- 1Department of Pathology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Chengdu, Sichuan, China
- 3Department of Gynecology and Obstetrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
Objective: Microsatellite instability (MSI)/mismatch repair (MMR) protein testing is important for Lynch syndrome (LS) identification, prognostic stratification, and immune checkpoint inhibitor screening in many solid malignancies. MSH6, an MMR protein, is less studied in LS, and the exact mechanism of inconsistent MSI and MMR results among endometrial cancer (EC) patients who are carriers of MSH6 mutations remains unclear. The aim of this study was to identify the molecular patterns and clinicopathological characteristics of MSH6 protein-deficient LS-related EC and to further investigate possible causes of discordant MSI and IHC results in MSH6 variant carriers.
Methods: Twenty-seven patients who were diagnosed with EC with only MSH6 protein deficiency from 2021 to 2023 at West China Second University Hospital were enrolled. PCR capillary electrophoresis (PCR-CE) was performed in all cases and further next-generation sequencing (NGS) was performed in non-MSI-high cases. Data on immunohistochemistry (IHC) markers, microsatellite shift patterns, and molecular profiles were further reviewed by an experienced molecular pathologist.
Results: Among the 27 patients, 14 (51.9%) cases were found to be non-MSI-high, while only 8 of 14 (57%) cases successfully underwent NGS and ultimately incorporated into our study. All patients who were MSH6 protein negative were diagnosed with early-stage endometrioid carcinoma (EC), with a median age of 55 years (range 48–67 years). We reanalyzed the shift of all microsatellite loci and found one case with an additional unstable locus. Minimal microsatellite shifts (one to three nucleotide shift) were observed in all cases (100%), which occurred in mononucleotide markers from BAT 25 or BAT 26. Nevertheless, 3 of the 8 patients (37.5%) displayed MSI-H by NGS, which revealed truncating mutations in the MSH6 gene in exon 4 in 62.5% (5/8) of the patients, including nonsense mutations (37.5%), frameshift insertions (12.5%), and frameshift deletions (12.5%). The proportion of cases correctly classified (as determined via IHC markers) by MMR genomic status was greater (100%) than that correctly classified by PCR-CE (12.5%) in cases of MSH6 truncating variation. In addition, NGS (37.5%) had a higher MSI-H detection rate than PCR-CE (12.5%) in evaluating MSI status.
Conclusion: Carriers of a germline pathogenic MSH6 variant are more likely to develop EC at an advanced age, and a non-MSI-H phenotype with minimal microsatellite shift is frequently observed only when the MSH6 protein is lost. This atypical MSI pattern is often overlooked, potentially increasing the risk of underdiagnosis of LS.
Introduction
Lynch syndrome (LS) is an autosomal dominant inherited disorder characterized by an increased risk of developing colorectal cancer (CRC) in affected families, as well as extracolonic tumors in the endometrium, ovary, stomach, small intestine, urothelium, hepatobiliary system, and other organs (1, 2). Endometrial cancer (EC) is the most common extraintestinal tumor in women with LS, with a lifetime risk of approximately 40-60% (3, 4). The pathogenesis of LS is related to pathogenic germline variations in DNA mismatch repair (MMR) genes (MLH1, MSH2, PMS2 and MSH6) (5). The MMR system can involve multiple mismatch repair proteins in DNA repair, including the MutS (MSH2, MSH3, MSH6, etc.) and MutL (MLH1, MLH3, PMS1, and PMS2) families. Among them, MLH1, MSH2, MSH6, and PMS2 are the dominant proteins of MMR. Mutations in DNA repair genes result in the accumulation of errors in microsatellite sequences so that they become either longer or shorter. The insertion or deletion of repetitive units and deficient mismatch repair (dMMR) during the DNA replication process can cause changes in the length of microsatellite alleles; this molecular phenotype is called microsatellite instability (MSI). Therefore, MSI and MMR are considered hallmarks of LS-related CRC and EC.
The vast majority of LS-associated tumors presents MSI due to their DNA MMR deficiency. A large number of studies have shown that tumors with dMMR/MSI molecular characteristics exhibit increased tumor antigen load due to high-frequency gene mutations, inducing infiltration of killing T lymphocytes and high expression of corresponding immunosuppressive molecules, resulting in a good response to corresponding immunotherapy (6). Therefore, dMMR/MSI detection for EC molecular classification, Lynch syndrome screening and immunotherapy prediction are crucial. Currently, universal screening for Lynch syndrome of all newly diagnosed endometrial cancer patients has been advocated in several clinical recommendations, including The Society of Gynecologic Oncology (SGO) and American College of Obstetricians and Gynecologists (ACOG). Specifically, they recommend the process of molecular evaluation of patients at risk for Lynch syndrome as follows: molecular tumor screening with immunohistochemistry (IHC) for MMR genes expression and/or microsatellite instability followed by germline genetic testing if the screening test is positive (7).
Although immunohistochemistry (IHC) and PCR capillary electrophoresis (PCR-CE) have a high concordance of results in CRC, several studies have shown that the PCR assay used for LS screening has a high false negativity rate and, particularly, a low detection sensitivity for the loss of MSH6 protein (8, 9). This may be related to the function of the MSH6 protein, which is involved in the repair of both single-base mismatches and insertion/deletion loops but is not absolutely required for MMR activity (10). In the absence of MSH6, the MSH3 protein can partially replace the MSH6 repair function and protect against DNA accumulation (11). On the other hand, up to 80% of dMMR tumors are attributable to somatic events and are, therefore, unrelated to LS (12, 13). Germline mutations in the MSH6 gene account for approximately 15-30% of cases of hereditary nonpolyposis colorectal cancer (HNPCC), whereas MSH6 germline mutations are seemed to be more common in EC than in CRC in some existing studies (14). Goodfellow et al. also showed that mutations in mismatch repair genes, especially MSH6, are closely related to the occurrence of EC (15). However, MSH6 is less studied in molecular LS screening, the frequency of mutations in this gene may be largely underestimated, and the frequency and exact mechanism of inconsistent MSI and MMR results among carriers of MSH6 mutations are still unclear. LS screening in EC patients is crucial for identifying which patients should be offered genetic counseling and genetic testing to prevent further LS-related cancers. Given the phenomenon of inconsistency of IHC and PCR-CE results, especially the relatively low sensitivity of MSI testing in EC patients with MSH6 germline mutations, which means tumors with MSH6 variants are particularly prone to discordant MMR/MSI, LS families with MSH6 mutations may be underdiagnosed using traditional diagnostic criteria.
Therefore, in this study, we conducted an in-depth analysis of eight LS-related EC patients who were MSH6 protein negative and had germline MSH6 truncating mutations, and described the molecular and clinical findings, aiming to explore and analyze the inconsistency and reasons for the loss of MSH6 protein and MSI PCR-CE, as well as the patterns of microsatellite shifting in these patients.
Materials and methods
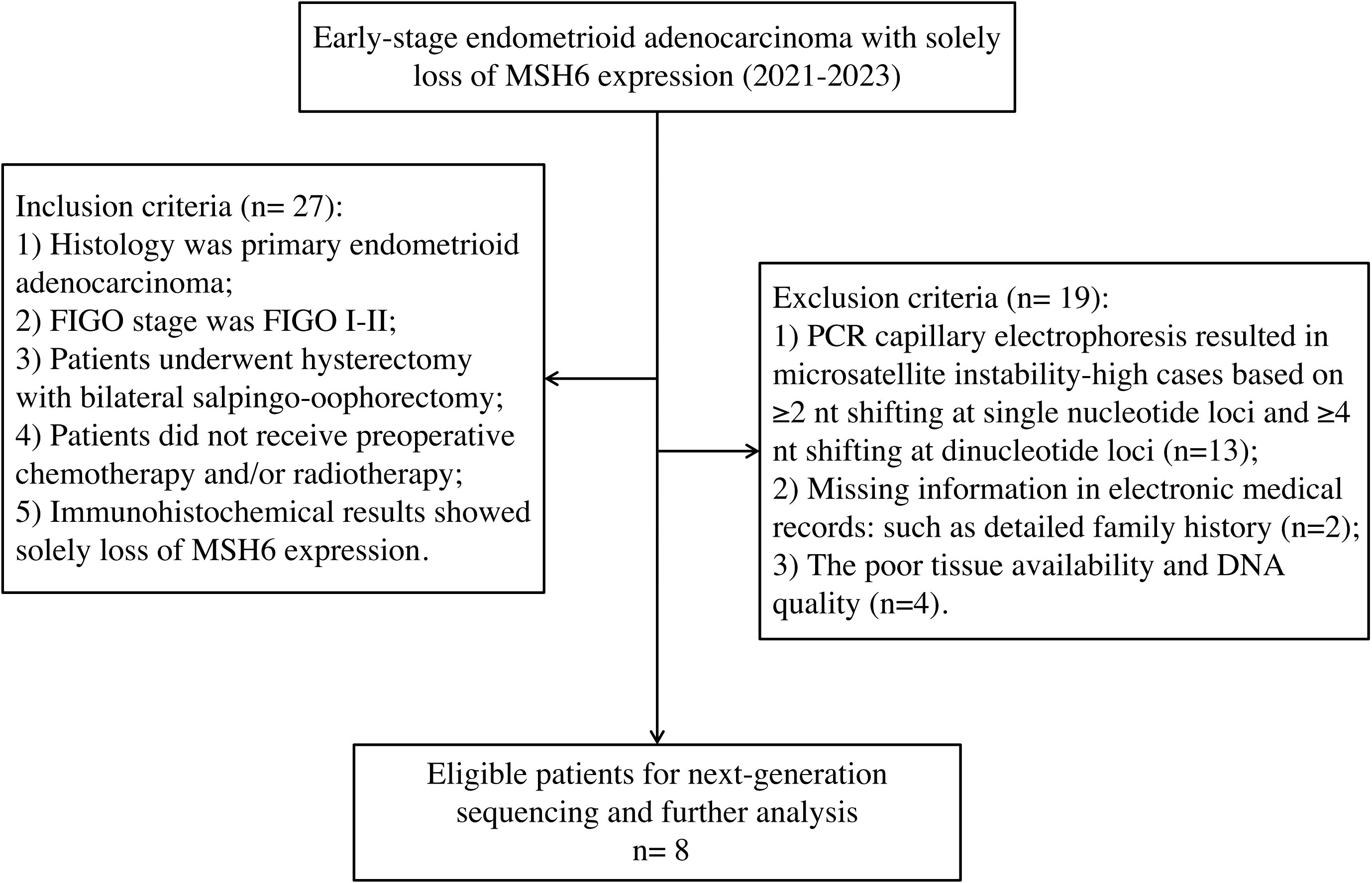
Study population
Patients with early-stage endometrioid carcinoma diagnosed with solely loss of MSH6 protein between January 2021 to December 2023 at West China Second University Hospital of Sichuan University were selected (n=27). All patients underwent hysterectomy with bilateral salpingo-oophorectomy and did not receive any preoperative chemotherapy and/or radiotherapy. Cases with non-MSI-H results by PCR-CE analysis were selected to further undergo NGS. Finally, only 8 cases successfully underwent NGS and ultimately incorporated into our study. The remaining non-MSI-H samples were failed to undergo NGS, mainly due to the lack of a detailed family medical histology, a low tumor tissue component that did not reach sufficient tumor concentration even after tumor cell enrichment, or poor DNA quality. According to the Amsterdam II criteria and Bethesda guidelines, electronic medical records, and detailed family history, oncologists made the clinical diagnosis of LS. The inclusion and exclusion criteria of our study were shown in Figure 1. The study was performed under a protocol approved by the institutional review board of the Ethics Committee of West China Second University Hospital of Sichuan University (protocol 2023037). Written informed consent was obtained from all the participants prior to the publication of this study.
IHC staining
Representative formalin-fixed, paraffin-embedded (FFPE) tumor tissue blocks were used to make 4-μm sections. The EnVision system was used for visualization as previously described (16). The IHC staining antibodies used included MLH1, PMS2 (1:100; clone EP51), MSH2 (1:1000; clone MX061), MSH6 (1:1400; clone MX056), ER (ready-to-use, clone SP1), PR (ready-to-use, clone SP2), HER-2 (ready-to-use, clone 4B5), and p53 (1:600, clone MX008) antibodies. The results of the expression of MMR proteins were further interpreted as ‘retained’ and ‘lost’ by determining the intensity of staining in tumor cells relative to internal controls (lymphocytes, mesothelial cells, and glandular cells). Tumor tissues with surrounding normal tissues showed nuclear staining, which was considered retained staining. Complete loss of staining was defined as tumor cell nuclei without staining and normal nuclei of surrounding tissues with staining.
Analysis of MSI by PCR-CE
MSI status was assessed by comparing tumor and matched normal DNA samples via PCR-CE analysis. In brief, the NCI-recommended panel was used for MSI analysis and contained two mononucleotide loci (BAT25 and BAT26) and three dinucleotide loci (D2S123, D5S346, and D17S250). All slices of FFPE tumor tissue specimens were used for testing after confirmation and enrichment of adequate tumor cells (>30%). Microsatellite instability high (MSI-H) was defined as the presence of two or more instability loci; microsatellite instability low (MSI-L) was defined as the presence of only one locus; and MSS was defined as the absence of instability at five loci. Two molecular pathologists reanalyzed the shift of all microsatellite loci. As previously study described, minimal microsatellite shift was further defined as a 1–3 nucleotide or base pair shift in the tumor DNA relative to the matched normal tissue at an involved locus, and a major shift was more than 3 microsatellite repeat shifts (14).
Massively parallel sequencing
Paired germline and somatic tumor sequencing were performed on tumor and matched normal samples via 1021-gene panel targeted sequencing (Supplementary Table). In brief, somatic single nucleotide variants (SNVs) and insertions and deletions (InDels) were detected via MuTect (v1.14) and GATK (the Genome Analysis Toolkit, v3.4-46-gbc02625), respectively (17). Copy number alterations (CNVs) in the tumor were identified with CONTRA (Copy Number Targeted Resequencing Analysis, v2.0.8). The MSIsensor algorithm for the detection of somatic microsatellite changes computes the length distributions of microsatellites per site in paired tumor-normal sequence data, subsequently yielding a quantitative MSIsensor score; MSIsensor scores ≥10 presented with MSI-H, 3–9 presented with MSI-L, <3 presented with MSS (18).The tumor mutational burden (TMB) was defined as the number of somatic nonsynonymous SNVs and InDels per megabase (mut/Mb), TMB-H defined as ≥10 muts/Mb, <10 muts/Mb presented with TMB-L. The identified genetic variants were manually assessed via IGV (v2.13.1), and interpreted via current standards for variant classification according to American College of Medical Genetics and Genomics (ACMG) guidelines, and all germline mutations in patients in our cohort were classified as likely pathogenic or pathogenic according to ACMG criteria.
Results
Clinicopathologic characteristics
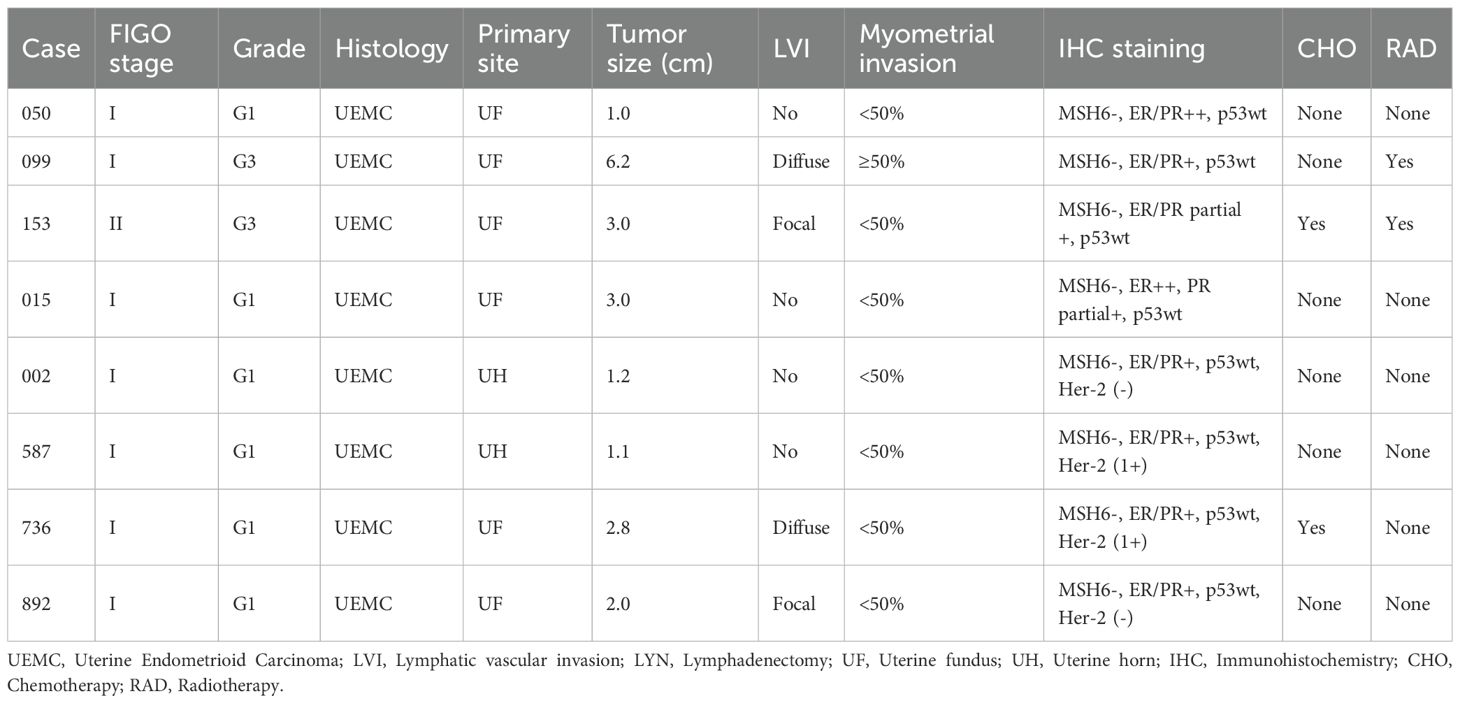
A total of 8 of 27 patients with solely loss of MSH6 protein successfully completed further massively parallel sequencing and had confirmatory germline testing to screen for LS. Among the 8 patients, pathogenic germline variants were identified in MSH6 (100%), 4 (50%) harbored a frameshift mutation and 4 (50%) harbored a nonsense mutation. Seven (87.5%) patients had EC as their sole malignancy, and 1(12.5%) patient with a history of CRC was diagnosed with LS. Six patients (75%) had a family history of LS-related neoplasia (Table 1). All patients had been diagnosed with early stage endometrioid carcinoma (FIGO I-II), 6 (75%) were histologic grade 1, and 2 (25%) were grade 2 or 3. Additionally, 2 (25%) cases occurred in the uterine horn, and 6 (75%) in the uterine fundus. The median age of our cohort was 55 years (range 48–67 years), and the median tumor size was 2.5 cm (range 1.0-6.2) (Table 2).
Immunohistochemistry results
Among the 8 tumors, 7 (87.5%) were ER/PR/p53-positive, and the remaining tumor (12.5%) was ER/PR partially positive and p53 positive. Four tumors had available HER-2 test results, 2 (50%) were positive, and the remaining (50%) were negative. An isolated protein loss of MSH6 was present in all patients (100%), with truncating mutations impairing protein function. Patient 153, harboring an MSH6 c.5341dupA frameshift insertion, demonstrated complete loss of MSH6 (Figure 2A) and partial ER/PR positive (Figure 2C).
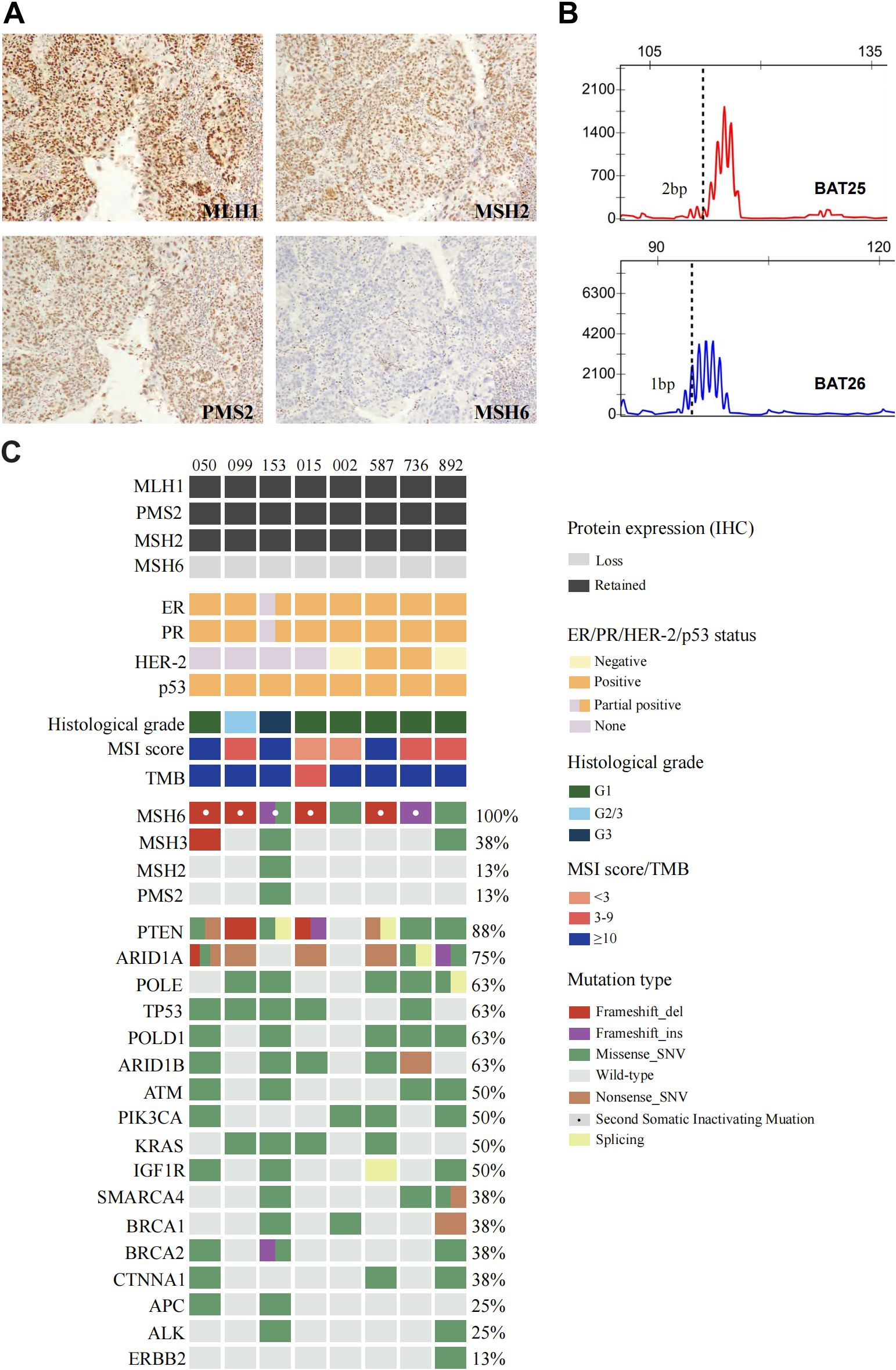
Figure 2. Positive staining results of (A) MLH1/PMS2/MSH2 and absence of MSH6 protein in endometrioid carcinoma of Case 153. (B) Microsatellite instability testing by PCR capillary electrophoresis (2B/3D panel). (C) Clinicopathologic and genomic characterization of endometrioid carcinoma in patients with Lynch syndrome related germline pathogenic variants.
Microsatellite instability analysis
PCR-CE analysis of 8 EC patients revealed a change of any length due to microsatellite repeat unit insertions or deletions in tumor tissue compared with normal tissue. The most representative MSI pattern was non-MSI. Specifically, 7 of the 8 (87.5%) patients presented with MSI-L status, which was inconsistent with the IHC results. A microsatellite repeated unit change (ranging from 1bp to 3bp) at mononucleotide markers from BAT25 or BAT26 was observed in these patients (Table 3). Minimal microsatellite shifts were found in all cases (100%) of microsatellite repeat unit changes that occurred in mononucleotides, including the only MSI-H case (case 153, Figure 2B), which slightly shifted at BAT25 and BAT26. Five of the 7 MSI-L cases (71.4%) with minimal microsatellite shifts occurred at the BAT25 locus of the single nucleotide marker and 2 (28.6%) occurred at BAT26.
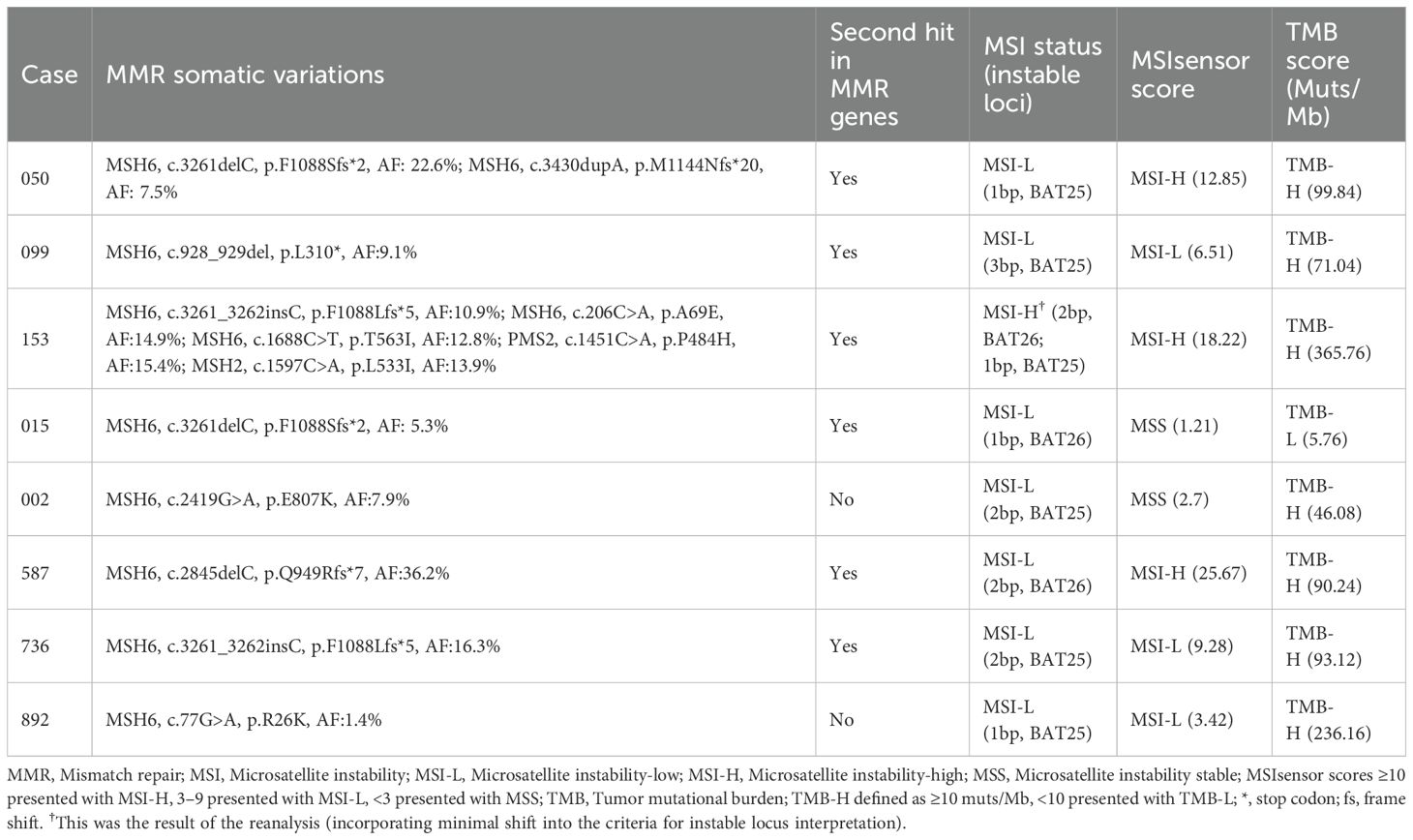
Table 3. Summary of MMR gene mutations, MSI status (based on PCR), tumor mutational burden and MSI score (based on NGS) in our cohort.
Genomic features
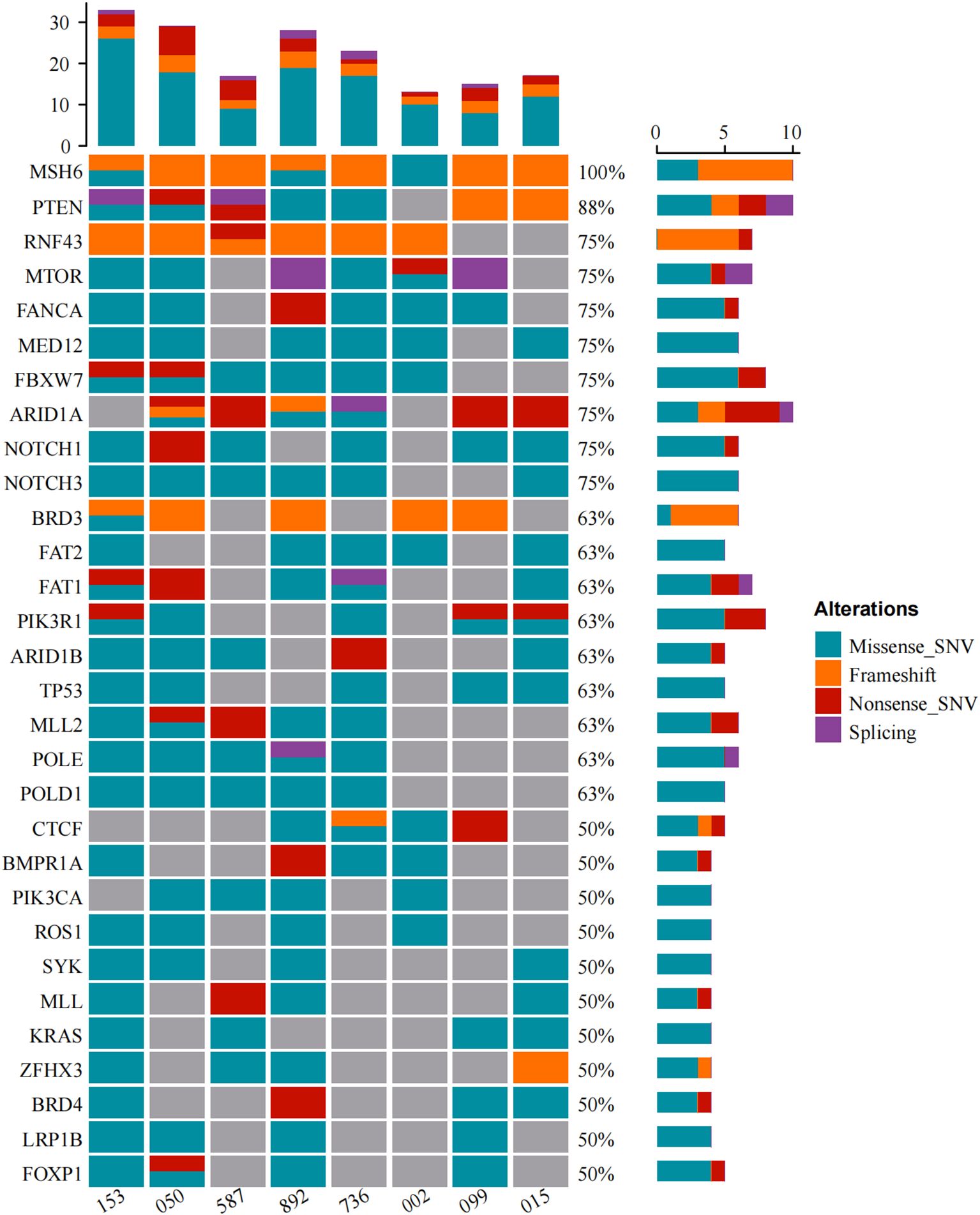
Alterations in a repertoire of somatic mutations were confirmed via targeted sequencing of 1021 genes, including PTEN (88%), ARID1A (75%), POLE/TP53/POLD1/ARID1B (63%), and ATM/PI3KCA/KRAS/IGF1R (50%) (Figure 2C). All the EC patients whose MSH6 protein was abnormal according to IHC showed a second hit in the proposed LS-related gene. Four patients (cases 015, 050, 099 and 587) harbored frameshift deletions (p.F1088Sfs*, p.L310*, and p.Q949Rfs*), resulting in second somatic inactivation of MSH6, and 2 patients (cases 153 and 736) harbored frameshift insertion (p.F1088Lfs*). Recurrent passenger mutation in an MSH6 exon 5 coding microsatellite, MSH6 F1088fs*, was observed in 50% (4/8) of all patients (Table 3, Figure 3).
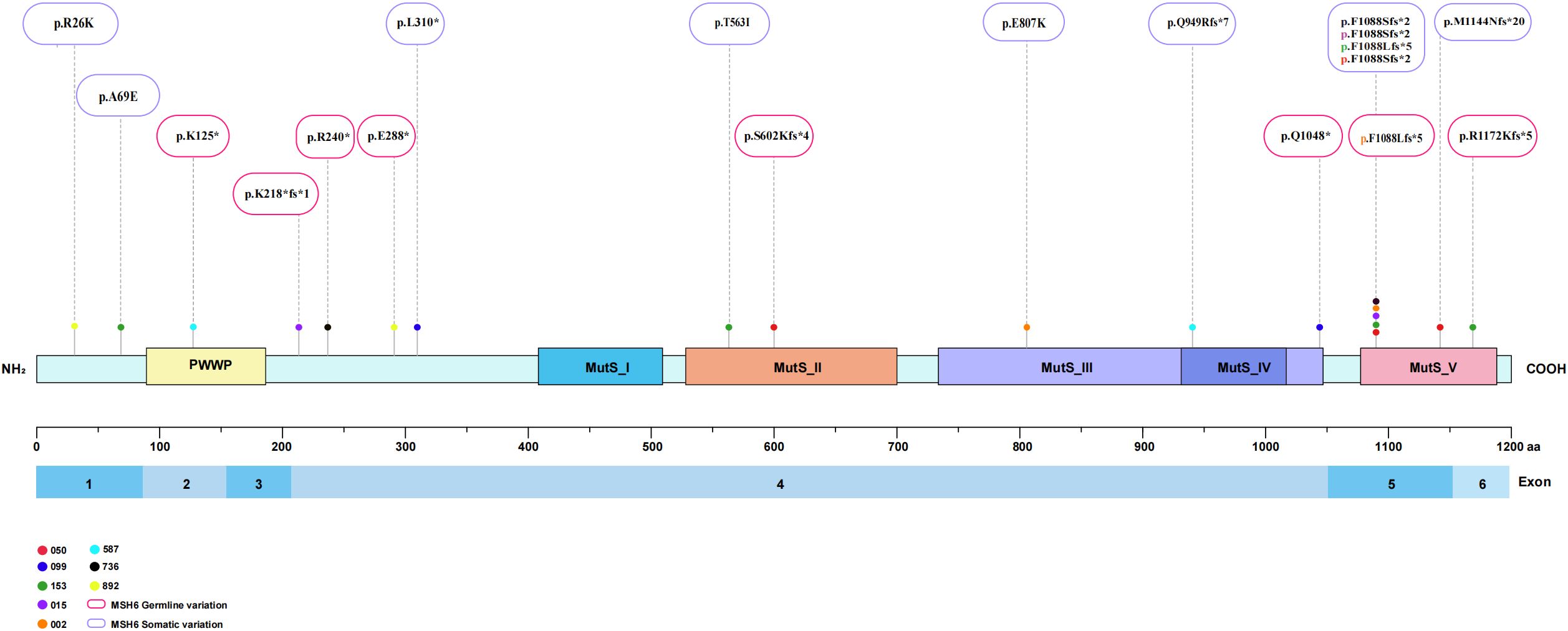
Figure 3. Structure of the MSH6 protein showing function and variants located in the study cohort. MSH6 germline variations and somatic variations are marked red boxes and purple boxes.
MSI status was further analyzed via next-generation sequencing (NGS), and 3 of 8 cases (37.5%) displayed MSI-H (Figure 2C), whereas cases 050 and case 587 were defined as MSI-L when one unstable mononucleotide marker was detected via PCR-CE. Patient 099 presented evidence of MSI-L by PCR analysis but had a low MSIsensor score of 6.51, which was deemed MSS. The sole case (case 015, Table 3) considered to be MSI-L/MSS with TMB-L was a 48-year-old woman with a personal history of colorectal and breast cancer and family members who had a history of LS-related cancer. The tumor was a well-differentiated EC occurring at the uterine fundus with a low tumor mutational burden (TMB score=5.76 Muts/Mb) and MSS (MSIsensor score=1.21). In our cohort, the TMB was > 10 in all patients except patient 015, where patients 153 and 892 had a high TMB or ultramutated phenotypes (TMB score>100 Muts/Mb). A minority (25%, 2/8) of the tumors had multiple MSH6 mutation types, many tumors had only one mutation, and the molecular landscape of all the patients is presented in Figure 4. Taking IHC as the reference, the proportion of cases correctly classified by MMR genomic status was greater (100%) than classified by PCR-CE (12.5%) in cases of MSH6 truncating variation. In addition, NGS (37.5%) testing had a higher MSI-H detection rate than PCR-CE (12.5%) in the evaluation of MSI status.
Discussion
In the current study, we performed NGS of 8 out of 27 LS-EC patients with only MSH6 protein negative from the largest gynecological oncology center in Southwest China. Of the 8 LS-EC patients, 7 had MSI-L by PCR-CE and 5 had MSI-L/MSS by NGS. The rate of discordance between IHC-MMR and PCR-MSI in ECs was 87.5%, which was significantly higher than that of the NGS-MSI strategy (62.5%). MSH6 mutations were demonstrated by next-generation sequencing (NGS) in 8 of 27 patients with loss of MSH6 protein expression, and all the mutations were truncating mutations, which are generally classified as pathogenic. The evidence from EC patients with LS in our cohort indicated that pathogenic germline mutations in MSH6 are more frequently present as non-MSI-H status (including MSI-L and/or MSS). This may reveal a pitfall for LS individuals with MSI identified by either PCR or multigene panel testing in ECs with MSH6 germline truncating mutations.
MSH6, an MMR gene also known as G/T mismatch binding protein, is located at 2p16, a site not far from MSH2, with a total DNA length of 23,806 bp and 10 exons (19). Compared with those in CRC, MSH6 germline mutations are more common in EC, as demonstrated in many studies (20). Previous studies have shown that the incidence of EC is 26-fold greater in women who carry MSH6 pathogenic variants than in the general population (21). In a study including 22 EC patients with LS, who typically harbor MMR genetic germline mutations, 55% had MSH6 and PMS2 mutations, which is higher than previously reported (22). However, given the scarcity of reports of mutations in the MSH6 gene in molecular LS screenings, mutations in this gene could be largely underestimated. More importantly, more evidences has indicated that the risk for EC is significantly greater in women with MSH6 pathogenic variants than in those with MLH1 or MSH2 variants, and the cumulative risk for the diagnosis of EC throughout their lifetime is 16%-49% (23, 24). Therefore, determining MSH6 variant pathogenicity is of significant clinical importance, particularly for predicting cancer risk. According to the available data, the most frequent MSH6 mutation occurs in exon 4, and the remaining exons have a lower mutation frequency (25). Truncating mutations in MSH6 have been identified in patients with hereditary LS and are generally classified as pathogenic. Truncating mutations can introduce a premature stop codon that results in a C-terminal truncated form of the protein, including partial or complete deletion of the highly conserved MutS structural domain. Total or partial loss of this structural domain results in loss of ATPase activity, which impairs DNA binding and mismatch repair functions (26, 27). In our study, massively parallel sequencing analysis revealed truncating mutations in the MSH6 gene in exon 4 in 62.5% (5/8) of patients, including nonsense mutations (37.5%), frameshift insertions (12.5%) and frameshift deletions (12.5%). What draws more of our attention is that these cases presented a non-MSI-H pattern on PCR-CE analysis, or displayed a relatively lower degree of MSI, which is consistent with the recent research results in CRC by Helderman et al (28).
Previous studies in yeast have shown that mutations in MSH6 do not lead to dinucleotide repeat instability but instead lead to weak single nucleotide repeat instability and significantly increase the accumulation rate of base substitution mutations (29). In addition, studies of MSH6 mutant mice have shown that these mice have significantly increased cancer susceptibility and that the tumors in these mice do not exhibit repeat instability, similar to the results observed in yeast (30). Researchers have reported that mice harboring MSH6 mutations not only have a reduced life expectancy, but also develop a variety of unrelated tumors involving multiple organ systems. In the following years, several in vitro and in vivo studies established the association of HNPCC-related tumors with low MSI with germline mutations in MSH6, further confirming that mutations in MSH6 alone were not sufficient to cause MSI in CRC cell lines (31, 32). These results suggest that MSH6 mutations may contribute to cancer susceptibility, but the tumors produced may differ from those observed in kinases that inherit MSH2 and MLH1 mutations, at least in terms of their microsatellite instability phenotype. For eukaryotes, in the process of DNA mismatch repair, MutS homologous dimer (with mismatch binding activity) and MutL homologous dimer (capable of interacting with proteins) combine to form a tetramer complex and work synergistically. And there are a number of heterodimeric MutS homologs, the most important of which are MutSα (MSH2/MSH6) and MutSβ (MSH2/MSH3); the former recognizes one or two base unpaired sites, while the latter recognizes longer insertion-deletion loops with up to more than ten nucleotides (19). The MSH6 protein is involved in the repair of both single-base mismatches and insertion/deletion loops but is not absolutely required for the MMR system. In the absence of MSH6, the MSH3 protein can partially replace the MSH6 repair function, and the MSI profile is not present as MSI-H. Therefore, non-MSI-H (MSS/MSI-L) phenotype could not be considered as an exclusion criterion for MMR gene monitoring, especially for MSH6.
PCR-CE is the gold standard for assessing MSI, including 5 or 7 loci, whereas NGS-based methods can examine hundreds to thousands of target microsatellite loci, allowing for a more comprehensive assessment. Our data revealed that 7 (87.5%) LS individuals with solely MSH6 loss presented non-MSI-H disease by PCR, but 5 cases (62.5%) were identified by NGS. In our cohort, we identified only one patient with MSI-H status (using PCR-CE assay), and the BAT25 locus may not be a truly unstable locus if a 2bp changing is used as a criterion for unstable loci interpretation; thus, Patient 153 exhibited an MSI-L pattern. Our findings may provide direct evidence that a subset of primary ECs that develop in the context of an MSH6 germline pathogenic mutation harbor features of non-MSI-H, including MSS or MSI-L, which is inconsistent with previous observations (16). An MSIsensor score of 10 reliably identifies the MSI-H status of solid tumors at various primary sites, and again, we used this criterion (33). TMB-H was found in all three cases with MSIsensor scores >10 (cases 050, 153 and 587), and MSH3 variants were found in 2 of them, suggesting that MSIsensor performs well in MSH6-deficient ECs. Middha et al. reliably assessed pan-cancer microsatellite instability using MSK-IMPACT assay and found that MSIsensor may be sensitive for the MSH6-equivocal EC, suggesting that MSIsensor performs well in MSH6-deficienct tumors. Our massively parallel sequencing was also similar to MSHK-IMPACT, and therefore, we adopted MSIscore greater than or equal to 10 as a criterion for MSI-H interpretation (18). Compared with PCR methods, Simultaneous MSI detection by NGS not only saves resources efficiently, but also may be more sensitive to dMMR and may identify MSI-H in a wide range of cancers not typically screened.
Notably, among different solid tumors, the number of microsatellite instability nucleotide shifts varies by PCR-CE, including major microsatellite repeat shifts and minimal shifts (34). At present, although there is no unified guideline for the definition of minimal microsatellite shift, it was defined by shift of 1 to 3 microsatellite nucleotide repeats at an involved locus in most studies. Moreover, MSI-H ECs have a significantly greater frequency (52%) of minimal microsatellite shift (14, 35). A comparing the MSI status of EC and CRC revealed that 53% of MSI-H-type EC cases presented an average of 1–2 small nucleotide changes, whereas approximately 80% of MSI-H-type CRC cases presented an average of 6 nucleotide changes (36). Therefore, minimal microsatellite shifts are more likely to occur in EC. The subtle changes caused by minimal microsatellite shifts are easily overlooked when interpreting PCR-CE results, leading to false-negative results. Therefore, minimal microsatellite shifts are also considered one of the main reasons for the high inconsistency rates of IHC and PCR-CE in EC. According to the criteria of minimal shifting, minimal microsatellite shifts were found in all cases (100%) of tumors with only MSH6 loss, and instability loci occurred only at single nucleotide sites in our study. Our results are higher than those of previous studies (30%) (14, 37), suggesting that ECs with the loss of MSH6 alone had a greater chance of minimal shift. Therefore, the identification of minimal microsatellite shifts is crucial for accurate interpretation of microsatellite instability PCR data in EC in terms of clinical diagnosis.
The major limitation of MSI testing is that it is less accurate in identifying EC patients with the MSH6 mutations. Minimal microsatellite shifts are may be another major reason; if the MSI status is evaluated on the basis of minimal microsatellite shifts, the detection sensitivity of PCR-CE and its consistency with IHC can be improved. Since the MSH6 protein is not involved in the repair of mismatches of dinucleotides in length, and consequently, the 2B/3D panel (NCI recommended) often shows an MSS in MSH6-deficient tumors, mononucleotide repeats are recognized as being more sensitive and specific for determining of the MSI status in these tumors (38). MSH6-deficient tumors are, therefore at risk of being misclassified as MSI-L or MSS, depending on the markers chosen. Some investigators have recommended assessing the MSI status of MSH6-deficient tumors via a panel of 5 mononucleotide markers, including NR21, BAT25, BAT26, NR24 and NR22 (Pentaplex Assay) (39). In addition, for ECs with superficial muscle layer infiltration, collecting a sufficient proportion of tumor cells during the detection process is difficult, which may also negatively affect the MSI results. Overall, researchers need to be aware that PCR-CE testing is challenging when used to detect MSH6 truncated variant carriers.
We explored the clinicopathological characteristics of ECs in LS patients. All patients were diagnosed with endometrioid carcinoma at an early stage (100%), and the majority were well differentiated (75%). Notably, the median age of the 8 LS individuals with an isolated germline MSH6 mutation in our cohort was 56.5 years (range from 48 to 67 years). A published study revealed that EC patients with MSH6 gene mutations had a greater mean age of onset (58 years) than did patients with MLH1 or MSH2 gene mutations (49 years) (40). Despite the small number of cases in our study cohort, a delay in the age of onset of EC, which is characteristic of MSH6 mutations, is also well supported. LS patients are usually clinically diagnosed according to the Amsterdam or Bethesda criteria. However, the current clinical criteria for patients with LS (who typically harbor MSH2 and/or MLH1 germline mutations) were deemed insensitive for identifying MSH6 mutation carriers. Furthermore, owing to potential challenges in gathering family information and the absence of distinctive clinical phenotypes, the diagnostic rate may be much lower than the actual incidence. Thus, criteria for a diagnosis of MSH6-related LS were established that differed from the Amsterdam major and/or minor criteria and should incorporate the unusual phenotypes of patients with an isolated germline MSH6 mutation, such as those patients who are older at the time of diagnosis of the primary malignancy (41). IHC is still the initial screening tool for the detection of the involved MMR protein. With respect to the retention of MSH2 and MLH1 expression and the lack of MSH6 alone, MSI analysis using a panel composed of mononucleotides alone (Pentaplex Assay) is recommended instead of the standard 2B/3D panel. In the case of non-MSI-H, germline mutation analysis of MSH6 is needed, especially in the context of a positive family history. Even if germline mutation analysis of MSH6 is negative, the proband and his family members will still require strict cancer surveillance.
A limitation of this study was the retrospective, highly selective nature of this cohort. Additionally, this study is based on a relatively small sample size from a single center, and a well-designed multicenter study is still needed to demonstrate the incidence of MSH6 gene mutations and the clinical phenotype, which is one of our ongoing studies in China. The MSIsensor score cutoff value varies somewhat from cancer to cancer, and further large-sample studies are needed. Our study expands the spectrum of the known germline mutations of the MSH6 gene in EC patients who are MSH6 protein negative. A multicenter trial with a larger number of EC patients with LS is needed to clarify the biological impact of these mutations on susceptibility to LS and their impact on the effectiveness of anti-PD-1 treatment.
In conclusion, this study highlights that the diagnosis of LS caused by pathogenic germline MSH6 variants may be complicated by inconsistent results in terms of the IHC and PCR-CE phenotypes. The molecular and clinical data of these patients add to our understanding of the clinical implications of MSH6 germline variants. We explored a variety of causes for discordant MSI and IHC results in MSH6 variant carriers. Compared with other methods, IHC is widely available and not expensive and may confer an advantage over PCR-CE due to the lower sensitivity of PCR-CE for MSH6-deficient tumors. In addition, MSI analysis using a panel composed of mononucleotides is recommended instead of the standard 2B/3D panel when tumors lack MSH6 alone, and germline mutation analysis of MSH6 is mandated.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, Sequence Read Archive (SRA) database (PRJNA990462).
Ethics statement
The studies involving humans were approved by the Medical Ethical Committee of West China Second University Hospital of Sichuan University Institutional (protocol 2023037). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. DL: Funding acquisition, Investigation, Supervision, Writing – review & editing. WW: Funding acquisition, Supervision, Writing – review & editing. WK: Data curation, Resources, Writing – review & editing. JZo: Methodology, Resources, Writing – review & editing. JZe: Project administration, Resources, Visualization, Writing – original draft. MF: Funding acquisition, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by key research and development project of cadre health care in Sichuan Province Research on diagnostic strategy and clinicopathological study of endometrial cancer molecular typing (Project No: ZH2023-1701), the Clinical Discipline Development Fund of West China Second Hospital of Sichuan University (Clinical Application Research on the Latest Molecular Typing Pathological Diagnosis Strategy and Scheme of Endometrial Cancer) (Project No: KS336), Beijing Jingjian Pathology Development Foundation, Tongshu Microsatellite Instability Research Fund Project (Primary endometrial cancer microsatellite instability detection) (Project No: JJTS2020-014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1520500/full#supplementary-material
References
1. Vasen HFA, Möslein G, Alonso A, Bernstein I, Bertario L, Blanco I, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet. (2007) 44:353–62. doi: 10.1136/jmg.2007.048991
2. Biller LH, Creedon SA, Klehm M, and Yurgelun MB. Lynch syndrome-associated cancers beyond colorectal cancer. Gastrointest Endosc Clin N Am. (2022) 32:75–93. doi: 10.1016/j.giec.2021.08.002
3. Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. (1999) 81:214–8. doi: 10.1002/(SICI)1097-0215(19990412)81:2<214::AID-IJC8>3.0.CO;2-L
4. Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. (1997) 6:105–10. doi: 10.1093/hmg/6.1.105
5. Kuiper RP, Vissers LELM, Venkatachalam R, Bodmer D, Hoenselaar E, Goossens M, et al. Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat. (2011) 32:407–14. doi: 10.1002/humu.21446
6. Dudley JC, Lin M-T, Le DT, and Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. (2016) 22:813–20. doi: 10.1158/1078-0432.CCR-15-1678
7. Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. (2017) 146:217–24. doi: 10.1016/j.ygyno.2017.06.002
8. van der Werf-’t Lam A-S, Terlouw D, Tops CM, van Kan MS, van Hest LP, Gille HJP, et al. Discordant staining patterns and microsatellite results in tumors of MSH6 pathogenic variant carriers. Mod Pathol. (2023) 36:100240. doi: 10.1016/j.modpat.2023.100240
9. Pan S, Cox H, Willmott J, Mundt E, Gorringe H, Landon M, et al. Discordance between germline genetic findings and abnormal tumor immunohistochemistry staining of mismatch repair proteins in individuals with suspected Lynch syndrome. Front Oncol. (2023) 13:1069467. doi: 10.3389/fonc.2023.1069467
10. Hsieh P and Zhang Y. The Devil is in the details for DNA mismatch repair. Proc Natl Acad Sci U S A. (2017) 114:3552–4. doi: 10.1073/pnas.1702747114
11. Buza N, Ziai J, and Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. (2016) 16:591–604. doi: 10.1586/14737159.2016.1156533
12. Haraldsdottir S, Hampel H, Tomsic J, Frankel WL, Pearlman R, de la Chapelle A, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. (2014) 147:1308–1316.e1. doi: 10.1053/j.gastro.2014.08.041
13. Seppälä TT, Latchford A, Negoi I, Sampaio Soares A, Jimenez-Rodriguez R, Sánchez-Guillén L, et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg. (2021) 108:484–98. doi: 10.1002/bjs.11902
14. Wu X, Snir O, Rottmann D, Wong S, Buza N, and Hui P. Minimal microsatellite shift in microsatellite instability high endometrial cancer: a significant pitfall in diagnostic interpretation. Mod Pathol. (2019) 32:650–8. doi: 10.1038/s41379-018-0179-3
15. Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. (2003) 100:5908–13. doi: 10.1073/pnas.1030231100
16. Wang C, Kuang W, Zeng J, Ren Y, Liu Q, Sun H, et al. A retrospective study of consistency between immunohistochemistry and polymerase chain reaction of microsatellite instability in endometrial cancer. PeerJ. (2023) 11:e15920. doi: 10.7717/peerj.15920
17. Qiu T, Zhang J, Lu R, and Zhu Z. Genome segment S8 of grass carp hemorrhage virus encodes a virion protein. Intervirology. (2001) 44:317–20. doi: 10.1159/000050064
18. Middha S, Zhang L, Nafa K, Jayakumaran G, Wong D, Kim HR, et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol. (2017) 2017):84. doi: 10.1200/PO.17.00084
19. Sharma M, Predeus AV, Kovacs N, and Feig M. Differential mismatch recognition specificities of eukaryotic MutS homologs, MutSα and MutSβ. Biophys J. (2014) 106:2483–92. doi: 10.1016/j.bpj.2014.04.026
20. Metcalf AM and Spurdle AB. Endometrial tumor BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Fam Cancer. (2014) 13:1–12. doi: 10.1007/s10689-013-9671-6
21. Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. (2010) 102:193–201. doi: 10.1093/jnci/djp473
22. Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman A-R, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. (2016) 29:1381–9. doi: 10.1038/modpathol.2016.135
23. Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. (2018) 67:1306–16. doi: 10.1136/gutjnl-2017-314057
24. Yang C, Misyura M, Kane S, Rai V, Latham A, and Zhang L. Characterization of a germline variant MSH6 c.4001G > C in a Lynch syndrome family. Mol Genet Genom Med. (2023) 11:e2104. doi: 10.1002/mgg3.2104
25. Nirwal S, Kulkarni DS, Sharma A, Rao DN, and Nair DT. Mechanism of formation of a toroid around DNA by the mismatch sensor protein. Nucleic Acids Res. (2018) 46:256–66. doi: 10.1093/nar/gkx1149
26. Iaccarino I, Marra G, Palombo F, and Jiricny J. hMSH2 and hMSH6 play distinct roles in mismatch binding and contribute differently to the ATPase activity of hMutSalpha. EMBO J. (1998) 17:2677–86. doi: 10.1093/emboj/17.9.2677
27. Thompson BA, Spurdle AB, Plazzer J-P, Greenblatt MS, Akagi K, Al-Mulla F, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. (2014) 46:107–15. doi: 10.1038/ng.2854
28. Helderman NC, Strobel F, Bohaumilitzky L, Terlouw D, van der Werf-’t Lam A-S, van Wezel T, et al. Lower degree of microsatellite instability in colorectal carcinomas from MSH6-associated lynch syndrome patients. Mod Pathol. (2025) 38:100757. doi: 10.1016/j.modpat.2025.100757
29. Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. (1996) 10:1433–42. doi: 10.1101/gad.10.12.1433
30. Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, et al. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell. (1997) 91:467–77. doi: 10.1016/s0092-8674(00)80433-x
31. Wu Y, Berends MJ, Mensink RG, Kempinga C, Sijmons RH, van der Zee AG, et al. Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet. (1999) 65:1291–8. doi: 10.1086/302612
32. Wagner A, Hendriks Y, Meijers-Heijboer EJ, de Leeuw WJ, Morreau H, Hofstra R, et al. Atypical HNPCC owing to MSH6 germline mutations: analysis of a large Dutch pedigree. J Med Genet. (2001) 38:318–22. doi: 10.1136/jmg.38.5.318
33. Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. (2016) 34:2141–7. doi: 10.1200/JCO.2015.65.1067
34. Ferreira AM, Westers H, Wu Y, Niessen RC, Olderode-Berends M, van der Sluis T, et al. Do microsatellite instability profiles really differ between colorectal and endometrial tumors? Genes Chromosomes Cancer. (2009) 48:552–7. doi: 10.1002/gcc.20664
35. Libera L, Sahnane N, Pepe F, Pisapia P, De Luca C, Russo G, et al. Critical aspects of microsatellite instability testing in endometrial cancer: a comparison study. Hum Pathol. (2022) 128:134–40. doi: 10.1016/j.humpath.2022.07.014
36. Wang Y, Shi C, Eisenberg R, and Vnencak-Jones CL. Differences in microsatellite instability profiles between endometrioid and colorectal cancers: A potential cause for false-negative results? J Mol Diagn. (2017) 19:57–64. doi: 10.1016/j.jmoldx.2016.07.008
37. Liu Y, Wang M, Chen Q, Zheng Q, Li G, Cheng Q, et al. A novel heterozygous large deletion of MSH6 gene in a Chinese family with Lynch syndrome. Gene. (2019) 704:103–12. doi: 10.1016/j.gene.2019.04.011
38. You J-F, Buhard O, Ligtenberg MJL, Kets CM, Niessen RC, Hofstra RMW, et al. Tumors with loss of MSH6 expression are MSI-H when screened with a pentaplex of five mononucleotide repeats. Br J Cancer. (2010) 103:1840–5. doi: 10.1038/sj.bjc.6605988
39. Pagin A, Zerimech F, Leclerc J, Wacrenier A, Lejeune S, Descarpentries C, et al. Evaluation of a new panel of six mononucleotide repeat markers for the detection of DNA mismatch repair-deficient tumors. Br J Cancer. (2013) 108:2079–87. doi: 10.1038/bjc.2013.213
40. Cederquist K, Emanuelsson M, Göransson I, Holinski-Feder E, Müller-Koch Y, Golovleva I, et al. Mutation analysis of the MLH1, MSH2 and MSH6 genes in patients with double primary cancers of the colorectum and the endometrium: a population-based study in northern Sweden. Int J Cancer. (2004) 109:370–6. doi: 10.1002/ijc.11718
Keywords: Lynch syndrome, mismatch repair, endometrial carcinoma, microsatellite instability, minimal microsatellite shift
Citation: Wang C, Liang D, Wang W, Kuang W, Zou J, Zeng J and Feng M (2025) Early-stage endometrioid carcinoma with MSH6 protein deficiency: pitfalls in the diagnostic interpretation of microsatellite instability. Front. Oncol. 15:1520500. doi: 10.3389/fonc.2025.1520500
Received: 19 December 2024; Accepted: 30 April 2025;
Published: 21 May 2025.
Edited by:
Kunqi Chen, Fujian Medical University, ChinaReviewed by:
Xiaojuan Wang, Shanxi Province Cancer Hospital, ChinaMingming Lu, Yale University, United States
Qingru Xu, Yale University, United States, in collaboration with reviewer ML
Copyright © 2025 Wang, Liang, Wang, Kuang, Zou, Zeng and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Feng, aHVheGlwYXRoZm1AMTYzLmNvbQ==
 Cheng Wang
Cheng Wang Dongni Liang1,2
Dongni Liang1,2 Wei Kuang
Wei Kuang