- 1Department of Pathology, Yaan People’s Hospital, Yaan, Sichuan, China
- 2Department of Pathology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 3School of Basic Medical Sciences, Southwest Medical University, Luzhou, Sichuan, China
- 4School of Clinical Medical Sciences, Southwest Medical University, Luzhou, Sichuan, China
Background: The expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) in residual lesions may be different compared with primary tumors of the breast after neoadjuvant therapy (NAT). Given the clinical implications of hormone receptor expression for breast cancer management, we assessed conversions in ER, PR, and HER2 in breast cancer patients after NAT.
Methods: Our study comprised 589 individuals with aggressive breast cancer who underwent NAT. We examined the ER, PR, and HER2 statuses in primary and residual breast cancers and investigated the relationship between receptor conversion and clinicopathological variables.
Results: The pathologic complete response (pCR) rate for the overall cohort was 38.7%, with pCR rates of 57.0%, 13.1%, and 33.3% for HER2-positive, Luminal, and triple-negative breast cancer (TNBC), respectively. Cases with negative hormone receptor expression were more likely to achieve pCR than positive cases. The highest pCR rates were seen in HER2-positive breast cancers, followed by HER2-zero and HER2-low tumors. After NAT, there were 26 (7.8%) cases of ER status conversion and 53 (16.0%) cases of PR status conversion. The conversion of hormone receptors was mainly from positive to negative. When cases were categorized as HER2-negative or positive, 15 (5.1%) cases had a conversion of HER2 status, predominantly positive to negative. When cases were classified as HER2-zero, -low, or -positive, HER2 status conversion happened in 54 (18.6%) cases and was mostly happened between HER2-zero and HER2-low. HER2 status before NAT correlated with ER and HER2 conversion.
Conclusion: Some breast cancer patients may show ER, PR, or HER2 status conversion after NAT. Residual lesions need to be immunohistochemically re-tested to reassess the patient’s receptor expression status and to adjust the subsequent treatment regimen.
1 Introduction
According to data released by the International Agency for Research on Cancer, approximately 2.3 million new cases of breast cancer were reported globally in 2022, ranking first in the incidence of female malignant tumors (1). Breast cancer is a systemic disease that is usually treated with surgery, radiotherapy, chemotherapy, immunotherapy, endocrine therapy, and targeted therapy (2). Neoadjuvant therapy (NAT) is a systemic adjuvant therapy administered prior to surgical resection of the neoplasm that aims to reduce the size of the primary lesion, decrease axillary staging, improve breast retention, determine drug sensitivity, and guide subsequent treatment and prognostic analysis (3).
Prior to NAT, ultrasound-guided core needle biopsy (CNB) is commonly used to obtain the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 in the lesions of patients with breast cancer. Based on receptor expression, breast cancers are classified into the following four molecular subtypes: Luminal A, Luminal B, HER2-overexpressing, and triple-negative breast cancers (TNBC) (4). The pathologic complete response (pCR) rate is a major factor in response to NAT efficacy, and pCR is used as an early surrogate endpoint for predicting overall survival (OS) (5). Breast cancer patients who achieve pCR after NAT have a better prognosis (6). With advances in technology and medicine, systemic therapy has been developed, and the percentage of patients with pCR after NAT has increased significantly (7). However, some patients still have residual lesions after NAT. Determining the molecular subtype of the remaining tumor foci following NAT in non-pCR patients is crucial for prognosis and treatment regimen optimization (8).
Owing to the highly heterogeneous nature of breast cancer, preoperative CNB may not fully reveal the true nature of the neoplasm. ER, PR, and HER2 expression in residual lesions may be different compared with the primary tumors after NAT (9–11). In the subset of patients with receptor status conversion, diagnostic strategies and the effect on prognosis are controversial. Receptor conversion has a prognostic value and may influence clinicians’ therapeutic decisions. As a result, this study retrospectively examined variations in ER, PR, and HER2 expression in 589 breast cancer patients treated with NAT to assess the effect of NAT on the status of these biomarkers and to investigate the clinicopathological causes of these variations.
2 Materials and methods
2.1 Patient selection
Clinical and pathological data on invasive breast cancer patients treated at the Southwest Medical University Affiliated Hospital in Sichuan Province between January 2019 and June 2023 were collected. Inclusion criteria included female breast cancer patients with primary invasive cancer diagnosed by CNB prior to treatment, with at least four cycles of NAT and complete clinical and pathological data. Male patients or those with bilateral breast cancer, radiological evidence of distant metastases, a history of additional malignancies, prior endocrine treatment, tumor-targeted radiation, or local excision were excluded from the study. pCR is defined as the absence of residual invasive cancer in the completely resected breast specimen and all sampled regional lymph nodes (ypT0/Tis ypN0 according to AJCC staging criteria) (12).
2.2 Immunohistochemistry staining interpretation and grouping standards
Immunohistochemistry (IHC) was performed to assess ER, PR, HER2, and Ki-67 status in pre-treatment CNB and surgical resection specimens. IHC were detected using the Roche Ventana Benchmark automated IHC system. The detection of ER, PR, and HER2 was performed using antibodies from Roche (ER clone: SP1; PR clone: 1E2; HER2 clone: 4B5). The Ki-67 antibody (clone: MIB.I) was provided by Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. ER/PR tumor cell nuclear staining <1% indicated ER/PR negativity and ≥1% indicated positivity. Hormone receptor (HR) positivity was defined as ER or PR positivity (13).
. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines (14), HER2 IHC staining results were graded as follows: HER2 0: no staining or ≤10% of invasive carcinoma cells show incomplete, weak cell membrane staining; HER2 1+: >10% of invasive carcinoma cells show incomplete, weak cell membrane staining; HER2 2+: >10% of invasive carcinoma cells show complete, weak to moderately intense cell membrane staining or ≤10% of invasive carcinoma cells show strong and complete cell membrane staining; and HER2 3+: >10% of invasive cancer cells show strong, complete, and uniform cell membrane staining. Fluorescence in situ hybridization (FISH) was performed to detect HER2 gene amplification in IHC 2+ tumors, utilizing the HER2 gene amplification kit from Beijing Jinpujia Company.HER2-positive was defined as IHC 3+ or IHC 2+ with FISH amplification. HER2-low was defined as IHC 1+ or IHC 2+ without FISH amplification, and HER2-zero tumors were IHC 0. HER2-negative comprised HER2-zero and HER2-low tumors. The percentage of tumor nuclei stained in the examined IHC sections was used to compute the Ki67 expression levels. The International Ki67 in Breast Cancer Working Group consensus is that Ki-67 <5% or >30% can be used to estimate prognosis (15). Therefore, in this study, 15% was used as a threshold to categorize Ki-67 into low expression (Ki-67 <15%) and high expression (Ki-67 ≥15%).
The following categories were applied to the cases based on their IHC status: Luminal (HR-positive and HER2-negative); HER2-overexpressing (HER2-positive, regardless of HR status); and TNBC (HR-negative and HER2-negative).
2.3 Statistical analysis
SPSS 25.0 was used for data processing and analysis. The count data are displayed as the total number of instances and the percentage (%). Pearson’s chi-square test and Fisher’s exact test were used for group comparisons. Cohen’s kappa test was used in the consistency analysis. P < 0.05 was considered statistically significant.
3 Results
3.1 Patient cohorts and clinicopathologic features
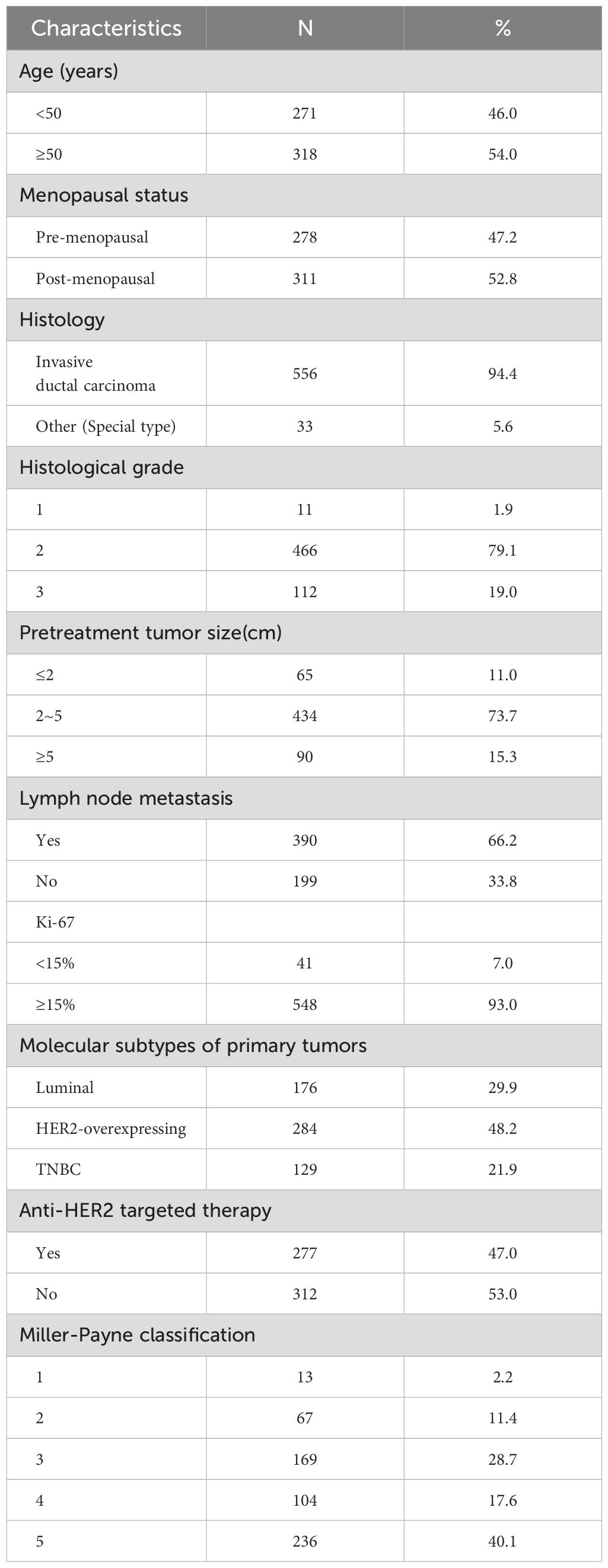
We collected clinical and pathological data from 589 breast cancer patients who underwent NAT. Table 1 illustrates the major clinicopathological characteristics of the entire cohort. The median age was 51 (29–78) years old. In CNB specimens, the majority of patients had invasive ductal carcinoma (n = 556, 94.4%) with a histologic grade of 2 (n = 466, 79.1%). In most patients, the maximum diameter of the tumor before treatment was 2–5 cm (n = 434, 73.7%). The immunophenotypes of all cases before treatment were as follows: Luminal 29.9% (n = 176), HER2-overexpressing 48.2% (n = 284), and TNBC 21.9% (n = 129). Nearly half of the patients received anti-HER2 targeted therapy (n = 277, 47.0%).
3.2 Rate of pCR
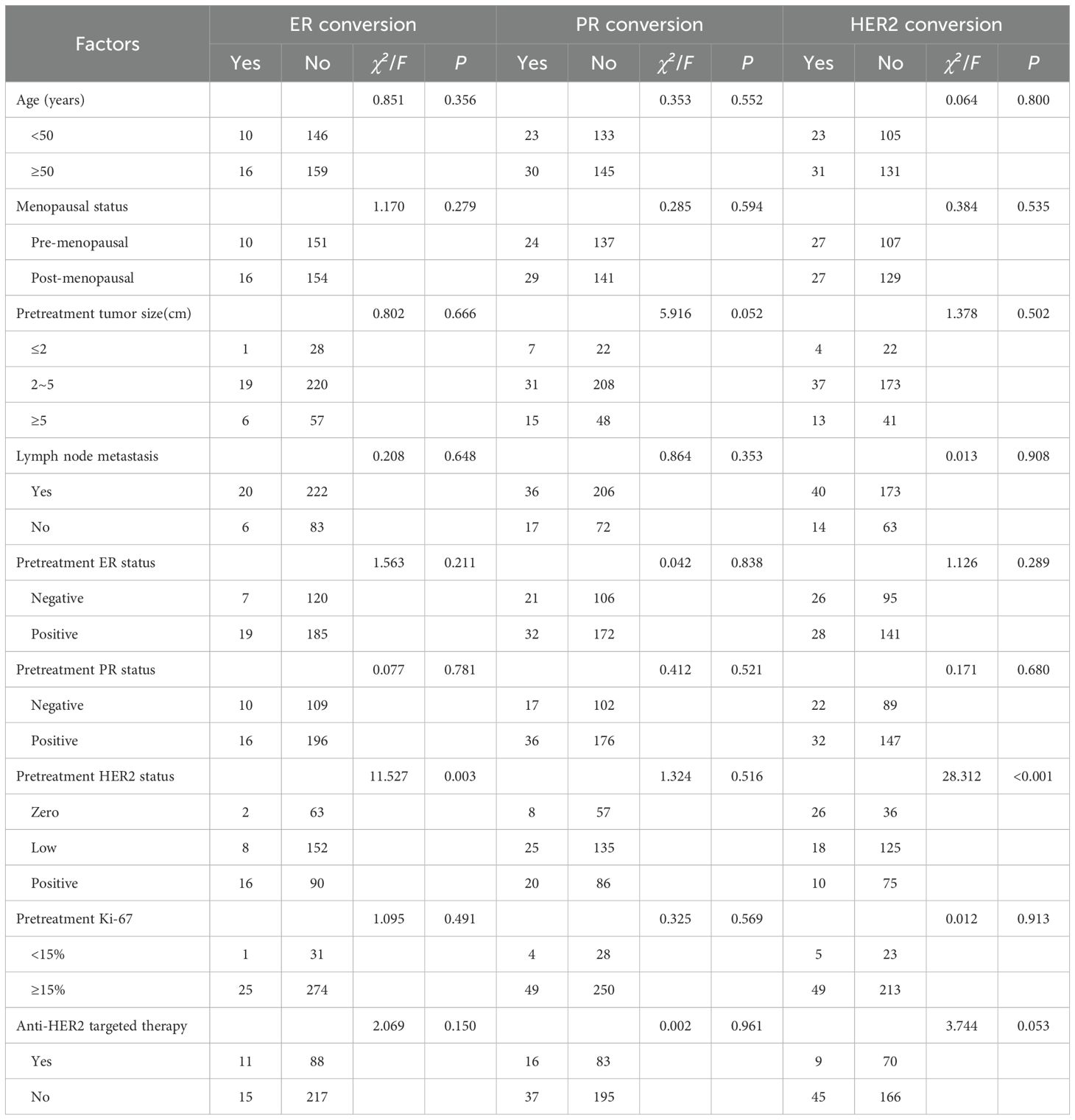
Of the 236 patients with Miller-Payne (MP) grade 5, intravascular thrombus was detected in the specimen after surgery in one patient, and invasive cancer cells were detected in axillary lymph nodes after surgery in seven patients. Therefore, pCR was achieved in 228 cases, and the pCR rate for the overall population was 38.7% (228/589). The rate of pCR was 13.1% (23/176) for Luminal cancer, 57.0% (162/284) for HER2-overexpressing cancer, and 33.3% (43/129) for TNBC (Figure 1A). Thus, HER2-overexpressing tumors had a higher rate of pCR than Luminal or TNBC (P < 0.001). Further stratification of the HER2-overexpressing cohort by HR status revealed that the pCR rate in the HER2+/HR+ group was 50.5% (101/200), significantly lower than the 72.6% (61/84) observed in the HER2+/HR− group, with a statistically significant difference (P < 0.001). HR-negative patients were more likely to achieve pCR than positive cases (pCR rates in ER-negative vs. ER-positive: 49.3% vs. 29.3%, P < 0.001; PR-negative vs. PR-positive: 49.2% vs. 30.2%, P < 0.001; Figure 1B). There was a significant association between HER2 expression and pCR rates. The highest pCR rates were found in HER2-positive breast cancers, followed by tumors with HER2-zero and HER2-low (pCR rates in HER2-positive vs. HER2-zero vs. HER2-low: 57.0% vs. 33.7% vs. 15.4%, P < 0.001; Figure 1C).
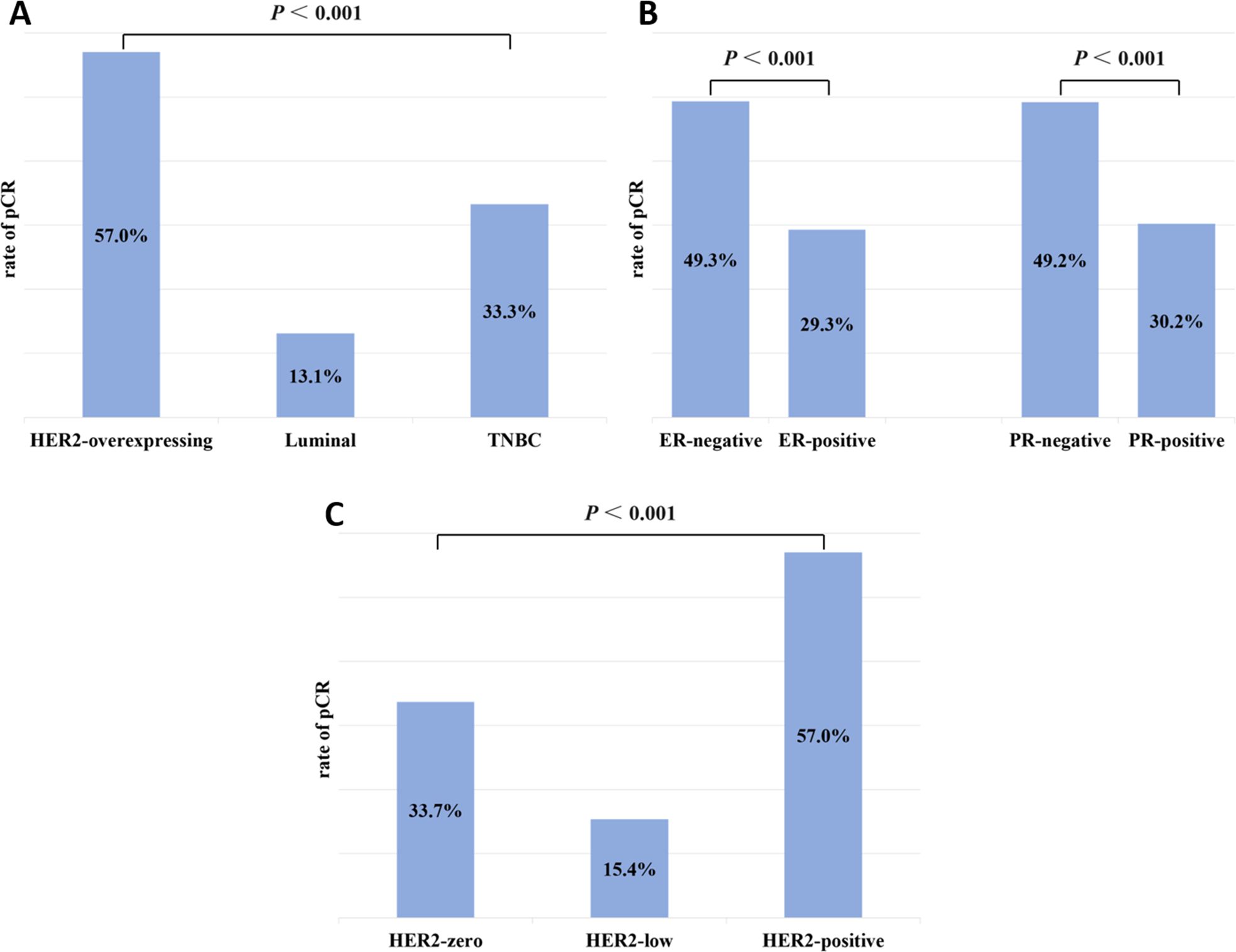
Figure 1. Rates of pCR by (A) subtype (HER2-overexpressing, Luminal, and TNBC); (B) hormone receptor status (ER-negative and ER-positive; PR-negative and PR-positive); and (C) HER2 status (zero, low, and positive).
3.3 Conversion of ER and PR
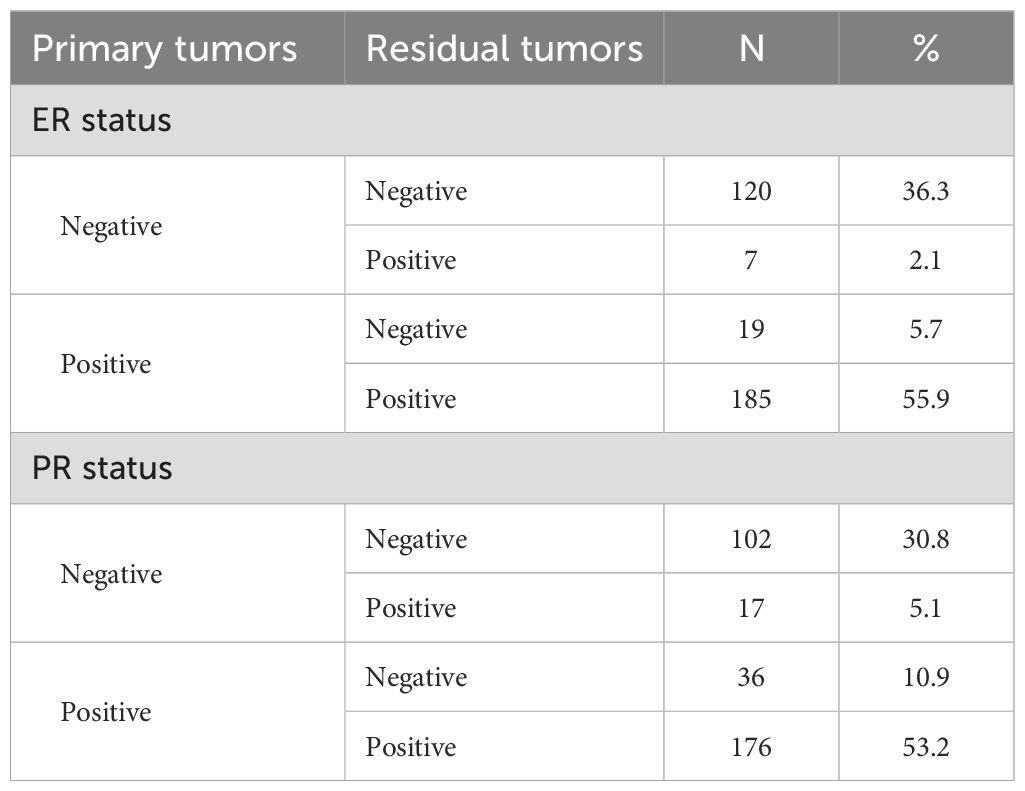
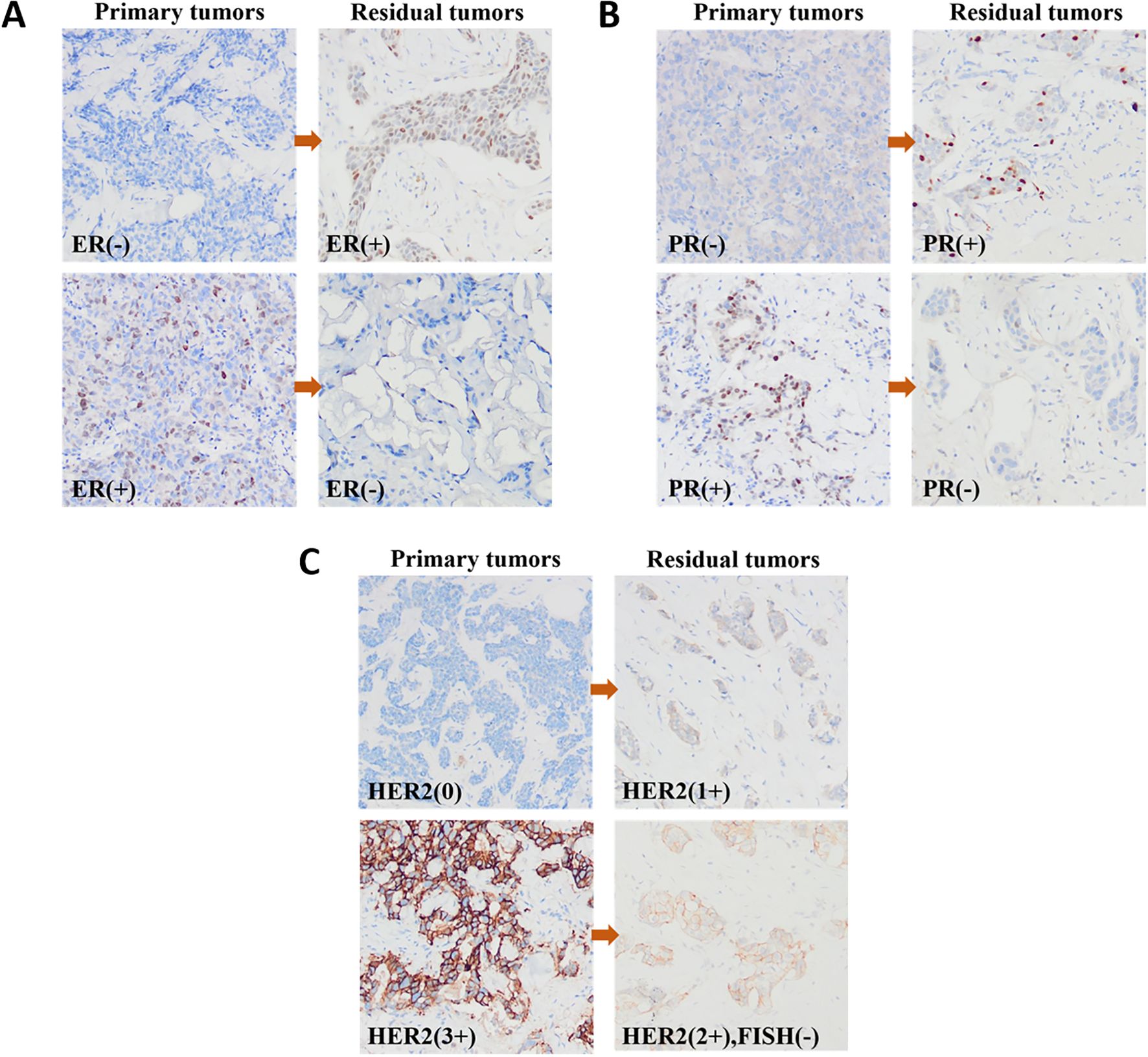
Of the 361 non-pCR patients, 30 patients were not tested for IHC after NAT because of the low number of residual tumor cells. Therefore, 331 patients were included in the evaluation of ER and PR statuses. Table 2 summarizes the conversion of ER and PR statuses of breast cancers from CNB specimens to residual lesions after specimen removal. Figures 2A, B show IHC images of patients with ER and PR conversion.
The primary breast cancer was ER-negative in 127 (38.4%) cases and ER-positive in 204 (61.6%) cases. Among residual lesions, 139 (42.0%) were ER-negative, and 192 (58.0%) were ER-positive. After NAT, 7 (2.1%) ER-negative cases converted to ER-positive, and 19 (5.7%) ER-positive cases converted to ER-negative (Figure 3A). The rate of ER conversion was 7.8% (n = 26), and Cohen’s kappa coefficient was 0.837, indicating that the ER status after NAT was highly consistent with that before treatment.
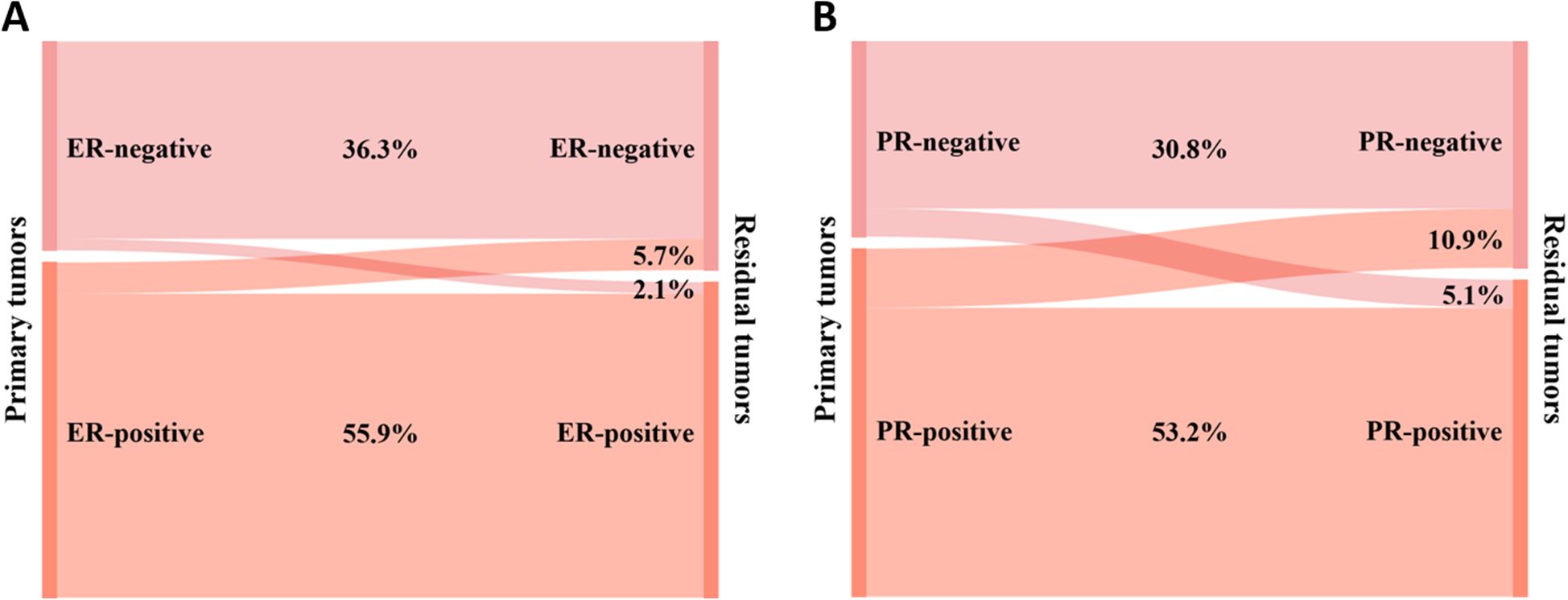
Figure 3. Conversion of hormone receptor expression between primary and residual tumors: (A) ER and (B) PR.
Among primary breast cancers, 119 (36.0%) were PR-negative, and 212 (64.0%) were PR-positive. Among the residual lesions, 138 (41.7%) cases were PR-negative, and 193 (58.3%) cases were PR-positive. As shown in Figure 3B, after NAT, PR-negative converted to PR-positive in 17 (5.1%) cases, and PR-positive converted to PR-negative in 36 (10.9%) cases. The PR conversion rate was 16.0% (n = 53), and Cohen’s kappa coefficient was 0.664, indicating that the consistency of PR status was relatively high.
3.4 Conversion of HER2
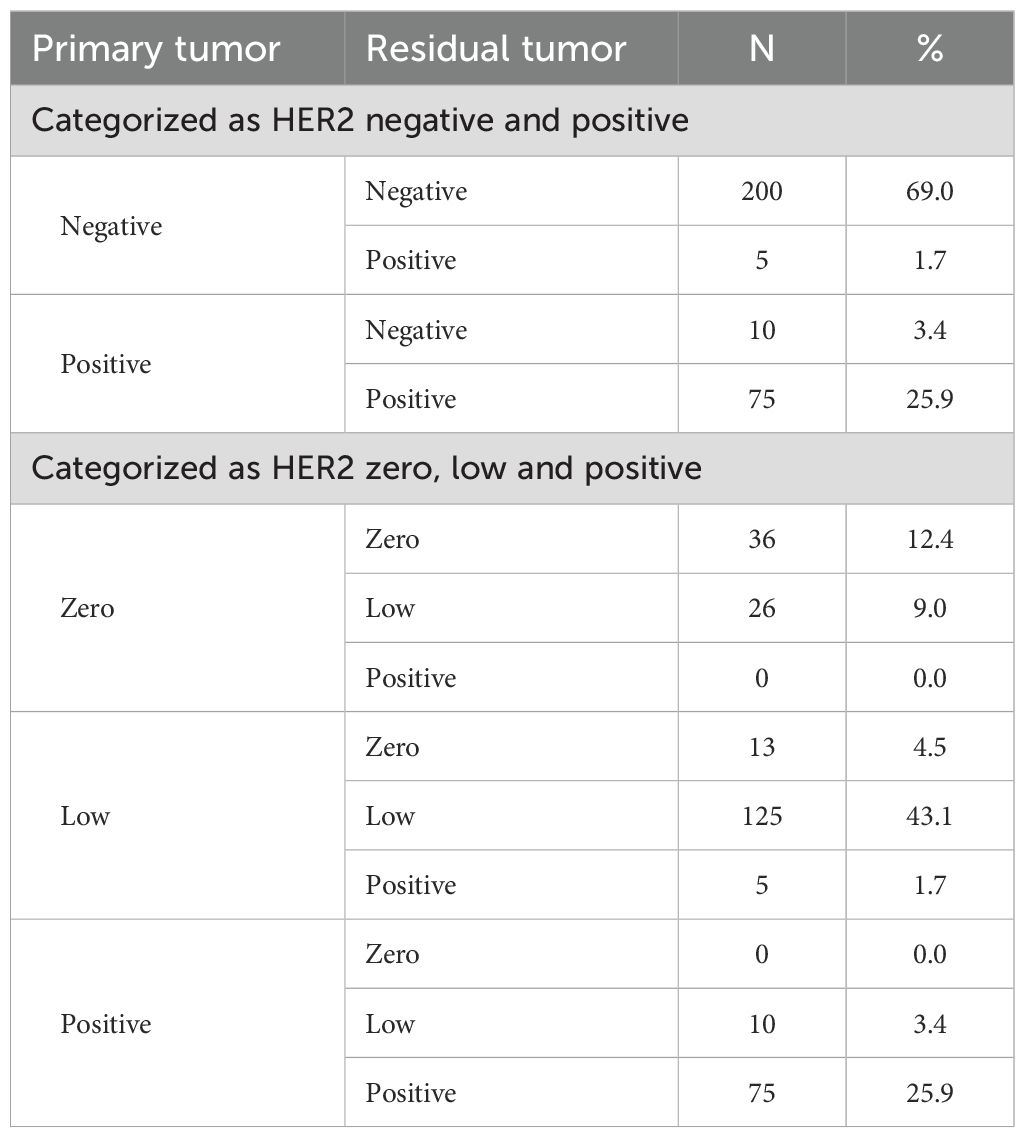
After NAT, 41 patients were HER2 IHC 2+ but did not undergo FISH testing to verify HER2 amplification status. therefore, the assessment of HER2 status included 290 patients. Primary breast cancer was HER2-negative in 205 (70.7%) cases and HER2-positive in 85 (29.3%) cases. HER2-negative cases included 143 (49.3%) HER2-low cases and 62 (21.4%) HER2-zero cases. Residual lesions were HER2-positive in 80 (27.6%) and negative in 210 (72.4%) cases. HER2-negative cases included 161 (55.5%) HER2-low cases and 49 (16.9%) HER2-zero cases. Table 3 summarizes the HER2 status conversion of breast cancers from CNB specimens to residual lesions after specimen removal. Figure 2C shows IHC images of patients with HER2 conversion.
Figure 4A shows the conversion of HER2 status in cases categorized as HER2-positive or HER2-negative. Of the primary breast cancers to residual lesions, HER2-negative converted to HER2-positive in 5 (1.7%) cases, and HER2-positive converted to HER2-negative in 10 (3.4%) cases. The rate of HER2 conversion was 5.1% (n = 15), and Cohen’s kappa coefficient was 0.873, indicating that the HER2 status was highly consistent.
Figure 4. Conversion of HER2 expression between primary and residual tumors: (A) HER2 is categorized as negative or positive; (B) HER2 is categorized as zero, low, or positive.
Figure 4B shows the conversion of HER2 status in cases categorized as HER2-zero, HER2-low, or HER2-positive. After NAT, HER2-zero converted to HER2-low in 26 (9.0%) cases; HER2-low converted to HER2-zero in 13 (4.5%) cases, and HER2-low converted to HER2-positive in 5 (1.7%) cases; HER2-positive was converted to HER2-low in 10 (3.4%) cases; and no conversion was seen between HER2-zero and HER2-positive. The rate of HER2 conversion was 18.6% (n = 54), and Cohen’s kappa coefficient was 0.694, indicating that the consistency of HER2 status was relatively high.
There were 205 HER2-negative patients before NAT, comprising 129 (62.9%) with Luminal cancer and 76 (37.1%) with TNBC. Figure 5 depicts the conversion of HER2 expression in the HER2-negative group based on the breast cancer phenotype. 24 (18.6%) patients with primary breast cancer of the Luminal type had a conversion of the HER2 status of the residual disease (Figure 5A). Of these, 14 (10.9%) converted from HER2-zero to HER2-low, 7 (5.4%) converted from HER2-low to HER2-zero, and 3 (2.3%) converted from HER2-low to HER2-positive. As shown in Figure 5B, 20 (26.3%) patients whose primary breast cancer was TNBC showed HER2 status conversion in residual tumors. 12 (15.8%) HER2-zero patients converted to HER2-low, 6 (7.9%) HER2-low patients converted to HER2-zero, and 2 (2.6%) HER2-low patients converted to HER2-positive. Patients with TNBC had a higher rate of HER2 conversion than those with the Luminal type; however, the difference was not statistically significant (χ2 = 1.687, P = 0.194).
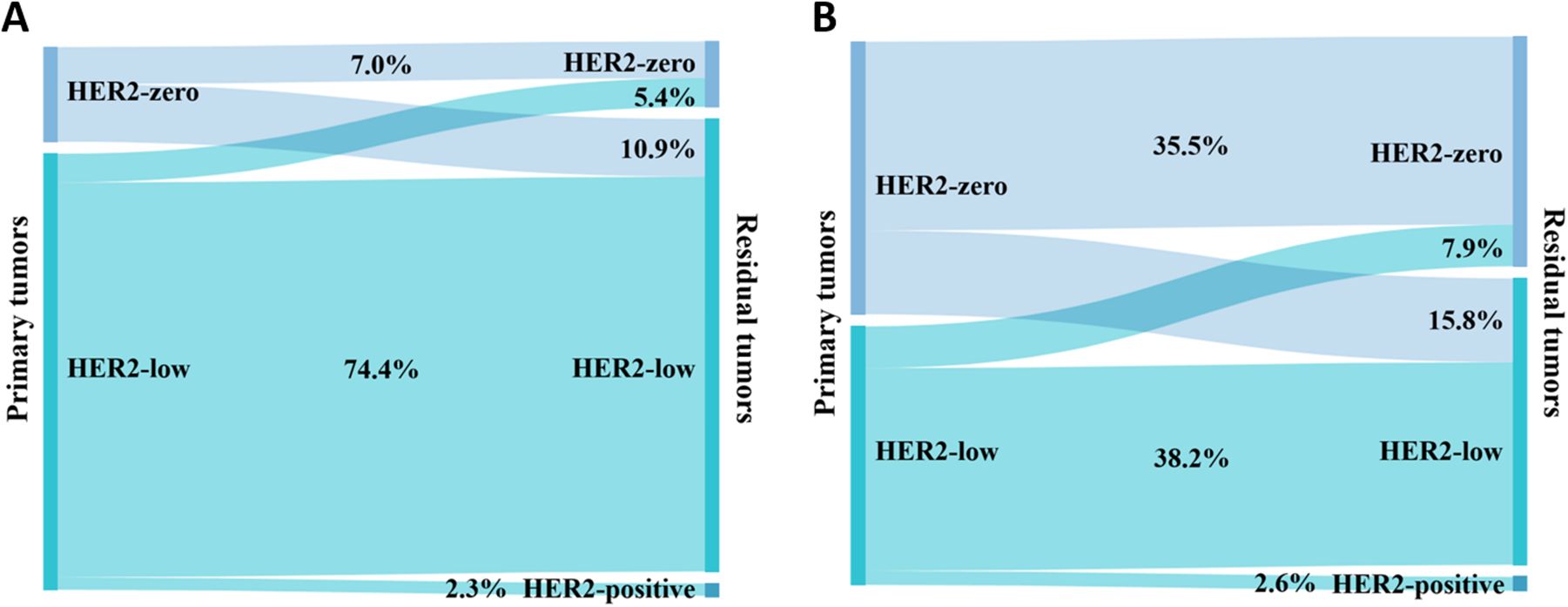
Figure 5. Conversion of expression in HER2-negative patients between primary and residual tumors: (A) Luminal and (B) TNBC.
3.5 Clinicopathological factors associated with ER, PR, and HER2 conversion
Table 4 shows clinicopathologic factors associated with ER, PR, and HER2 conversions. ER conversion correlated with pre-NAT HER2 status (χ2 = 11.527, P = 0.003), with HER2-positive individuals having a higher likelihood of undergoing ER conversion. HER2 conversion correlated with pre-NAT HER2 status (χ2 = 28.312, P < 0.001), with HER2-zero patients showing a higher likelihood of HER2 conversion. We found no clinicopathologic features associated with PR conversion.
4 Discussion
In our cohort of 589 breast cancer patients receiving NAT, comprehensive analysis revealed significant correlations between baseline HR and HER2 expression levels with pathological treatment response. Notably, receptor status conversion was observed in a subset of patients following NAT completion. pCR is an alternative endpoint to disease-free survival (DFS) and OS in clinical studies of NAT in breast cancer and is critical to NAT efficacy (16). In our study, the pCR rates of patients with HER2-zero, -low, and -positive were 33.7%, 15.4%, and 57.0%, respectively, which are similar to the results of Zhang et al. (17). We observed a relatively low pCR rate in patients with TNBC, which may be associated with our treatment strategy (18). The NAT regimen in our study primarily consisted of anthracycline plus taxane-based therapy with or without cyclophosphamide, while published evidence demonstrates that the addition of platinum-based agents or immunotherapy can significantly improve pCR rates (19). In clinical practice, HER2-negative tumors receive only neoadjuvant endocrine therapy or chemotherapy, not anti-HER2 targeted therapy, and thus, HER2-negative patients exhibit the lowest pCR rate. HER2-low breast cancers are predominantly HR-positive, and hence, the distribution of molecular subtypes results in different pCR rates between HER2-zero and HER2-low (16). Several retrospective studies have shown that the percentage of ER- and PR-negative breast cancer patients receiving NAT who achieve pCR after chemotherapy is significantly higher than that of ER- and PR-positive breast cancer patients (20, 21). Our study showed similar results. This phenomenon may be explained by the fact that ER- and PR-negative breast tumors are poorly differentiated, have high dividing and proliferative activity, and are consequently more sensitive to chemotherapeutic agents (16). Molecular typing of breast cancers based on receptor status showed a pCR rate of 57.0% for HER2-positive breast cancers, which is similar to the results of recent studies (22, 23) but slightly higher than the result of a previous study (11). The higher pCR rate may be attributed to the ability of neoadjuvant targeted therapy containing trastuzumab to increase the pCR rate of HER2-positive breast carcinoma compared with conventional extra NAT (24). Moreover, we observed a lower pCR rate in the HER2+/HR+ cohort compared to the HER2+/HR− group. From a biological perspective, this may be attributed to bidirectional crosstalk between the HR and HER2 signaling pathways, which can contribute to resistance against both HER2-targeted therapies and endocrine treatments (25).
Our results revealed that the ER and PR conversion rates in residual lesions following NAT were 7.8% and 16.0%, respectively, which are comparable with the results of He et al. (26). The proportion of ER and PR undergoing conversion varies considerably across studies. A review of 32 publications showed that the HR status conversion rate between pre-NAT CNB specimens and post-NAT surgical specimens ranged from approximately 8% to 33%, the ER status conversion rate from approximately 2.5% to 17.0%, and the PR status conversion rate from approximately 5.9% to 51.7% (27). Several studies have shown that PR status is more likely to undergo conversion after NAT compared with ER (8, 10, 11, 26). This phenomenon is explained by the fact that PR expression often depends on intact signaling pathways, and therefore, PR exhibits a more heterogeneous spread within tumor cells (28).
There are no uniform conclusions about the factors influencing the conversion of ER and PR statuses. In a multifactorial logistic regression analysis by Yilmaz et al. (29), lower ER expression and smaller tumor size were found to be independent influences on ER and PR conversions, respectively. We found that pre-NAT HER2 status was an influential factor in ER conversion and did not find a correlation between clinicopathologic features and PR conversion. A study by Colleoni et al. (17) concluded that there was no significant association between conversions in HR status and clinicopathological characteristics of patients. The mechanisms by which conversions in HR status occur after NAT are complex. CNB and surgical resection biopsies are commonly considered to be highly concordant in detecting ER and PR expression (30). Possible reasons for changes in HR expression include fewer tumor cells in the CNB sample, which may not fully reflect the microenvironment inside the tumor, and technical problems in the assay. ASCO/CAP analyzed factors affecting receptor conversion, such as specimen handling, tissue fixation, and analytical assay methods; used 1% as the optimal cutoff for ER/PR positivity; and recommended that endocrine therapy for this subset of breast cancer patients could help mitigate biomarker changes and their possible adverse effects (31–33). In addition to this, Zhang et al. (34) showed that cases receiving NAT had a significantly higher incidence of discordant pre- and postoperative ER and PR statuses than cases not receiving NAT, implying that NAT drug administration may result in receptor status conversion. Sensitivity of tumor cells to chemotherapy correlates with HR status. HR-negative tumor cells are more sensitive to chemotherapy than HR-positive tumor cells, and therefore, tumor cells in residual lesions predominantly show HR positivity (35). Another explanation for the change from negative to positive HR could be due to the cells initially originating from HR-positive breast carcinoma cells and returning to their previous state under the influence of chemotherapy. In contrast, the conversion from positive to negative HR may be because the chemotherapy suppresses ovarian function, decreases circulating hormone levels, and downregulates ER and PR expression levels in premenopausal women (27). The different directions of HR conversion lead to prognostic differences in patients. Patients who converted to positive HR and received adjuvant endocrine therapy achieved better DFS and OS than HR-negative patients (36). Tacca et al. (35) analyzed the variations in ER and PR expression and their influence on survival and showed that the receptor status of the residual lesion influences the patient’s prognosis, not the subtype assessed at the time of the first biopsy. Therefore, it is clinically important to reassess the ER and PR statuses of the specimen after NAT. In particular, endocrine therapy is essential for HR-negative patients converted to HR-positive.
When we categorized HER2 as negative versus positive, the HER2 conversion rate was 5.1%, and decreased HER2 expression was more common than increased HER2 expression. It has been proposed that anti-HER2 targeted medications may be the cause of the lack of HER2 expression following NAT (11). Ignatov et al. (37) reported that 47.3% of HER2 conversion was from positive to negative with trastuzumab in NAT, and when the trastuzumab combined with the pertuzumab regimen was used, the rate of HER2 conversion from positive to negative increased to 63.2%. Here, we show that HER2 conversion was only connected with HER2 status in pre-NAT lesions and that individuals who had HER2-zero expression were most likely to undergo HER2 conversion; a similar conclusion was reported by Bo et al. (8). In addition, the HER2 conversion may be associated with tumor heterogeneity, inter- and intra-observer variability, variability in tissue handling and fixation, and sampling error (38, 39). The prognostic significance of HER2 expression variations is uncertain. A study reported that patients with absent HER2 status had worse DFS than those for whom HER2 status remained positive after NAT (40). In contrast, Yoshida et al. (41) performed a retrospective analysis and showed that variations in HER2 expression were not associated with patient prognosis.
The development of antibody-drug conjugates has brought HER2-low breast cancers to the forefront of research (42). The HER2 conversion rate before and after NAT increased to 18.6% when we included HER2-low in the subgroup. Shang et al. (43) demonstrated that the HER2 conversion rate was 21.42%, and this phenomenon was mainly caused by the conversion between HER2-zero and HER2-low. The results of Miglietta et al. (44) confirmed the instability of HER2-low in various environments. In the HER2-negative cohort, the rate of HER2 conversion was greater in TNBC than in Luminal breast cancer, but this difference was not statistically significant, which is comparable to the findings of Shang et al. (43).
As the conversion from HER2-zero to HER2-positive between pre-NAT primary breast cancers and post-NAT residual lesions is uncommon, and little has been learned about the clinical implications of the change in HER2 status after NAT, the present standard of treatment is determined by the receptor expression of the patient’s primary breast cancer (45). A study of the relationship between repeat biopsies and HER2-low presented at ASCO 2023 found that the percentage of cases with HER2-low expression increased alongside the number of repeat biopsies, with 59%, 73%, 83%, 83%, and 100% of cases with HER2-low expression at 1, 2, 3, 4, and ≥5 biopsies, respectively (46). In conjunction with the existing literature and the results of this study, it is significant to re-examine the HER2 status of post-NAT samples to better assess which patients may experience a conversion from HER2-zero to HER2-low, as well as informing clinical trials for patients with low HER2 so that these patients can be treated with novel drugs that may be effective.
This research has several noteworthy limitations. First, the single-center retrospective design, coupled with a relatively small sample size and incomplete follow-up data, precluded robust survival analysis. Second, the study protocol did not systematically evaluate the correlation between neoadjuvant therapeutic regimens and receptor status conversion, which constrains the clinical applicability of our findings. Finally, selection bias was inevitable as our HER2 conversion analysis was restricted to patients with definitively documented HER2 status.
5 Conclusion
Some breast cancer patients may have conversions of ER, PR, and HER2 status after NAT. Residual lesions must be immunohistochemically re-evaluated to determine the patient’s receptor expression status and adjust the future therapy plan.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Clinical Trial Ethics Committee of The Affiliated Hospital of Southwest Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JW: Conceptualization, Formal Analysis, Writing – original draft. XL: Methodology, Writing – original draft. MT: Supervision, Writing – review & editing. XX: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Costa B, Amorim I, Gärtner F, and Vale N. Understanding breast cancer: from conventional therapies to repurposed drugs. Eur J Pharm Sci. (2020) 151:105401. doi: 10.1016/j.ejps.2020.105401
3. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:452–78. doi: 10.6004/jnccn.2020.0016
4. Balic M, Thomssen C, Würstlein R, Gnant M, and Harbeck N. St. Gallen/vienna 2019: A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel). (2019) 14:103–10. doi: 10.1159/000499931
5. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the ctneobc pooled analysis. Lancet. (2014) 384:164–72. doi: 10.1016/S0140-6736(13)62422-8
6. Chen J, Jin L, Chen L, Bian Z, Li Z, Cao S, et al. Patients achieved pcr during neoadjuvant chemotherapy had better outcome than adjuvant chemotherapy setting in breast cancer: A comparative study. Cancer Treat Res Commun. (2023) 36:100719. doi: 10.1016/j.ctarc.2023.100719
7. Asaoka M, Narui K, Suganuma N, Chishima T, Yamada A, Sugae S, et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur J Surg Oncol. (2019) 45:2289–94. doi: 10.1016/j.ejso.2019.08.001
8. Bo J, Yu B, Bi R, Xu X, Cheng Y, Tu X, et al. Conversion of er and her2 status after neoadjuvant therapy in chinese breast cancer patients. Clin Breast Cancer. (2023) 23:436–46. doi: 10.1016/j.clbc.2023.03.002
9. Jin X, Jiang YZ, Chen S, Yu KD, Shao ZM, and Di GH. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: A prospective observational study. Oncotarget. (2015) 6:9600–11. doi: 10.18632/oncotarget.3292
10. Rey-Vargas L, Mejia-Henao JC, Sanabria-Salas MC, and Serrano-Gomez SJ. Effect of neoadjuvant therapy on breast cancer biomarker profile. BMC Cancer. (2020) 20:675. doi: 10.1186/s12885-020-07179-4
11. Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, et al. Changes in tumor expression of her2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. (2016) 27:480–7. doi: 10.1093/annonc/mdv611
12. Litton JK, Regan MM, Pusztai L, Rugo HS, Tolaney SM, Garrett-Mayer E, et al. Standardized definitions for efficacy end points in neoadjuvant breast cancer clinical trials: neosteep. J Clin Oncol. (2023) 41:4433–42. doi: 10.1200/jco.23.00435
13. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: asco/cap guideline update. J Clin Oncol. (2020) 38:1346–66. doi: 10.1200/jco.19.02309
14. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. Her2-low breast cancer: pathological and clinical landscape. J Clin Oncol. (2020) 38:1951–62. doi: 10.1200/jco.19.02488
15. Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, et al. Assessment of ki67 in breast cancer: updated recommendations from the international ki67 in breast cancer working group. J Natl Cancer Inst. (2021) 113:808–19. doi: 10.1093/jnci/djaa201
16. Cortazar P and Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. (2015) 22:1441–6. doi: 10.1245/s10434-015-4404-8
17. Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. (2022) 20:142. doi: 10.1186/s12916-022-02346-9
18. Vidra R, Nemes A, Vidrean A, Pintea S, Tintari S, Deac A, et al. Pathological complete response following cisplatin or carboplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: A systematic review and meta-analysis. Exp Ther Med. (2022) 23:91. doi: 10.3892/etm.2021.11014
19. Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the parp inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (Brightness): A randomised, phase 3 trial. Lancet Oncol. (2018) 19:497–509. doi: 10.1016/s1470-2045(18)30111-6
20. Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: A study of preoperative treatment. Clin Cancer Res. (2004) 10:6622–8. doi: 10.1158/1078-0432.Ccr-04-0380
21. Klein J, Tran W, Watkins E, Vesprini D, Wright FC, Look Hong NJ, et al. Locally advanced breast cancer treated with neoadjuvant chemotherapy and adjuvant radiotherapy: A retrospective cohort analysis. BMC Cancer. (2019) 19:306. doi: 10.1186/s12885-019-5499-2
22. Chen XC, Jiao DC, Qiao JH, Wang CZ, Sun XF, Lu ZD, et al. De-escalated neoadjuvant weekly nab-paclitaxel with trastuzumab and pertuzumab versus docetaxel, carboplatin, trastuzumab, and pertuzumab in patients with her2-positive early breast cancer (Helen-006): A multicentre, randomised, phase 3 trial. Lancet Oncol. (2025) 26:27–36. doi: 10.1016/s1470-2045(24)00581-3
23. Tarantino P, Ajari O, Graham N, Vincuilla J, Parker T, Hughes ME, et al. Evolution of her2 expression between pre-treatment biopsy and residual disease after neoadjuvant therapy for breast cancer. Eur J Cancer. (2024) 201:113920. doi: 10.1016/j.ejca.2024.113920
24. Kurozumi S, Inoue K, Takei H, Matsumoto H, Kurosumi M, Horiguchi J, et al. Er, pgr, ki67, P27(Kip1), and histological grade as predictors of pathological complete response in patients with her2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer. (2015) 15:622. doi: 10.1186/s12885-015-1641-y
25. Chen H, Gui X, Zhou Z, Su F, Gong C, Li S, et al. Distinct er and pr expression patterns significantly affect the clinical outcomes of early her2-positive breast cancer: A real-world analysis of 871 patients treated with neoadjuvant therapy. Breast. (2024) 75:103733. doi: 10.1016/j.breast.2024.103733
26. He Y, Zhang J, Chen H, Zhou Y, Hong L, Ma Y, et al. Clinical significance and prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients. Front Surg. (2022) 9:1037215. doi: 10.3389/fsurg.2022.1037215
27. van de Ven S, Smit VT, Dekker TJ, Nortier JW, and Kroep JR. Discordances in er, pr and her2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. (2011) 37:422–30. doi: 10.1016/j.ctrv.2010.11.006
28. Clark BZ, Onisko A, Assylbekova B, Li X, Bhargava R, and Dabbs DJ. Breast cancer global tumor biomarkers: A quality assurance study of intratumoral heterogeneity. Mod Pathol. (2019) 32:354–66. doi: 10.1038/s41379-018-0153-0
29. Yilmaz C and Cavdar DK. Biomarker discordances and alterations observed in breast cancer treated with neoadjuvant chemotherapy: causes, frequencies, and clinical significances. Curr Oncol. (2022) 29:9695–710. doi: 10.3390/curroncol29120761
30. Dekker TJ, Smit VT, Hooijer GK, Van de Vijver MJ, Mesker WE, Tollenaar RA, et al. Reliability of core needle biopsy for determining er and her2 status in breast cancer. Ann Oncol. (2013) 24:931–7. doi: 10.1093/annonc/mds599
31. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch Pathol Lab Med. (2018) 142:1364–82. doi: 10.5858/arpa.2018-0902-SA
32. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Nccn guidelines® Insights: breast cancer, version 4.2021. J Natl Compr Canc Netw. (2021) 19:484–93. doi: 10.6004/jnccn.2021.0023
33. Hammond ME, Hayes DF, Wolff AC, Mangu PB, and Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. (2010) 6:195–7. doi: 10.1200/jop.777003
34. Zhang N, Moran MS, Huo Q, Haffty BG, and Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: A meta-analysis. Cancer Invest. (2011) 29:594–8. doi: 10.3109/07357907.2011.621913
35. Tacca O, Penault-Llorca F, Abrial C, Mouret-Reynier MA, Raoelfils I, Durando X, et al. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. (2007) 12:636–43. doi: 10.1634/theoncologist.12-6-636
36. Li C, Fan H, Xiang Q, Xu L, Zhang Z, Liu Q, et al. Prognostic value of receptor status conversion following neoadjuvant chemotherapy in breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res Treat. (2019) 178:497–504. doi: 10.1007/s10549-019-05421-7
37. Ignatov T, Gorbunow F, Eggemann H, Ortmann O, and Ignatov A. Loss of her2 after her2-targeted treatment. Breast Cancer Res Treat. (2019) 175:401–8. doi: 10.1007/s10549-019-05173-4
38. Asogan AB, Hong GS, and Arni Prabhakaran SK. Concordance between core needle biopsy and surgical specimen for oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 status in breast cancer. Singapore Med J. (2017) 58:145–9. doi: 10.11622/smedj.2016062
39. Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K, et al. Examination of low erbb2 protein expression in breast cancer tissue. JAMA Oncol. (2022) 8:1–4. doi: 10.1001/jamaoncol.2021.7239
40. Tural D, Karaca M, Zirtiloglu A, MH B, Sendur MA, and Ozet A. Receptor discordances after neoadjuvant chemotherapy and their effects on survival. J buon. (2019) 24:20–5.
41. Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, and Yamauchi H. Change in her2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. (2017) 116:1021–8. doi: 10.1002/jso.24762
42. Eiger D, Agostinetto E, Saúde-Conde R, and de Azambuja E. The exciting new field of her2-low breast cancer treatment. Cancers (Basel). (2021) 13(5):1015. doi: 10.3390/cancers13051015
43. Shang J, Sun X, Xu Z, Cai L, Liu C, Wu S, et al. Evolution and clinical significance of her2-low status after neoadjuvant therapy for breast cancer. Front Oncol. (2023) 13:1086480. doi: 10.3389/fonc.2023.1086480
44. Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, et al. Her2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. (2022) 8:66. doi: 10.1038/s41523-022-00434-w
45. Luo QQ, Huang JB, Wu YT, Li X, Zhao CX, Wu H, et al. Tidal chemotherapy in premenopausal patients with hormone receptor positive breast cancer. Med Hypotheses. (2017) 102:4–7. doi: 10.1016/j.mehy.2017.03.003
Keywords: breast cancer, neoadjuvant therapy, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2
Citation: Wang J, Long X, Tang M and Xiao X (2025) HER2 and hormone receptor conversion after neoadjuvant therapy for breast cancer. Front. Oncol. 15:1522460. doi: 10.3389/fonc.2025.1522460
Received: 04 November 2024; Accepted: 20 May 2025;
Published: 09 June 2025.
Edited by:
Xiaoyun Mao, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Ioannis Boutas, National and Kapodistrian University of Athens, GreeceKonstantinos Papazisis, Interbalkan Medical Center, Greece
Nan Niu, China Medical University, China
Copyright © 2025 Wang, Long, Tang and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxi Tang, bXh0YW5nNjlAMTYzLmNvbQ==; Xiuli Xiao, eGlhb3hpdWxpbHlAc2luYS5jb20=
†Present address: Xiuli Xiao, Department of Pathology, The Fourth Affiliated Hospital of Southwest Medical University, Meishan, Sichuan, China
‡These authors have contributed equally to this work
 Jing Wang
Jing Wang Xin Long3,4‡
Xin Long3,4‡ Mingxi Tang
Mingxi Tang