- 1Department of Otorhinolaryngology-Head and Neck Surgery, University Hospitals Leuven, Leuven, Belgium
- 2Department of Pathology, University Hospitals Leuven, Leuven, Belgium
- 3Department of General Medical Oncology, University Hospitals Leuven, Leuven Cancer Institute, Leuven, Belgium
- 4Department of Radiology, University Hospitals Leuven, Leuven, Belgium
- 5Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium
- 6Research Group Experimental Neurosurgery and Neuroanatomy and Leuven Brain Institute, Catholic University of Leuven, Leuven, Belgium
- 7Department of Radiotherapy–Oncology, University Hospitals Leuven, Leuven, Belgium
- 8Laboratory of Experimental Radiotherapy, Department of Oncology, Catholic University Leuven, Leuven, Belgium
- 9Section of Head and Neck Oncology, Department of Oncology, Catholic University Leuven, Leuven, Belgium
- 10Department of Neurosciences, Experimental Otorhinolaryngology, Rhinology Research, Catholic University Leuven, Leuven, Belgium
- 11Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Group, Catholic University Leuven, Leuven, Belgium
Background: Sinonasal intestinal-type adenocarcinoma (ITAC) is a rare disease entity. In contrast to most previous studies, this cohort study consists of a substantial number of uniformly treated patients undergoing endoscopic surgery and adjuvant radiotherapy and provides updated insights into survival outcomes and tumor and treatment-related prognostic factors.
Material and methods: We retrospectively analyzed the medical records of 200 patients primarily treated for ITAC between 1992 and 2022 in our tertiary referral center. Descriptive statistics were applied using Kaplan-Meier method. Cox models were used for univariable and multivariable data analysis.
Results: The 5-year overall survival (OS), disease-specific survival (DSS), and local recurrence-free survival (LRFS) rates were 71.4%, 85.1%, and 55.2% respectively. At 10 years, the numbers decreased to 48.2%, 76.2%, and 32.2% respectively. Significant differences were found in OS and DSS between T-groups. Poorly differentiated tumors had decreased DSS compared to well-differentiated tumors (HR: 3.38 [95% CI: 1.20–9.51], p=.0209). Signet-cell differentiation was associated with the poorest survival among poorly differentiated tumors although not reaching significance. In 34.0% of patients, there was local recurrence, with half of the cases detected within the first two years of follow-up but over 10% of recurrence occurring after 10 years. Positive surgical margins (HR: 2.95 [95% CI: 1.29–6.74], p=.0106), local recurrence (HR: 12.28 [95% CI: 5.59–26.99], p<.0001), and distant metastasis (HR: 41.17 [95% CI: 21.58–78.55], p<.0001) negatively affected DSS. Distant metastasis occurred more frequently in poorly differentiated tumors (25.6%) compared to moderately differentiated (9.5%) and well-differentiated tumors (2.5%) (p=.002).
Conclusions: This extensive study focusing on sinonasal ITAC primarily managed through endoscopic resection and radiotherapy, demonstrates that T-classification and tumor differentiation are independent prognostic factors influencing survival. Furthermore, local recurrence, distant metastasis, and positive surgical margins negatively affect OS and DSS.
1 Introduction
According to data from the International Agency for Research on Cancer (IARC), malignant tumors affecting the sinonasal cavity are considered rare, compromising only 0.4% of all malignancies worldwide (1). However, in Belgium, these malignancies constitute a larger proportion, comprising 4.6% of newly incident head and neck carcinomas in 2017 (2). Among these, intestinal-type adenocarcinoma (ITAC) emerges as one of the most frequently observed histopathological subtypes. This notable incidence of ITAC is primarily attributed to occupational exposure to hardwood dust (3, 4).
Primary ITAC is histopathologically similar to adenocarcinomas of the intestinal mucosa. Sinonasal ITAC can be further subclassified morphologically and according to histological grade of differentiation into well-differentiated (papillary, tubular, and papillary-tubular type), moderately differentiated (papillary-mucinous and papillary-tubular-mucinous type) and poorly differentiated (mucinous, alveolar goblet cell, and signet-ring type) tumors (4).
Presenting symptoms in patients with ITAC are usually nonspecific, leading to a delay in diagnosis. Diagnosis of sinus tumors is primarily based on endoscopic visualization and biopsy. Cross-sectional imaging through CT and MRI is recommended to evaluate tumor extent and regional spread. Additionally, further diagnostic tests, such as abdominal ultrasound and chest X-ray, as well as more recent methods like chest and abdominal CT scans, and PET-CT in selected cases, are commonly employed to assess for distant metastasis (5).
Historically, the main treatment for sinonasal malignancies consisted of external approaches like craniofacial resection or lateral rhinotomy with medial maxillectomy, possibly combined with adjuvant radiotherapy. However, over the past two decades, endoscopic endonasal surgery has emerged as the predominant approach, propelled by enhanced surgeon expertise, refined surgical techniques, and technological innovations such as high-definition endoscopes and image-guided procedures, thereby pushing the boundaries of what is achievable (6–9). Several studies have demonstrated that extended endoscopic surgery for ITAC is oncologically safe for most patients up to selected T4b cases, with limited morbidity (10, 11). Moreover, extensive evidence has consistently shown the superiority of this approach compared to external techniques in terms of margin status, local recurrence, and disease-specific survival (12–14). After endoscopic surgical treatment, adjuvant radiotherapy is frequently administered. Advances in radiotherapy, shifting from 3D conformal radiotherapy to intensity-modulated radiotherapy (IMRT), have minimized damage to surrounding healthy tissues (15).
The prognosis for sinonasal ITAC is relatively favorable, with reported 5-year overall survival (OS) and disease-specific survival (DSS) rates of 72.7 (67.3%-78.5%) and 80.0% (75.1%-85.5%) respectively (16). Nonetheless, recurrence rates of sinonasal ITAC remain as high as 50% in some cohorts (16).
The current study updates our previous experience (17, 18), and involves a substantial cohort with prolonged follow-up to further substantiate the efficacy of a treatment protocol consisting of endoscopic surgery followed by radiotherapy.
2 Materials and methods
2.1 Patient selection
This study spans thirty-one years from 1992 to 2022, during which 200 individuals with sinonasal ITAC were referred to our Department of Otorhinolaryngology, Head and Neck Surgery. All these patients underwent a state-of-the-art endoscopic resection in an academic tertiary reference center setting, followed by radiotherapy (RT) in the majority of cases. Those who received a different primary treatment, such as craniofacial resection (CFR) or lateral rhinotomy, or were deemed inoperable were excluded from the analysis. Among the 200 patients, 171 (85.5%) received their primary treatment at our department, while the remaining 29 patients underwent incomplete resection at a regional hospital before being referred to our institution for revision endoscopic surgery, conducted by one of our three senior anterior skull base surgeons (L.V.G., M.J. and V.V.P.). Data on a subset of 44 patients and later 123 patients were previously reported by Van Gerven et al. and Camp et al. (17, 18). This current cohort of 200 patients further updates the most recently reported experience on 123 patients (18).
Patient selection for endoscopic resection was based on a shared decision by the multidisciplinary Head and Neck tumor board and the patient, considering tumor extent, patient comorbidity, reconstructive requirements, and anticipated surgical morbidity and mortality. In summary, endoscopic resection was considered for all patients lacking dural, brain, or orbital invasion on preoperative imaging, consisting of CT and MRI. Exceptions included 9 T4b lesions exhibiting limited dural invasion, 2 of which showed minimal intracranial invasion, that were treated endoscopically.
2.2 Preoperative assessment
Diagnostic workup included histopathological examination of endoscopic biopsy specimens, with postoperative confirmation. Tumor extent was assessed preoperatively using contrast-enhanced CT primarily for bone evaluation and multiplanar MRI with gadolinium for further soft tissue evaluation. Tumors were staged according to the 8th edition of the International Union for Cancer Control TNM classification of 2017 (19). Evaluation for regional and distant metastasis involved routine blood tests, liver function tests, in the early period chest X-rays and abdominal ultrasound scans, and more recently CT scans of the chest and abdomen, as well as FDG-positron emission tomography (PET) scans in selected patients.
2.3 Surgical approach
Endoscopic resection for T1 tumors involved en bloc removal following optimal exposure and identification of the tumor’s origin. For middle-sized tumors (T2), a phased resection approach was employed. Initially, the tumor bulk was visualized, and its origin was pinpointed using atraumatic suction during a preparatory phase. Subsequently, the bulk of the tumor was excised using either cold instruments or a microdebrider. Following tumor bulk reduction the identified tumor origin was broadly circumscribed and where possible and in the absence of critical anatomical structures a 1 cm mucosal margin was aimed at.
For larger tumors (T3–T4), a more extended “tumor disassembly” and subperiosteal centripetal resection implied a fronto-sphenoethmoidectomy and a resection of the superior segment of the nasal septum. In cases of unilateral tumors, tumors with a unilateral origin with a volume confined to one nasal cavity, contralateral radical surgery was not undertaken. Control biopsies at the cribriform plate were routinely conducted at the end of each surgery. In tumors abutting to but not clearly invading the cribriform plate and olfactory groove, the macroscopically uninvolved olfactory mucosa and olfactory fibers at the cribriform plate were routinely sampled. On rare occasions, this implied a limited CSF leakage that spontaneously stopped or was covered with fibrin sealant and fascia lata. Endoscopic reconstruction at the level of the ethmoid roof using a multilayer closure technique involving fascia lata and mucosa was exclusively performed in cases where limited dural invasion required dural resection.
Given the estimated regional metastasis prevalence at presentation of 1% or even lower, and the absence of evidence in the literature supporting prophylactic treatment of first-echelon lymph nodes (5), elective neck treatment was not performed.
2.4 Postoperative management
Adjuvant RT was preferred in most cases, except for select small T1 tumors with negative resection margins and patients with numerous comorbidities or advanced age. Intensity-modulated RT has been the standard since 2003, offering improved tissue sparing over conventional RT (15). For patients radiated postoperatively at our center, the prescribed total dose was 60 Gy in 30 daily fractions of 2 Gy (5 fractions per week). An additional boost volume up to 66-70 Gy was delineated in case of incomplete surgical resection (either microscopic or macroscopic). No elective irradiation of the cervical lymph nodes was performed (20). One patient was diagnosed with positive bilateral cervical lymph nodes, including a retropharyngeal adenopathy (N2c), on pre-radiation CT. This patient subsequently received adjuvant chemoradiotherapy.
2.5 Follow-up
Follow-up intervals were scheduled every two months during the first two years, every three months in the third year, and every four months in the fourth year, transitioning to bi-annual evaluations during the fifth year and annually thereafter. Each follow-up included a comprehensive clinical and endoscopic assessment of the nasal cavity, supplemented by imaging at predefined intervals. MRI of the sinonasal region was conducted every six months during the first five years. Whole-body imaging, such as PET-CT, was reserved for specific clinical indications or performed prior to initiating systemic therapy.
2.6 Statistical analysis
In this study, comprehensive clinical data, preoperative imaging records, surgical and histopathological reports, details regarding adjuvant therapy, and follow-up information were extracted from the electronic medical file. The Statistical Package for Social Sciences (SPSS, version 29.0.1.0, Chicago, IL) was utilized to process the collected data. Survival analyses were conducted to evaluate overall survival (OS), defined as the time to death from any cause; disease-specific survival (DSS), defined as the time to death specifically from ITAC; and local recurrence-free survival (LRFS), defined as the time to local tumor recurrence or death from any cause, using the Kaplan–Meier method. The time to each event was calculated from the date of primary surgery. Group comparisons in univariate analyses were performed using the Log-rank test, with statistical significance defined as a p-value of ≤.05. Cox proportional hazard models were applied to estimate the hazard ratios (HR) for individual variables, reported along with their corresponding 95% confidence intervals (CI). A forward selection procedure was employed to construct a multivariable model of independent predictors, requiring a significance level of 5% for variables to be included in the model.
3 Results
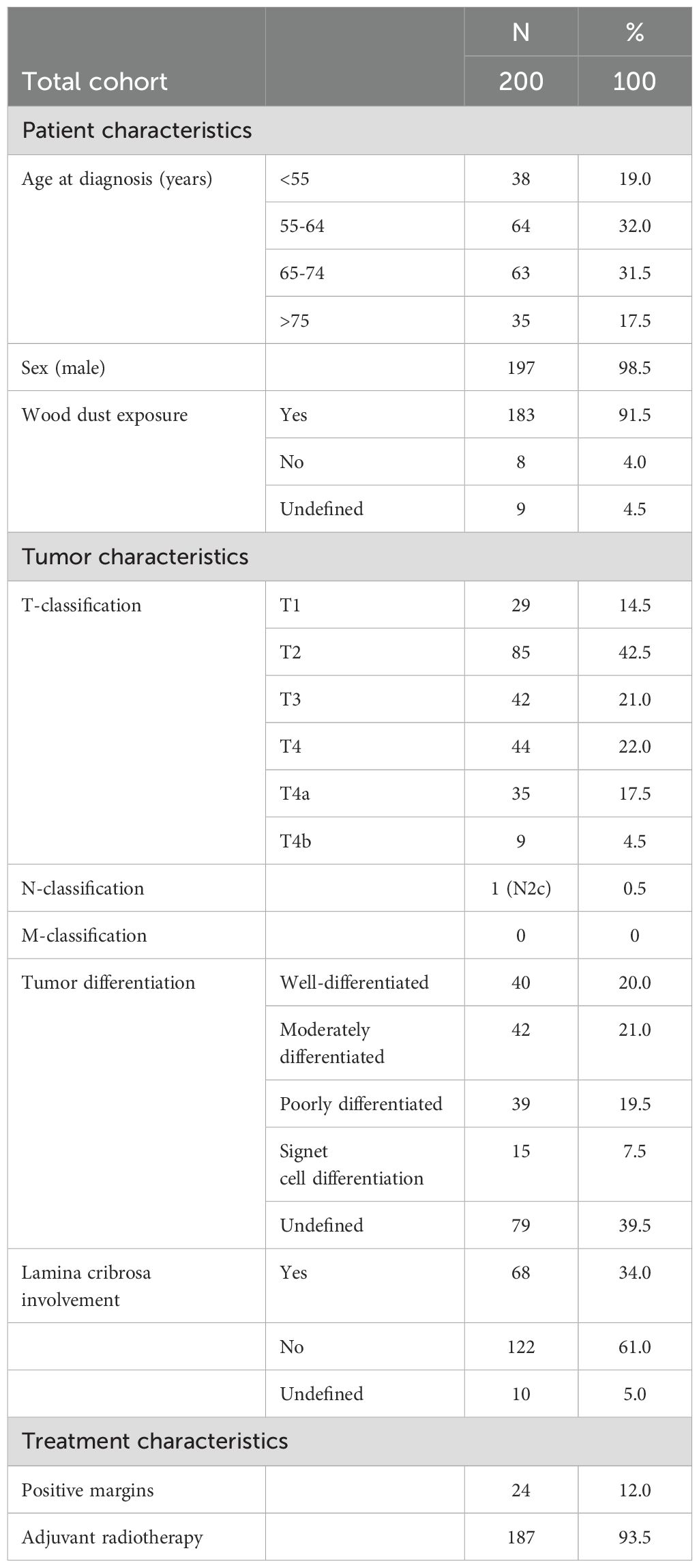
A total of 200 patients were included in the study. The age at diagnosis ranged from 39 to 93 years, mean 64.3, median 64 years. Patients were followed for a mean and median of 93.2 and 78 months respectively (range = 1–336 months). The mean and median follow-up time for local recurrence-free patients was 80.7 and 64 months respectively (range = 1–329 months). Wood dust exposure emerged as the primary etiological factor for ITAC, with only eight patients (4%) claiming not to have been exposed to wood dust in our cohort. The occupational hazard led to a significant disparity in the gender distribution, with a male-to-female ratio of 197:3. The olfactory cleft was the most commonly affected site, with 81.5% of tumors originating in this region. Cribriform plate involvement was reported in 68 patients.
The TNM classification at initial diagnosis is summarized in Table 1. None of the patients presented with distant metastatic disease at first presentation. However, one patient, with a T4a primary tumor, had regional metastatic disease, involving the contralateral neck, classified as N2c. During follow-up, distant metastases developed in 20 cases (10.0%), occurring at multiple sites (n=7), or isolated in the lung (n=5), brain (n=4), bone (n=2), liver (n=1), or spinal cord (n=1). The mean and median time between first treatment and development of distant metastasis was 34.6 and 20.5 months respectively (range 1-114 months). Only two patients (1.0%) developed cervical lymph node metastasis during follow-up.
Local recurrence occurred in 68 patients (34.0%), predominantly in the posterior ethmoid (46.2%), fovea ethmoidalis (33.8%), and posterior septum (32.3%). Unlike the primary tumor, the first local recurrence less frequently originated in the olfactory cleft and relatively more in the sphenoid sinus (13.6% vs 3.0% for the primary tumor). Thirty-two patients developed a second recurrence, and seventeen had a third recurrence. The mean time to first local recurrence was 50.7 months, while the median time interval was 26 months (range 5-247 months) (Figure 1). Therapy of the first local recurrence was predominantly surgery (91.2%), followed by radiotherapy in cases with no previous radiation, and by chemotherapy in 3 other patients. A combined endoscopic and external approach resection was performed in six of these patients (8.8%). Chemotherapy alone was administered in another three patients. Two patients underwent debulking surgeries to alleviate discomfort caused by recurrent tumors that were deemed inoperable.
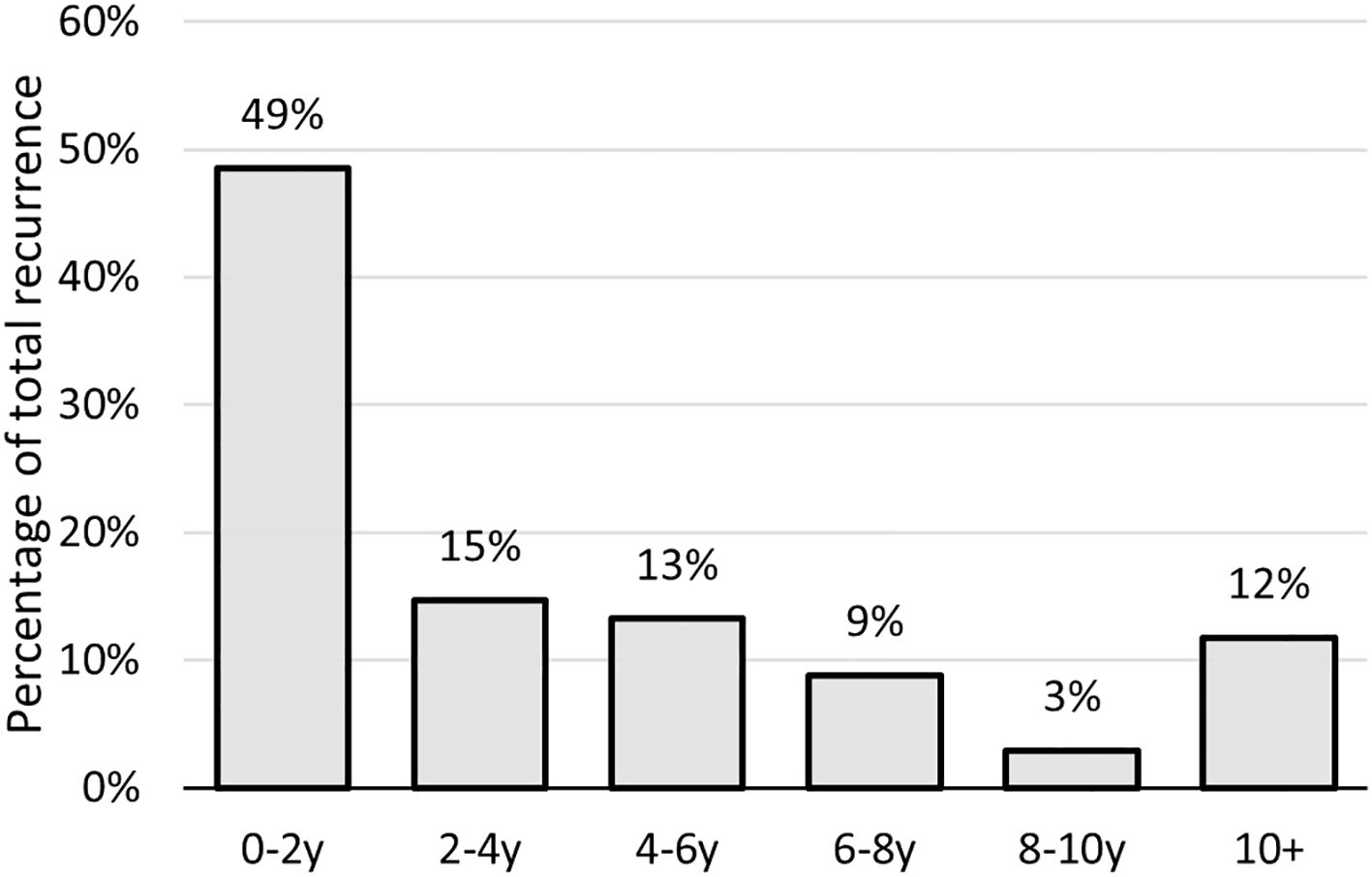
Figure 1. Time to recurrence/second primary of sinonasal ITAC in years after treatment; ITAC, intestinal-type adenocarcinoma.
At the last follow-up, 47.5% of patients were alive, 20.5% had succumbed to the disease and 32.0% to intercurrent illnesses. The 5-year and 10-year overall survival (OS), disease-specific survival (DSS), and local recurrence-free survival (LRFS) rates were analyzed, with the respective rates reported as follows: 5-year OS: 71.4% (± 3.4%), 10-year OS: 48.2% (± 4.1%); 5-year DSS 85.1% (± 2.8%), 10-year DSS: 76.2% (± 3.8%); 5-year LRFS: 55.2% (± 3.7%), 10-year LRFS: 32.2% (± 3.8%).
In univariate analysis, significant differences in OS and DSS were observed among different T-classifications at presentation (Figure 2). Specifically, T1 tumors demonstrated significantly better OS compared to T3 (HR: 2.34 [95% CI: 1.08–5.09], p=.032) and T4 tumors (HR: 3.96 [95% CI: 1.87–8.37], p=.0003), as well as improved DSS compared to T3 (HR: 4.63 [95% CI: 1.02–20.90], p=.047) and T4 tumors (HR: 6.27 [95% CI: 1.41–27.69], p=.016). In terms of LRFS, T1, T2, and T3 tumors showed a more favorable prognosis compared to T4 tumors (p=.027, p<.001, and p=.028, respectively). When categorizing tumors into small (T1–T2) and large (T3–T4) groups, T-classification had a pronounced significant effect on OS (HR: 2.19 [95% CI: 1.48–3.24], p<.0001), DSS (HR: 2.57 [95% CI: 1.37–4.79], p=.0031), and LRFS (HR: 1.61 [95% CI: 1.15–2.27], p=.0061).
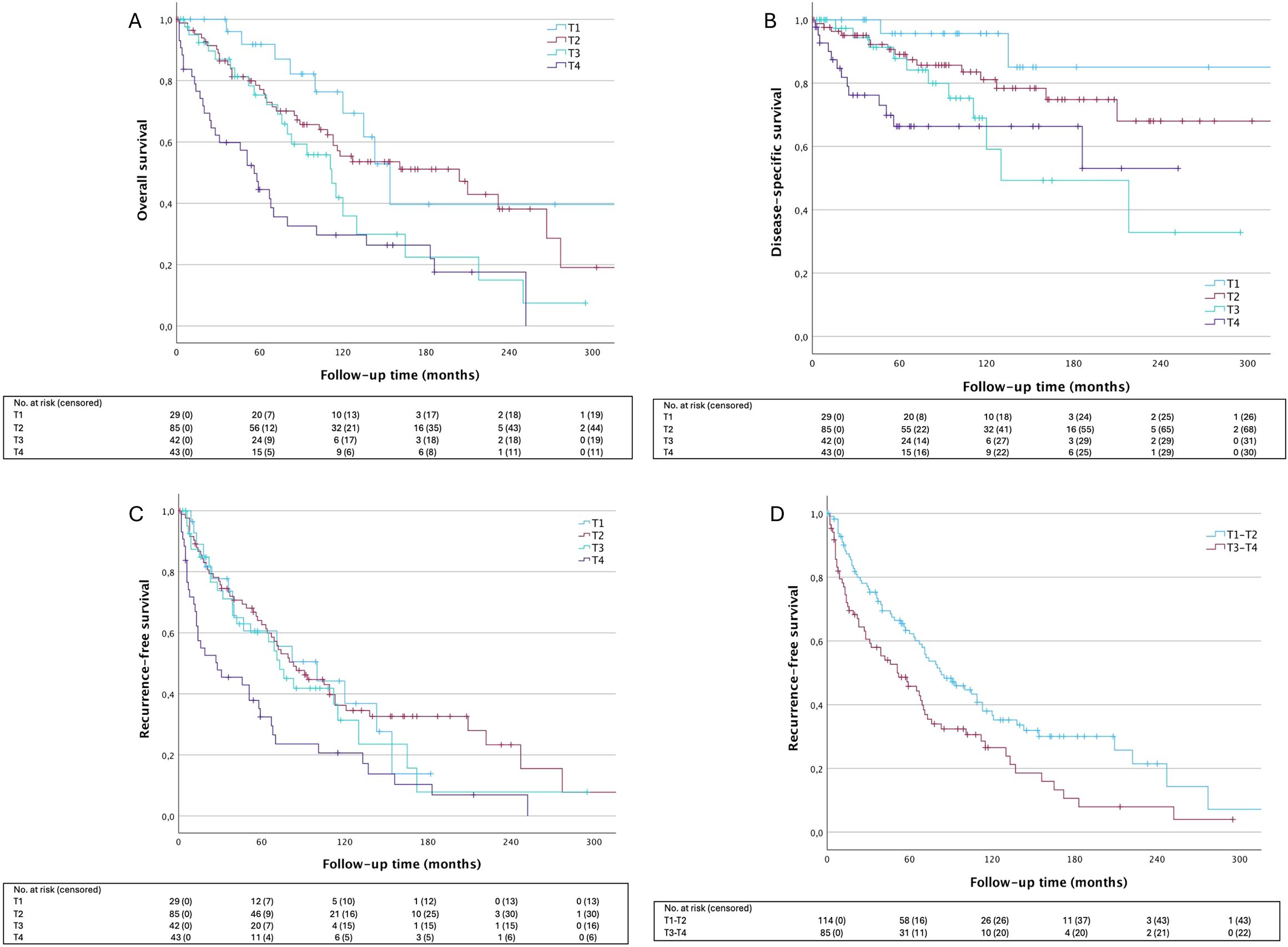
Figure 2. Survival outcomes according to T-lassification. (A) OS HRT2 1.53 [95% CI: 0.74-3.17], p=.249; HRT3 2.34 [95% CI: 1.08-5.09], p=.32; HRT4 3.96 [95% CI:1.87-8.37], p=.0003; (B) DSS HRT2 2.459 [95% CI: 0.56-10.77], p=.2324; HRT3 4.63 [95% CI: 1.02-20.90], p=.0465; HRT4 6.24 [95% CI: 1.41-27.69], p=.0161; (C) LRFS OS HRT2 0.94 [95% CI: 0.53-1.64], p=.8185; HRT3 1.12 [95% CI: 0.60- 2.10], p=.7132; HRT4 2.06 [95% CI: 1.14-3.71], p=.0163; (D) LRFS HRT3-4 1.61 [95% CI: 1.15-2.27], p=.0061. T1 is used as reference for analysis in (A–C), T1-2 is used as reference in (D). OS, overall survival; DDS, disease-specific survival; LRFS, local recurrence-free survival.
Local recurrence had a significant negative impact on OS (HR: 1.96 [95% CI: 1.27–3.02], p=.0023), and DSS (HR: 12.28 [95% CI: 5.59–26.99], p<.0001). An even stronger association was observed between the development of distant metastasis and OS (HR: 11.96 [95% CI: 7.23–19.76], p<.0001) and DSS (HR: 41.17 [95% CI: 21.58–78.55], p<.0001).
Well-differentiated tumors exhibited significantly better OS and DSS compared to poorly differentiated tumors (HR: 2.08 [95% CI: 1.12–3.84], p=.0202 and HR: 3.38 [95% CI: 1.20–9.51], p=.0209, respectively) (Figure 3). Similarly, moderately differentiated tumors had improved OS compared to poorly differentiated ones (p=.048). When analyzing signet-ring differentiation independently, we observed a trend toward worse DSS compared to other poorly differentiated tumors with respective 5-year DSS rates of 67.7% (± 14.0%) and 80.6% (± 8.7%). Nonetheless, this difference did not achieve significance overall (p=.456). No significant differences were seen in the local recurrence rate in relation to the histological grade of differentiation (p=.330), however, distant metastasis occurred more frequently in poorly differentiated tumors (25.6%) compared to moderately differentiated (9.5%) and well-differentiated tumors (2.5%) (p=.006).
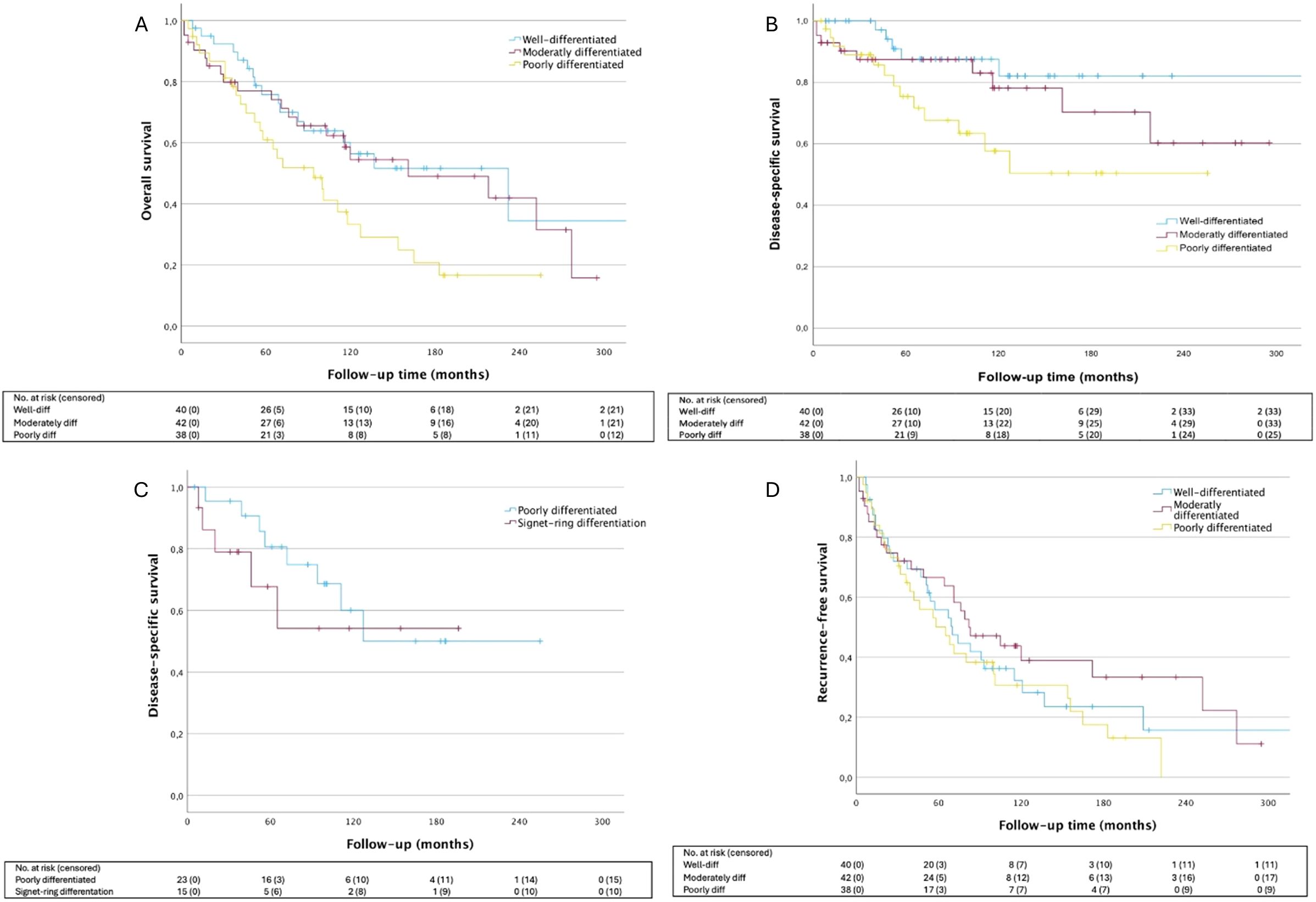
Figure 3. Survival outcomes according to tumor differentiation. (A) OS HRmod 1.16 [95% CI: 0.61-2.22], p=.6497; HRpoor 2.08 [95% CI: 1.12-3.84], p=.0202; (B) DSS HRmod 1.80 [95% CI: 0.60-5.39], p=.2940; HRpoor 3.38 [95%CI: 1.20-9.51, p=.0209; (C) DSS HRpoor 2.96 [95% CI: 0.97-9.05], p=.0575; HRsign 4.42[95% CI: 1.27-15.39], p=.0197; (D) LRFS HRmod 0.78 [95% CI: 0.46-1.35], p=.3802; HRpoor 1.18 [95% CI: 0.70-1.99], p=.5351. Well-differentiated tumor group is used as the reference for all analysis. OS, overall survival; DSS, disease-specific survival; LRFS, local recurrence-free survival.
Moreover, the status of the cribriform plate was investigated regarding resection margins. Tumor involvement at this site was associated with reduced OS (HR: 2.24 [95% CI: 1.49–3.38], p=.0001), DSS (HR: 3.04 [95% CI: 1.60–5.78], p=.0007), and LFRS (HR: 1.752 [95% CI: 1.22–2.51], p=.0023), and a greater need for salvage surgical procedures.
In terms of treatment-related factors, we found that patients who underwent a non-radical resection followed by radiotherapy had lower OS (HR: 1.89 [95% CI: 1.00–3.58], p=.0491), DSS (HR: 2.95 [95% CI: 1.29–6.74], p=.0106), and LRFS (HR: 2.15 [95% CI: 1.26–3.67], p=.0051) compared to patients with negative section margins and adjuvant radiotherapy. Distant metastasis was also more frequent in this group (p=.019). Conversely, our survival analysis could not demonstrate a significant difference regarding OS, DSS, or LRFS between patients who previously underwent an incomplete resection in a regional hospital before being referred to our department for completion of endoscopic surgery.
OS is described by a multivariable model in which independent predictors include age at diagnosis, T-status, and tumor differentiation (Table 2). For DSS, after adjusting for other variables, independent prognostic factors include pooled tumor size (small, T1-T2, versus large tumors, T3-T4) and positive surgical margins. Age at diagnosis, T-status, and positive surgical margins were identified as independent predictors for LRFS.
The majority of patients (93.5%) received postoperative RT (60 or 66Gy in most cases). One patient received pre-operative RT at a regional hospital before referral and two patients were treated with adjuvant concomitant chemoradiotherapy, one because of bilateral neck involvement (N2c) and one because of synchronous esophageal cancer. When examining the patients who did not receive radiotherapy, we noticed an increased local recurrence rate of 38.5% compared to 34.1% in the surgery plus radiotherapy group (p=.059), despite a selection bias of more extended primary tumors being selected for postoperative RT.
4 Discussion
Previous research demonstrated favorable 5-year DSS and LRFS for endoscopically treated ITAC (11, 13, 21–23). Our findings, indicating a 5-year DSS of 85.1% and a 5-year LRFS of 55.2%, align closely with these established reports. Whereas previously we could not find a statistically significant influence of T-classification (18), the present study, through multivariable analysis, establishes T-classification as an independent prognostic factor for OS, DSS, and LRFS.
The literature has identified pathological prognostic factors. The presence of signet-ring cells has been shown to correlate with unfavorable prognosis and overall poorly differentiated tumors are associated with shorter recurrence-free survival compared to well- and moderately differentiated tumors and are prone to distant relapse (13, 21, 24–26). Indeed, our results confirm that poorly differentiated tumors have a trend toward a reduced OS and DSS compared to well-differentiated tumors. After adjusting for other variables, tumor differentiation remained a significant predictor of OS. Although not reaching statistical significance, the signet-ring cell subtype appeared to be associated with the poorest DSS among poorly differentiated tumors. Distant metastasis occurred most frequently in poorly differentiated tumors. Among the 15 patients with metastatic disease and documented differentiation status, 10 were poorly differentiated, 4 were moderately differentiated, and 1 was well-differentiated. Conversely, contrary to what has been reported prior to this study (21), no difference in local recurrence was observed between histological subgroups in our cohort.
Lastly, we examined treatment-related factors. Positive margins have been demonstrated to be a poor prognostic factor for local recurrence (11, 13). This finding was confirmed in our study with 5-year LRFS dropping from 89.0% (± 2.6%) to 61.2% (± 14.8%) in case of positive tumor margin status. For DSS and LRFS, incomplete resection before adjuvant radiotherapy was independently associated with a worse prognosis. Cribriform plate involvement, a common contributor to positive margins, is a well-established prognostic factor in sinonasal cancer. Numerous studies have demonstrated its association with reduced survival and higher recurrence rates (18, 23, 27). This is likely due to the inherent difficulties of achieving clear resection margins in this anatomically complex region, which significantly elevates the risk of local recurrence, a finding corroborated by the results of our study. Studies published over the last two decades have demonstrated the feasibility and efficacy of more radical strategies, such as the endoscopic endonasal transcribriform approach (EETA), for achieving clear margins in locally advanced sinonasal malignancies. EETA extends surgery to the anterior skull base, enabling precise en bloc resections and reducing the risk of microscopic residual disease (10, 28, 29). Although the detailed evaluation of EETA is beyond the scope of this paper, our findings underline the pressing need for innovative approaches to improve the radicality of surgery in patients with cribriform plate involvement. Ensuring negative margins before adjuvant therapy is crucial and therefore treatment of ITAC should be reserved for centers of excellence capable of performing extensive resections when needed.
We analyzed data from 29 patients who initially underwent incomplete resection at a regional hospital before being referred to our center for completion surgery. In these patients, unilateral polyps were identified as benign pathology, and conventional endoscopic surgery was performed. Following the pathology reports indicating malignancy, patients were referred for further treatment. This subgroup did not exhibit significantly inferior outcomes in terms of OS and DSS compared to the 171 patients initially treated at our tertiary center, and contrary to what we demonstrated in our previous research LRFS is also not affected. Thus, we were unable to demonstrate the negative impact of possible transtumoral dissection and tumor seeding in the sinonasal tract. This association remained non-significant even after adjustment in the multivariable analysis. Possible explanations for this could be the lack of statistical power in our analysis due to a small number of patients in the referral group.
The role of adjuvant radiotherapy for ITAC has been a topic of discussion. The existing evidence advocates for adjuvant radiotherapy in large tumors (T3-T4), positive margins, and poor histological differentiation (17). At our center, adjuvant radiotherapy was generally recommended for most patients by the multidisciplinary Head and Neck tumor board. Only 13 patients did not undergo radiation, mostly because of advanced age or co-morbidities rather than T- or margin status. Consequently, no pertinent conclusions could be drawn as to the contribution of postoperative radiotherapy to survival. Nonetheless, a trend toward increased local recurrence was observed in the non-radiation group, without impacting overall or disease-specific survival. Further research is warranted to elucidate this matter and derive relevant conclusions.
We found great heterogeneity in the time of local recurrence in our cohort. Although roughly half of the recurrences occurred within the first 2 years after treatment, more than 10% were diagnosed more than a decade after the initial follow-up period. This finding underscores that late recurrences or second primaries are not uncommon, highlighting the need for extended and vigilant long-term monitoring of patients. In our study population, local recurrence significantly affected overall survival.
The tendency of metastatic expansion is well documented in ITAC. Our data reveals that 10.0% of patients develop metastasis during the follow-up period, with a median duration from initial diagnosis to detection of distant disease being only 20.5 months. This finding could argue for more surveillance during the follow-up period, perhaps tailored to the differentiation status of the primary tumor. Moreover, based on our data, additional risk factors for metastasis include advanced-stage tumors and positive margins. In this context, it might be advisable to implement a customized surveillance plan that is adjusted to the specific characteristics of the tumor and treatment, rather than adopting a one-size-fits-all approach. The impact of this on survival may be limited, however.
The study is subject to several limitations, including the retrospective collection of data, the exclusion of inoperable patients, the prolonged duration of inclusion, and the potential classification bias due to the extended follow-up period.
5 Conclusion
In conclusion, our study focused exclusively on patients diagnosed with ITAC who underwent endoscopic treatment followed by radiotherapy. Our findings align closely with previous reports regarding favorable 5-year OS, DSS, and LRFS for endoscopically treated ITAC. Notably, we observed reduced OS and DSS for poorly differentiated tumors, with the signet-ring cell subtype showing a trend toward the poorest DSS. Moreover, poorly differentiated tumors exhibited a higher incidence of metastatic disease. Positive margins are a poor prognostic factor for local recurrence and a risk factor for distant metastasis. Ensuring negative margins should set the benchmark for surgical best practices. Nearly half of all local recurrences occur within the first two years of follow-up, but since over 10% occur after 10 years, lifelong follow-up is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board (or Ethics Committee) of UZ Leuven (protocol code S64142). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study protocol was a retrospective study.
Author contributions
Q-AP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. MDW: Data curation, Writing – original draft, Writing – review & editing. EH: Supervision, Writing – original draft, Writing – review & editing. PC: Supervision, Writing – original draft, Writing – review & editing. RH: Supervision, Writing – original draft, Writing – review & editing. TD: Supervision, Writing – original draft, Writing – review & editing. JVL: Supervision, Writing – original draft, Writing – review & editing. SN: Supervision, Validation, Writing – original draft, Writing – review & editing. MJ: Supervision, Validation, Writing – original draft, Writing – review & editing. VVP: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. LVG: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. LVG was supported by Research Foundation Flanders (FWO): Senior Clinical Investigator Fellowship 18B2222N. VVP disclosed financial support for the publication of this article from the Walter Vandeputte Head and Neck Cancer Fund (KU Leuven, Leuven, Belgium).
Acknowledgments
Part of this data was presented at the 2024 EUSICA meeting in London, UK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, et al. Cancer incidence in five continents. Vol. XI (2021). Lyon, France: International Agency for Research on Cancer (IARC).
2. Belgian Cancer Registry. Cancer burden in Belgium 2004-2017. (2020). Brussels, Belgium: Belgian Cancer Registry
3. Acheson ED, Hadfield EH, Macbeth RG. Carcinoma of the nasal cavity and accessory sinuses in woodworkers. Lancet. (1967) 289:311–2. doi: 10.1016/S0140-6736(67)91243-3
4. Kleinsasser O, Schroeder H. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otolaryngol - Head Neck Surgery. (1988) 245:1–15. doi: 10.1007/BF00463541
5. Vander Poorten V, Jorissen M. A comprehensive update on intestinal- and non-intestinal-type adenocarcinomas. Adv Otorhinolaryngol. (2020) 84:137–53. doi: 10.1159/000457934
6. Rampinelli V, Ferrari M, Nicolai P. Intestinal-type adenocarcinoma of the sinonasal tract: an update. Curr Opin Otolaryngol Head Neck Surg. (2018) 26:115–21. doi: 10.1097/MOO.0000000000000445
7. Roxbury CR, Ishii M, Richmon JD, Blitz AM, Reh DD, Gallia GL. Endonasal endoscopic surgery in the management of sinonasal and anterior skull base Malignancies. Head Neck Pathol. (2016) 10:13–22. doi: 10.1007/s12105-016-0687-8
8. Goffart Y, Jorissen M, Daele J, Vander Poorten V, Born J, Deneufbourg JM, et al. Minimally invasive endoscopic management of Malignant sinonasal tumours. Acta Otorhinolaryngol Belg. (2000) 54:221–32.
9. Ferrari M, Mattavelli D, Tomasoni M, Raffetti E, Bossi P, Schreiber A, et al. The MUSES∗: a prognostic study on 1360 patients with sinonasal cancer undergoing endoscopic surgery-based treatment. Eur J Cancer. (2022) 171:161–82. doi: 10.1016/j.ejca.2022.05.010
10. Castelnuovo P, Turri-Zanoni M, Battaglia P, Antognoni P, Bossi P, Locatelli D. Sinonasal Malignancies of anterior skull base: histology-driven treatment strategies. Otolaryngol Clin North Am. (2016) 49:183–200. doi: 10.1016/j.otc.2015.09.012
11. Nicolai P, Schreiber A, Bolzoni Villaret A, Lombardi D, Morassi L, Raffetti E, et al. Intestinal type adenocarcinoma of the ethmoid: Outcomes of a treatment regimen based on endoscopic surgery with or without radiotherapy. Head Neck. (2016) 38: E996–E1003. doi: 10.1002/hed.v38.S1
12. Nicolai P, Battaglia P, Bignami M, Villaret AB, Delù G, Khrais T, et al. Endoscopic surgery for Malignant tumors of the sinonasal tract and adjacent skull base: A 10-year experience. Am J Rhinol. (2008) 22:308–16. doi: 10.2500/ajr.2008.22.3170
13. de Gabory L, Waubant A, Verillaud B, Michel J, Malard O, Rumeau C, et al. Multicenter study to assess surgical treatments of 452 sinonasal intestinal-type adenocarcinomas: A REFCOR study. Eur J Surg Oncol. (2023) 49:39–46. doi: 10.1016/j.ejso.2022.07.021
14. Vermassen T, De Keukeleire S, Saerens M, Heerwegh S, Debacker JM, Huvenne W, et al. Choice of surgery in intestinal-type adenocarcinoma of the sinonasal tract: a long-term comparative study. Eur Arch Oto-Rhino-Laryngology. (2024) 281(6):2993–3004. doi: 10.1007/s00405-024-08447-w
15. Dirix P, Vanstraelen B, Jorissen M, Vander Poorten V, Nuyts S. Intensity-modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiat OncologyBiologyPhysics. (2010) 78:998–1004. doi: 10.1016/j.ijrobp.2009.09.067
16. Kuan EC, Wang EW, Adappa ND, Beswick DM, London NR, Su SY, et al. International consensus statement on allergy and rhinology: sinonasal tumors. Int Forum Allergy Rhinol. (2024) 14:149–608. doi: 10.1002/alr.23262
17. Van Gerven L, Jorissen M, Nuyts S, Hermans R, Vander Poorten V. Long–term follow-up of 44 patients with adenocarcinoma of the nasal cavity and sinuses primarily treated with endoscopic resection followed by radiotherapy. Head Neck. (2011) 33:898–904. doi: 10.1002/hed.21556
18. Camp S, Van Gerven L, Vander PV, Nuyts S, Hermans R, Hauben E, et al. Long-term follow-up of 123 patients with adenocarcinoma of the sinonasal tract treated with endoscopic resection and postoperative radiation therapy. Head Neck. (2016) 38:294–300. doi: 10.1002/hed.23900
19. Brierley J, Gospodarowicz M, Wittekind Ch. The TNM classification of Malignant tumours. 8th Edition. Oxford, England: Wiley (2017).
20. Dirix P, Nuyts S, Geussens Y, Jorissen M, Vander Poorten V, Fossion E, et al. Malignancies of the nasal cavity and paranasal sinuses: long-term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiat OncologyBiologyPhysics. (2007) 69:1042–50. doi: 10.1016/j.ijrobp.2007.04.044
21. Turri-Zanoni M, Battaglia P, Lambertoni A, Giovannardi M, Schreiber A, Volpi L, et al. Treatment strategies for primary early-stage sinonasal adenocarcinoma: A retrospective bi-institutional case-control study. J Surg Oncol. (2015) 112:561–7. doi: 10.1002/jso.24038
22. Vergez S, du Mayne MD, Coste A, Gallet P, Jankowski R, Dufour X, et al. Multicenter study to assess endoscopic resection of 159 sinonasal adenocarcinomas. Ann Surg Oncol. (2014) 21:1384–90. doi: 10.1245/s10434-013-3385-8
23. Russel A, Nguyen DT, Vigouroux C, Gallet P, Vignaud J, Rumeau C, et al. Compartmentalized endoscopic resection of the olfactory cleft for nasal intestinal adenocarcinomas. Head Neck. (2018) 40:2389–98. doi: 10.1002/hed.v40.11
24. Nicolai P, Villaret AB, Bottazzoli M, Rossi E, Valsecchi MG. Ethmoid Adenocarcinoma—From Craniofacial to Endoscopic Resections: a single-institution experience over 25 years. Otolaryngology–Head Neck Surgery. (2011) 145:330–7. doi: 10.1177/0194599811403873
25. Castillo C, Buob D, Mortuaire G, Chevalier D, Aubert S, Copin MC, et al. Signet-ring cell adenocarcinoma of sinonasal tract: an immunohistochemical study of the mucins profile. Arch Pathol Lab Med. (2007) 131:961–4. doi: 10.5858/2007-131-961-SCAOST
26. Franchi A, Miligi L, Palomba A, Giovannetti L, Santucci M. Sinonasal carcinomas: Recent advances in molecular and phenotypic characterization and their clinical implications. Crit Rev Oncol Hematol. (2011) 79:265–77. doi: 10.1016/j.critrevonc.2010.08.002
27. Arosio AD, Bernasconi DP, Valsecchi MG, Pacifico C, Battaglia P, Bignami M, et al. Patterns of recurrences in sinonasal cancers undergoing an endoscopic surgery-based treatment: Results of the MUSES* on 940 patients. Oral Oncol. (2022) 134:106123. doi: 10.1016/j.oraloncology.2022.106123
28. Schreiber A, Ferrari M, Mattavelli D, Rampinelli V, Taboni S, Vural A, et al. Unilateral endoscopic resection with transnasal craniectomy for sinonasal intestinal-type adenocarcinoma: A bi-institutional case-control study on 54 patients. Oral Oncol. (2018) 87:89–96. doi: 10.1016/j.oraloncology.2018.10.027
Keywords: sinonasal tumor, intestinal-type adenocarcinoma, endoscopic sinus surgery, skull base, radiotherapy
Citation: Parys Q-A, De Witte M, Hauben E, Clement PM, Hermans R, Decramer T, van Loon J, Nuyts S, Jorissen M, Vander Poorten V and Van Gerven L (2025) Long-term outcomes of endoscopic resection and tailored adjuvant radiotherapy for sinonasal intestinal-type adenocarcinoma: a historical single-center cohort study in 200 patients. Front. Oncol. 15:1522113. doi: 10.3389/fonc.2025.1522113
Received: 03 November 2024; Accepted: 10 March 2025;
Published: 28 March 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Marco Ferrari, University of Padua, ItalyMartin Leu, University Medical Center Göttingen, Germany
Copyright © 2025 Parys, De Witte, Hauben, Clement, Hermans, Decramer, van Loon, Nuyts, Jorissen, Vander Poorten and Van Gerven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Van Gerven, bGF1cmEudmFuZ2VydmVuQHV6bGV1dmVuLmJl
†These authors share senior authorship
‡ORCID: Quentin-Alexandre Parys, orcid.org/0000-0001-6209-4266
Matthias De Witte, orcid.org/0009-0006-7737-9997
Esther Hauben, orcid.org/0000-0001-8688-9231
Paul M. Clement, orcid.org/0000-0001-7600-0806
Robert Hermans, orcid.org/0000-0002-9935-5543
Thomas Decramer, orcid.org/0000-0002-2585-581X
Johannes van Loon, orcid.org/0000-0002-4684-0051
Sandra Nuyts, orcid.org/0000-0002-5540-4796
Mark Jorissen, orcid.org/0000-0002-1719-5989
Vincent Vander Poorten, orcid.org/0000-0003-1341-829X
Laura Van Gerven, orcid.org/0000-0002-5325-7956
 Quentin-Alexandre Parys
Quentin-Alexandre Parys Matthias De Witte
Matthias De Witte Esther Hauben2‡
Esther Hauben2‡ Paul M. Clement
Paul M. Clement Robert Hermans
Robert Hermans Johannes van Loon
Johannes van Loon Sandra Nuyts
Sandra Nuyts Mark Jorissen
Mark Jorissen Vincent Vander Poorten
Vincent Vander Poorten Laura Van Gerven
Laura Van Gerven