- 1Department of Urology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Urology, Urologic Surgery Center, Xinqiao Hospital, Third Military Medical University (Army Medical University), Chongqing, China
Background: Regarding the comparison of cardiovascular disease risk between gonadotropin-releasing hormone (GnRH) antagonists and GnRH agonists, there are discrepancies in results from different studies. Therefore, this meta-analysis was conducted to investigate whether degarelix could reduce cardiovascular disease risk.
Methods: We systematically searched the PubMed, Embase, Web of Science, and Cochrane Library databases with a search time limit of up to December 2023 for articles focusing on the use of degarelix, a GnRH antagonist, in prostate cancer, with an emphasis on articles comparing degarelix to GnRH agonists. Study endpoints included major adverse cardiovascular events, stroke, all-cause mortality, myocardial infarction, heart failure, and arrhythmia.
Results: A total of 1320 articles were retrieved, of which eight met our inclusion criteria and involved 138–065 patients. The pooled results showed no difference in the risk of major adverse cardiovascular events (hazard ratio [HR]=0.94, 95% confidence interval [CI]: 0.65–1.35; P=0.73), stroke (HR=0.89, 95% CI: 0.62–1.27; P=0.52), myocardial infarction (HR=0.98, 95% CI: 0.70–1.37; P=0.91), all-cause mortality (HR=1.09, 95% CI: 0.73–1.65; P=0.67), and arrhythmia (risk ratio=0.64, 95% CI: 0.15–2.76; P=0.55) between degarelix and GnRH agonists. However, degarelix reduced the risk of heart failure (HR=0.56, 95% CI: 0.36–0.88; P=0.01).
Conclusion: Further clarification on the effects of different androgen deprivation therapy modalities on cardiovascular disease is needed from future and larger prospective randomized controlled trials.
1 Introduction
With the exception of non-melanoma skin cancer, prostate cancer is the most common type of cancer diagnosed in males and the second largest cause of cancer-related deaths in the United States (US) (1). The incidence of prostate cancer was estimated to increase by 2–3% per year between 2015 and 2019; thus, the number of newly diagnosed prostate cancer cases in the US in 2024 is estimated to exceed 290 000, and the number of predicted deaths is estimated to exceed 35 000 (2).
The development of prostate cancer depends on androgens and androgen receptors; therefore, androgen deprivation therapy (ADT) is a commonly used treatment for the disease (3). ADT can be categorized into two main groups: drug treatment and surgical castration. Surgical castration is an orchiectomy, and the drugs used for therapy include gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists (4, 5). Owing to the irreversibility of orchiectomy and its psychological impact on patients, it is gradually being replaced with drug therapy. Currently, the commonly used GnRH agonists include leuprorelin, goserelin, buserelin, and triptorelin, whereas GnRH antagonists include degarelix and relugolix, the former being administered via subcutaneous injection and the latter administered orally (6). Some studies have suggested that ADT increases the risk of cardiovascular disease (7, 8), which is the most common cause of death in patients with prostate cancer (9).
Degarelix inhibits the excitatory effects of endogenous GnRH on the pituitary gland by competitively binding to GnRH receptors, thereby inhibiting follicle-stimulating hormone (FSH) and luteinizing hormone (LH) production and directly decreasing testosterone levels such that no testosterone surge occurs. Results from a 1-year, randomized, open-label phase III trial (CS21) showed that degarelix was similar to leuprorelin in inducing and maintaining low serum testosterone levels (≤0.5 ng/mL); it significantly induced prostate-specific antigen and testosterone suppression faster than leuprorelin (10). GnRH agonists, however, regulate testosterone levels through a negative feedback pathway mechanism of the hypothalamic-pituitary-gonadal axis; the initial use of the drug can lead to a sharp increase in testosterone levels, and the increase in testosterone may induce or exacerbate urinary retention, bone pain, and spinal cord compression, leading to worsening of clinical symptoms (6, 11, 12). It has been suggested that the transient increase in testosterone induced by GnRH agonists promotes angiogenesis and neutrophil aggregation in atherosclerotic plaques, leading to plaque instability and an increased likelihood of rupture (13), which may be one of the reasons why GnRH agonists are associated with a greater risk of cardiovascular disease than GnRH antagonists. Additionally, it has been proposed that GnRH antagonists inhibit both LH and FSH, whereas GnRH agonists primarily inhibit LH, and that the difference in FSH between the two may explain the difference in cardiovascular disease risk (5, 14). Although GnRH agonists cause testosterone levels to fluctuate, both GnRH agonists and antagonists suppress serum testosterone levels, which are independent predictors of metabolic syndrome in men (15, 16), and increase the risk of cardiovascular disease.
The main mechanisms of using GnRH agonists in clinical practice include the initial “Flare-up effect” and long-term effects (continuous excitation leads to pituitary desensitization and eventually inhibits testosterone to castration levels (<50 ng/dL)). The main mechanisms of GnRH antagonists include direct receptor blocking, rapid testosterone reduction (to castration levels within 24 hours), and sustained inhibition. The advantages of GnRH antagonists in cardiovascular integrity have been supported by some studies, and they are suitable for patients with concurrent cardiovascular diseases or those requiring rapid testosterone suppression. However, GnRH agonists remain the standard choice for most patients in the stable stage due to their relatively low cost. Clinical decisions need to take into account the disease stage, complications, economic factors and patient preferences comprehensively, and be dynamically adjusted with reference to the latest guidelines. There is still controversy regarding the risk of cardiovascular disease between GnRH antagonists and agonists, with some studies suggesting similar risk (17–19), and others suggesting that GnRH antagonists reduce the risk of cardiovascular disease (20–25). Owing to this, we conducted a review and meta-analysis of published results to explore whether degarelix, a GnRH antagonist, reduces the risk of cardiovascular disease.
2 Materials and methods
2.1 Search strategy
We conducted and report this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and registered it with the International Prospective Register of Systematic Reviews (ID: CRD42024503998). We systematically searched the PubMed, Embase, Web of Science, and Cochrane Library databases with a search time limit up to December 2023 for articles focusing on the use of degarelix in patients with prostate cancer. We searched the following combination of Medical Subject Headings (MeSH) and related keywords: ‘Prostatic Neoplasms [Mesh] or Prostate Neoplasms or Prostate Cancer or Prostatic Cancer’ and ‘degarelix’.
2.2 Inclusion and exclusion criteria
We developed inclusion criteria on the basis of the PICOS principles: (1) population, patients diagnosed with prostate cancer by histopathologic examination; (2) intervention, treatment of prostate cancer with degarelix; (3) comparison, treatment of prostate cancer with GnRH agonists; (4) outcome, comparison of the risk of cardiovascular disease between degarelix and GnRH agonists, including major adverse cardiovascular events (MACEs, defined as the composite endpoint of stroke, myocardial infarction, or death from any cause), stroke, all-cause mortality, myocardial infarction, heart failure, and arrhythmia; and (5) study design, we had no restrictions on the article study design. The exclusion criteria were as follows: lack of relevant outcome indicators, studies that did not discuss cardiovascular disease risk, reviews, commentaries, letters, conference abstracts, and animal studies.
2.3 Quality assessment and data extraction
Two independent researchers reviewed the titles and abstracts of the studies. Then, a full-text search of articles meeting the inclusion criteria was performed, and quality assessment and data extraction were completed. In cases of disagreement, a decision was made after discussion with a third researcher. Two independent researchers extracted the following data from the articles based on a pre-designed table: authors, date of publication, country, study design, sample size, and treatment. For randomized controlled trials, the Risk of Bias tool (RoB 2) was used for quality assessment, while the Newcastle–Ottawa Scale (NOS) was used for the quality assessment of non-randomized controlled trials. Disagreements between the researchers were resolved through negotiation.
2.4 Statistical analysis
Study effect indicators are presented as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) or relative risks (RRs) and corresponding 95% CIs. For our meta-analysis, we calculated the overall HR or RR and 95% CI using Stata (version 15.0; StataCorp, College Station, TX, USA). The I2 test was used to assess heterogeneity across studies, using a random-effects model if I2 > 50% and a fixed-effects model if I2 < 50%. If heterogeneity was evident, a subgroup analysis was performed to determine the source. We used the Egger test to assess publication bias, which suggested the presence of publication bias if the P-value was <0.05. We also performed sensitivity analysis using the literature-by-exclusion method to assess the robustness of the results.
3 Results
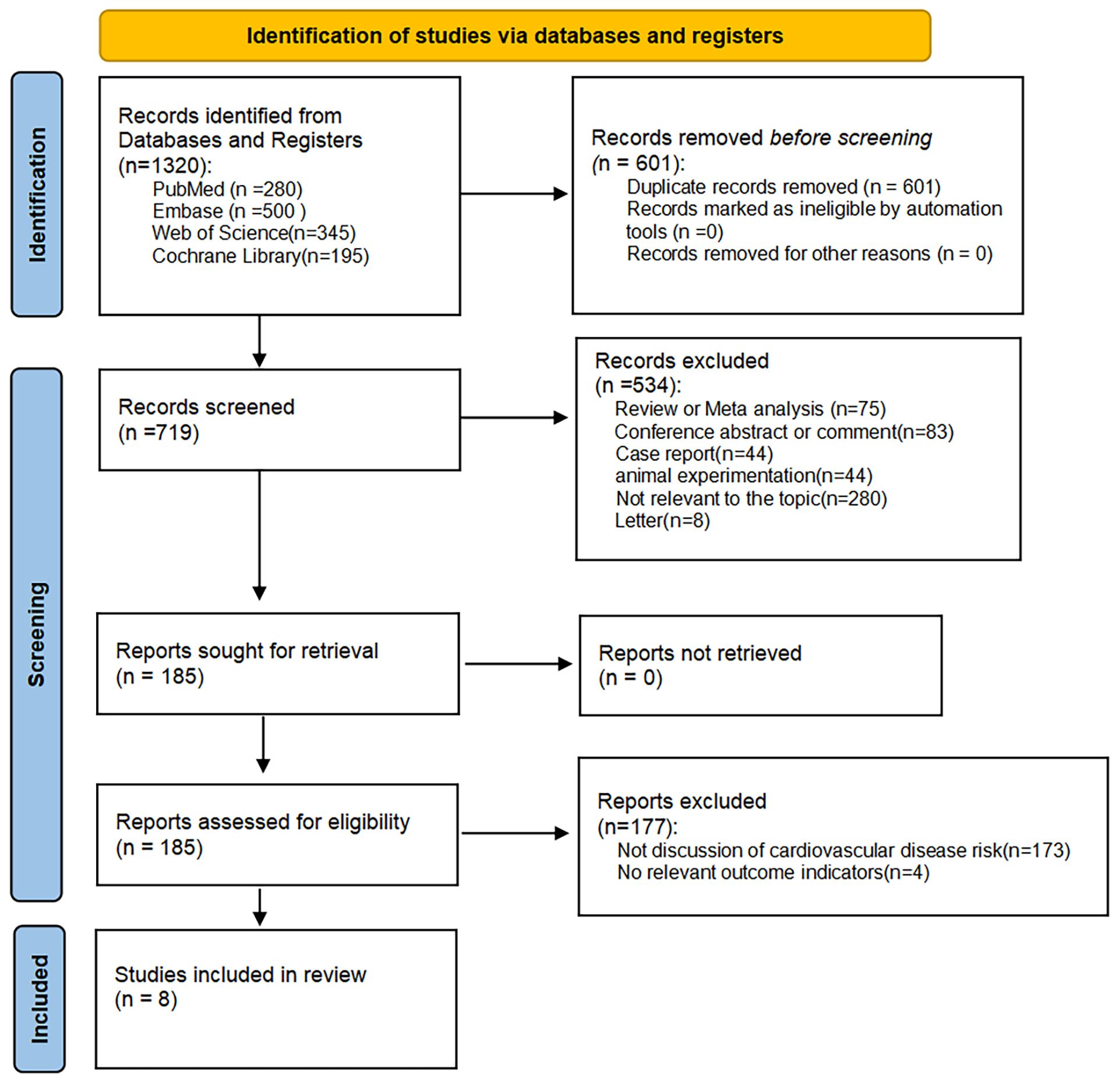
We obtained 1320 articles by searching multiple databases; 601 articles were excluded because of duplication, and 534 articles were excluded for the following reasons after reading the titles and abstracts: irrelevance to the topic of our study, systematic review, meta-analysis, conference abstracts, case reports, letters, and animal studies. Of the remaining 185 articles, 177 were excluded because they did not focus on cardiovascular disease risk and did not have relevant outcome indicators; thus, eight articles were included in our meta-analysis (23, 26–32) (Figure 1).
3.1 Study characterization and quality assessment
We included eight studies from five countries, including 138–065 patients (23, 26–32). The articles were published between 2021 and 2023: three from the US, two from China, and three from Italy, the United Kingdom, and Canada. One of these was a randomized controlled study and the remaining seven were retrospective cohort studies. Cardiovascular disease risks of interest for inclusion in the study included MACEs, stroke, all-cause mortality, myocardial infarction, heart failure, arrhythmia, and ischemic heart disease. We used the RoB 2 to assess the quality of the randomized controlled trial (29), which assessed some risk for both the randomization process and deviation from the established intervention components. This was due to differences in the mode of administration (subcutaneous versus [vs.] intramuscular) and frequency of administration (monthly vs. every 3 months) between degarelix and leuprorelin during the trial, which made it impossible to blind the patients and nurses who administered the drugs. For non-randomized controlled trials (23, 26–28, 30–32), we assessed study quality using the NOS, which showed that all studies scored between 7 and 9 and were of high quality. The characteristics of every included study are shown in Table 1.
3.2 Synthesis of results
Of all the included studies, five of which had MACEs as the endpoint (26, 29–32), our pooled results showed that the risk of MACEs was similar for both degarelix and GnRH agonists compared to each other (HR=0.94, 95% CI: 0.65–1.35; P=0.73). Because there was heterogeneity across studies (I2=70.8%, P=0.01, Figure 2A), a random-effects model was used, and a subgroup analysis was conducted to identify sources of heterogeneity. Of the five included studies, two compared degarelix with leuprorelin, triptorelin, goserelin, and buserelin (26, 31), and three compared degarelix with leuprorelin (29, 30, 32). We categorized the former into subgroup 1 and the latter into subgroup 2. The results suggested no heterogeneity within the two subgroups (subgroup 1: I2=0.0%, P=0.38; subgroup 2: I2=0.0%, P=0.86; Figure 3A); therefore, the difference in the contrasting drugs was considered a source of heterogeneity.
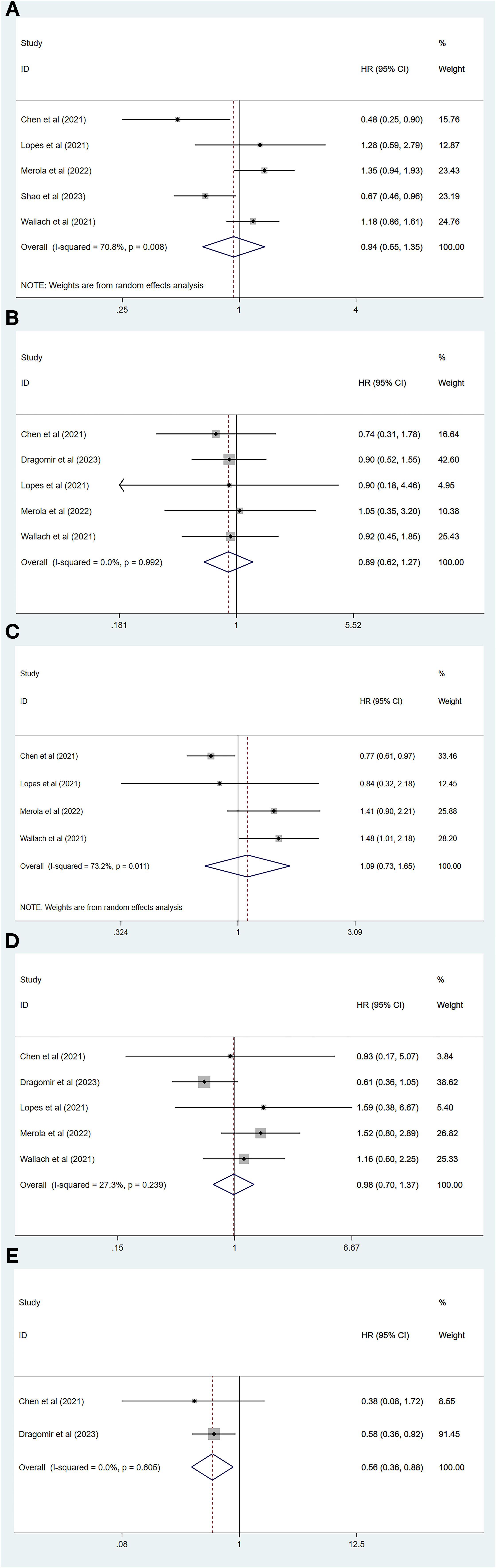
Figure 2. Forest plot comparing cardiovascular disease risk between degarelix and gonadotropin-releasing hormone agonists. (A) Forest plot comparing risk of major adverse cardiovascular event between degarelix and GnRH agonists. (B) Forest plot comparing risk of stroke between degarelix and GnRH agonists. (C) Forest plot comparing risk of all-cause mortality between degarelix and GnRH agonists. (D) Forest plot comparing risk of myocardial infarction between degarelix and GnRH agonists. (E) Forest plot comparing risk of heart failure between degarelix and GnRH agonists. HR, hazard ratio; CI, confidence interval.
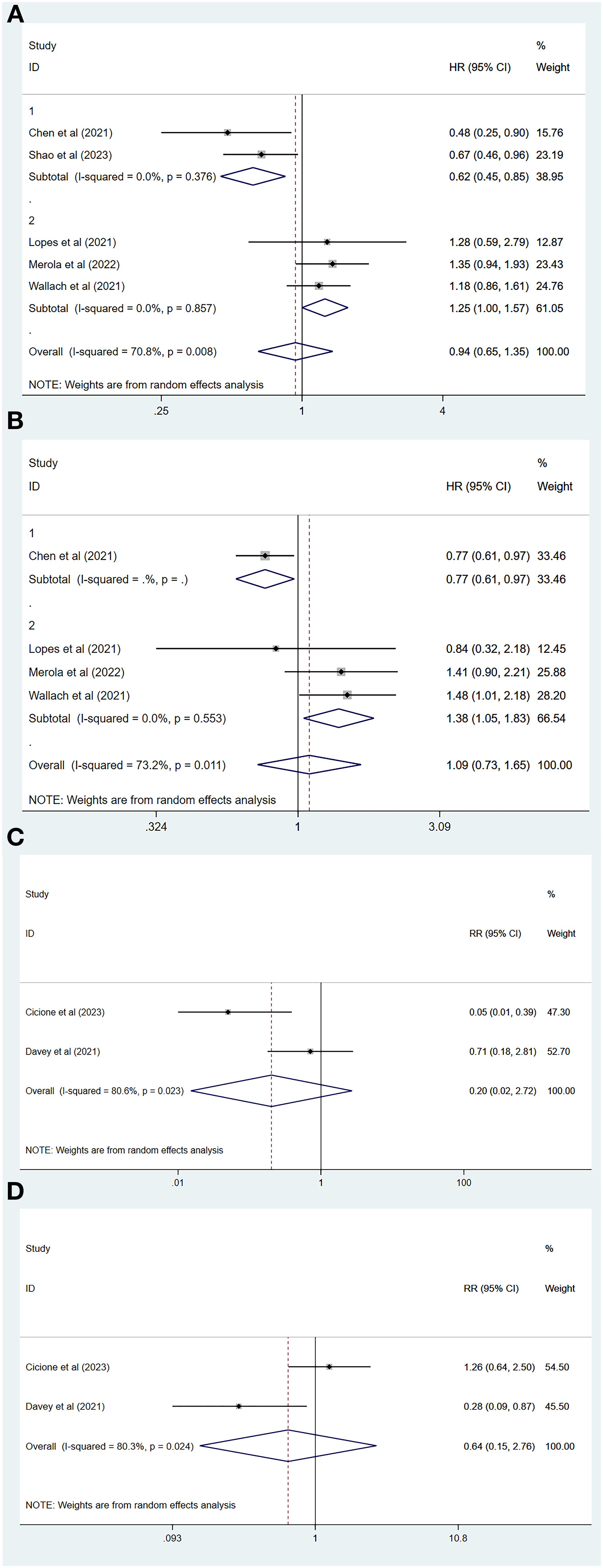
Figure 3. Subgroup analysis and forest plot with RR as a summary indicator. (A) Subgroup analysis comparing major adverse cardiovascular event between degarelix and GnRH agonists. (B) Subgroup analysis comparing all-cause mortality between degarelix and GnRH agonists. (C) Forest plot comparing risk of myocardial infarction between degarelix and GnRH agonists. (D) Forest plot comparing risk of arrhythmia between degarelix and GnRH agonists. RR, relative risk; CI, confidence interval.
Five of all studies focused on stroke as the endpoint (26, 28–30, 32), and our combined results showed no significant difference in the risk of stroke between degarelix and GnRH agonists (HR=0.89, 95% CI: 0.62–1.27, P=0.52). A fixed-effects model was used because there was no heterogeneity among the five studies (I2 = 0.0%, P=0.99, Figure 2B).
A total of four studies had an endpoint of all-cause mortality (26, 29, 30, 32), and the pooled results suggested a similar risk of all-cause mortality between degarelix and GnRH agonists (HR=1.09, 95% CI: 0.73–1.65, P=0.67). We used a random-effects model to pool the results because of the significant heterogeneity among the studies (I2=73.2%, P=0.01, Figure 2C). Three of these studies compared degarelix to leuprorelin (29, 30, 32), and were included in a subgroup, with pooled results suggesting no heterogeneity among studies within this subgroup (I2=0.0%, P=0.55; Figure 3B). Therefore, the consideration of heterogeneity came from comparing degarelix with different GnRH agonists.
Five studies focused on myocardial infarction as the endpoint (26, 28–30, 32) and the combined results suggested that degarelix did not show a lower risk of myocardial infarction than GnRH agonists (HR=0.98, 95% CI: 0.70–1.37, P=0.91). Heterogeneity between the studies was not significant (I2=27.3%, P=0.24); therefore, a fixed-effects model was used (Figure 2D).
Two of all the articles focused on heart failure as a study endpoint (26, 28), and the combined results suggested that degarelix reduces the risk of heart failure (HR=0.56, 95% CI: 0.36–0.88, P=0.01). There was no heterogeneity among the studies (I2=0.0%, P=0.61); therefore, a fixed-effects model was used (Figure 2E). Because of the small number of included studies, publication bias and sensitivity analysis were not performed.
Two studies used RR as the outcome metric (23, 27), with the common endpoints of interest being myocardial infarction and arrhythmia, and the pooled results suggesting that degarelix and GnRH agonists have a similar risk of myocardial infarction (RR=0.20, 95% CI: 0.02–2.72, P=0.23; Figure 3C) and arrhythmia (RR=0.64, 95% CI: 0.15–2.76, P=0.55; Figure 3D). We combined the data using a random-effects model because of the heterogeneity between the two studies regarding myocardial infarction (I2=80.6%, P=0.02) and the two studies concerning arrhythmia (I2=80.3%, P=0.02). As there were not enough included studies, subgroup analysis, sensitivity analysis, and publication bias evaluations could not be performed.
3.3 Publication bias
The Egger test showed no significant publication bias in studies with the following endpoints: MACEs (P=0.59), stroke (P=0.93), all-cause mortality (P=0.51), and myocardial infarction (P=0.57) (Figure 4).
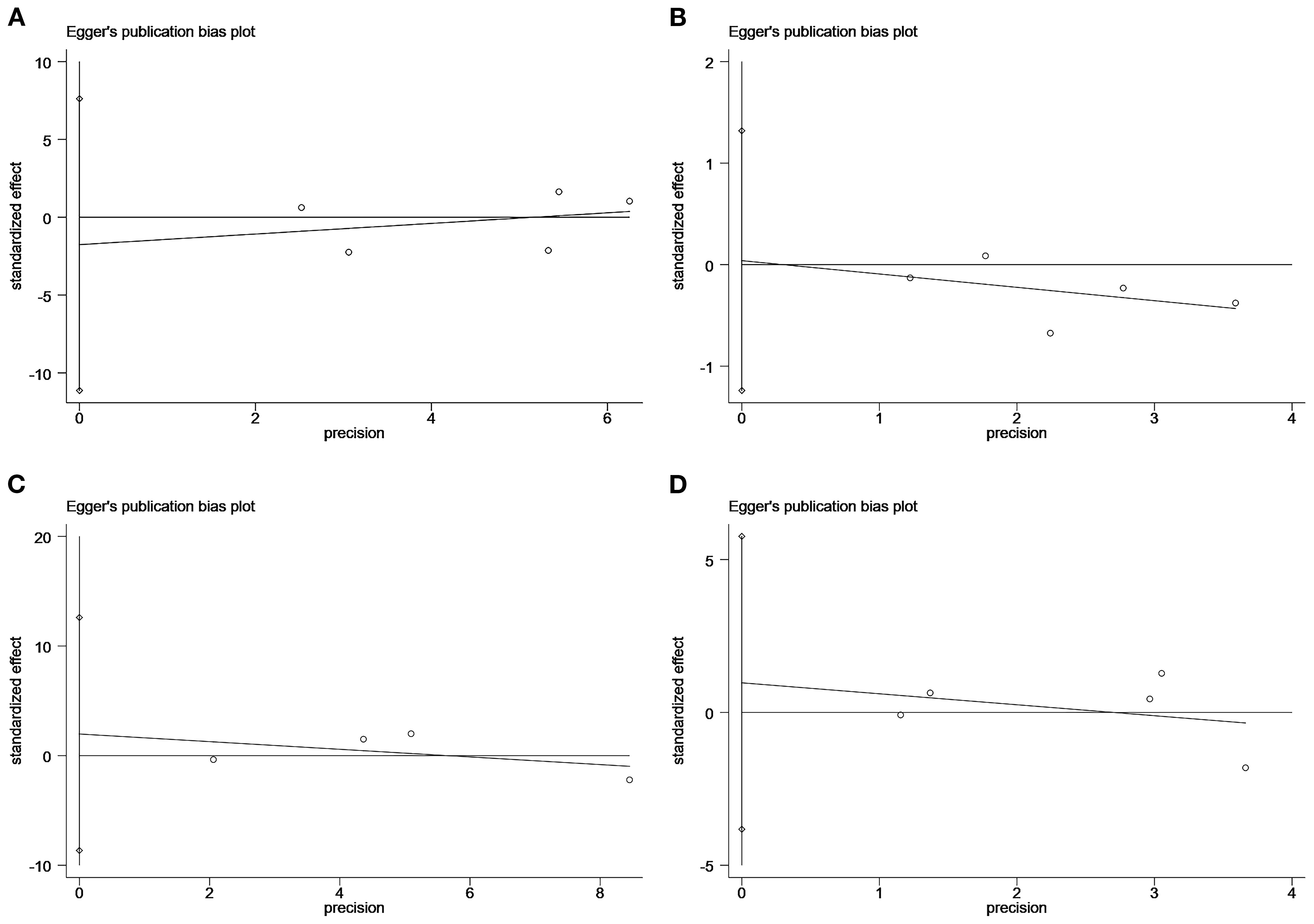
Figure 4. Plot of the Egger’s test for publication bias: major adverse cardiovascular event (A); stroke (B); all-cause mortality (C); myocardial infarction (D).
3.4 Sensitivity analysis
We performed sensitivity analyses of articles with MACEs, stroke, all-cause mortality, and myocardial infarction as the endpoints using the literature-by-exclusion method. We found that the exclusion of any of the studies had no effect on the pooled results (Figure 5), suggesting that our results are reliable and robust.
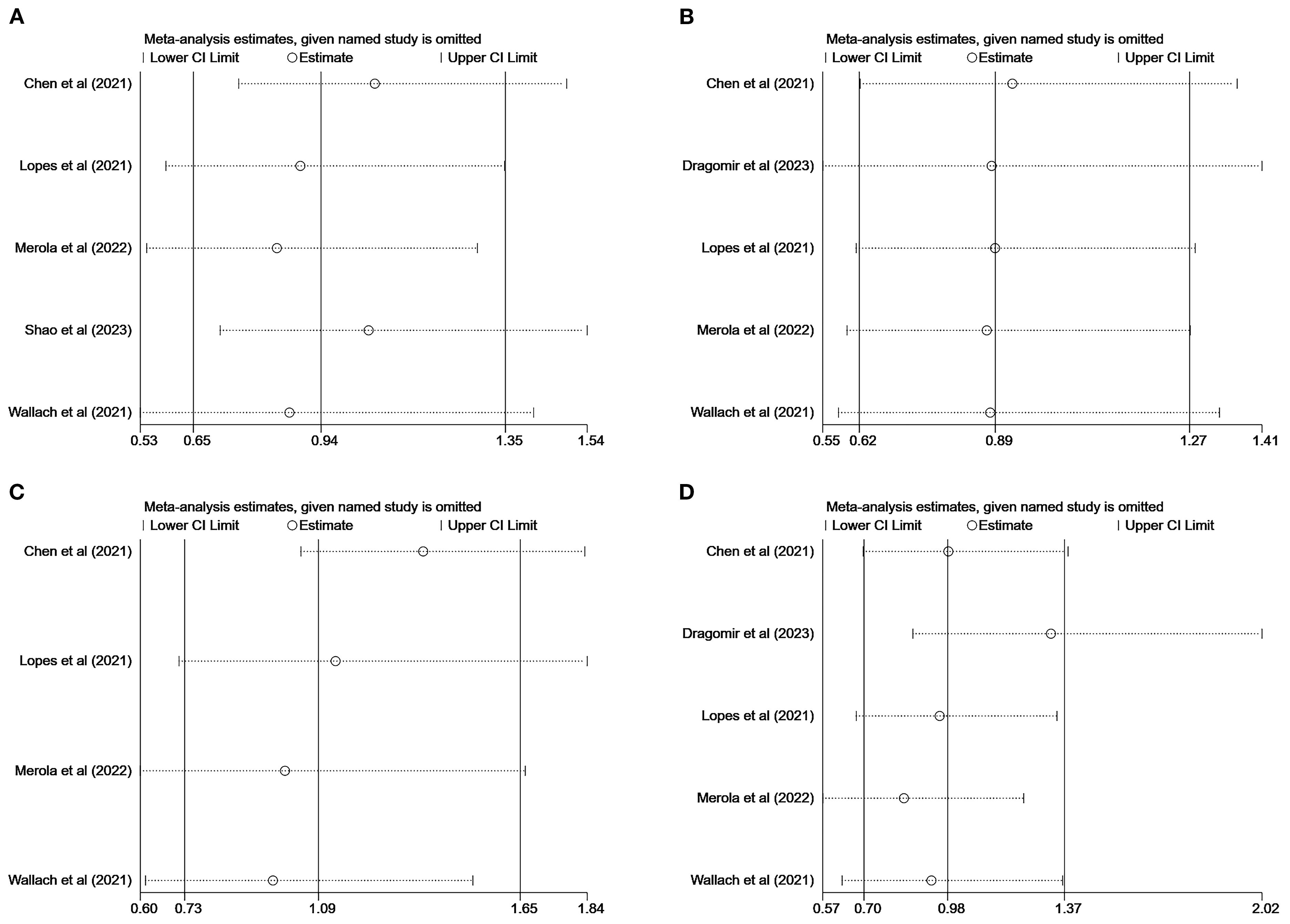
Figure 5. Sensitivity analysis: major adverse cardiovascular event (A); stroke (B); all-cause mortality (C); myocardial infarction (D).
4 Discussion
Comparing the risk of cardiovascular disease between degarelix and GnRH agonists was our main study objective, and the meta-analysis of the included studies suggested that there was no difference in the risk of MACEs, stroke, myocardial infarction, all-cause mortality, or arrhythmia between degarelix and GnRH agonists; however, degarelix was shown to reduce the risk of heart failure. These results are similar to those of a prospective international randomized clinical trial (PRONOUNCE trial) (29).
There are currently conflicting views regarding whether ADT in patients with prostate cancer increases the risk of cardiovascular disease. A pooled analysis of the results of eight randomized trials by Nguyen et al. (33) showed that the risk of cardiovascular death was similar in patients who received ADT compared to controls (RR=0.93, 95% CI: 0.79–1.10, P=0.41). Similarly, an opinion by Alibhai et al. (34) suggests that the continuous use of ADT for at least 6 months is linked to a higher risk of diabetes mellitus (HR=1.16, 95% CI: 1.11–1.21) and fragility fracture (HR=1.65, 95% CI: 1.53–1.77), but there is no increased risk of sudden cardiac death (HR=0.96, 95% CI: 0.83–1.10) or acute myocardial infarction (HR=0.91, 95% CI: 0.84–1.00). However, Cardwell et al. (7) reported that ADT leads to a 30% increased risk of cardiovascular events (HR=1.30, 95% CI: 1.20–1.40) and suggested that both GnRH agonists (HR=1.30, 95% CI: 1.20–1.40) and degarelix (HR=1.50, 95% CI: 1.20–1.90) lead to an increased risk of cardiovascular events. In addition, Taylor et al. (8) reported a 17% increase in cardiovascular-related mortality with the use of ADT in patients with prostate cancer (HR=1.17, 95% CI: 1.07–1.29).
GnRH agonists and antagonists induce and maintain testosterone suppression, and there is a positive correlation between physiologic testosterone levels and vascular health; low testosterone levels are associated with hypertension, decreased bone density, abnormal glucose metabolism, and increased cardiovascular risk (35, 36). These adverse effects are part of metabolic syndrome. Muller et al. (15) conducted a cross-sectional study and found that higher testosterone levels in men were independently associated with increased insulin sensitivity and reduced risk of metabolic syndrome. Similarly, a longitudinal study by Laaksonen et al. (16) showed that low testosterone levels in men led to an increased risk of metabolic syndrome and diabetes mellitus. The use of ADT in patients with prostate cancer leads to a higher percentage of abdominal obesity and a higher prevalence of hyperglycemia, which may lead to increased body mass index, dyslipidemia, and decreased insulin sensitivity (37–39). Men with metabolic syndrome have an increased risk of cardiovascular disease and all-cause mortality even in the absence of baseline cardiovascular disease or diabetes mellitus (40, 41). Although GnRH agonists and antagonists have different mechanisms of action, they both suppress testosterone, which may explain the similarity in cardiovascular disease risk between the two.
Some studies have suggested that GnRH agonists are associated with a higher risk of cardiovascular disease than antagonists, possibly because of the differences in FSH levels between the two. GnRH agonists activate the expression of GnRH receptors in pituitary cells, leading to elevated FSH levels, which begin to decrease when GnRH receptors in pituitary cells are gradually desensitized (5), whereas GnRH antagonists directly inhibit FSH and LH production by rapidly and competitively binding to the GnRH receptor and blocking GnRH from binding to its receptor. FSH levels in patients treated with GnRH agonists do not fall as low as those in patients treated with GnRH antagonists because the former primarily inhibit LH, whereas the latter inhibit both LH and FSH (14). Based on the differences in FSH levels, some researchers have hypothesized that FSH affects cardiovascular diseases. The results of an animal study by Han et al. (5) suggested that FSH leads to the progression of atherosclerosis and destabilizes plaques by promoting the inflammatory response and migration of macrophages. Similarly, Wang et al. (14) reported that FSH accelerates atherosclerosis by exacerbating endothelial inflammation and promoting endothelial adhesion of monocytes, thereby contributing to ADT-associated cardiovascular disease. We speculate that degarelix’s reduction of the risk of heart failure may be related to the following mechanisms: Firstly, as a GnRH antagonist, degarelix can rapidly and directly lower testosterone levels, which may reduce the direct adverse effects of androgens on the heart. Secondly, degarelix may improve cardiovascular function by regulating inflammatory responses and enhancing endothelial function. Moreover, the mechanism and hormonal level changes of degarelix differ from those of GnRH agonists, which may be the reason for the differences in cardiovascular endpoint risks. For example, GnRH agonists have a “flare-up” phenomenon, which may have adverse effects on the cardiovascular system.
We compared the risk of cardiovascular disease between degarelix and GnRH agonists by performing a systematic and comprehensive search of databases, and subgroup and sensitivity analyses demonstrated the reliability and stability of the results. The results of this study may have certain significance for clinical treatment decisions: First, in terms of risk assessment, a comprehensive cardiovascular risk assessment was conducted for all prostate cancer patients, including medical history, physical examination and necessary laboratory tests; Secondly, in terms of treatment options, for patients with a history of cardiovascular diseases or a high risk of cardiovascular events, digarec may be a better choice. Thirdly, in terms of risk management, all prostate cancer patients receiving ADT should receive active cardiovascular risk management, including lifestyle intervention and drug treatment. Closely monitor the cardiovascular conditions of patients receiving degarix treatment.
In addition, this article also has potential utility in other fields: First, in oncology and endocrine therapy, the methods of this study can be extended to the drug safety assessment of other hormone-dependent cancers (such as breast cancer), and compare the cardiovascular risks of different endocrine therapies; Secondly, in terms of cardiovascular drug safety research, similar methods can be used to evaluate the cardiovascular effects of new hypoglycemic drugs or immune checkpoint inhibitors; Thirdly, in terms of drug regulation and clinical guideline formulation, regulatory agencies (such as the FDA and EMA) can refer to such meta-analyses to optimize drug safety warnings or indication recommendations, and clinical guidelines (such as NCCN and ESC) can adjust treatment recommendations based on high-quality evidence, such as giving priority to drugs with lower cardiovascular risks. Fourth, in terms of integrating real-world evidence (RWE), in the future, randomized controlled trials (RCTS) and real-world data (such as electronic health records) can be combined to further verify the conclusions of meta-analyses. However, there are some limitations to our study. Among the included studies, only one was a randomized controlled trial (RCT), and the remaining seven were retrospective cohort studies. Retrospective studies are vulnerable to selection bias, information bias and confounding factors (for example, factors such as patients’ baseline cardiovascular risk, comorbidities, lifestyle, etc. may affect the research results), which may affect the reliability of the research results. The evidence level of RCT is higher, but this study has some risks in terms of deviations in the randomization process and intervention measures, which may affect the interpretation of the research results. At present, the RCT studies for diagnosing Degarelix are limited and a sufficient number have not been included in this article.
5 Conclusion
Overall, the risks of MACEs, stroke, myocardial infarction, all-cause mortality, and arrhythmia were similar between degarelix and GnRH agonists; however, degarelix reduced the risk of heart failure. There is a need to monitor the potential side effects of ADT, especially in patients with cardiovascular disease at baseline. Regarding the effects of different ADT modalities on cardiovascular disease, larger prospective randomized controlled trials are needed for further clarification.
Author contributions
WL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. ZL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. LS: Conceptualization, Formal Analysis, Investigation, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. HZ: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft. YL: Conceptualization, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. JZ: Conceptualization, Formal Analysis, Project administration, Supervision, Validation, Writing – original draft. SS: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. DW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the doctoral program of the first affiliated hospital of Chongqing Medical University (CYYY-BSYJSCXXM-202332).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GnRH, Gonadotropin-releasing hormone; MACE, Major adverse cardiovascular event; HR, Hazard ratio; CI, Confidence interval; ADT, Androgen deprivation therapy; FSH, Follicle-stimulating hormone; LH, Luteinizing hormone; MeSH, Medical Subject Headings; NOS, Newcastle–Ottawa Scale; RR, Relative risk; RoB 2, Risk of Bias.
References
1. Pinsky PF and Parnes H. Screening for prostate cancer. N Engl J Med. (2023) 388:1405–14. doi: 10.1056/NEJMcp2209151
2. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. Ca-Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. Debes JD and Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. (2004) 351:1488–90. doi: 10.1056/NEJMp048178
4. Schröder F, Crawford ED, Axcrona K, Payne H, and Keane TE. Androgen deprivation therapy: past, present and future. Bju Int. (2012) 109 Suppl 6:1–12. doi: 10.1111/j.1464-410X.2012.11215.x
5. Han JL, Song YX, Yao WJ, Zhou J, and Du Y. Xu, T. Follicle-stimulating hormone provokes macrophages to secrete il-1β contributing to atherosclerosis progression. J Immunol. (2022). doi: 10.4049/jimmunol.2200475
6. Liu YF, Fu SQ, Yan YC, Gong BB, Xie WJ, Yang XR, et al. Progress in clinical research on gonadotropin-releasing hormone receptor antagonists for the treatment of prostate cancer. Drug Des Devel Ther. (2021) 15:639–49. doi: 10.2147/DDDT.S291369
7. Cardwell CR, O’Sullivan JM, Jain S, Harbinson MT, Cook MB, Hicks BM, et al. The risk of cardiovascular disease in prostate cancer patients receiving androgen deprivation therapies. Epidemiology. (2020) 31:432–40. doi: 10.1097/EDE.0000000000001132
8. Taylor LG and Canfield SE. Du XL Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. (2009) 115:2388–99. doi: 10.1002/cncr.24283
9. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–7. doi: 10.1093/annonc/mdw604
10. Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase iii study in patients with prostate cancer. Bju Int. (2008) 102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x
11. Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, and Kold OT. Degarelix: a novel gonadotropin-releasing hormone (gnrh) receptor blocker–results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol. (2008) 54:805–13. doi: 10.1016/j.eururo.2008.04.065
12. Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N, Crawford ED, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (cs21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol. (2010) 57:836–42. doi: 10.1016/j.eururo.2009.11.029
13. Tisseverasinghe S, Tolba M, Saad F, Gravis G, Bahoric B, and Niazi T. Should prostate cancer patients with history of cardiovascular events be preferentially treated with luteinizing hormone-releasing hormone antagonists? J Clin Oncol. (2022) 40:4173–7. doi: 10.1200/JCO.22.00883
14. Wang Q, Han J, Liang Z, Geng X, and Du Y. Zhou, J.; Yao, W.; Xu, T. Fsh is responsible for androgen deprivation therapy-associated atherosclerosis in mice by exaggerating endothelial inflammation and monocyte adhesion. Arterioscler Thromb Vasc Biol. (2024) 44:698–719. doi: 10.1161/ATVBAHA.123.319426
15. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, and van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. (2005) 90:2618–23. doi: 10.1210/jc.2004-1158
16. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. (2004) 27:1036–41. doi: 10.2337/diacare.27.5.1036
17. Smith MR, Klotz L, Persson BE, Olesen TK, and Wilde AA. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase iii trial in patients with prostate cancer. J Urol. (2010) 184:2313–9. doi: 10.1016/j.juro.2010.08.012
18. Scailteux LM, Vincendeau S, Balusson F, Leclercq C, Happe A, Le Nautout B, et al. Androgen deprivation therapy and cardiovascular risk: no meaningful difference between gnrh antagonist and agonists-a nationwide population-based cohort study based on 2010–2013 french health insurance data. Eur J Cancer. (2017) 77:99–108. doi: 10.1016/j.ejca.2017.03.002
19. George G, Garmo H, Scailteux LM, Balusson F, De Coster G, De Schutter H, et al. Risk of cardiovascular disease following gonadotropin-releasing hormone agonists vs antagonists in prostate cancer: real-world evidence from five databases. Int J Cancer. (2021) 148:2203–11. doi: 10.1002/ijc.33397
20. Cirne F, Aghel N, Petropoulos JA, Klotz L, Lenihan DJ, Saad F, et al. The cardiovascular effects of gonadotropin-releasing hormone antagonists in men with prostate cancer. Eur Heart J.-Cardiovasc Pharmacother. (2022) 8:253–62. doi: 10.1093/ehjcvp/pvab005
21. Sciarra A, Busetto GM, Salciccia S, Del GF, Maggi M, Crocetto F, et al. Does exist a differential impact of degarelix versus lhrh agonists on cardiovascular safety? Evidences from randomized and real-world studies. Front Endocrinol. (2021) 12:695170. doi: 10.3389/fendo.2021.695170
22. Margel D, Peer A, Ber Y, Shavit-Grievink L, Tabachnik T, Sela S, et al. Cardiovascular morbidity in a randomized trial comparing gnrh agonist and gnrh antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. (2019) 202:1199–208. doi: 10.1097/JU.0000000000000384
23. Davey P and Kirby MG. Cardiovascular risk profiles of gnrh agonists and antagonists: real-world analysis from uk general practice. World J Urol. (2021) 39:307–15. doi: 10.1007/s00345-020-03433-3
24. Higano CS, Crawford ED, Shore ND, Bosnyak Z, Malmberg A, Neijber A, et al. Risk of cardiovascular events with degarelix versus leuprolide after biochemical relapse of prostate cancer: exploratory analysis of a randomized controlled trial. J Clin Oncol. (2015) 33. doi: 10.1200/jco.2015.33.7_suppl.151
25. Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, and Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. (2014) 65:565–73. doi: 10.1016/j.eururo.2013.10.032
26. Chen DY, Su PJ, See LC, Liu JR, Chuang CK, Pang ST, et al. Gonadotropin-releasing hormone antagonist associated with lower cardiovascular risk compared with gonadotropin-releasing hormone agonist in prostate cancer: a nationwide cohort and in vitro study. Prostate. (2021) 81:902–12. doi: 10.1002/pros.24187
27. Cicione A, Nacchia A, Guercio A, Gravina C, Franco A, Grimaldi MC, et al. Cardiovascular adverse events-related to gnrh agonists and gnrh antagonists: analysis of real-life data from eudra-vigilance and food and drug administration databases entries. Prostate Cancer Prostatic Dis. (2023) 26:765–71. doi: 10.1038/s41391-022-00640-4
28. Dragomir A, Touma N, Hu J, Perreault S, and Aprikian AG. Androgen deprivation therapy and risk of cardiovascular disease in patients with prostate cancer based on existence of cardiovascular risk. J Natl Compr Cancer Netw. (2023) 21:163–71. doi: 10.6004/jnccn.2022.7083
29. Lopes RD, Higano CS, Slovin SF, Nelson AJ, Bigelow R, Sørensen PS, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the pronounce randomized trial. Circulation. (2021) 144:1295–307. doi: 10.1161/CIRCULATIONAHA.121.056810
30. Merola D, Schneeweiss S, Sreedhara SK, Zabotka LE, Quinto K, Concato J, et al. Real-world evidence prediction of a phase iv oncology trial: comparative degarelix vs leuprolide safety. Jnci Cancer Spectr. (2022) 6. doi: 10.1093/jncics/pkac049
31. Shao YJ, Hong JH, Chen CK, and Huang CY. Cardiovascular risk of gonadotropin-releasing hormone antagonist versus agonist in men with prostate cancer: an observational study in Taiwan. Prostate Cancer Prostatic Dis. (2023) 26:722–9. doi: 10.1038/s41391-022-00555-0
32. Wallach JD, Deng Y, Mccoy RG, Dhruva SS, Herrin J, Berkowitz A, et al. Real-world cardiovascular outcomes associated with degarelix vs leuprolide for prostate cancer treatment. JAMA Netw Open. (2021) 4:e2130587. doi: 10.1001/jamanetworkopen.2021.30587
33. Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. Jama-J Am Med Assoc. (2011) 306:2359–66. doi: 10.1001/jama.2011.1745
34. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. (2009) 27:3452–8. doi: 10.1200/JCO.2008.20.0923
35. Saad F. Androgen therapy in men with testosterone deficiency: can testosterone reduce the risk of cardiovascular disease? Diabetes-Metab Res Rev. (2012) 28 Suppl 2:52–9. doi: 10.1002/dmrr.2354
36. Barone B, Napolitano L, Abate M, Cirillo L, Reccia P, Passaro F, et al. The role of testosterone in the elderly: what do we know? Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23073535
37. Tsai HK, D’Amico AV, Sadetsky N, Chen MH, and Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. Jnci-J Natl Cancer Inst. (2007) 99:1516–24. doi: 10.1093/jnci/djm168
38. Conteduca V, Di Lorenzo G, Tartarone A, and Aieta M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: an unresolved controversy. Crit Rev Oncol./Hematol. (2013) 86:42–51. doi: 10.1016/j.critrevonc.2012.09.008
39. Basaria S, Muller DC, Carducci MA, Egan J, and Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. (2006) 106:581–8. doi: 10.1002/cncr.21642
40. Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. (2006) 24:3979–83. doi: 10.1200/JCO.2006.05.9741
Keywords: prostate cancer, degarelix, GnRH agonists, androgen deprivation therapy, meta-analysis
Citation: Liu W, Liu Z, Song L, Zhu H, Luo Y, Zhang J, Su S and Wang D (2025) Comparing the risk of cardiovascular disease between degarelix and gonadotropin-releasing hormone agonists:a systematic review and meta-analysis. Front. Oncol. 15:1523794. doi: 10.3389/fonc.2025.1523794
Received: 06 November 2024; Accepted: 16 September 2025;
Published: 01 October 2025.
Edited by:
Hongbing Zhang, Tianjin Medical University General Hospital, ChinaReviewed by:
Dimple Modi, GlaxoSmithKline, United StatesDaniel Vargas Pivato De Almeida, Oncoclinicas Group, Brazil
Karen Abboud, Houston Methodist Hospital, United States
Copyright © 2025 Liu, Liu, Song, Zhu, Luo, Zhang, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Wang, ZGx3YW5nd3NAc2luYS5jb20=; Shuai Su, c3VzaHVhaTkzMDgwOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wencong Liu1†
Wencong Liu1† Zhenyu Liu
Zhenyu Liu Huixuan Zhu
Huixuan Zhu Jindong Zhang
Jindong Zhang Delin Wang
Delin Wang