- 1Department of Pharmacy, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, China
- 2Department of Pathology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, China
Background: Glioblastoma (GBM) is the most common and aggressive primary brain malignancy in adults. Diagnosis primarily relies on imaging techniques like CT scan and MRI, while pathological biopsy remains the diagnostic gold standard. Standard of care for newly diagnosed GBM includes maximal safe resection followed by radiotherapy and chemotherapy, although prognosis remains poor. GBM patients are at heightened risk for venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), with chemotherapy and targeted therapy further elevating this risk.
Case summary: We report a case of a patient with atypical cranial imaging findings, where initial assessments at both an external hospital and our institution were equivocal. A definitive GBM diagnosis was achieved only after biopsy. GBMs are highly vascularized malignant tumors. Anlotinib, an anti-angiogenic multi-kinase inhibitor, has been used to treat GBM. Following diagnosis, the patient received anlotinib therapy and subsequently developed PE, suspected as an anlotinib-induced adverse event.
Conclusion: Anlotinib may cause PE and should be used with caution. Clinicians should close coagulation monitoring following anlotinib treatment, including D-dimer testing and imaging (eg, CT), to ensure prompt diagnosis and timely treatment for PE. This case highlights the critical need for vigilant PE monitoring and prompt management in GBM patients on anlotinib therapy.
1 Introduction
Glioblastoma (GBM), a high-grade glioma, is the most aggressive primary brain tumor in adults, classified as grade IV by the WHO (1–3). Diagnosis relies heavily on imaging—primarily CT and MRI—followed by histopathological and molecular analyses from tumor biopsy or resection to confirm grade and subtype (3). This diagnostic process can be protracted, delaying treatment. Standard of care for GBM includes maximal safe surgical resection, followed by radiotherapy and chemotherapy, though prognosis remains poor, with a five-year survival rate around 6% (3, 4). GBM patients also face an elevated risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), risks exacerbated by chemotherapy and targeted therapy (5, 6). We report on a case of a patient with atypical neuroimaging findings and a protracted diagnostic course, ultimately diagnosed with GBM after biopsy and treated with anlotinib, after which the patient developed PE. The case details are as follows.
2 Case report
A 64-year-old Asian male presented with recurrent right-sided weakness in September 2023. His history was unremarkable for major diseases. Initial cranial CT showed mild cerebral atrophy, and MRI indicated infiltrative lesions in the brain (Figure 1A), suggestive of inflammation or low-grade glioma. Treated with butylphthalide, edaravone, aspirin, and clopidogrel, his symptoms persisted. At our neurosurgery department, extensive testing, including lumbar puncture and whole-body PET/CT, failed to reveal a definitive diagnosis. Stereotactic brainstem biopsy performed in October 2023 confirmed GBM (WHO Grade IV, IDH wild-type). The molecular pathology showed IDH1 wild-type, TERT promoter mutation, and CDKN2A homozygous deletion. The immunohistochemistry showed Oligo-2 (+), P53 (–), ATRX (+, no loss), GFAP (+), KI-67 (approximately 80%+), Vim (focal+), H3-K27M (-), IDH-1 (-), MGMT (-), Syn (partially+). The patient had an Eastern Cooperative Oncology Group (ECOG) performance status score of 1.The patient received radiotherapy (DT60Gy) with concurrent anlotinib (12 mg daily for two weeks on, one week off). After 3 months of anlotinib treatment, MRI indicated disease progression (Figure 1B), and anlotinib was discontinued in February 2024.
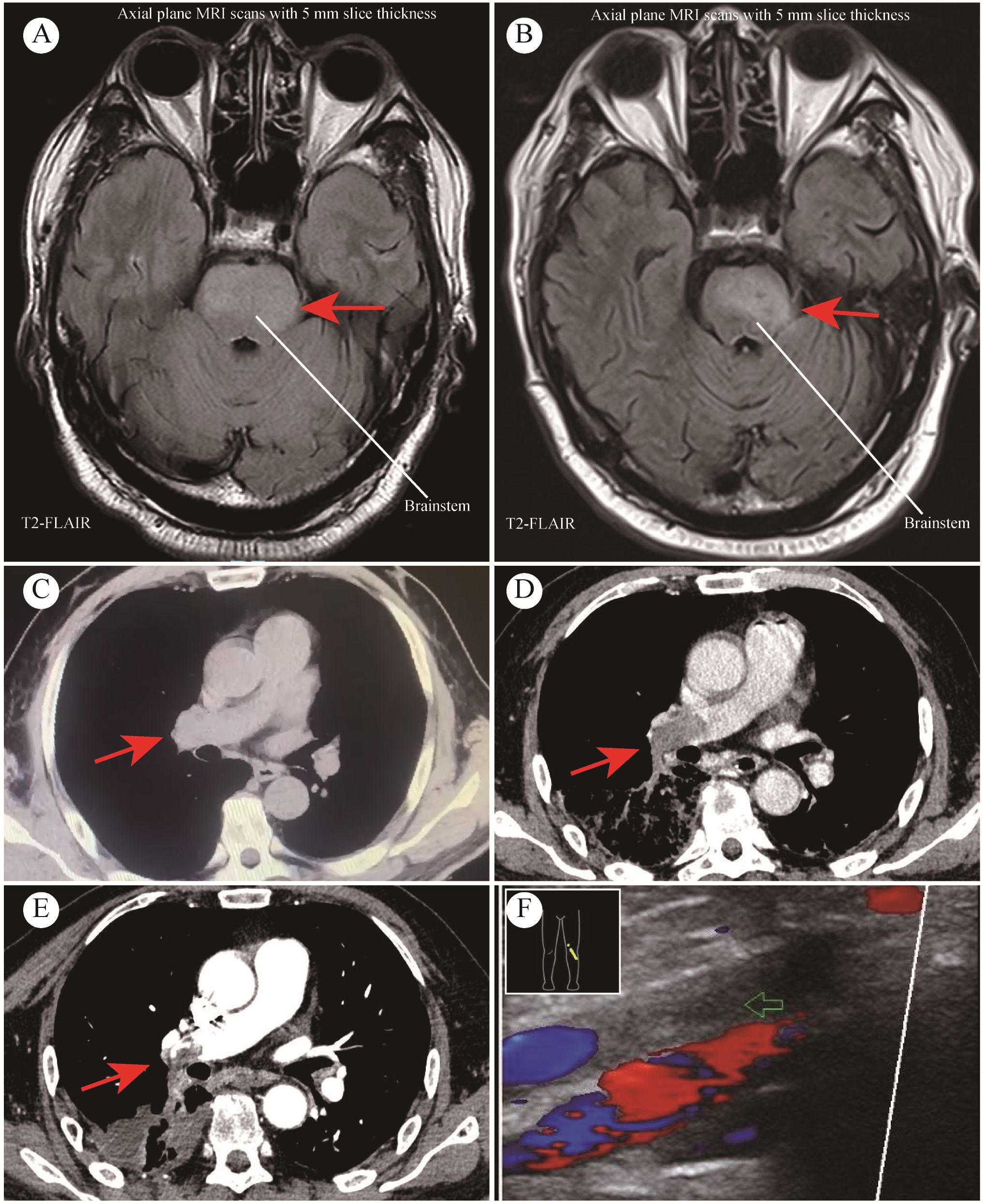
Figure 1. MRI and CT Imaging from case study. (A) Preoperative MRI findings; (B) MRI findings after 3 months of anlotinib treatment; (C) CT indicated no PE before initiating anlotinib treatment; (D) CT indicated extensive PE after 3 months of anlotinib treatment; (E) CT scan after 2 months of anticoagulant therapy; (F) Thromboses detected in lower leg veins.
At the same time, he was confirmed by CT of thromboses in both pulmonary arteries (Figure 1D), and with associated deep vein thrombosis in the lower extremities (Figure 1F). Before initiating anlotinib treatment, CT revealed no PE occurred (Figure 1C). Key laboratory test results were demonstrated as follows: oxygen saturation (SpO2) 97.7% when oxygen therapy, platelet count (PLT) 208×10^9/L, D-dimer 3.61↑μg/L, antithrombin (AT) 56%↓. Anticoagulation therapy was initiated, shifting from heparin (due to heparin resistance) to argatroban. But the treatment effect was suboptimal and he was given fondaparinux. After 2 months of anticoagulant treatment, the patient CT scan showed the thrombus in the left pulmonary artery had almost completely disappeared, and the thrombus in the right pulmonary artery had decreased (Figure 1E). Anlotinib initially achieved disease stabilization; however, the subsequent PE significantly compromised the patient’s functional status and overall well-being. Despite aggressive treatment, he experienced further complications and passed away five months post-diagnosis. The timeline is shown in Figure 2.
3 Discussion
This case illustrates the diagnostic challenges in atypical GBM presentations and highlights the limitations of imaging alone. When GBM exhibits atypical imaging characteristics, including morphology, lesion number, location, signal intensity, it is prone to misdiagnosis as other diseases. In this report, the tumor’s brainstem location and overlapping MRI signal characteristics delayed the diagnosis, ultimately confirmed through biopsy, the diagnostic gold standard, though it imposes substantial physical, psychological, and economic burdens on patients.
The current standard of care for GBM is the Stupp regimen—surgical resection followed by radiotherapy with concurrent and adjuvant temozolomide (TMZ)—yet median progression-free survival (PFS) is 6.9 months, and median overall survival (OS) is 14.6 months (3, 4, 7). Novel approaches, including targeted therapy(eg, angiogenesis inhibitors), immunotherapy(eg, immune checkpoint blockade), and physical therapy (eg, Tumor Treating Fields, TTF) hold promise but are largely experimental (8, 9). GBMs are highly vascularized malignant tumors that produce VEGF (7, 10). Robust aberrant angiogenesis renders GBM potentially amenable to anti-angiogenic therapy (7, 10). Anlotinib, an anti-angiogenic multi-kinase inhibitor, has been used to treat GBM. Our patient’s tumor was IDH wild-type, MGMT promoter unmethylated and TERT-mutated suggesting a poor prognosis, particularly given MGMT promoter unmethylation, indicating limited TMZ efficacy. For such patients, clinical trials or alternative regimens may be prioritized. The patient received radiotherapy with concurrent and adjuvant anlotinib.
Anlotinib, a tyrosine kinase inhibitor (TKI), targets onco-angiogenesis and suppresses tumor growth by simultaneously blocking vascular endothelial growth factor receptor (VEGFR)1/2/3, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and c-kit (11). Li et al. discovered that anlotinib effectively inactivated the JAK3/STAT3 pathway to inhibit growth and induce apoptosis in malignant glioma cells independent of MGMT expression. Meanwhile, anlotinib alone or in combination with radiation was effective and safe in vivo evaluation (12). A phase II study of anlotinib combined with the Stupp regimen in patients with newly diagnosed GBM reported the median PFS was 10.9 months, median OS was17.4 months, and the 12 months PFS rate was 48.5% (7). Anlotinib has shown efficacy in GBM, though its role in increasing VTE risk warrants attention. While VEGFR TKIs are generally safe, cases of PE have been reported in cancer patients receiving anlotinib combination treatment. These cases underscore a possible link between VEGF inhibition, endothelial dysfunction, and thrombogenesis (6, 13–16). The mechanism underlying anlotinib-induced VTE may involve the inhibition of VEGF signaling pathway, leading to endothelial dysfunction, decreased production of nitric oxide and prostacyclin, increased endothelin-1 production, which facilitate platelet aggregation, thereby increasing the risk of VTE (6, 15). Additionally, angiogenesis inhibitors induce an increase in tissue factor levels and are associated with an increased incidence of VTE (6).
Managing VTE in GBM patients is challenging. The high intrinsic risk of VTE complicates treatment, especially with tumor-associated venous thromboembolism (TAVTE). Studies have shown that the risk of VTE in cancer patients is 9-fold higher than in general population (17). The occurrence of VTE has been reported to increase the likelihood of death for cancer patients by 2- to 6-fold (17, 18). Notably, the incidence rate of VTE is 39% in GBM patients, significantly higher than other tumor types and is correlated with an elevated risk of intracranial hemorrhage (5).Besides cancer-related factors, both chemotherapy and targeted therapy are associated with an increased risk of VTE in cancer patients (6). Guidelines regarding the treatment of VTE in GBM patients are lacking. The current management for VTE in patients with GBM follows the established guidelines for VTE in patients with other tumor types. There is a CSCO guideline for VTE prophylaxis (19). The guideline does not recommend routine anticoagulation for VTE prophylaxis in all cancer patients. However, pharmacological thromboprophylaxis may be offered in high-risk case such as medical treatment patients with cancer who have acute medical illness or reduced mobility, and high-risk cancer patients (Khorana score of 2 or higher). Moreover, Current guidelines recommend anticoagulation for VTE treatment, yet heparin resistance in low antithrombin (AT) conditions, as seen in this patient, necessitates alternatives like argatroban or direct thrombin inhibitors (17, 18, 20, 21).
It is necessary to strengthen awareness and management of VTE following angiogenesis inhibitors treatment for GBM, especially in patients with comorbidities such as cardiovascular diseases or a prior history of VTE (5, 15, 22). Neuro-oncologists close coagulation monitoring and early anticoagulation could prevent treatment discontinuation due to VTE adverse event. Moreover, a close collaboration between neuro-oncologists and cardiovascular specialists should be emphasized.
4 Conclusion
This case underscores the critical role of biopsy in GBM diagnosis, particularly in cases with atypical imaging findings. Despite a comprehensive treatment approach, GBM remains highly aggressive, with limited treatment efficacy and survival gains. Early, accurate diagnosis and multimodal treatment are essential for improving outcomes. Anlotinib may cause PE and should be used with caution. Therefore, Clinicians should have a high index of suspicion for prompt diagnosis and timely treatment. Meanwhile, use of anlotinib in patients with a prior history of thrombotic events or other known risk factors for VTE warrants careful consideration. The therapeutic potential of anlotinib in GBM requires validation through large-scale clinical trials, particularly to elucidate its role in VTE risk.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
J-WL: Writing – review & editing. J-LZ: Writing – original draft. Y-LZ: Writing – original draft. K-JQ: Writing – original draft. Y-YJ: Writing – review & editing. J-LL: Writing – review & editing. JZ: Writing – review & editing. S-TW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
2. Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, et al. Epidemiology of glioblastoma multiforme-literature review. Cancers (Basel). (2022) 14:2412. doi: 10.3390/cancers14102412
3. National Health Commission Medical Administration, Chinese Anti-Cancer Association Glioma Professional Committee, Chinese Medical Doctor Association Glioma Professional Committee. Guidelines for the diagnosis and treatment of glioma (2022 edition). Chin J Neurosurg. (2022) 38:757–77. doi: 10.3760/cma.j.cn112050-20220510-00239
4. Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O, Brem S. Central nervous system cancers. Version 2.2024. National Comprehensive Cancer Network (NCCN) (2024). NCCN Clinical Practice Guidelines in Oncology.
5. Lu JW, Liu ZM, Xia JS, Tang Z, Li X, Chen J. Progress in diagnosis and treatment of glioblastoma combined with venous thromboembolism. J Pract Cardiovasc Cerebrovascular Diseases. (2023) 31:7–11. doi: 10.3969/j.issn.1001-5075.2023.01.002
6. Pantazi D, Tselepis AD. Cardiovascular toxic effects of antitumor agents: Pathogenetic mechanisms. Thromb Res. (2022) 213 Suppl 1:S95–S102. doi: 10.1016/j.thromres.2021.12.017
7. Lai S, Li P, Liu X, Liu G, Xie T, Zhang X, et al. Efficacy and safety of anlotinib combined with the STUPP regimen in patients with newly diagnosed glioblastoma: a multicenter, single-arm, phase II trial. Cancer Biol Med. (2024) 21:433–44. doi: 10.20892/j.issn.2095-3941.2023.0373
8. Chen Q. Research progress of diagnosis and treatment of glioblastoma. Cancer Res Prev Treat. (2022) 49:1103–6. doi: 10.3971/j.issn.1000-8578.2022.22.0090
9. Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. (2022) 41:142. doi: 10.1186/s13046-022-02349-7
10. Wang Z, Du F, Ren Y, Jiang W. Treatment of MGMT promoter unmethylated glioblastoma with PD-1 inhibitor combined with anti-angiogenesis and epidermal growth factor receptor tyrosine kinase inhibitor: a case report. Ann Transl Med. (2021) 9:1508. doi: 10.21037/atm-21-4625
11. Li S, Wang H. Research progress on mechanism and management of adverse drug reactions of anlotinib. Drug Des Devel Ther. (2023) 17:3429–37. doi: 10.2147/DDDT.S426898
12. Li PJ, Lai SZ, Jin T, Ying HJ, Chen YM, Zhang P, et al. Radiotherapy opens the blood-brain barrier and synergizes with anlotinib in treating glioblastoma. Radiother Oncol. (2023) 183:109633. doi: 10.1016/j.radonc.2023.109633
13. Shi Y, Ji M, Jiang Y, Yin R, Wang Z, Li H, et al. A cohort study of the efficacy and safety of immune checkpoint inhibitors plus anlotinib versus immune checkpoint inhibitors alone as the treatment of advanced non-small cell lung cancer in the real world. Transl Lung Cancer Res. (2022) 11:1051–68. doi: 10.21037/tlcr-22-350
14. Li T, Chang K, Qiu X, Lai Z, Luo Y, Chen J, et al. A pilot study of anlotinib with third-generation epidermal growth factor receptor tyrosine kinase inhibitors in untreated EGFR-mutant patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. (2023) 12:1256–63. doi: 10.21037/tlcr-23-175
15. Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol. (2016) 32:852–62. doi: 10.1016/j.cjca.2015.12.023
16. Muñoz Martín AJ, Ramírez SP, Morán LO, Zamorano MR, Benéitez MCV, Salcedo IA, et al. Pharmacological cancer treatment and venous thromboembolism risk. Eur Heart J Suppl. (2020) 22 Suppl C:C2–C14. doi: 10.1093/eurheartj/suaa004
17. Falanga A, Ay C, Di Nisio M, Gerotziafas G, Jara-Palomares L, Langer F, et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann Oncol. (2023) 34:452–67. doi: 10.1016/j.annonc.2022.12.014
18. Streiff MB, Holmstrom B, Angelini D, Ashrani A, Buckner T, Diep R. Cancer-associated venous thromboembolic disease version:2.2023.National Comprehensive Cancer Network (NCCN) (2023). NCCN clinical practice guidelines in oncology.
19. Chinese Society of Clinical Oncology Guidelines Committee. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Prevention and Treatment of Venous Thromboembolism in Cancer Patients 2024. Beijing, China: People’s Medical Publishing House. (2024).
20. Men JL, Xu FY, Zhai ZG. Mechanism and clinical treatment strategy of heparin resistance. Zhonghua Yi Xue Za Zhi. (2023) 103:707–13. doi: 10.3760/cma.j.cn112137-20220830-01838
21. Levy JH, Connors JM. Heparin resistance-clinical perspectives and management strategies. N Engl J Med. (2021) 385:826–32. doi: 10.1056/NEJMra2104091
22. Vallerio P, Orenti A, Tosi F, Maistrello M, Palazzini M, Cingarlini S, et al. Major adverse cardiovascular events associated with VEGF-targeted anticancer tyrosine kinase inhibitors: a real-life study and proposed algorithm for proactive management. ESMO Open. (2022) 7:100338. doi: 10.1016/j.esmoop.2021.100338
Keywords: anlotinib, glioblastoma, pulmonary embolism, targeted therapy, biopsy
Citation: Zhao J-L, Zhang Y-L, Qu K-J, Jiang Y-Y, Li J-L, Zhou J, Wu S-T and Li J-W (2025) Anlotinib-associated pulmonary embolism in brainstem glioblastoma treatment: a case report. Front. Oncol. 15:1526337. doi: 10.3389/fonc.2025.1526337
Received: 11 November 2024; Accepted: 25 March 2025;
Published: 09 April 2025.
Edited by:
Veronica Aran, Instituto Estadual do Cérebro Paulo Niemeyer (IECPN), BrazilReviewed by:
Bruno Marques Vieira, Instituto Estadual do Cérebro Paulo Niemeyer (IECPN), BrazilAlejandro Rodríguez Camacho, Departamento de Radioneurocirugía del Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez, Mexico
Copyright © 2025 Zhao, Zhang, Qu, Jiang, Li, Zhou, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Wei Li, bGlqdW53ZWlAc3R1LmhudWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Jia-Lan Zhao1†
Jia-Lan Zhao1† Jun-Wei Li
Jun-Wei Li