- 1Department of Medical Oncology, Xi ‘an No.3 Hospital, the Affiliated Hospital of Northwest University, Xi’an, China
- 2Cancer Center, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Department of Thyroid Breast Surgery, Xi’an NO.3 Hospital, the Affiliated Hospital of Northwest University, Xi’an, China
- 4Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Introduction: The incidence of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) is increasing. To improve patient outcomes, it is essential to develop integrated treatment strategies based on tumor characteristics. Researchers need a rapid visualization of global research trends in GEP-NETs. However, there is currently no bibliometric analysis of GEP-NETs available. This paper aims to fill this gap by using bibliometric methods to quantitatively visualize the current status and research hotspots of GEP-NETs from 2000 to 2023, thereby providing a reference for future research.
Methods: We analyzed 1,140 English publications on GEP-NETs from 2000 to 2023, sourced from the Web of Science Core Collection (WOSCC). Microsoft Excel 2021, CiteSpace, and VOSviewer were used for bibliometric analysis and visualization.
Results: From 2000 to 2023, the number of annual publications on GEP-NETs steadily increased. We identified 1,140 articles published in 401 journals by 5,751 authors from 55 countries. The United States emerged as a leading contributor to GEP-NETs research. Erasmus University Rotterdam, the journal Neuroendocrinology, and the author De Herder WW had the highest number of publications. The most frequently cited reference was by Dasari A. A co-word analysis of keywords revealed five research clusters within the field of GEP-NETs. Immunotherapy and peptide receptor radionuclide therapy (PRRT) are prominent research trends. The terms “carcinoid tumors” and “Lu 177 dotatate” showed significant burst strength.
Conclusions: With the rising incidence of GEP-NETs, there is an increasing focus on their diagnosis and treatment. This bibliometric analysis spotlights the current status, key contributors, top journals, influential publications, and the trends of research topics on GEP-NETs. It provides a comprehensive overview of GEP-NETs research from 2000 to 2023. By providing this quantitative analysis, our study aims to guide future research efforts and support the development of more effective diagnosis and treatment strategies, ultimately advancing the field of GEP-NETs. Our study can help researchers understand global research trends and future directions in GEP-NETs.
1 Introduction
GEP-NETs are heterogeneous tumors originating from the neuroendocrine cells of the gastrointestinal tract and pancreas. These tumors exhibit neuroendocrine differentiation and express specific biomarkers (1). The incidence of GEP-NETs is increasing globally (2), with higher rates observed in European and American populations compared to Asian populations (3–5). The exact causes of GEP-NETs remain unclear. Minnetti et al. (6) suggest a genetic predisposition. The incidence is higher in males than females (7, 8), which may be related to differences in diet and hormone secretion (9–12). Clinical manifestations depend on the tumor’s ability to store and secrete biologically active hormones. For instance, insulinomas, which secrete insulin, are associated with hypoglycemia symptoms such as panic attacks, heart palpitations, and altered mental status (13). Effective treatment strategies for GEP-NETs are limited. Surgical treatment is crucial for localized non-metastatic disease, while the role of primary tumor resection in metastatic GEP-NETs remains debated (14). For metastatic cases, integrated treatment strategies including surgery, chemotherapy, targeted therapy, or immunotherapy are recommended. In recent years, PRRT has been approved for the treatment of GEP-NETs (15). Despite advancements in basic and clinical research, clinical outcomes and survival rates remain unsatisfactory (16).
Bibliometrics involves quantitative analysis of literature to assess research hotspots and trends in different fields. Using tools like VOSviewer and CiteSpace, we analyzed authors, institutions, countries/regions with high publication output, as well as their collaborative relationships (17). We also examined keyword co-occurrence, keyword bursts, and literature co-citation. This visual analysis helps researchers understand the development, challenges, and prospects in the field of GEP-NETs.
While numerous reviews on GEP-NETs have systematically explored their epidemiology, diagnosis, treatment, and prognosis, there has been no quantitative analysis of this field. To address this gap, we collected 1,140 publications from 2000 to 2023 and employed bibliometric methods to analyze research trends in GEP-NETs. Our study aims to provide researchers with a comprehensive understanding of the current status and development of research in this area. Ultimately, we hope that ongoing research will refine treatment strategies and enhance patient outcomes.
2 Materials and methods
2.1 Ethics statement
This study did not involve any human or animal experimentation. Data were obtained from the WOSCC (https://www.webofscience.com/wos/woscc). Therefore, Institutional Review Board approval was not necessary.
2.2 Data sources and collection
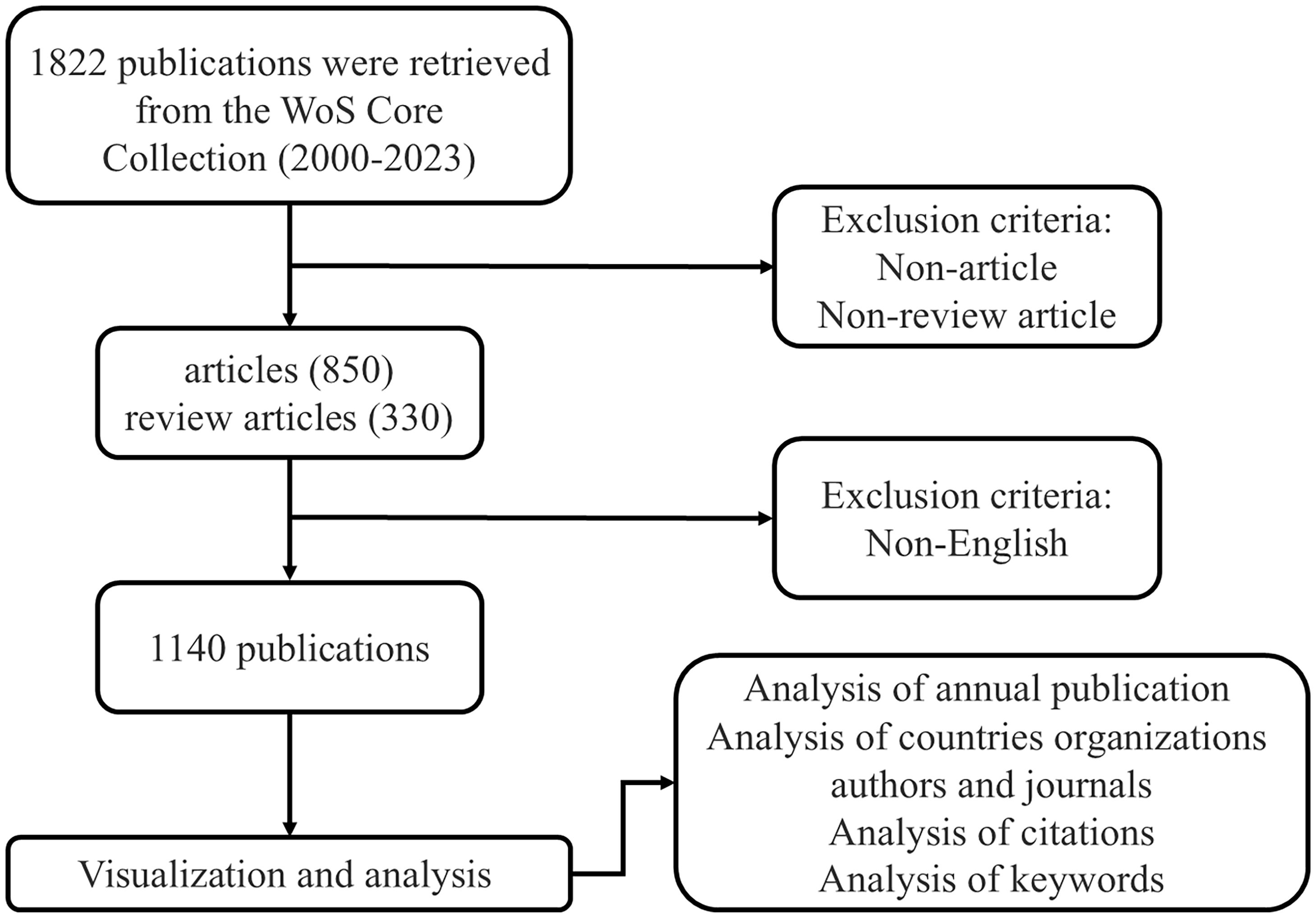
On January 27, 2024, we retrieved publications on GEP-NETs from WOSCC. We referred to relevant literature on PubMed and considered plurals, abbreviations, and other variations to finalize the search keywords. We used the following search strategies: TS = (“gastroenteropancreatic neuroendocrine tumors” OR “gastroenteropancreatic neuroendocrine tumor” OR “GEP-NETs” OR “GEP-NET” OR “gastrointestinal and pancreatic neuroendocrine tumors” OR “gastrointestinal and pancreatic neuroendocrine tumor”) OR AB = (“gastroenteropancreatic neuroendocrine tumors” OR “gastroenteropancreatic neuroendocrine tumor” OR “GEP-NETs” OR “GEP-NET” OR “gastrointestinal and pancreatic neuroendocrine tumors” OR “gastrointestinal and pancreatic neuroendocrine tumor”), document type = article or review article, language = English, publication year = 2000-2023. Figure 1 illustrates the details. We collected information on authors, institutions, countries/regions, keywords, references, etc. A total of 1,140 publications met the inclusion criteria and were downloaded in TXT format (Supplementary Materials).
2.3 Data analysis
WOSCC, developed by Clarivate Analytics, is an information retrieval platform that includes the most influential journals and is one of the most authoritative sources for scientific and technical literature. We used it to obtain publication counts by different years, authors, journals, institutions, and countries/regions. We also gathered the H-index and the count of citations excluding self-citations (Nc) based on the citation report in Web of Science (WoS) (18). Additionally, we employed the Impact Factor (IF) and Journal Impact Factor Quartile (JIF Quartile) from the Journal Citation Reports to assess journal impact.
Microsoft Excel 2021 was used to plot the growth trend in the number of publications from 2000 to 2023.
CiteSpace (https://citespace.podia.com/download, v.6.2.R7) is a Java-based visual analysis tool commonly used in scientific papers to analyze the structure and research trends of a field. It helps researchers identify research priorities and discover potential directions. In this study, CiteSpace was used for reference co-citation, reference burst analysis, and keyword burst analysis. The chosen parameters were: Selection criteria: g-index (K = 25), Years per slice: 1, e = 1.0, L/N = 10, link retaining factor (LRF = 3), Pruning mode: pathfinder.
VOSviewer (https://www.vosviewer.com/, v.1.6.19) is a free software for bibliometric analysis (19). In this study, it was used to map the cooperation among institutions and countries/regions. We also employed VOSviewer to analyze keyword co-occurrence. It calculated the total link strength between different countries/regions, institutions, and keywords. Additionally, it was used to obtain average publication year (APY) of each node.
3 Results
3.1 Analysis of global research trends
To analyze publication trends in GEP-NETs, we examined 1,140 documents from the 1,822 records retrieved from the WoSCC database. We excluded conference abstracts, letters, book chapters, editorial materials, and non-English publications (Figure 1).
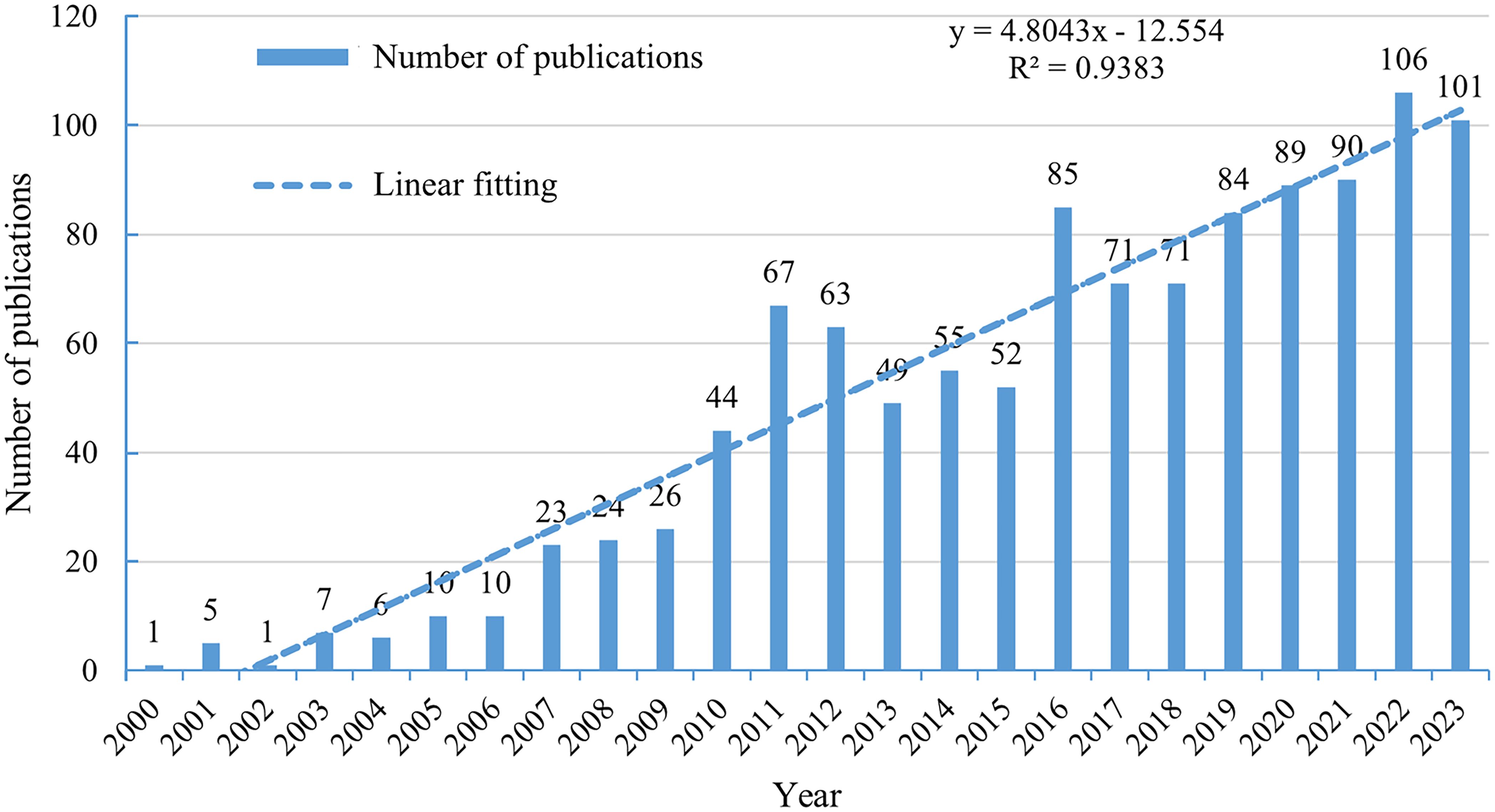
From 1 January 2000 to 31 December 2023, the number of articles and reviews showed a consistent increase (Figure 2). This linear growth (y=4.8043x-12.544, R²=0.9383) in GEP-NET research indicated rising interest in this field.
3.2 Analysis of cooperation of countries/regions
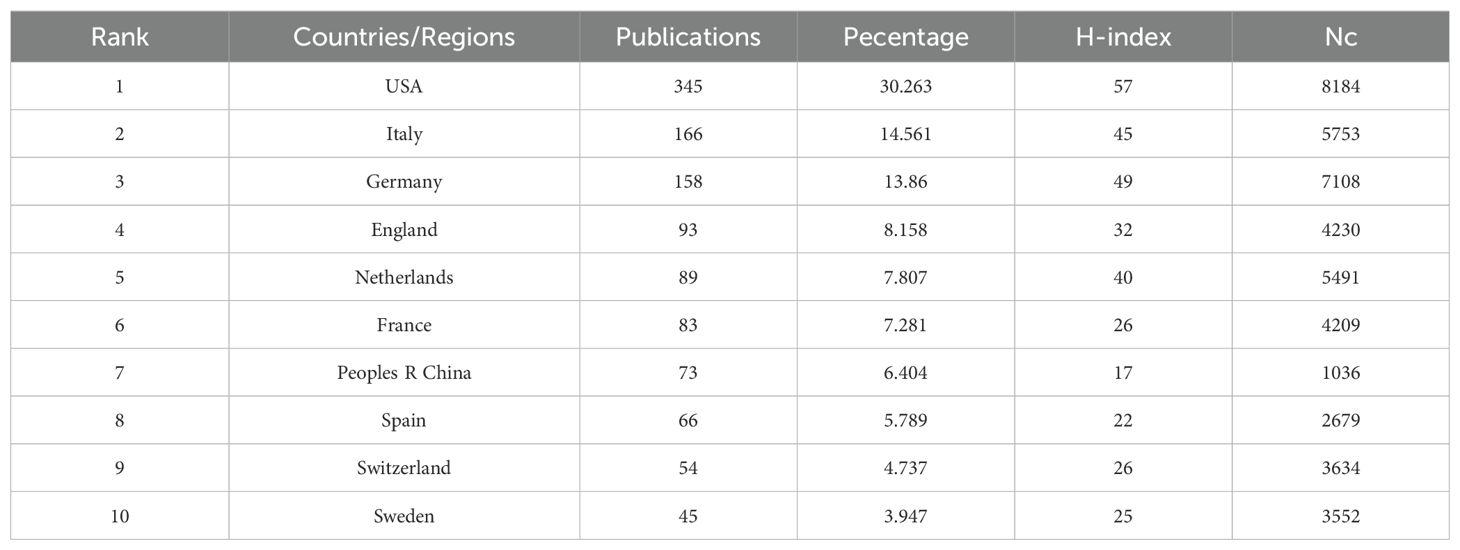
A total of 55 countries/regions have published research on GEP-NETs, with 31 of them having more than five publications each. The USA led with the most publications (345, 30.263%), followed by Italy (166, 14.561%), Germany (158, 13.86%), England (93, 8.158%), and the Netherlands (89, 7.807%). The United States (H-index=57, Nc=8184) led in terms of H-index and Nc, followed by Germany (H-index=49, Nc=7,108), Italy (H-index=45, Nc=5,753), the Netherlands (H-index=40, Nc=5,491), and England (H-index=32, Nc=4,230). This data suggests that the United States had the greatest influence on GEP-NET research. Research was primarily concentrated in Europe and the United States (Table 1).
We used VOSviewer to visualize co-authorship among countries/regions with at least five publications each (Figure 3A). Nodes represented the number of publications, while lines indicated co-authorship between countries/regions. As shown in Figure 3A, the United States held a central role in this field, collaborating with numerous countries such as Italy, England, Germany, the Netherlands, and Spain. We also found that the United States (264), Germany (181), and England (179) exhibited the greatest total link strength. Despite having the second-largest number of publications, the total link strength of Italy was just 172. As shown in Figure 3B, the overlay visualization map showed that certain countries, like Germany, experienced earlier advancements in this area with an APY of 2013.91, while India had a later progression with an APY of 2018.14.
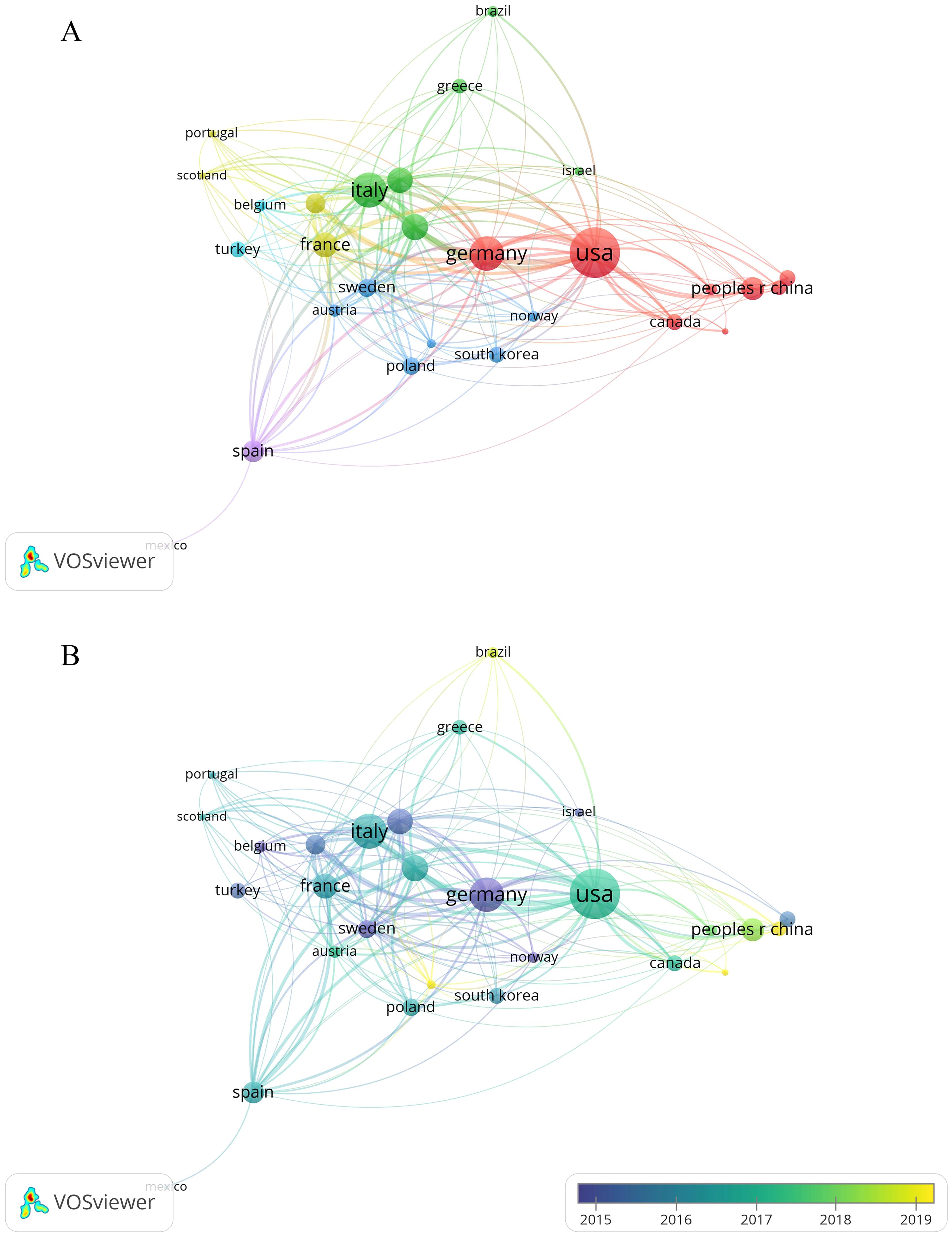
Figure 3. Co-occurrence map of countries/regions. (A) Visualization of the network of cooperation between countries/regions. (B) Visualization of the overlay of cooperation between countries/regions.
3.3 Analysis of cooperation of institutions
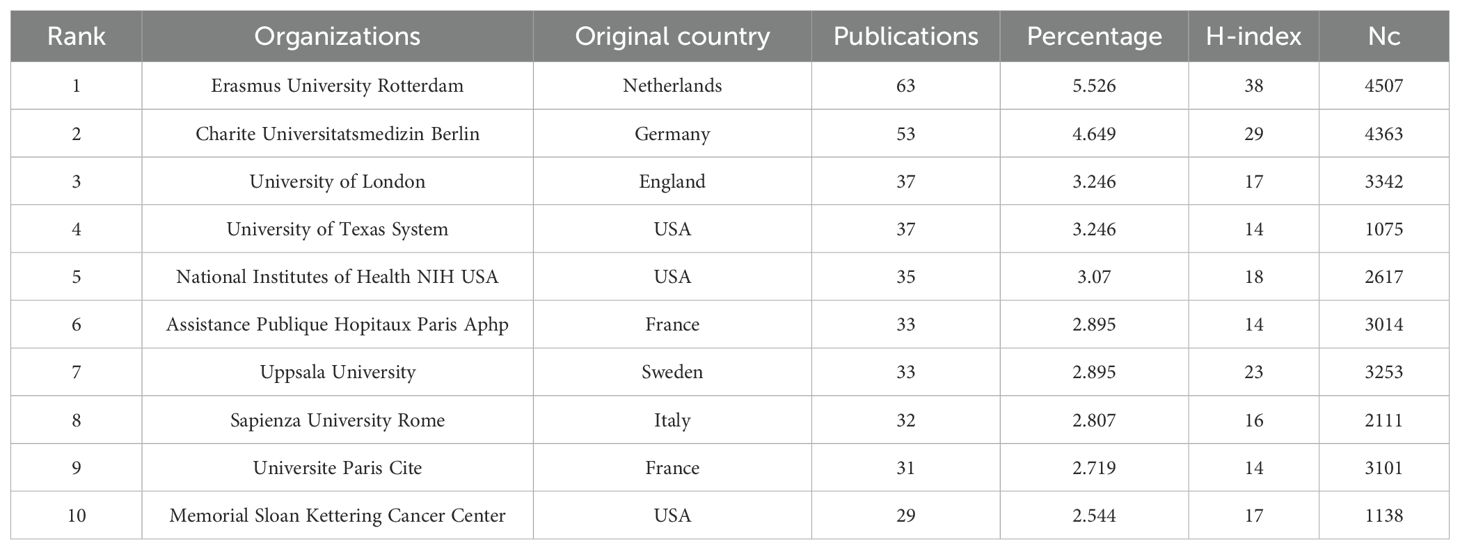
A total of 1,679 institutions contributed to publishing GEP-NET-related papers. The five most productive organizations were Erasmus University Rotterdam (63 papers, 5.526%), Charite Universitatsmedizin Berlin (53 papers, 4.649%), University of London (37 papers, 3.246%), University of Texas System (37 papers, 3.246%), and National Institutes of Health (NIH) USA (35 papers, 3.07%). However, in terms of H-index and Nc, the top five institutions were Erasmus University Rotterdam (H-index=38, Nc=4,507), Charite Universitatsmedizin Berlin (H-index=29, Nc=4,363), Uppsala University (H-index=23, Nc=3,253), National Institutes of Health (NIH) USA (H-index=18, Nc=2,617), and University of London (H-index=17, Nc=3,342) (Table 2).
We also used VOSviewer to visualize co-authorship among institutions with at least seven papers each (Figure 4A). Figure 4A shows that Erasmus University Rotterdam played a leading role, collaborating with many institutions such as Memorial Sloan Kettering Cancer Center and Yale University. Additionally, the top three institutions with the strongest total link strength were Memorial Sloan Kettering Cancer Center (62), Emory University (60), and Vanderbilt University (57). Despite producing the highest number of publications, Erasmus University Rotterdam’s total link strength was just 53. The overlay visualization demonstrated that research at the University of Michigan and the Sapienza University of Rome had emerged mainly in recent years (Figure 4B).
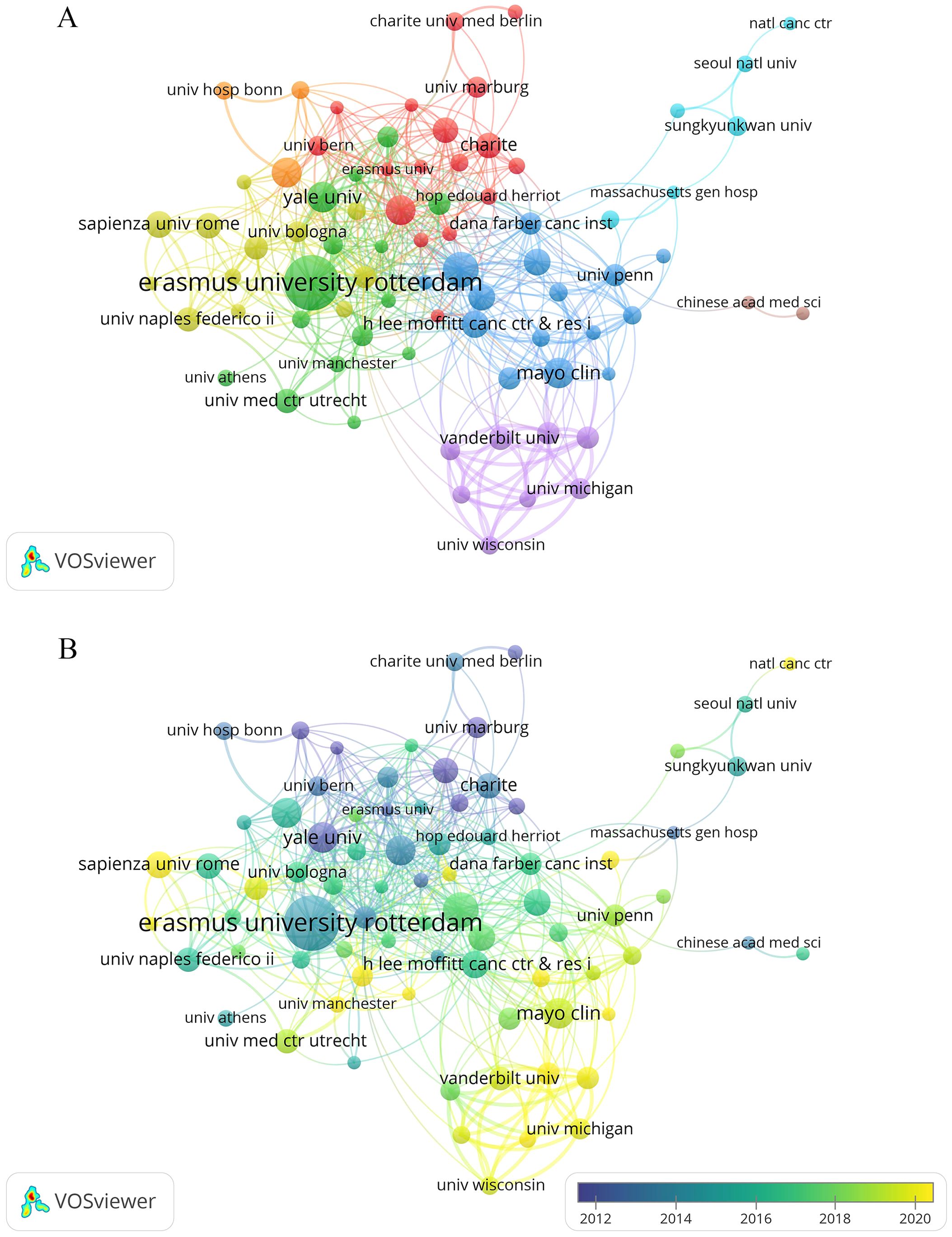
Figure 4. Co-occurrence map of institutions. (A) Visualization of the network of cooperation between institutions. (B) Visualization of the overlay of cooperation between institutions.
3.4 Analysis of journals and authors
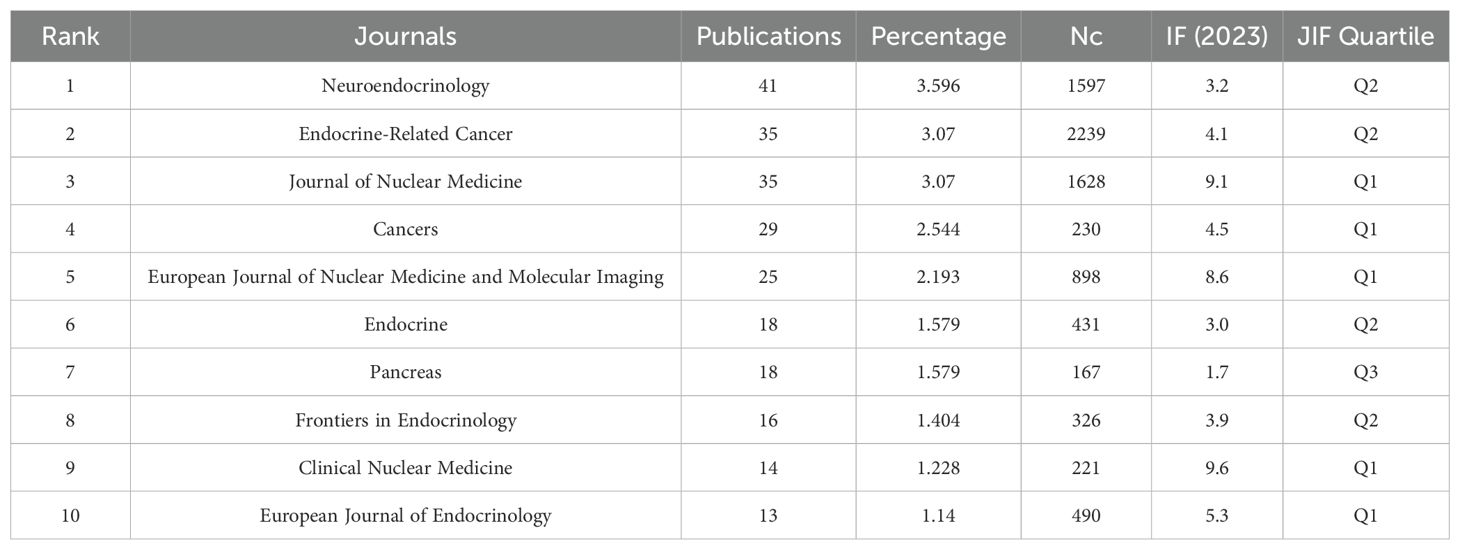
A total of 401 journals have published articles on GEP-NETs. Table 3 demonstrates that the top three journals with the largest number of papers are Neuroendocrinology (41 papers, 3.596%), Endocrine-Related Cancer (35 papers, 3.07%), and the Journal of Nuclear Medicine (35 papers, 3.07%). However, in terms of Nc, Endocrine-Related Cancer led with 2,239 citations, followed by the Journal of Nuclear Medicine with 1,628 citations, and Neuroendocrinology with 1,597 citations. Among the top ten productive journals, the most influential is Clinical Nuclear Medicine (Impact Factor = 9.6, Q1), followed by the Journal of Nuclear Medicine (Impact Factor = 9.1, Q1) and the European Journal of Nuclear Medicine and Molecular Imaging (Impact Factor = 8.6, Q1).
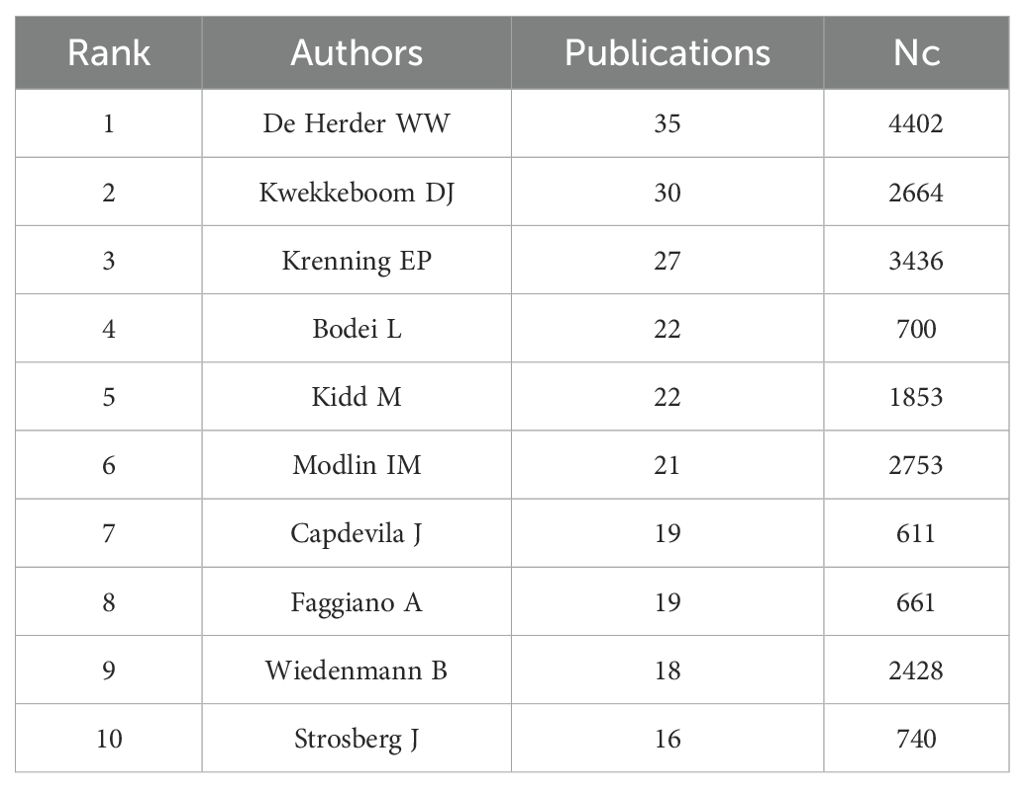
The research in the GEP-NET field involved 5,751 authors. De Herder WW ranked first with 35 papers, followed by Kwekkeboom DJ (30 papers) and Krenning EP (27 papers). However, in terms of Nc, the top three authors were De Herder WW (4,420 citations), Modlin IM (2,753 citations), and Kwekkeboom DJ (2,664 citations), indicating their significant citation impact (Table 4). Additionally, the co-authorship map of the authors is shown in Supplementary Figure 1.
3.5 Analysis of co-cited references
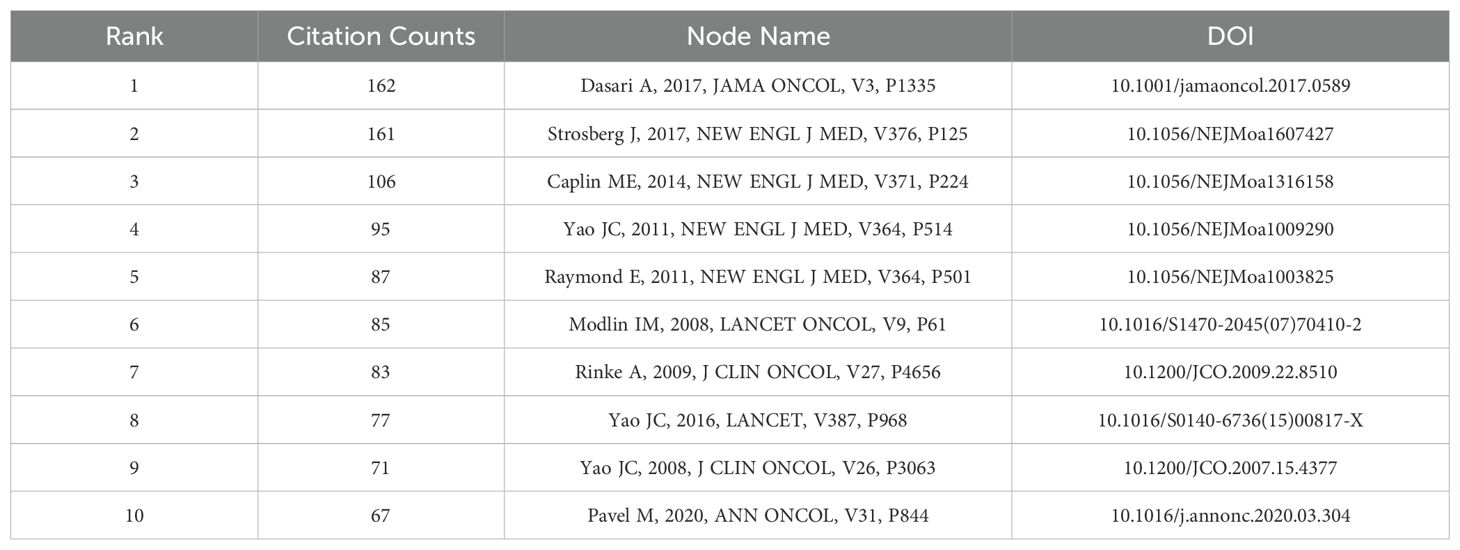
We performed a co-citation analysis to identify the core literature, prominent topics, and emerging trends in GEP-NETs. Figure 5A illustrates the network of co-cited references, consisting of 1,199 nodes and 2,900 links. The top three publications with the most citations were by Dasari A (2017) (162 citations), Strosberg J (2017) (161 citations), and Caplin ME (2014) (106 citations) (Table 5).
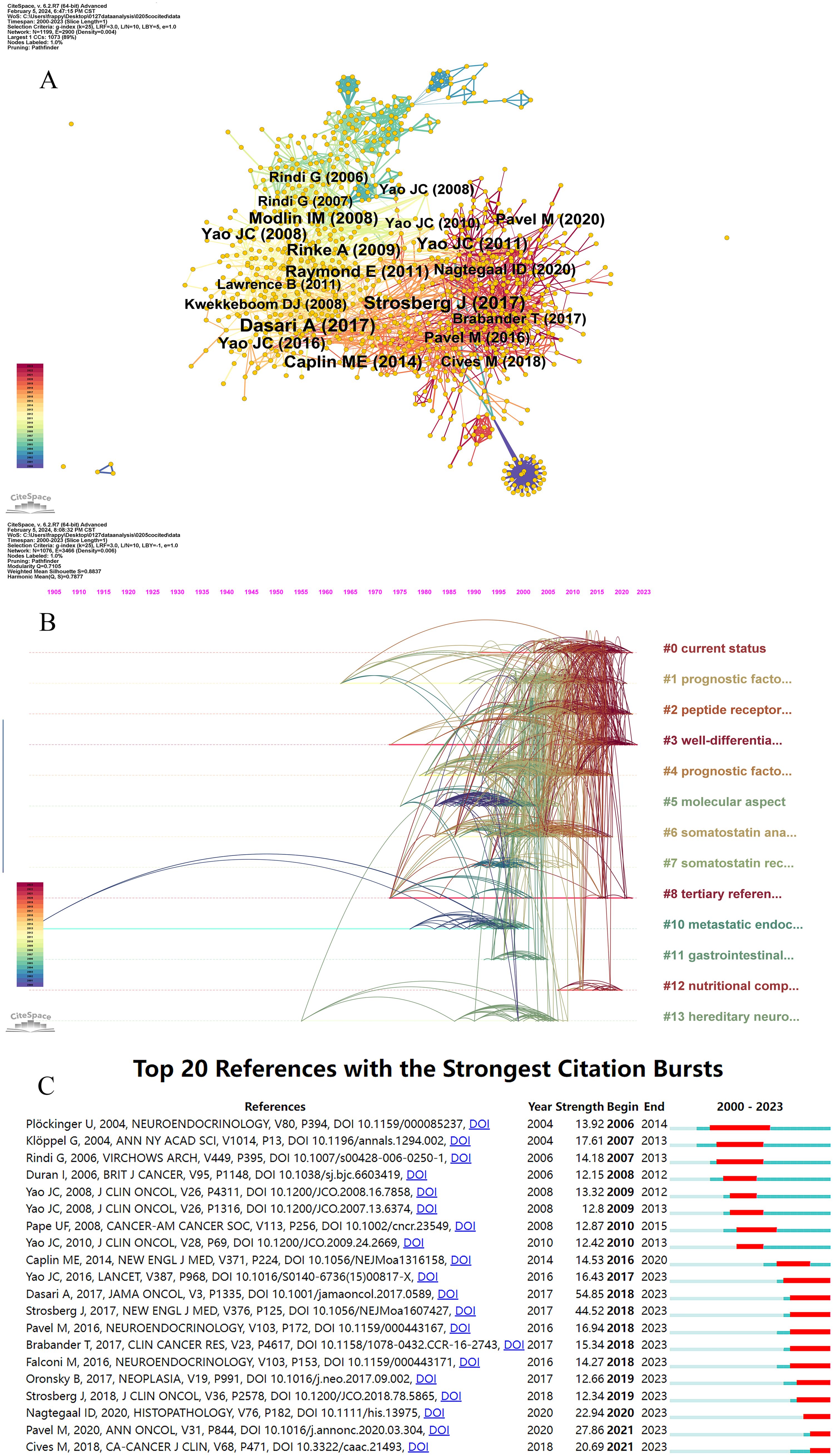
Figure 5. Visualization of reference co-citation network and timeline. (A) Visualization of the network of reference co-citation. (B) Visualization of the reference timeline. (C) Visualization of citation bursts.
Additionally, we conducted a reference timeline analysis using the log-likelihood ratio (LLR) algorithm. CiteSpace summarized cluster labels using the core terms found in each cluster. This bibliometric analysis produced 16 clusters, including current status (cluster #0), prognostic factor (cluster #1), peptide receptor radionuclide therapy (cluster #2), well-differentiated grade (cluster #3), molecular aspect (cluster #5), somatostatin analogue (cluster #6), somatostatin receptor scintigraphy (cluster #7), tertiary reference center (cluster #8), metastatic endocrine tumor (cluster #10), gastrointestinal carcinoid (cluster #11), nutritional complication (cluster #12), and hereditary neuroendocrine tumor (cluster #13). Silhouette values are a measure used in cluster analysis to determine how similar an object is to its own cluster compared to other clusters. In the context of bibliometric analysis, these values help assess the consistency of the clustering results, indicating how well the articles are grouped together based on their citation patterns or other relevant metrics. The average silhouette values of clusters exceeded 0.8, indicating consistent and significant clustering quality. As shown in Figure 5B, each co-cited reference cluster had a distinct active period.
Figure 5C illustrates the citation bursts of the references, showing the burst period of the top 20 references. “Dasari A, 2017” (54.85), “Strosberg J, 2017” (44.52), and “Pavel M” (27.86) emerged as the top three references in terms of strength. “Ploeckinger U, 2004” was the first reference to spur a citation burst and also had the longest burst period (2006-2014). This publication focused on diagnosing and treating neuroendocrine gastrointestinal tumors. Additionally, recent burst references include “Pavel M, 2020” (27.86), and “Cives M” (20.69), representing current research frontiers and hotspots.
3.6 Analysis of keywords
Keywords provide a concise and comprehensive overview of GEP-NET-related papers. To identify research hotspots and potential directions, we used VOSviewer to analyze the high-frequency keywords. In Figure 6A, the node size indicates the frequency of keywords, and the links show the relationships between them.
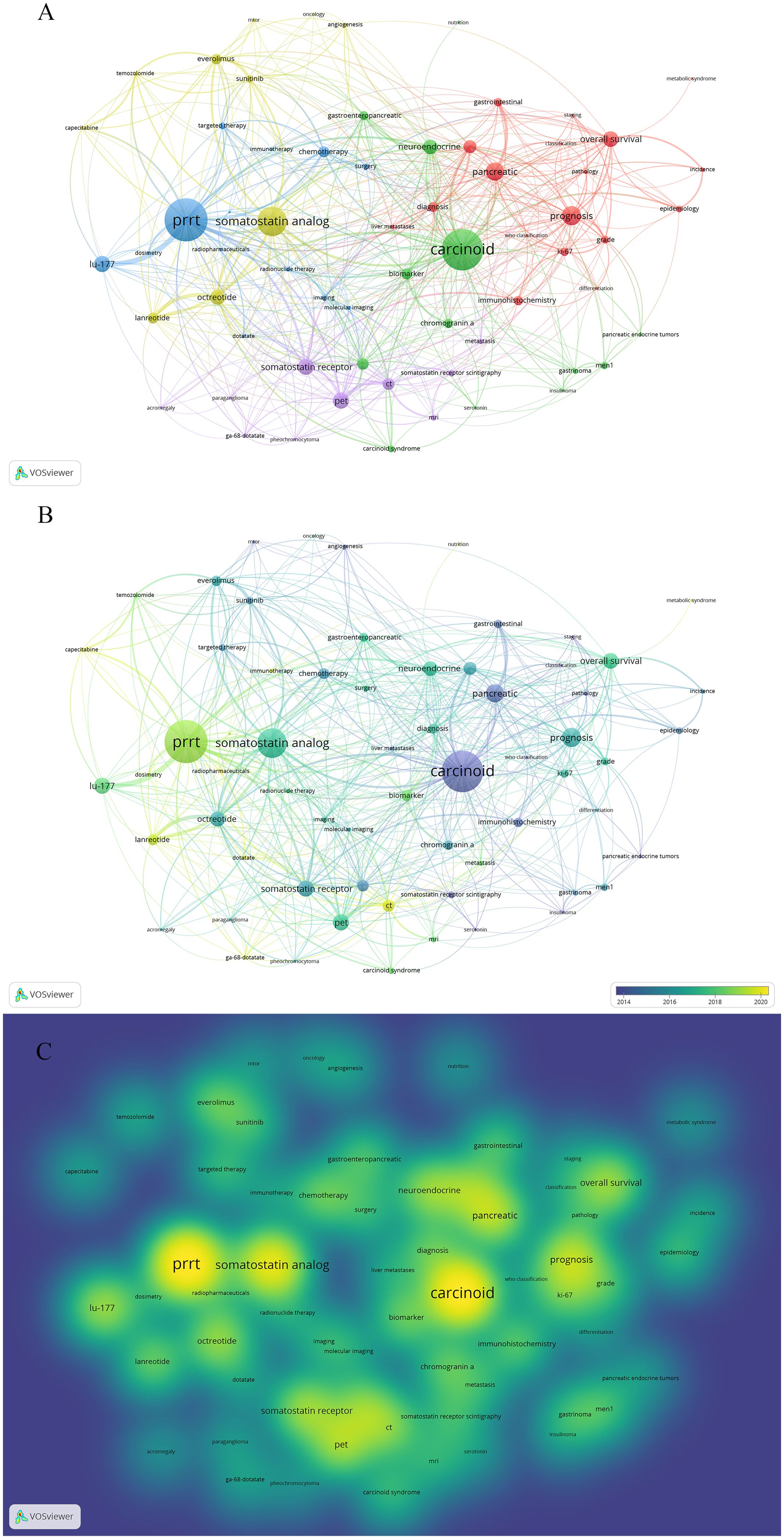
Figure 6. Co-occurrence map of keywords. (A) Visualization of the network of keywords. (B) Visualization of the overlay of keywords. (C) Visualization of the density of keywords.
In this author keyword co-occurrence analysis, we analyzed and visualized 112 keywords that appeared more than seven times. For clarity and accuracy, we merged keywords such as “peptide receptor radionuclide therapy” and “PRRT” (Supplementary Materials). Additionally, we removed some keywords, including “neuroendocrine tumor” and “neuroendocrine tumors” (Supplementary Materials). Ultimately, we obtained 64 keywords in the network analysis.
After clustering analysis using VOSviewer, five clusters were identified (Figure 6A). Cluster 1 (red) focused on the epidemiologic, clinical, and pathological features of GEP-NETs; Cluster 2 (green) on the investigation of molecular mechanisms like biomarkers; Cluster 3 (blue) on integrated tumor treatment strategies, including chemotherapy, targeted therapy, immunotherapy, and PRRT; Cluster 4 (yellow) on specific therapeutic drugs like everolimus; and Cluster 5 (purple) on the various diagnostic methods of GEP-NETs. Unlike traditional reviews, the cluster analysis of keywords using bibliometric methods quantitatively visualizes research hotspots in GEP-NET studies. As shown in the overlay visualization map (Figure 6B), immunotherapy (APY=2020.80), PRRT (APY= 2018.82) have recently emerged as research hotspots. Figure 6C illustrates the density of keywords. The top 10 keywords with the highest weights were carcinoid (45), somatostatin analog (43), PRRT (39), neuroendocrine (36), prognosis (33), pancreatic (31), overall survival (27), pet (27), chemotherapy (26), octreotide (26).
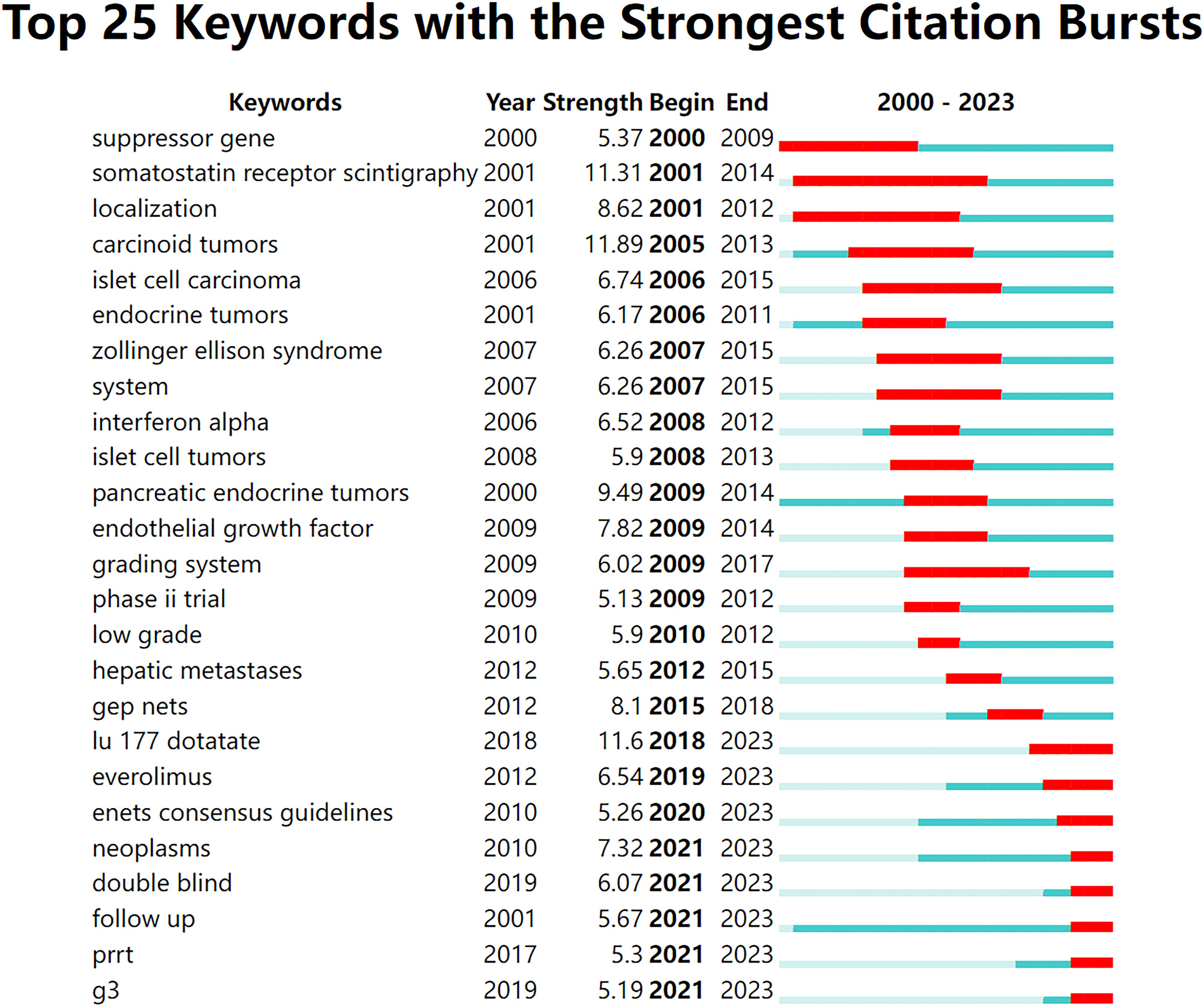
Additionally, we analyzed the top 25 keywords with the most significant citation bursts (Figure 7). From 2000 to 2004, research on GEP-NETs focused on suppressor genes, somatostatin receptor scintigraphy, and localization. From 2005 to 2018, the three keywords with the strongest citation bursts were carcinoid tumors, pancreatic endocrine tumors, and GEP-NETs. During this period, islet cell carcinoma (2006-2015) had the longest burst. Since 2019, research has shifted toward treatment strategies, including lu-177-dotatate, everolimus, and PRRT.
4 Discussion
As the prevalence of GEP-NETs increases (20, 21), the number of studies on their causes, diagnosis, treatment, and outcomes is also rising (22). With a vast amount of publications available, researchers need to visualize the evolution of GEP-NET-related research quickly. Using visualization software like VOSviewer and CiteSpace, we conducted a bibliometric analysis of the relevant research papers, aiding researchers in comprehensively evaluating current status, understanding research directions, and identifying hotspots for future research. This study is the first bibliometric analysis of GEP-NET studies from 2000 to 2023.
4.1 General information on GEP-NET-related literature
In general, the incidence of GEP-NETs has increased from 2000 to 2023 globally (2, 3, 23, 24). In line with this, publications on GEP-NETs also show a linear growth trend over the past few years. The top ten countries in terms of the number of publications were mainly European and American countries, led by the United States. This indicated the contribution of these countries to research in this field. It may also be related to the higher prevalence in Europe and the United States compared to Asia (2). More developed medical infrastructure in Europe and the US makes it easier for the population to access cancer screening tests compared to Asia. In addition, well-developed health insurance systems and registries facilitate the collection and study of cases (2, 3). Several collaborative networks have been formed between different countries, indicating that GEP-NETs have attracted attention and collaborative research from researchers around the world. However, existing research collaborations were mainly concentrated between European and American countries. Therefore, it is necessary to strengthen the exchanges and cooperation with Asian countries. With the improvement of economic levels, medical and health conditions, scientific research, and cancer registration in Asia, more research on GEP-NETs will be published by Asian countries such as China (25). This will also facilitate the development of international multicentre clinical trials to further investigate the differences in treatment and prognosis between different races and regions.
The top ten institutions in terms of publications were all from Europe and the United States. Erasmus University Rotterdam was the leading institution with the largest number of publications. Erasmus University Rotterdam’s medical school is the top-ranked medical school in the Netherlands. The Chinese Academy of Medical Sciences (CAMS) from Asia also appears in the collaborative network, but its cooperation with other institutions remains relatively limited. Academic exchanges and cooperation need to be strengthened in the future.
The top-ranked journal in terms of publications was Neuroendocrinology, which publishes original research in Neuroendocrinology, including basic and clinical research. This journal explores the complex bidirectional interactions between the nervous and endocrine systems in physiological and pathological states. Additionally, numerous research results were published in Journal of Nuclear Medicine and European Journal of Nuclear Medicine and Molecular Imaging. This may be due to the fact that molecular imaging and PRRT of GEP-NETs have become hotspots in recent years. For example, 177Lu-DOTATATE, which is one of the drugs commonly used in PRRT can significantly enhance progression free survival (PFS) (26). 177Lu-DOTATATE has been approved by the Food and Drug Administration (FDA) for the treatment of GEP-NETs.
The top three authors were De Herder WW, Kwekkeboom DJ and Krenning EP, all from Erasmus University Rotterdam, confirmed the strength of the university’s research on GEP-NETs. The most productive author, De Herder WW, focuses on the fields of endocrinology and metabolism, neuroscience and neurology, oncology, radiology, nuclear medicine and medical imaging. De Herder WW published 35 articles with a total of 4402 citing articles (without self-citations) in this area. The review Gastroenteropancreatic Neuroendocrine Tumors, co-authored by De Herder WW, has been cited 1324 times. Wiedenmann B’s research focuses on gastroenterology and hepatology oncology, endocrinology and metabolism, biochemistry and molecular biology. Although he has only 19 publications on GEP-NETs, his articles have been cited 2,428 times (without self-citations). This demonstrates his significant contribution to the field of GEP-NETs.
The timeline view of the references illustrates the progression of GEP-NET research. Before 2000: The pathogenesis and molecular characteristics of GEP-NETs were still unclear and diagnostic methods needed to be improved. For example, Schillaci et al. (27) found that somatostatin receptor scintigraphy could be used to detect liver metastases in GEP-NETs. At that time, molecular aspects and hereditary neuroendocrine tumors were mainly used as reference terms. From 2000 to 2010: Prognostic factors of patients with GEP-NETs became a hot topic of research. Surgery was considered one of the main treatment options for GEP-NETs. Plöckinger et al. (28) summarized the therapeutic strategies for GEP-NETs and suggested that a team of surgeons, radiologists, endocrinologists, gastroenterologists, etc. should be involved in a multidisciplinary treatment group for GEP-NETs patients. At that time, the main reference terms were prognostic factors and somatostatin analogue. Since 2010, the diagnosis and treatment of GEP-NETs have advanced significantly. Chauhan et al. (29) released new evidence that supported changes to each staging system. Treatments such as PRRT have prolonged patient survival. Amir Sabet suggested that a combination of cytotoxic or radiosensitising drugs and PRRT drugs appears to significantly improve the prognosis of pancreatic NETs (30). Well-differentiated grade and PRRT are the main terms used at this stage.
We also performed a co-citation analysis of the references, which helped to analyze the degree of association between the publications. This allowed us to identify significant papers and authors in the field, and to discern common themes and findings across the publications. The most co-cited reference is a clinical study by Dasari A, published in JAMA Oncology in 2017. The study was based on the Surveillance, Epidemiology, and End Results (SEER) database and included 64,971 patients with neuroendocrine tumors (NETs). The results of the study showed that the incidence and prevalence of NETs were steadily increasing. Survival rates have improved over time for all NETs, especially distant gastrointestinal NETs and pancreatic NETs (31). The increase in prevalence may be related to the advancement of medical technologies like endoscopy, biopsy, and so on (32). Advances in therapy strategies such as surgery, chemotherapy, targeted therapies, PRRT, and immunotherapy have improved the outcomes of patients with GEP-NETs (33).
4.2 Hotspots and frontiers of GEP-NET research
Keywords are widely used for document categorization and publication retrieval as they can reflect the main content or key techniques of study (34). Based on keyword co-occurrence clustering and keyword burst analysis, this bibliometric analysis identifies two main research trends in this field: immunotherapy and PRRT. Research hotspots are identified through keyword analysis, which enables clinicians to stay updated on the latest therapeutic strategies and formulate precise treatment plans for patients.
The first popular topic is immunotherapy. There is a lack of biomarkers to predict its efficacy (35). Clinical trials have shown that single agents like pembrolizumab, spartalizumab, and toripalimab have limited efficacy in GEP-NETs (36–38). Current research focuses on combination regimens, including combinations of immune checkpoint inhibitors with different targets and combinations of immune checkpoint inhibitors with other types of drugs. For example, CA209–538 is a prospective, multicenter clinical trial in patients with advanced rare cancers. This trial enrolled 29 patients with advanced NETs. 43% of these patients had pancreatic neuroendocrine neoplasms (NENs). The median progression-free survival (mPFS) was 4.8 months, and overall survival (OS) was 14.8 months. Combination immunotherapy with ipilimumab and nivolumab showed significant clinical activity in a subgroup of patients with advanced NETs, including atypical bronchial carcinoid tumors and high-grade pancreatic NENs (39). Furthermore, NCT03728361 is a nonrandomized, phase II study of nivolumab and temozolomide in patients with NENs. The study included 28 patients with NENs from different primary sites, including 11 gastrointestinal neuroendocrine neoplasms (GI-NENs) and 3 pancreatic neuroendocrine neoplasms (PanNENs). The objective response rate (ORR) was 32.1% (n = 9), and the mPFS was 8.8 months. Nivolumab and temozolomide combination therapy showed promising activity in NENs (40). Additionally, a single-arm, open-label, nonrandomized clinical study demonstrated that the combination of bevacizumab and atezolizumab is effective for patients with advanced, progressive grade 1 to 2 NETs. In this study, the ORR was 20%, and PFS was 14.9 months in a subgroup of patients with pancreatic neuroendocrine tumors (pNETs) (41). In recent years, researchers have also been trying to find new targets for immunotherapy, such as delta like canonical Notch ligand 3 (DLL3) (42). Overall, the most valuable research in immunotherapy for GEP-NETs includes new immunotherapy targets and biomarkers for predicting immunotherapy efficacy. Additionally, the efficacy of combination regimens such as dual immunotherapy, immunotherapy combined with chemotherapy, or anti-angiogenic drugs warrants further investigation.
The second major research focus is PRRT. Recent studies aim to position PRRT as a frontline treatment option and expand its range of indications. One commonly used drug for PRRT is 177Lu-DOTATATE, which damages DNA by releasing β radiation. Compared to octreotide long-acting repeatable (LAR), 177Lu-DOTATATE significantly improves PFS. It is widely used in patients with unresectable or metastatic GEP-NETs. Additionally, PRRT is employed as a neoadjuvant therapy in patients with resectable tumors. A recent phase II single-arm trial reported that neoadjuvant PRRT with 177Lu-DOTATATE was safe and effective for patients with resectable high-risk nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). The study enrolled 31 patients with high-risk recurrence factors, including tumor size > 4 cm, Ki67 >10%, nearby organ invasion, vascular invasion, nodal involvement, and single liver metastasis. Twenty-six patients tolerated four cycles of 177Lu-DOTATATE therapy, and 18 patients showed a partial radiological response without disease progression. Ultimately, 29 patients underwent surgery, with 24 R0 resections and 4 R1 resections (43, 44). Although 177Lu-DOTATATE has shown promising clinical efficacy in NETs, most patients only achieve tumor stabilization and rare but serious long-term hematological toxicity has been reported. Consequently, researchers are developing new drugs, such as 225Ac-DOTATOC, which damages DNA by releasing α radiation. In an animal study, 225Ac-DOTATOC demonstrated good efficacy in a mouse model of hepatic micrometastatic pancreatic NETs (45). PRRT has shifted from being a later-line therapy to a more integral part of the treatment strategy for GEP-NETs. Clinicians should carefully determine the timing of PRRT based on tumor size, stage, and pathological features. Furthermore, selecting appropriate drugs is essential to reduce the risk of adverse effects, such as myelosuppression and renal impairment. Research into novel drugs for PRRT is likely to become a growing priority.
In addition to the above-mentioned hotspots, attention should also be paid to some keywords that have a low frequency in this network. For example, artificial intelligence (AI) and nutritional therapy have also emerged as hot topics of research in recent years. The emergence of AI has helped clinicians to establish more accurate prognostic models to predict patients’ prognoses and to guide clinical decisions (46–51). Bevilacqua et al. (46) established a non-invasive model based on preoperative 68Ga-DOTANOC positron emission tomography/computed tomography (PET/CT) and conventional diagnostic methods. The model can accurately predict primary grade 1 or 2 pNETs and provide a reference for clinicians. With the support of the Bevilacqua model and other diagnostic models (46, 47), clinicians can predict tumor grade and select appropriate personalized treatment, follow-up strategy, or surgical resection for low-grade pNET. However, these studies have many limitations. For example, these studies were retrospective and included small numbers of cases. Therefore, the value of AI needs to be further evaluated in prospective clinical trials. Furthermore, some GEP-NET patients have an overproduction of gastrointestinal hormones, peptides, and amines, which can lead to malabsorption, diarrhea, and steatorrhoea. In addition, the surgery and the medication may have an impact on the diet and the nutrition. Several studies have suggested the Mediterranean diet and the ketogenic diet as nutritional therapies for patients with GEP-NETs (10, 52). The ketogenic diet puts the body into a glucose starvation state, which regulates several signaling pathways to inhibit tumor growth, such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway and the mammalian target of rapamycin (mTOR) pathway (53). Therefore, early nutritional intervention is necessary for GEP-NET patients.
4.3 Strengths and limitations
Our bibliometric analysis has several strengths. Firstly, compared to previous narrative reviews, bibliometrics is a quantitative analysis whose results can be presented visually. Therefore, it is more helpful for researchers to understand the current status of research and future research perspectives. Secondly, this study analyzes a larger number of publications, including a total of 1,140 literature from 2000 to 2003. This can comprehensively reflect the development of GEP-NET-related research. In addition, this study provides analysis from multiple dimensions, which can reflect the contributions of authors, institutions, countries, etc. to this area and the collaborative relationships among them.
This study also has some limitations: 1) Although WoSCC is the most commonly used database for bibliometric analysis, obtaining the literature only from this database may miss some relevant publications. 2) Only English articles were collected for this study, which may result in relevant studies in other languages not being recorded, thus affecting the outcomes. 3) Some of the results of this study are based on analyses of citation counts. More recently published articles may have lower citation rates, which can lead to analytical bias. 4) Unstandardized information, such as the names and affiliations of some article authors, may impact the accuracy of the analysis.
5 Conclusion
This study analyzed global research trends and future directions in GEP-NETs by reviewing 1,140 publications from 2000 to 2023. European and American countries remain the primary contributors to this field, with close cooperation among these countries. However, Asian countries are increasingly playing a significant role. Erasmus University Rotterdam produced the most publications among 1,679 institutions. The most frequently cited reference was authored by Dasari A. The analysis of references and keywords indicates that immunotherapy and PRRT have been prominent research topics in recent years. Continued research in these areas is needed to enhance our understanding of GEP-NET characteristics and inform treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LW: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Software. TW: Data curation, Supervision, Writing – review & editing. SJ: Investigation, Software, Writing – original draft. JZ: Methodology, Software, Writing – original draft. MZ: Formal Analysis, Project administration, Writing – original draft. HG: Data curation, Resources, Writing – review & editing. HW: Conceptualization, Software, Writing – review & editing. YZ: Formal Analysis, Supervision, Writing – review & editing. RR: Resources, Validation, Writing – review & editing. DD: Investigation, Supervision, Visualization, Writing – review & editing. JY: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1515893/full#supplementary-material
Supplementary Figure 1 | Co-authorship map of the authors. (A) Visualization of the network of cooperation between the authors. (B) Visualization of the overlay of cooperation between the authors.
References
1. Aggarwal P, Satapathy S, Sood A, Singh H, Mittal BR, Lal S, et al. Safety and efficacy of 177 Lu-DOTATATE in children and young adult population: A single-center experience. Clin Nucl Med. (2024) 49:e312–e8. doi: 10.1097/rlu.0000000000005233
2. Das S and Dasari A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: are there global differences? Curr Oncol Rep. (2021) 23:43. doi: 10.1007/s11912-021-01029-7
3. Zheng R, Zhao H, An L, Zhang S, Chen R, Wang S, et al. Incidence and survival of neuroendocrine neoplasms in China with comparison to the United States. Chin Med J (Engl). (2023) 136:1216–24. doi: 10.1097/cm9.0000000000002643
4. Masui T, Ito T, Komoto I, and Uemoto S. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: A population-based study. BMC Cancer. (2020) 20:1104. doi: 10.1186/s12885-020-07581-y
5. Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, and Tsai HJ. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci Rep. (2021) 11:7881. doi: 10.1038/s41598-021-86839-2
6. Minnetti M and Grossman A. Somatic and germline mutations in nets: implications for their diagnosis and management. Best Pract Res Clin Endocrinol Metab. (2016) 30:115–27. doi: 10.1016/j.beem.2015.09.007
7. Jann H, Krieg S, Krieg A, Eschrich J, Luedde T, Kostev K, et al. Analyses of sex-based clinicopathological differences among patients with gastrointestinal neuroendocrine neoplasms in Europe. J Cancer Res Clin Oncol. (2023) 149:7557–63. doi: 10.1007/s00432-023-04711-4
8. Poleé IN, Hermans BCM, van der Zwan JM, Bouwense SAW, Dercksen MW, Eskens F, et al. Long-term survival in patients with gastroenteropancreatic neuroendocrine neoplasms: A population-based study. Eur J Cancer. (2022) 172:252–63. doi: 10.1016/j.ejca.2022.06.003
9. Altieri B, Barrea L, Modica R, Bottiglieri F, de Cicco F, Muscogiuri G, et al. Vitamin D deficiency and tumor aggressiveness in gastroenteropancreatic neuroendocrine tumors. Endocrine. (2022) 75:623–34. doi: 10.1007/s12020-021-02869-w
10. Barrea L, Altieri B, Muscogiuri G, Laudisio D, Annunziata G, Colao A, et al. Impact of nutritional status on gastroenteropancreatic neuroendocrine tumors (GEP-NET) aggressiveness. Nutrients. (2018) 10(12):1854. doi: 10.3390/nu10121854
11. Qiu W, Christakis I, Stewart AA, Vodopivec DM, Silva-Figueroa A, Chen H, et al. Is estrogen exposure a protective factor for pancreatic neuroendocrine tumours in female patients with multiple endocrine neoplasia syndrome type 1? Clin Endocrinol (Oxf). (2017) 86:791–7. doi: 10.1111/cen.13324
12. Abdel-Rahman O and Fazio N. Sex-based differences in prognosis of patients with gastroenteropancreatic-neuroendocrine neoplasms: A population-based study. Pancreas. (2021) 50:727–31. doi: 10.1097/mpa.0000000000001821
13. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. Enets consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. (2016) 103:153–71. doi: 10.1159/000443171
14. Wu Z, Wang W, Zhang K, Fan M, and Lin R. The impact of surgery and survival prediction in patients with gastroenteropancreatic neuroendocrine tumors: A population-based cohort study. Int J Surg. (2023) 109:1629–38. doi: 10.1097/js9.0000000000000336
15. Kashyap R, Raja S, Adusumilli A, Gopireddy MMR, Loveday BPT, Alipour R, et al. Role of neoadjuvant peptide receptor radionuclide therapy in unresectable and metastatic gastro-entero-pancreatic neuroendocrine neoplasms: A scoping review. J Neuroendocrinol. (2024) 37(3):e13425. doi: 10.1111/jne.13425
16. Liu H, Xie R, Zhao Z, Xu D, Yang K, Ding M, et al. An 11-year retrospective study: clinicopathological and survival analysis of gastro-entero-pancreatic neuroendocrine neoplasm. Med (Baltimore). (2020) 99:e21682. doi: 10.1097/md.0000000000021682
17. Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. (2016) 18:296–309. doi: 10.4103/1008-682x.171582
18. Jones T, Huggett S, and Kamalski J. Finding a way through the scientific literature: indexes and measures. World Neurosurg. (2011) 76:36–8. doi: 10.1016/j.wneu.2011.01.015
19. van Eck NJ and Waltman L. Software survey: vosviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
20. Cives M and Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. (2018) 68:471–87. doi: 10.3322/caac.21493
21. Zhong Q, Chen QY, Xie JW, Wang JB, Lin JX, Lu J, et al. Incidence trend and conditional survival estimates of gastroenteropancreatic neuroendocrine tumors: A large population-based study. Cancer Med. (2018) 7:3521–33. doi: 10.1002/cam4.1598
22. Sedlack AJH, Varghese DG, Naimian A, Yazdian Anari P, Bodei L, Hallet J, et al. Update in the management of gastroenteropancreatic neuroendocrine tumors. Cancer. (2024) 130:3090–105. doi: 10.1002/cncr.35463
23. Michael M, Thursfield V, Te Marvelde L, Kong G, and Hicks RJ. Incidence, prevalence, and survival trends for neuroendocrine neoplasms in victoria, Australia, from 1982 to 2019: based on site, grade, and region. Asia Pac J Clin Oncol. (2022) 18:e306–e17. doi: 10.1111/ajco.13671
24. Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie JW, et al. Incidence and survival trends for gastric neuroendocrine neoplasms: an analysis of 3523 patients in the seer database. Eur J Surg Oncol. (2018) 44:1628–33. doi: 10.1016/j.ejso.2018.01.082
25. Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. (2020) 21:e342–e9. doi: 10.1016/s1470-2045(20)30073-5
26. Nicolini S, Severi S, Ianniello A, Sansovini M, Ambrosetti A, Bongiovanni A, et al. Investigation of receptor radionuclide therapy with (177)Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. (2018) 45:923–30. doi: 10.1007/s00259-017-3925-8
27. Schillaci O, Spanu A, Scopinaro F, Falchi A, Danieli R, Marongiu P, et al. Somatostatin receptor scintigraphy in liver metastasis detection from gastroenteropancreatic neuroendocrine tumors. J Nucl Med. (2003) 44:359–68.
28. Plöckinger U and Wiedenmann B. Endocrine tumours of the gastrointestinal tract. Management of metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. (2005) 19:553–76. doi: 10.1016/j.bpg.2005.02.013
29. Chauhan A, Chan K, Halfdanarson TR, Bellizzi AM, Rindi G, O’Toole D, et al. Critical updates in neuroendocrine tumors: version 9 American joint committee on cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. (2024) 74:359–67. doi: 10.3322/caac.21840
30. Sabet A, Biersack HJ, and Ezziddin S. Advances in peptide receptor radionuclide therapy. Semin Nucl Med. (2016) 46:40–6. doi: 10.1053/j.semnuclmed.2015.09.005
31. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
32. Rossi RE, Elvevi A, Gallo C, Palermo A, Invernizzi P, and Massironi S. Endoscopic techniques for diagnosis and treatment of gastro-entero-pancreatic neuroendocrine neoplasms: where we are. World J Gastroenterol. (2022) 28:3258–73. doi: 10.3748/wjg.v28.i26.3258
33. Garcia-Carbonero R, Anton-Pascual B, Modrego A, Del Carmen Riesco-Martinez M, Lens-Pardo A, Carretero-Puche C, et al. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: are we moving forward? Endocr Rev. (2023) 44:724–36. doi: 10.1210/endrev/bnad006
34. Ma J, Cheng J, and Zhang Y. A novel keyword generation model based on topic-aware and title-guide. Comput Intell Neurosci. (2022) 2022:1787369. doi: 10.1155/2022/1787369
35. García-Torralba E, Garcia-Lorenzo E, Doger B, Spada F, and Lamarca A. Immunotherapy in neuroendocrine neoplasms: A diamond to cut. Cancers (Basel). (2024) 16(14):2530. doi: 10.3390/cancers16142530
36. Mehnert JM, Bergsland E, O’Neil BH, Santoro A, Schellens JHM, Cohen RB, et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the keynote-028 study. Cancer. (2020) 126:3021–30. doi: 10.1002/cncr.32883
37. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II keynote-158 study. Clin Cancer Res. (2020) 26:2124–30. doi: 10.1158/1078-0432.Ccr-19-3014
38. Lu M, Zhang P, Zhang Y, Li Z, Gong J, Li J, et al. Efficacy, safety, and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: A multiple-center phase Ib trial. Clin Cancer Res. (2020) 26:2337–45. doi: 10.1158/1078-0432.Ccr-19-4000
39. Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: A subgroup analysis of the CA209–538 clinical trial for rare cancers. Clin Cancer Res. (2020) 26:4454–9. doi: 10.1158/1078-0432.Ccr-20-0621
40. Owen DH, Benner B, Wei L, Sukrithan V, Goyal A, Zhou Y, et al. A phase ii clinical trial of nivolumab and temozolomide for neuroendocrine neoplasms. Clin Cancer Res. (2023) 29:731–41. doi: 10.1158/1078-0432.Ccr-22-1552
41. Halperin DM, Liu S, Dasari A, Fogelman D, Bhosale P, Mahvash A, et al. Assessment of clinical response following atezolizumab and bevacizumab treatment in patients with neuroendocrine tumors: A nonrandomized clinical trial. JAMA Oncol. (2022) 8:904–9. doi: 10.1001/jamaoncol.2022.0212
42. Choudhury N, Jain P, Dowlati A, Thompson J, Johnson ML, Mamdani H, et al. 698p interim results from a phase I/II study of HPN328, a tri-specific, half-life (T1/2) extended DLL3-targeting T cell engager in patients (Pts) with small cell lung cancer (SCLC) and other neuroendocrine neoplasms (NEN). Ann Oncol. (2023) 34:S486. doi: 10.1016/j.annonc.2023.09.1884
43. Partelli S, Landoni L, Bartolomei M, Zerbi A, Grana CM, Boggi U, et al. 1186mo a prospective phase II single-arm trial on neoadjuvant peptide receptor radionuclide therapy (PRRT) with 177lu-dotatate followed by surgery for pancreatic neuroendocrine tumors (Neolupanet). Ann Oncol. (2023) 34:S703. doi: 10.1016/j.annonc.2023.09.719
44. Partelli S, Landoni L, Bartolomei M, Zerbi A, Grana CM, Boggi U, et al. Neoadjuvant 177Lu-DOTATATE for non-functioning pancreatic neuroendocrine tumours (Neolupanet): multicentre phase II study. Br J Surg. (2024) 111(9):znae178. doi: 10.1093/bjs/znae178
45. Lugat A, Chouin N, Chocteau F, Esnault M, Marionneau-Lambot S, Gouard S, et al. Survival impact of [(225)Ac]Ac-DOTATOC alpha-therapy in a preclinical model of pancreatic neuroendocrine tumor liver micrometastases. Eur J Nucl Med Mol Imaging. (2024) 52(2):730–43. doi: 10.1007/s00259-024-06918-0
46. Bevilacqua A, Calabrò D, Malavasi S, Ricci C, Casadei R, Campana D, et al. A [68ga]Ga-DOTANOC PET/CT radiomic model for non-invasive prediction of tumour grade in pancreatic neuroendocrine tumours. Diagnostics (Basel). (2021) 11(5):870. doi: 10.3390/diagnostics11050870
47. Panzuto F, Merola E, Pavel ME, Rinke A, Kump P, Partelli S, et al. Stage IV gastro-entero-pancreatic neuroendocrine neoplasms: A risk score to predict clinical outcome. Oncologist. (2017) 22:409–15. doi: 10.1634/theoncologist.2016-0351
48. Fang C, Wang W, Feng X, Sun J, Zhang Y, Zeng Y, et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer. (2017) 117:1544–50. doi: 10.1038/bjc.2017.315
49. Xu G, Xiao Y, Hu H, Jin B, Wu X, Wan X, et al. A nomogram to predict individual survival of patients with liver-limited metastases from gastroenteropancreatic neuroendocrine neoplasms: A us population-based cohort analysis and chinese multicenter cohort validation study. Neuroendocrinology. (2022) 112:263–75. doi: 10.1159/000516812
50. Wu ZQ, Li Y, Sun NN, Xu Q, Zhou J, Su KK, et al. Nomogram for preoperative estimation of histologic grade in gastrointestinal neuroendocrine tumors. Front Endocrinol (Lausanne). (2022) 13:991773. doi: 10.3389/fendo.2022.991773
51. Fazio N and La Salvia A. Precision medicine in gastroenteropancreatic neuroendocrine neoplasms: where are we in 2023? Best Pract Res Clin Endocrinol Metab. (2023) 37:101794. doi: 10.1016/j.beem.2023.101794
52. Muscogiuri G, Barrea L, Campolo F, Sbardella E, Sciammarella C, Tarsitano MG, et al. Ketogenic diet: A tool for the management of neuroendocrine neoplasms? Crit Rev Food Sci Nutr. (2022) 62:1035–45. doi: 10.1080/10408398.2020.1832955
Keywords: gastroenteropancreatic neuroendocrine tumors, bibliometrics, visual analysis, cooccurrence, global research trends
Citation: Wu L, Wang T, Jiang S, Zhang J, Zhang M, Gao H, Wang H, Zhou Y, Ran R, Dong D and Yang J (2025) Global research trends in gastroenteropancreatic neuroendocrine tumors: a bibliometric analysis from 2000 to 2023. Front. Oncol. 15:1515893. doi: 10.3389/fonc.2025.1515893
Received: 04 December 2024; Accepted: 30 April 2025;
Published: 22 May 2025.
Edited by:
Alex Giakoustidis, Aristotle University of Thessaloniki, GreeceReviewed by:
Ali-Farid Safi, Craniologicum - Center for Craniomaxillofacial Surgery, SwitzerlandMarie Louise Ndzie Noah, Shandong First Medical University, China
Richard Mprah, Xuzhou Medical University, China
Weipang Ho, Linkou Chang Gung Memorial Hospital, Taiwan
Copyright © 2025 Wu, Wang, Jiang, Zhang, Zhang, Gao, Wang, Zhou, Ran, Dong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Yang, eWFuZ2ppbkBtYWlsLnhqdHUuZWR1LmNu
 Lei Wu
Lei Wu Tongfei Wang1
Tongfei Wang1 Siyuan Jiang
Siyuan Jiang Juan Zhang
Juan Zhang Huan Gao
Huan Gao Hui Wang
Hui Wang Ran Ran
Ran Ran Danfeng Dong
Danfeng Dong Jin Yang
Jin Yang