- 1Health Management Center, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2Department of Gastrointestinal Surgery, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 3Department of Neurology, Shandong Provincial Third Hospital, Shandong University, Jinan, Shandong, China
- 4Department of Gastrointestinal Surgery, Xintai City People’s Hospital, Xintai, Shandong, China
- 5Department of General Surgery, The First Affiliated Hospital of Shandong First Medical University, Jinan, Shandong, China
- 6Department of Gastrointestinal, Shandong Provincial Third Hospital, Shandong University, Jinan, China
Background: Visceral fat area (VFA) has been suggested as an alternative to body mass index (BMI) for evaluating the effects of obesity and fat distribution. Although VFA has shown potential as a predictor of perioperative complications and surgical duration, its prognostic value in gastric cancer patients remains contentious. This meta-analysis aimed to elucidate the prognostic significance of VFA in this patient population.
Methods: We searched the PubMed, Cochrane Library, CNKI, and EMBASE databases, including literature published up to July 31, 2024, to investigate the prognostic implications of VFA in postoperative and non-surgical gastric cancer patients. Outcome measures encompassed overall survival (OS), relapse-free survival (RFS), disease free survival (DFS).
Results: A total of 6,054 eligible patients were selected from 12 studies. The results indicated a significant association between elevated VFA and improved OS and DFS/RFS (OS: HR 0.76, 95% CI 0.65-0.90, p = 0.001; DFS: HR 0.84, 95% CI 0.75-0.95, p = 0.004). Subgroup analyses were conducted to assess the robustness of these findings.
Conclusions: This meta-analysis demonstrated that among patients with gastric cancer who underwent surgery or other treatments, elevated VFA was significantly associated with improved OS and DFS/RFS. Consequently, VFA may serve as a useful prognostic indicator for assessing the prognosis of gastric cancer patients following treatment. However, further prospective studies are necessary to validate these findings and confirm the reliability of VFA as a prognostic marker.
Systematic review registration: https://inplasy.com/, identifier INPLASY202490078.
1 Introduction
Gastric cancer is a prevalent malignancy, accounting for 6.8% of cancer-related mortality worldwide (1). It is the fifth most commonly diagnosed cancer globally and ranks third in cancer-associated deaths (2). Gastric cancer is characterized by its asymptomatic onset, rapid progression, high degree of malignancy, and poor prognosis (3–5). The majority of patients are diagnosed at an advanced stage, significantly limiting the benefits of surgical intervention; many may even lose the opportunity for curative resection (6, 7). This highlights the necessity for more sophisticated and comprehensive therapeutic strategies aimed at disease control, symptom palliation, and improving overall survival and quality of life. While multimodal treatments such as combination chemotherapy (8) and immunotherapy (9) have demonstrated some efficacy in extending survival and enhancing quality of life, their overall clinical impact remains suboptimal.
Notably, with economic development, advancements in healthcare infrastructure, increased public health awareness, and the widespread use of antibiotics, the incidence of gastric cancer has declined compared to historical trends (1). However, an alarming rise in incidence is being observed in younger individuals, particularly those with obesity (10). Multiple studies have identified obesity as a significant risk factor not only for cardiovascular diseases but also for various malignancies, negatively influencing oncological outcomes (11, 12). Specifically, visceral obesity may facilitate tumorigenesis through the induction of adipose tissue inflammation and systemic inflammatory responses, leading to dysregulation of key signaling pathways in the adipose microenvironment and the tumor microenvironment (TME) (13, 14). Body mass index (BMI), defined as the ratio of body weight (kg) to the square of height (m²), is a widely utilized metric for evaluating excess adiposity and its severity (15, 16). While BMI is advantageous due to its simplicity, cost-effectiveness, and reproducibility, it fails to consistently reflect body fat distribution across individuals. Significant variability exists in body fat percentage at the same BMI across different age groups, genders, and ethnicities (17–19). In contrast, VFA is a more precise indicator of adipose tissue dysfunction and has been strongly linked to a range of obesity-related comorbidities (20, 21). Additionally, VFA has been proposed as a superior metric to BMI in assessing perioperative risk (22). Several studies have reported that increased VFA is associated with higher rates of postoperative complications in gastric cancer surgery, such as pancreatic fistula, anastomotic leakage (23), and surgical site infections (24). Moreover, elevated VFA has been correlated with worse clinical outcomes in patients with breast cancer, pancreatic cancer, and hepatocellular carcinoma (25–27).
Despite these findings, the prognostic significance of VFA in gastric cancer patients undergoing surgical or non-surgical treatment remains a matter of debate. Thus, elucidating the impact of visceral fat area on the prognosis of gastric cancer patients is of paramount importance. Quantitative measurement of body composition, including muscle and fat distribution, through computed tomography (CT) has been established as a reliable method for evaluating body composition (28–31), and has become a burgeoning area of research in oncology. Clinically, patients typically undergo axial abdominal CT scans in the supine position, with measurements commonly taken at the levels of L3, L4, and the umbilicus. Image analysis software is then employed to delineate visceral and subcutaneous fat compartments. The density of adipose tissue on CT images generally ranges between -150 and -50 Hounsfield units (HU), allowing for accurate identification and quantification of fat areas (measured in cm²).
This study employs a systematic review and meta-analysis to evaluate the association between VFA and the prognosis of gastric cancer patients, providing clinicians with a novel prognostic tool for use in clinical decision-making.
2 Materials and methods
2.1 Search strategy
This systematic review and meta-analysis were conducted in accordance with the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (32). Two independent researchers systematically searched PubMed, Embase, CNKI, and the Cochrane Library to identify studies related to the prognostic significance of VFA in both postoperative and non-surgical gastric cancer patients. The search encompassed relevant studies from the inception of these databases until July 31, 2024. We used the following keywords to investigate the predictive significance of VFA in gastric cancer patients: “Gastric Cancer” or “Stomach Neoplasms” or “Gastric Neoplasms” or “Gastric Adenocarcinomas” or “Stomach Cancer” or “Stomach carcinoma” or “Gastric malignancy,” and “Visceral Fat” or “Abdominal Fat” or “Intra-Abdominal Fat” or “Adipose Tissue” or “Visceral fat area” or “Intra-abdominal fat area” or “Visceral adiposity area.” In addition to using free-text terms and Medical Subject Headings (MeSH) for searching within titles and abstracts, we screened the references of selected articles to ensure a comprehensive search.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
(1) Patients were diagnosed with gastric cancer through a comprehensive evaluation, including imaging studies, serum tumor marker tests, and histopathological biopsy; (2) Visceral fat area (VFA) was calculated using the L3-CT imaging level; (3) Studies provided long-term survival data such as overall survival, relapse-free survival, or disease-free survival; (4) Hazard ratios (HR) and 95% confidence intervals (CI) could be obtained either directly from the literature or indirectly through calculation.
2.2.2 Exclusion criteria
(1) reviews, case reports, case series, conference abstracts, or commentaries; (2) studies with insufficient data; (3) nonclinical or nonhuman studies; (4) studies with overlapping or duplicate data; (5) studies without preoperative CT-confirmed visceral fat area.
2.3 Data extraction and quality assessment
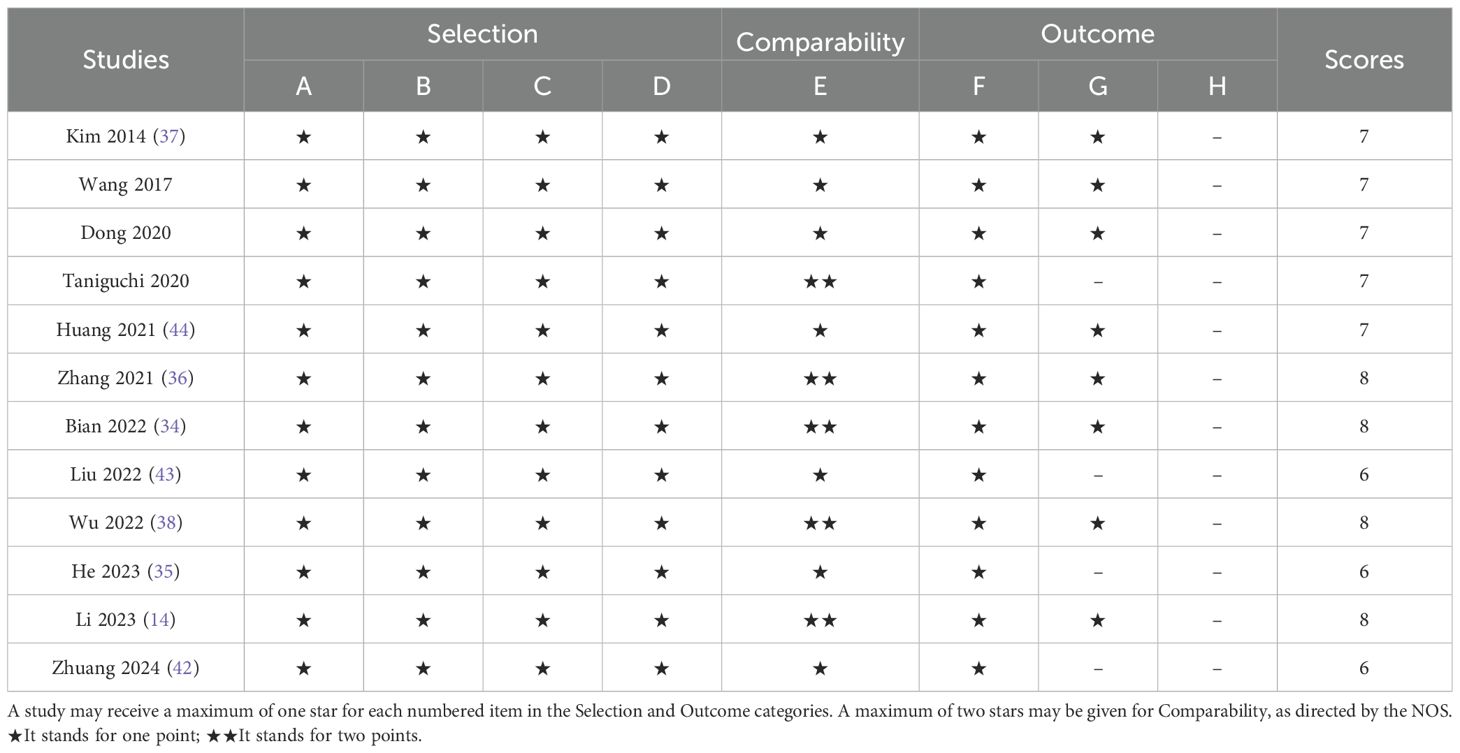
Two researchers independently extracted data from eligible studies. Any discrepancies were resolved through discussion or consultation with a third researcher. The extracted data included: first author’s name, publication year, country of study, study design, sample size, mean or median age of included patients, gender distribution, treatment modalities, and survival analyses (including hazard ratios and corresponding 95% confidence intervals for overall survival and disease-free survival). The quality of the studies was assessed using the Newcastle-Ottawa Scale (NOS), which covers three aspects: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Each of the two researchers scored the three aspects across eight questions, with a total score ranging from 0 to 9. Studies with a score above 6 were considered high-quality studies (33).
2.4 Data statistics
Statistical analysis was conducted using Stata SE (version 12.0; StataCorp, College Station, Texas, USA). Heterogeneity among the studies was assessed with Cochran’s Q-test and I² statistics. When heterogeneity was not significant (P ≥ 0.10 or I² < 50%), a fixed-effects model was used; otherwise, a random-effects model was applied in the presence of significant heterogeneity (P < 0.10 or I² ≥ 50%). For survival data, including OS and DFS or RFS, hazard ratios (HR) and their 95% confidence intervals (CI) were calculated. Statistical significance was defined as P < 0.05. Publication bias was evaluated by examining the symmetry of the funnel plot and using methods such as Egger’s linear regression and Begg’s rank correlation test, with a P-value < 0.05 suggesting potential publication bias. Sensitivity analysis was performed to determine the impact of individual studies on OS and DFS or RFS. To investigate the sources of heterogeneity, subgroup analyses were conducted based on treatment modalities, sample sizes, cutoff values, and analytical models.
3 Results
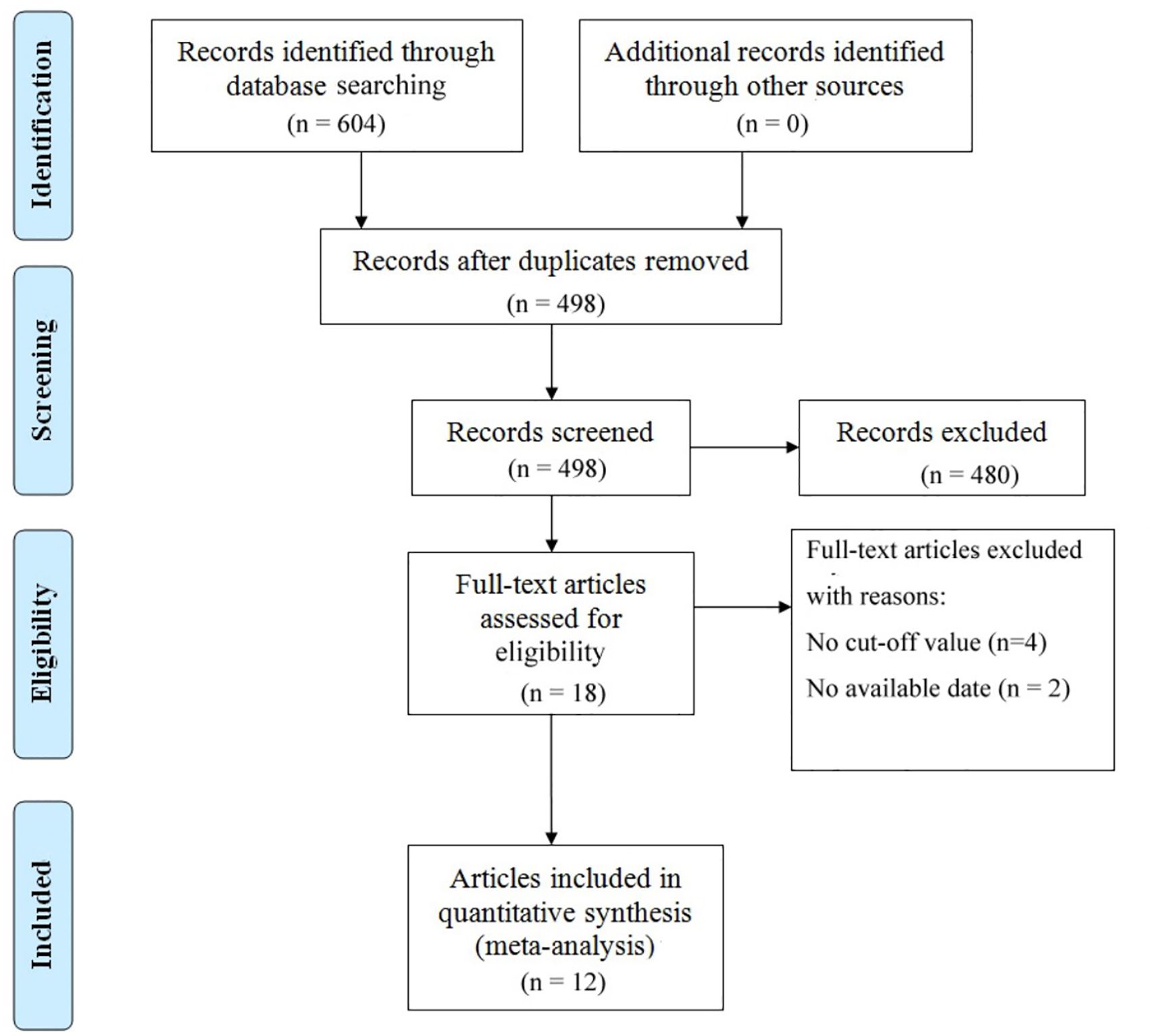
3.1 Study selection and characteristics
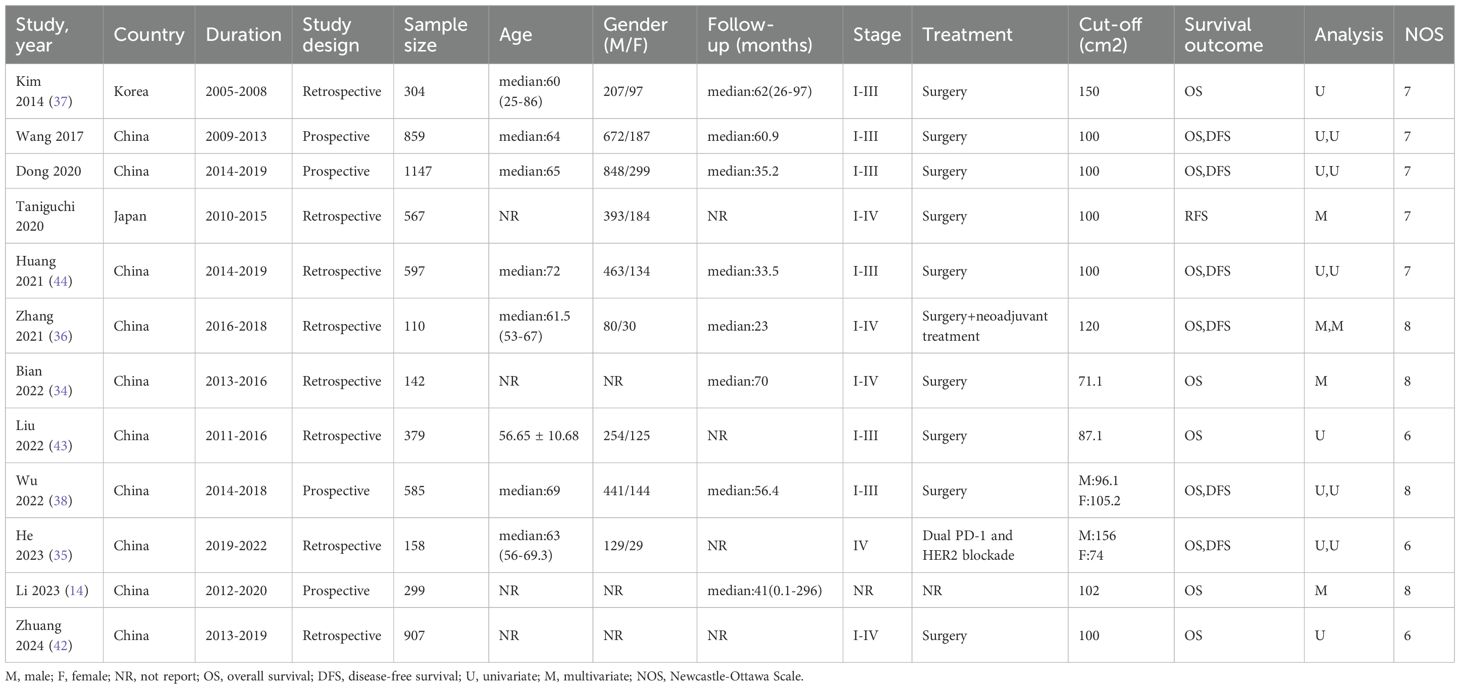
The literature selection process is comprehensively outlined in Figure 1. In accordance with the previously established search strategy, an initial pool of 604 articles was identified. After the removal of duplicates, 498 unique studies remained. Subsequently, a rigorous screening of titles and abstracts based on predefined inclusion and exclusion criteria resulted in the exclusion of 480 studies. An additional six studies were excluded due to the unavailability of full-text access. Ultimately, 12 studies assessing the correlation between VFA and gastric cancer prognosis were included in the final analysis (14, 34–44). Table 1 provides a summary of the key characteristics of these studies. Published between 2014 and 2024, ten studies were conducted in China, while the remaining two originated from Japan and South Korea. Of these, eight were retrospective in design, while four were prospective. The sample sizes across the studies ranged from 110 to 1,147, with a cumulative total of 6,054 patients. Nine studies focused on surgical interventions alone, while two included combination regimens involving ICIs; one study did not comprehensively report treatment details. Disease-free survival was reported in seven studies, while overall survival (OS) was documented in 11 studies. Quality assessment using the Newcastle-Ottawa Scale (NOS) yielded scores ranging from 6 to 8 across the included studies, reflecting a generally high level of methodological rigor. The detailed NOS scores are presented in Table 2.
3.2 Association of VFA with OS and DFS/RFS
A total of 11 studies, comprising 5,487 gastric cancer patients, investigated the association between VFA and OS. Heterogeneity analysis revealed statistically significant heterogeneity (P=0.028 < 0.1, I² = 50.4% > 50%), indicating that a random-effects model was the most appropriate for this meta-analysis. The pooled Hazard Ratio (HR) and corresponding 95% Confidence Interval (CI) were: HR=0.76, 95% CI=0.65–0.90, P=0.001, suggesting that elevated VFA correlates with improved OS in GC patients undergoing surgical or other therapeutic interventions (Figure 2A). Furthermore, seven studies, encompassing 4,023 patients, assessed the relationship between VFA and DFS/RFS in GC patients following surgery or other treatments. Heterogeneity testing indicated no significant heterogeneity (P=0.207 > 0.1, I² = 29.2% < 50%). The aggregated data demonstrated an HR of 0.84, 95% CI=0.75–0.95, P=0.004, indicating that higher VFA levels are associated with superior DFS or RFS outcomes in GC patients (Figure 2B). The prognostic value of VFA for OS in gastric cancer patients varied significantly across subgroups, with notable heterogeneity. Large-sample studies (>500 patients) demonstrated a significant association between lower VFA and improved OS (HR = 0.85, 95% CI: 0.76–0.96, P = 0.009), supported by low heterogeneity (I² = 0%), indicating robust results. In contrast, smaller studies (<500 patients) showed a lower HR (HR = 0.62, P = 0.01) but with higher heterogeneity (I² = 61.8%), necessitating cautious interpretation. When a cutoff of 100 cm² was applied, VFA significantly correlated with OS (HR = 0.86, P = 0.021) and minimal heterogeneity. However, studies using non-100 cutoffs suggested stronger protective effects (HR = 0.64, P = 0.004) but with moderate heterogeneity (I² = 54.7%), reflecting potential variability in cutoff selection. Prospective studies (HR = 0.77, P < 0.001) and multivariate-adjusted analyses (HR = 0.40, P < 0.001) further validated VFA as an independent prognostic factor. Retrospective studies, however, did not reach statistical significance (P = 0.058), possibly due to bias or confounding factors. Overall, lower VFA was consistently linked to prolonged OS, particularly in standardized, large-sample prospective cohorts. (Tables 3). The association between VFA and DFS/PFS was less consistent, with greater variability across subgroups. Large-sample studies (>500 patients) revealed a significant benefit of lower VFA for DFS (HR = 0.85, P < 0.001) and low heterogeneity (I² = 0%). However, smaller studies (<500 patients) showed a wide confidence interval (HR = 0.65, 95% CI: 0.27–1.57, P < 0.001) and extreme heterogeneity (I² = 75.7%), indicating unstable results. A cutoff of 100 cm² did not yield statistical significance for DFS (P = 0.07), while non-100 cutoffs showed a significant protective effect (HR = 0.75, P = 0.01) but with moderate heterogeneity (I² = 51.6%). Prospective studies supported a clear association (HR = 0.83, P = 0.005), whereas retrospective analyses were nonsignificant (P = 0.319). Notably, multivariate-adjusted results for DFS were inconclusive (HR = 0.65, P = 0.336), likely due to limited sample size (only 2 studies) or variability in adjusted confounders. These findings suggest that the prognostic role of VFA in DFS/PFS may depend on endpoint definitions, study design, and methodological heterogeneity. Future studies should prioritize standardized cutoffs, larger cohorts, and prospective designs to clarify its clinical utility for DFS/PFS (Tables 4).
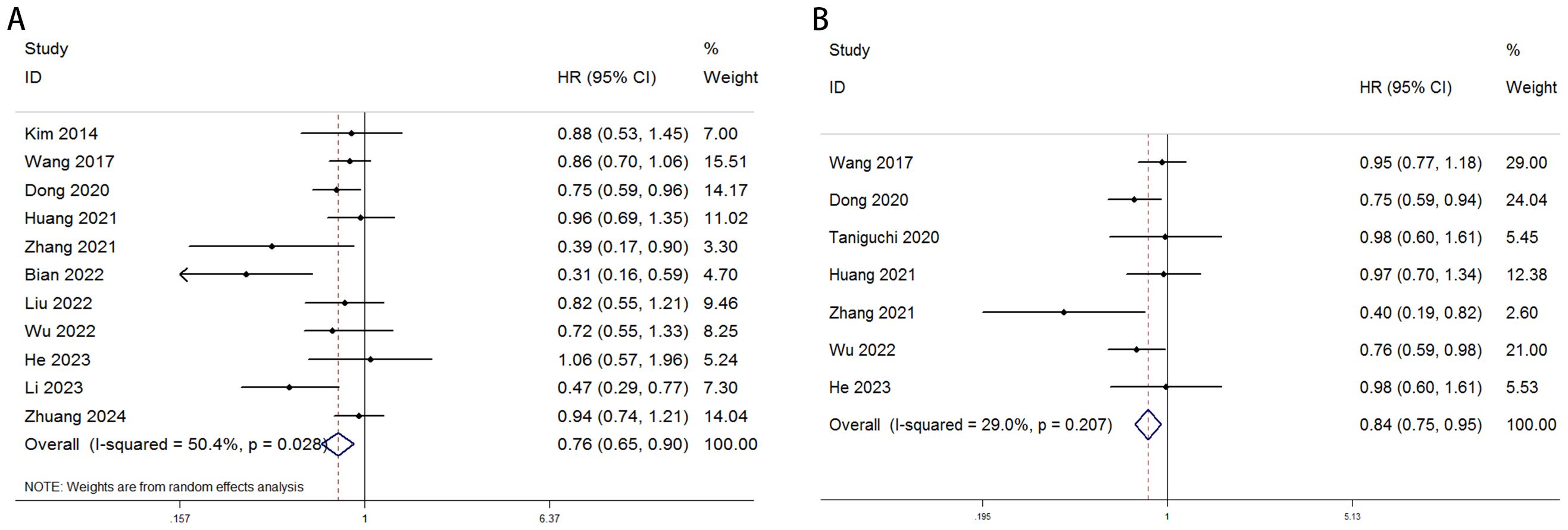
Figure 2. Forest plot illustrating the association between visceral fat area (VFA) and (A) overall survival (OS) and (B) disease-free survival (DFS) or recurrence-free survival (RFS) in gastric cancer patients.
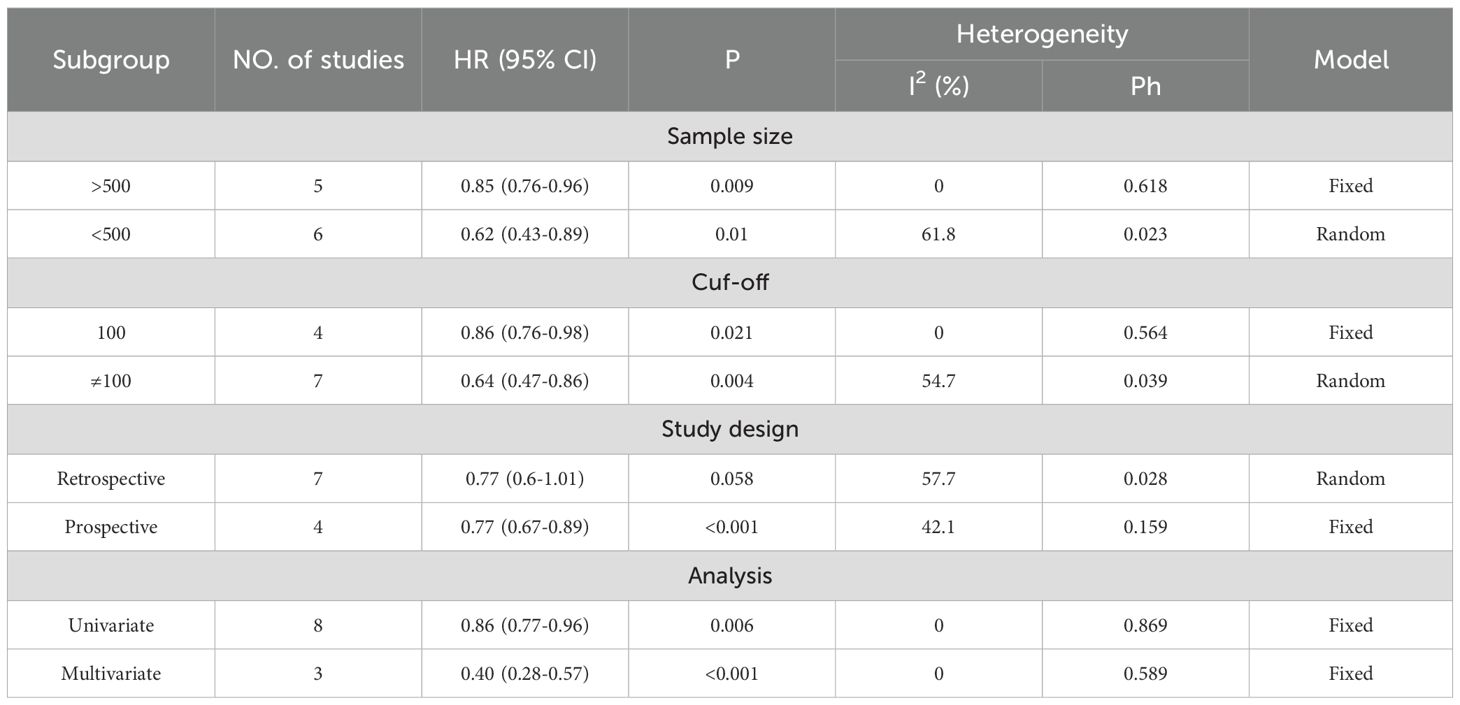
Table 3. Subgroup analysis evaluating the prognostic significance of VFA for OS in gastric cancer patients undergoing surgery or other treatments.
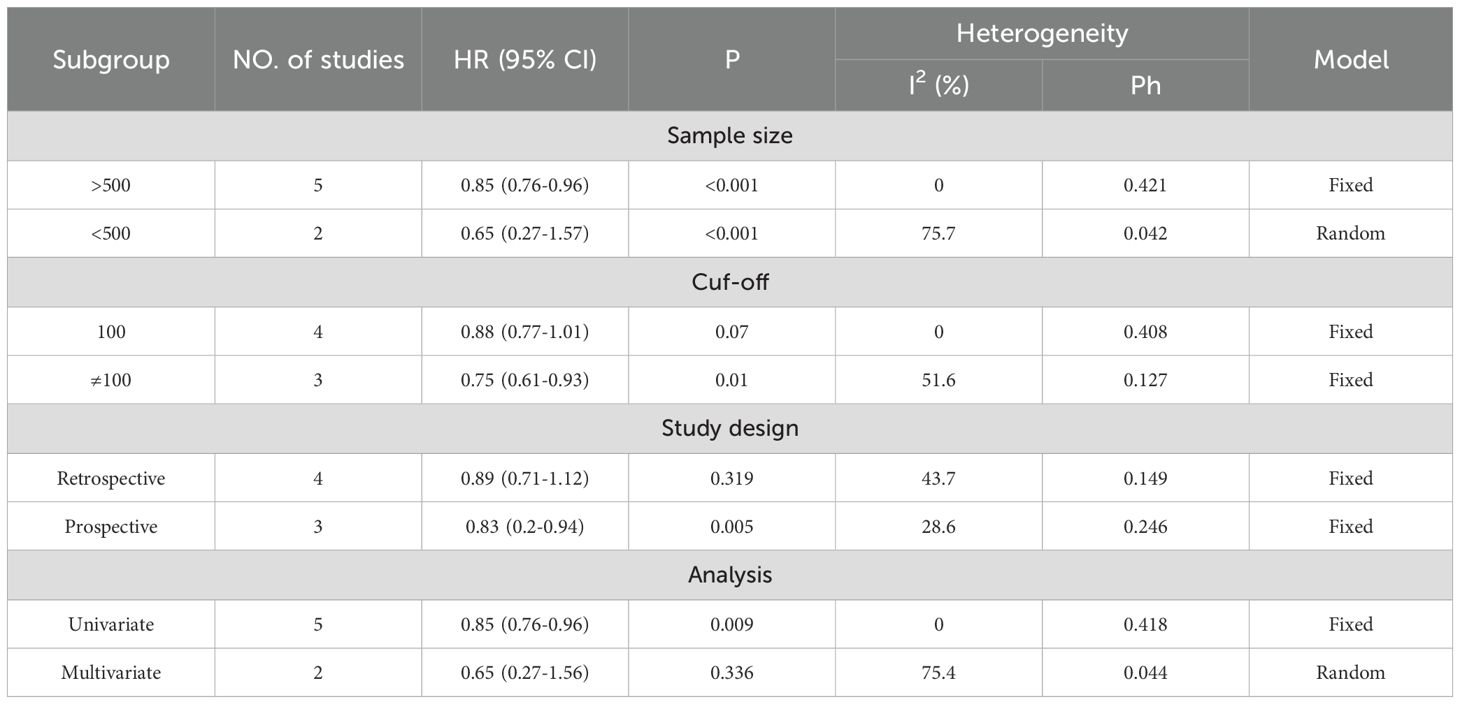
Table 4. Subgroup analysis evaluating the prognostic significance of VFA for DFS/RFS in gastric cancer patients undergoing surgery or other treatments.
3.3 Publication bias and sensitivity analysis
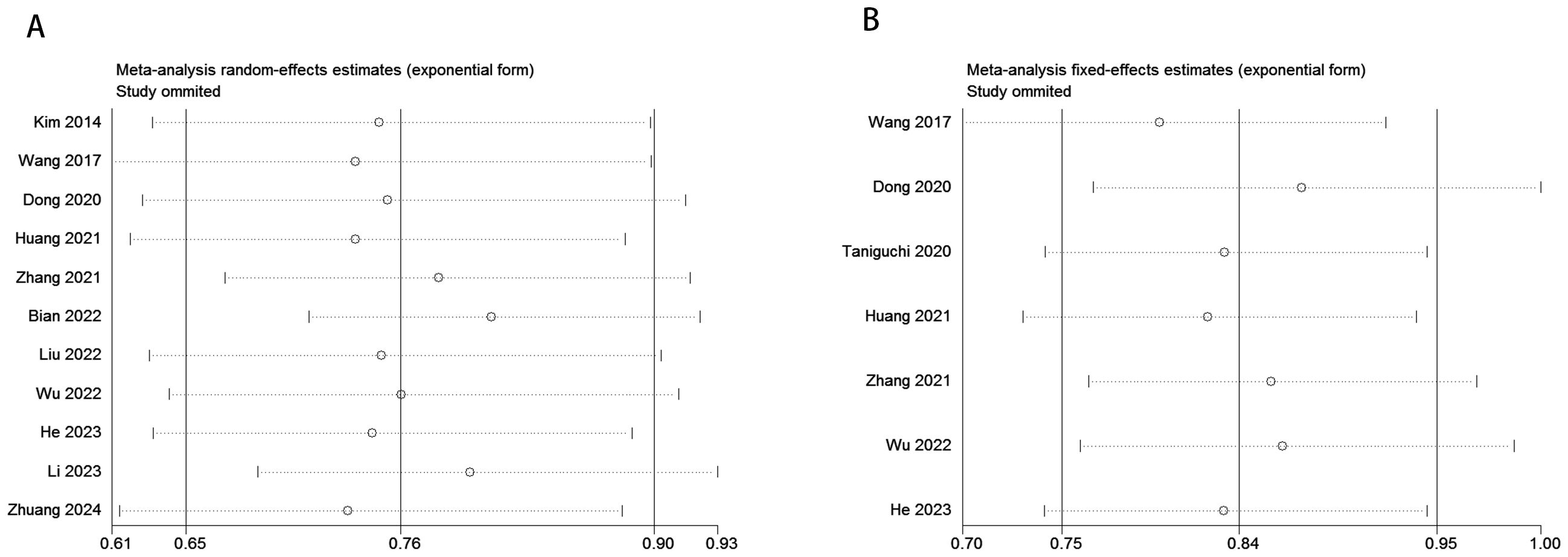
To evaluate potential publication bias, a combination of funnel plots, Begg’s test, and Egger’s test was utilized. The funnel plots for OS (Figure 3A) and DFS/RFS (Figure 3B) both displayed relatively symmetrical distributions. The results of Begg’s test indicated no significant publication bias for either OS or DFS/RFS (OS, p = 0.161; Figure 4A; DFS/RFS, p = 0.368; Figure 4B). Egger’s test further corroborated these findings, showing no evident publication bias in the studies related to OS and DFS/RFS (OS, p = 0.067; Figure 5A; DFS/RFS, p = 0.609; Figure 5B). To further assess the possibility of publication bias, we conducted a sensitivity analysis by sequentially excluding each study and performing a cumulative analysis to determine its impact on the overall results. The analysis demonstrated that no individual study had a significant effect on the relationship between VFA and OS or DFS/RFS in gastric cancer patients (Figure 6). This finding underscores the robustness of the observed associations, as the results remained consistent and unaffected by the exclusion of individual studies.
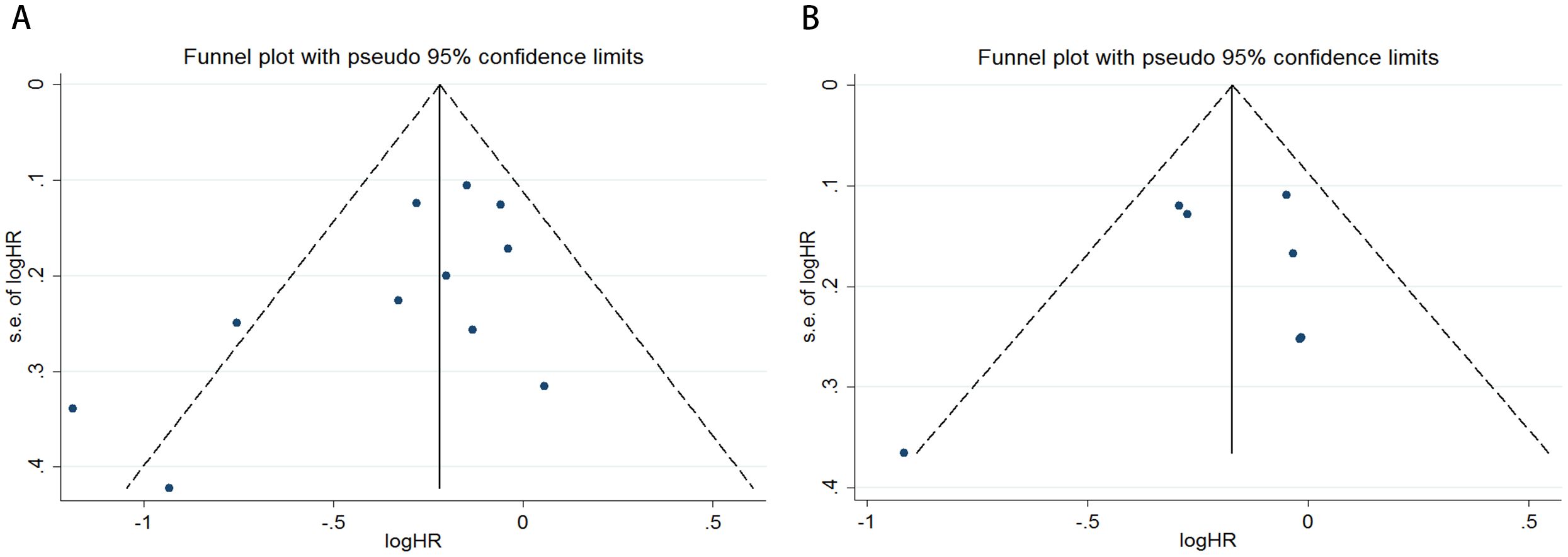
Figure 3. Funnel plots are utilized to assess the presence of publication bias in (A) overall survival (OS) and (B) disease-free survival (DFS) or recurrence-free survival (RFS).
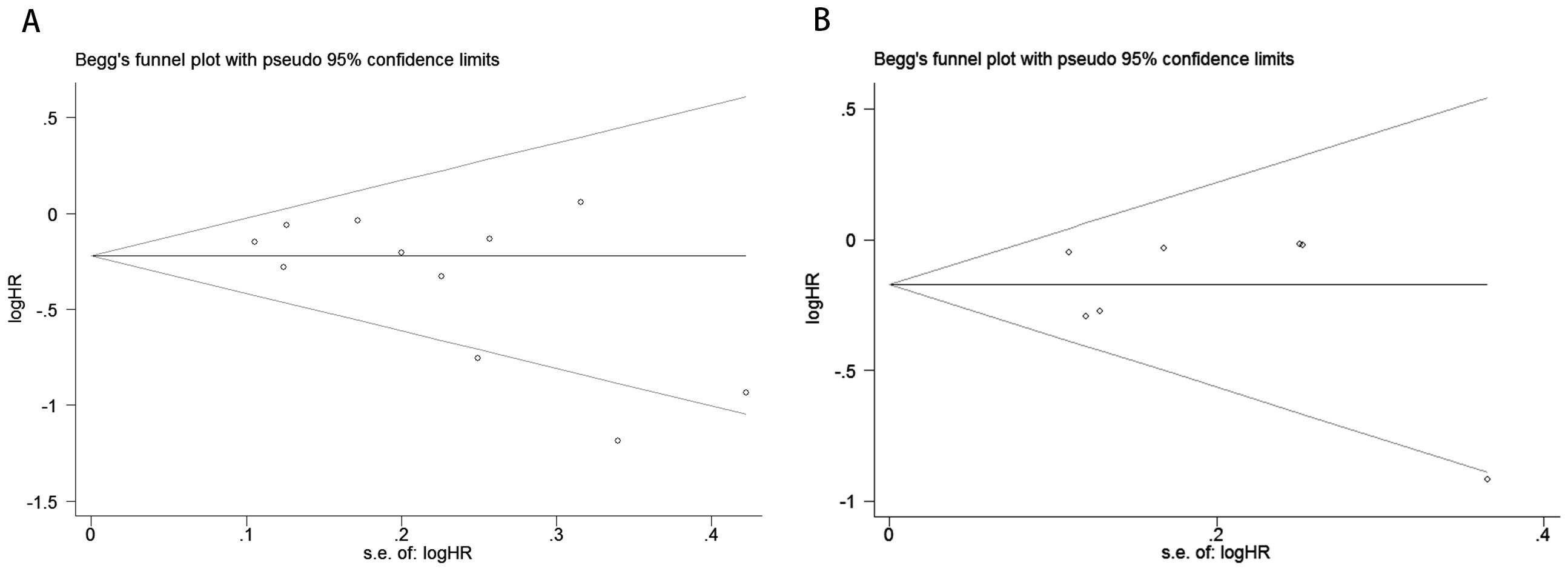
Figure 4. Publication bias test. (A) Begg tests for OS, p = 0.161; (B) Begg tests for DFS/RFS, p = 0.368.

Figure 5. Publication bias test. (A) Egger’s test for OS, p = 0.067; (B) Egger’s test for DFS/RFS, p = 0.609.
4 Discussion
Gastric cancer (GC) is a leading cause of cancer-related mortality worldwide. Despite significant advances in treatment over recent years, the recurrence and mortality rates for gastric cancer remain high (45, 46). Complications following surgical treatment, along with adverse events from chemotherapy, immunotherapy, and targeted therapy, severely impact patient survival outcomes. Multiple studies have indicated that excessive abdominal visceral fat not only affects metabolic function but is also closely associated with postoperative complications and adverse events in gastric cancer patients (38–41). Additionally, adipose tissue secretes various factors, such as tumor necrosis factor (TNF) and interleukin-6 (IL-6) (47, 48). These factors are not only associated with the mechanical effects of obesity, such as gastroesophageal reflux, but are also considered to play a significant pathological role in the development and progression of gastrointestinal cancers.Therefore, accurate prognostic assessment is crucial before performing radical gastric surgery or initiating non-surgical treatments such as combination chemotherapy.
BMI (Body Mass Index) is a commonly used indicator for assessing obesity, but it has significant limitations, particularly regarding accuracy across different ethnic groups. For instance, Asians often face a higher risk of cardiovascular diseases or metabolic syndrome at lower BMI levels (49). This discrepancy is largely due to differences in body fat distribution and percentage. BMI reflects only the ratio of weight to height, failing to distinguish between muscle and fat composition, and it does not effectively differentiate between peripheral obesity (fat accumulation in the limbs) and abdominal obesity (visceral fat accumulation), the latter of which is associated with greater health risks. Consequently, relying solely on BMI to assess health can overlook potential metabolic risks and variations in body composition. In contrast, visceral fat is a more reliable indicator of disease risk, with CT assessment considered the gold standard for detecting visceral fat obesity. Since Asians tend to accumulate visceral fat more readily, using visceral fat area (VFA) as an obesity assessment standard is more accurate than BMI and better reflects health risks.
Previous studies have shown that patients with higher intra-abdominal fat, such as those with pancreatic or colorectal cancer, tend to have poorer overall survival rates. This may be due to visceral fat increasing serum levels of inflammatory cytokines, angiogenic factors, and oxidative stress markers, thereby affecting the tumor microenvironment (50–52). However, in contrast to these findings, Harada et al. (53) demonstrated that low visceral fat could significantly increase overall mortality in patients with upper gastrointestinal cancers. Many researchers believe that high BMI or high VFA correlates with poor prognosis in gastric cancer patients, as previous studies have shown a strong association between high BMI or VFA and decreased postoperative morbidity and lymph node retrieval (54–56). Feng et al. (57) found that preoperative low VFA and significant postoperative VFA loss predicted shorter progression-free survival and overall survival in metastatic gastric cancer patients. Similarly, Park et al. (58) reported that a marked reduction in postoperative VFA was indicative of shorter progression-free and overall survival. Furthermore, Matsui et al. (59) identified low VFA as an independent prognostic factor for poor adherence to adjuvant chemotherapy in advanced gastric cancer patients requiring neoadjuvant treatment. Likewise, Zhang et al. (36) noted that lower VFA before and after neoadjuvant chemotherapy correlated with worse progression-free and overall survival. In summary, the impact of high VFA on survival outcomes in gastric cancer patients post-surgery or after non-surgical treatment remains unclear.
The purpose of this study was to determine whether VFA can serve as a prognostic indicator for assessing the prognosis of gastric cancer patients after treatment. To clarify the impact of high VFA on survival outcomes in gastric cancer patients following surgery or non-surgical treatment, we conducted a comprehensive meta-analysis of data from 12 relevant trials involving 6,054 patients from three countries. Our analysis confirmed the favorable impact of higher visceral fat on the prognosis of gastric cancer patients. Patients with higher visceral fat had longer overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS) compared to those with lower visceral fat. The pooled hazard ratio (HR) for OS was 0.76 (95% CI = 0.65–0.90, P = 0.001), and the pooled HR for DFS or RFS was 0.84 (95% CI = 0.75–0.95, P = 0.004). This suggests that the beneficial impact of visceral fat on the prognosis of gastric cancer patients outweighs its potential harmful effects. In addition, our subgroup analysis indicated that higher VFA was predictive of more favorable OS and DFS or RFS outcomes regardless of sample size (≥500 or <500), cut-off value (100 or ≠100), study design (retrospective or prospective), and analysis type (multivariate or univariate). Notably, heterogeneity in OS might be attributed to differences in studies with sample sizes of <500, cut-off values ≠100, or retrospective designs. Heterogeneity in DFS or RFS might be due to differences in sample sizes <500 or studies using multivariate analysis. To assess potential publication bias, we employed several methods, including funnel plot analysis, Begg’s test, and Egger’s test. Sensitivity analysis and assessment of publication bias further corroborated the robustness of the conclusions drawn in this meta-analysis. Some clinical trials found no significant correlation between VFA and prognosis in gastric cancer patients. We consider the following possible reasons for this inconsistency: First, insufficient sample sizes in some trials may have led to result bias (35). Second, differences in baseline characteristics among patients could lead to variations in treatment outcomes. For example, in Taniguchi’s study, a higher proportion of patients with low visceral fat content received neoadjuvant or adjuvant therapy, which improved the long-term prognosis of this group (40). Third, treatment protocols varied across different centers. When the negative impact of higher VFA outweighs its benefits, the correlation with favorable prognosis may be obscured. There are several limitations to our study. First, there was heterogeneity among the included studies. Patient baseline characteristics, VFA cut-off values, and treatment protocols varied across trials. Second, the sample size in this analysis was relatively limited, with most data derived from Asian countries. Therefore, the value of VFA in European and other populations requires further exploration to determine its applicability across different demographics. Third, despite differences in body composition and hormones between men and women, we were unable to conduct a gender-based analysis due to the limitations of the original literature. Consequently, more high-quality, large-sample, prospective studies are needed in the future to validate and refine our findings.
5 Conclusions
This meta-analysis demonstrated that among patients with gastric cancer who underwent surgery or other treatments, elevated VFA was significantly associated with improved OS and DFS. Consequently, VFA may serve as a useful prognostic indicator for assessing the prognosis of gastric cancer patients following treatment. However, further prospective studies are necessary to validate these findings and confirm the reliability of VFA as a prognostic marker.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SH: Data curation, Formal analysis, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. BY: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. RH: Conceptualization, Data curation, Formal analysis, Investigation, Software, Supervision, Writing – original draft. LiL: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. DS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. LeL: Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, and Lordick F. Gastric cancer. Lancet (London England). (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
3. Lopez MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. (2023) 181:103841. doi: 10.1016/j.critrevonc.2022.103841
4. Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, et al. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. (2023) 29:2452–68. doi: 10.3748/wjg.v29.i16.2452
5. Le J, Pan G, Zhang C, Chen Y, Tiwari AK, and Qin JJ. Targeting ferroptosis in gastric cancer: Strategies and opportunities. Immunol Rev. (2024) 321:228–45. doi: 10.1111/imr.13280
6. Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: A review. Med Sci Monit. (2019) 25:3537–41. doi: 10.12659/MSM.916475
7. Wang Y, Zhang L, Yang Y, Lu S, and Chen H. Progress of gastric cancer surgery in the era of precision medicine. Int J Biol Sci. (2021) 17:1041–9. doi: 10.7150/ijbs.56735
8. Patel TH and Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol. (2020) 21:70. doi: 10.1007/s11864-020-00774-4
9. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, and Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z
10. Avgerinos KI, Spyrou N, Mantzoros CS, and Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
11. Elagizi A, Kachur S, Carbone S, Lavie CJ, and Blair SN. A review of obesity, physical activity, and cardiovascular disease. Curr Obes Rep. (2020) 9:571–81. doi: 10.1007/s13679-020-00403-z
12. Iyengar NM, Gucalp A, Dannenberg AJ, and Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/JCO.2016.67.4283
13. O'Sullivan J, Lysaght J, Donohoe CL, and Reynolds JV. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol. (2018) 15:699–714. doi: 10.1038/s41575-018-0069-7
14. Li L, Li W, Xu D, et al. Association between visceral fat area and cancer prognosis: A population-based multicenter prospective study. Am J Clin Nutr. (2023) 118:507–17. doi: 10.1016/j.ajcnut.2023.07.001
15. Sweatt K, Garvey WT, and Martins C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What is the Path Forward? [published correction appears in Curr Obes Rep. Curr Obes Rep. (2024) 13:584–95. doi: 10.1007/s13679-024-00580-1
16. Nevill AM, Duncan MJ, and Myers T. BMI is dead; long live waist-circumference indices: But which index should we choose to predict cardio-metabolic risk? Nutr Metab Cardiovasc Dis. (2022) 32:1642–50. doi: 10.1016/j.numecd.2022.04.003
17. Liu C, Cheng KY, Tong X, Cheung WH, Chow SK, Law SW, et al. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front Endocrinol (Lausanne). (2023) 14:1077255. doi: 10.3389/fendo.2023.1077255
18. Van Cauwenberge J, Van Baelen K, Maetens M, Geukens T, Nguyen HL, Nevelsteen I, et al. Reporting on patient's body mass index (BMI) in recent clinical trials for patients with breast cancer: a systematic review. Breast Cancer Res. (2024) 26:81. doi: 10.1186/s13058-024-01832-7
19. Kim T, Shin SH, Kim H, Im Y, Cho J, Kang D, et al. Longitudinal BMI change and outcomes in Chronic Obstructive Pulmonary Disease: a nationwide population-based cohort study. Respir Res. (2024) 25:150. doi: 10.1186/s12931-024-02788-0
20. Tchernof A and Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. (2013) 93:359–404. doi: 10.1152/physrev.00033.2011
21. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. (2010) 33:920–2. doi: 10.2337/dc09-1825
22. Tokunaga M, Hiki N, Fukunaga T, Ohyama S, Yamaguchi T, and Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients, Ann. Surg Oncol. (2009) 16:3245e3251. doi: 10.1245/s10434-009-0645-8
23. Tanaka K, Miyashiro I, Yano M, Kishi K, Motoori M, Seki Y, et al. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol. (2009) 16:1520–5. doi: 10.1245/s10434-009-0391-y
24. Nishigori T, Tsunoda S, Okabe H, Tanaka E, Hisamori S, Hosogi H, et al. Impact of sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann Surg Oncol. (2016) 23:524–31. doi: 10.1245/s10434-016-5385-y
25. Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O'Brien KM, et al. Adult weight change and premenopausal breast cancer risk: A prospective pooled analysis of data from 628,463 women. Int J Cancer. (2020) 147:1306–14. doi: 10.1002/ijc.v147.5
26. Tan Y, Zhang X, Zhang W, Tang L, Yang H, Yan K, et al. The influence of metabolic syndrome on the risk of hepatocellular carcinoma in patients with chronic hepatitis B infection in mainland China. Cancer Epidemiol Biomarkers Prev. (2019) 28:2038–46. doi: 10.1158/1055-9965.EPI-19-0303
27. Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes i n obese adults. JAMA. (2012) 308:1150–9. doi: 10.1001/2012.jama.11132
28. Meyer HJ, Wienke A, Pech M, and Surov A. Computed tomographydefined fat composition as a prognostic marker in gastric adenocarcinoma: a systematic review and meta-analysis. DigDis. (2023) 41:177–86. doi: 10.1159/000527532
29. Matsui R, Inaki N, Tsuji T, and Fukunaga T. Relationship between fat mass indices and postoperative complications after laparoscopic gastrectomy in patients with gastric cancer: a propensity score matching analysis. Anticancer Res. (2022) 42:4841–8. doi: 10.21873/anticanres.15989
30. Nishimura E, Irino T, Matsuda S, Fukuda K, Nakamura R, Kawakubo H, et al. Comparison of changes in body-fat mass and reflux esophagitis among reconstruction methods for proximal gastrectomy. Asian J Surg. (2023) 46:394–8. doi: 10.1016/j.asjsur.2022.04.110
31. Lin GT, Huang JB, Lin JL, Lin JX, Xie JW, Wang JB, et al. Body composition parameters for predicting the efficacy of neoadjuvant chemotherapy with immunotherapy for gastric cancer. Front Immunol. (2022) 13:1061044. doi: 10.3389/fimmu.2022.1061044
32. Moher D, Liberati A, Tetzlaff J, Altman DG, and Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
33. Moskalewicz A and Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in crosssectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
34. Li L, Li W, Xu D, He H, Yang W, Guo H, et al. Associations of radiological features of adipose tissues with postoperative complications and overall survival of gastric cancer patients. Eur Radiol. (2022) 32:8569–78. doi: 10.1007/s00330-022-08918-w
35. Bian L, Wu D, Chen Y, Ni J, Qu H, Li Z, et al. Associations of subcutaneous fat area and Systemic Immune-inflammation Index with survival in patients with advanced gastric cancer receiving dual PD-1 and HER2 blockade. J Immunother Cancer. (2023) 11:e007054. doi: 10.1136/jitc-2023-007054
36. He M, Chen ZF, Zhang L, Gao X, Chong X, Li HS, et al. Impact of body composition on clinical outcomes in people with gastric cancer undergoing radical gastrectomy after neoadjuvant treatment. Nutrition. (2021) 85:111135. doi: 10.1016/j.nut.2020.111135
37. Kim JH, Chin HM, Hwang SS, and Jun KH. Impact of intra-abdominal fat on surgical outcome and overall survival of patients with gastric cancer. Int J Surg. (2014) 12:346–52. doi: 10.1016/j.ijsu.2014.01.010
38. Kim JH, Chin HM, Hwang SS, and Jun KH. Impact of metabolic syndrome on the short- and long-term outcomes for the elderly patients with gastric cancer after radical gastrectomy. Clin Res Hepatol Gastroenterol. (2022) 46:102041. doi: 10.1016/j.clinre.2022.102041
39. Wu H, Jiang HJ, Wang SL, Chen XY, Ma LL, Yu Z, et al. Impact of visceral fat on surgical complications and long-term survival of patients with gastric cancer after radical gastrectomy. Eur J Clin Nutr. (2018) 72:436–45. doi: 10.1038/s41430-017-0032-7
40. Wang SL, Ma LL, Chen XY, Zhou DL, Li B, Huang DD, et al. Impacts of preoperative psoas muscle mass and visceral fat area on postoperative short- and long-term outcomes in patients with gastric cancer. World J Surg. (2021) 45:815–21. doi: 10.1007/s00268-020-05857-9
41. Taniguchi Y, Kurokawa Y, Takahashi T, Saito T, Yamashita K, Tanaka K, et al. Influence of body composition, muscle strength, and physical performance on the postoperative complications and survival after radical gastrectomy for gastric cancer: A comprehensive analysis from a large-scale prospective study. Clin Nutr. (2021) 40:3360–9. doi: 10.1016/j.clnu.2020.11.007
42. Dong QT, Cai HY, Zhang Z, Zou HB, Dong WX, Wang WB, et al. Muscle attenuation, not skeletal muscle index, is an independent prognostic factor for survival in gastric cancer patients with overweight and obesity. Nutrition. (2024) 122:112391. doi: 10.1016/j.nut.2024.112391
43. Zhuang CL, Wu HF, Jiang HJ, Zhang FM, Shi HP, Yu Z, et al. Preoperative computed tomography-determined sarcopenia is a reliable prognostic factor in patients with gastric cancer after radical gastrectomy: A sex-specific analysis. Front Nutr. (2022) 9:884586. doi: 10.3389/fnut.2022.884586
44. Liu T, Yi X, Ge J, Zhang J, Tan F, Song K, et al. The relationship between the GLIM-defined malnutrition, body composition and functional parameters, and clinical outcomes in elderly patients undergoing radical gastrectomy for gastric cancer. Eur J Surg Oncol. (2021) 47:2323–31. doi: 10.1016/j.ejso.2021.02.032
45. O'Connor A, O'Morain CA, and Ford AC. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. (2017) 14:230–40. doi: 10.1038/nrgastro.2016.195
46. O'Connor A and O'Moráin C. Helicobacter pylori infection in Europe: current perspectives. Expert Rev Gastroenterol Hepatol. (2013) 7:541–8. doi: 10.1586/17474124.2013.824707
47. van Kruijsdijk RC, van der Wall E, and Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. (2009) 18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372
48. van Kruijsdijk RC, van der Wall E, and Visseren FL. Metabolic syndrome, obesity, and gastrointestinal cancer. Gastroenterol Res Pract. (2012) 2012:483623. doi: 10.1155/2012/483623
49. Allott EH and Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. (2015) 22:R365–86. doi: 10.1530/ERC-15-0400
50. Balentine CJ, Enriquez J, Fisher W, Hodges S, Bansal V, Sansgiry S, et al. Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg. (2010) 14:1832–7. doi: 10.1007/s11605-010-1297-5
51. Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. (2008) 15:1918–22. doi: 10.1245/s10434-008-9891-4
52. Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Role of tissue microenvironment resident adipocytes in colon cancer. World J Gastroenterol. (2017) 23:5829–35. doi: 10.3748/wjg.v23.i32.5829
53. Tabuso M, Homer-Vanniasinkam S, Adya R, and Arasaradnam RP. Low visceral fat content is associated with poor prognosis in a database of 507 upper gastrointestinal cancers. Ann Surg Oncol. (2015) 22:3946–53. doi: 10.1245/s10434-015-4432-4
54. Harada K, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, Izumi D, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc. (2011) 25:3825–30. doi: 10.1007/s00464-011-1798-7
55. Yoshikawa K, Shimada M, Kurita N, Iwata T, Nishioka M, Morimoto S, et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. (2000) 59:18–23. doi: 10.1159/000012131
56. Go JE, Kim MC, Kim KH, Oh JY, and Kim YM. Effect of visceral fat area on outcomes of laparoscopyassisted distal gastrectomy for gastric cancer: subgroup analysis by gender and parameters of obesity. Ann Surg Treat Res. (2015) 88:318–24. doi: 10.4174/astr.2015.88.6.318
57. Go JE, Kim MC, Kim KH, Oh JY, and Kim YM. Severe loss of visceral fat and skeletal muscle after chemotherapy predicts poor prognosis in metastatic gastric cancer patients without gastrectomy. J Cancer. (2020) 11:3310–7. doi: 10.7150/jca.37270
58. Feng W, Huang M, Zhao X, Chen S, Wang C, Chang J, et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: single-center study from the CLASSIC trial. Ann Surg Oncol. (2018) 25:3222–30. doi: 10.1245/s10434-018-6624-1
Keywords: gastric cancer, visceral fat area, overall survival, disease-free survival, meta -analysis
Citation: Liu L, Hou S, Yan B, Hao R, Li L and Song D (2025) Visceral fat area as a prognostic biomarker in gastric cancer: a systematic review and meta-analysis of survival outcomes. Front. Oncol. 15:1532200. doi: 10.3389/fonc.2025.1532200
Received: 22 November 2024; Accepted: 22 July 2025;
Published: 08 September 2025.
Edited by:
Javad Sharifi-Rad, Espiritu Santo University, EcuadorReviewed by:
Denis Fedorinov, Russian Medical Academy of Postgraduate Education, RussiaDina Keumala Sari, Universitas Sumatera Utara, Indonesia
Copyright © 2025 Liu, Hou, Yan, Hao, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Song, c29uZ2RhbmRhbjA4QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Lei Liu1†
Lei Liu1† Shufu Hou
Shufu Hou Ruiqi Hao
Ruiqi Hao Linchuan Li
Linchuan Li Dandan Song
Dandan Song