- 11st Department of Surgery, Aristotle University of Thessaloniki, Papageorgiou General Hospital, Thessaloniki, Greece
- 24th Academic Department of Surgery, Aristotle University of Thessaloniki, G.Papanikolaou General Hospital, Thessaloniki, Greece
- 3Département de Gynécologie-Obstétrique, Hôpital Bichat, Paris, France
- 4Division of Hematology and Oncology, Department of Medicine, University of Washington, Seattle, WA, United States
Hydropic leiomyoma (HLM) is a rare subtype of uterine leiomyoma characterized by significant interstitial fluid accumulation, often mimicking malignant tumors due to its imaging features. Although most uterine leiomyomas are benign and commonly occur in women of reproductive age, HLM can grow to an unusually large size, leading to diagnostic challenges. In this case report, we present a case of a 59-year-old postmenopausal woman with a giant HLM exhibiting extensive cystic hydropic degeneration resembling an aggressive abdominopelvic tumor. The tumor measured 35 × 27 × 17 cm and caused a significant mass effect on surrounding organs. Surgical management involved a total abdominal hysterectomy with right salpingo-oophorectomy via midline laparotomy. Intraoperative findings included displacement of the small bowel, transverse colon, and greater omentum by the tumor, with adherence of the left adnexa to the external surface of the uterus. A left ureteral transection occurred during tumor dissection and was successfully repaired with ureteral reanastomosis and placement of a pigtail stent. The operation lasted 4 hours 11 minutes, and the patient had an uncomplicated postoperative recovery. Histopathological examination confirmed the diagnosis of HLM with extensive cystic degeneration. Based on available literature, this case appears to represent the largest HLM reported to date, highlighting the importance of accurately distinguishing benign from malignant tumors to guide appropriate clinical management. This case underscores the complexities associated with diagnosing and surgically treating large, degenerating uterine leiomyomas.
Introduction
Uterine leiomyomas, also known as fibroids or uterine myomas, are the most common benign neoplasm in women, occurring in 20%–30% of women, mainly in the 30–50 year age group (1). They are non-cancerous monoclonal tumors arising from smooth muscle cells and fibroblasts of the uterine wall (2).
In 80%–90% of cases, leiomyomas are of the conventional or usual type, with monotonous spindle cells, rare mitoses, and benign biological behavior. However, leiomyoma variants constitute a heterogeneous group with the same symptoms and signs as classic leiomyomas, but with malignant or uncertain potential (3). In addition, uterine leiomyomas can undergo different types of degeneration, including hyaline, cystic, hydropic, myxoid, fatty, hemorrhagic degeneration, or presentation of calcifications (4). These degenerative changes have been attributed to relative local ischemia during mass enlargement (5) and are of paramount importance in avoiding pitfalls in the differential diagnosis of uterine sarcoma and other ovarian tumors (6).
Uterine leiomyomas are classified by the International Federation of Gynaecology and Obstetrics (FIGO) into nine subtypes (0–8) based on their location within the uterus (7). These range from submucosal (types 0–2) and intramural (types 3–4) to subserosal (types 5–7) and those leiomyomas that do not relate to the myometrium at all, such as cervical, parasitic (type 8) (7). While hydropic leiomyomas (HLMs) are not explicitly categorized in this system, their large size and extensive degeneration often make them comparable to FIGO types 7 or 8, particularly when they grow exophytically or involve adjacent structures.
According to the 5th edition of the World Health Organization (WHO) classification of female genital tumors, HLM is a distinct, extremely rare leiomyoma subtype with significant interstitial fluid or stromal watery edema leading to the separation and compartmentalization of smooth muscle cells, increased vascularity, and arrangement of tumor cells in nodules or cords (8, 9). HLM tumors are significantly larger than usual-type leiomyomas, with a mean size of 14.4 cm (10). Presentation of imaging characteristics that can resemble malignancy renders differential diagnosis from malignant uterine tumors like leiomyosarcoma quite challenging. However, this distinction is critical for guiding treatment choices and ensuring appropriate patient follow-up. The literature contains a limited number of HLM cases, while reports of giant HLMs are extremely rare.
Hydropic degeneration refers to the accumulation of interstitial fluid and stromal edema within the uterine leiomyoma, leading to the expansion and softening of the tumor (10). While focal hydropic change is relatively common, certain rare and more extensive subtypes have been described, including diffuse hydropic degeneration and perinodular hydropic degeneration (PHD). Diffuse hydropic degeneration results in the widespread disruption of the classic smooth muscle architecture of the leiomyoma, often with prominent cystic spaces, vascular congestion, and soft, gelatinous consistency. PHDL is characterized by excessive interstitial fluid accumulation around smooth muscle bundles, creating a multinodular pattern that can mimic more aggressive or infiltrative tumors, such as intravenous leiomyomatosis or low-grade endometrial stromal sarcoma (8). These degenerative patterns are particularly important because they can significantly alter both the imaging characteristics and gross morphology of leiomyomas, complicating preoperative diagnosis and raising suspicion for malignancy. For this reason, early and precise recognition of such variants is essential in guiding appropriate surgical and pathological management.
Herein, we report an unusual case of a 59-year-old postmenopausal woman who presented with a giant uterine HLM with massive cystic hydropic degeneration mimicking an aggressive abdominopelvic tumor. The case underscores the diagnostic challenges, therapeutic considerations, and surgical complexities associated with the management of such massive tumors.
Case description
A 59-year-old nulliparous woman presented to the outpatient clinic of our general surgery department complaining of an abdominopelvic mass that had been gradually increasing in size for the past 5 years. The patient also reported abdominal distension without any other symptoms. The patient had no history of chronic medical conditions, prior abdominal or pelvic surgeries, or hormone therapy. Her gynecologic history included regular menstrual cycles until menopause at age 49. She was nulliparous, with no history of infertility evaluation or assisted reproduction. There were no prior reports of abnormal uterine bleeding, pelvic pain, or known uterine pathology. She was a non-smoker, reported no alcohol or drug use, and had no family history of gynecologic or colorectal malignancies. Surgical history included an open appendectomy in childhood. Investigation of the abdominal mass began 1 month ago during hospital admission for renal colic of the right kidney.
The patient had first noticed gradual abdominal distension approximately 5 years ago but attributed it to aging, dietary habits, and minor weight gain. Because she remained largely asymptomatic and experienced no significant pain, bleeding, or gastrointestinal or urinary disturbances, she did not seek medical evaluation during that period. She had not undergone any imaging or gynecologic evaluation prior to the recent hospitalization. The presence of right-sided renal colic led to her first abdominal ultrasound, which incidentally revealed the large abdominopelvic mass and initiated further diagnostic investigation.
On physical examination, the patient was emaciated and frail with normal vital signs. Abdominal examination revealed significant abdominal distension due to a large, irregular, non-tender, and immobile lesion occupying the entire abdominal and pelvic cavity. Physical examination also revealed a dullness to percussion and a shifting dullness, indicating the presence of ascites. The overlying skin showed striae and dilated abdominal wall veins, consistent with abdominal distension and venous congestion (Figure 1). No abnormal lymph nodes were palpable.
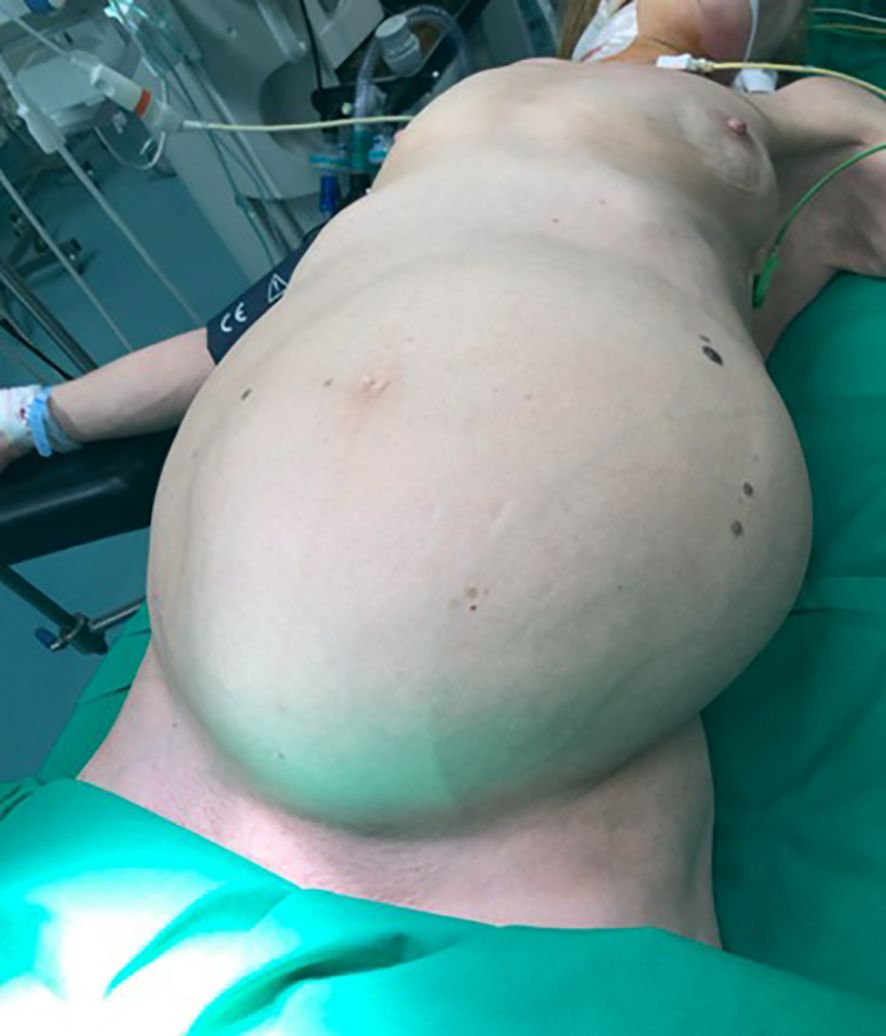
Figure 1. Significant distension of the abdomen, with overlying skin striae and dilated abdominal wall veins.
Laboratory tests, including the tumor markers Cancer Antigen 125 (CA-125), Alpha-fetoprotein (AFP), Cancer Antigen 15-3 (CA15-3), and Cancer Antigen 19-9 (CA19-9), were within the normal range, whereas Carcinoembryonic Antigen (CEA) was slightly elevated (8.04 ng/mL).
The tumor markers were obtained as part of the preoperative evaluation to assess for potential gynecologic or gastrointestinal malignancy, given the presence of ascites and complex imaging features. While the CA-125, CA19-9, and AFP levels were within normal limits, CEA was mildly elevated. This finding was interpreted with caution, as low-level CEA elevation may occur in benign conditions involving chronic compression or inflammation of the bowel. In the absence of radiologic or clinical findings suggestive of gastrointestinal cancer, CEA was considered non-specific and not indicative of malignancy in this case.
Abdominal ultrasound (US) showed a huge cystic mass occupying most of the abdominal cavity. Fatty liver infiltration with inhomogeneous parenchyma without dilatation of the intrahepatic biliary system, without cirrhosis, as well as microlithiasis of the kidneys with pelvicalyceal dilatation, especially on the right side, and abundant ascitic fluid in the peri-hepatic, hepato-renal, and peri-splenic spaces and in the pouch of Douglas were additional US findings. Computed tomography (CT) of the chest, abdomen, and pelvis showed a large complex cystic mass with calcifications occupying the entire abdomen and pelvis, causing compression of the adjacent abdominal organs. Intravenous contrast showed thick, enhancing septations. The ovaries and cervix were not visible due to displacement and compression. There was no evidence of intra-thoracic metastases or enlarged lymph nodes. Magnetic resonance imaging (MRI) of the abdomen and the pelvis revealed occupation of the greatest part of the abdomen and the pelvis by a huge abdominopelvic complex cystic mass, with a small number of solid parts and thick septations, as well as ascitic fluid in the pouch of Douglas. The mass measured approximately 27.7 cm in transverse diameter, 19 cm in anterolateral diameter, and 32 cm in cephalocaudal diameter (Figures 2A–C). Cytological examination was negative for malignancy, showing only rare mesothelial cells and sparse lymphocytes.
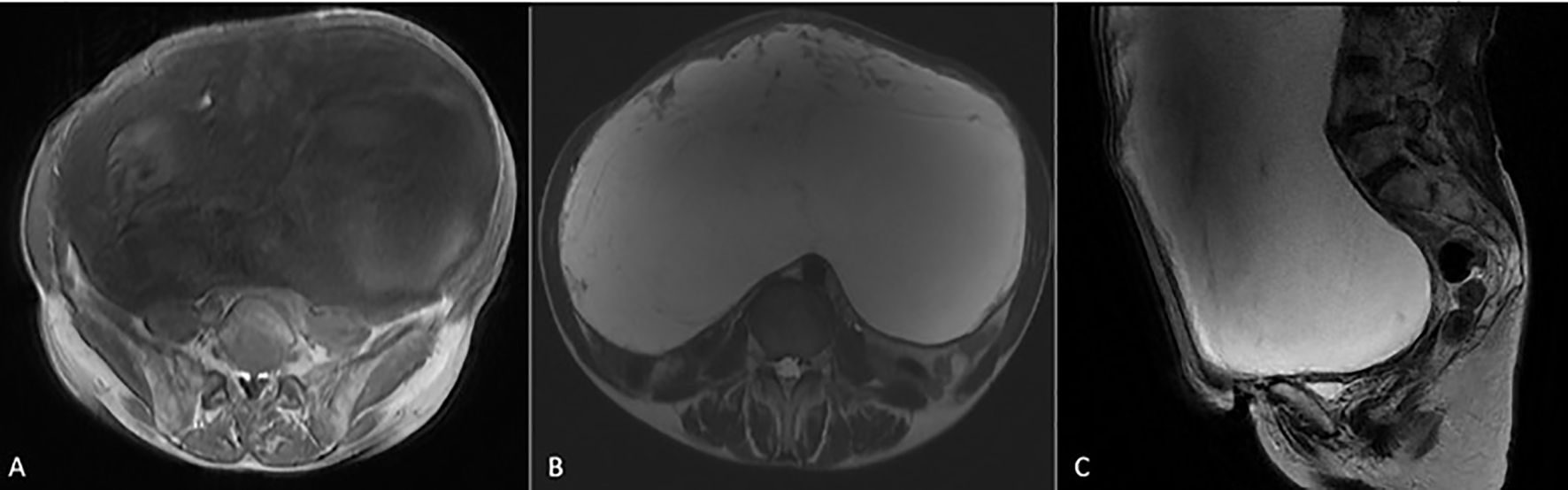
Figure 2. MRI images of the giant hydropic leiomyoma (HLM). (A) T1-weighted image showing the leiomyoma with low-to-intermediate signal intensity, reflecting the tumor’s solid components. (B) T2-weighted image demonstrating high signal intensity within the leiomyoma, indicative of significant fluid accumulation due to hydropic degeneration. (C) T2-weighted MRI in the sagittal plane showing a large HLM with high signal intensity. The mass causes significant mass effect on adjacent organs, displacing the bladder anteriorly and compressing the rectum posteriorly.
Despite the presence of imaging findings that raised suspicion for malignancy—such as the tumor’s large size, thick internal septations, and the presence of ascites—several characteristics supported a benign diagnosis. On MRI, the lesion demonstrated well-circumscribed margins without infiltrative behavior, and there were no signs of lymphadenopathy, necrosis, or peritoneal deposits. The solid components showed no irregular or intense enhancement, and the T2-weighted hyperintensity correlated with significant fluid accumulation rather than tumoral aggressiveness. These findings, when combined with normal CA-125 and the absence of systemic symptoms, favored a diagnosis of a benign but atypically degenerating uterine mass. Nonetheless, due to the size, mass effect, and residual diagnostic uncertainty, surgical resection was deemed necessary by the multidisciplinary team.
Based on preoperative imaging, the differential diagnosis included ovarian mucinous cystadenoma, uterine leiomyosarcoma, endometrial stromal sarcoma, and mesenteric cyst, and after multidisciplinary tumor board consultation and patient consent, surgical resection was decided. The case was reviewed by a multidisciplinary tumor board comprising a general surgeon, a gynecologic oncologist, a radiologist, and a pathologist. Imaging findings—including a large cystic mass with enhancing septations, absence of visible ovaries, and associated ascites—raised concern for malignancy, particularly ovarian epithelial tumors and uterine sarcomas. However, the absence of intrathoracic metastases, normal CA-125 levels, and a well-circumscribed border on MRI tempered suspicion for overt malignancy. Due to the tumor’s massive size, increasing abdominal discomfort, and diagnostic uncertainty, the consensus was to proceed with exploratory surgery and resection for both therapeutic and diagnostic purposes.
Under general anesthesia, the patient was placed in the supine position, and a midline laparotomy was performed via a midline incision. After entry into the peritoneal cavity, a giant cystic lesion was recognized, displaying the small bowel, the transverse colon, and the greater omentum to the left hypochondrium, while the body of the uterus and the right ovary were found in the right lateral abdomen. Investigation of the liver and the peritoneal cavity was negative for metastatic disease or lymphadenopathy. The left ovary was not recognized intraoperatively. Dissection of the cystic mass was performed in combination with total hysterectomy and right salpingo-oophorectomy, using a LigaSure™ vessel-sealing device (Medtronic, Covidien) for hemostasis and tissue sealing.
Both ureters were recognized intraoperatively, while the left ureter was transected during the dissection of the posterior surface of the tumor. The transection occurred despite prior identification of the ureter due to dense adhesions and anatomic distortion caused by the tumor’s size and posterior extension. The injury was immediately recognized and repaired intraoperatively with ureteral spatulation, reanastomosis using interrupted 4–0 PDS sutures, and placement of a 6-Fr pigtail stent. Preoperative prophylactic ureteral stenting was not performed, as the anatomy was presumed navigable on imaging; however, this approach may be reconsidered in similar future cases. Estimated blood loss was approximately 300 mL, and no blood transfusion was required. No other intraoperative complications occurred.
During anterior dissection, the tumor was successfully dissected off the bladder, and the cervix was identified. After ligation and division of the round, broad, sacro-uterine, and transverse cervical ligaments of the uterus, vaginal transection was performed. The specimen of the tumor en bloc, along with the uterus and the right ovary, was sent for histopathological examination (Figures 3A, B). The total operative time was 4 hours 11 minutes, and the patient was transferred to the ward without postoperative administration in the intensive care unit.
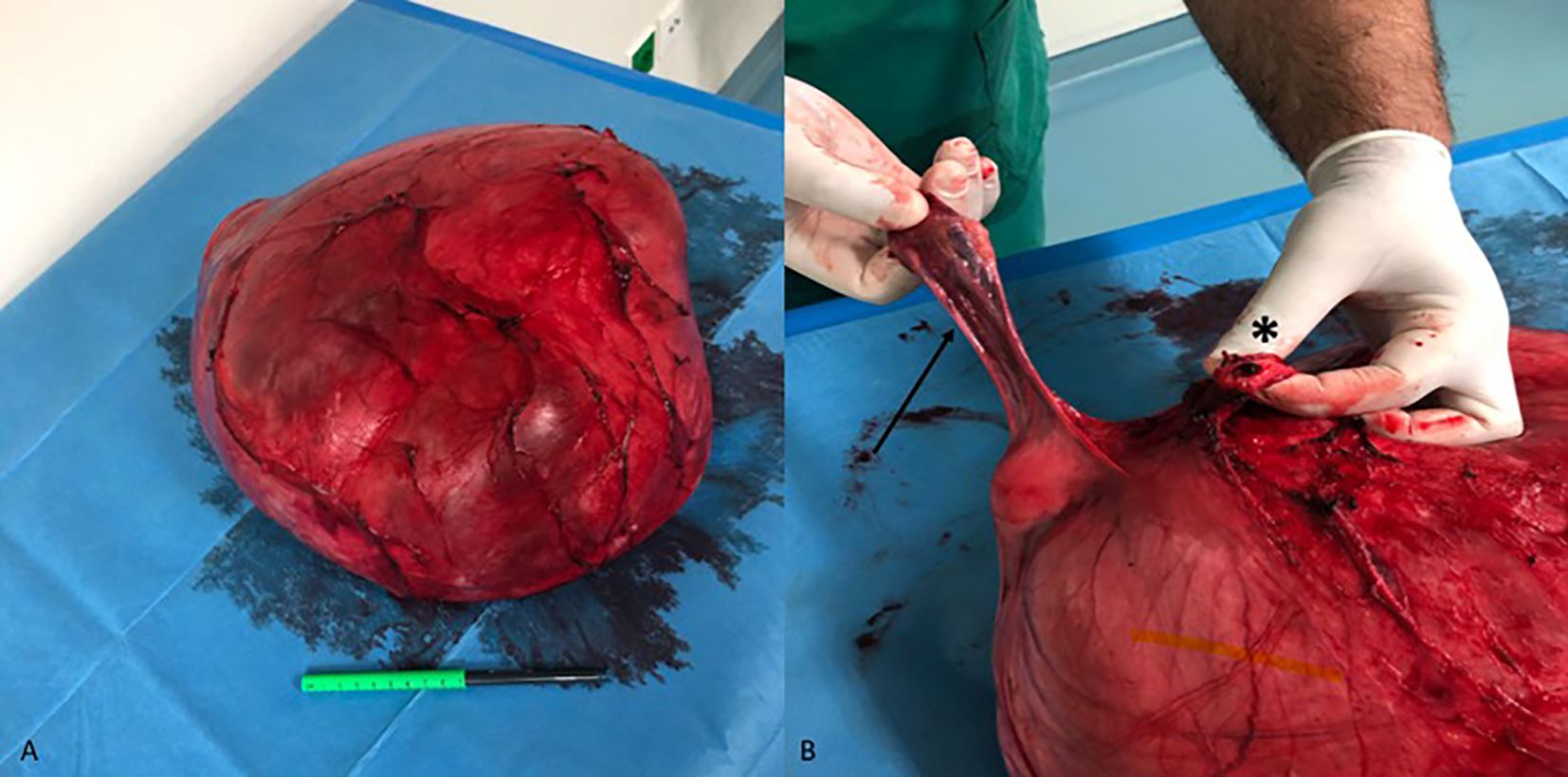
Figure 3. Uterine leiomyoma with diffuse hydropic degeneration. (A) Surgical specimen after total hysterectomy with right salpingo-oophorectomy. (B) The right adnexa (arrow) and the vaginal stump (asterisk) are recognizable on the specimen. Although the left ovary was not recognized intraoperatively, it was found to be completely adherent to the outer surface of the tumor on histological examination.
The mass measured 35 × 27 × 17 cm in diameter. On macroscopic examination, the specimen consisted of an encapsulated multilocular white tumor, predominantly cystic with gelatinous content and partly solid with fibrous-elastic structure. Its external surface was smooth and intact. The tumor was attached to the uterus and both adnexa. The left adnexa (ovary and fallopian tube) was completely adherent to the outer surface of the tumor. The tumor presented microscopic morphology suggestive of HLM with extensive cystic hydropic degeneration. Focal ischemic-type necroses were observed, while no cellular atypia or increased mitotic activity was noted. The cell proliferation index (Ki-67) reached up to 2%. Immunohistochemical stains were also indicative of HLM, with the following immunophenotype: desmin (+), smooth muscle actin (SMA) (+), calponin (+), Wilms tumor 1 (WT-1) (+), Estrogen Receptor (ER) +, Progesterone Receptor (PR) +), AE1/AE3 (−), inhibin (−), melan-A (−), and calretinin (−). The histological appearance of the ovaries and the fallopian tubes was age-consistent.
The patient had an uncomplicated postoperative course and was discharged on the fourth postoperative day, with no signs of complications during outpatient visits. The pigtail stent was removed following an ascending urethrocystography, which confirmed ureteral anastomotic integrity and the absence of leakage. A subsequent cystoscopy was then performed to complete the evaluation and facilitate the stent’s removal. On 6-month follow-up, she remained symptom-free, with no evidence of recurrence on ultrasound. A concise overview of the patient’s course is presented in Table 1.
Discussion
Uterine leiomyomas are the most common neoplasm in premenopausal women, with a lifetime incidence of approximately 70%, and they are more common in patients on progesterone therapy (11). Although the majority of cases are asymptomatic, approximately 30% of women with leiomyomas present with a range of serious symptoms, including abnormal uterine bleeding, anemia, pelvic or low back pain, urinary frequency and retention, constipation, obstetric complications, or infertility, particularly in the case of submucosal leiomyomas (12). Leiomyomas are the most common indication for hysterectomy in the United States, resulting in an annual economic burden of approximately $5.9–34.4 billion (USD) (13). To our knowledge, the present case represents the largest HLM reported in the literature, contributing to the limited body of literature on this rare clinical entity.
The classic (conventional or typical) spindled type of uterine leiomyoma is the most common form of leiomyoma, accounting for 80%–90% of leiomyomas (14). In contrast, leiomyoma variants are extremely rare, with an incidence of 1% to 2% (4). Most classic-type leiomyomas have a mitotic index of less than five mitotic figures per 10 high-power fields, mild cytological atypia, and no evidence of tumor cell necrosis (15). In contrast, leiomyoma variants are extremely rare, with an incidence of 1% to 2% (4). In addition, classic-type leiomyomas do not present a diagnostic dilemma on MRI and US imaging (16). On US, classic-type non-degenerated leiomyomas present as a round or oval, well-circumscribed, hypoechoic solid lesion, often accompanied by posterior shadowing due to the presence of calcifications or the interface of the leiomyoma-normal uterine wall (4). On MRI, a classical leiomyoma usually presents as a well-circumscribed mass with isointense or mildly low signal intensity (SI) on T1-weighted (T1W) images, characteristically low SI on T2-weighted (T2W) images, and enhancement after contrast administration (14). Histologically, leiomyomas are characterized by large amounts of extracellular matrix containing collagen, proteoglycan, and fibronectin and have a thin pseudocapsule containing areolar tissue and compressed muscle fibers (2).
Degenerative changes of leiomyomas are common, with an incidence of 10%, even within the same tumor, and are caused by the inadequate blood supply of leiomyomas, which are usually large or have ramified growth (6, 17). Degenerative changes result in a heterogeneous appearance on imaging, with minimal or irregular enhancement (18). Conventional-type leiomyomas may present a series of various degenerative changes. Hyaline is the most common type of degeneration (in 60% of cases) and is characterized by the presence of eosinophilic bands or plaques in the extracellular space (6, 19). Hemorrhagic (carneous or red) degeneration is common in pregnant women or women taking oral contraceptives and is caused by massive hemorrhagic infarction due to venous thrombosis in the periphery of the tumor (20). Myxoid degeneration is extremely rare and is characterized by smooth muscle cells with a significant concentration of cell-rich acid mucin (21). Fatty degeneration or lipoleiomyoma is a rare type of tumor caused by fatty metamorphogenesis of smooth muscle cells and is composed of adipocytes and smooth muscle cells separated by thin fibrous septa (22). Finally, hydropic degeneration results from extensive fluid accumulation and watery edema within the leiomyoma (23).
Focal hydropic degeneration is commonly encountered, occurring in up to 50% of fibroids (23). However, diffuse hydropic cystic degeneration and PHDL are also two rare presentation subtypes of hydropic degeneration (6). More specifically, diffuse hydropic cystic degeneration leads to extensive obliteration of the common fibroid architecture, with the presence of numerous thick-walled blood vessels distorting its smooth muscle origin (8). PHDL is caused by increased fluid accumulation around the fascicles of the smooth muscle bundles, resulting in a multinodular growth pattern. The microscopic and macroscopic features of PHDLs pose a significant challenge in their differential diagnosis with intravenous leiomyomatosis, endometrial stromal sarcoma, or myxoid leiomyosarcoma (24). The aforementioned rare types of hydropic degeneration can lead to an excessive increase in tumor size due to massive intra-tumoral watery edema, with consequent diagnostic difficulties regarding their origin and biological behavior, similar to our patient (25). The extensive accumulation of interstitial fluid, as well as the cystic degeneration, can alter classic leiomyoma features, making differential diagnosis difficult. The risk of misdiagnosis is particularly high when evaluating tumor size, vascularity, and cellular morphology. In such cases, immunohistochemical analysis holds a crucial role in distinguishing HLM from other malignant lesions. Markers such as desmin, SMA, and calponin confirm smooth muscle origin, while a low Ki-67 proliferation index (<5%) supports a benign diagnosis (26). In this case, a broad immunohistochemical panel was applied not only to confirm smooth muscle differentiation but also to exclude other potential differential diagnoses given the tumor’s size, cystic appearance, and the patient’s postmenopausal status. AE1/AE3 was used to exclude an epithelial neoplasm, while inhibin and melan-A were employed to rule out sex cord stromal tumors, such as granulosa cell tumors. Calretinin and WT-1, although sometimes positive in smooth muscle tumors, were also useful in excluding a mesothelial origin or serous carcinoma, particularly given the presence of ascites and adnexal adhesions. This comprehensive panel supports the pathological conclusion of a benign hydropic leiomyoma, despite its unusual gross and radiologic features. The use of Ki-67 as a proliferation marker was of particular diagnostic value. According to existing histopathological criteria, leiomyomas typically exhibit a Ki-67 index of <5%, whereas higher indices (>10%–15%) are suggestive of malignant smooth muscle tumors such as leiomyosarcoma or smooth muscle tumors of uncertain malignant potential (STUMP) (15, 27). In our case, the Ki-67 index was 2%, consistent with low proliferative activity and reinforcing the benign diagnosis. The absence of cytological atypia, tumor cell necrosis, and increased mitotic activity further supported this interpretation. Thus, Ki-67 served as a practical and clinically meaningful tool to distinguish this degenerating leiomyoma from its malignant mimics. Consultation of a specialized pathologist with expertise in gynecologic pathology is advisable in cases with ambiguous histological features to ensure diagnostic accuracy and avoid unnecessary overtreatment.
The imaging and histologic findings in this case show a clear radiologic–pathological correlation. On MRI, the lesion demonstrated marked T2-weighted hyperintensity (Figures 2B, C), which corresponds to the extensive stromal edema and cystic fluid accumulation seen histologically—hallmark features of hydropic degeneration. Additionally, the compartmentalized, nodular architecture identified on microscopic examination explains the septated and multilocular appearance of the tumor on imaging. Grossly, the tumor’s smooth, lobulated external surface (Figure 3A) supports its benign, encapsulated nature. Immunohistochemical staining further confirmed a smooth muscle origin (desmin+, SMA+, and calponin+) with low proliferative activity (Ki-67 < 5%), consistent with a diagnosis of benign hydropic leiomyoma and excluding high-grade malignancy.
HLMs may present on MRI with imaging features that overlap with malignant tumors, particularly leiomyosarcoma, endometrial stromal sarcoma, and ovarian cystic malignancies. Common features that raise suspicion for malignancy include large size, thick septations, cystic-solid architecture, and associated ascites. In our case, these characteristics necessitated careful preoperative evaluation and tumor board discussion (11). However, several radiologic findings were more suggestive of a benign process. These included the well-defined tumor margins, lack of infiltrative behavior, absence of intratumoral necrosis, and no evidence of pelvic or para-aortic lymphadenopathy or peritoneal nodules. Additionally, there was no enhancement of solid components in a pattern typical of aggressive sarcomas. The T2-weighted hyperintensity corresponded histologically to interstitial fluid accumulation and stromal edema, which are hallmark features of hydropic degeneration. Together, these imaging characteristics—although atypical—favored the diagnosis of a benign but degenerative leiomyoma, supporting the decision to proceed with surgical management. To further assist in differential diagnosis, a comparative summary of radiologic and clinical features distinguishing HLM from other cystic abdominopelvic masses is presented in Table 2.
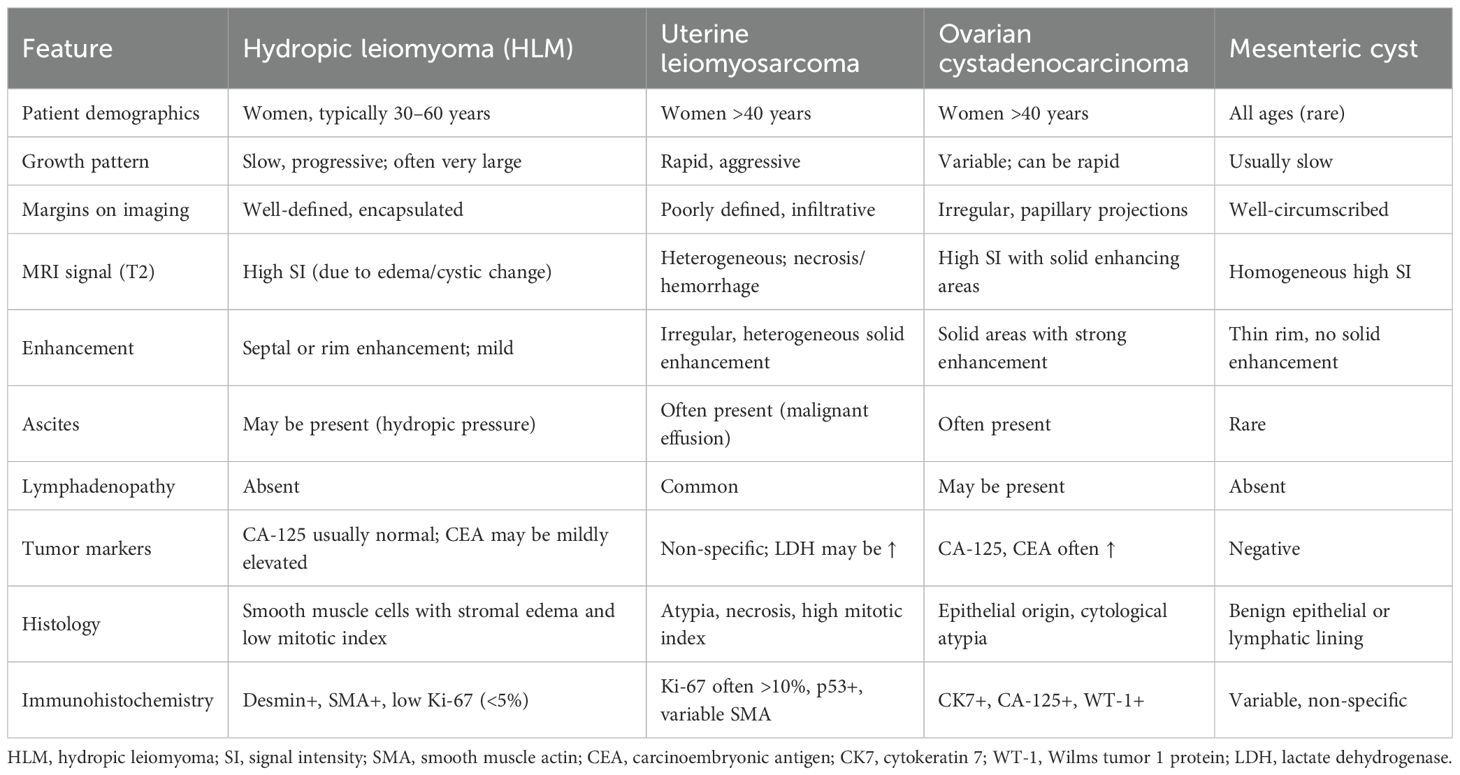
Table 2. Differential diagnostic features of hydropic leiomyoma and other cystic abdominopelvic tumors.
Based on its clinical and radiologic features, the HLM in this case aligns most closely with a FIGO type 7 or 8 leiomyoma, given its predominantly subserosal and exophytic growth pattern, with significant distortion of the uterus and involvement of adjacent structures (7). The extensive hydropic degeneration further complicates classification, as it contributes to the tumor’s massive size and fluid accumulation, features that are not explicitly addressed in the current FIGO system. This case underscores the need for enhanced classification criteria for rare leiomyoma variants such as HLM, particularly those presenting with aggressive mass effects and atypical degenerative changes. Although the FIGO classification system is a valuable tool for categorizing uterine leiomyomas based on their anatomic location, it does not account for critical characteristics such as tumor volume, extent of degeneration, or displacement into extrauterine spaces, all of which are highly relevant in cases of giant or hydropically degenerating leiomyomas (7). In our case, the tumor demonstrated extensive exophytic growth, stromal edema, and cystic changes, which are not explicitly addressed within the current FIGO types (0–8). We suggest that future iterations of the FIGO system could incorporate modifiers or subclassifications to reflect tumor size, degeneration type (e.g., hydropic, cystic, and myxoid), and anatomic distortion, as these factors have important implications for surgical strategy, differential diagnosis, and reporting consistency across studies.
The clinical presentation of HLMs is similar to that of classical leiomyomas, as reported by Clement et al. in the first case series of 10 patients (8). However, there are reports in the literature of HLMs presenting as pseudo-Meigs syndrome with dyspnea, pleural effusion, and ascites, which is a diagnostic pitfall in the differential diagnosis with other uterine and ovarian malignancies (28, 29). In addition, several cases of pregnant women with HLMs have been described (30, 31). Lai et al. described a case of retroperitoneal HLM preoperatively diagnosed as an ovarian cyst in a 46-year-old woman (32).
When evaluating large abdominopelvic masses, the differential diagnosis should extend beyond malignant conditions such as leiomyosarcoma or ovarian neoplasms to include also some benign but potentially complex presentations of large uterine leiomyomas, which may mimic more aggressive pathology. Among the latter, leiomyoma torsion is a rare but severe complication, occurring when a pedunculated subserosal fibroid twists around its vascular stalk, leading to impaired blood flow and necrosis (33). Clinical symptomatology often includes severe abdominopelvic pain, and imaging findings are suggestive of compromised blood flow, although definitive diagnosis is frequently made intraoperatively (33). Furthermore, leiomyoma torsion has been described in patients with syndromic conditions, such as in Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome. In such cases, leiomyomas may arise in rudimentary uterine structures and undergo torsion, leading to acute abdominal pain and requiring prompt surgical intervention (34). Finally, large degenerative cystic leiomyomas often mimic ovarian neoplasms due to their mixed solid and cystic components. These lesions may cause significant mass effect to adjacent organs, leading to symptoms such as dyspnea, abdominal distension, and pressure-related organ dysfunction, which can complicate preoperative differential diagnosis from malignancy (35). Given the potential overlap in clinical presentation among these conditions, a thorough diagnostic approach incorporating detailed imaging, intraoperative assessment, and histopathological confirmation is essential for accurate differentiation.
HLMs are generally characterized by a larger size compared to classic-type leiomyomas. According to Griffin et al., HLMs have a mean size of 14.4 ± 8.2 cm, compared to a mean size of 6.7 ± 0.8 cm for classic-type leiomyomas (10). The literature reports limited data on giant HLMs. The first relative case report dates back to 1994, when Moore et al. reported a giant uterine leiomyoma with focal hydropic degeneration in a young pregnant woman (31). Horta et al. described a rare case of a 35-year-old patient with a giant pedunculated uterine leiomyoma with diffuse hydropic degeneration measuring approximately 20 × 30 × 8 cm who was treated with fertility-sparing myomectomy (36). Akkour et al. in 2021 reported a case of a 32-year-old woman with large pelvoabdominal masses measuring 33 × 24 × 15 cm in total who was treated with myomectomy with transposition of the ovaries to the lateral abdominal wall. Histopathology revealed a uterine leiomyoma with massive cystic hydropic degeneration (6). In a case report by Ye et al., a degenerated leiomyoma with extensive edema measuring 30 × 25 × 16 cm causing uterine torsion was resected in a postmenopausal woman who was treated with total abdominal hysterectomy (37). Based on published reports available in the literature, the present case appears to represent one of the largest HLMs documented to date.
As of today, there is no consensus on the optimal management of HLMs, which mainly includes the options of myomectomy and total abdominal hysterectomy with or without bilateral salpingo-oophorectomy (10). The decision between myomectomy and hysterectomy is influenced by various parameters such as patient age, tumor size and macroscopic appearance, willingness for fertility preservation, and intraoperative findings. In younger patients with fertility concerns, myomectomy should be preferred, provided that the tumor is well-demarcated and malignancy is not suspected. On the contrary, in postmenopausal women or cases of enlarged tumors with extensive adhesions rendering uterine preservation challenging, hysterectomy is proposed to ensure complete, margin-negative tumor resection and to reduce the risk of recurrence. In our case, a total abdominal hysterectomy was chosen due to the enlarged abdominopelvic tumor, its extensive cystic degeneration, and its mass effect on adjacent organs. Given the patient’s postmenopausal status and the increased risk of future adnexal pathology, right salpingo-oophorectomy was performed to facilitate complete tumor excision and optimize long-term outcomes. Postoperative course of HLMs was uncomplicated in the case series of Clement et al. and in a few case reports reported in the literature (8, 28, 30–32, 36, 37). Surgical management of uterine fibroids must be tailored to tumor size and complexity, particularly in rare cases like hydropic leiomyoma. Recent reviews highlight the evolving role of both open and minimally invasive approaches in fibroid treatment (38). The growing spectrum of available medical and surgical options for uterine fibroids underscores the importance of individualized treatment planning. Even in rare variants like hydropic leiomyoma, awareness of evolving therapeutic strategies can aid in selecting the most appropriate and safe approach (39).
This case report provides valuable insights into the rare presentation of giant HLM, contributing to the limited literature on this uncommon subtype of uterine leiomyoma. Based on available reports in the literature, this case appears to represent one of the largest HLMs documented to date. It contributes meaningfully to the limited body of knowledge on this rare clinical entity and serves as a reminder that even benign tumors may present with aggressive features, necessitating individualized surgical planning and multidisciplinary management. Surgeons managing large abdominopelvic masses should maintain a broad differential diagnosis and be prepared for unexpected findings, ensuring optimal patient outcomes through precise surgical execution and postoperative care.
A major strength is the detailed clinical and surgical documentation, which emphasizes the diagnostic challenges and surgical considerations required for such large, hydropic tumors, especially when they mimic malignancies. The report also highlights the successful management of intraoperative complications, underscoring the importance of multidisciplinary planning and expertise in managing complex cases. In this case, a total abdominal hysterectomy with right salpingo-oophorectomy was performed, along with ureteral reconstruction following iatrogenic injury, highlighting the complexities inherent to these extensive surgical procedures.
The operative management of giant HLMs poses significant technical challenges. Distorted anatomy, particularly the displacement of ureters and adnexa, increases the risk of intraoperative complications. In our case, an iatrogenic ureteral transection occurred despite careful dissection, highlighting the need for heightened vigilance in such anatomically complex settings. Although preoperative ureteral stenting was not performed, it may be advisable in similar cases with anticipated pelvic distortion. Intraoperative bleeding was moderate, and no transfusion was needed; however, surgeons must be prepared for substantial hemorrhage and potential visceral injury during resection of such massive tumors.
The patient’s successful postoperative recovery reinforces the value of a multidisciplinary approach and careful intraoperative techniques. However, a limitation is that the report represents a single case, limiting the generalizability of findings. Further research is needed in this field to better understand the pathophysiology and optimal management strategies for HLMs, particularly those of massive size.
In conclusion, HLMs, particularly those of considerable size, pose unique diagnostic and therapeutic challenges for surgeons. The potential for these tumors to mimic malignant processes underscores the critical importance of preoperative imaging and thorough histopathological assessment in order to guide surgical decision-making. In cases of giant HLMs, as presented in this report, the significant mass effect on surrounding organs requires meticulous preoperative planning and a highly skilled surgical approach to ensure complete resection while minimizing complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because it is not applicable for case reports in my institution. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor PC declared a past co-authorship with the author EA.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li B, Wang F, Chen L, and Tong H. Global epidemiological characteristics of uterine fibroids. Arch Med Sci. (2023) 19:1802–10. doi: 10.5114/aoms/171786
2. Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, and Rogers PAW. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod. (2014) 20:250–9. doi: 10.1093/molehr/gat083
3. Alran L and Rychlik Agnieszka CS. Leiomyoma-General . Available online at: https://www.pathologyoutlines.com/topic/uterusleiomyoma.html (Accessed May 13, 2025).
4. Arleo EK, Schwartz PE, Hui P, and McCarthy S. Review of leiomyoma variants. Am J Roentgenol. (2015) 205:912–21. doi: 10.2214/AJR.14.13946
5. Dancz CE and Macdonald HR. Massive cystic degeneration of a pedunculated leiomyoma. Fertil Steril. (2008) 90:1180–1. doi: 10.1016/j.fertnstert.2007.12.046
6. Akkour K, Alhulwah M, Alqahtani N, and Arafah MAA. Giant leiomyoma with massive cystic hydropic degeneration mimicking an aggressive neoplasm: A challenging case with a literature review. Am J Case Rep. (2021) 22:1–5. doi: 10.12659/AJCR.929085
7. Gomez E, Nguyen M-LT, Fursevich D, Macura K, and Gupta A. MRI-based pictorial review of the FIGO classification system for uterine fibroids. Abdom Radiol (N Y). (2021) 46:2146–55. doi: 10.1007/s00261-020-02882-z
8. Clement PB, Young RH, and Scully RE. Diffuse, perinodular, and other patterns of hydropic degeneration within and adjacent to uterine leiomyomas. Problems in differential diagnosis. Am J Surg Pathol. (1992) 16:26–32. doi: 10.1097/00000478-199201000-00004
9. Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, and Horn L-C. 2020 WHO classification of female genital tumors. Geburtshilfe Frauenheilkd. (2021) 81:1145–53. doi: 10.1055/a-1545-4279
10. Griffin BB, Ban Y, Lu X, and Wei J-J. Hydropic leiomyoma: A distinct variant of leiomyoma closely related to HMGA2 overexpression. Hum Pathol. (2019) 84:164–72. doi: 10.1016/j.humpath.2018.09.012
11. Lameira P, Filipe J, Cabeçadas J, and Cunha TM. Hydropic leiomyoma: A radiologic pathologic correlation of a rare uterine tumor. Radiol Case Rep. (2022) 17:3151–6. doi: 10.1016/j.radcr.2022.06.008
12. Giuliani E, As-Sanie S, and Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynecol Obstet. (2020) 149:3–9. doi: 10.1002/ijgo.13102
13. Styer AK and Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. (2016) 34:3–12. doi: 10.1016/j.bpobgyn.2015.11.018
14. Tu W, Yano M, Schieda N, Krishna S, Chen L, Gottumukkala RV, et al. Smooth muscle tumors of the uterus at MRI: focus on leiomyomas and FIGO classification. Radiographics. (2023) 43. doi: 10.1148/rg.220161
15. Bell SW, Kempson RL, and Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. (1994) 18:535–58. doi: 10.1097/00000478-199406000-00001
16. Murase E, Siegelman ES, Outwater EK, Perez-Jaffe LA, and Tureck RW. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. RadioGraphics. (1999) 19:1179–97. doi: 10.1148/radiographics.19.5.g99se131179
17. Bura V, Pintican RM, David RE, Addley HC, Smith J, Jimenez-Linan M, et al. MRI findings in-between leiomyoma and leiomyosarcoma: A rad-path correlation of degenerated leiomyomas and variants. Br J Radiol. (2021) 94:20210283. doi: 10.1259/bjr.20210283
18. Han SC, Kim M-D, Jung DC, Lee M, Lee MS, Park S, et al. Degeneration of leiomyoma in patients referred for uterine fibroid embolization: incidence, imaging features and clinical characteristics. Yonsei Med J. (2013) 54:215–9. doi: 10.3349/ymj.2013.54.1.215
19. Ueda H, Togashi K, Konishi I, Kataoka ML, Koyama T, Fujiwara T, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiogr Rev Publ Radiol Soc North Am Inc. (1999) 19:S131–45. doi: 10.1148/radiographics.19.suppl_1.g99oc04s131
20. Takeuchi M, Matsuzaki K, Bando Y, and Harada M. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. (2019) 18:158–62. doi: 10.2463/mrms.mp.2018-0074
21. Panda A, Mahoorkar DN, Reddy N, and Reddy BM. Myxoid degeneration of leiomyoma-a masquerader. Int J Reprod Contraception Obstet Gynecol. (2022) 11:3415. doi: 10.18203/2320-1770.ijrcog20223145
22. Nazir HM, Mehta S, Seena CR, and Kulasekaran N. Uterine Lipoleiomyoma: A report of two cases. J Clin Imaging Sci. (2017) 7:26. doi: 10.4103/jcis.JCIS_13_17
23. Patil AR, Nandikoor S, and Padilu R. Hydropic degeneration of leiomyoma in nongravid uterus: the “Split fiber” Sign on magnetic resonance imaging. Indian J Radiol Imaging. (2018) 28:182–6. doi: 10.4103/ijri.IJRI_472_17
24. Kim S-N, Jang J, and Kim K-R. Uterine leiomyomas with perinodular hydropic degeneration: A report of two cases. Korean J Pathol. (2002) 36:257–61.
25. El Ghali EH, Meziyane J, Auragh S, Saadi H, Taheri H, and Mimouni A. Hydropic leiomyoma, a considerable differential diagnosis: A case series. Int J Reprod Contraception Obstet Gynecol. (2022) 11:1767. doi: 10.18203/2320-1770.ijrcog20221455
26. Miettinen M. Immunohistochemistry of soft tissue tumours - review with emphasis on 10 markers. Histopathology. (2014) 64:101–18. doi: 10.1111/his.12298
27. Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. (2016) 29:S104–20. doi: 10.1038/modpathol.2015.139
28. Zou L, Lou J, Huang H, and Xu L. Pseudo-meigs syndrome caused by a rapidly enlarging hydropic leiomyoma with elevated CA125 levels mimicking ovarian Malignancy: A case report and literature review. BMC Womens Health. (2024) 24:445. doi: 10.1186/s12905-024-03285-8
29. Pauls M, MacKenzie H, and Ramjeesingh R. Hydropic leiomyoma presenting as a rare condition of pseudo-meigs syndrome: literature review and a case of a pseudo-meigs syndrome mimicking ovarian carcinoma with elevated CA125. BMJ Case Rep. (2019) 12. doi: 10.1136/bcr-2018-226454
30. Heffernan E, Köbel M, and Spielmann A. Case report: hydropic leiomyoma of the uterus presenting in pregnancy: imaging features. Br J Radiol. (2009) 82:e164–7. doi: 10.1259/bjr/50866065
31. Moore L, Wilson S, and Rosen B. Giant hydropic uterine leiomyoma in pregnancy: unusual sonographic and doppler appearance. J Ultrasound Med. (1994) 13:416–8. doi: 10.7863/jum.1994.13.5.416
32. Lai PH and Ding DC. Retroperitoneal hydropic leiomyoma mimicking an ovarian cyst. Case Rep Obstet Gynecol. (2022) 2022:1–4. doi: 10.1155/2022/2012376
33. Khaoula M, Slama F, Dhaoui S, Khayati W, Sana M, and Abir K. Uterine leiomyoma torsion: A rare cause of acute abdominal pain. Int J Surg Case Rep. (2024) 122:109788. doi: 10.1016/j.ijscr.2024.109788
34. Romano F, Carlucci S, Stabile G, Mirenda G, Mirandola M, Mangino FP, et al. The rare, unexpected condition of a twisted leiomyoma in mayer-rokitansky-küster-hauser (MRKH) syndrome: etiopathogenesis, diagnosis and management. Our experience and narrative review of the literature. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph18115895
35. Hoang VT, Hoang TH, Van HAT, Pham NTT, Chansomphou V, Nguyen TTT, et al. Giant degenerative uterine leiomyoma mimicking an ovarian neoplasm: case report. SAGE Open Med Case Rep. (2025) 13:2050313X251315066. doi: 10.1177/2050313X251315066
36. Horta M, Cunha TM, Oliveira R, and Magro P. Hydropic leiomyoma of the uterus presenting as a giant abdominal mass. BMJ Case Rep. (2015) 2015:1–5. doi: 10.1136/bcr-2015-211929
37. Ye Z, Jiang Y, Yan K, and Yu C. Uterine torsion with degeneration and infarction of giant leiomyoma in a postmenopausal woman: A case report. Medicine (Baltimore). (2023) 102:e35964. doi: 10.1097/MD.0000000000035964
38. Cianci S, Gulino FA, Palmara V, La Verde M, Ronsini C, Romeo P, et al. Exploring surgical strategies for uterine fibroid treatment: A comprehensive review of literature on open and minimally invasive approaches. (2024) 60(1):64. doi: 10.3390/medicina60010064
Keywords: uterine leiomyoma, hydropic degeneration, cystic degeneration, abdominopelvic mass, benign uterine tumors, hysterectomy, surgical resection
Citation: Giannios K, Anestiadou E, Liampou E, Spilioti LK, Bakaloudi DR and Papadopoulos V (2025) Case Report: Giant hydropic leiomyoma of the uterus presenting as an aggressive abdominopelvic tumor. Front. Oncol. 15:1529793. doi: 10.3389/fonc.2025.1529793
Received: 15 January 2025; Accepted: 23 September 2025;
Published: 22 October 2025.
Edited by:
Pasquale Cianci, ASL Bari, ItalyReviewed by:
Marco La Verde, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyStefania Carlucci, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), Italy
Chiara Ripepi, University of Trieste, Italy
Copyright © 2025 Giannios, Anestiadou, Liampou, Spilioti, Bakaloudi and Papadopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elissavet Anestiadou, ZWxpc3NhdmV0eGF0ekBnbWFpbC5jb20=
 Konstantinos Giannios
Konstantinos Giannios Elissavet Anestiadou
Elissavet Anestiadou Eftychia Liampou1
Eftychia Liampou1 Vasileios Papadopoulos
Vasileios Papadopoulos