- 1Department of Neuro-oncology, Cancer Center, China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2National Institute for Data Science in Health and Medicine, Capital Medical University, Beijing, China
Background: This study investigates survival disparities and prognostic factors in patients with brain metastases originating from various primary cancers to facilitate risk stratification and enhance precision in diagnosis and treatment.
Methods: Patients diagnosed with brain metastases between 2010 and 2018 were identified from the SEER database for analysis. Overall survival (OS) was evaluated using Kaplan-Meier curves and log-rank tests, complemented by multivariate Cox regression analysis. The impact of age on the risk and survival of brain metastases was examined using Restricted Cubic Splines (RCS) in Cox regression models.
Results: A total of 55,094 patients diagnosed with brain metastases between 2010 and 2018 were retrospectively identified from the SEER database for inclusion in this study. It was found that the median survival times were 2 months (95% CI: 2–3 months) for liver cancer, 3 months (95% CI: 3–4 months) for stomach cancer, and 5 months (95% CI: 4–5 months) for lung cancer. Survival was influenced by factors such as sex, age, primary cancer site, race, income, marital status, and treatment approaches. Surgical treatment notably decreased the mortality risk, with a hazard ratio (HR) of 0.49 (95% CI: 4–5 months) for lung cancer, 0.43 (95% CI:3–4 months) for kidney cancer, and 0.63 (95% CI: 5–7 months) for breast cancer. The predictive model created with these variables achieved a C-index of 0.723 and 0.722 in the training and test sets, respectively, indicating vital accuracy. Calibration curves displayed minimal errors, and the area under the curve (AUC) values showed excellent performance at 3 months (training: 0.83, test: 0.83), 6 months (training: 0.80, test: 0.80), and 12 months (training: 0.77, test: 0.76).
Conclusion: Brain metastases from liver, stomach, and lung cancers are linked to a poor prognosis. Surgical intervention significantly lowers mortality risk. The predictive model, which incorporates vital survival factors, demonstrates high accuracy and reliable performance, supporting the clinical management of patients with brain metastases.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD420251054176.
Introduction
Approximately 20% of cancer patients are diagnosed with brain metastases during their disease course (1–3), though this estimate is likely conservative. Autopsy studies suggest a higher incidence, with brain metastases identified in 30–40% of cancer patients (4, 5). Primary tumours most commonly linked to brain metastases include lung cancer, which affects 20–56% of these patients, breast cancer (5–20%), and melanoma (7–16%) (6–8). The presence of brain metastases is typically indicative of advanced disease and is correlated with a poor prognosis (8–11). For individuals diagnosed with brain metastases, OS rates are alarmingly low, with only 5-24% surviving up to two years and a mere 2.4-15% reaching the five-year mark, regardless of the type of primary tumour (12–15). In addition to sex, tumour origin, and molecular subtype, the development of brain metastases is also affected by ethnicity, geographic location, age, and treatment methods (1). A comprehensive examination of these known and unidentified factors that may influence the occurrence and prognosis of brain metastases could significantly improve clinical management and enhance treatment outcomes for affected patients (16). For instance, research conducted by Kuksis et al. has shown a high prevalence of brain metastases in patients with HER2-positive and triple-negative metastatic breast cancer (MBC). Improving screening for brain metastases in patients with HER2-positive and triple-negative MBC could enable earlier detection and treatment, ultimately enhancing therapeutic outcomes (17). Additionally, Tsai et al. found that survival was significantly reduced in patients with brain metastases originating from gastroesophageal adenocarcinoma who did not receive surgery or radiotherapy, according to multivariable analyses (14).
Furthermore, research conducted by K. Salari et al. has demonstrated that, in patients with brain-only metastatic non-small cell lung cancer (NSCLC), definitive treatment of the thoracic primary site after intracranial radiosurgery was linked to slower disease progression and improved survival (18). These studies offer valuable insights and recommendations for treating brain metastases. Still, they focus exclusively on metastases from specific primary sites without comprehensively analysing those originating from various primary locations. The research by W. Sperduto et al. addresses this gap by performing a multifactorial analysis of factors that influence prognosis in individuals with brain metastases, which led to the development of the Graded Prognostic Assessment (GPA) for brain metastases from multiple primary sites (19). This allows for risk stratification and treatment guidance using the GPA, effectively overcoming the limitations of the studies mentioned above. However, when constructing the GPA, the researchers did not consider the primary site as a covariate, instead opting for a generalized analysis. This approach is inadequate because prognoses vary significantly based on the primary site (20, 21). In the latest iteration of the GPA (22), the authors introduced stratification to mitigate the influence of the primary site. However, this approach created separate scoring scales for each type of brain metastasis, rendering the GPA calculation overly complex.
This study aims to utilize data from patients with brain metastases originating from various primary sites to develop a tool for risk stratification. By analysing relevant prognostic factors, the study seeks to assist clinicians in delivering precise diagnoses and tailored treatments for patients affected by brain metastases.
Materials and methods
Data extraction
Study data were extracted using SEER*Stat software (version 8.3.9), utilising the “Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018)” dataset. All cases were initially identified using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology and site codes relevant to the study population.
Due to the absence of the “SEER Combined Mets at DX-brain” variable for patients diagnosed before 2010, it was impossible to determine the presence of brain metastases in these cases. Therefore, the study included only patients diagnosed with brain metastases between 2010 and 2018. Those with missing data on age at diagnosis, race, sex, marital status, or incomplete follow-up were excluded. The primary endpoint of this study was OS, defined as the time from diagnosis to death from any cause or last follow-up.
Study design
The overall study design is illustrated in a flowchart (Figure 1). A total of 55,094 patients diagnosed with brain metastases between 2010 and 2018 were retrospectively identified from the SEER database. After data cleaning and selection based on predefined inclusion and exclusion criteria, eligible patients were randomly assigned to training and testing sets in a 7:3 ratio. A multivariable Cox regression model was applied to identify independent prognostic factors, and a nomogram was constructed based on variables including age, sex, race, income, marital status, primary site, surgery, radiotherapy, and chemotherapy to predict 1-year OS. The performance of the nomogram was assessed using the concordance index (C-index), time-dependent Receiver Operating Characteristic (ROC) curves, and calibration plots at 3, 6, and 12 months in both training and testing sets. The total nomogram score for each patient was calculated, and the corresponding AUC was used to compare the predictive accuracy of the nomogram with the TNM clinical staging system.
Statistical analysis
The primary endpoint of this study was OS. Categorical variables included sex, race (White, Black, Other), and marital status (married, single, divorced/separated, widowed). Age was treated both as a categorical variable (using clinically meaningful age groups) and as a continuous variable in selected models.
Kaplan-Meier curves and the log-rank test were used to assess the survival rate. To evaluate prognostic factors associated with OS, univariate Cox proportional hazards regression was first performed. Variables with p < 0.05 in univariate analysis were included in a multivariate Cox regression model to identify independent predictors of survival. HR and 95% confidence interval (CI) were reported.
The effect of age on the likelihood of developing brain metastases and related survival outcomes was analysed continuously through restricted cubic splines RCS within Cox regression models, with three to five knots placed at percentiles of the age distribution. Model performance was evaluated using the concordance index (C-index) to assess discriminative ability, and time-dependent ROC curves were utilized to evaluate predictive accuracy at different time points (e.g., 3, 6, 12 months). Calibration plots comparing predicted versus observed survival probabilities were used to assess model calibration.
All statistical analyses were carried out using R software (version 4.3.1) with relevant packages including “survival”, “rms”, “timeROC”, and “ggplot2”.
Results
Patient characteristics
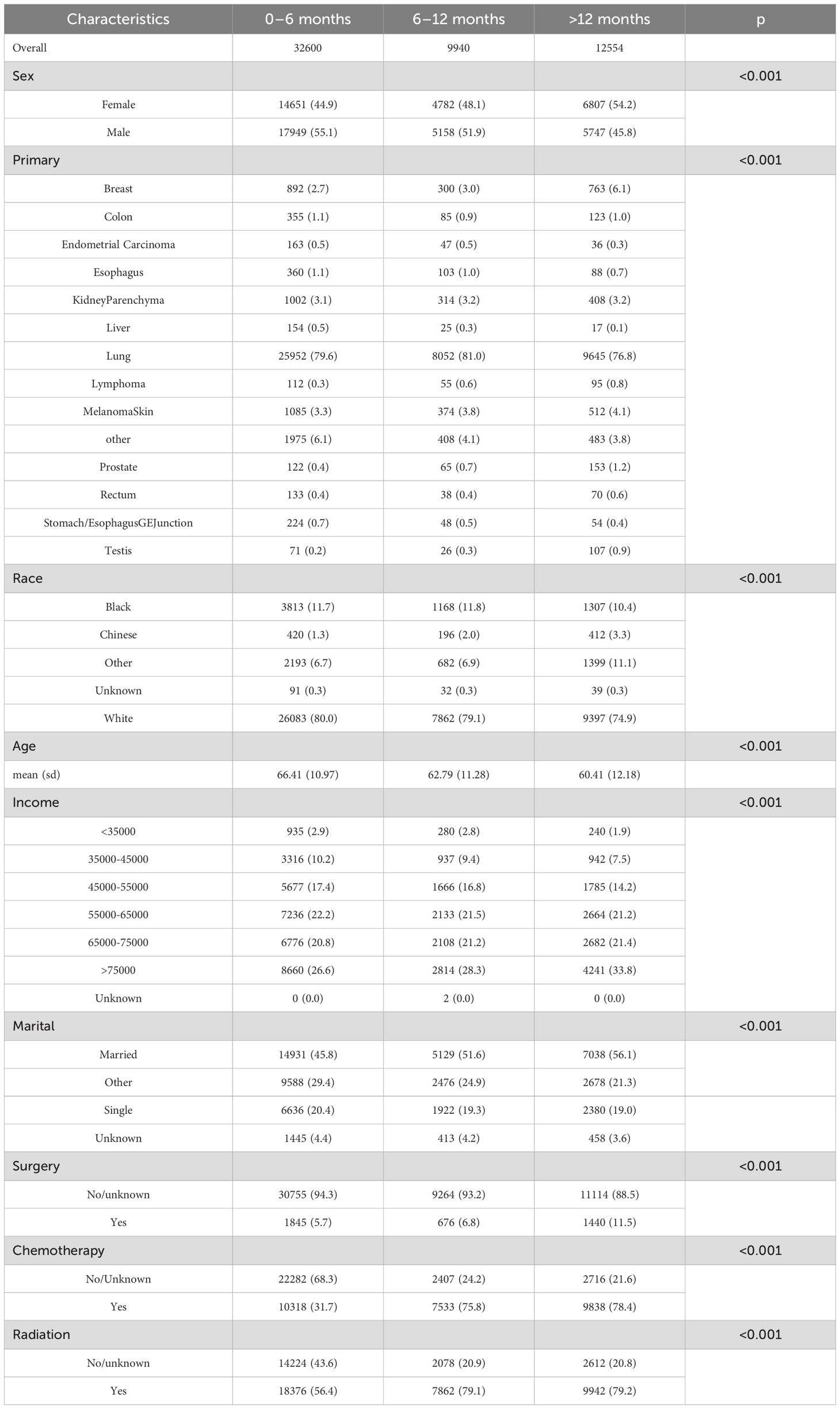
55,094 patients diagnosed with brain metastases between 2010 and 2018 were enrolled in this study (Table 1). The survival distribution of the patients followed a U-shaped pattern, with a higher proportion of patients having survival times of less than six months or more significant than twelve months. Patients having survival times of between six and 12 months were less common. Specifically, 59.1% of patients with brain metastases had an OS of less than six months, including 4.2% who did not reach the endpoint. In contrast, 22.8% of the population had survival times exceeding 12 months. Among patients with survival times less than 12 months, the proportion of males was higher than females. However, the trend reversed in those with survival times more significant than 12 months, with a significantly higher proportion of females (p < 0.001).
Among all primary sites of brain metastases, lung cancer had the highest incidence (79.2%), followed by skin cancer (3.8%), breast cancer (3.5%), and kidney parenchyma cancer (3.1%). At the initial diagnosis of brain metastases, a younger age was associated with a better subsequent prognosis. The mean age at initial diagnosis for patients with survival times of 0–6 months was 66.41 years. The mean ages for the better survival groups were significantly lower, at 62.79 and 60.41 years, respectively.
In addition to the factors above of age, sex, and primary site, other variables such as race, income, marital status, and treatment modalities also significantly influenced the prognosis of brain metastases. All observed differences were statistically significant (p < 0.001). However, due to the retrospective nature of the SEER database, certain data points may be incomplete or missing, potentially impacting the accuracy and generalizability of these findings.
The p-values in Table 1 were calculated based on grouping the population into three categories according to survival time. For categorical variables, we applied the chi-square test; for continuous variables, one-way ANOVA was used. When the assumptions of the chi-square test or ANOVA were not met, non-parametric tests were employed to assess differences among the survival groups.
Survival analysis
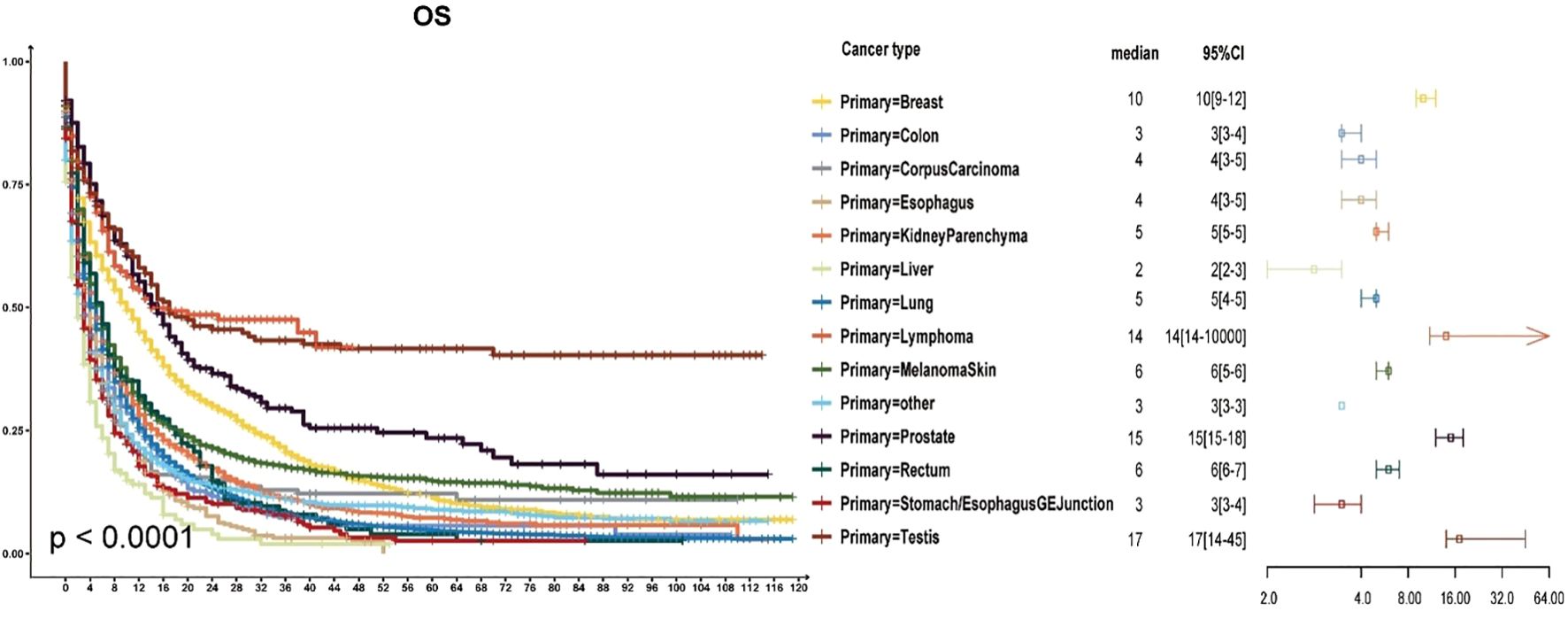
As shown in Figure 1, most patients with brain metastases from various primary sites had a median survival time of less than six months. The poorest prognosis was observed in patients with brain metastases from liver cancer, where the median survival time was two months (95% CI: 2–3 months). Brain metastases from stomach cancer had a median survival time of three months (95% CI: 3–4 months). Lung cancer, which represented the most significant proportion of brain metastases, also had a poor prognosis with a median survival time of five months (95% CI: 4–5 months). Conversely, patients with brain metastases from breast cancer, lymphoma, prostate cancer, and testicular cancer had a median survival time exceeding ten months. Among these, testicular cancer had the most favourable prognosis, with a median survival time surpassing 17 months (95% CI: 14–45 months).
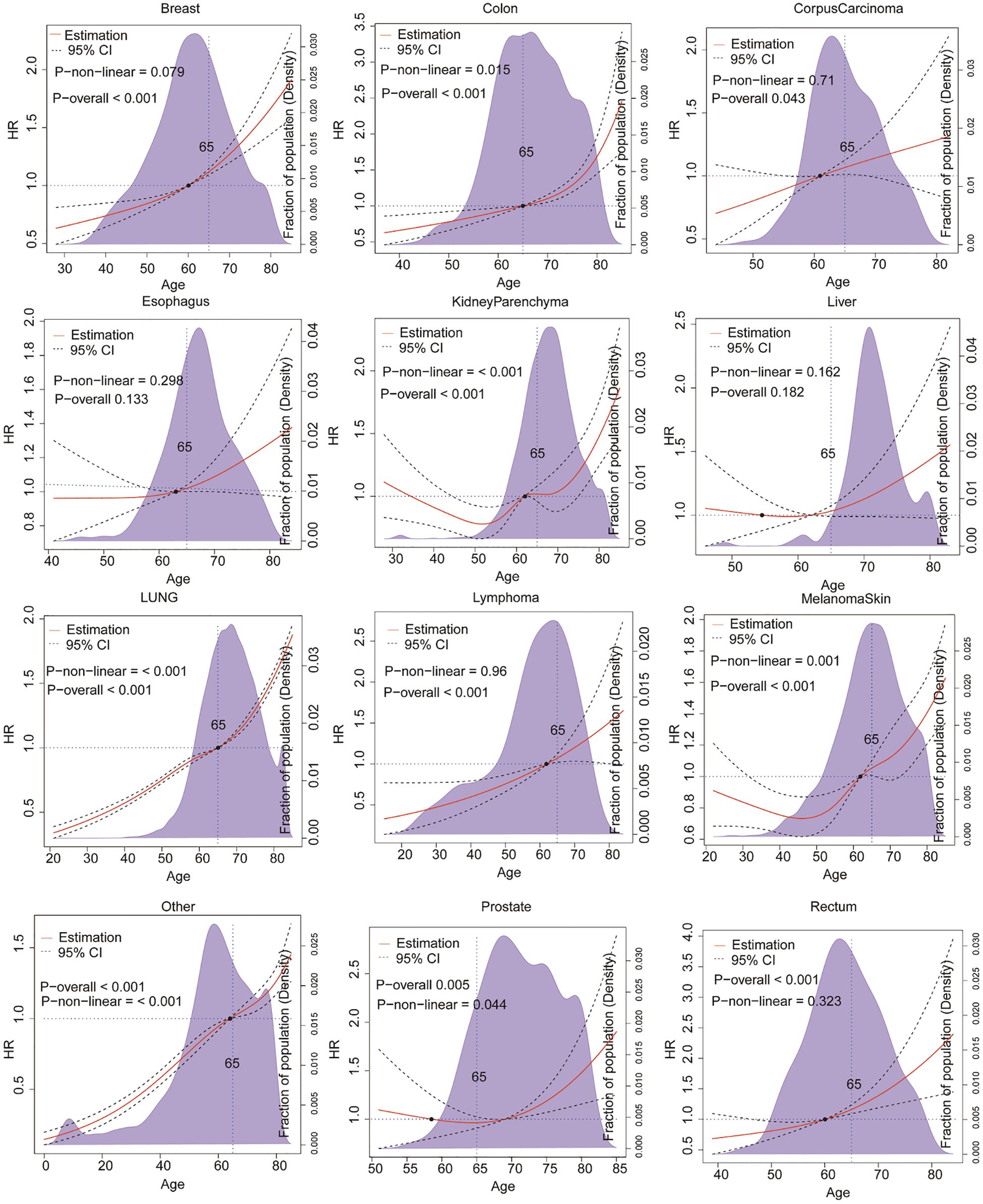
In addition to the primary site of origin, age is a critical factor influencing the prognosis of patients with brain metastases. Cancer is often considered a disease of aging, with its occurrence and outcomes closely linked to the aging process (23). We employed RCS to model these effects and explore further the relationship between the primary site of brain metastases and the age-related risk variation in survival.RCS is a flexible statistical method for modelling non-linear relationships between variable (24). By applying RCS in our analysis, we were able to examine how the risk of mortality changes with age among patients with brain metastases from different primary cancers. This approach provides a nuanced understanding of the impact of age on survival, taking into account the varying risks associated with other primary sites.
As illustrated in Figure 2, the findings of our study indicate that the risk of survival following the development of brain metastases from the majority of primary sites increases with age. However, the pattern of this age-related risk varies among different primary cancers. For patients with brain metastases originating from lung cancer, breast cancer, and lymphoma, the mortality hazard exhibits a rapid increase with age across all age groups (P < 0.001). In contrast, for patients with brain metastases from testicular cancer, colorectal cancer, and EndometrialCarcinoma, the risk of mortality due to brain metastases remains relatively stable until the age of 65, after which it increases sharply. It is noteworthy that patients with brain metastases from liver cancer and rectal cancer also exhibit an age-related increase in mortality risk. However, this trend does not reach statistical significance (P>0.05). These findings emphasise the necessity of considering both the primary site of cancer and the patient’s age when evaluating prognosis and developing treatment strategies for brain metastases.
The decision between radiotherapy and surgical resection (”surgical resection” in this manuscript specifically refers to neurosurgical resection of metastatic brain lesions, not resection of the primary tumours.)can be influenced by several factors, including the size and diversity of the tumour (25–27). While the majority of contemporary studies concentrate on tumour size to ascertain the necessity of surgery for brain metastases, there has been a lack of research investigating whether the primary location of the tumour influences the decision to operate. As illustrated in Figure 3, the results of our study indicate that surgical intervention for brain metastases originating from various primary sites generally reduces the risk of death. For instance, the mortality risk for lung cancer metastases following surgery is reduced by nearly half, with an HR of 0.49 [95% CI 0.46-0.53]. After adjusting for age and gender, the HR is 0.52 [95% CI 0.48-0.56]. Similarly, the HR for kidney parenchyma metastases is 0.43 [95% CI 0.37-0.49] and 0.44 [95% CI 0.38-0.50] after adjustment. The HR for breast cancer metastases is 0.63 [95% CI 0.54-0.74] and 0.66 [95% CI 0.56-0.77] after adjustment. Nevertheless, it should be noted that not all tumours with brain metastases are suitable for surgical treatment. For instance, patients with brain metastases from liver cancer, bladder cancer, or esophageal cancer do not exhibit a significant survival benefit post-surgery (P>0.05).
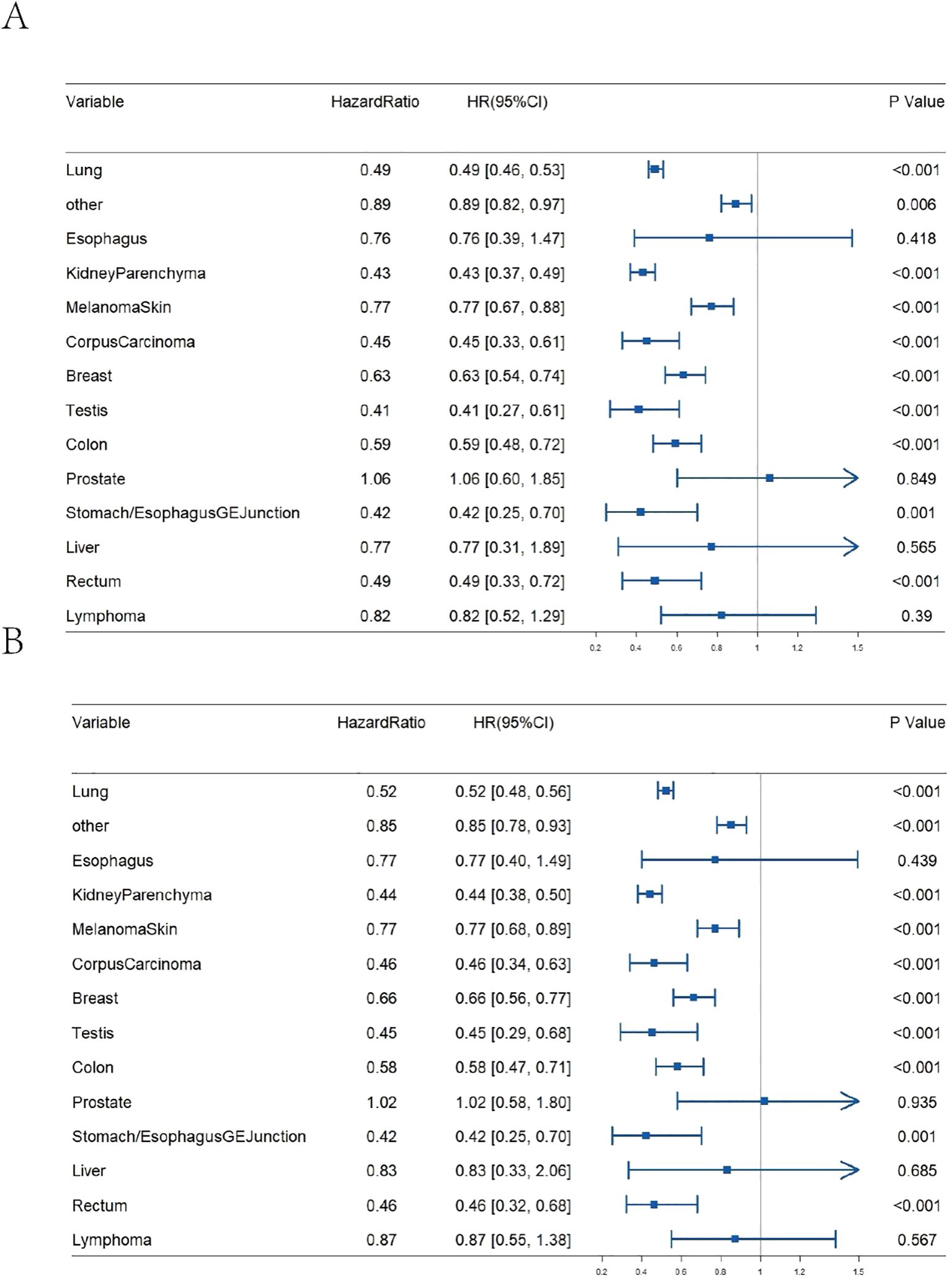
Figure 3. Impact of surgical resection on OS for brain metastases from various primary tumour sites. (A) Un-adjusted age and gender; (B) Adjusted age and gender.
Development and validation of a survival prediction model for patients with brain metastases
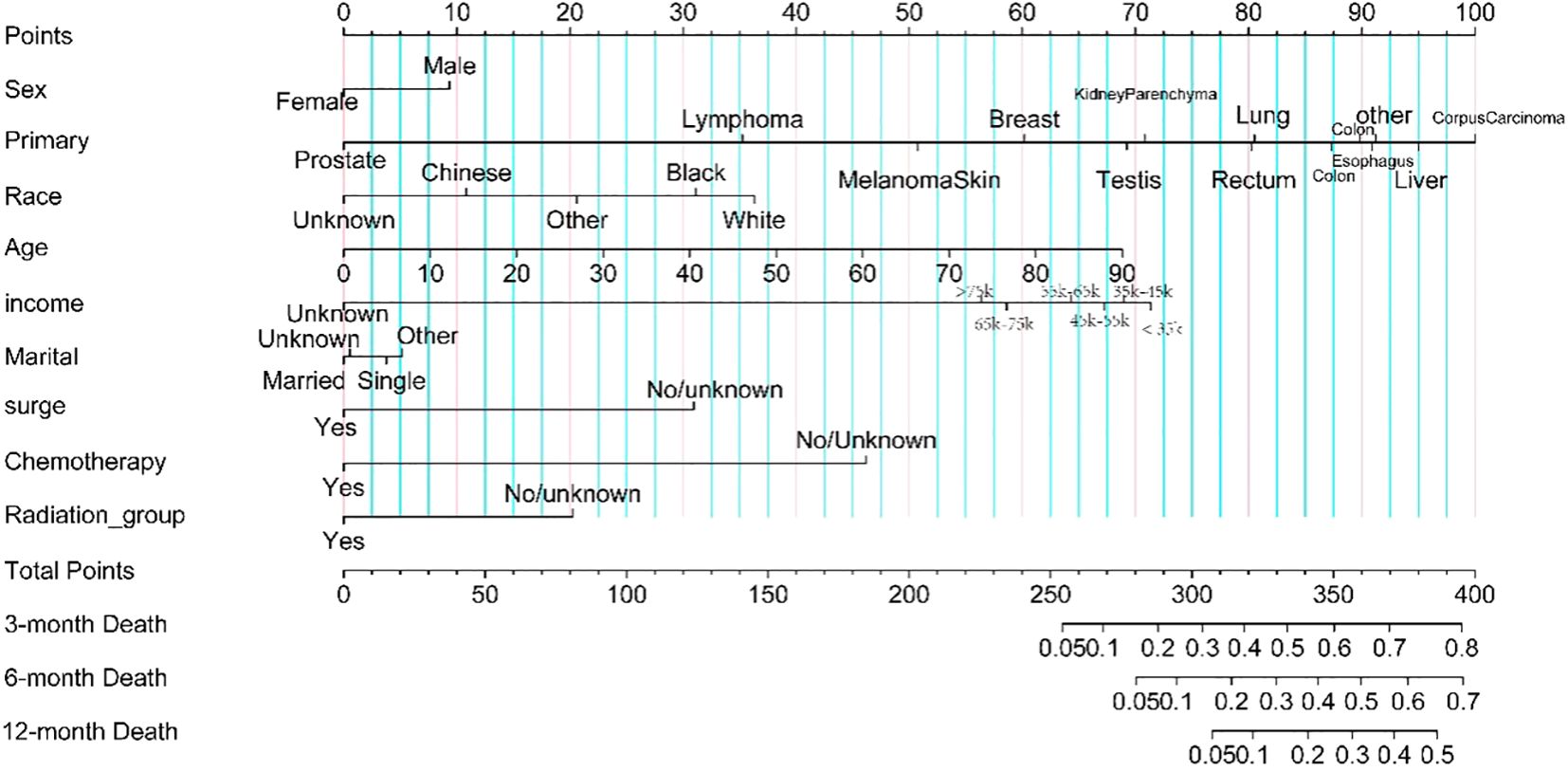
Seventy percent of patients with brain metastases were randomly assigned to the training set, while 30% were assigned to the test set. The prediction model incorporated variables related to survival, such as sex, age, race, income, marital status, surgery, radiotherapy, and chemotherapy. To enhance usability, we created a nomogram (see Figure 4) that assesses the importance of these variables in predicting patient survival. Higher scores indicate a more significant impact on survival. The model’s robustness was evaluated using the C-index, ROC, and calibration curves. It showed excellent performance in both sets, with calibration curves in Figures 5A, B indicating that predicted survival values at 3, 6, and 12 months closely matched observed values, reflecting high stability. The C-index was 0.723 for the training set and 0.722 for the test set. The time-dependent AUC for the training set was 0.83, 0.80, and 0.77 for the respective time points, while the test set yielded values of 0.83, 0.80, and 0.76, as shown in Figures 5C, D.
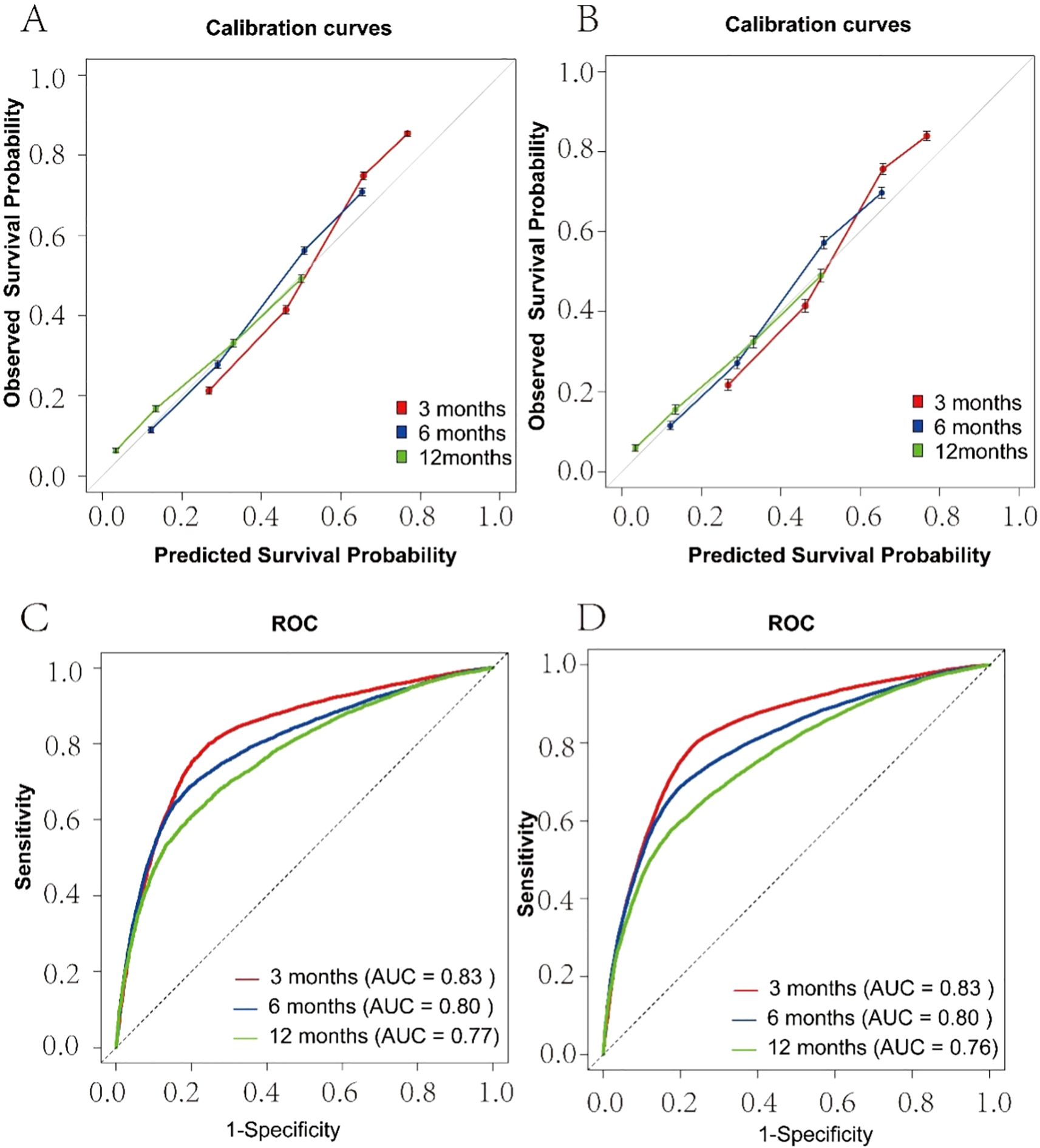
Figure 5. Calibration Plot and ROC for the Prediction of 3,6,12-months Survival Rate. (A) Training Set Calibration Curve; (B) Test Set Calibration Curve; (C) Training Set ROC; (D) Test Set ROC.
Discussion
Brain metastases are the most prevalent form of intracranial tumour (28). Historically, the prognosis for brain metastasis has been poor, with over half of the patients dying within 3 to 27 months of diagnosis (6, 29). This is consistent with our study findings, which underscore the significant burden brain metastases impose on patient survival. It is, therefore, crucial to analyse and explore the factors influencing survival in this context to inform subsequent treatments and reduce patient mortality risk. However, current research on brain metastasis primarily focuses on metastasis from a single primary site or lacks a practical tool for timely assessment of mortality hazard in patients with brain metastasis. Our study comprehensively compared risk factors for patients with brain metastases from different primary sites. This allowed us to identify commonalities and differences among brain metastases from various origins. For example, our study revealed that patients with brain metastases from liver cancer, lung cancer, colorectal cancer, and gastric cancer face the highest mortality risks, consistent with earlier research findings (30–32). This indicates the need for increased vigilance when treating patients with brain metastases from these primary sites. The survival differences in brain metastasis patients based on primary tumour location may be due to genetic differences associated with different primary tumour sites and types (33). For example, third-generation tyrosine kinase inhibitors, such as Osimertinib, have shown exceptional efficacy in lung adenocarcinoma patients with brain metastases and alterations in the EGFR or ALK genes (34). Likewise, for patients with HER2-positive breast cancer who progress to CNS involvement after initial local treatment, various HER2-directed therapies have been shown to provide the possibility of long-lasting responses in some cases (35, 36). Additionally, randomized phase II data suggest that dual immune checkpoint inhibition using ipilimumab and nivolumab could be a promising treatment option for patients with brain metastases from melanoma (37).
Furthermore, among the different types of brain metastases, those originating from lung cancer are the most prevalent, a finding that aligns with results from other research studies (38). Unfortunately, the prognosis for brain metastases from lung cancer is also poor. This highlights the necessity for enhanced surveillance of the risk of brain metastases in lung cancer patients, particularly those aged 65 and above. Furthermore, our study revealed that the mortality risk for lung cancer patients with brain metastases increases significantly after the age of 65. A similar age-related risk pattern is observed in brain metastases from both breast cancer and lymphoma. Surgery is a critical treatment option for patients with brain metastases. The decision to proceed with surgery is typically based on the size of the metastases and several other comprehensive factors. While it is widely believed that neurosurgical resection offers an OS benefit, some studies suggest that surgical resection significantly enhances OS and functional status compared to whole-brain radiotherapy in treating brain metastases (39, 40). However, it is generally believed that neurosurgical resection provides an OS benefit (41, 42). Some studies indicate that surgical resection is associated with significantly improved OS and functional status compared to whole-brain radiotherapy for treating brain metastases (43). In the present study, a stratified analysis was conducted to assess the survival benefits of surgery for each type of brain metastasis, focusing on different primary sites. The study results showed that most brain metastases responded positively to surgical intervention. However, patients with brain metastases from liver cancer, bladder cancer, or esophageal cancer did not demonstrate a significant survival benefit following surgery. This indicates that brain metastases from different primary sites may necessitate the implementation of bespoke, personalized treatment plans.
To enhance the standardized management and prognostic analysis of patients with brain metastases, we have diverged from earlier studies that developed distinct risk-scoring models for metastases originating from various primary sites (44–47). In contrast, the primary site of the brain tumour was included as a covariate in the model. This approach addresses the need for different risk prediction models for brain metastases from various origins and avoids the bias associated with treating all brain metastases as a homogeneous category (48). In conclusion, although our study provides valuable epidemiological insights into prognostic factors influencing survival in brain metastases, several limitations—such as the retrospective design, data quality constraints, lack of detailed treatment information, and absence of molecular profiling—highlight the need for well-designed prospective studies. Incorporating molecular and genetic determinants into predictive models represents an essential next step to improve risk stratification, treatment personalization, and overall clinical outcomes for patients with brain metastases.
Limitations of the study
This study has several limitations that should be acknowledged. First, its retrospective design based on data from the SEER database may introduce selection biases and preclude establishing definitive causal relationships. Second, the quality and completeness of data within the SEER database are inherently limited, with certain variables potentially incomplete or missing, thus potentially impacting the accuracy and generalizability of our findings. Additionally, detailed treatment information, including specifics regarding chemotherapy, radiotherapy, and immunotherapy, was not available, which could significantly influence patient survival outcomes. Furthermore, since the SEER database predominantly includes data from specific geographic regions, the generalizability of our results may be restricted by variations in race, socioeconomic status, and healthcare access. Moreover, the study did not incorporate potentially important prognostic factors such as genetic biomarkers, molecular characteristics, treatment responses, nor did it account for recurrence or progression of brain metastases, which might limit the precision of the predictive model. The primary focus on short-term survival analysis, with limited medium- and long-term follow-up data, further restricts the assessment of long-term survival outcomes and quality of life. Finally, the absence of detailed analysis regarding variations in surgical techniques, timing of surgical intervention, and institutional expertise may impact the robustness of our conclusions. Therefore, future research through large-scale prospective cohort studies is essential to validate and enhance the predictive accuracy of our findings.
Conclusions
The findings of our study indicate that brain metastases originating from different primary sites exhibit distinct survival patterns and age-specific mortality risks. Several clinical factors, including sex, age, primary cancer site, income level, race, and therapeutic interventions (e.g., surgery, chemoradiotherapy), were identified as significant prognostic factors influencing patient survival.Furthermore, we developed a stable, accurate, validated risk prediction model for brain metastases. Although our risk prediction model demonstrated stable and validated predictive performance, it remains limited by reliance on retrospective SEER database data, lacking detailed molecular markers, genetic profiles, and comprehensive treatment information. Future research should incorporate prospective cohort designs, integrate genetic and molecular biomarkers, and apply advanced analytical approaches to enhance the clinical applicability and accuracy of predictive models for patients with brain metastases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZY: Writing – original draft, Writing – review & editing. QJ: Writing – original draft, Writing – review & editing. XK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. LZ: Data curation, Writing – review & editing. FC: Supervision, Writing – review & editing. WL: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We are grateful for the financial support from the Talent Introduction Foundation of Tiantan Hospital (No. RCYJ-2020-2025-LWB to WL) and the Clinical Major Specialty Projects of Beijing (2-1-2-038 to WL). The study funders did not participate in the design, data collection, analysis, interpretation, or report writing. National Natural Science Foundation of China (Grant No. 32471036).
Acknowledgments
The authors thank the National Cancer Institute (USA) staff for contributing to the SEER program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Steeg PS et al: Brain metastases. Nat Rev Dis Primers. (2019) 5(1):5. doi: 10.1038/s41572-018-0055-y
2. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, and Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. (2012) 32(4):4655–62. Available online at: https://ar.iiarjournals.org/content/32/11/4655.
3. Kim H, Park S, Jung HA, Sun JM, Lee SH, Ahn JS, et al. Long-term survival in non-small cell lung cancer patients with metachronous brain-only oligorecurrence who underwent definitive treatment. Cancer Res Treat. (2022) 54:150–6. doi: 10.4143/crt.2021.306
4. Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, et al. Childhood brain tumour epidemiology: a brain tumour epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. (2014) 23:2716–36. doi: 10.1158/1055-9965.EPI-14-0207
5. Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. (2008) 10:R20. doi: 10.1186/bcr1870
6. Nayak L, Lee EQ, and Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. (2012) 14:48–54. doi: 10.1007/s11912-011-0203-y
7. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, and Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. (2004) 22:2865–72. doi: 10.1200/JCO.2004.12.149
8. Berghoff AS, Schur S, Füreder LM, Gatterbauer B, Dieckmann K, Widhalm G, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. (2016) 1:e000024. doi: 10.1136/esmoopen-2015-000024
9. Gori S, Rimondini S, De Angelis V, Colozza M, Bisagni G, Moretti G, et al. Central nervous system metastases in HER-2–positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. (2007) 12:766–73. doi: 10.1634/theoncologist.12-7-766
10. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, and Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. (2012) 23:103–12. doi: 10.1007/s10552-011-9859-8
11. Frinton E, Tong D, Tan J, Read G, Kumar V, Kennedy S, et al. Metastatic melanoma: prognostic factors and survival in patients with brain metastases. J Neurooncol. (2017) 135:507–12. doi: 10.1007/s11060-017-2591-9
12. Lauko A, Rauf Y, and Ahluwalia MS. Medical management of brain metastases. Neuro-Oncol Adv. (2020) 2:vdaa015. doi: 10.1093/noajnl/vdaa015
13. Chao ST, Barnett GH, Liu SW, Reuther AM, Toms SA, Vogelbaum MA, et al. Five-year survivors of brain metastases: a single-institution report of 32 patients. Int J Radiat Oncol Biol Phys. (2006) 66:801–9. doi: 10.1016/j.ijrobp.2006.05.015
14. Tsai C, Nguyen B, Luthra A, Chou JF, Feder L, Tang LH, et al. Coit DG et al: Outcomes and molecular features of brain metastasis in gastroesophageal adenocarcinoma. JAMA Netw Open. (2022) 5(9):e2228083–e2228083. doi: 10.1001/jamanetworkopen.2022.28083
15. Park K, Bae GH, Kim WK, Yoo C-J, Park CW, Kim S-K, et al. Radiotherapy for brain metastasis and long-term survival. Sci Rep. (2021) 11:8046. doi: 10.1038/s41598-021-87357-x
16. Mitchell D, Kwon H, Kubica P, Huff W, O’regan R, and Dey M. Brain metastases: An update on the multi-disciplinary approach of clinical management. Neurochirurgie. (2022) 68:69–85. doi: 10.1016/j.neuchi.2021.04.001
17. Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, et al. Chan KK et al: The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro-Oncology. (2020) 23:894–904. doi: 10.1093/neuonc/noaa285
18. Salari K, Lee JS, Ye H, Seymour ZA, Lee KC, Chinnaiyan P, et al. Long-term survival in patients with brain-only metastatic non-small cell lung cancer undergoing upfront intracranial stereotactic radiosurgery and definitive treatment to the thoracic primary site. Radiother Oncol. (2024) 196:110262. doi: 10.1016/j.radonc.2024.110262
19. Sperduto PW, Berkey B, Gaspar LE, Mehta M, and Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat OncolBiolPhys. (2008) 70:510–4. doi: 10.1016/j.ijrobp.2007.06.074
20. Stelzer KJ. Epidemiology and prognosis of brain metastases. Surg Neurol Int. (2013) 4:S192. doi: 10.4103/2152-7806.111296
21. Di Lorenzo R and Ahluwalia MS. Targeted therapy of brain metastases: latest evidence and clinical implications. Ther Adv Med Oncol. (2017) 9:781–96. doi: 10.1177/1758834017736252
22. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. (2020) 38:3773–84. doi: 10.1200/JCO.20.01255
23. Badiola I, Santaolalla F, Garcia-Gallastegui P, Unda F, and Ibarretxe G. Biomolecular bases of the senescence process and cancer. A new approach to oncological treatment linked to ageing. Ageing Res Rev. (2015) 23:125–38. doi: 10.1016/j.arr.2015.03.004
24. Harrell FE. “Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis, ” Springer Series in Statistics. New York, NY: Springer-Verlag (2001). doi: 10.1007/978-1-4757-3462-1
25. Ene CI and Ferguson SD. Surgical management of brain metastasis: challenges and nuances. Front Oncol. (2022) 12:847110. doi: 10.3389/fonc.2022.847110
26. Churilla TM, Chowdhury IH, Handorf E, Collette L, Collette S, Dong Y, et al. Comparison of local control of brain metastases with stereotactic radiosurgery vs surgical resection: a secondary analysis of a randomized clinical trial. JAMA Oncol. (2019) 5:243–7. doi: 10.1001/jamaoncol.2018.4610
27. Krist DT, Naik A, Thompson CM, Kwok SS, Janbahan M, Olivero WC, et al. Management of brain metastasis. Surgical resection versus stereotactic radiotherapy: a meta-analysis. Neurooncol Adv. (2022) 4(1):vdac033. doi: 10.1093/neuoncadv/vdac033
28. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: A Cancer J Clinicians. (2008) 58:71–96. doi: 10.3322/CA.2007.0010
29. Han YM, Cai G, Chai WM, Xu C, Cao L, Ou D, et al. Radiological distribution of brain metastases and its implication for the hippocampus avoidance in whole brain radiotherapy approach. Br J Radiol. (2017) 90:20170099. doi: 10.1259/bjr.20170099
30. Zhu Y, Zhou M, Li C, Kong W, and Hu Y. Gastric cancer with brain metastasis: from molecular characteristics and treatment. Front Oncol. (2024) 14:1310325. doi: 10.3389/fonc.2024.1310325
31. Menis J, Fontanella C, Follador A, Fasola G, and Aprile G. Brain metastases from gastrointestinal tumours: Tailoring the approach to maximize the outcome. Crit Rev Oncology/Hematol. (2013) 85:32–44. doi: 10.1016/j.critrevonc.2012.04.001
32. Mani K, Deng D, Lin C, Wang M, Hsu ML, and Zaorsky NG. Causes of death among people living with metastatic cancer. Nat Commun. (2024) 15:1519. doi: 10.1038/s41467-024-45307-x
33. Dagogo-Jack I, Gill CM, Cahill DP, Santagata S, and Brastianos PK. Treatment of brain metastases in the modern genomic era. Pharmacol Ther. (2017) 170:64–72. doi: 10.1016/j.pharmthera.2016.10.011
34. Wu Y-L, Ahn M-J, Garassino MC, Han J-Y, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. (2018) 36:2702–9. doi: 10.1200/JCO.2018.77.9363
35. Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol. (2019) 37:1081–9. doi: 10.1200/JCO.18.01511
36. Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. (2017) 3:1069–77. doi: 10.1001/jamaoncol.2017.0001
37. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
38. Stella GM, Corino A, Berzero G, Kolling S, Filippi AR, and Benvenuti S. Brain metastases from lung cancer: is MET an actionable target? Cancers. (2019) 11(3):271. doi: 10.3390/cancers11030271
39. Ratnaike TE, Das S, Gregson BA, and Mendelow AD. A review of brain abscess surgical treatment—78 years: aspiration versus excision. World Neurosurg. (2011) 76:431–6. doi: 10.1016/j.wneu.2011.03.048
40. Lamba N, Cagney DN, Brigell RH, Martin AM, Besse LA, Catalano PJ, et al. Neurosurgical resection and stereotactic radiation versus stereotactic radiation alone in patients with a single or solitary brain metastasis. World Neurosurg. (2019) 122:e1557–61. doi: 10.1016/j.wneu.2018.11.100
41. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1040–8. doi: 10.1016/S1470-2045(17)30414-X
42. Thapa B, Borghei-Razavi H M, Mohammadi A, and Ahluwalia M. An excellent clinical outcome with stereotactic radiosurgery in a geriatric patient with multiple and recurrent brain metastases. Cureus. (2017) 9:e1979. doi: 10.7759/cureus.1979
43. Mishra MV, Louie AV, Gondi V, and Slotman B. The evolving role of radiotherapy in the management of small cell lung cancer. J Thoracic Dis. (2018) 10:S2545–s2554. doi: 10.21037/jtd.2018.06.98
44. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Braunstein S et al: Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol. (2020) 38:3773–84. doi: 10.1200/JCO.20.01255
45. Nie Y, Ying B, Lu Z, Sun T, and Sun G. Predicting survival and prognosis of postoperative breast cancer brain metastasis: a population-based retrospective analysis. Chin Med J. (2023) 136:1699–707. doi: 10.1097/CM9.0000000000002674
46. Zhao Y, Gu S, Li L, Zhao R, Xie S, Zhang J, et al. Zhang S et al: A novel risk signature for predicting brain metastasis in patients with lung adenocarcinoma. Neuro Oncol. (2023) 25:2207–20. doi: 10.1093/neuonc/noad115
47. Deng Q, Wang F, Song L, Chen L, Huang Y, Guo Z, et al. Proteomics-based model for predicting the risk of brain metastasis in patients with resected lung adenocarcinoma carrying the EGFR mutation. Int J Med Sci. (2024) 21:765–74. doi: 10.7150/ijms.92993
48. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Shih H et al: Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. (2010) 77:655–61. doi: 10.1016/j.ijrobp.2009.08.025
Keywords: brain metastases, risk factors, prediction model, SEER, prognosis
Citation: Ji Q, Yang Z, Kang X, Zhou L, Chen F and Li W (2025) Analysis of prognostic factors and risk prediction in brain metastases: a SEER population-based study. Front. Oncol. 15:1523069. doi: 10.3389/fonc.2025.1523069
Received: 06 November 2024; Accepted: 18 April 2025;
Published: 21 May 2025.
Edited by:
Weimin Gao, Barrow Neurological Institute (BNI), United StatesReviewed by:
Eswari Dodagatta-Marri, University of California, San Francisco, United StatesAhmed Gilani, University of Colorado Hospital, United States
Copyright © 2025 Ji, Yang, Kang, Zhou, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Chen, Y2hlbmZlbmc0MDZAc2luYS5jb20=; Wenbin Li, bGl3ZW5iaW5AY2NtdS5lZHUuY24=
†These authors have contributed equally to this work
 Qiang Ji1,2†
Qiang Ji1,2† Zixuan Yang
Zixuan Yang Wenbin Li
Wenbin Li