- Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
Currently, there is no definitive and effective treatment strategy for relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). In recent years, studies on brentuximab vedotin (BV) and programmed cell death-1 (PD-1) monotherapy for R/R DLBCL have demonstrated significant clinical benefits. Based on this, this article retrospectively analyzes a case of R/R DLBCL with secondary hemophagocytic syndrome successfully treated with BV combined with a PD-1 monoclonal antibody and reviews the relevant literature. The patient was a 55-year-old woman who was diagnosed with stage IIE diffuse large B-cell lymphoma in June 2020. She failed to achieve complete remission during first-line treatment with the R-CHOP (rituximab, cyclophosphamide, doxorubicin/epirubicin, vincristine, and prednisone) regimen. After switching to the R2-GDP regimen for second-line salvage therapy, her condition continued to progress, and recurrent hemophagocytic syndrome developed. Subsequent treatment with BV combined with a PD-1 monoclonal antibody resulted in significant relief of her symptoms. As of the follow-up on 8 March 2025, the patient maintained a normal life and had no intolerable immune-related adverse effects. This study suggests that BV combined with PD-1 monoclonal antibody may exert a synergistic effect in the treatment of R/R DLBCL complicated with hemophagocytic lymphohistiocytosis (HLH).
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 33% of NHL cases in developed countries (1). Although first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin/epirubicin, vincristine, and prednisone) therapy can cure the majority of patients with DLBCL, approximately 40% eventually experience relapse or refractoriness, progressing to relapsed/refractory DLBCL (R/R DLBCL) (2, 3). Notably, patients with R/R DLBCL are highly prone to developing hemophagocytic lymphohistiocytosis (HLH) as a complication, driven by uncontrolled inflammatory responses triggered by malignant cells. Once HLH develops, conventional HLH treatment regimens often yield limited efficacy. Given these limitations of standard therapies, exploring novel therapeutic approaches with distinct mechanisms of action is imperative. In this context, monoclonal antibodies targeting the programmed cell death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway offer a promising strategy: by reversing tumor-mediated immune suppression, they reactivate anti-tumor immune responses. Such agents have shown considerable therapeutic value in the management of R/R DLBCL complicated by HLH.
PD-1 is a receptor protein expressed on immune T cells, which primarily inhibits immune system activation through binding to PD-L1. PD-L1 is expressed in 11%–30% of DLBCL cells (4–7). The response rate to PD-1 monotherapy in DLBCL is only 10% (8), which may be associated with the low PD-L1 expression levels in DLBCL cells (9). Despite the poor prognosis of patients with DLBCL receiving anti-PD-1 therapy, the efficacy of PD-1 monoclonal antibodies in combination with other agents for treating DLBCL warrants further investigation.
With deeper insights into the disease and advances in science and technology, the treatment of DLBCL has entered the era of precision targeting. Numerous studies have indicated that while high CD30 expression is a hallmark of classical Hodgkin lymphoma (cHL) and anaplastic large cell lymphoma (ALCL), its expression in DLBCL is relatively low and heterogeneous, with a positive rate typically ranging from 20% to 30% (10). Despite the overall low CD30 expression in DLBCL, its tendency to be upregulated in relapsed/refractory populations—coupled with the availability of brentuximab vedotin (BV), a highly effective, mechanistically distinct targeted agent that has demonstrated significant clinical activity in pivotal trials (e.g., Study SG035-0004)—renders CD30 an important therapeutic target for R/R DLBCL (11–16). As an antibody–drug conjugate (ADC) targeting CD30, BV was officially approved by China’s National Medical Products Administration (NMPA) in 2020 for the treatment of CD30-positive relapsed/refractory cHL and ALCL, thereby providing a novel therapeutic option for CD30-positive lymphomas (17, 18).
In this article, we retrospectively analyzed the clinical features, treatment course, and disease regression of a patient with R/R DLBCL complicated by HLH who received BV in combination with a PD-1 monoclonal antibody. Additionally, we reviewed relevant literature to explore the efficacy and safety of this combined regimen in the management of R/R DLBCL with concurrent HLH.
Case report
A 55-year-old female patient was admitted to the hospital on 15 June 2020, with a 5-month history of right lower quadrant abdominal pain and a 20-day history of diarrhea. She first developed recurrent dull pain in the right lower abdomen in January 2020, which was not taken seriously at the time. Starting from 18 May 2020, the pain in the right lower quadrant became frequent and evolved into a tearing sensation, accompanied by yellowish diarrhea (approximately two to three episodes per day) and significant weight loss (B symptom). She denied experiencing fever, night sweats, or other discomfort. There were no notable findings in her past medical history, personal history, family history, or marital and reproductive history. Physical examination revealed no abnormalities.
Comprehensive relevant examinations were performed during hospitalization:
1. Pathological examination (Figure 1): The results confirmed non-germinal center B-cell-like (non-GCB) DLBCL, with sparse CD30 expression. Genetic testing revealed mutations in TP53 (51.80%), IRF4 (19.29%), BIG1 (35.18%), TMSB4X (8.33%), IGLL5 (51.80%), and PCL0 (17.07%).
2. Laboratory tests: Red blood cell (RBC) count, 3.24×10¹²/L; hemoglobin (Hb) count, 88.50 g/L; neutrophil (Neu), count 1.08×109/L; lactate dehydrogenase (LDH), 205 U/L. Fecal occult blood test was positive.
3. Bone marrow aspiration and biopsy: No evidence of lymphoma infiltration.
4. PET/CT: (i) Multiple lymphomatous lesions involving the cecum, ascending colon, colon, and right para-aortic region [maximum standardized uptake value (SUVmax) 7.5]; (ii) mild lymph node hyperplasia in both inguinal regions.
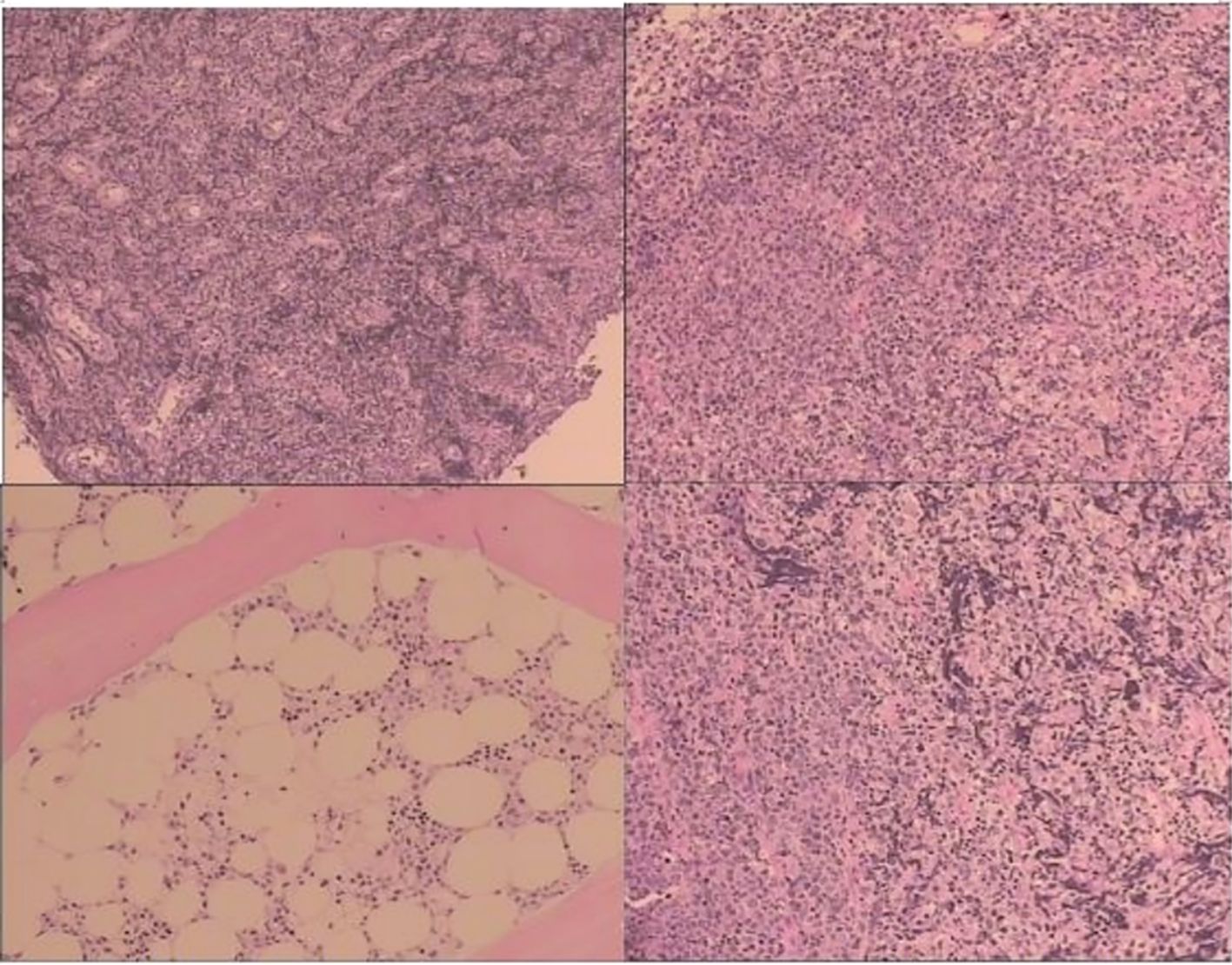
Figure 1. Pathological examination (mass in the terminal ileum and the ileocecal region): the positive expression of CD20*, CD79a, CD30, MUM-1, Bcl-2, Bc1-6, P53, Ki-67#, and c-Myc was identified using immunohistochemistry; CD10, CD21/CD23, CD3, CD5, CyclinD1, and CK all express negatively using immunohistochemistry.
Based on the 2016 WHO Classification of Lymphoid Neoplasms, the patient was diagnosed with DLBCL. According to the Hans algorithm, she was classified as having the non-germinal center B-cell-like subtype. Per the Ann Arbor staging system, her disease was staged as IIE, with B symptoms; her International Prognostic Index (IPI) score was 1.
In summary, the patient was categorized into the low-intermediate-risk group. She was suspected to have double-expressor DLBCL and met the criteria for first-line R-CHOP therapy. Therefore, she initiated a six-cycle first-line R-CHOP regimen on 19 June 2020 (rituximab 600 mg on day 0; cyclophosphamide 1.2 g, vincristine 2 mg, and pirarubicin 80 mg on day 1; dexamethasone 15 mg on days 1–5). During treatment, she developed grade II neutropenia after the first cycle of chemotherapy.
To assess treatment response, a PET/CT scan was performed on 23 December 2020, which showed residual tumor tissue in the ileocecal and ascending colon regions, though with a reduced area compared to prior imaging. Since complete remission (CR) was not achieved, a six-cycle second-line R2-GDP regimen was initiated on 24 December 2020 (rituximab 600 mg on day 1; gemcitabine 1.6 g on days 1 and 7; cisplatin 60 mg on days 1 and 2; dexamethasone 40 mg on days 1–4; lenalidomide 25 mg on days 1–10).
Post-treatment follow-up PET/CT revealed that most lesions had improved, with a significant reduction in SUV values of a 1.6-cm lymph node adjacent to the ascending colon compared to the original ileocecal lesion. Overall, the patient achieved a partial remission (PR). It was recommended that she receive local radiotherapy targeting the residual small lesions near the ascending colon after completing six cycles of the second-line chemotherapy.
In June 2021, the patient presented with fever and a palpable right abdominal mass, prompting readmission for further evaluation. Relevant examinations revealed the following: (1) Laboratory tests: White blood cell (WBC) count, 3.12×109/L; RBC count, 2.05×10¹²/L; platelet (PLT) count, 58.20×109/L; LDH, 382 U/L; CA125, 47.20 U/mL; serum ferritin, 3,104.9 ng/mL; and triglycerides, 3.09 mmol/L. (2) Bone marrow aspiration: Active bone marrow hyperplasia with hemophagocytic cells was observed throughout the smear (Figures 2A, B). (3) PET/CT (Figure 3): Post-chemotherapy findings for lymphoma included the following: (i) Progression of lesions in the ileocecal region, colon, and mesentery compared to prior scans; and (ii) newly emerging right anterior diaphragmatic lymph node lesions. Given the patient’s failure to achieve CR with prior first-line and second-line therapies, coupled with progressive abdominal lesions, she was diagnosed with R/R DLBCL complicated by hemophagocytic syndrome.

Figure 2. Bone marrow cytomorphologic findings in a patient with relapsed/refractory diffuse large B-cell lymphoma with hemophagocytic syndrome. Rhett’s staining (A) ×20, (B) ×100. Bone marrow hyperplasia is active and hemophagocytic cells are seen on the whole film.
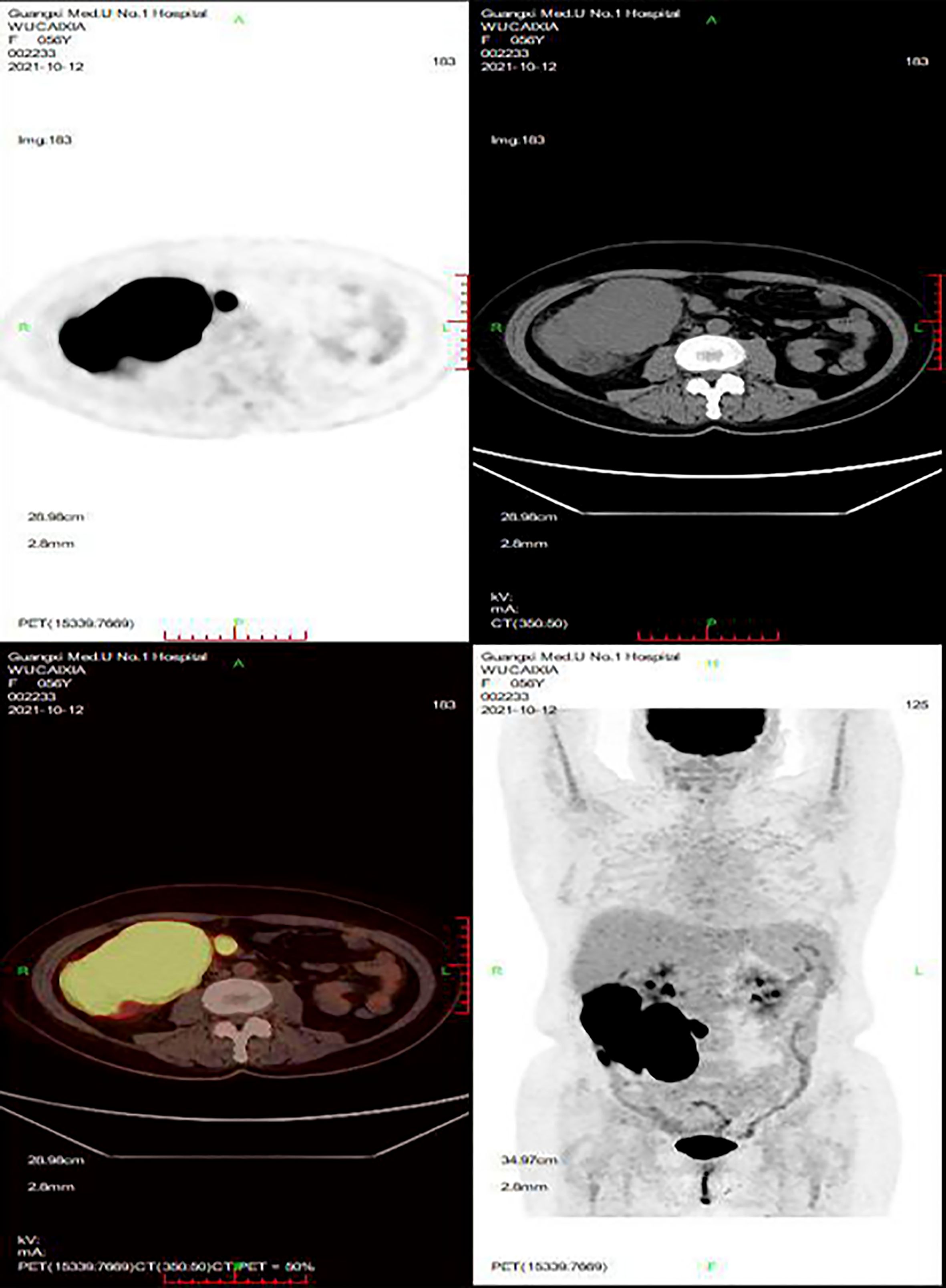
Figure 3. PET/CT. After lymphoma chemotherapy: (A) The lesions in the ileocecal region, the colon, and the mesentery were more advanced than before. (B) The newly developed right anterior lymph node lesions of the diaphragm.
On 5 November 2021, the patient initiated the first cycle of chemotherapy comprising liposomal doxorubicin (60 mg on day 1), zanubrutinib (160 mg twice daily orally on days 1–14), paclitaxel (0.4 g on day 5), decitabine (10 mg on days 1–5), and lenalidomide. Following chemotherapy, she developed grade IV myelosuppression, accompanied by multiple perineal and sacrococcygeal skin ulcerations with localized purulence. On 27 December 2021, the patient presented with recurrent fever; her condition was assessed as progressive lymphoma and recurrent hemophagocytic syndrome. On 3 January 2022, she commenced third-line DEP chemotherapy to control the hemophagocytic syndrome. However, the patient continued to experience recurrent fevers, and hemophagocytic markers failed to normalize. Given the previously detected positive CD30 expression, she was deemed eligible for BV-targeted therapy. On 19 January 2022, the patient initiated a regimen of BV combined with a PD-1 monoclonal antibody (BV 200 mg on day 1 + PD-1 monoclonal antibody 200 mg on day 1, every 3 weeks) for five cycles. Post-treatment, her symptoms improved significantly. A repeat PET/CT on 7 May 2022 (Figure 4) confirmed a PR. Follow-up was conducted until 8 March 2025, during which no recurrence of HLH or disease progression was observed. The patient attended regular hospital visits to receive maintenance therapy with the BV plus PD-1 regimen. By the final follow-up, she had resumed a normal life with no grade 3 or higher immune-related adverse events (Table 1).

Figure 4. PET/CT. After chemotherapy and targeted treatment of lymphoma: (A) The right anterior lymph node lesions of the protodiaphragm have been absorbed and inactivated. (B) The primary ileocecal, colon, and mesenteric lesions were obviously improved; most of them were absorbed and inactivated, but tumor active tissues were still found in the ileocecal region. (C) No definite new lesions were found (Deauville score: 4 points).
Discussion
R/R DLBCL shows low sensitivity to conventional chemotherapy and radiotherapy. Even with salvage chemotherapy ± radiotherapy and autologous stem cell transplantation (ASCT), the long-term relapse rate remains as high as approximately 80%. Although CD19-targeted chimeric antigen receptor T-cell (CAR-T) therapies and bispecific antibodies have significantly improved survival outcomes, their exorbitant treatment costs far exceed the financial means of average patients, resulting in severely limited global accessibility. Thus, exploring novel agents and therapeutic strategies is particularly urgent and crucial.
Both the tumor microenvironment and cancerous tissues in DLBCL are highly complex, with both expressing PD-L1 (19)—a factor associated with treatment responses and prognosis in R/R DLBCL (9). Currently, monoclonal antibodies to the PD-1/PD-L1 pathway for the treatment of R/R DLBCL are still in early-stage clinical studies, and their safety and efficacy need to be validated by more studies and longer follow-ups. The ORR of R/R DLBCL was 25% by pembrolizumab and CDK9 regimen (20). Another study observed that 27 of 30 patients with DLBCL obtained remission by pembrolizumab and R-CHOP therapy (21). The 2-year progression-free survival (PFS) was 83%, and the median follow-up duration was 25.5 months (21). This study strongly demonstrated that pembrolizumab combined with R-CHOP therapy significantly improved the CR rate and 2-year PFS in DLBCL, and the toxicity of the combination therapy was the same as that of the R-CHOP regimen. These findings establish an important foundation for future research on PD-1-based immunotherapy combined with other therapeutic agents.
It is well known that BV can significantly improve the prognosis of patients with CD30-positive peripheral T-cell lymphoma, cHL, and ALCL. Therefore, BV has been clinically approved for the treatment of these tumors. A meta-analysis on the efficacy of BV in the treatment of R/R cHL found that BV was significantly effective in the treatment of R/R cHL by reviewing 32 studies (ORR: 62.2%, 95% CI: 56.0–68.9, I2 = 9.7%; 5-year PFS: 31.9%–33%) (22). Other studies have attained similar consequences (23, 24). In recent years, clinical studies on BV treatment of R/R DLBCL are gaining popularity, and more attempts have been made in creating new combination therapy programs, expanding indications, and exploring adverse reactions. A clinical research demonstrated that BV was strongly responsive to R/R DLBCL (OR: 44%), which was not particularly correlated with CD30 expression in tumor cells (17). In addition, this study further revealed that BV can significantly improve the prognosis of patients with R/R DLBCL, including 17% of patients (8 cases) having a CR and 27% of patients (13 cases) having a PR, with a median duration of 16.6 months (25). Later, further studies found that BV combined with other treatment regimens can significantly improve the prognosis of patients with R/R DLBCL compared with BV alone. A phase I/II multicenter trial found that patients with R/R DLBCL were significantly improved by BV combined with R-CHOP chemotherapy. Furthermore, another phase I clinical study found that BV combined with lenalidomide also improved the prognosis of patients with R/R DLBCL, with a complete response rate of 35% (95% CI, 20.7–52.6) and an overall survival of 14.3 months (95% CI, 10.2–35.6) (26).
In 2021, the first report on the combination of BV and PD-1 monoclonal antibody for relapsed/refractory Hodgkin lymphoma (R/R HL) demonstrated superior efficacy, with a PFS rate of 77%, an ORR of 85%, and a CR rate of 67%. Notably, no grade 3 or higher immune-related adverse events were observed (27). Other studies also further confirmed this conclusion (28, 29). In addition, based on its unique high CD30 expression (>80%) and the aberrant molecular features of the PD-L1 pathway, relapsed/refractory primary mediastinal large B-cell lymphoma (R/R PMBL) was shown to be treated with vibutuximab in combination with navulizumab to achieve an ORR of 73% and a CR of 70% in a controlled safety profile (≥grade 3 in the phase II CheckMate-436 trial treatment-related adverse event rate <30%) and significantly better efficacy than historical controls (30). In view of the previous studies on BV and PD-1 monoclonal antibody alone in the treatment of R/R DLBCL, they all showed controllable toxicity and significant clinical benefits. Reviewing this case, the patient was resistant to the first-line and second-line chemotherapy schemes and lenalidomide, and developed hemophagocytic syndrome. Considering that the efficacy of BV alone may be poor, we had to boldly try to treat the patient with BV combined with PD-1 monoclonal antibody. Surprisingly, the patient gained a better prognosis. Recently, we found a similar conclusion in another patient with DLBCL (31). Therefore, we speculate that BV and PD-1 monoclonal antibody may have a synergistic effect on patients with R/R DLBCL, and they show promise as first-line or second-line treatment for patients with R/R DLBCL. At the same time, we found that the patient’s CD30 expression was low. Therefore, we speculate that the therapeutic efficacy of BV may not necessarily be related to tumor CD30 expression, but this speculation needs to be confirmed by further studies.
Conclusion
R/R DLBCL with secondary hemophagocytic syndrome (R/R DLBCL-sHLH) has an extremely poor prognosis. This case suggests that BV in combination with a PD-1 inhibitor may be a therapeutic option; however, a high degree of caution is required—immune checkpoint inhibitors by themselves may induce sHLH through hyperactivation of the T cell–macrophage axis (incidence, 0.1%–0.7%; lethality, >40%). This regimen should only be considered when ICI-related HLH is strictly excluded (serum IFN-γ >500 pg/mL or sCD25 >10,000 U/mL) and there is clear evidence of lymphoma-driven sHLH, and its risk–benefit ratio still needs to be validated in future studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Guangxi Medical University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. HW: Data curation, Formal analysis, Methodology, Writing – original draft. WB: Conceptualization, Resources, Supervision, Writing – review & editing. ZP: Methodology, Resources, Supervision, Validation, Writing – review & editing. FH: Resources, Supervision, Validation, Writing – review & editing. SZ: Methodology, Supervision, Validation, Writing – review & editing. QM: Supervision, Validation, Writing – review & editing. YL: Supervision, Validation, Writing – review & editing. YB: Supervision, Validation, Writing – review & editing. DL: Supervision, Validation, Writing – review & editing. HY: Formal analysis, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate the contributions made by our other colleagues to the current case. The data used during the current report are available from the corresponding author on reasonable request. We attest to the originality of the submission and confirm that the manuscript has been submitted solely to this journal and has not been published elsewhere.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:203. doi: 10.3322/caac.21830
2. Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. (2011) 12:1013–22. doi: 10.1016/S1470-2045(11)70235-2
3. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. (2010) 116:2040–5. doi: 10.1182/blood-2010-03-276246
4. Xing W, Dresser K, Zhang R, Evens AM, Yu H, Woda BA, et al. PD-L1 expression in EBV-negative diffuse large B-cell lymphoma: clinicopathologic features and prognostic implications. Oncotarget. (2016) 7:59976–86. doi: 10.18632/oncotarget.11045
5. Menter T, Bodmer-Haecki A, Dirnhofer S, and Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. (2016) 54:17–24. doi: 10.1016/j.humpath.2016.03.005
6. Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. (2016) 68:1079–89. doi: 10.1111/his.12882
7. Georgiou K, Chen L, Berglund M, Ren W, de Miranda NF, Lisboa S, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. (2016) 127:3026–34. doi: 10.1182/blood-2015-12-686550
8. Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J Clin Oncol. (2019) 37:481–9. doi: 10.1200/JCO.18.00766
9. Fang X, Xiu B, Yang Z, Qiu W, Zhang L, Zhang S, et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Med (Baltimore) Med. (2017) 96:e6398. doi: 10.1097/MD.0000000000006398
10. Wang XJ, Seegmiller AC, Reddy NM, and Li S. CD30 expression and its correlation with MYC rearrangement in de novo diffuse large B-cell lymphoma. Eur J Haematol European J Haematol. (2016) 97:39–47. doi: 10.1111/ejh.12680
11. Rodrigues-Fernandes CI, Abreu LG, Radhakrishnan R, Perez D, Amaral-Silva GK, Gondak RO, et al. Prognostic significance of CD30 expression in diffuse large B-cell lymphoma: A systematic review with meta-analysis. J Oral Pathol Med. (2021) 50:587–93. doi: 10.1111/jop.13208
12. Campuzano-Zuluaga G, Cioffi-Lavina M, and Lossos IS. Chapman-Fredricks J R.Frequency and extent of CD30 expression in diffuse large B-cell lymphoma and its relation to clinical and biologic factors: a retrospective study of 167 cases. Leuk Lymphoma. (2013) 54:2405–11. doi: 10.3109/10428194.2013.778407
13. Hao X, Wei X, Huang F, Wei Y, Zeng H, Xu L, et al. The expression of CD30 based on immunohistochemistry predicts inferior outcome in patients with diffuse large B-cell lymphoma. PloS One. (2015) 10:e126615. doi: 10.1371/journal.pone.0126615
14. Salas MQ, Climent F, Tapia G, DomingoDomenech E, Mercadal S, Oliveira AC, et al. Clinicopathologic features and prognostic significance of CD30 expression in de novo diffuse large B-cell lymphoma (DLBCL): results in a homogeneous series from a single institution. Biomarkers. (2020) 25:69–75. doi: 10.1080/1354750X.2019.1691656
15. Gong QX, Lu TX, Liu C, Wang Z, Liang JH, Xu W, et al. Prevalence and clinicopathologic features of CD30-positive de novo diffuse large B-cell lymphoma in Chinese patients: a retrospective study of 232 cases. Int J Clin Exp Pathol. (2015) 8:15825–35. doi: 10.1177/1040638715586578
16. Qu Y, Lu XZ, Wang RX, Hei XF, Li J, Xiao BT, et al. Expression of CD30 in patients with diffuse large B-cell lymphoma and clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2024) 32:450–7. doi: 10.19746/j.cnki.issn.1009-2137.2024.02.020
17. Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. (2015) 125:1394–402. doi: 10.1182/blood-2014-09-598763
18. Camicia R, Winkler HC, and Hassa PO. Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review. Mol Cancer. (2015) 14:207. doi: 10.1186/s12943-015-0474-2
19. Bao F and Ke XY. Expression of PD-L1 and PD-1 in pathological tissue of patients newly diagnosed with diffuse large B-cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2022) 30:778–83. doi: 10.19746/j.cnki.issn.1009-2137.2022.03.019
20. Gregory GP, Kumar S, Wang D, Mahadevan D, Walker P, Wagner-Johnston N, et al. Pembrolizumab plus dinaciclib in patients with hematologic Malignancies: the phase 1b KEYNOTE-155 study. Blood Adv. (2022) 6:1232–42. doi: 10.1182/bloodadvances.2021005872
21. Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. (2020) 189:1119–26. doi: 10.1111/bjh.16494
22. Plattel WJ, Bergamasco A, Trinchese F, Gavini F, Bent-Ennakhil N, Zomas A, et al. Effectiveness of brentuximab vedotin monotherapy in relapsed or refractory Hodgkin lymphoma: a systematic review and meta-analysis. Leuk Lymphoma. (2021) 62:3320–32. doi: 10.1080/10428194.2021.1957865
23. Zinzani PL, Sasse S, Radford J, Shonukan O, and Bonthapally V. Experience of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma and relapsed/refractory systemic anaplastic large-cell lymphoma in the Named Patient Program: Review of the literature. Crit Rev Oncol Hematol. (2015) 95:359–69. doi: 10.1016/j.critrevonc.2015.03.011
24. Zinzani PL, Sasse S, Radford J, Gautam A, and Bonthapally V. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma: An updated review of published data from the named patient program. Crit Rev Oncol Hematol. (2016) 104:65–70. doi: 10.1016/j.critrevonc.2016.04.019
25. Svoboda J, Bair SM, Landsburg DJ, Dwivedy NS, Nagle SJ, Barta SK, et al. Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas. Haematologica. (2021) 106:1705–13. doi: 10.3324/haematol.2019.238675
26. Ward JP, Berrien-Elliott MM, Gomez F, Luo J, Becker-Hapak M, Cashen AF, et al. Phase 1/dose expansion trial of brentuximab vedotin and lenalidomide in relapsed or refractory diffuse large B-cell lymphoma. Blood. (2022) 139:1999–2010. doi: 10.1182/blood.2021011894
27. Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. (2021) 138:427–38. doi: 10.1182/blood.2020009178
28. Ma H, Li X, Lin M, Lv K, Zhang M, and Wu X. Advances in CD30- and PD-1-targeted therapies for relapsed or refractory Hodgkin lymphoma. Am J Transl Res. (2021) 13:12206–16. doi: 10.1186/s13045-018-0601-9
29. Massaro F, Meuleman N, Bron D, Vercruyssen M, and Maerevoet M. Brentuximab vedotin and pembrolizumab combination in patients with relapsed/refractory hodgkin lymphoma: A single-centre retrospective analysis. Cancers (Basel). (2022) 14:982. doi: 10.3390/cancers14040982
30. Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the phase II checkMate 436 study. J Clin Oncol J Clin Oncol: Off J Am Soc Clin Oncol. (2019) 37:3081–9. doi: 10.1200/JCO.19.01492
Keywords: HLH, relapsed/refractory diffuse large B-cell lymphoma, hemophagocytic syndrome, brentuximab vedotin, sintilimab
Citation: Cheng S, Wu H, Bao W, Peng Z, Huang F, Zhou S, Mo Q, Li Y, Bin Y, Lan D and Yang H (2025) Efficacy of brentuximab vedotin combined with sintilimab in relapsed/refractory DLBCL patient with secondary hemophagocytic syndrome: a case report and literature review. Front. Oncol. 15:1531713. doi: 10.3389/fonc.2025.1531713
Received: 20 November 2024; Accepted: 20 August 2025;
Published: 23 September 2025.
Edited by:
Giuliana Giardino, University of Naples Federico II, ItalyReviewed by:
Enrica Antonia Martino, Cosenza Hospital, ItalyMarco Cavaco, Gulbenkian Institute of Science (IGC), Portugal
Copyright © 2025 Cheng, Wu, Bao, Peng, Huang, Zhou, Mo, Li, Bin, Lan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Yang, eXlhYW56aUAxNjMuY29t
 Siyu Cheng
Siyu Cheng Huihong Wu
Huihong Wu Zhigang Peng
Zhigang Peng