- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
Introduction: Solitary Fibrous Tumor (SFT) is a rare mesenchymal tumor characterized by CD34-positive dendritic stromal cells that can differentiate into fibroblasts or myofibroblasts. Although commonly found in the pleura, these tumors can also occur in other locations, including the retroperitoneum, where a subset may show malignant behavior, leading to local invasion and metastasis.
Case presentation: We report the case of a 60-year-old woman with progressive abdominal distension (3 months) and dysuria (2 months). Imaging revealed a 7.6cm × 10.1cm × 10.7cm right pelvic mass compressing the ureter and bladder. Surgical resection of the firm, hypervascular uterine mass with infiltrative margins was achieved. Immunohistochemistry demonstrated diffuse positivity for CD34, STAT6, Bcl-2, CD99, SW-1 and ER, with focal TP53 expression and 10% Ki-67 index, confirming malignant solitary fibrous tumor. Early postoperative surveillance MRI at one month detected local recurrence with regional lymph node metastases. Given the tumor’s aggressive biology, high operative risk for re-intervention, and lack of effective systemic therapy options, a multidisciplinary team recommended transition to palliative care. The patient remains alive after following up 8 months.
Conclusion: Neoadjuvant radiotherapy may benefit high-risk SFT cases nearing the limits of resectability, but current evidence highlights the challenges in managing aggressive pelvic malignant SFTs and emphasizes the need for ongoing research into effective treatment options while also aiming to stimulate discussion among scholars and encourage the sharing of experiences from similar cases to provide valuable insights for future diagnosis and treatment of malignant SFTs.
Introduction
Solitary Fibrous Tumor (SFT) is a rare type of mesenchymal tumor initially identified in the pleura, but it can also develop in various other sites, including the pelvis, retroperitoneum, and meninges (1). These tumors are primarily composed of CD34-positive stromal cells, which can differentiate into fibroblasts or myofibroblasts (2). While SFTs generally grow slowly, malignant variants pose significant clinical challenges. These aggressive tumors are characterized by local infiltration, distant metastasis, and a high rate of recurrence, accounting for about 15-20% of all SFT cases (3). Notably, occurrences in extrathoracic locations, particularly in the pelvis, are infrequent, with only 11 documented cases of pelvic malignant SFTs to date. This paper presents a case of a pelvic malignant SFT that exhibited suspected recurrence and lymphatic metastasis one month after surgery. Furthermore, it reviews existing literature to discuss treatment strategies for managing such patients, aiming to enhance clinical understanding and management of this challenging condition.
Case presentation
This case involves a 60-year-old woman who presented with a three-month history of abdominal distension and a two-month history of dysuria. An ultrasound examination indicated a mass in the adnexal region. Screening for cervical cancer and tumor marker tests revealed no significant abnormalities. A CT scan of the pelvis and abdomen revealed a right-sided mass measuring approximately 7.6cm × 10.1cm × 10.7cm, with indistinct tumor borders between the lesion, the uterine wall, and the posterior bladder. Additionally, the lower segment of the right ureter was infiltrated, leading to hydronephrosis and dilation of the right ureter and kidney. The right adnexa appeared ambiguous, raising suspicion of a malignant ovarian tumor. During surgery, a solid mass measuring approximately 15cm × 15cm × 10cm was palpated in the lower anterior wall of the uterus and anterior to the vagina. The mass was hard, with prominent blood vessels and a rich blood supply, displacing the bladder and extending toward the right pelvic wall, pelvic floor, and vagina, with indistinct boundaries with the bladder, right ureter, and right pelvic wall. Intraoperative examination confirmed complete macroscopic resection; Histopathology confirmed complete tumor excision with negative margins, including the pelvic sidewall (R0 resection). Intraoperative blood loss was significant, totaling around 3000 ml. However, no abnormalities were detected in routine coagulation tests before surgery, our conclusion that the hemorrhage was primarily associated with the tumor’s biological behavior. The tumor showed aggressive features with vascularity and invasion into adjacent structures. Radical resection would have required bladder resection and ureteral reimplantation. After consulting the family about the risks and quality-of-life impacts, they declined procedures that could affect genitourinary function. Pathological examination confirmed a malignant SFT. (Figure 1) Immunohistochemical analysis showed positive results for CD34, STAT6, Bcl-2, CD99, SW-1, and ER, with occasional positivity for TP53, and Ki67 (10%). Other markers were negative, including HMB45, S-100, SMA, Caldesmon, Malan, Desmin, CD177, and DOG. One-month post-surgery, a follow-up MRI revealed an abnormal mass on the right side of the pelvis measuring approximately 6.9cm × 7.1cm × 6.0cm, accompanied by enlarged right obturator lymph nodes (about 2.4cm × 2.0cm), indicating a high likelihood of tumor recurrence and lymph node metastasis (Figure 2).
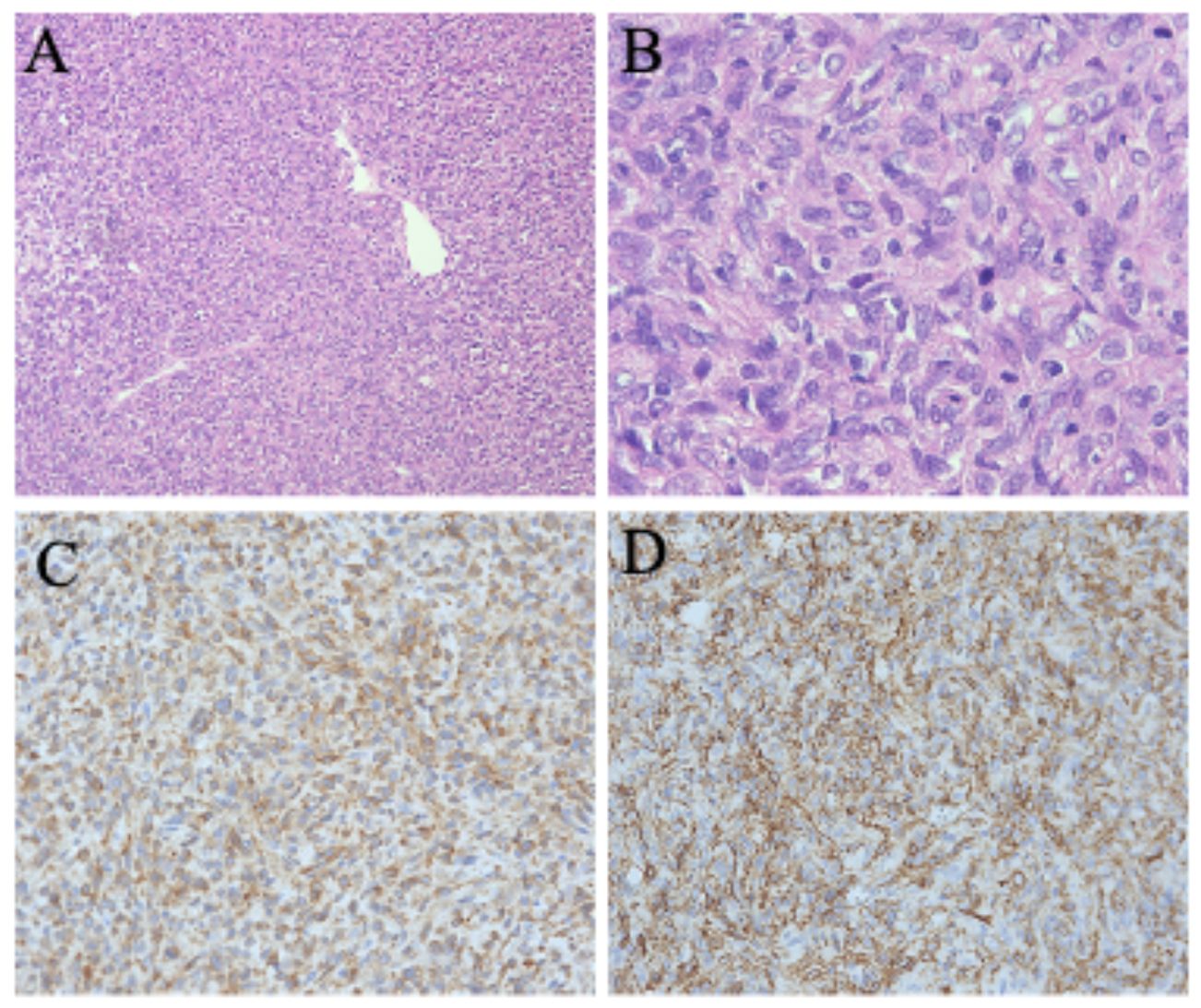
Figure 1. (A) Hematoxylin and eosin stain (100×): the tumor contains a considerable amount of collagen fibers with an increased cell density. (B) Hematoxylin and eosin stain (400×): there is evidence of cellular atypia, nuclear mitotic figures, and focal tumor necrosis Immunohistochemical stains (200×) are positive for CD34 (C) and Bcl-2 (D).
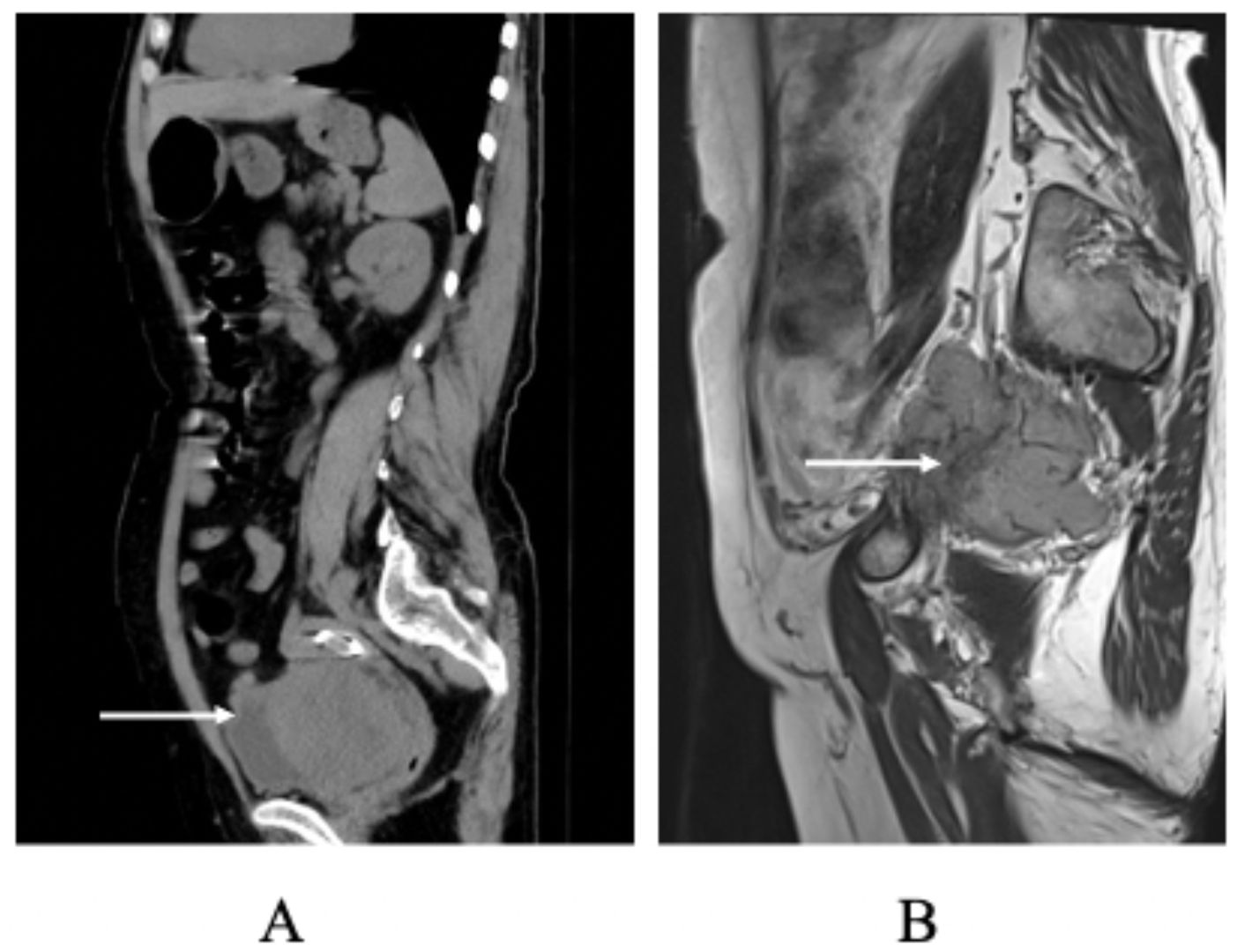
Figure 2. (A) Preoperative Non-contrast Abdominal CT Scan: this image depicts the non-contrast abdominal CT scan of the patient obtained before surgical intervention. (B) Postoperative MRI Scan at One Month: MRI was performed one month postoperatively to consider tumor recurrence.
Given the patient’s history, we chose not to proceed with a second surgery for three primary reasons. First, the significant bleeding experienced during the initial operation heightened the risk of hemorrhage in any subsequent procedure. Second, the patient developed lymphatic metastases rapidly, indicating a progression that made reoperation inadvisable in the near term. Lastly, we organized a multidisciplinary team (MDT) discussion involving the gynecology, oncology, pathology, and imaging departments, and it was not recommended to undergo surgery again. After the discussion, we concluded that the malignancy of this disease is extremely high and progresses rapidly, and our hospital currently has no suitable radiotherapy or chemotherapy options. We recommended that the patient seek possible clinical trials at the specialized cancer centers. Subsequently, we followed up with the patient by phone. However, due to the lack of suitable radiotherapy or chemotherapy options in the specialized cancer centers, the patient received two cycles of an investigational chemotherapy regimen combining azithromycin and dacarbazine, which showed limited clinical response. Due to the lack of therapeutic efficacy, the chemotherapy regimen was discontinued, and the patient was subsequently transitioned to palliative care. Over the 8-month follow-up period, the patient remains alive.
Discussion
This study presents a malignant SFT case with distinctive clinical characteristics. Through a comprehensive review of malignant SFTs reported across various anatomical locations, (Table 1) we identified that our case demonstrates an exceptionally aggressive clinical course, characterized by unexpected rapid recurrence within just one month post-operation. This accelerated disease progression challenges current clinical surveillance protocols for high-risk SFTs. Studies have identified several factors as reliable indicators of poor prognosis in malignant SFTs (11, 15). These include nuclear atypia, a high mitotic count (greater than 4 per 10 high-power fields), tumor size exceeding 10 cm, and the presence of necrosis or hemorrhage. Additionally, specific immunohistochemical markers such as CD34, CD99, Bcl-2, and STAT-6 have demonstrated high sensitivity and specificity for SFTs, while TP53 positivity is linked to malignant cases (16, 17). In the present case, the intraoperative specimen appeared as a spherical mass measuring 15cm × 15cm × 10cm. Histopathological examination revealed an increased mitotic count (7/10 HPFs), marked nuclear atypia, and focal necrosis. Immunohistochemical analysis demonstrated diffuse positivity for CD34, CD99, Bcl-2, and STAT-6, with focal TP53 expression. These findings are consistent with previously reported features of malignant solitary fibrous tumors. Currently, there is no conclusive literature addressing how the presence or absence of specific immunohistochemical markers may influence future clinical decision-making. Investigating this potential correlation represents a promising direction for further research.
The primary treatment for SFTs is complete surgical resection, as incomplete removal significantly increases the risk of recurrence (18, 19). In some instances, patients have undergone multiple surgical resections over extended periods, with reports of as many as six procedures spanning 24 years (6). The tumor showed aggressive features including marked vascularity, invasion into adjacent structures (bladder/ureter/pelvic wall), and loss of tissue planes. Despite suspected organ infiltration, resection was limited due to critical location and significant bleeding (3000 mL), following family’s refusal of radical surgery. However, in the case presented here, the tumor exhibited rapid regrowth within just one month following surgery, a phenomenon not commonly documented in the English literature. The tumor demonstrated aggressive characteristics with radiologic and intraoperative evidence suggesting invasion into adjacent structures (bladder, ureter, and pelvic wall). While histopathological examination confirmed R0 resection margins, the anatomical constraints imposed by the tumor’s proximity to these vital structures - combined with the family’s decision against more radical resection - may have permitted residual microscopic disease despite negative margins, potentially contributing to the observed recurrence pattern. Pelvic SFTs are often larger than their thoracic counterparts at diagnosis, increasing the likelihood of complications due to their vascularity and proximity to critical structures such as the bladder, ureter, and pelvic vasculature.
During follow-up at another institution, the patient underwent two cycles of an investigational chemotherapy regimen comprising azithromycin and dacarbazine, which demonstrated limited efficacy. Consequently, chemotherapy was discontinued, and the patient transitioned to palliative care. Regarding soft tissue tumors, evidence for chemotherapy mainly supports anthracycline-based regimens, which have shown a progression-free survival (PFS) of 3 to 5 months (20, 21). The combination of doxorubicin and ifosfamide may extend PFS further (21). Trabectedin has also demonstrated effectiveness against metastatic soft tissue sarcomas, with PFS varying between 2.3 to 11.6 months (22, 23). Additionally, the combination of temozolomide and bevacizumab has shown anti-angiogenic properties in SFTs (24). Tyrosine kinase inhibitors offer hope for patients with unresectable or recurrent disease (25). While tyrosine kinase inhibitors hold promise for patients with unresectable or recurrent disease, their routine clinical application requires further validation. For SFT cases near the limits of resectability, particularly those with a high mitotic rate, neoadjuvant radiotherapy may offer potential benefits (26). As summarized in Table 1, the primary treatment for SFT remains surgical resection. However, this patient was not a suitable candidate for repeat surgery. Currently, there is no standardized chemotherapy regimen for malignant SFT. While existing literature suggests that azithromycin-based combinations—commonly used for other soft tissue sarcomas—may represent a potential therapeutic option, the impact of tumor location on treatment efficacy remains unexplored. Establishing effective adjuvant or maintenance therapies post-surgery remains a critical challenge in managing this disease.
In summary, malignant SFTs are rare. Their unpredictable recurrence rates, local invasiveness, and potential for metastasis present significant challenges in treatment. These factors underscore the complexity of managing these tumors in clinical practice. As such, long-term follow-up and monitoring for patients with malignant SFT are crucial for the early detection of recurrences and the timely implementation of appropriate interventions. The aggressive nature of pelvic malignant SFTs necessitates careful consideration in terms of management and prognosis. Current treatment guidelines do not provide comprehensive strategies tailored to these tumors, highlighting a gap in the clinical framework. Future research should aim to optimize treatment approaches to enhance patient outcomes and address the unique challenges posed by malignant SFTs. In our current research and practice, we recognize that the complexity of this case presents many unresolved mysteries in the treatment process. Therefore, through this case report, we aim to engage more scholars in discussion and invite them to share their experiences and insights from similar cases, providing valuable references for our future diagnosis and treatment.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
D-ML: Data curation, Writing – original draft. DT: Investigation, Writing – review & editing. M-RQ: Methodology, Writing – review & editing. M-MH: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our gratitude to the Department of Obstetrics and Gynecology at West China Second Hospital for providing the platform and to the Departments of Radiology and Pathology for their support in analyzing this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Insabato L, Siano M, Somma A, Gentile R, Santangelo M, and Pettinato G. Extrapleural solitary fibrous tumor: A clinicopathologic study of 19 cases. Int J Surg Pathol. (2009) 17:250–4. doi: 10.1177/1066896909333779
2. Anderson WJ and Doyle LA. Updates from the 2020 world health organization classification of soft tissue and bone tumours. Histopathology. (2021) 78:644–57. doi: 10.1111/his.14265
3. Salas S, Resseguier N, Blay JY, Le Cesne A, Italiano A, Chevreau C, et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the french sarcoma group (Fsg) database. Ann Oncol. (2017) 28:1979–87. doi: 10.1093/annonc/mdx250
4. Munoz E, Prat A, Adamo B, Peralta S, Ramon y Cajal S, and Valverde C. A rare case of Malignant solitary fibrous tumor of the spinal cord. Spine (Phila Pa 1976). (2008) 33:E397–9. doi: 10.1097/BRS.0b013e31817343dc
5. Cui Y, Li B, Liang J, He L, and Zhang H. Case report: A study on disease diagnosis and treatment outcome of a case of bilateral Malignant solitary fibrous tumor of the kidneys. Front Oncol. (2025) 15:1436015. doi: 10.3389/fonc.2025.1436015
6. Park CK, Lee DH, Park JY, Park SH, and Kwon KY. Multiple recurrent Malignant solitary fibrous tumors: long-term follow-up of 24 years. Ann Thorac Surg. (2011) 91:1285–8. doi: 10.1016/j.athoracsur.2010.08.074
7. Wang H, Shen D, and Hou Y. Malignant solitary tumor in a child: A case report and review of the literature. J Pediatr Surg. (2011) 46:e5–8. doi: 10.1016/j.jpedsurg.2010.11.025
8. Dozier J, Jameel Z, McCain DA, Hassoun P, and Bamboat ZM. Massive Malignant solitary fibrous tumor arising from the bladder serosa: A case report. J Med Case Rep. (2015) 9:46. doi: 10.1186/s13256-014-0505-4
9. Iorio B, Ronchi A, Montella M, Cozzolino I, De Luca R, Rusciano M, et al. Malignant extrapleural solitary fibrous tumor arising in the sublingual gland: A case report and review of literature. Oncol. (2019) 90:141–4. doi: 10.1016/j.oraloncology.2018.12.013
10. Kumar P, Jindal A, Bhalgat B, Swain PK, and Sharma RG. Malignant solitary fibrous tumor of maxilla presenting as proptosis: A case report. J Cancer Res Ther. (2023) 19:S991–S3. doi: 10.4103/jcrt.jcrt_2329_21
11. Wang Y, Wang L, Huang J, Liu X, Sun H, Sui X, et al. A giant breast Malignant solitary fibrous tumor: A rare case report and brief review. Oncol Lett. (2023) 25:249. doi: 10.3892/ol.2023.13835
12. Bunajem F, Al Taei T, Mujbel N, Al Shaikh A, and Al Mail S. Malignant solitary fibrous tumor of the inguinal region: A case report. Cureus. (2023) 15:e47123. doi: 10.7759/cureus.47123
13. Wang XJ, Zhou JP, Pan Y, and Yu RS. Case report: A rare case of Malignant solitary fibrous tumor within the joint cavity with review of the literature. Front Oncol. (2024) 14:1463362. doi: 10.3389/fonc.2024.1463362
14. Arias Rivera AS, Nesme Vara M, Brener Chaoul M, de la Rosa Abaroa MA, and Padilla Longoria R. A giant Malignant solitary fibrous tumor of the pancreas: A case report and review of the literature. Cureus. (2024) 16:e61467. doi: 10.7759/cureus.61467
15. Yuan X, Liu Y, Wang X, Chen Y, Zhang L, and Wei J. Clinicopathological analysis of retroperitoneal solitary fibrous tumours: A study of 31 cases. Histol Histopathol. (2022) 37:43–50. doi: 10.14670/HH-18-392
16. Bieg M, Moskalev EA, Will R, Hebele S, Schwarzbach M, Schmeck S, et al. Gene expression in solitary fibrous tumors (Sfts) correlates with anatomic localization and nab2-stat6 gene fusion variants. Am J Pathol. (2021) 191:602–17. doi: 10.1016/j.ajpath.2020.12.015
17. Park HK, Yu DB, Sung M, Oh E, Kim M, Song JY, et al. Molecular changes in solitary fibrous tumor progression. J Mol Med (Berl). (2019) 97:1413–25. doi: 10.1007/s00109-019-01815-8
18. Haas RL, Walraven I, Lecointe-Artzner E, van Houdt WJ, Strauss D, Schrage Y, et al. Extrameningeal solitary fibrous tumors-surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the sarcoma patients euronet. Cancer. (2020) 126:3002–12. doi: 10.1002/cncr.32911
19. Ricciuti B, Metro G, Leonardi GC, Sordo RD, Colella R, Puma F, et al. Malignant giant solitary fibrous tumor of the pleura metastatic to the thyroid gland. Tumori. (2016) 102:S16–21. doi: 10.5301/tj.5000514
20. Constantinidou A, Jones RL, Olmos D, Thway K, Fisher C, Al-Muderis O, et al. Conventional anthracycline-based chemotherapy has limited efficacy in solitary fibrous tumour. Acta Oncol. (2012) 51:550–4. doi: 10.3109/0284186X.2011.626450
21. Levard A, Derbel O, Meeus P, Ranchere D, Ray-Coquard I, Blay JY, et al. Outcome of patients with advanced solitary fibrous tumors: the centre leon berard experience. BMC Cancer. (2013) 13:109. doi: 10.1186/1471-2407-13-109
22. Chaigneau L, Kalbacher E, Thiery-Vuillemin A, Fagnoni-Legat C, Isambert N, Aherfi L, et al. Efficacy of trabectedin in metastatic solitary fibrous tumor. Rare Tumors. (2011) 3:e29. doi: 10.4081/rt.2011.e29
23. Kobayashi H, Iwata S, Wakamatsu T, Hayakawa K, Yonemoto T, Wasa J, et al. Efficacy and safety of trabectedin for patients with unresectable and relapsed soft-tissue sarcoma in Japan: A Japanese musculoskeletal oncology group study. Cancer. (2020) 126:1253–63. doi: 10.1002/cncr.32661
24. Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and Malignant solitary fibrous tumor. Cancer. (2011) 117:4939–47. doi: 10.1002/cncr.26098
25. Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, et al. Pazopanib as first line treatment for solitary fibrous tumours: the royal marsden hospital experience. Clin Sarcoma Res. (2015) 5:5. doi: 10.1186/s13569-015-0022-2
26. Martin-Broto J, Hindi N, Lopez-Pousa A, Peinado-Serrano J, Alvarez R, Alvarez-Gonzalez A, et al. Assessment of safety and efficacy of combined trabectedin and low-dose radiotherapy for patients with metastatic soft-tissue sarcomas: A nonrandomized phase 1/2 clinical trial. JAMA Oncol. (2020) 6:535–41. doi: 10.1001/jamaoncol.2019.6584
Keywords: malignant solitary fibrous tumor, recurrence, treatment, lymph node metastasis, case report
Citation: Li D-M, Tang D, Qie M-R and Hou M-M (2025) Exploring intraoperative hemorrhage and early recurrence in pelvic malignant solitary fibrotic tumors: a case report and literature review. Front. Oncol. 15:1531597. doi: 10.3389/fonc.2025.1531597
Received: 20 November 2024; Accepted: 02 May 2025;
Published: 27 May 2025.
Edited by:
Cristina Secosan, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Giuseppe D’Ermo, Sapienza University of Rome, ItalyRazvan Ciortea, County Emergency Hospital Cluj-Napoca, Romania
Marius Preda, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2025 Li, Tang, Qie and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min-Min Hou, bW1pbm5oNzg5QDE2My5jb20=
 Dong-Mei Li
Dong-Mei Li Dan Tang1,2
Dan Tang1,2 Ming-Rong Qie
Ming-Rong Qie Min-Min Hou
Min-Min Hou