- 1Department of Pathology, Zhuhai People’s Hospital (Zhuhai Clinical Medical College of Jinan University), Zhuhai, Guangdong, China
- 2Department of Pathology, Foshan Hospital of Traditional Chinese Medicine, Foshan, Guangdong, China
ALK rearrangements are rarely documented in superficial soft tissue neoplasms exhibiting an infantile fibrosarcoma-like spindle cell tumor (IFS) pattern or stromal, resembling Neurotrophic Tyrosine Kinase Receptor(NTRK)rearranged spindle cell tumors. Here, we present two cases of pediatric cutaneous soft tissue tumors with an IFS pattern, in which ALK fusions involving related partner genes were identified. The tumors in both cases demonstrated similar morphology and consisted of ovoid and spindle cells with infiltrative boundaries. The spindle cells exhibited either a fascicular growth pattern or a haphazard pattern and stromal hyalinization. Both cases involved inflammatory cell infiltration, brisk mitosis, and CD34, S100, and ALK-D5F3 immunoreactivity. Next-generation sequencing identified ALK fusion with different partner genes (STRN and PLEKHH2). The fluorescence in situ hybridization break-apart assay confirmed ALK rearrangements in both cases. In case 1, no indications of disease progression or metastasis was observed within the limited follow-up (36 months). However, the patient in case 2 experienced a rapid recurrence and metastasis.
Introduction
The ALK gene on chromosome 2p23 encodes a receptor tyrosine kinase crucial for brain development and the functioning of certain nervous system neurons (1). ALK gene fusions are mutually exclusive oncogenic drivers and have been extensively documented in ALK-positive anaplastic large-cell lymphoma (ALCL), inflammatory myofibroblastic tumors (IMT) (2), non-small cell lung cancer (NSCLC), Spitz tumors, and Merkel cell carcinoma. ALK-rearranged soft tissue tumors were recently reported and involve various nomenclatures that have not been standardized. These include ALK-rearranged inflammatory myofibroblastic tumors (2), ALK-rearranged low-grade spindle cell tumor (3), superficial ALK-rearranged myxoid spindle cell tumors (4), ALK-rearranged infantile fibrosarcoma-like (IFS) tumors (5), ALK-rearranged histiocytosis (6), and ALK-rearranged cutaneous epithelioid fibrous histiocytomas (7). These tumors may exhibit benign, low-intermediate, or high-grade biological behaviors. ALK-rearranged low-grade spindle cell tumors share morphological and immunohistochemical features with ALK-rearranged IFS tumors without essential differences, indicating that the two tumors have highly identical low-grade characteristics, and can be considered the same lesion. Hence, additional cases are required to identify the inherent nature of such soft tissue tumors harboring ALK rearrangements and standardize their categorization.
In this study, we analyzed two IFS tumors with ALK gene rearrangements in children and summarized the clinicopathological characteristics of these kinase fusion-positive mesenchymal neoplasms, hoping to identify new approaches.
Case presentation
Clinicopathological findings
Case 1 was a 6-day-old male newborn who presented with multiple subcutaneous nodules in the lumbar back region, which were first detected at 30 weeks of gestation by trimester ultrasound. The pregnancy was otherwise uncomplicated, aside from fetal distress necessitating cesarean delivery at 35 weeks. Gross examination at birth revealed multiple subcutaneous nodules on his lumbar back with dense little black spots in the surrounding skin (Figure 1A). The clinical presentation and imaging characteristics indicated that the initial clinical impression was congenital nevus or neurogenic tumor. The largest mass (2 cm × 2 cm × 1 cm) was resected and part of the surface was ulcerated. Microscopic examination revealed congenital melanocytic nevi in the epidermis and superficial dermis with no obvious mitosis in the epidermal ulceration area (Figure 1B). The subcutaneous adipose tissue and dermis contained a nodular lesion, characterized by spindle to epithelioid cells lacking melanin arranged in long fascicles or randomly in a myxoid-to-collagenous stroma (Figure 1C). Furthermore, some ovoid tumor cells mixed with slight infiltrating inflammatory cells (Figure 1D), closely resembling an epithelioid inflammatory myofibroblastic tumors (IMT). Some epithelioid cells presented plentiful cytoplasm and small nucleoli. The tumor periphery exhibited hemangiopericytoma (HPC)-like vasculature (Figure 1E). The cellular region contained abundant mitotic figures (MFs), with approximately 3–8 MFs observed per 10 high-power fields (HPFs). Based on the grading system of Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) (8), the tumor exhibited low-grade features, corresponding to grade 1, with a differentiation score of 2, a mitotic count score of 1, and a necrosis score of 0. Immunohistochemistry demonstrated that the superficial nevus cells expressed S100, SOX10, and melanocytic markers (HMB45, Melan-A, and MiTF), while they were negative for ALK-D5F3 and CD34. In contrast, the deep neoplastic cells revealed strong positivity for ALK-D5F3 (Figure 1F) and S100 (Figure 1G), exhibited partial positivity for desmin and CD34 (Figure 1H), and were negative for SOX10, HMB45, Melan-A, MiTF, MyOD1, myogenin, SMA, Actin, pan-TRK, BRAF-V600E, and CK. The average Ki-67 index was 30%. The parents of the baby rejected therapy after the pathological diagnosis. There were no signs of progression or metastasis with limited follow-up information (36 months).
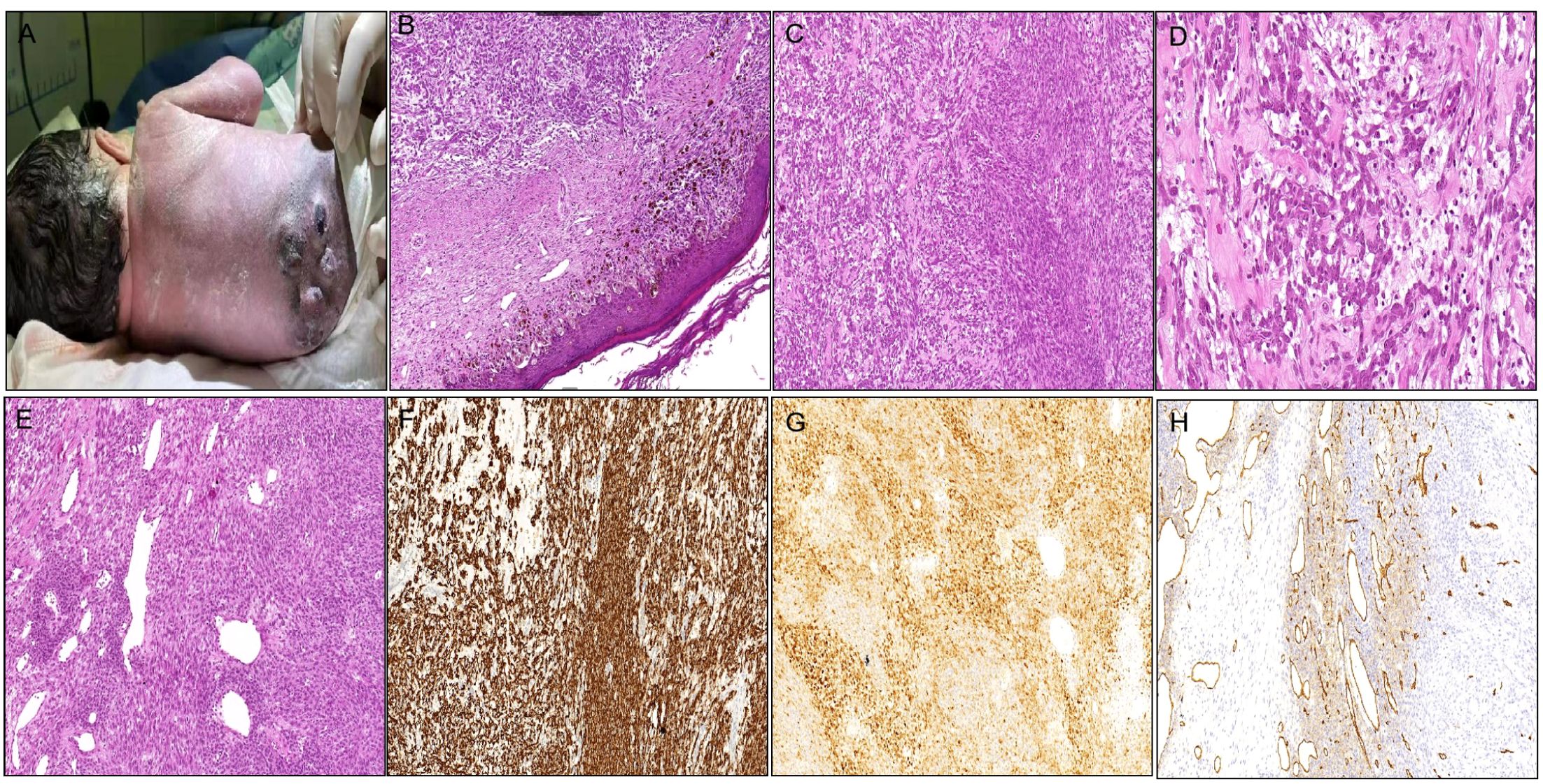
Figure 1. Clinicopathological findings of the tumor in Case 1. (A) Multiple subcutaneous nodules on the lumbar back with small black spots in the surrounding skin. (B) Nevus cell clusters in the epidermis and superficial dermis [(B) H&E staining×200]. (C) Spindle to epithelioid cells in long fascicles or haphazardly in myxoid-to-collagenous stroma [(C) H&E staining×200]. (D) Some ovoid tumor cells mixed with mild infiltrating inflammatory cells [(D) H&E staining×400]. (E) The tumor periphery demonstrating HPC-like vasculature [(E) H&E staining×200]. (F) Tumor cells densely cytoplasmic positive for ALK-D5F3. (G) Tumor cells densely positive for S100. (H) Tumor cells focally positive for CD34.
Case 2 was a 9-year-old girl with a mass on her left index finger (Figure 2A). Under gross examination, the biopsy specimen consisted of a 0.6 cm × 0.4 cm × 0.3 cm skin fragment. Microscopically, the lesion consisted of spindle and ovoid cells infiltrating the fat tissue, and microvascular proliferation and focal ectatic vessels (Figure 2B). The spindle cells were organized in a fascicular or random pattern, with indistinct cytoplasmic borders and mild to moderate-variability nuclear pleomorphism (Figure 2C). Furthermore, moderate inflammatory cell infiltration (Figure 2D) and brisk mitosis were observed (~5–6 MFs/10 HPFs). FNCLCC grading corresponded to histologic grade 1, with a differentiation score of 2, a mitotic count score of 1, and a necrosis score of 0. Immunohistochemical staining of the tumor cells in the finger was positive for ALK-D5F3 (Figure 2E), S100, CD34 (Figure 2F) and negative for AE1/3, SMA, desmin, STAT6, SOX10, and pan-TRK. A 96-month clinical follow-up revealed that the girl experienced double recurrences and required partial finger amputation due to metastases to the left axilla. Initially, the girl had a small red nodule, resembling a millet seed, protruding from the skin of the left index finger. This was not considered significant at the time. Two years later, as the nodule increased in size, the patient underwent cryotherapy supplemented with topical corticosteroid ointment. However, there was no improvement. A biopsy of the mass was then performed, and the pathology revealed infantile cellular hemangioma. Subsequently, the patient underwent surgical resection of the mass with negative postoperative margins. The pathology suggested a soft tissue tumor, but the specific diagnosis remained unclear. Four months after the surgery, the tumor recurred, prompting another resection. The pathology was reviewed by multiple hospitals, but opinions varied, and a definitive diagnosis could not be established. No further treatment was administered after this surgery. Four years later, the tumor recurred once more, and a metastatic lesion was discovered in the left axilla. The patient then underwent partial finger amputation and excision of the metastatic lesion. As of the latest follow-up, the patient remained alive with no recurrence. Pathologically, the relapsed and metastasized tumor exhibited similar morphology to the original tumor and was characterized by more compact tumor cells interspersed in collagen matrix (Figure 2G). Surprisingly, the tumor cells of the left axilla were immunohistochemically negative for ALK-D5F3 (Figure 2H), which differed from the primary tumor. The other immunophenotype of the metastasized tumor was similar to that of the original tumor.

Figure 2. Clinicopathological findings of the tumor in Case 2. (A) A subcutaneous mass on the left index finger. (B) Microvascular proliferation and focal ectatic vessels [(B) H&E staining×200]. (C) Spindle cells arranged in a fascicular growth or haphazard pattern with indistinct cytoplasmic borders and mild to moderate nuclear pleomorphism [(C) H&E staining×400]. (D) Moderate inflammatory cell infiltration [(D) H&E staining×100]. (E) Tumor cells of the finger immunohistochemically cytoplasmic positive for ALK-D5F3. (F) Tumor cells of the finger immunohistochemically positive for CD34. (G) Metastasized tumor demonstrating similar morphology to the original tumor, with more compact tumor cells interspersed in collagen matrix [(G) H&E staining×200]. (H) The tumor cells of the left axilla were immunohistochemically negative for ALK-D5F3.
Next-generation sequencing targeted genomic profiling
Genomic RNA was extracted from formaldehyde-fixed paraffin-embedded (FFPE) tumor tissues using magnetic beads. RNA was reverse-transcribed to complementary DNA using reverse transcriptase. Because the case series were from two regional hospitals, the NGS of the two patients involved two different approaches, panels, and sequencing companies. For Case 1, an AmpliSeq RNA SARC Fusion panel consisting of 204 primer pairs specific for 64 fusion gene pairs and primers specific for 10 internal reference genes was used to amplify soft tissue tumor-associated fusion genes and build amplicons. Sequencing was performed using an Ion PGM system kit (conducted by KingMed Center, Guangzhou, China).
For Case 2, comprehensive gene sequencing was conducted using a capture-based targeted sequencing panel (provided by Geneseeq Biotech, Nanjing, China) to detect RNA-based fusions and other genomic alterations. This panel included 506 genes and was capable of identifying single base substitutions, short and long insertions/deletions, copy number variations, gene fusions, and rearrangements.
NGS of Case 1 revealed a fusion transcript between STRN exon 3 and ALK exon 20 (Figure 3A), while Case 2 carried a fusion transcript between PLEKHH2 exon 6 and ALK exon 20 (Figure 3B). No additional pathogenic variants were identified in both cases. The predicted chimeric proteins comprised a coiled-coil domain in the STRN or PLEKHH2 N-terminus and a complete kinase domain in the ALK C-terminus.
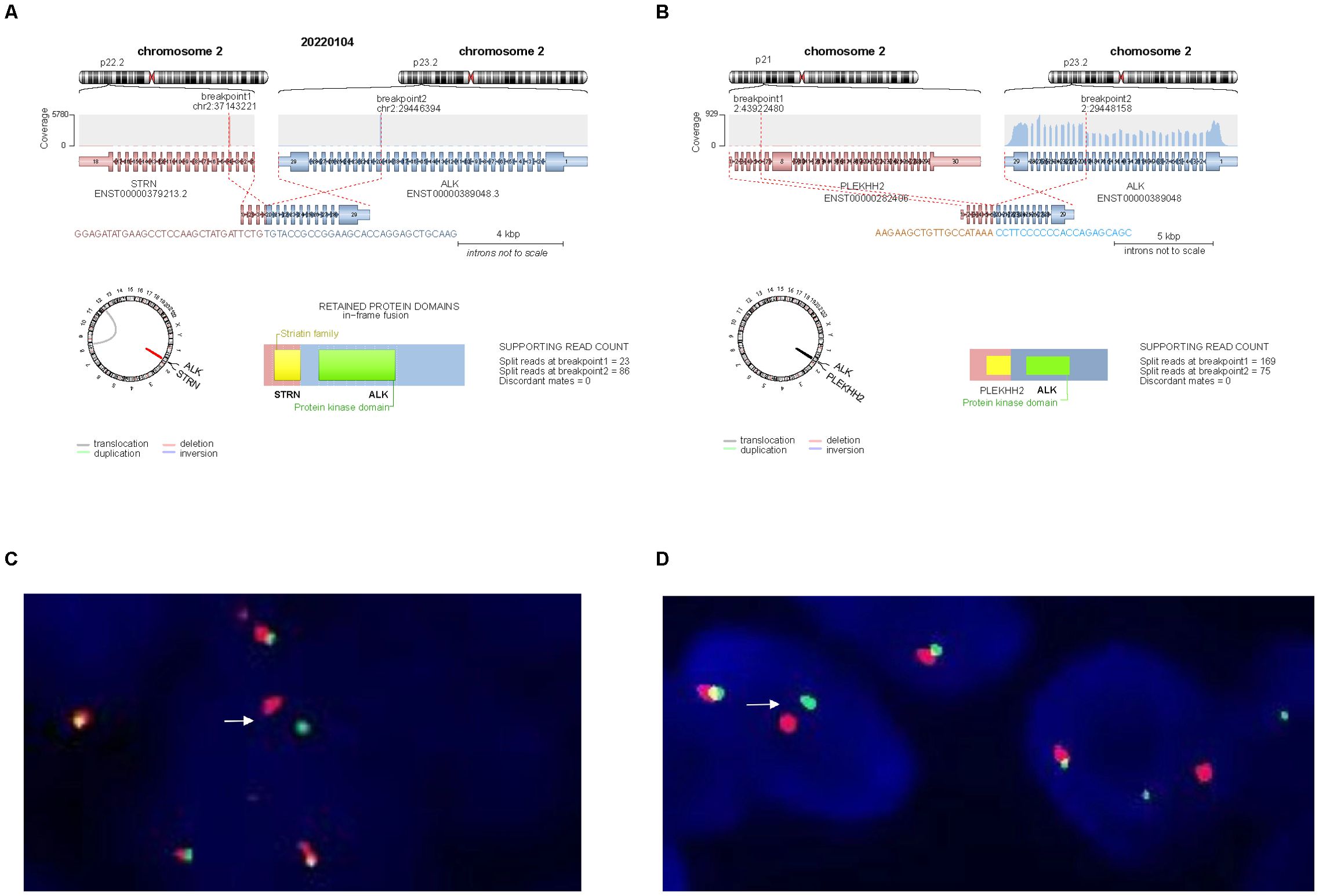
Figure 3. Molecular findings. (A) NGS identified a transcript comprising STRN exon 3 and ALK exon 20 in Case 1. (B) NGS identified a transcript comprising PLEKHH2 exon 6 and ALK exon 20 in Case 2. (C, D) ALK break-apart FISH test was positive in Case 1 (C) and Case 2 (D), demonstrating a signal pattern consisting of isolated 5′ (green), isolated 3′ (red), and fused 3′/5′ signals.
Fluorescence in situ hybridization
FISH was performed on 3-µm thick FFPE tumor sections using an ALK dual-color break-apart probe (Vysis, Abbott Molecular). More than 15% of the tumor cells with abnormal signals were considered positive for gene rearrangement based on the scoring of 100 non-overlapping cells. FISH confirmed that both cases carried ALK gene rearrangements (Figures 3C, D).
Discussion
We describe two pediatric ALK-rearranged spindle tumors with significant clinicopathological similarities to IFS. Histologically, both cases exhibited typical IFS morphology, including spindle to oval cells arranged either in long fascicles or haphazardly within a myxoid-to-collagenous stroma. Case 1 also exhibited HPC-like vessels. The immunohistochemistry of both cases demonstrated cytoplasmic ALK expression, with partly co-expressed CD34 and S100. This staining pattern is most commonly reported in NTRK-rearranged spindle cell neoplasms (9), whereas CD34 and/or S100 expression has been less frequently reported in IFS.
ALK rearrangements in pediatric spindle cell neoplasms often suggest the diagnosis of IMT (10), but the tumors in this study displayed clinicopathological features more consistent with IFS. Unlike IMTs, the two cases did not demonstrate significant lymphoplasmacytic or eosinophilic inflammation, nor contained ganglion-like mesenchymal cells. Furthermore, IMTs typically arise in the body cavities, lungs, soft tissues, and viscera of young patients (11, 12), with cutaneous IMTs being exceptionally rare. IMTs are fibroblastic/myofibroblastic tumors with strong SMA expression, with approximately 50–60% of cases carrying ALK gene fusions (11). Various ALK gene partners have been reported in IMTs, such as A2M, ATIC, CARS, CLTC, DCTN1, DES, EML4, FN1, HNRNPA1, IGFBP5, LMNA, PPFIBP1, PRKAR1A, RANBP2, RRBP1, SEC31L1, TFG, THBS1, TIMP3, TNS1, TPM3, and TPM4 (12, 13). The ALK::STRN fusions (14) and ALK::PLEKHH2 fusions (15) in the present study have also been described in IMT, but it is rare for these fusions to occur in cutaneous IFS-like tumors.
Interestingly, the clinical presentation and H&E-stained morphology led us to exclude a diagnosis of melanocytic tumors, such as melanomas arising in congenital naevi, atypical Spitzoid melanocytic tumor or Spitzoid melanoma with ALK fusion, Melanocytic myxoid spindle cell tumor with ALK rearrangement (MMySTAR) in case 1. Immunohistochemistry revealed the absence of SOX10 and melanocytic markers in the deep neoplastic cells, suggesting that these cells were non-melanocytic. Furthermore, we performed NGS testing, which covered a comprehensive panel of melanoma-associated molecular markers, including BRAF, NRAS, HRAS, CCND1, RET, KIT, CDKN2A, and the TERT promoter. However, no mutations were detected, further substantiating the absence of melanoma-related malignancies.
We detected two ALK fusions, each with a unique fusion partner, including one fusion (Case 1: STRN::ALK) and another fusion gene (Case 2: PLEKHH2::ALK). STRN without its coiled-coil domain or ALK with a tyrosine kinase domain mutation results in the absence of protein expression, and ALK with a tyrosine kinase sequence mutation does not lead to carcinogenesis (16). To the best of our knowledge, 46 cancer cases carried STRN exon 3 to ALK exon 20 fusion have been reported (17), including the present case, which comprises 3 malignant peritoneal mesothelioma cases, 31 cases of thyroid cancer, 5 cases of lung cancer, 3 cases of colorectal cancer, 2 cases of renal cancer, and 1 case of pancreatic cancer. PLEKHH2 encodes an intracellular protein highly enriched in renal glomerular podocytes and supports the podocyte foot processes (18). The N-terminus of PLEKHH2 contained a putative ahelical coiled-coil domain. PLEKHH2::ALK gene fusion has been reported in lung adenocarcinoma (19) and dermatofibrosarcoma protuberans (DFSP) (20). The positive response to ALK inhibitors in lung tumors with PLEKHH2::ALK fusion proteins further confirms their oncogenic potential. Commonly, ALK fusions activate the ALK kinase domain through autophosphorylation resulting from dimerization without requiring ligands (21). The fusion genes in this study contained the entire ALK intracellular kinase domain and the coiled-coil domain of the fusion partner genes, which mediated ALK dimerization and activation. Accordingly, we assumed that the fusion proteins were oncogenic.
In the second case, the metastatic lesion in the left axilla exhibited a loss of ALK expression on immunohistochemical analysis, while other immunophenotypic features remained consistent with those of the primary lesion. However, both lesions demonstrated ALK rearrangements with the same fusion partner, PLEKHH2, in NGS testing. The mutation abundance was 59.74% in the primary lesion and 42.47% in the metastatic lesion, and the tumor mutation burden (TMB) was 0 in both lesions. We hypothesize that this discrepancy may be due to the following potential mechanisms: First, metastatic clones may gain selective advantages by suppressing ALK expression through epigenetic modifications or other mechanisms, and may activate alternative signaling pathways to compensate for the loss of ALK signaling. Second, the microenvironment of the metastatic site, influenced by factors such as immune cell infiltration, cytokines, or hypoxia, may differ from that of the primary tumor and affect ALK expression. Third, metastatic cells may exhibit altered transcriptional regulation or RNA stability, with changes in transcription factors or miRNAs targeting ALK mRNA, leading to reduced ALK protein expression. Fourth, metastatic cells may enhance ALK protein degradation via the ubiquitin-proteasome pathway or inactivate ALK through dephosphorylation. Fifth, tumor heterogeneity and sampling bias in the metastatic lesion might result in the analysis of areas with lower ALK expression. Finally, epigenetic modifications, such as DNA methylation and histone modification, may silence ALK gene expression despite the presence of ALK rearrangements.
ALK fusion-driven soft tissue tumors exhibit varying specific histologic features across a broad histopathological spectrum (15). These neoplasms range from low- to intermediate-grade and are characterized by different underlying kinase fusions, which may demonstrate a pattern resembling the lipofibromatosis-like neural tumor (22) or IFS phenotype (5), or may resemble malignant peripheral nerve sheath tumors (23) and frequently contain regions with stromal and/or perivascular hyalinization (7). A small minority of kinase-fused neoplasms may exhibit malignant characteristics such as high cellularity, diffuse hyperchromasia, increased mitotic activity, and necrosis, leading to aggressive clinical behavior with distant metastases and fatal outcomes. Mesenchymal neoplasms with kinase fusion consistently exhibit cytologic monotony, regardless of where they are on this spectrum.
Despite their diagnostic terminology variations, IMT and IFS demonstrate similar outcomes, with a 25% recurrence risk and low rates of distant metastatic disease (12, 24). One of the most striking aspects of these pediatric kinase-rearranged mesenchymal tumors is the disconnect between traditional histologic grade based on FNCLCC and clinical outcome. As illustrated by Case 2, which is “low-grade” by FNCLCC, but the patient developed rapid recurrence and metastasis. This phenomenon may be caused by a variety of factors, including the genetic characteristics of the tumor, the microenvironment, and individual differences among patients. Reports on this aspect are scarce, we currently lack reliable prognostic markers for this category of tumors and need further research to understand their long-term clinical outcomes. Notably, ALK fusions in IFS-like tumors indicate a positive response to ALK inhibitor treatment, such as crizotinib. Furthermore, targeted therapeutics reduce mortality from aggressive tumors and alleviate morbidity from indolent tumors in challenging anatomical locations. Unfortunately, neither of our two cases was treated with ALK inhibitors. Particularly in Case 2, because the initial pathological diagnoses were inconclusive, effective treatment was not initiated, leading to multiple recurrences and ultimately necessitating amputation of the finger. Had we more accurately recognized the nature of such tumor and initiated early diagnosis and treatment, especially with ALK inhibitors, amputation might have been avoided.
Tan SY et al (5). described a series of four pediatric ALK-rearranged mesenchymal spindle cell tumors that exhibited clinicopathological features similar to IFS. Two tumors originated in the kidney, while the other two developed in soft tissues. Histologically, all cases demonstrated a morphological spectrum typical of IFS, characterized by cellular, spindle to ovoid cells arranged either in long fascicles or haphazardly within a collagenized to myxoid stroma. These tumors also featured HPC-like vessels and focal perivascular hyalinosis. ALK fusions were identified in all four tumors, each with a unique fusion partner. One renal tumor showed co-expression of S100 and CD34 and developed liver and lung metastases 1–2 months after presentation. On this basis, we have additionally reported two cases that share similar morphological, immunohistochemical, molecular, and clinical features with the previously described case series. This expanded case series highlights the considerable genetic overlap among inflammatory myofibroblastic tumors (IMT), cellular congenital mesoblastic nephroma (cCMN)/IFS, and NTRK-rearranged spindle cell neoplasms. These findings suggest that these entities may represent a continuum of kinase-related mesenchymal tumors or distinct but morphologically, immunophenotypically, and genetically overlapping groups of tumors. This insight underscores the potential for a more refined reclassification in the future.
Conclusion
Herein, we report two additional cases of ALK-rearranged mesenchymal neoplasms, each exhibiting distinct characteristics that support the overlap between IFS-like tumors and NTRK-rearranged spindle cell neoplasms. These findings suggest that ALK is involved in the development of these tumors. Identifying spindle cell tumors within this spectrum may have significant therapeutic implications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhuhai People’s Hospital (ZYEC(R)2024-067). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
QO: Writing – original draft, Writing – review & editing, Conceptualization, Software. XG: Data curation, Investigation, Writing – review & editing. RM: Conceptualization, Funding acquisition, Project administration, Supervision, Data curation, Software, Validation, Visualization, Writing – review & editing. ZC: Supervision, Funding acquisition, Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the patients for permitting us to use their data to complete this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, et al. Molecular characterization of alk, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. (1997) 14:439–49. doi: 10.1038/sj.onc.1200849
2. Fordham AM, Xie J, Gifford AJ, Wadham C, Morgan LT, Mould EVA, et al. Cd30 and alk combination therapy has high therapeutic potency in ranbp2-alk-rearranged epithelioid inflammatory myofibroblastic sarcoma. Br J Cancer. (2020) 123:1101–13. doi: 10.1038/s41416-020-0996-2
3. Kikawa C, Ketterl TG, Liu Ph DY, Reed RC, and Dahl JP. Pediatric low-grade spindle cell neoplasm with a novel ak5::Alk fusion: A case report. Ann Otol Rhinol Laryngol. (2023) 132:470–5. doi: 10.1177/00034894221092207
4. Dermawan JK, Azzato EM, Goldblum JR, Rubin BP, Billings SD, and Ko JS. Superficial alk-rearranged myxoid spindle cell neoplasm: A cutaneous soft tissue tumor with distinctive morphology and immunophenotypic profile. Mod Pathol. (2021) 34:1710–8. doi: 10.1038/s41379-021-00830-w
5. Tan SY, Al-Ibraheemi A, Ahrens WA, Oesterheld JE, Fanburg-Smith JC, Liu YJ, et al. Alk rearrangements in infantile fibrosarcoma-like spindle cell tumours of soft tissue and kidney. Histopathology. (2022) 80:698–707. doi: 10.1111/his.14603
6. Rossi S, Gessi M, Barresi S, Tamburrini G, Giovannoni I, Ruggiero A, et al. Alk-rearranged histiocytosis: report of two cases with involvement of the central nervous system. Neuropathol Appl Neurobiol. (2021) 47:878–81. doi: 10.1111/nan.12739
7. Kazlouskaya V, Ho J, Jedrych J, and Karunamurthy A. Spindle cell variant of epithelioid cell histiocytoma (Spindle cell histiocytoma) with alk gene fusions: cases series and review of the literature. J Cutan Pathol. (2021) 48:837–41. doi: 10.1111/cup.13923
8. Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. (2006) 130:1448–53. doi: 10.5858/2006-130-1448-GOSTSR
9. Gao X, Xu S, Zhu P, Lao IW, Yu L, and Wang J. Primary ntrk -rearranged spindle cell neoplasm of the gastrointestinal tract: A clinicopathological and molecular analysis of 8 cases. Am J Surg Pathol. (2024) 48:623–31. doi: 10.1097/PAS.0000000000002202
10. Bowman CJ, Medeiros F, Fadare O, Sangoi AR, Horvai AE, Devine WP, et al. Alk immunoexpression is specific for inflammatory myofibroblastic tumor among vulvovaginal mesenchymal neoplasms. Int J Gynecol Pathol. (2023) 42:1–10. doi: 10.1097/PGP.0000000000000858
11. Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, et al. Inflammatory myofibroblastic tumor: the experience of the european pediatric soft tissue sarcoma study group (Epssg). Eur J Cancer. (2020) 127:123–9. doi: 10.1016/j.ejca.2019.12.021
12. Coffin CM, Hornick JL, and Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including alk expression in atypical and aggressive cases. Am J Surg Pathol. (2007) 31:509–20. doi: 10.1097/01.pas.0000213393.57322.c7
13. Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. (2014) 4:889–95. doi: 10.1158/2159-8290.CD-14-0377
14. Li X, Zheng J, Li X, Chen Y, Liu K, Li F, et al. Case report: ensartinib for gastric epithelioid inflammatory myofibrosarcoma with strn-alk fusion. Front Oncol. (2023) 13:1252221. doi: 10.3389/fonc.2023.1252221
15. Dermawan JK, DiNapoli SE, Mullaney KA, Sukhadia P, Agaram NP, Dickson BC, et al. Alk-rearranged mesenchymal neoplasms: A report of 9 cases further expanding the clinicopathologic spectrum of emerging kinase fusion positive group of tumors. Genes Chromosomes Cancer. (2023) 62:75–84. doi: 10.1002/gcc.23097
16. Moqrich A, Mattei MG, Bartoli M, Rakitina T, Baillat G, Monneron A, et al. Cloning of human striatin cdna (Strn), gene mapping to 2p22-P21, and preferential expression in brain. Genomics. (1998) 51:136–9. doi: 10.1006/geno.1998.5342
17. Miyagawa C, Takaya H, Sakai K, Nishio K, Konishi M, Minamiguchi S, et al. A novel Malignant peritoneal mesothelioma with strn exon 2 and alk exon 20: A case report and literature review. Oncologist. (2021) 26:356–61. doi: 10.1002/onco.13714
18. Perisic L, Lal M, Hulkko J, Hultenby K, Onfelt B, Sun Y, et al. Plekhh2, a novel podocyte protein downregulated in human focal segmental glomerulosclerosis, is involved in matrix adhesion and actin dynamics. Kidney Int. (2012) 82:1071–83. doi: 10.1038/ki.2012.252
19. Nagasaka M, Fisher A, Chowdhury T, Ge Y, and Sukari A. Plekhh2-alk: A novel in-frame fusion with durable response to alectinib: utilizing rna sequencing in search for hidden gene fusions susceptible to targeted therapy. Clin Lung Cancer. (2021) 22:e51–e3. doi: 10.1016/j.cllc.2020.07.017
20. Ward RE, Stultz TW, Billings SD, and Vidimos AT. Mohs micrographic surgery for congenital scalp dermatofibrosarcoma protuberans with novel plekhh2-alk gene fusion. Dermatol Surg. (2024) 50:291–3. doi: 10.1097/DSS.0000000000004052
21. Lu L, Ghose AK, Quail MR, Albom MS, Durkin JT, Holskin BP, et al. Alk mutants in the kinase domain exhibit altered kinase activity and differential sensitivity to small molecule alk inhibitors. Biochemistry. (2009) 48:3600–9. doi: 10.1021/bi8020923
22. Kao YC, Suurmeijer AJH, Argani P, Dickson BC, Zhang L, Sung YS, et al. Soft tissue tumors characterized by a wide spectrum of kinase fusions share a lipofibromatosis-like neural tumor pattern. Genes Chromosomes Cancer. (2020) 59:575–83. doi: 10.1002/gcc.22877
23. Suurmeijer AJ, Dickson BC, Swanson D, Zhang L, Sung YS, Huang HY, et al. The histologic spectrum of soft tissue spindle cell tumors with ntrk3 gene rearrangements. Genes Chromosomes Cancer. (2019) 58:739–46. doi: 10.1002/gcc.22767
24. Orbach D, Sparber-Sauer M, Laetsch TW, Minard-Colin V, Bielack SS, Casanova M, et al. Spotlight on the treatment of infantile fibrosarcoma in the era of neurotrophic tropomyosin receptor kinase inhibitors: international consensus and remaining controversies. Eur J Cancer. (2020) 137:183–92. doi: 10.1016/j.ejca.2020.06.028
Keywords: case report, cutaneous, spindle tumor, infantile fibrosarcoma-like tumor, ALK rearrangement
Citation: Ouyang Q, Guo X, Mao R and Cao Z (2025) Case Report: ALK-rearranged mesenchymal neoplasms with S100 and CD34 co-expression: additional cases with distinct characteristics. Front. Oncol. 15:1516491. doi: 10.3389/fonc.2025.1516491
Received: 24 October 2024; Accepted: 09 May 2025;
Published: 29 May 2025.
Edited by:
Valdir Sabbaga Amato, University of São Paulo, BrazilReviewed by:
Qi-Xing Gong, First Affiliated Hospital of Nanjing Medical University, ChinaKathleen Bone, Medical College of Wisconsin, United States
Hiba Mechahougui, University Hospitals of Geneva, Switzerland
Copyright © 2025 Ouyang, Guo, Mao and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhixing Cao, dHNhb3p4QDE2My5jb20=; Rongjun Mao, MzA0MDg5MTA3QHFxLmNvbQ==
 Qi Ouyang
Qi Ouyang Xiaohong Guo1
Xiaohong Guo1