- 1Department of Bone and Soft Tissue, Affiliated Tumor Hospital of Xinjiang Medical University, Xinjiang, China
- 2Department of Cardiovascular Medicine, General Hospital of Xinjiang Military Region, Urumqi, Xinjiang, China
- 3Department of Neurosurgery, Neurosurgery Centre, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
Background: Melanoma has the third highest rate of brain metastases among all cancers and is associated with poor long-term survival. This study aimed to develop machine learning models to predict early death in melanoma brain metastasis (MBM) patients to guide clinical decision-making
Methods: We analyzed MBM patients from the SEER database and Xinjiang Medical University. Patients were randomly divided into training and testing cohorts (7:3 ratio). Seven machine learning models were developed and validated using cross-validation, ROC analysis, decision curve analysis, and calibration curves to predict cancer-specific early death (CSED) and all-cause early death (ACED) within 3 months of diagnosis.
Results: Among 1,547 MBM patients, 531 (34.3%) experienced CSED, and 554 (35.8%) experienced ACED. Key predictive factors included age, treatment modalities (radiation, chemotherapy, surgery), tumor characteristics (ulceration), and extracranial metastases (bone, liver). XGBoost achieved the best performance for ACED prediction (AUC=0.776), while logistic regression performed best for CSED prediction (AUC=0.694). External validation confirmed model reliability with comparable performance.
Conclusion: These machine learning models demonstrate strong predictive performance and may assist clinicians in early risk stratification and treatment planning for MBM patients. The models provide objective risk assessment tools that could improve patient counseling and guide aggressive versus palliative care decisions.
1 Introduction
Melanoma is the most lethal form of skin cancer, known for its aggressive spread to multiple organs (1, 2). Malignant melanoma ranks third in brain metastasis incidence among all cancers, followed by lung and breast cancer (3). Central nervous system involvement is evident in 40-60% of advanced melanoma patients (4, 5), and is the direct cause of death in 60-70% of melanoma patients (1). The long-term survival rate of MBM patients has been under 10% (6). Without treatment, MBM advances quickly, with a 5-year survival rate of less than 10%, and most patients succumb within 7.5 months (7). According to previous studies across multiple cancer types, early death (ED) was defined as patient death within 3 months of the first diagnosis (8, 9), a definition that has been consistently validated in gastric cancer, lung cancer with brain metastasis, and specifically in melanoma populations (10, 11), demonstrating its clinical relevance across diverse cancer types and treatment eras. More than 95% of early melanoma deaths were metastatic melanoma and resulted in cancer-specific death (12). Previous studies have reported factors influencing the incidence and prognosis of MBM, but the conclusions remain disputed (12–14). Therefore, it’s necessary to recognize the risk factors associated with ED from the large sample data and develop models to assess the likelihood of early death in MBM patients accurately.
Retrospective studies using historical data play a crucial role in clinical decision-making (15). Artificial intelligence has been widely utilized in both cardiovascular disease (16) and oncology (17) to develop predictive models. Machine learning (ML), a branch of artificial intelligence, can automatically analyze large datasets, identify hidden relationships between factors and survival outcome, and build highly effective models to predict outcomes for new data. This study aimed to retrospectively analyze the clinicopathological characteristics associated with ED and build ML models to predict the ED of MBM patients. The predictive ML models may assist patients and doctors in making optimal clinical decisions.
2 Materials and methods
2.1 Data collection
The SEER*Stat software (www.seer.cancer.gov, version 8.43)was used to extract data on MBM patients from the SEER database. The International Classification of Oncology Diseases and Version 3 (ICD-O-3) criteria were used to determine the primary cancer site, and the diagnosis was finally histologically confirmed. CSED was defined as patients dying due to MBM within three months. ACED was defined as patients dying due to all causes within three months. For the SEER database component, ethics approval was not required as SEER data is publicly available and de-identified.
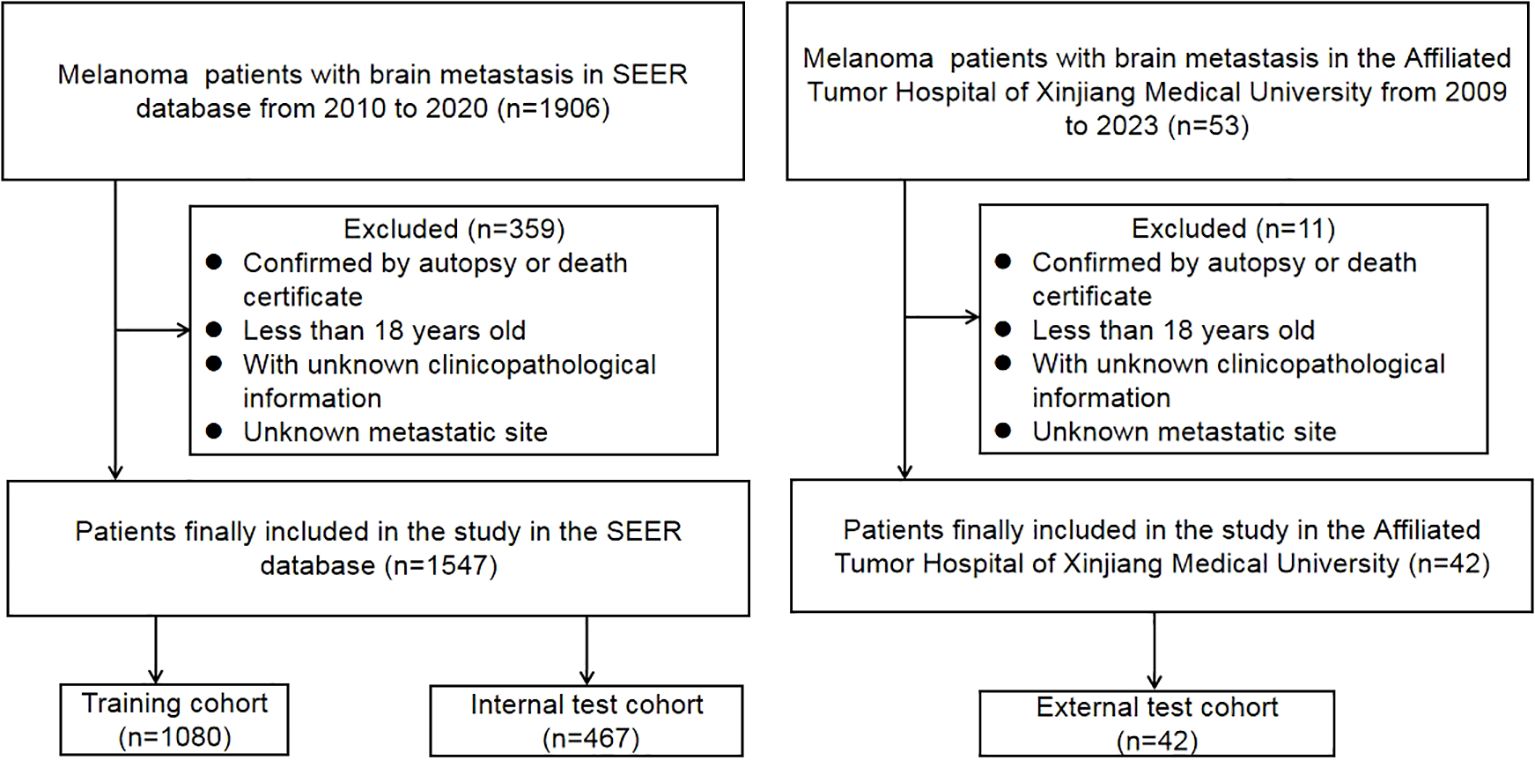
The inclusion criteria were as follows: (1) primary cancer only, (2) patients histologically confirmed with MBM from 2010 to 2020 in the SEER database. The exclusion criteria were as follows: (1) diagnosis confirmed by autopsy or death certificate only, (2) age less than 18 years, (3) with unknown clinicopathological information, (4) Unknown metastatic site. For metastatic site identification, we utilized the Collaborative Stage (CS) metastatic site codes in the SEER database to identify patients with brain metastasis and to determine the presence of other distant metastases, including liver, lung, and bone. It’s important to note that the SEER database records these metastases as binary variables (Yes/No) without providing further anatomical subdivision. This limitation prevented more granular analyses of metastatic patterns in our study.
Regarding missing data handling, we implemented a conservative approach to maintain data integrity. For categorical variables where “Unknown” was explicitly coded as a category in the SEER database (such as ulceration status, marital status, etc.), we retained these patients and analyzed “Unknown” as a separate category rather than excluding them. This approach is consistent with previous SEER-based studies and preserves the sample size while acknowledging data limitations transparently (18). This strategy allowed us to develop more robust machine learning models that can provide predictions even when certain data points might be missing in clinical practice.
To confirm the reliability of the data, the included patients from the SEER database were randomly divided into training and testing cohorts in a ratio of 7:3. The validation data cohort was extracted from the Affiliated Tumor Hospital of Xinjiang Medical University from 2010 to 2023, and follow-up lasted until October 31, 2023(Figure 1). Approval for this study was obtained from the Institutional Review Board of the Affiliated Tumor Hospital of Xinjiang Medical University.
2.2 Study design
Independent risk factors were identified through a systematic variable selection process using univariate and multivariate logistic regression analysis. Initially, all candidate variables were evaluated using univariate logistic regression. Features with p values <0.05 in the univariate analysis were then entered into a backward stepwise multivariate logistic regression model. The backward elimination process started with all significant univariate variables included in the full model and sequentially removed the variable with the highest p-value (least significant) at each step, provided that p>0.10. This process continued iteratively until all remaining variables in the model achieved statistical significance (p<0.05). The final multivariate model retained only variables that remained statistically significant after controlling for other factors through this backward elimination process. These independently significant risk factors identified through backward stepwise regression were then used as input features to construct the machine learning models. Seven ML models were developed, including logistic regression (LR), K-nearest neighbor (KNN), support vector machine (SVM), decision tree (DT), random forest (RF), XGBoost, and LightGBM. The training cohort was used for building ML models and cross-validation, while testing and validation cohorts were used to evaluate the predictive performance of the models.
2.3 Statistical analysis
Categorical variables are presented as numbers (%), and comparisons were made using the Pearson Chi-square test. Univariate and multivariate logistic regression analyses were conducted to determine risk factors. All statistical analysis, model development, and validation are implemented in R software (version 4.3.0). A two-sided p-value <0.05 was considered statistically significant.
Cross-validation methodology: All models were evaluated using stratified 10-fold cross-validation to ensure robust performance estimation while maintaining outcome distribution balance across folds. For the logistic regression model, which requires no hyperparameter tuning, cross-validation was used solely for unbiased performance evaluation. For the remaining six models (KNN, SVM, DT, RF, XGBoost, and LightGBM), a nested cross-validation approach was implemented where hyperparameter optimization occurred independently within each fold to prevent data leakage and ensure valid generalizability estimates.
Hyperparameter optimization: Each model’s hyperparameters were tuned using random grid search within the cross-validation framework. KNN optimized the number of neighbors (3-11); SVM tuned cost (-5 to 5) and RBF sigma (-4 to -1); DT optimized tree depth (3-7), minimum node size (5-10), and cost complexity (-6 to -1); RF tuned the number of variables per split (2-10), number of trees (200-500), and minimum node size (20-50); XGBoost optimized six parameters including learning rate, tree depth, and regularization terms; LightGBM similarly tuned six key parameters. For models requiring data preprocessing (KNN, SVM), normalization and dummy coding were performed within each fold using the tidymodels recipe framework to prevent data leakage.
Model validation strategy: Model performance was evaluated through a three-tier validation approach: (1) Internal validation using 10-fold cross-validation on the training cohort to assess model stability and select optimal hyperparameters; (2) Temporal validation using an internal test set (30% holdout from the same institution/database) that was never used during model development or cross-validation; and (3) External validation using an independent cohort from a different institution/database/time period to evaluate model generalizability across different populations and clinical settings. This comprehensive validation strategy ensures robust assessment of model performance and clinical applicability across diverse patient populations.
ROC analysis, calibration curves, and DCA curves were used to evaluate the models’ accuracy.
3 Results
3.1 Patient characteristics
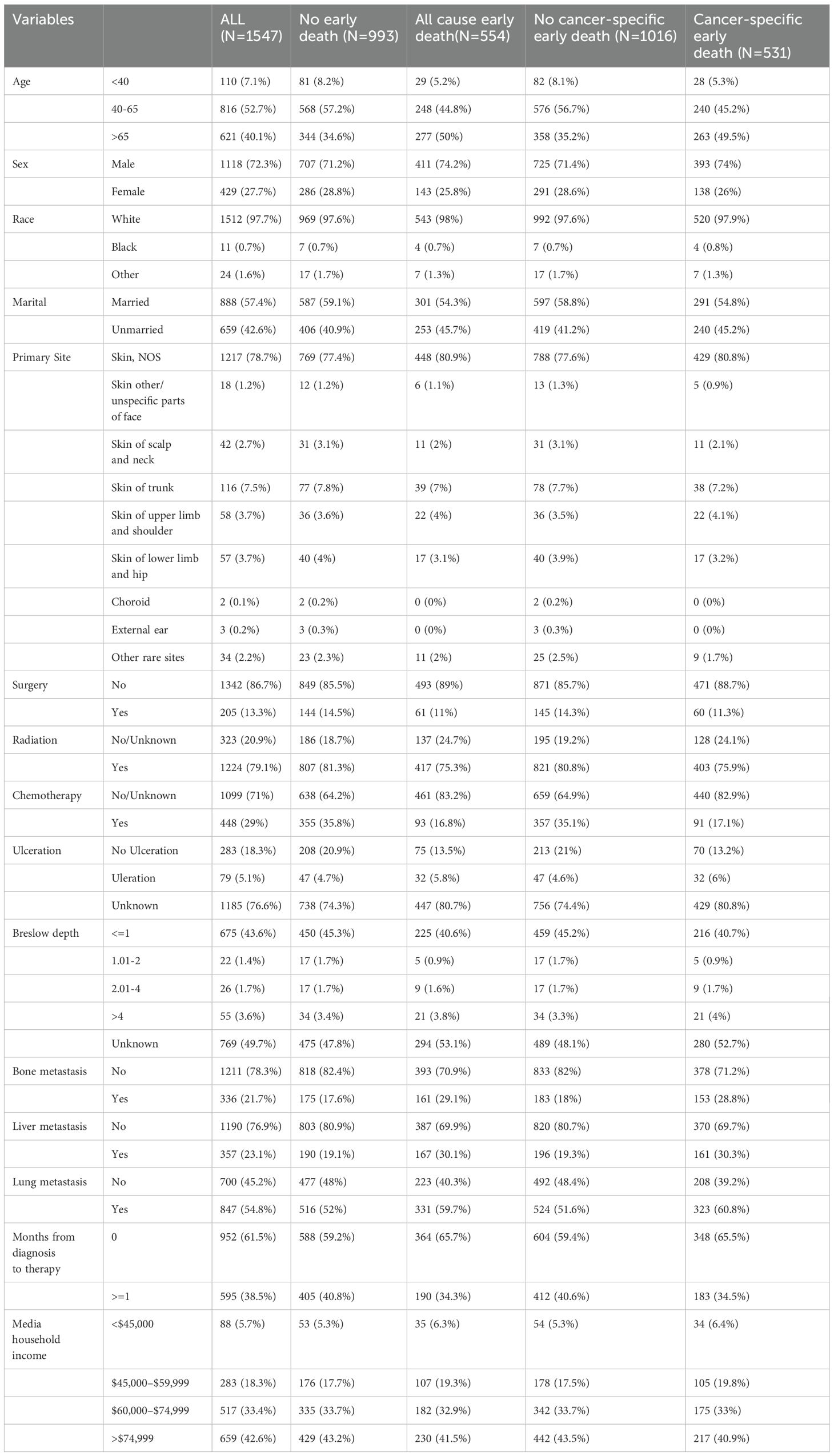
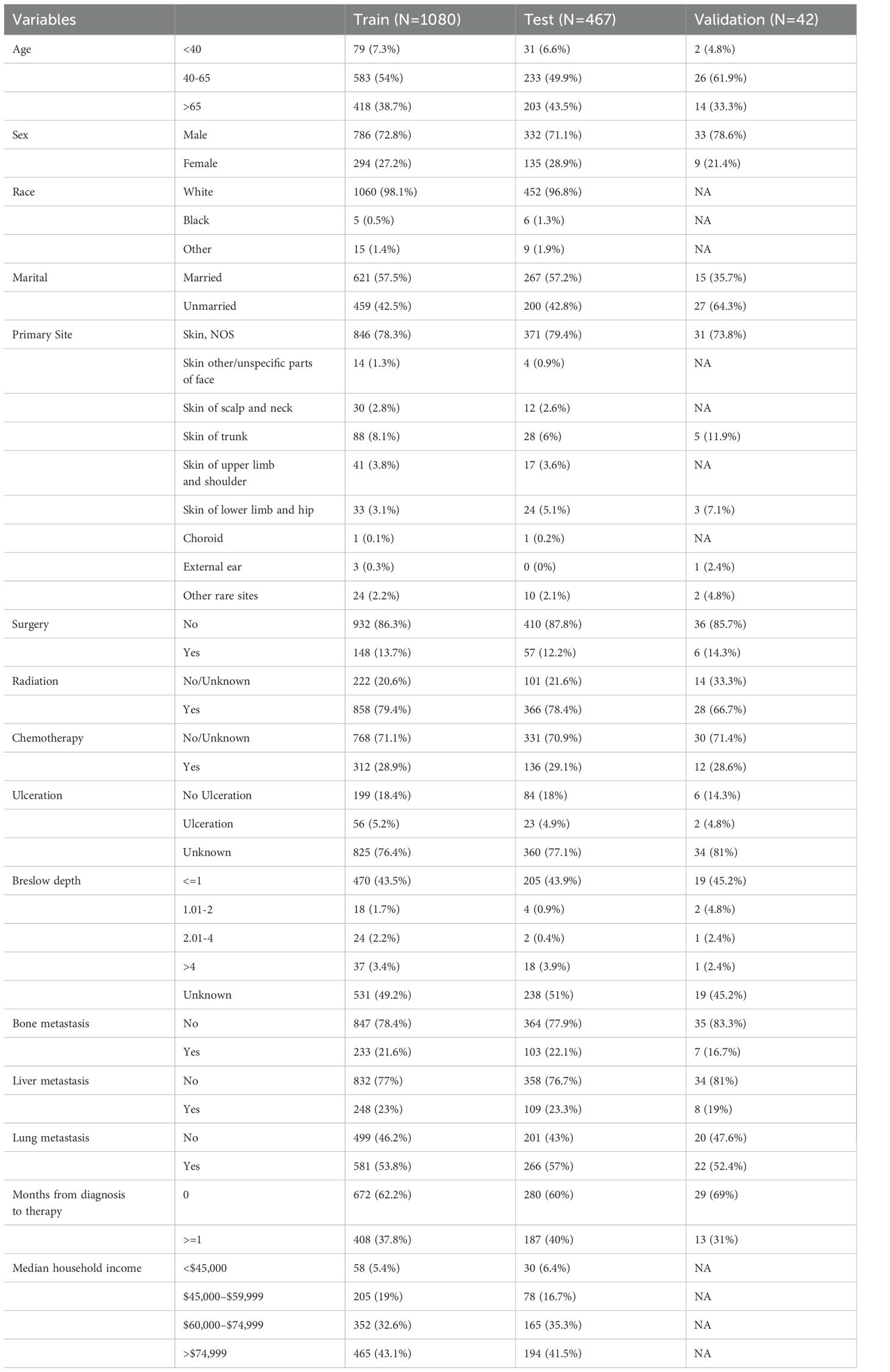
A total of 1547 MBM patients from the SEER database were finally included in this study. Among them, 531 (34.3%) cases suffered from CSED, and 554 (35.8%) cases suffered from ACED. The details of the patients are displayed in Table 1. For ACED, most patients were > 60 years old (50%), male (74.2%), white (98%), did not receive surgery (89%), received radiation (75.3%), and did not receive or had unknown chemotherapy status (83.2%). As for CSED, most of the patients > 60 years old (49.5%), male (74%), white (97.9%), without surgery (88.7%), with radiation (75.9%), without or unknown whether perform chemotherapy (82.9%). The demography of patients in the training cohort (n=1080), test cohort (n=467), and validation (n=42) cohort is shown in Table 2.
3.2 Univariate and multivariate logistic regression analyses
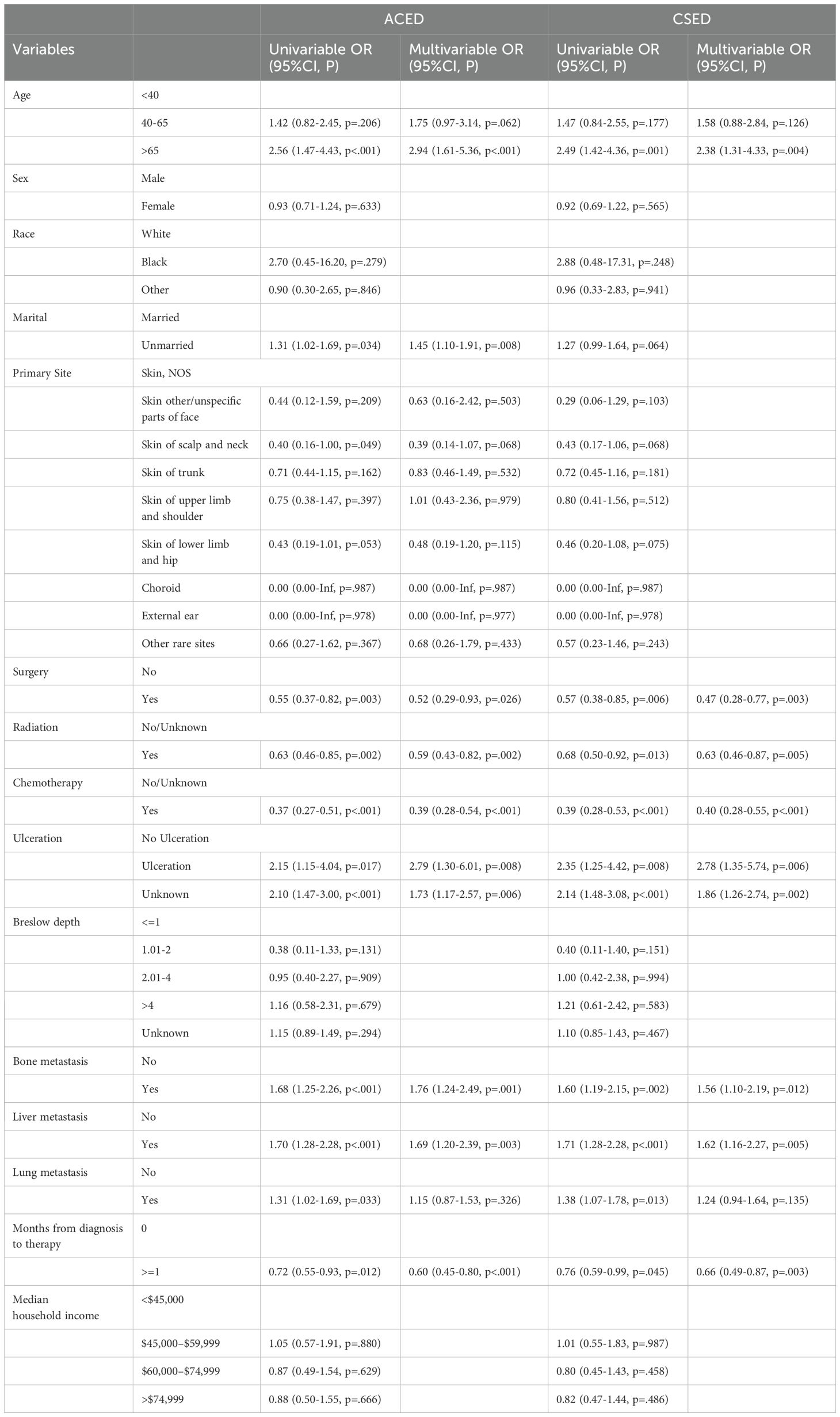
According to the univariate logistic regression analyses, age, marital status, primary site, radiation, chemotherapy, ulceration, surgery, bone metastasis, liver metastasis, lung metastasis, and months from diagnosis to therapy may be risk factors for the ACED of MBM patients. Age, radiation, chemotherapy, ulceration, surgery, bone metastasis, liver metastasis, lung metastasis, and months from diagnosis to therapy may associated with the CSED of MBM patients.
Multivariate logistic regression analyses revealed that age, marital status, radiation, chemotherapy, ulceration, surgery, bone metastasis, liver metastasis, and months from diagnosis to therapy were significant risk factors for the ACED of MBM patients. Age, radiation, chemotherapy, ulceration, surgery, bone metastasis, liver metastasis, and months from diagnosis to therapy were significant risk factors for the CSED of MBM patients. (Table 3)
3.3 Model performance
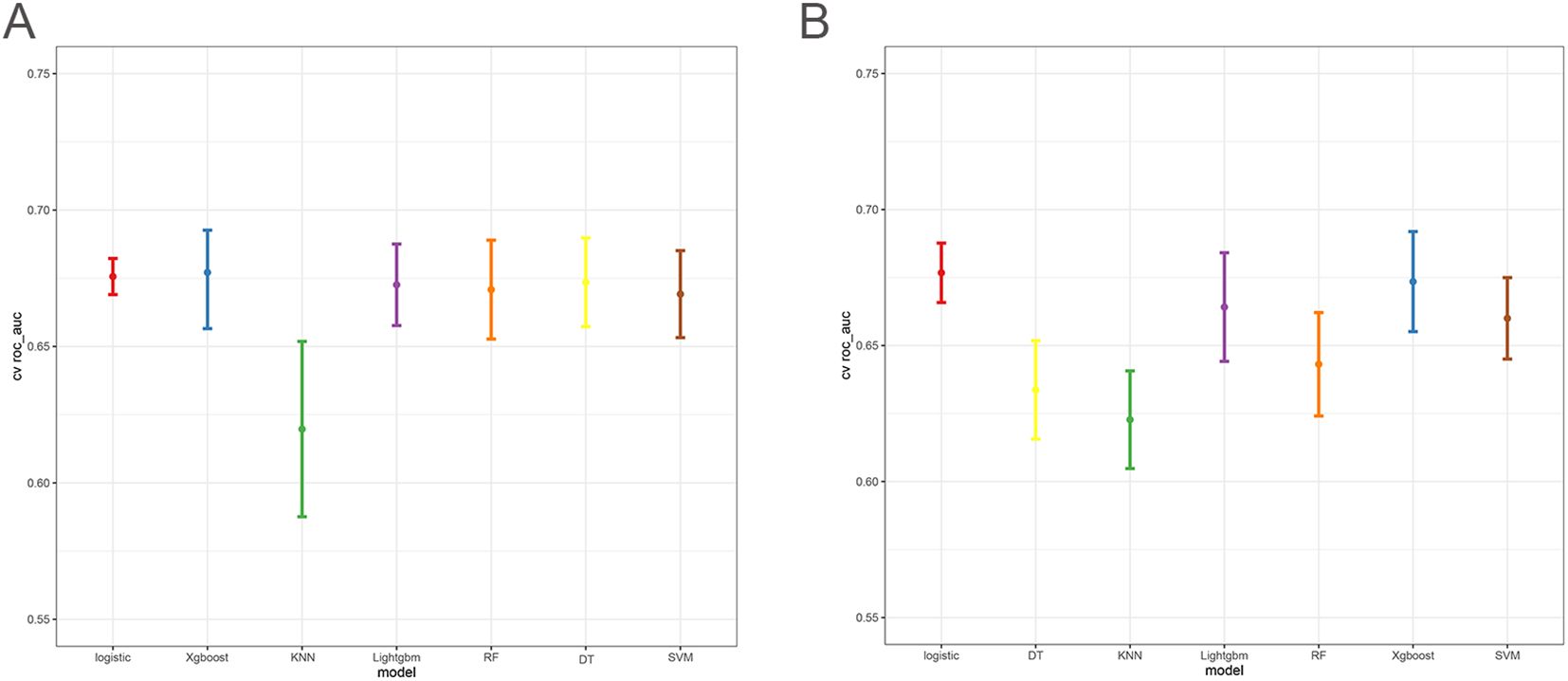
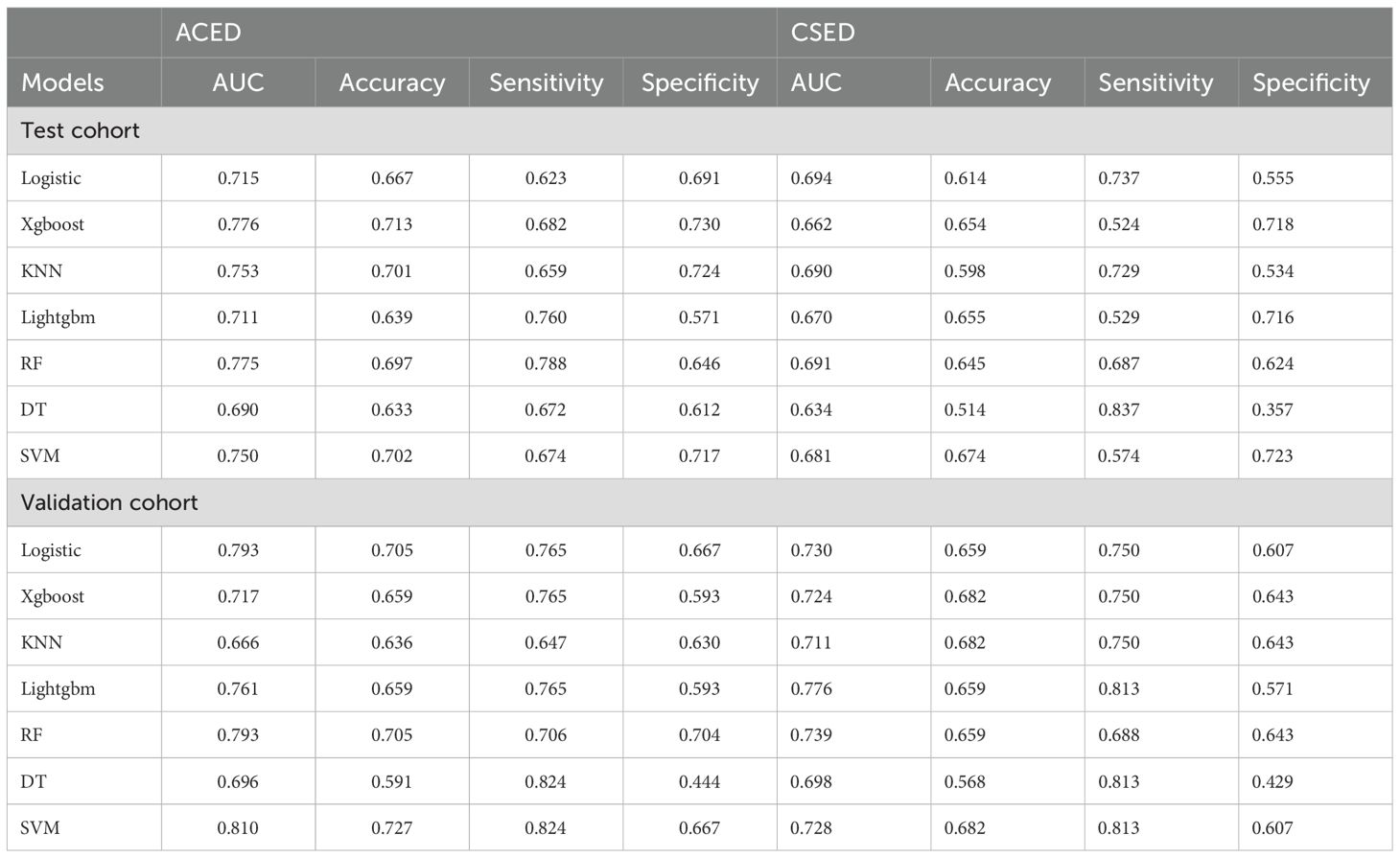
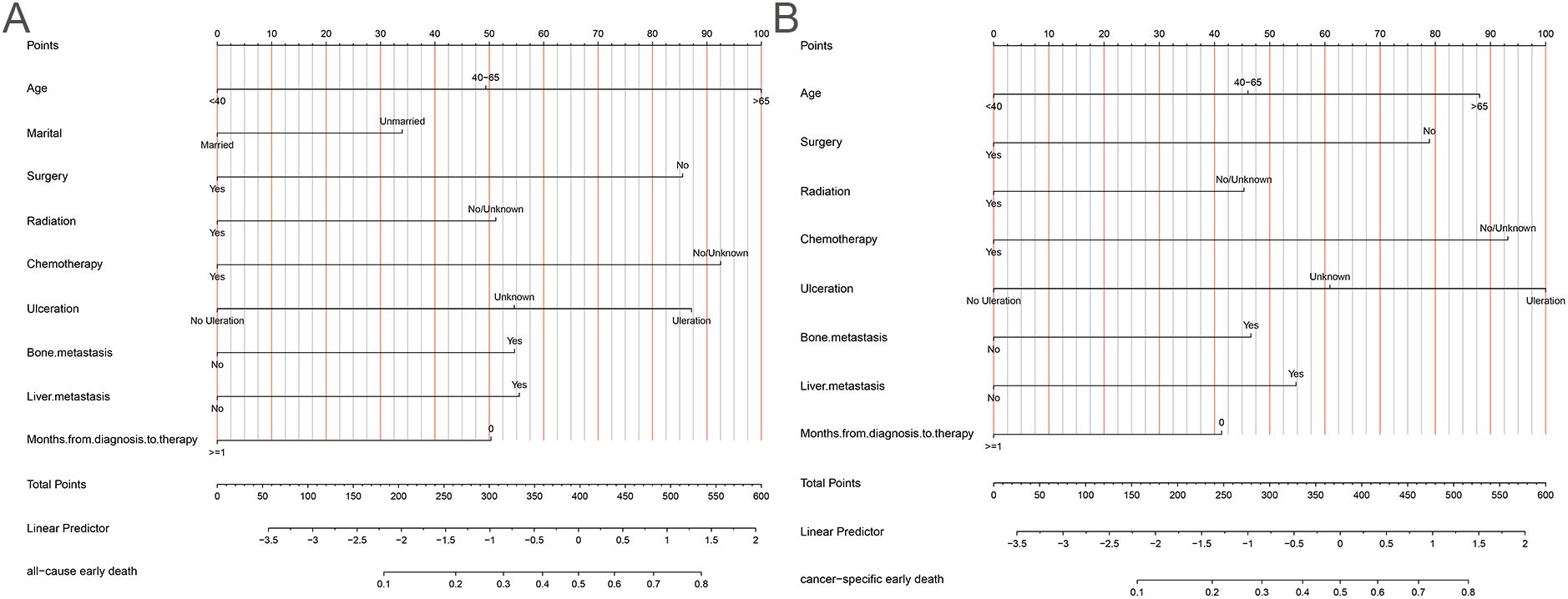
The 10-fold cross-validation analysis revealed that the XGBoost model performed best in predicting ACED in MBM patients (Figure 2A). In the testing cohort, Xgboost model showed the best with area under curve (AUC)=0.776, accuracy =0.713, sensitivity = 0.682, and specificity = 0.730 (Figures 3A, B; Table 4). In the external validation cohort, Xgboost model was also shown excellent prediction performance, with AUC=0.717, accuracy =0.659, sensitivity = 0.765, and specificity = 0.593 (Figures 4A, B; Table 4). Calibration curve analysis revealed that Xgboost model has a more accurate prediction performance (Figures 3C, 4C). DCA curves revealed that Xgboost model clinical application value (Figures 3D, 4D). Besides that, a nomogram based on the Xgboost model showed that age contributes most to the ACED of MBM patients, followed by chemotherapy, ulceration, surgery, liver metastasis, bone metastasis, radiation, months from diagnosis to therapy, and marital status (Figure 5A).
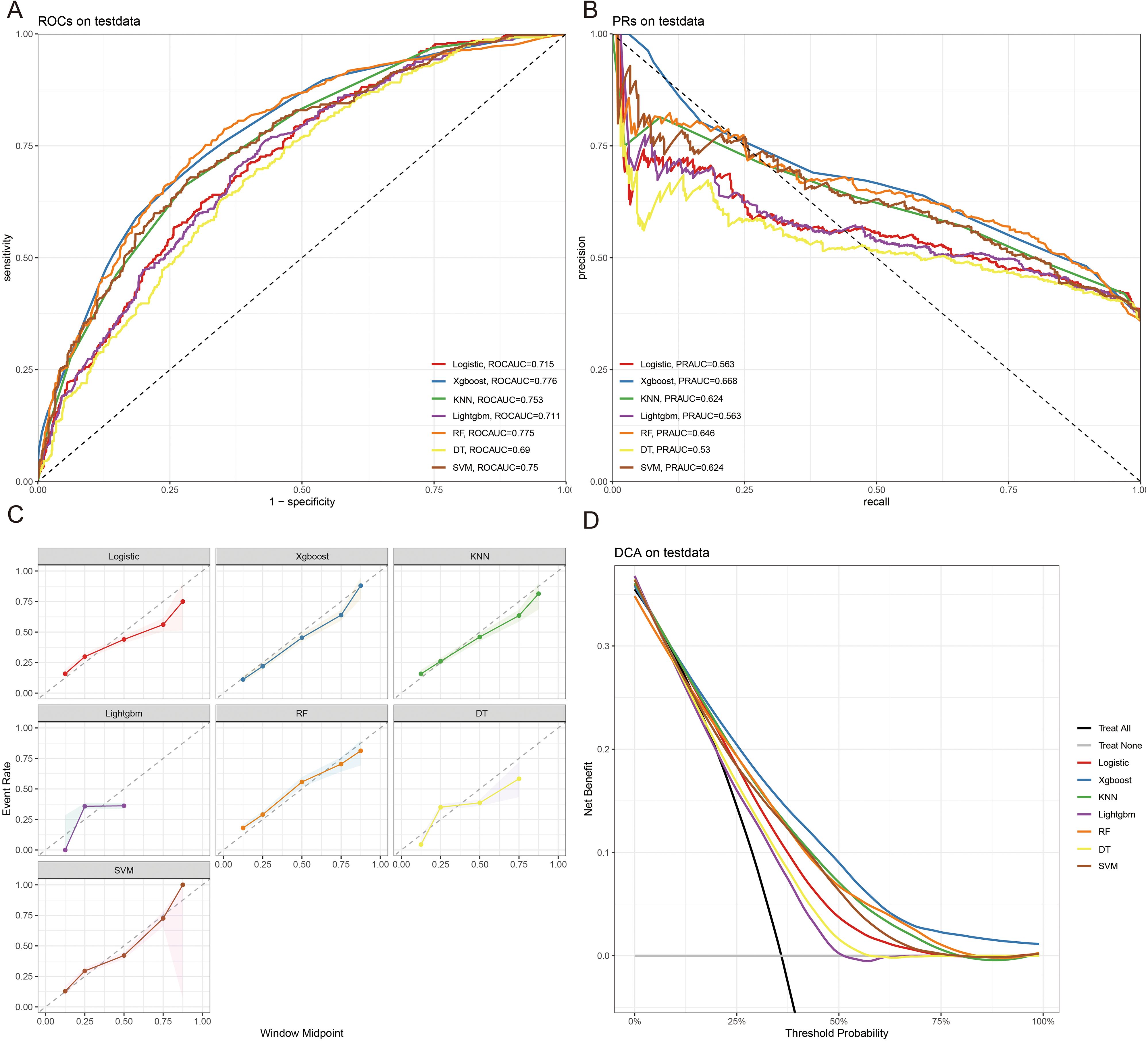
Figure 3. The ROCAUC (A), PRAUC (B), calibration curve (C), and DCA analysis (D) of ACED in the test cohort.
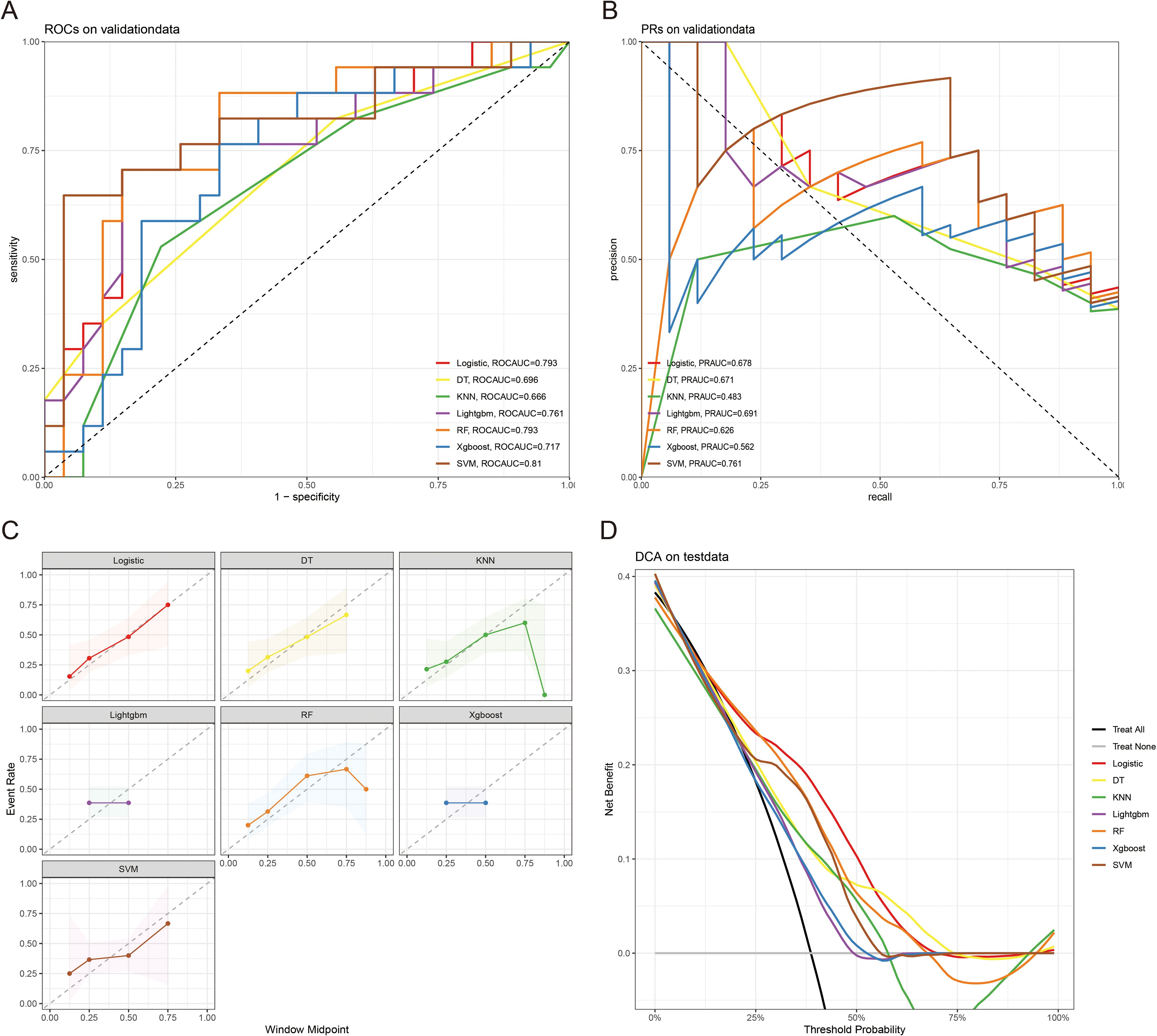
Figure 4. The ROCAUC (A), PRAUC (B), calibration curve (C), and DCA analysis (D) of ACED in the external validation cohort.
The 10x cross-validation analysis revealed that the LR model performs best in predicting the CSED of MBM patients (Figure 2B). In the testing cohort, the LR model showed the best performance with AUC=0.694, accuracy =0.614, sensitivity = 0.737, and specificity = 0.555 (Figures 6A, B; Table 4). In the external validation cohort, the LR model also showed excellent prediction performance, with AUC=0.730, accuracy =0.659, sensitivity = 0.750, and specificity = 0.609 (Figures 7A, B; Table 4). Calibration curve analysis revealed that the LR model has a more accurate prediction performance (Figures 6C, 7C). DCA curves revealed the LR model clinical application value (Figures 6D, 7D). Besides that, a nomogram based on the LR model revealed that ulceration contributes most to the CSED of MBM patients, followed by chemotherapy, age, surgery, liver metastasis, bone metastasis, radiation, and months from diagnosis to therapy (Figure 5B).
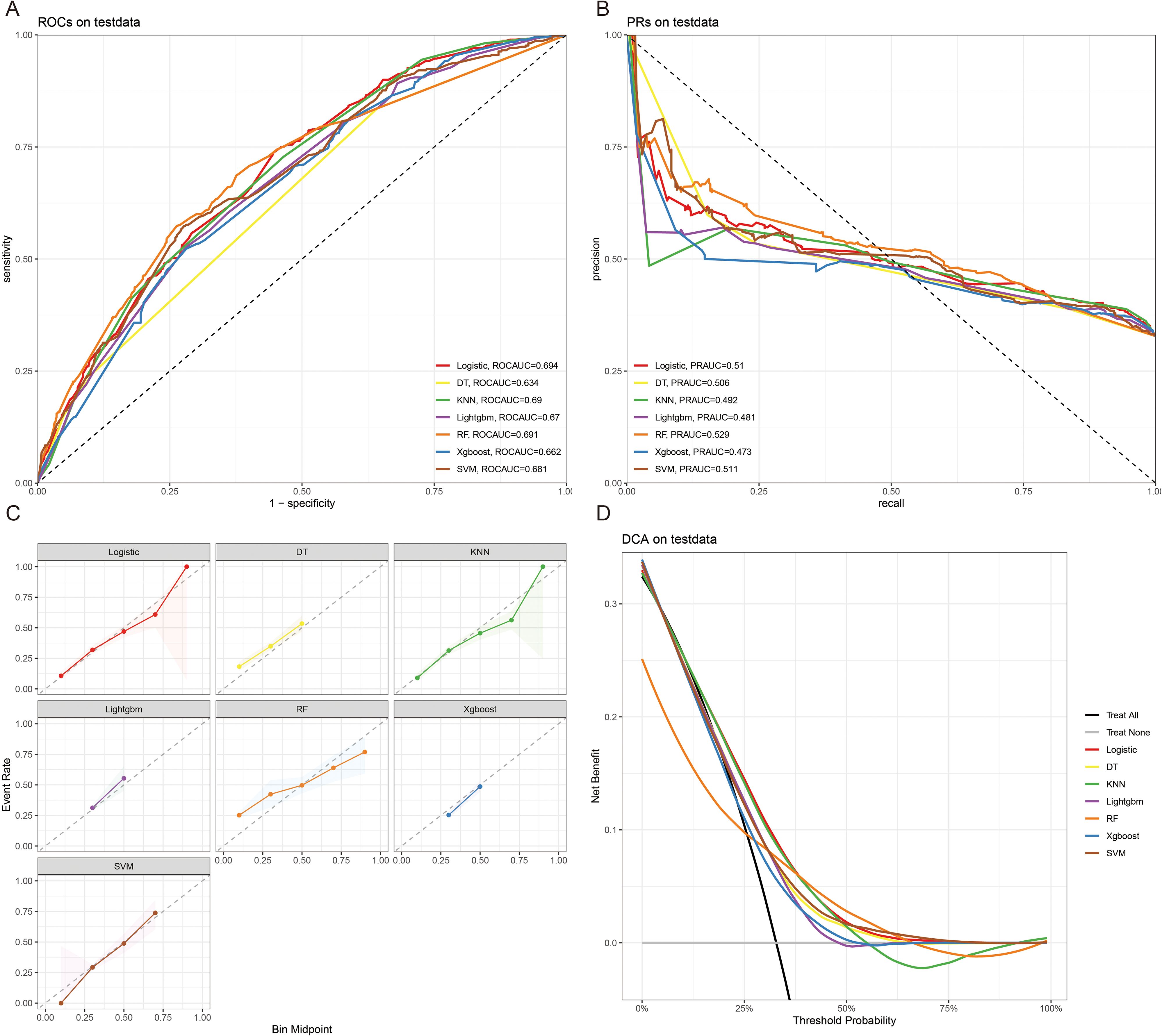
Figure 6. The ROCAUC (A), PRAUC (B), calibration curve (C), and DCA analysis (D) of CSED in the test cohort.
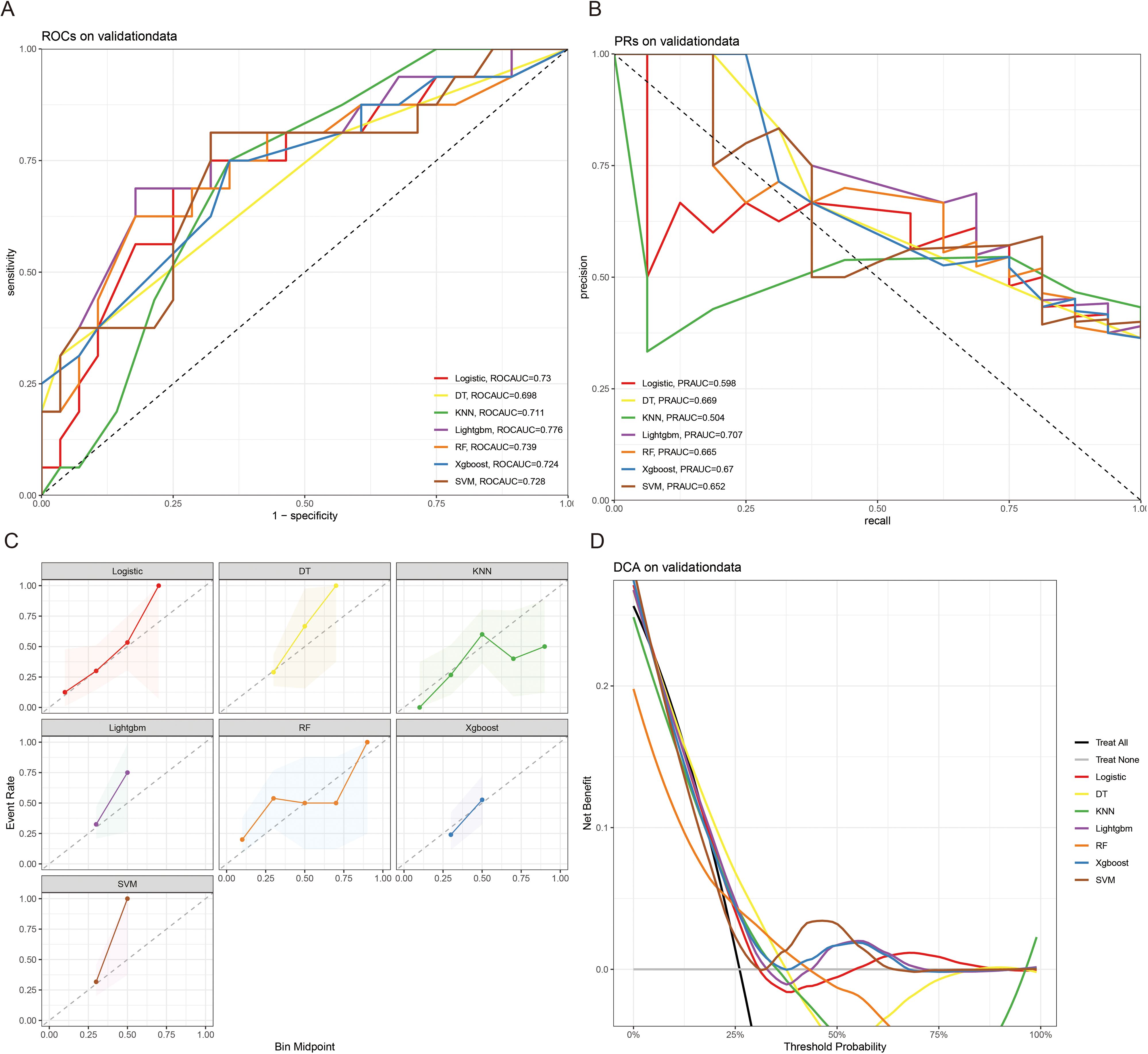
Figure 7. The ROCAUC (A), PRAUC (B), calibration curve (C), and DCA analysis (D) of CSED in the external validation cohort.
3.4 SHAP analysis results
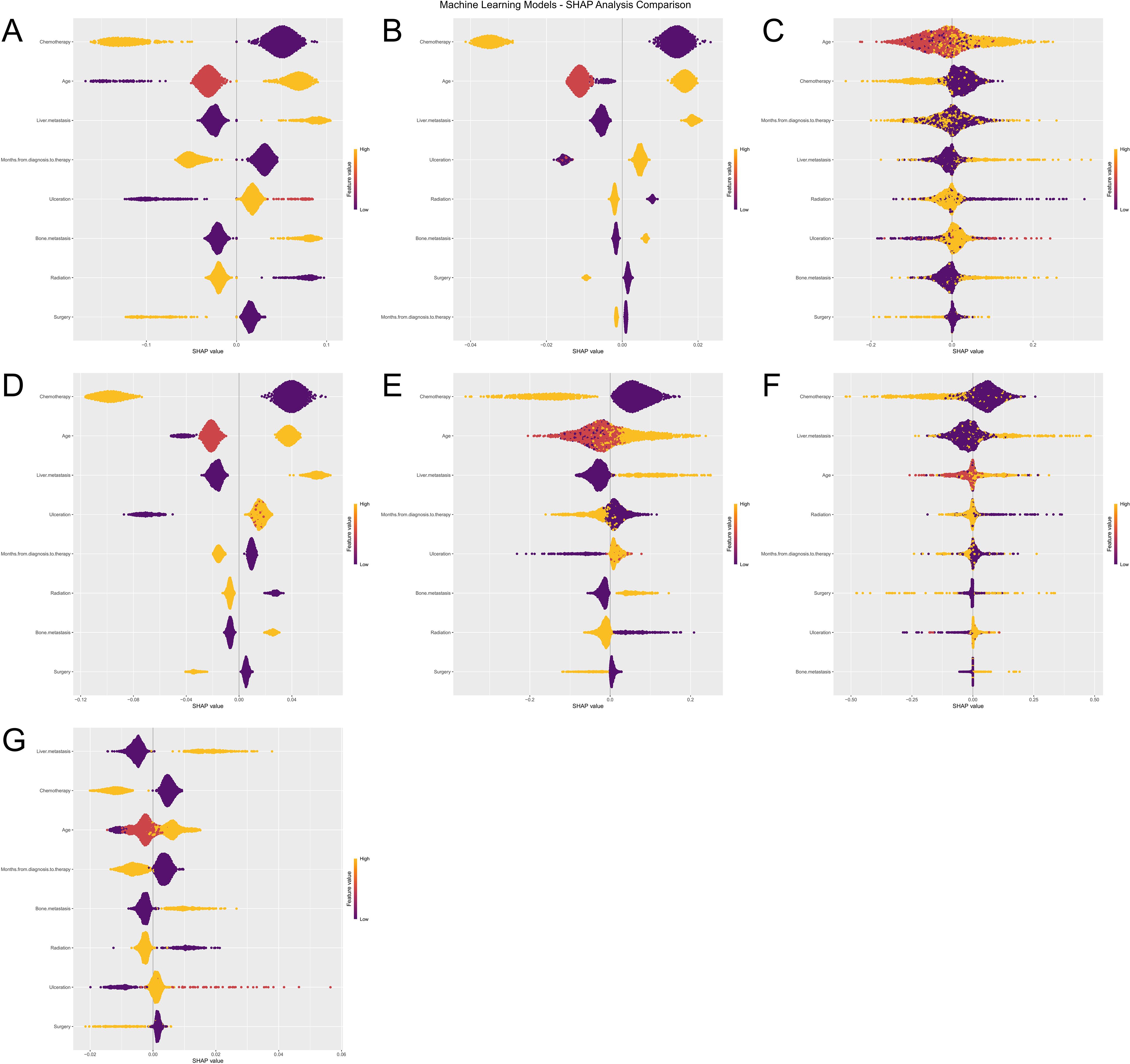
To further interpret the machine learning models, we visualized the feature importance using the Shapley Additive Explanations (SHAP) analysis for all seven models predicting both ACED and CSED outcomes (Figures 8A–G), (Figures 9A–G). In the SHAP plots, variables are arranged in descending order of importance based on their mean absolute SHAP values.
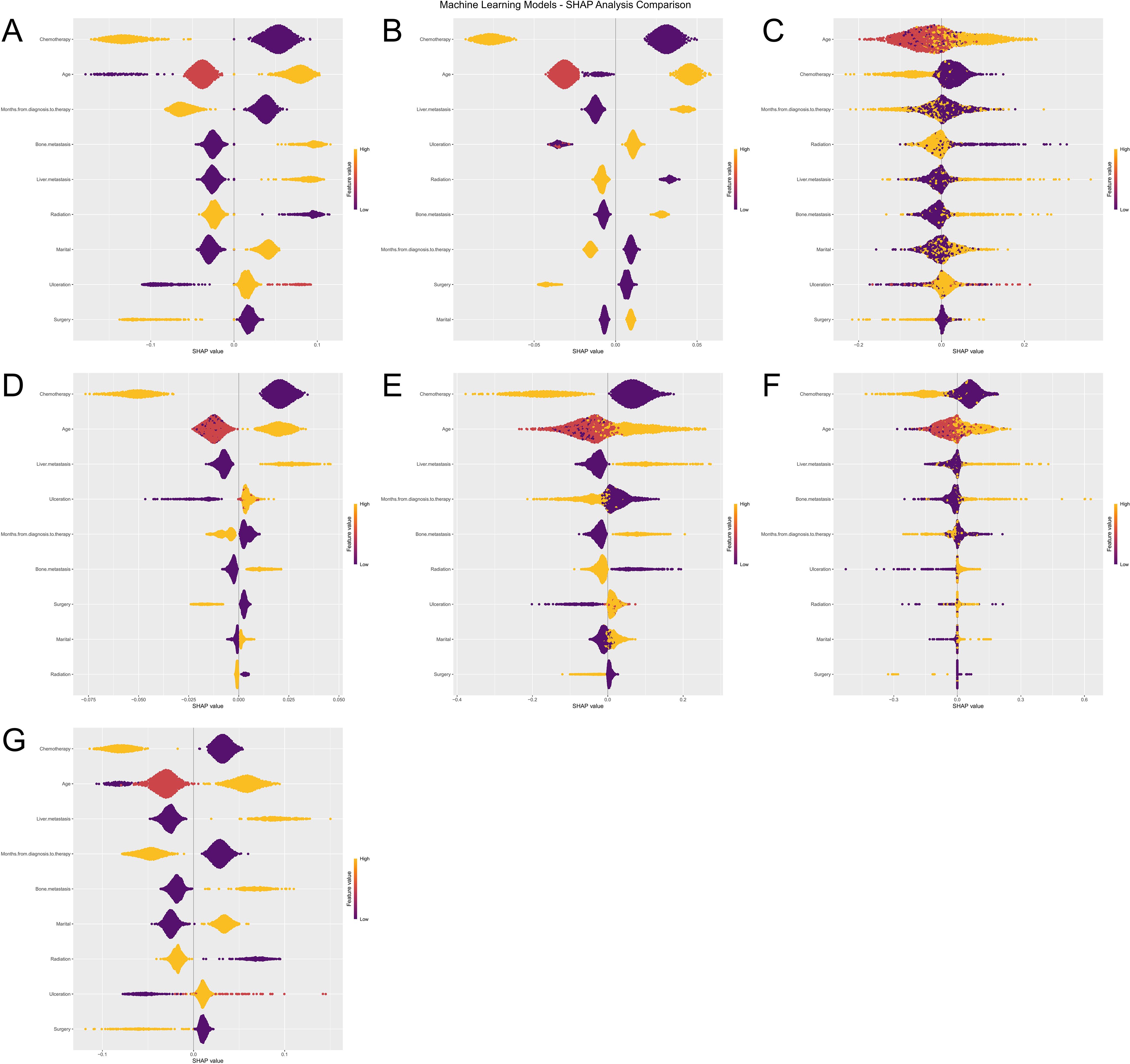
Figure 8. Machine Learning SHAP Analysis Comparison for ACED Outcomes. Subfigures (A–G) illustrate the influence of various features (e.g., Age, Chemotherapy, Surgery) on the ACED predictions of seven machine learning models (Logistic Regression, XGBoost, KNN, LightGBM, Random Forest, Decision Tree, and SVM). The violin plots in each subfigure display the distribution of SHAP values for each feature, with color indicating different feature values. Deeper colors represent higher SHAP values, signifying a greater impact of the feature on the prediction results.
Despite algorithmic differences and different outcome definitions (ACED vs CSED), there was remarkable consistency in feature importance rankings across all seven models (Logistic Regression, XGBoost, KNN, LightGBM, Random Forest, Decision Tree, and SVM). Chemotherapy, age, liver metastasis, and months from diagnosis to therapy consistently emerged as the top four most important features across both ACED and CSED prediction models, highlighting their critical role in predicting early death in melanoma brain metastasis patients regardless of death cause classification.
The consistent ranking of these four variables across different algorithms and outcome definitions suggests their robust prognostic value. The SHAP values provide insights into both the magnitude and direction of each feature’s contribution to the prediction, with higher absolute SHAP values indicating greater influence on the model’s output. This consistency between ACED and CSED models reinforces the reliability of these predictive factors in clinical practice.
4 Discussion
Melanoma is a frequent cause of brain metastases, and the survival rate of MBM patients was low (19). Despite the successful development of targeted therapies and immunotherapies that have significantly improved overall survival for MBM patients, the management of MBM remains challenging (20, 21). Brain metastases often lead to poor quality of life (22). Early detection of brain metastases can reduce treatment-related toxicity, mortality, and morbidity (23). In this research, more than a third of patients suffered from ED. Therefore, it is crucial to develop more effective predictive models.
ML is usually used for prognosis prediction and disease diagnosis (24). With the improvement of ML, ML models show great potential in the prediction of treatment and cancer prognosis, refinement of clinical care, and medical research (25). Yi Zhao et al. revealed that combined ML with disulfide ptosis-related signatures showed great significance in predicting the immunotherapy effectiveness and prognosis in cutaneous melanoma (26). Yi Xu et al. identify novel circadian biomarkers for melanoma diagnosis and prognosis through ML approaches (27). In this research, we predicted the ED of MBM patients through clinicopathologic features via ML algorithms, and the AUC value of the Xgboost model of ACED in the test and validation cohort were 0.776, and 0.717 respectively. The AUC values of the LR model of CSED in the test and validation cohort were 0.694, and 0.730 respectively. All AUC values >0.7 indicate that the nomograms have good predictive accuracy. The calibration curve fits the diagonal well. The closer the black solid line is to 45°, the better the prediction effect is and the better the predicted results are in agreement with the actual results. The results show that the predicted early deaths are highly consistent with the actual results. DCA curve revealed that the models have high clinical application value.
Our study presents several noteworthy and novel findings. First, to our knowledge, this study includes the largest cohort of patients, drawing data not only from the SEER database but also from external hospital records. Second, Results revealed that the Xgboost model performs best in predicting the ACED and the LR model performs best in predicting the CSED in MBM patients. In this study, the predictive ML models offered a practical tool for assessing ED risk factors in MBM, aiding physicians in making optimal clinical decisions to improve patient outcomes. ML is being increasingly utilized in clinical settings to assist physicians in making more informed recommendations (28, 29). Compared to traditional data analytics, ML is better suited for handling complex, multi-dimensional big data (30). Notably, lung metastasis was excluded from the final CSED model during backward stepwise regression despite showing univariate significance, likely due to collinearity with other metastatic variables (liver and bone metastases) that demonstrated stronger independent predictive value in the multivariate analysis. Seven ML algorithms were constructed based on the SEER database and hospital data to develop an effective ED prediction tool for MBM patients. Third, in this study, nomograms were developed to quantitatively support individualized prediction of ED based on MBM-related risk factors. Elderly melanoma patients tend to have thicker tumors, a greater likelihood of ulceration, and higher mitotic rates (31). We found that the point of elderly patients in the ACED nomogram is 100, and in the CSED nomogram is about 88, this phenomenon may be linked to the increased sensitivity of the elderly population to the adverse effects of tumor treatment. The presence of ulceration may indicate the relatively rapid progression of melanoma (31). The 5-year survival rate for melanoma significantly decreases in the presence of ulceration (32, 33), and our results showed that patients with ulcers have important significance in promoting ED. Previous studies have shown that radiation, surgery, and chemotherapy are still relatively ideal treatments for MBM, they can reduce the risk of recurrence and prolong the survival of patients (34). However, due to the lack of information on targeted therapies, and immunotherapy in the SEER database, further exploration of treatment options is limited. Given the heterogeneity of MBM patients, accurate risk stratification is crucial, and nomograms offer a convenient tool for this purpose.
This study has several important limitations. First, the information on detailed treatment plans, other treatments, aggregate brain tumor volume, comorbidities, karnofsky performance status that suggest being potential important prognostic factors (35, 36) is unavailable from the SEER database. Additionally, the SEER database does not contain imaging data, precluding the inclusion of important radiological features such as tumor size, shape, location, edema, and enhancement patterns. These imaging characteristics could potentially enhance model performance, as demonstrated in previous studies. Second, this retrospective analysis did not utilize real clinical external data for prospective validation, which will need to be addressed in future research. Third, our dataset is geographically limited to SEER populations (representing selected US regions) and a single regional Chinese hospital, which may limit the generalizability of our findings to other global populations. Population genetics, tumor biology, healthcare system differences, and socioeconomic factors may vary significantly across different geographical regions, potentially affecting model performance and clinical applicability. While our two-population validation using both Western and Asian cohorts demonstrates some cross-population validity, broader validation across diverse geographical regions, healthcare systems, and ethnic populations is essential before widespread clinical implementation. Fourth, our external validation cohort from the Affiliated Cancer Hospital of Xinjiang Medical University was relatively small (n=42) compared to the training and testing cohorts, which may limit the robustness of our generalizability assessment. While this sample size reflects the relative rarity of MBM cases at a single institution and still demonstrated consistent model performance, larger multi-institutional external validation studies are needed to more definitively establish model reliability across diverse clinical settings. Future studies should prioritize multi-continental validation and consider population-specific model recalibration to optimize performance for local clinical use.
5 Conclusion
Seven ML algorithms were used to establish ED predictive models of MBM patients by demographic characteristics. Xgboost model exhibited the best predictive ability for ACED, and LR model performs best in predicting the CSED, indicating potential clinical application value.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by All experiments were performed in accordance with relevant guidelines and regulations.This study was approved by the Ethics Review Board of Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, China. All patients provided informed consent prior to their participation in the treatment and this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MM: Writing – original draft, Writing – review & editing. MK: Writing – review & editing. AM: Writing – review & editing. JW: Writing – review & editing. ZD: Writing – review & editing. RJ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gutzmer R, Vordermark D, Hassel JC, Krex D, Wendl C, SChadendorf D, et al. Melanoma brain metastases - Interdisciplinary management recommendations 2020. Cancer Treat Rev. (2020) 89:102083. doi: 10.1016/j.ctrv.2020.102083
2. Conway JW, Rawson RV, Lo S, Ahmed T, Vergara IA, Gide TN, et al. Unveiling the tumor immune microenvironment of organ-specific melanoma metastatic sites. J Immunother Cancer. (2022) 10(9):e004884. doi: 10.1136/jitc-2022-004884
3. Ascha MS, Ostrom QT, Wright J, Kumthekar P, Bordeaux JS, Sloan AE, et al. Lifetime occurrence of brain metastases arising from lung, breast, and skin cancers in the elderly: A SEER-medicare study. Cancer Epidemiol Biomarkers Prev. (2019) 28:917–25. doi: 10.1158/1055-9965.EPI-18-1116
4. Cohen JV, Tawbi H, Margolin KA, Amravadi R, Bosenberg M, Brastianos PK, et al. Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. (2016) 29:627–42. doi: 10.1111/pcmr.12538
5. Sadetsky N, Hernandez A, Wallick CJ, McKenna EF, Surinach A, and Colburn DE. Survival outcomes in an older US population with advanced melanoma and central nervous system metastases: SEER-Medicare analysis. Cancer Med. (2020) 9:6216–24. doi: 10.1002/cam4.3256
6. Liu H, Xu YB, Guo CC, Li MX, Ji JL, Dong RR, et al. Predictive value of a nomogram for melanomas with brain metastases at initial diagnosis. Cancer Med. (2019) 8:7577–85. doi: 10.1002/cam4.2644
7. Sundararajan S, Thida AM, Yadlapati S, Mukkamalla SKR, and Koya S. Metastatic melanoma. In: StatPearls. StatPearls Publishing LLC, Treasure Island (FL (2024).
8. Zhu Y, Fang X, Wang L, Zhang T, and Yu D. A predictive nomogram for early death of metastatic gastric cancer: A retrospective study in the SEER database and China. J Cancer. (2020) 11:5527–35. doi: 10.7150/jca.46563
9. Shen H, Deng G, Chen Q, and Qian J. The incidence, risk factors and predictive nomograms for early death of lung cancer with synchronous brain metastasis: a retrospective study in the SEER database. BMC Cancer. (2021) 21:825. doi: 10.1186/s12885-021-08490-4
10. Koch Hein EC, Vilbert M, Hirsch I, et al. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: real-world experience from a Canadian Comprehensive Cancer Centre. Cancers (Basel). (2023) 15(17):4312. doi: 10.3390/cancers15174312
11. Nieder C, Aanes SG, and Haukland EC. Survival and early death within three months from the start of immune checkpoint inhibitors in patients with different types of cancer. Anticancer Res. (2022) 42:3061–6. doi: 10.21873/anticanres.15793
12. Li S, Yin C, Yang X, Lu Y, Wang C, and Liu B. Risk factors and predictive models for early death in patients with advanced melanoma: A population-based study. Med (Baltimore). (2023) 102:e35380. doi: 10.1097/MD.0000000000035380
13. Li S, Li J, Yang Q, Yin C, and Liu B. Construction and validation of prediction models of risk factors for early death in patients with metastatic melanoma. Sichuan Da Xue Xue Bao Yi Xue Ban. (2024) 55:367–74. doi: 10.12182/20240360101
14. Zhang D, Wang Z, Shang D, Yu J, and Yuan S. Incidence and prognosis of brain metastases in cutaneous melanoma patients: a population-based study. Melanoma Res. (2019) 29:77–84. doi: 10.1097/CMR.0000000000000538
15. Zhou SN, Jv DW, Meng XF, Zhang JJ, Liu C, Wu ZY, et al. Feasibility of machine learning-based modeling and prediction using multiple centers data to assess intrahepatic cholangiocarcinoma outcomes. Ann Med. (2023) 55:215–23. doi: 10.1080/07853890.2022.2160008
16. Ainiwaer A, Hou WQ, Qi Q, Kadier K, Qin L, Rehemuding R, et al. Deep learning of heart-sound signals for efficient prediction of obstructive coronary artery disease. Heliyon. (2024) 10:e23354. doi: 10.1016/j.heliyon.2023.e23354
17. Lynch CM, Abdollahi B, Fuqua JD, de Carlo AR, Bartholomai JA, Balgemann RN, et al. Prediction of lung cancer patient survival via supervised machine learning classification techniques. Int J Med Inform. (2017) 108:1–8. doi: 10.1016/j.ijmedinf.2017.09.013
18. Zhang Y, Zhang Z, Wei L, and Wei S. Construction and validation of nomograms combined with novel machine learning algorithms to predict early death of patients with metastatic colorectal cancer. Front Public Health. (2022) 10:1008137. doi: 10.3389/fpubh.2022.1008137
19. Switzer B, Puzanov I, Skitzki JJ, Hamad L, and Ernstoff MS. Managing metastatic melanoma in 2022: A clinical review. JCO Oncol Pract. (2022) 18:335–51. doi: 10.1200/OP.21.00686
20. Sperduto PW, Salama AKS, and Anders C. Progress for patients with melanoma brain metastases. Neuro Oncol. (2023) 25:1321–2. doi: 10.1093/neuonc/noad050
21. Fateeva A, Eddy K, and Chen S. Overview of current melanoma therapies. Pigment Cell Melanoma Res. (2023) 37(5):562–8. doi: 10.1111/pcmr.13154
22. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. Jama. (2016) 316:401–9. doi: 10.1001/jama.2016.9839
23. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009) 10:1037–44. doi: 10.1016/S1470-2045(09)70263-3
24. Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, and Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. (2018) 284:603–19. doi: 10.1111/joim.12822
25. Li J, Dan K, and Ai J. Machine learning in the prediction of immunotherapy response and prognosis of melanoma: a systematic review and meta-analysis. Front Immunol. (2024) 15:1281940. doi: 10.3389/fimmu.2024.1281940
26. Zhao Y, Wei Y, Fan L, Nie Y, Li J, Zeng R, et al. Leveraging a disulfidptosis-related signature to predict the prognosis and immunotherapy effectiveness of cutaneous melanoma based on machine learning. Mol Med. (2023) 29:145. doi: 10.1186/s10020-023-00739-x
27. Xu Y, Zeng C, Bin J, Tang H, and Li W. Identifying novel circadian rhythm biomarkers for diagnosis and prognosis of melanoma by an integrated bioinformatics and machine learning approach. Aging (Albany NY). (2024) 16:11824–42. doi: 10.18632/aging.205961
28. Miao R, Chen HH, Dang Q, Xia LY, Yang ZY, He MF, et al. Beyond the limitation of targeted therapy: Improve the application of targeted drugs combining genomic data with machine learning. Pharmacol Res. (2020) 159:104932. doi: 10.1016/j.phrs.2020.104932
29. Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. (2020) 31:1491–505. doi: 10.1016/j.annonc.2020.07.014
30. Deo RC. Machine learning in medicine. Circulation. (2015) 132:1920–30. doi: 10.1161/CIRCULATIONAHA.115.001593
31. Shen W, Sakamoto N, and Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic Malignant melanoma: a population-based analysis. BMC Cancer. (2016) 16:413. doi: 10.1186/s12885-016-2438-3
32. Huang JN, Yu H, Wan Y, Ming WK, Situ F, Zhu L, et al. A prognostic nomogram for the cancer-specific survival of white patients with invasive melanoma at BANS sites based on the Surveillance, Epidemiology, and End Results database. Front Med (Lausanne). (2023) 10:1167742. doi: 10.3389/fmed.2023.1167742
33. Maurichi A, Miceli R, Camerini T, Mariani L, Patuzzo R, Ruggeri R, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol. (2014) 32:2479–85. doi: 10.1200/JCO.2013.54.2340
34. Gao M, Wu B, and Bai X. Establishment and validation of a nomogram model for predicting the specific mortality risk of melanoma in upper limbs based on the SEER database. Sci Rep. (2024) 14:9623. doi: 10.1038/s41598-024-57541-w
35. Hauswald H, Stenke A, Debus J, and Combs SE. Linear accelerator-based stereotactic radiosurgery in 140 brain metastases from Malignant melanoma. BMC Cancer. (2015) 15:537. doi: 10.1186/s12885-015-1517-1
Keywords: melanoma, brain metastasis, early death, machine learning, prognosis
Citation: Maihemuti M, Kamaierjiang M, Maimaiti A, Wu J, Dai Z and Jiang R (2025) Development and validation of machine learning model to predict early death of melanoma brain metastasis patients. Front. Oncol. 15:1517961. doi: 10.3389/fonc.2025.1517961
Received: 27 October 2024; Accepted: 20 June 2025;
Published: 08 July 2025.
Edited by:
Vladimir Spiegelman, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Aikeliyaer Ainiwaer, Maastricht University, NetherlandsSuhrud Panchawagh, Mayo Clinic, United States
Copyright © 2025 Maihemuti, Kamaierjiang, Maimaiti, Wu, Dai and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renbing Jiang, emx5eWd5cnp6a0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Maierdanjiang Maihemuti
Maierdanjiang Maihemuti Maiheliya Kamaierjiang2†
Maiheliya Kamaierjiang2† Aierpati Maimaiti
Aierpati Maimaiti Zhibing Dai
Zhibing Dai Renbing Jiang
Renbing Jiang