- 1Department of Hematology, Jiujiang First People’s Hospital, Jiujiang, Jiangxi, China
- 2Department of Cardiovascular Medicine, Jiujiang First People’s Hospital, Jiujiang, Jiangxi, China
Most cases of acute promyelocytic leukemia (APL) are driven by the PML::RARA fusion gene, which is sensitive to differentiation induction therapy comprising of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). Treatment with ATRA plus ATO has achieved remarkable clinical outcome in patients with typical APL. However, 5% of patients still died from relapsed/refractory disease, predominantly high-risk APL and variant APL. The diagnosis and treatment of variant APL remain challenging. Here, we report a case of TFG::RARA variant APL recognized by targeted RNA sequencing. The patient achieved a sustained complete response following treatment with venetoclax combined with ATRA. Currently, the overall survival (OS) of the patient has exceeded 30 months, and the progression-free survival (PFS) has reached 29 months. Our results suggest that venetoclax combined with ATRA may be an ideal treatment option for patients with TFG::RARA variant APL.
Background
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) with unique morphological and cytogenetic features. It is classified as AML-M3 in the French-American-British (FAB) classification system and accounts for 10–15% of newly diagnosed AML (1). Classic APL is characterized by a specific chromosomal balanced reciprocal translocation t(15;17) (q24.1;q21.2) that generates the oncogenic fusion protein PML::RARA, resulting in abnormally proliferation of immature promyelocytes in the bone marrow (2–6). The past 30 years have seen rapid progress in classical APL treatment by using all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) to target the PML::RARA fusion protein, converting the disease from highly lethal to highly curable (7, 8).
However, approximately 2% of patients are variant APL, characterized by atypical rearrangements, including two scenarios: RARA is fused to other partners instead of PML and PML::RARA fusion protein is negative, or the translocation refers to other RAR family members rather than RARA (8, 9). RARB rearrangement and RARG rearrangement were also found to cause APL (2, 9). In addition to PML, RARA has many other partner genes that interact to produce fusion proteins, such as PLZF::RARA, NPM1::RARA, NUMA::RARA, STAT5B::RARA, and BCOR::RARA (10–17). Apart from high-risk APL, primary variants of APL that are mostly resistant to ATRA and ATO (9). The identification, diagnosis, and treatment of patients with APL variants present considerable challenges for clinicians.
Herein, we report a patient with TFG::RARA variant APL treated with venetoclax plus ATRA to achieve a favorable curative effect. To the best of our knowledge, this is the second report of such a variant APL till submission. However, we employed a distinct therapeutic schedule and performed a longer follow-up period.
Case presentation
A 56-year-old female patient presenting with general pain for 3 months visited the outpatient clinic. Blood routine examination showed a white blood cell (WBC) count of 2.71×109/L (ref. 3.5–9.5×109/L) without abnormal immature cells, neutrophil ratio of 43.9%(ref. 40%-75%), hemoglobin level of 91g/L (ref.115–150 g/L), and platelet count of 87×109/L (ref.125-350×109/L). And then the patient was admitted to the hematology department because of pancytopenia. The blood clotting function displayed a fibrinogen level of 4.65 g/L (ref. 2.00–4.00 g/L), D-dimer level of 5.76 ug/mL (ref. 0.00–0.55 ug/mL), along with prothrombin time and activated partial thromboplastin time were 13.0 seconds (ref. 10.0–14.0 s) and 26.6 s (ref. 24.0–34.0 s), respectively. Lactate dehydrogenase (LDH) was 334 U/L (ref. 135–225U/L) and alpha-hydroxybutyrate dehydrogenase (HBDH) was 278U/L (ref. 72–182U/L). There were no abnormalities in liver and kidney function. Bone marrow smear showed 50.6% abnormal hypergranular promyelocytes without Auer rods (Figure 1A). Immunophenotypes of the blasts/promyelocytes by flow cytometric were as follows: CD34-, CD117+, CD33+, CD13+, HLA-DR (weak) +, CD64+, CD56+, CD9+, CD123(dim) +, CD14-, CD2-, CD5-, CD7-, CD19-, CD20- (Figure 1B). An initial diagnosis of APL was established, and 25 mg/m2 ATRA was administered on day 1 of admission.
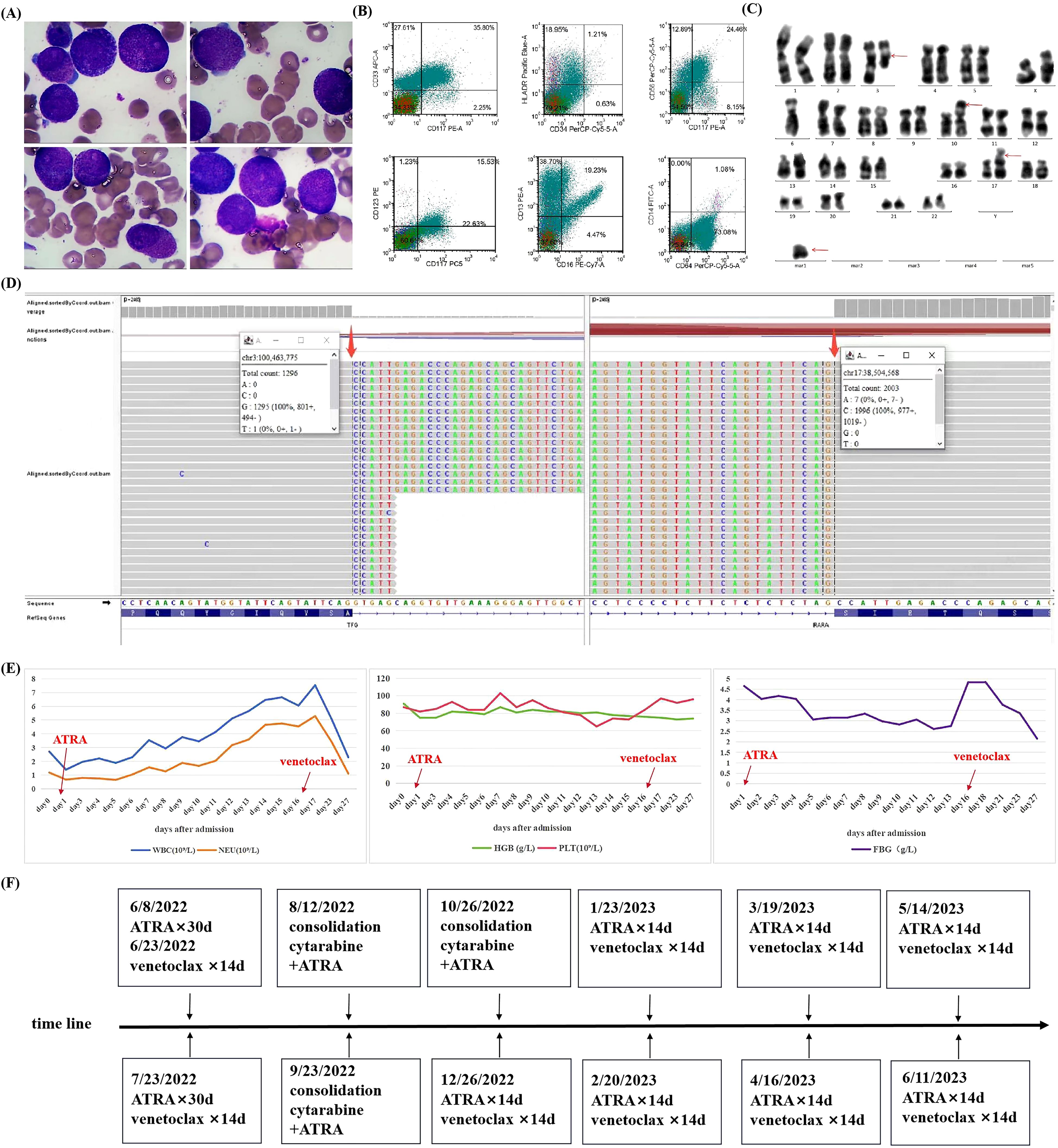
Figure 1. Clinical characteristics of a patient with variant acute promyelocytic leukemia with TFG::RARA rearrangement (A) Wright–Giemsa staining of bone marrow smear (×1000). (B) Immunophenotype of blasts/promyelocyte by flow cytometry. (C) Chromosome G band analysis, with red arrows indicating abnormal karyotypes. (D) Targeted RNA sequencing of TFG::RARA fusion gene, with positive fusion genes identified by thick red arrows. Targeted RNA sequencing for blood tumors involves isolation of RNA, conversion into cDNA, enrichment of tumor-specific genes, and sequencing to identify mutations and gene expression changes. (E) Changes in blood cells and fibrinogen during the first course of induction therapy. (F) Timeline with the main treatment of the clinical episode. WBC, white blood cell; NEU, neutrophil; HGB, hemoglobin; PLT, platelet; FBG, fibrinogen; ATRA, all-trans retinoic acid.
The PML::RARA rearrangement was negative upon quantitative polymerase chain reaction (q-PCR) and fluorescence in situ hybridization (FISH). Chromosome analysis revealed a 46, XX, t (3;17) (q21; q25), -6, add (10) (p13), +mar (6)/46, XX (12) karyotype (Figure 1C). Next-generation sequencing identified Shwachman-Bodian-Diamond syndrome (SBDS) gene mutations, although clinical relevance was not established. Thereafter, the specimen was subjected to targeted RNA sequencing, a technique focusing on a specific RNA molecule or a set of genes. The sequencing results revealed the presence of the TFG::RARA fusion gene with a breakpoint in exon 7 of the TFG gene and exon 3 of the RARA gene (Figure 1D). The patient was eventually diagnosed with a variant APL. Owing to the lack of therapeutic target for ATO, induction therapy for the TFG::RARA variant APL comprised venetoclax and ATRA. The specific protocols used were ATRA 20 mg twice daily (30 days), venetoclax 100 mg once daily (14 days), and voriconazole 200 mg q12h to enhance the blood concentration of venetoclax. During induction therapy, the patient did not develop notable differentiation syndrome or bleeding, nor warrant transfusions of blood products. Specific changes in blood cell counts and fibrinogen levels are shown in Figure 1E. Follow-up of bone marrow morphology and measurable residual disease (MRD) by flow cytometric after one course of induction therapy indicated complete remission (CR). Subsequently, the same regimen was repeated for another course of induction therapy. In addition to bone marrow morphology and MRD, targeted RNA sequencing was negative for the TFG::RARA rearrangement, and the chromosomal karyotype returned to normal. After two courses of ATRA and venetoclax therapy, the patient achieved genetic remission.
Consolidation therapy comprised three courses of cytarabine (1.5 g/m2 every 12 hours, on day 1, day 3, and day 5, respectively) combined with ATRA (20 mg twice daily for 30 days). Maintenance regimen included ATRA 20 mg twice daily and venetoclax 100 mg once daily for 14 days, with voriconazole 200 mg administered every 12 hours to increase the blood concentration of venetoclax (18). The treatment process of the patient is illustrated in Figure 1F. The patient remained in continuous CR at follow-up, as determined by bone marrow morphology, MRD, targeted RNA sequencing and chromosome karyotype analysis. The last reexamination was conducted on 7 November 2024. The results of the bone marrow morphology, MRD, and TFG::RARA fusion gene were negative, with normal blood routine, coagulation function, and the chromosome karyotype. Currently, the patient has an overall survival (OS) exceeding 30 months.
Discussion
In addition to high-risk APL, the existence of variant APL has been identified as an underlying factor for APL relapse and refractoriness. If bone marrow morphology and immunotypes reveal a typical APL phenotype but a negative PML::RARA fusion gene, variant APL needs to be identified through fusion gene screening and/or targeted RNA-sequencing as early as possible. In the current case, the patient who presented with pancytopenia without bleeding, differing from the clinical manifestations of typical APL. A missed diagnosis was feasible owing to the normal coagulation function and absence of abnormal cells in the peripheral blood smear. Bone marrow smears showed classic abnormal hypergranular promyelocytes, while flow cytometry revealed a typical APL immunophenotype. However, neither q-PCR nor FISH detected the PML::RARA rearrangement. Accordingly, targeted RNA sequencing of hematological tumors is particularly important. Therefore, the patient was eventually diagnosed with TFG::RARA variant APL.
TFG::RARA was first reported by Chong et al. in 2018 (19). The protein encoded by the TFG gene plays a role in maintaining the dynamic state of the endoplasmic reticulum and its associated microtubules (20, 21). Likewise, TFG can also form fusion genes together with other partner genes, such as TFG::TEC, TFG::RET, TFG::NTRK1, and TFG::ALK (22–25). The first case of TFG::RARA variant APL involved a 16-year-old male who presented with leukopenia, moderate anemia, normal platelet count, and normal fibrinogen levels. We hypothesized that the TFG::RARA variant APL exerts a relatively limited effect on coagulation function and results in less severe thrombocytopenia. Therefore, these two patients with TFG::RARA variant APL did not present notable bleeding symptoms or diffuse intravascular coagulation (DIC). Nevertheless, this requires validation using large-sample data. Treatment of variant APL remains challenging. Chong et al. (19) employed an ATRA single-drug induction therapy and verified in vitro that TFG::RARA is sensitive to ATRA in vitro. Subsequently, the authors administered two courses of ATRA combined with idarubicin followed by maintenance with ATRA alone. At the time of submission, their patient was still undergoing maintenance therapy; hence, the long-term survival of the patient remains unclear.
To date, only one case of TFG::RARA variant APL has been reported, and its overall prognosis remains poorly understood. Notably, our case belonged to the CD56-positive APL subtype associated with a poor prognosis (26), and treatment warranted careful consideration. Currently, experience in treating variant APL patients is insufficient, and chemotherapy with or without ATRA has been used for treatment in the past (9). Venetoclax, a type of selective B‐cell lymphoma 2 (BCL‐2) inhibitor, has been widely used in AML patients (27). Furthermore, venetoclax‐based treatment was reportedly effective in patients with ATRA/ATO‐resistant APL (28, 29). Additionally, some case reports revealed that venetoclax can achieve good results in ATRA/ATO-resistant variant APL patients (30–34). Xu et al. (35) reported that venetoclax can overcome resistance to ATRA in the case of TNRC18::RARA variant APL. BCL-2 functions in cooperation with PML-RARA fusion protein to promote acute leukemia and prevent neutrophil differentiation (36). BCL-2 may be associated with ATRA resistance in APL patients (37). Therefore, we performed induction therapy with two courses of venetoclax plus ATRA. The patient achieved CR after the first course of induction therapy. Moreover, TFG::RARA and chromosome tests were negative after two- courses induction therapy. Given the uncertain prognosis of this variant APL, we administered three courses of consolidation chemotherapy comprising cytarabine combined with ATRA referring to high-risk APL. We then performed maintenance therapy with venetoclax combined with ATRA for 7 months. The patient has been off treatment for more than one year and has maintained CR status. Currently, the OS is more than 30 months and the progression-free survival (PFS) has reached 29 months. Our case revealed that venetoclax combined with ATRA to treat of TFG::RARA variant APL could achieve excellent curative effects.
Conclusion
In this case report, we diagnosed and treated a patient with TFG::RARA variant APL, the second case reported till submission. Our results demonstrate that venetoclax combined with ATRA can be a good therapeutic option for patients with TFG::RARA variant APL. Nevertheless, data on the efficacy of ATRA plus venetoclax compared with that of ATRA alone are lacking to confirm the additional benefits of venetoclax on disease control and relapse.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committee of the G. Martino Hospital of Messina, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XO: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. JY: Writing – review & editing, Conceptualization, Methodology. SH: Data curation, Writing – review & editing, Validation. WZ: Data curation, Software, Writing – review & editing. QZ: Writing – review & editing, Data curation, Software. FH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks Professor Honghu Zhu from the Department of Hematology, Beijing Chaoyang Hospital, Capital Medical University, and Professor Yuping Gong from the Department of Hematology, West China Hospital, Sichuan University, for their suggestions and guidance on the diagnosis and treatment of this patient. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guarnera L, Ottone T, Fabiani E, Divona M, Savi A, Travaglini S, et al. Atypical rearrangements in APL-like acute myeloid leukemias: molecular characterization and prognosis. Front Oncol. (2022) 12:871590. doi: 10.3389/fonc.2022.871590
2. Geoffroy M-C, de Thé H. Classic and variants APLs, as viewed from a therapy response. Cancers. (2020) 12:1–22. doi: 10.3390/cancers12040967
3. de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. (1990) 347:558–61. doi: 10.1038/347558a0
4. de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A, et al. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. (1991) 66:675–84. doi: 10.1016/0092-8674(91)90113-d
5. Borrow J, Goddard AD, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Sci (New York NY). (1990) 249:1577–80. doi: 10.1126/science.2218500
6. Miller WH, Kakizuka A, Frankel SR, Warrell RP, DeBlasio A, Levine K, et al. Reverse transcription polymerase chain reaction for the rearranged retinoic acid receptor alpha clarifies diagnosis and detects minimal residual disease in acute promyelocytic leukemia. Proc Natl Acad Sci United States America. (1992) 89:2694–8. doi: 10.1073/pnas.89.7.2694
7. Wang Q-F, Zhu H-H. APL: Nemo finds its sea anemone. Blood. (2022) 140:2311–2. doi: 10.1182/blood.2022018020
8. Wang Z-Y, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. (2008) 111:2505–15. doi: 10.1182/blood-2007-07-102798
9. Zhang X, Sun J, Yu W, Jin J. Current views on the genetic landscape and management of variant acute promyelocytic leukemia. biomark Res. (2021) 9:33. doi: 10.1186/s40364-021-00284-x
10. Sainty D, Liso V, Cantù-Rajnoldi A, Head D, Mozziconacci MJ, Arnoulet C, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Blood. (2000) 96:1287–96.
11. Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. (1996) 87:882–6. doi: 10.1182/blood.V87.3.882.bloodjournal873882
12. Wells RA, Catzavelos C, Kamel-Reid S. Fusion of retinoic acid receptor alpha to NuMA, the nuclear mitotic apparatus protein, by a variant translocation in acute promyelocytic leukaemia. Nat Genet. (1997) 17:109–13. doi: 10.1038/ng0997-109
13. Wells RA, Hummel JL, Koven De A, Zipursky A, Kirby M, Dubé I, et al. A new variant translocation in acute promyelocytic leukaemia: molecular characterization and clinical correlation. Leukemia. (1996) 10:735–40.
14. Arnould C, Philippe C, Bourdon V, Grégoire MJ, Berger R, Jonveaux P. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor alpha in acute promyelocytic-like leukaemia. Hum Mol Genet. (1999) 8:1741–9. doi: 10.1093/hmg/8.9.1741
15. Iwanaga E, Nakamura M, Nanri T, Kawakita T, Horikawa K, Mitsuya H, et al. Acute promyelocytic leukemia harboring a STAT5B-RARA fusion gene and a G596V missense mutation in the STAT5B SH2 domain of the STAT5B-RARA. Eur J Haematol. (2009) 83:499–501. doi: 10.1111/j.1600-0609.2009.01324.x
16. Yamamoto Y, Tsuzuki S, Tsuzuki M, Handa K, Inaguma Y, Emi N. BCOR as a novel fusion partner of retinoic acid receptor alpha in a t(X;17)(p11;q12) variant of acute promyelocytic leukemia. Blood. (2010) 116:4274–83. doi: 10.1182/blood-2010-01-264432
17. Ichikawa S, Ichikawa S, Ishikawa I, Takahashi T, Fujiwara T, Harigae H. Successful treatment of acute promyelocytic leukemia with a t(X;17)(p11.4;q21) and BCOR-RARA fusion gene. Cancer Genet. (2015) 208:162–3. doi: 10.1016/j.cancergen.2015.01.008
18. Dong J, Liu S-B, Rasheduzzaman JM, Huang C-R, Miao L-Y. Development of physiology based pharmacokinetic model to predict the drug interactions of voriconazole and venetoclax. Pharm Res. (2022) 39:1921–33. doi: 10.1007/s11095-022-03289-9
19. Chong M-L, Cheng H, Xu P, You H, Wang M, Wang L, et al. TFG-RARA: A novel fusion gene in acute promyelocytic leukemia that is responsive to all-trans retinoic acid. Leukemia Res. (2018) 74:51–4. doi: 10.1016/j.leukres.2018.09.012
20. Kanadome T, Shibata H, Kuwata K, Takahara T, Maki M. The calcium-binding protein ALG-2 promotes endoplasmic reticulum exit site localization and polymerization of Trk-fused gene (TFG) protein. FEBS J. (2017) 284:56–76. doi: 10.1111/febs.2017.284.issue-1
21. Beetz C, Johnson A, Schuh AL, Thakur S, Varga R-E, Fothergill T, et al. Inhibition of TFG function causes hereditary axon degeneration by impairing endoplasmic reticulum structure. Proc Natl Acad Sci United States America. (2013) 110:5091–6. doi: 10.1073/pnas.1217197110
22. Lim B, Kim Jun A-y HJ, Kim S, Choi J, Kim J. The TFG-TEC fusion gene created by the t(3;9) translocation in human extraskeletal myxoid chondrosarcomas encodes a more potent transcriptional activator than TEC. Carcinogenesis. (2012) 33:1450–8. doi: 10.1093/carcin/bgs164
23. Krishnan A, Berthelet J, Renaud E, Rosigkeit S, Distler U, Stawiski E, et al. Proteogenomics analysis unveils a TFG-RET gene fusion and druggable targets in papillary thyroid carcinomas. Nat Commun. (2020) 11:2056. doi: 10.1038/s41467-020-15955-w
24. Pfeifer A, Rusinek D, Żebracka-Gala J, Czarniecka A, Chmielik E, Zembala-Nożyńska E, et al. Novel TG-FGFR1 and TRIM33-NTRK1 transcript fusions in papillary thyroid carcinoma. Genes Chromosomes Cancer. (2019) 58:558–66. doi: 10.1002/gcc.22737
25. Hernández L, Pinyol M, Hernández S, Beà S, Pulford K, Rosenwald A, et al. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood. (1999) 94:3265–8.
26. Testa U, Lo-Coco F. Prognostic factors in acute promyelocytic leukemia: strategies to define high-risk patients. Ann Hematol. (2016) 95:673–80. doi: 10.1007/s00277-016-2622-1
27. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. (2019) 133:1–29. doi: 10.1182/blood-2018-08-868752
28. Li Y, Yu J, Xu Q, Zhang K. Relapsed/refractory acute promyelocytic leukemia with RARA-LBD region mutation was salvaged by venetoclax: A case report. Medicine. (2021) 100:e28076. doi: 10.1097/MD.0000000000028076
29. Wang QQ, Wang H-F, Zhao J-Z, Naranmandura H, Jin J, Zhu H-H. Venetoclax for arsenic-resistant acute promyelocytic leukaemia. Br J Haematol. (2022) 197:e58–60. doi: 10.1111/bjh.v197.5
30. Liu M, Zhao X, Pan W, Qian Z, Du M, Wang L-M, et al. A novel HNRNPC-RARA fusion in acute promyelocytic leukaemia lacking PML-RARA rearrangement, sensitive to venetoclax-based therapy. Br J Haematol. (2021) 195:e123–8. doi: 10.1111/bjh.v195.2
31. Li H, Xiang X, Ding H, Yu J, Xu J, Yuan Y, et al. Differentiation therapy using low-dose venetoclax in a variant acute promyelocytic leukaemia carrying ZBTB16-RARA. Br J Haematol. (2022) 199:768–71. doi: 10.1111/bjh.v199.5
32. Zhang G, Song Y, Wan L, Liu K, Qiu S, Wang J, et al. Treatment of STAT5b-RARA positive acute promyelocytic leukemia by Venetoclax combining with homoharringtonine, cytarabine: A case report and literature review. Blood Sci (Baltimore Md). (2022) 4:93–6. doi: 10.1097/BS9.0000000000000111
33. Song B, Wang X, Kong X, Wang M, Yao L, Shen H, et al. Clinical response to venetoclax and decitabine in acute promyelocytic leukemia with a novel RARA-THRAP3 fusion: A case report. Front Oncol. (2022) 12:828852. doi: 10.3389/fonc.2022.828852
34. Ding W, Weng G, Wang Z, Guo Y, Wang M, Shen H, et al. Case report: Identification of a novel HNRNPC::RARG fusion in acute promyelocytic leukemia lacking RARA rearrangement. Front Oncol. (2022) 12:1028651. doi: 10.3389/fonc.2022.1028651
35. Xu J, Li H, Wang Z, Wang M, Li Q, Hang X, et al. Venetoclax overcomes resistance to all-trans retinoic acid in a variant acute promyelocytic leukemia with TNRC18::RARA fusion. Mol Carcinogenesis. (2024) 63:553–7. doi: 10.1002/mc.23671
36. Kogan SC, Brown DE, Shultz DB, Truong BT, Lallemand-Breitenbach V, Guillemin MC, et al. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARalpha) to block neutrophil differentiation and initiate acute leukemia. J Exp Med. (2001) 193:531–43. doi: 10.1084/jem.193.4.531
Keywords: acute promyelocytic leukemia, variant APL, TFG::RARA, venetoclax, all-trans retinoic acid, arsenic trioxide
Citation: Ouyang X, Yan J, Hu S, Zhu W, Zhou Q and Hu F (2025) Venetoclax combined with ATRA shows promising therapeutic potential for TFG:: RARA variant APL: a case report. Front. Oncol. 15:1529640. doi: 10.3389/fonc.2025.1529640
Received: 17 November 2024; Accepted: 09 April 2025;
Published: 02 May 2025.
Edited by:
Ezhilarasi Chendamarai, Washington University in St. Louis, United StatesReviewed by:
Michael Diamantidis, General Hospital of Larissa, GreeceJia Yin, First Affiliated Hospital of Soochow University, China
Copyright © 2025 Ouyang, Yan, Hu, Zhu, Zhou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Hu, MzEyNzQ5MTUyQHFxLmNvbQ==
 Xianfeng Ouyang
Xianfeng Ouyang Jianguo Yan1
Jianguo Yan1