and specificity. However, 3 h delayed imaging did significantly improve the accuracy of FDG for prostate cancer diagnosis. It provides an excellent complement to FDG for the diagnosis of prostate cancer. In the past, many studies have confirmed that PSMA PET/CT delayed imaging is of great help in the diagnosis of PCa (9), especially in the detection and diagnosis of metastatic foci. However, due to the fact that the sensitivity of FDG is not very high in PCa it has been neglected (14), and few studies were performed to confirm the usefulness of FDG delayed imaging. In this study, comparison of PSMA and FDG imaging proved that delayed imaging of FDG can be of great help in detection PCa primary foci.
In addition, we found some interesting cases. A patient with negative PSMA results but positive pathology and FDG results (Figure 4), which may be explained by some recently reported studies. Paschalis et al. observed inter-patient heterogeneity in PSMA expression, a significant number of PCa patients have undetectable PSMA expression (22). One study confirmed that PCa patients with suppressed PSMA expression have high expression of glucose transporter enzyme (23). These findings have been confirmed by numerous studies and cases (24–28). Another case with positive results with FDG and PSMA showed inconsistent lesions sites, which may be related to the heterogeneity of PSMA expression within the tumor (Figure 5) (29, 30).

Figure 4. (A) 53-year-old man with PCa, no abnormal uptake of the prostate (SUVmax, 3.9) was seen on FDG 1 h conventional imaging (B), and a high uptake foci (SUV max, 6.2) was seen at FDG 3 h delayed imaging on the left side of the prostate [(C), white arrows]. H&E staining results proved this early PCa (G). No abnormal uptake was seen at PSMA 1 h and 3 h PSMA delayed imaging (E, F). No obvious lesions were seen on the CT images either (D). (A, H) are MIP maps of whole-body PET scans of FDG and PSMA, respectively.
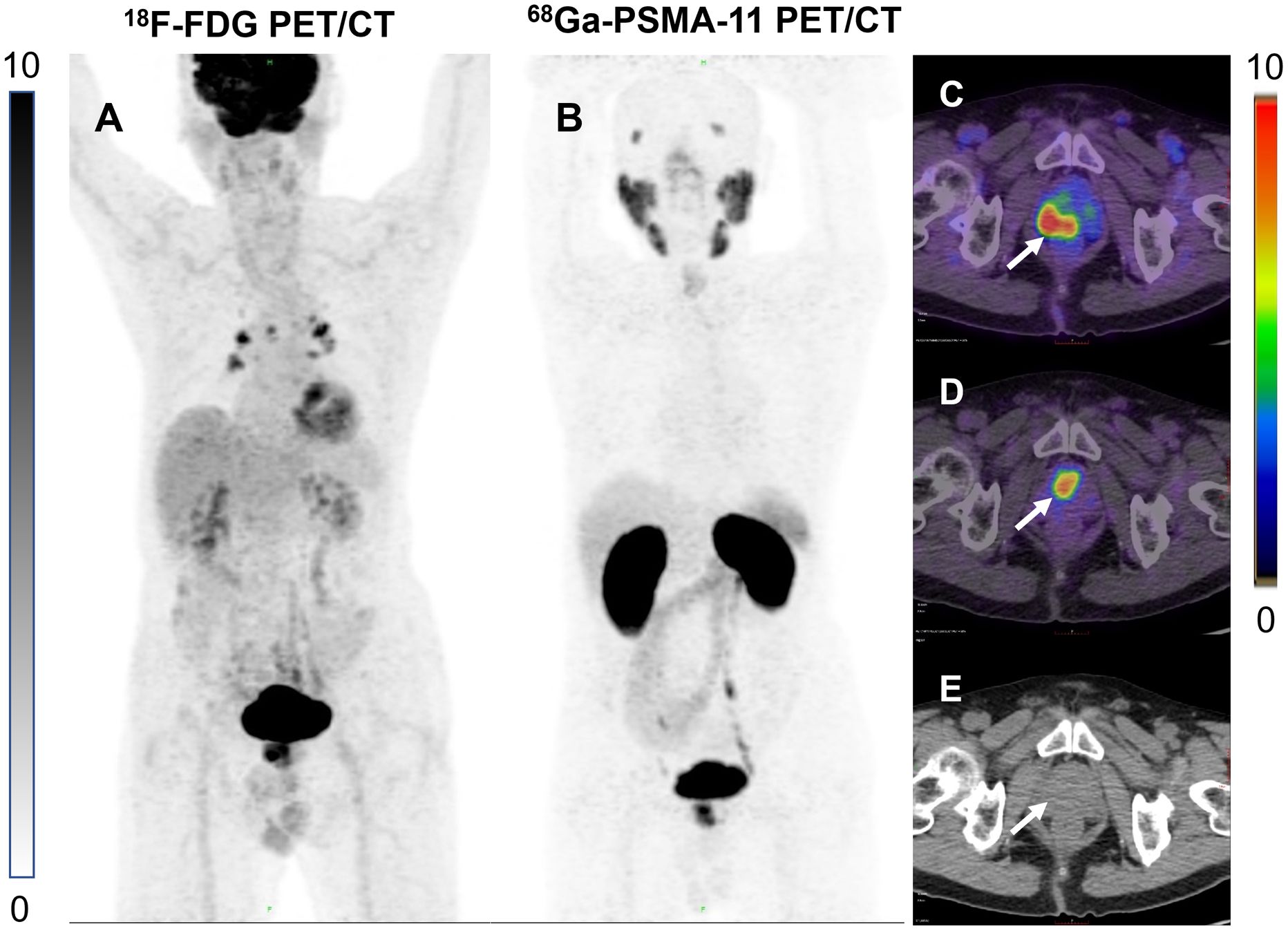
Figure 5. (A) 86-year-old man with a high uptake lesion in the right posterior aspect of the prostate was detected at FDG 3 h delayed imaging [(C), white arrows]. High uptake lesion in the central anterior aspect of the prostate was detected on PSMA 3 h delayed imaging [(D), white arrows]. No obvious lesions were seen on the CT images either (E). (A, B) are MIP maps of whole-body PET scans of FDG and PSMA, respectively.
In this study delayed FDG PCa imaging was showed a diagnostic accuracy of 73.91%. Because 5 patients diagnosed with PCa had positive results in the 1 h imaging with FDG, we did not perform the 3 h delayed imaging also considering the patient’s age and physical limitations. If the 3 h delayed imaging results of these 5 patients were positive, the sensitivity and accuracy of FDG delayed imaging might be better. We concluded that 3 h delayed FDG imaging is of significant in the detection of PCa primary foci.
The associated increased uptake of nutrients, including glucose, had been clearly demonstrated in PCa in a growing number of studies (31). Some studies confirmed in selected cases that FDG PET/CT may represent a useful tool in managing PCa patients (32). As a result, although conventional FDG imaging may currently have poor sensitivity in PCa diagnosis, in this study we found that FDG delayed imaging improves the sensitivity of PCa primary foci diagnosis.
Unlike other studies, this study failed to confirm the correlation between FDG and Gleason score (33, 34), which may be related to the slightly small sample size. We also analyzed the sensitivity of FDG and PSMA at different Gleason scores and in different PSA risk groups. And due to the lack of precision of the results due to the small amount of data, we put this in the supplemental information (Supplementary Table 1). In addition, we did not collect the patients’ MRI findings for comparison with PSMA delayed imaging. This is another limitation of this study.
5 Conclusions
PSMA PET/CT had a significant advantage over FDG PET/CT in the search for lymph node metastases as well as bone metastatic lesions in PCa. PSMA PET/CT at 3 h p.i. showed most lesions characteristic for primary PCa, and helps to identify suspicious lymph node metastases, also with a higher uptake and contrast than FDG. However, FDG delayed PET/CT imaging is still important in primary PCa diagnosis, especially for hospitals that do not have 68Ga-PSMA-11. The 3 h FDG PET/CT delayed imaging significantly improves the detection rate of PCa primary lesions and may find some lesions missed by PSMA. Further research with larger scale is warranted to corroborate its diagnostic performance, especially in comparison to PSMA PET/CT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. HNY: Methodology, Supervision, Writing – review & editing. MP: Software, Validation, Writing – review & editing. HLY: Conceptualization, Data curation, Formal analysis, Writing – original draft. XX: Investigation, Methodology, Project administration, Writing – original draft. DL: Writing – review & editing. SY: Writing – review & editing. QC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded in part by the National Natural Science Foundation of China (82171982). Natural Science Foundation of Fujian Province (2023J06031). Joint Funds for the Innovation of Science and Technology, Fujian Province (2024Y9138). Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-001A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1515653/full#supplementary-material
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. (2012) 61:1132–8. doi: 10.1016/j.eururo.2011.11.008
4. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: A systematic review and meta-analysis. Eur Urol. (2020) 77:403–17. doi: 10.1016/j.eururo.2019.01.049
5. Sprute K, Kramer V, Koerber SA, Meneses M, Fernandez R, Soza-Ried C, et al. Diagnostic accuracy of (18)F-PSMA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J Nucl Med. (2021) 62:208–13. doi: 10.2967/jnumed.120.246363
6. Tan N, Oyoyo U, Bavadian N, Ferguson N, Mukkamala A, Calais J, et al. PSMA-targeted Radiotracers versus (18)F Fluciclovine for the Detection of Prostate Cancer Biochemical Recurrence after Definitive Therapy: A Systematic Review and Meta-Analysis. Radiology. (2020) 296:44–55. doi: 10.1148/radiol.2020191689
7. Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of (68)Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J Nucl Med. (2020) 61:1793–9. doi: 10.2967/jnumed.120.242180
8. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. (2014) 41:11–20. doi: 10.1007/s00259-013-2525-5
9. Afshar-Oromieh A, Sattler LP, Mier W, Hadaschik BA, Debus J, Holland-Letz T, et al. The clinical impact of additional late PET/CT imaging with (68)Ga-PSMA-11 (HBED-CC) in the diagnosis of prostate cancer. J Nucl Med. (2017) 58:750–5. doi: 10.2967/jnumed.116.183483
10. Dai H, Huang S, Tian T, Hou N, Zeng H, Wei Q, et al. Clinical value of dual tracer PET imaging with (68)Ga-PSMA and (18)F-FDG in patients with metastatic prostate cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. (2024) 55:1063–70. doi: 10.12182/20240960201
11. Mainta IC, Neroladaki A, Wolf NB, Benamran D, Boudabbous S, Zilli T, et al. (68)Ga]Ga-PSMA-11 PET and prostate cancer bone metastases: diagnostic performance of available standardized criteria. J Nucl Med. (2024) 65:1376–82. doi: 10.2967/jnumed.124.267899
12. Thin P, Hotta M, Gafita A, Grogan T, Czernin J, Calais J, et al. Clinical factors that influence repeat (68)Ga-PSMA-11 PET/CT scan positivity in patients with recurrent prostate cancer under observation after a negative (68)Ga-PSMA-11 PET/CT scan: A single-center retrospective study. J Nucl Med. (2024) 65:1571–6. doi: 10.2967/jnumed.124.267591
13. Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of (18)F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. (2021) 48:428–48. doi: 10.1007/s00259-020-04967-9
14. Sutherland DEK, Azad AA, Murphy DG, Eapen RS, Kostos L, Hofman MS. Role of FDG PET/CT in management of patients with prostate cancer. Semin Nucl Med. (2024) 54:4–13. doi: 10.1053/j.semnuclmed.2023.06.005
15. Perez PM, Hope TA, Behr SC, van Zante A, Small EJ, Flavell RR. Intertumoral heterogeneity of 18F-FDG and 68Ga-PSMA uptake in prostate cancer pulmonary metastases. Clin Nucl Med. (2019) 44:e28–32. doi: 10.1097/rlu.0000000000002367
16. Parida GK, Tripathy S, Datta Gupta S, Singhal A, Kumar R, Bal C, et al. Adenocarcinoma prostate with neuroendocrine differentiation: potential utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT over 68Ga-PSMA PET/CT. Clin Nucl Med. (2018) 43:248–9. doi: 10.1097/rlu.0000000000002013
17. Yu L, Huang S, Wu S, Yue J, Yin L, Lin Z. Comparison of 18F-FDG PET/CT imaging with different dual time 18F-FDG PET/CT with forced diuresis in clinical diagnosis of prostate cancer. Med (Baltimore). (2023) 102:e32331. doi: 10.1097/md.0000000000032331
18. Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. (2018) 59:469–78. doi: 10.2967/jnumed.117.198119
19. Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. (2018) 38:200–17. doi: 10.1148/rg.2018170108
20. Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1. 0. Eur J Nucl Med Mol Imaging. (2017) 44:1014–24. doi: 10.1007/s00259-017-3670-z
21. Clark MS, Packard AT, Johnson DR, Johnson GB. Pitfalls of a mixed metabolic response at PET/CT. Radiographics. (2019) 39:1461–75. doi: 10.1148/rg.2019180093
22. Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. (2019) 76:469–78. doi: 10.1016/j.eururo.2019.06.030
23. Bakht MK, Lovnicki JM, Tubman J, Stringer KF, Chiaramonte J, Reynolds MR, et al. Differential expression of glucose transporters and hexokinases in prostate cancer with a neuroendocrine gene signature: A mechanistic perspective for (18)F-FDG imaging of PSMA-suppressed tumors. J Nucl Med. (2020) 61:904–10. doi: 10.2967/jnumed.119.231068
24. Badrane I, Castello A, Brunelli M, Cittanti C, Adamantiadis S, Bagni I, et al. Atypical metastases from prostate cancer: alpha-methylacyl-coenzyme A racemase (AMACR) as a potential molecular target in prostate-specific membrane antigen-negative prostate adenocarcinoma. Biomolecules. (2024) 15(1):17. doi: 10.3390/biom15010017
25. Jung T, Neureiter D, Schweighofer-Zwink G, Rendl G, Pirich C, Beheshti M. B-CLL with negative (18)F-FDG PET/CT and intensive solitary lesion on PSMA PET/CT mimicking prostate cancer bone metastases. EJNMMI Rep. (2025) 9:2. doi: 10.1186/s41824-024-00235-3
26. Doroudinia A, Wolfe T. FDG PET/CT scan still can help in evaluation of prostate cancer. Clin Nucl Med. (2025) 50:e41–e2. doi: 10.1097/rlu.0000000000005518
27. Bian L, Li P, Wang X, Zuo Y, Liu X, Bai L, et al. Dual-tracer 18 F-FDG and 68 ga-PSMA PET/CT imaging of heterogeneous phenotypes of metastatic castration-resistant prostate cancer for predicting response to novel hormone therapy. Clin Nucl Med. (2025) 50:143–9. doi: 10.1097/rlu.0000000000005587
28. Gondhane AI, Parghane RV, Basu S. Incidental detection of prostatic lesion on 18 F-FDG PET/CT and 68 ga-PSMA-11 PET/CT leading to diagnosis of second primary prostatic adenocarcinoma in a patient of differentiated thyroid cancer. Clin Nucl Med. (2025) 50:e120–e1. doi: 10.1097/rlu.0000000000005607
29. Sayar E, Patel RA, Coleman IM, Roudier MP, Zhang A, Mustafi P, et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight. (2023) 8(7):e162907. doi: 10.1172/jci.insight.162907
30. Bakht MK, Beltran H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat Rev Urol. (2024) 22:26–45. doi: 10.1038/s41585-024-00900-z
31. Meziou S, Ringuette Goulet C, Hovington H, Lefebvre V, Lavallée É, Bergeron M, et al. GLUT1 expression in high-risk prostate cancer: correlation with (18)F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. (2020) 23:441–8. doi: 10.1038/s41391-020-0202-x
32. Borea R, Favero D, Miceli A, Donegani MI, Raffa S, Gandini A, et al. Beyond the prognostic value of 2-[(18)F]FDG PET/CT in prostate cancer: A case series and literature review focusing on the diagnostic value and impact on patient management. Diagnostics (Basel). (2022) 12(3):581. doi: 10.3390/diagnostics12030581
33. Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging. (2013) 40 Suppl 1:S5–10. doi: 10.1007/s00259-013-2361-7
34. Lavallée E, Bergeron M, Buteau FA, Blouin AC, Duchesnay N, Dujardin T, et al. Increased prostate cancer glucose metabolism detected by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in localised gleason 8-10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur Urol Focus. (2019) 5:998–1006. doi: 10.1016/j.euf.2018.03.008
Keywords: prostate cancer, PET/CT, 68Ga-PSMA-11, 18F-FDG, delayed imaging
Citation: Hu B, Yu H, Pan M, Yang H, Xing X, Li D, Yao S and Chen Q (2025) Head-to-head comparison of 68Ga-PSMA-11 and 18F-FDG in delayed PET/CT imaging in prostate cancer diagnosis. Front. Oncol. 15:1515653. doi: 10.3389/fonc.2025.1515653
Received: 23 October 2024; Accepted: 14 March 2025;
Published: 11 April 2025.
Edited by:
Chao Li, University of Cambridge, United KingdomReviewed by:
Mahboubeh Nabavinia, The Research Institute at Nationwide Children’s Hospital, United StatesQiang Xie, Anhui Provincial Hospital, China
Copyright © 2025 Hu, Yu, Pan, Yang, Xing, Li, Yao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Li, ZHJfbGlkb25nQDE2My5jb20=; Shaobo Yao, eWFvc2hhb2JvMDA4QDE2My5jb20=; Qiusong Chen, cXNfYzhAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Biyao Hu1†
Biyao Hu1† Hailei Yang
Hailei Yang Shaobo Yao
Shaobo Yao Qiusong Chen
Qiusong Chen