- Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Qingdao University, Qingdao, China
The most common epidermal growth factor receptor (EGFR) mutation in non-small cell lung cancer (NSCLC) is exon 19 deletion (19del), which is sensitive to EGFR tyrosine kinase inhibitors (EGFR-TKIs). However, uncommon EGFR 19del mutations exhibit varied responses to EGFR-TKI treatment. Research and clinical data on these uncommon subtypes are limited. Additionally, resistance to EGFR-TKIs is inevitable. EGFR C797S is a frequent mechanism of resistance to third-generation EGFR-TKIs, usually occurs in cis with T790M and in 5% of patients in trans. Here, we report a patient diagnosed with lung adenocarcinoma harboring EGFR 19Del L747-A755delinsSKD mutation with co-occurring T790M and trans-C797S mutations, who showed a positive response to combination therapy with first- and third-generation TKIs. This case report suggests an effective treatment option for such patients.
Introduction
Epidermal growth factor receptor (EGFR) mutations are prevalent driver genes in the pathogenesis and progression of non-small cell lung cancer (NSCLC) (1). Among all EGFR mutations, exon 19 deletion (19del) is the most frequent (45%) and is generally more sensitive to tyrosine kinase inhibitors (TKIs) than other common EGFR mutations (2, 3). However, not all 19del alterations are considered as “golden” mutations. The predominant 19del subtype is E746-A750del (66.1%), followed by delL747-P753insS (9.7%) and L747-T751 (6.9%) (4). Moreover, there are over 70 uncommon subtypes and their response to TKIs is still unclear (5). After first- or second-generation EGFR-TKIs therapy, EGFR T790M is the most common secondary mutation. Although it can be overcome by osimertinib and other third generation inhibitors, resistance seems inevitable as well (6, 7). Resistance to the third-generation EGFR-TKIs can be mainly divided into EGFR-dependent and -independent ones. EGFR-dependent mechanism refers to manifold EGFR mutations while EGFR-independent mechanisms include bypass signal activation, histologic transformation and so on (8). The coexistence of T790M and C797S accounts for approximately 18% of all resistant mutations, with trans-mutations accounting for less than 15% (9). Clinical evidence of EGFR T790M and C797S in trans is still needed. Herein, we present a case report of lung adenocarcinoma harboring the rare EGFR L747_A755delinsSKD mutation. Additionally, EGFR T790M and C797S in trans were detected during treatment. In conjunction with the existing literature on C797S in trans, we conducted a comprehensive analysis to provide real-world data reference for these patients.
Case presentation
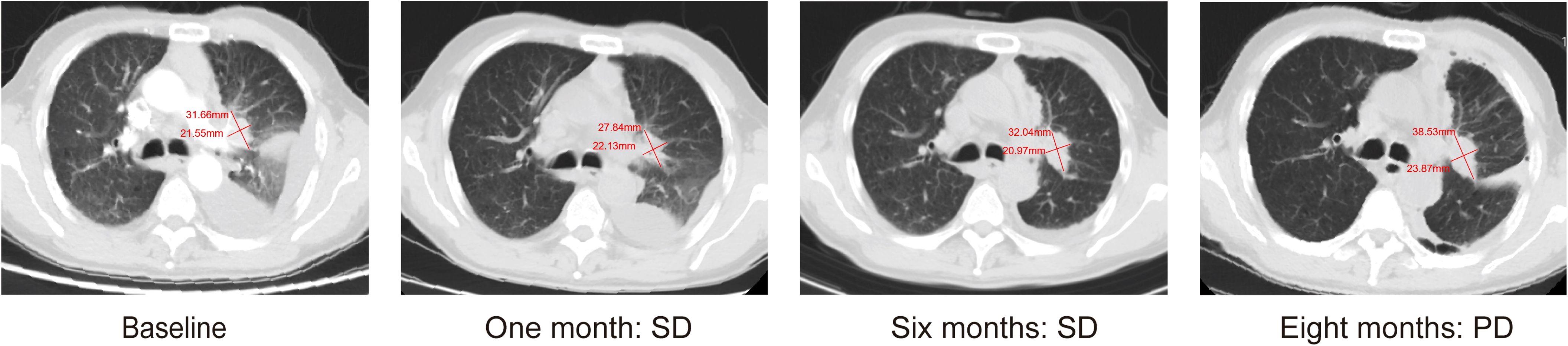
A 67-year-old man presented with a persistent dry cough for more than 3 months in July, 2019. The patient had a smoking history of 50 years with 30 cigarettes a day and he denied any other medical or family history. Contrast enhances chest computed tomography (CT) revealed a mass in the central left upper lobe, enlarged bilateral mediastinal lymph nodes and bilateral pulmonary nodules. A biopsy of the lesion under tracheoscopy confirmed lung adenocarcinoma histologically. Macroscopic metastases were observed in the bone by Whole Body Scan (WBS), and the patient was clinically classified as stage IVB (T4N0M1c) NSCLC. Zoledronic acid was administered to control bone destruction during the treatment. Owing to the deletion of EGFR exon 19 detected by Amplification Refractory Mutation System Polymerase Chain Reaction (ARMS-PCR) in the tumor tissue (Figure 1A), the patient began treatment with gefitinib 250 mg once daily, resulting in a radiological response and rapid clinical benefit lasting 11 months. Subsequent tissue biopsy at the time of relapse confirmed an acquired T790M in exon 20 (c.2369C>T, frequency as 61.9%) by droplet digital polymerase chain react (ddPCR) (Figure 1B). He was then switched to osimertinib 80mg once daily, achieving stable disease with shrinkage of hepatic lesions. Almost 10 months later, the patient was readmitted to the hospital because of malignant pleural effusion. Next generation DNA sequencing (NGS) using a plasma sample was conducted to identify potential targets, revealing an EGFR exon 20 mutation (p.T790M, c.2369C>T, frequency as 0.26%), an EGFR exon 20 mutation (p.C797S, c.2389T>A, frequency as 0.20%, Figure 2B), and an EGFR Exon19 alteration (p.L747-A755delinsSKD, c.2240-2264>CGAAAGA, frequency as 1.36%, Figure 2A, Supplementary Table S1). Based on these NGS results, the patient was started on gefitinib 250mg daily combined with osimertinib 80mg daily as a further-line treatment. Cisplatin and bevacizumab were added to control malignant pleural effusion. The patient experienced a significant improvement in dyspnea, cough, fatigue, and malaise within one week. However, 8 months after starting the combination TKI therapy, dyspnea worsened with evidence of increased left pleural effusion and disease progression in the lungs and lymph nodes (Figure 3, Supplementary Figure S1). The patient refused further biopsy owing to physical and financial conditions. He was then undergoing bevacizumab and pemetrexed for four cycles but was switched to a regimen of oral anlotinib 8mg and furmonertinib 160mg daily after progression. Again, target therapy resulted in a stable disease lasting five months. Adverse effects, including fatigue and gastrointestinal symptoms, were controlled with supportive care. Because of ongoing clinical benefit, the patient continued receiving anlotinib and furmonertinib for a further 3 months as salvage treatment. However, the patient’s condition continued to deteriorate and died in 2023 (45 months after the diagnosis of lung cancer) (Figure 4, Supplementary Figure S2).
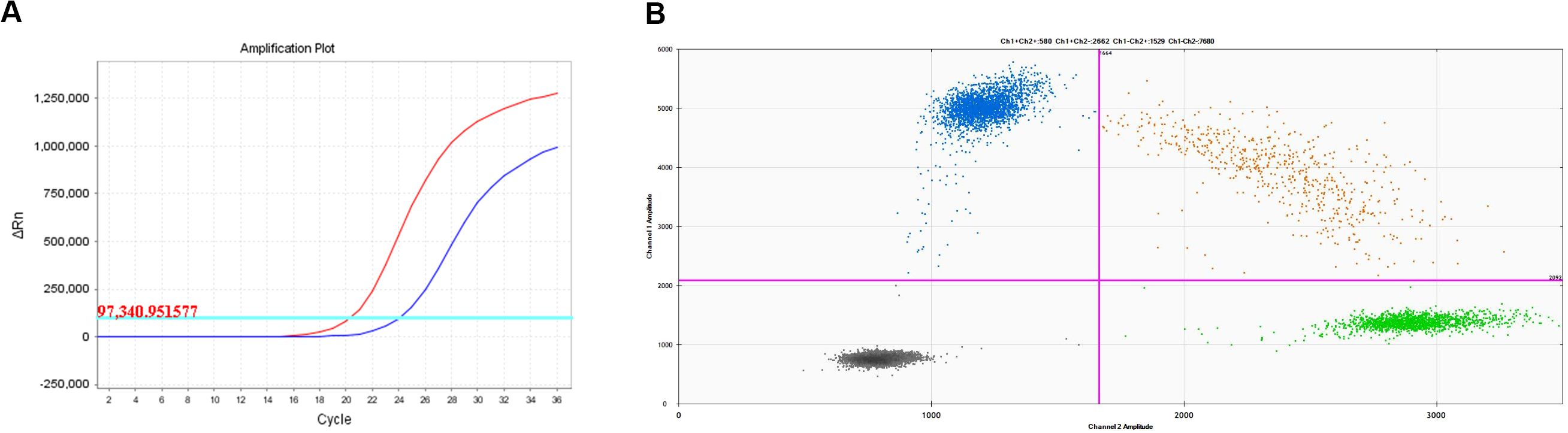
Figure 1. Molecular analysis on lung biopsy. (A) EGFR 19 deletion mutation; (B) EGFR T790M mutation.
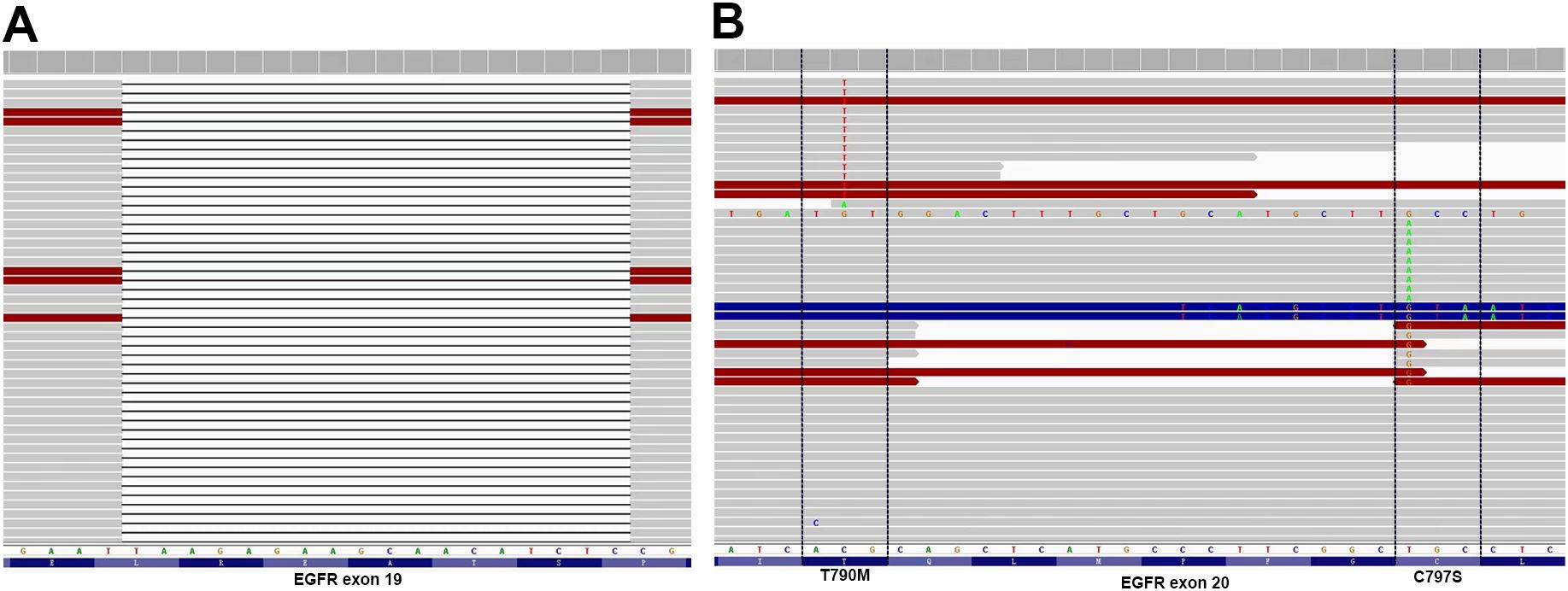
Figure 2. Allelic context on plasma before First- and Third-Generation EGFR TKIs combination therapy. (A) EGFR Exon19 alteration (p. L747-A755delinsSKD); (B) EGFR C797S located in trans with T790M.
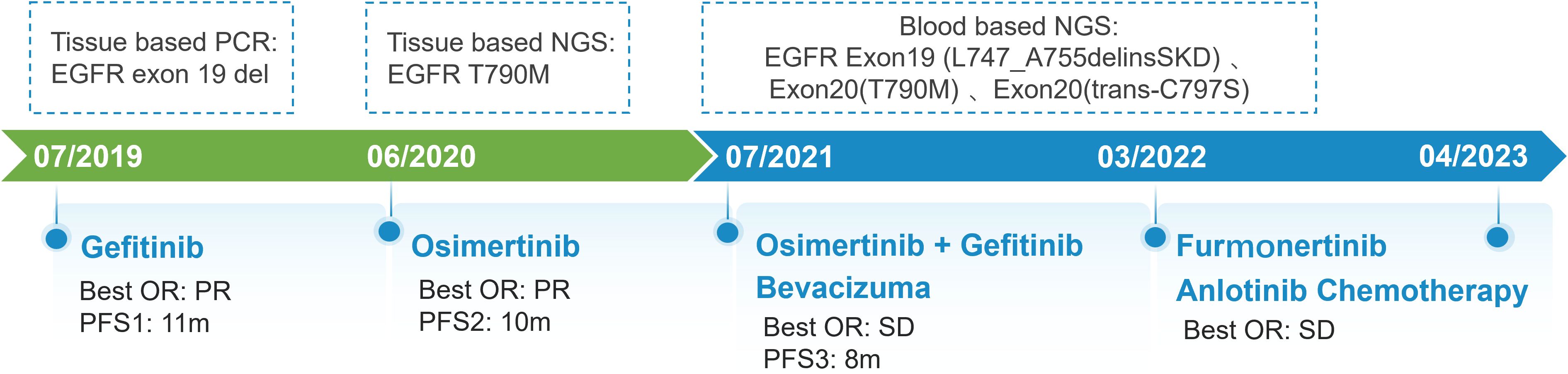
Figure 4. Schematic summary of treatment course. PCR, polymerase chain reaction; EGFR, epidermal growth factor receptor; OR, objective response; PFS, progression free of survival; NGS, next generation sequencing.
Discussion
Exon 19 predominantly harbors a deletion mutation within codons 746-752, resulting in the elimination of four highly conserved amino acids (LREA) from the amino acid sequence (10). Based on the number of deleted bases, initiation codon deletions, and common in-frame deletions, they can be classified into various subtypes (11). Chung et al. reported patients with LRE deletions in exon 19 had a better response to EGFR-TKIs than those with non-LRE deletions (12). Studies disclosed that patients with the exon 19 deletion starting on codon E746 had a better median PFS, compared to those starting on L747 (13, 14). However, Peng et al. indicated that patients with uncommon EGFR 19delins have better clinical outcomes (15). Another study found no statistically significant difference in PFS between the two groups (HR=0.89; P=0.468) (16). These differences may be due to the limited sample sizes of these studies. As for the L747-A755delinsSKD subtype observed in our case, all of the initiation codon, base pair deletion length, and amino acid insertions were uncommon. Studies have also demonstrated that deletions with amino acid insertions have a poorer prognosis compared to deletions without insertions(P=0.0244) (11, 17, 18). Fortunately, the deletion fragment in our patient includes the LREA sequence, which may explain the sensitivity EGFR-TKI drugs (19). Similarly, the reported PFS1 and PFS2 of our patient were shorter than those reported in clinical studies. In conclusion, further in vitro and clinical studies are still needed.
As for resistance mechanisms, studies have suggested that the E746-A750del subtype has a higher frequency of acquiring T790M mutation compared to other 19del subtypes (16). However, similarities in resistance patterns have been observed between the E746-initiated and L747-initiated subtypes (15, 16). The tertiary EGFR mutations are responsible for the third-generation EGFR TKIs resistance, in which C797S mutation is the major one. The cysteine is substituted by a less nucleophile serine in the EGFR C797 position, leading to the destruction of a covalent bond between the mutant receptor and inhibitor (8). In previous studies, only one case harboring L747-A755delinsSKD subtype was reported, which also acquired T790M/trans C797S after osimertinib resistance (20). Unfortunately, the patient soon succumbed to disease. The efficacy of 1st and 3rd generation TKIs has been confirmed in in vivo studies (21).Durable response have been observed in our patient after the combination therapy. Here, we reviewed previous reports on T790M/C797S in trans. The search strategy is detailed in Supplementary Table S2 and the study flowchart is provided in Supplementary Figure S3. Despite its safety profile, PFS varied from 6 weeks to 14 months among patients (Table 1), which is the same as demonstrated in a large retrospective study. Besides, the patients who had durable response to the combination regimen also tended to have better OS. Three factors may be at play in combination therapy: dynamic changes of EGFR C797S-carrying clones, resistance to third-generation TKIs, and whether T790M and C797X are in the same clone (22). Notably, previous investigations have often not mentioned the specific subtype of exon19 deletion. The roles distinct subtypes of exon 19 deletion paly in combination therapy await validation with larger, prospective studies such as the ORCHARD trial (NCT03944772). VEGFR inhibitors has demonstrated favorable therapeutic outcomes in patients with cis C797S/T790M mutations (23, 24). Cases from the table below also revealed the potential advantages for patients harboring the trans-C797S/T790M mutation (25, 26).
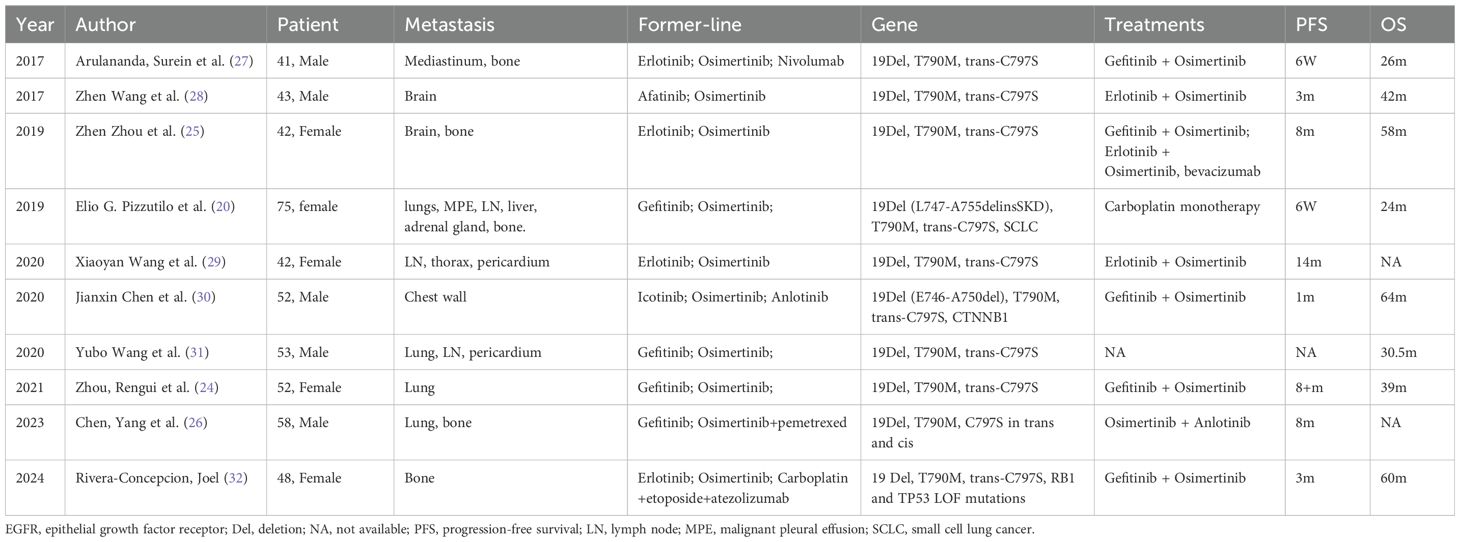
Table 1. Detailed clinical and molecular characteristics of each patient who acquired T790M and trans-C797S mutation after relapse from EGFR-TKIs therapy.
This study has certain limitations. Owing to the constraints of the available detection methods at that time, we were unable to identify the exact EGFR 19 subtype during the initial diagnosis. Furthermore, genetic testing was not repeated after the progression of the first- and third-generation TKI combination therapy because of the patient’s body function, family support, and economic conditions. Consequently, our knowledge regarding changes in genes following resistance development remains incomplete. Challenges remain in conducting repeated biopsies and genetic tests, especially in settings with limited resources. A balanced approach is crucial, requiring a comprehensive assessment of the patient’s health, treatment benefits and risks, and personal preferences. In summary, this study emphasized maintaining open communication with patients and striving for the best disease management and quality of life outcomes.
Conclusion
Briefly, we present a case of uncommon EGFR 19 Del(L747-A755delinsSKD) and T790M/trans-C797S mutations after resistance, showing a positive response to combination therapy with first- and third-generation EGFR-TKIs. Distinct subtypes of exon 19 deletion may influence both trans C797S production and response to combined first- and third-generation TKIs therapy. Angiogenesis inhibitors may further improve the efficacy of EGFR TKIs, and dynamic monitoring of gene mutations is necessary throughout the treatment process. However, further basic experiments and clinical studies are warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Review Committee of Qingdao University Affiliated Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YX: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. DR: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HB: Methodology, Writing – review & editing. YZ: Methodology, Writing – review & editing. YS: Methodology, Writing – review & editing. WH: Methodology, Writing – review & editing. NN: Methodology, Writing – review & editing. HW: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the China Health Promotion Foundation (Spark Program-Cancer Treatment Clinical Research Innovation and Development Project Fund).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1525885/full#supplementary-material
References
1. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin oncology: Off J Am Soc Clin Oncol. (2022) 40:611–25. doi: 10.1200/jco.21.01626
2. Grant MJ, Aredo JV, Starrett JH, Stockhammer P, van Alderwerelt van Rosenburgh IK, Wurtz A, et al. Efficacy of osimertinib in patients with lung cancer positive for uncommon EGFR exon 19 deletion mutations. Clin Cancer research: an Off J Am Assoc Cancer Res. (2023) 29:2123–30. doi: 10.1158/1078-0432.Ccr-22-3497
3. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. New Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
4. Gu W, Lu Z, Shi S, Ma J, Lu G, Deng W, et al. Molecular characteristics of EGFR exon 19 deletion subtypes in NSCLC patients. J Clin Oncol. (2021) 39:8530–0. doi: 10.1200/JCO.2021.39.15_suppl.8530
5. Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev. (2013) 39:839–50. doi: 10.1016/j.ctrv.2013.05.001
6. Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann oncology: Off J Eur Soc Med Oncol. (2020) 31:1536–44. doi: 10.1016/j.annonc.2020.08.2100
7. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. (2019) 121:725–37. doi: 10.1038/s41416-019-0573-8
8. Li S, Zhu S, Wei H, Zhu P, Jiao Y, Yi M, et al. The prospect of combination therapies with the third-generation EGFR-TKIs to overcome the resistance in NSCLC. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2022) 156:113959. doi: 10.1016/j.biopha.2022.113959
9. Chmielecki J, Mok T, Wu YL, Han JY, Ahn MJ, Ramalingam SS, et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat Commun. (2023) 14:1071. doi: 10.1038/s41467-023-35962-x
10. van Alderwerelt van Rosenburgh IK, Lu DM, Grant MJ, Stayrook SE, Phadke M, Walther Z, et al. Biochemical and structural basis for differential inhibitor sensitivity of EGFR with distinct exon 19 mutations. Nat Commun. (2022) 13:6791. doi: 10.1038/s41467-022-34398-z
11. Tokudome N, Koh Y, Akamatsu H, Fujimoto D, Okamoto I, Nakagawa K, et al. Differential significance of molecular subtypes which were classified into EGFR exon 19 deletion on the first line afatinib monotherapy. BMC Cancer. (2020) 20:103. doi: 10.1186/s12885-020-6593-1
12. Chung KP, Wu SG, Wu JY, Yang JC, Yu CJ, Wei PF, et al. Clinical outcomes in non-small cell lung cancers harboring different exon 19 deletions in EGFR. Clin Cancer research: an Off J Am Assoc Cancer Res. (2012) 18:3470–7. doi: 10.1158/1078-0432.Ccr-11-2353
13. Lee VH, Tin VP, Choy TS, Lam KO, Choi CW, Chung LP, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2013) 8:1148–55. doi: 10.1097/JTO.0b013e31829f684a
14. Truini A, Starrett JH, Stewart T, Ashtekar K, Walther Z, Wurtz A, et al. The EGFR exon 19 mutant L747-A750>P exhibits distinct sensitivity to tyrosine kinase inhibitors in lung adenocarcinoma. Clin Cancer research: an Off J Am Assoc Cancer Res. (2019) 25:6382–91. doi: 10.1158/1078-0432.Ccr-19-0780
15. Peng X, Long X, Liu L, Zeng L, Yang H, Jiang W, et al. Clinical impact of uncommon epidermal growth factor receptor exon 19 insertion-deletion variants on epidermal growth factor receptor-tyrosine kinase inhibitor efficacy in non-small-cell lung cancer. Eur J Cancer (Oxford England: 1990). (2020) 141:199–208. doi: 10.1016/j.ejca.2020.10.005
16. Huang LT, Zhang SL, Han CB, Ma JT. Impact of EGFR exon 19 deletion subtypes on clinical outcomes in EGFR-TKI-Treated advanced non-small-cell lung cancer. Lung Cancer (Amsterdam Netherlands). (2022) 166:9–16. doi: 10.1016/j.lungcan.2022.01.014
17. Zhao C, Jiang T, Li J, Wang Y, Su C, Chen X, et al. The impact of EGFR exon 19 deletion subtypes on clinical outcomes in non-small cell lung cancer. Trans Lung Cancer Res. (2020) 9:1149–58. doi: 10.21037/tlcr-19-359
18. Chen Y, Xu J, Zhang L, Song Y, Wen W, Lu J, et al. A multicenter-retrospective study of non-small-cell lung carcinoma harboring uncommon epidermal growth factor receptor (EGFR) mutations: different subtypes of EGFR exon 19 deletion-insertions exhibit the clinical characteristics and prognosis of non-small cell lung carcinoma. Trans Lung Cancer Res. (2022) 11:238–49. doi: 10.21037/tlcr-22-48
19. Wu SG, Gow CH, Chen YL, Liu YN, Tsai MF, Shih JY. Different treatment efficacies and T790M acquisition of EGFR-TKIs on NSCLC patients with variable Del-19 subtypes of EGFR. Int J Cancer. (2023) 153:352–63. doi: 10.1002/ijc.34507
20. Pizzutilo EG, Lauricella C, Cerea G, Giannetta LG, Tomasello G, Stabile S, et al. Concurrent small-cell transformation and emergence of trans-C797S and T790M mutations under sequential treatment with EGFR inhibitors in lung adenocarcinoma. JCO Precis Oncol. (2019) 3:1–5. doi: 10.1200/po.19.00229
21. Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer research: an Off J Am Assoc Cancer Res. (2015) 21:3924–33. doi: 10.1158/1078-0432.Ccr-15-0560
22. Lu C, Wei XW, Wang Z, Zhou Z, Liu YT, Zheng D, et al. Allelic context of EGFR C797X-mutant lung cancer defines four subtypes with heterogeneous genomic landscape and distinct clinical outcomes. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2024) 19:601–12. doi: 10.1016/j.jtho.2023.11.016
23. Zhao J, Zou M, Lv J, Han Y, Wang G, Wang G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. OncoTargets Ther. (2018) 11:5545–50. doi: 10.2147/ott.S170358
24. Zhou R, Song L, Zhang W, Shao L, Li X, Li X. Combination of osimertinib and anlotinib may overcome the resistance mediated by in cis EGFR T790M-C797S in NSCLC: A case report. OncoTargets Ther. (2021) 14:2847–51. doi: 10.2147/ott.S298655
25. Zhou Z, Zhao Y, Shen S, Gu L, Niu X, Xu Y, et al. Durable clinical response of lung adenocarcinoma harboring EGFR 19Del/T790M/in trans-C797S to combination therapy of first- and third-generation EGFR tyrosine kinase inhibitors. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2019) 14:e157–9. doi: 10.1016/j.jtho.2019.04.020
26. Chen Y, Hong H, Bao S, Tang H. Stevens-Johnson syndrome induced by toripalimab in a previously EGFR-TKI-treated advanced lung adenocarcinoma patient harboring EGFR mutations 19 del/T790M/C797S in trans and cis: a case report. Front Pharmacol. (2023) 14:1131703. doi: 10.3389/fphar.2023.1131703
27. Arulananda S, Do H, Musafer A, Mitchell P, Dobrovic A, John T. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung cancer. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2017) 12:1728–32. doi: 10.1016/j.jtho.2017.08.006
28. Wang Z, Yang JJ, Huang J, Ye JY, Zhang XC, Tu HY, et al. Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2017) 12:1723–7. doi: 10.1016/j.jtho.2017.06.017
29. Wang XY, Wang R, Yao YY, Xu LL, Wang AB. Osimertinib combined with erlotinib for the treatment of lung adenocarcinoma with EGFR-T790M and C797S transmutation: A case report and literature review. Chin J Cancer Biotherapy. (2020) 27:951–3. doi: 10.3872/j.issn.1007-385x.2020.08.017
30. Wang XY, Wang R, Yao YY, Xu LL, Wang AB. Primary resistance to combination therapy with first- and third-generation EGFR tyrosine kinase inhibitors of lung adenocarcinoma harboring EGFR 19Del/T790M/in trans-C797S mutations with co-occurring CTNNB1 alteration. OncoTargets Ther. (2020) 13:6749–53. doi: 10.2147/ott.S262594
31. Wang Y, Tian P, Xia L, Li L, Han R, Zhu MV, et al. The clinical efficacy of combinatorial therapy of EGFR-TKI and crizotinib in overcoming MET amplification-mediated resistance from prior EGFR-TKI therapy. Lung Cancer (Amsterdam Netherlands). (2020) 146:165–73. doi: 10.1016/j.lungcan.2020.06.003
Keywords: lung adenocarcinoma, uncommon EGFR mutations, T790M mutation, trans-C797S, EGFR-TKI resistance, combination therapy, case report, literature review
Citation: Xiao Y, Ren D, Bi H, Zhou Y, Shao Y, Han W, Na N and Wang H (2025) Advanced lung adenocarcinoma harboring uncommon EGFR 19 Del and T790M/trans-C797S mutations after resistance: a case report and literature review. Front. Oncol. 15:1525885. doi: 10.3389/fonc.2025.1525885
Received: 10 November 2024; Accepted: 28 March 2025;
Published: 16 April 2025.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
Shiyu Li, The Chinese University of Hong Kong, ChinaClaudia Scimone, University of Naples Federico II, Italy
Meng Fu, University of Science and Technology of China (USTC), China
Copyright © 2025 Xiao, Ren, Bi, Zhou, Shao, Han, Na and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Wang, ZG9yLndobUAxNjMuY29t
†These authors have contributed equally to this work
 Yuting Xiao
Yuting Xiao Dunqiang Ren
Dunqiang Ren Huanhuan Bi
Huanhuan Bi Yinxue Zhou
Yinxue Zhou Yanmei Shao
Yanmei Shao Weizhong Han
Weizhong Han Na Na
Na Na Hongmei Wang
Hongmei Wang