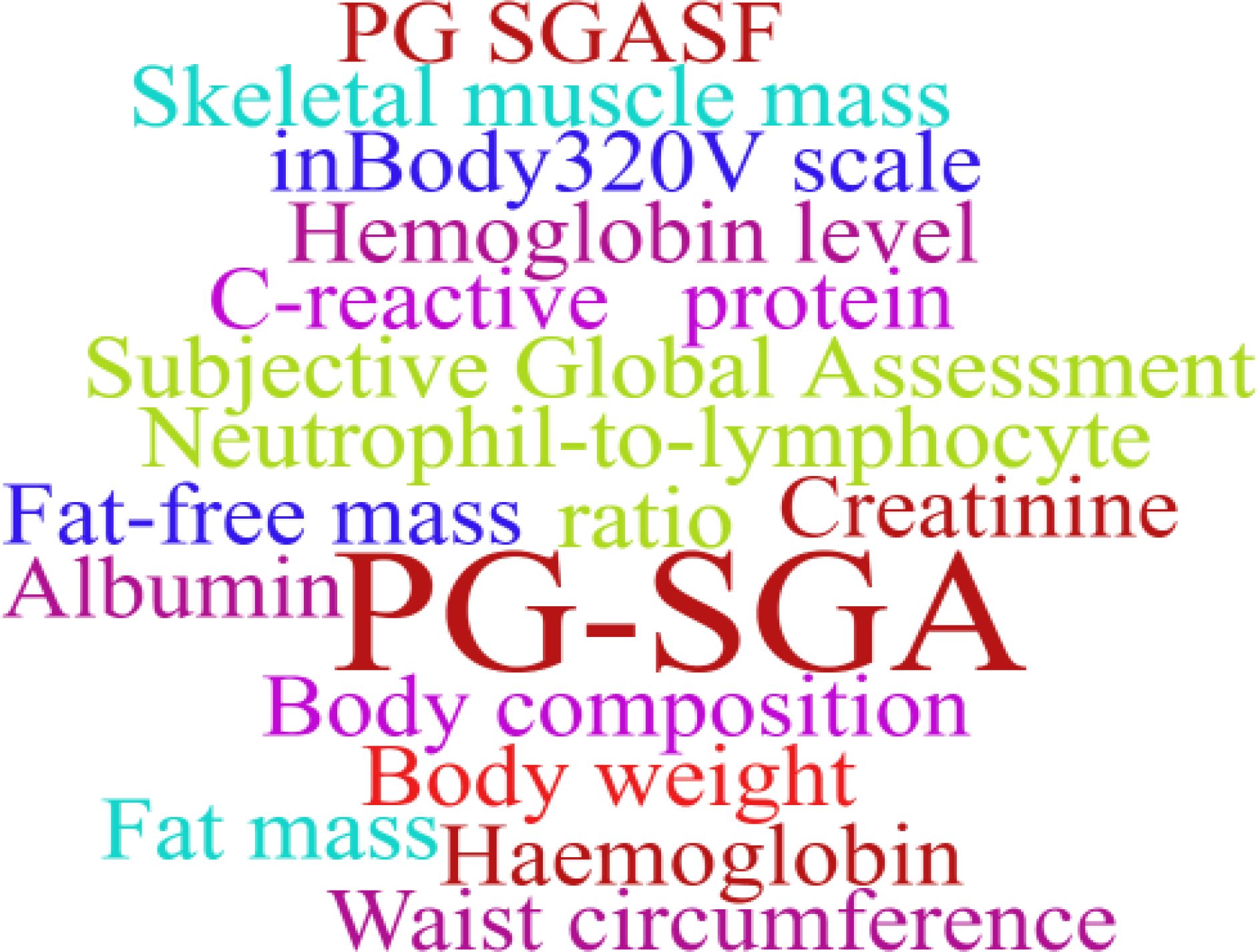- Colorectal Cancer Center, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, Sichuan, China
Objectives: Multimodal prehabilitation has been widely used in patients undergoing surgery for colorectal cancer and has improved clinical outcomes. The aim of this scoping review is to review the content and current state of clinical practice of multimodal prehabilitation programs.
Methods: A systematic literature review of multimodal prehabilitation studies in patients undergoing colorectal cancer surgery was conducted according to the PRISMA extension framework for scoping reviews. The literature was searched via the PubMed, Web of Science, SCOPUS, EMBASE and Cochrane Library databases. The results of the study included the components of multimodal prehabilitation (exercise, nutritional, and psychological interventions) and related evaluation indicators, duration, and compliance-related components.
Results: This review included 12 studies with 9 randomized controlled trials, 1 pilot intervention study, 1 cohort study, and 1 mock-target trial design. Specific protocols for multimodal rehabilitation training vary widely, ranging in duration from 2–8 weeks, and were implemented in healthcare facilities, the community, and at home. Adherence rates ranged from 50% to almost 100%. Common outcome indicators include the 6-minute walk test, comorbidities, length of hospitalization, health-related quality of life, and several anxiety assessment scales.
Conclusion: Current evidence suggests that multimodal preconditioning has a positive effect on the clinical prognosis of patients undergoing elective colorectal cancer surgery. However, owing to the heterogeneity of multimodal rehabilitation in terms of implementation protocols and evaluation metrics, many high-quality studies are still needed to explore the optimal model of multimodal rehabilitation and promote its standardization.
1 Introduction
Colorectal cancer (CRC) is the third most common cancer in the world and the second leading cause of cancer death globally, and is an important component of morbidity and mortality in the global population (1). The International Agency for Research on Cancer (IARC) reported (2) that in 2020 there were 1,932,000 new cases of CRC diagnosed globally, of which 930,000 will die. Unfortunately, 3.2 million new cases and 1.6 million deaths from CRC are expected annually by 2040, with the incidence increasing in younger people. Despite the fact that countries are actively promoting surgery-based comprehensive treatment modalities and have achieved some success, the morbidity and mortality rates of CRC remain high globally (3). The prevention and treatment experiences of some developed countries indicate that current CRC prevention strategies rely mainly on early screening and diagnosis, improvements in lifestyle and the environment, and polypectomy and aspirin administration, whereas CRC treatment modalities include surgery, chemotherapy, radiotherapy and immunotherapy (4). However, colorectal cancer surgical patients usually suffer from preoperative malnutrition, wasting and decreased fitness, and even cachexia and sarcopenia, all of which have been shown to be associated with poor clinical outcomes (5). In addition, surgical trauma triggers a series of metabolic changes in the body, including hormonal, hematologic, metabolic, and immunologic changes, a process known as surgical stress. The aim of prehabilitation is to optimize the various risk factors present in the patient during the preoperative waiting period and to improve the patient’s preoperative physiological and metabolic reserve to better withstand the trauma of surgery, reduce the incidence of perioperative complications and mortality, and optimize the patient’s clinical outcome (6).
Multimodal prehabilitation has been widely used in cancer patients, with initial preoperative rehabilitation focusing on a single exercise regimen to improve the patient’s functional reserve, followed by the addition of nutritional interventions to correct existing nutritional problems, and to help mitigate the patient’s perioperative trauma-related catabolic damage (7). Finally, psychological interventions were introduced to improve adherence to the treatment regimen while enhancing the patient’s intrinsic drive for exercise and nutritional interventions (8). Thus, a well-established multimodal prehabilitation program consists of three components: exercise, nutritional interventions, and psychological interventions, which can also be added to ameliorate poor lifestyles and anemia, among others. Despite the existence of a large body of data on multimodal prehabilitation programs, significant heterogeneity remains for each part of the intervention program, which has an important influence on further analysis.
Although the understanding of preoperative multimodal prehabilitation for rectal cancer is incomplete, the current growing body of clinical evidence gives us confidence in the future. This review will summarize the impact of multimodal prehabilitation on current clinical practice on the basis of the evidence for its plausibility and speculate on directions for further development.
2 Materials and methods
The PRISMA Extension Framework for Scoping Reviews (PRISMA-ScR) checklist was adopted in our study (9).
2.1 Search strategy
A comprehensive literature search of the PubMed, Web of Science, SCOPUS, EMBASE and Cochrane Library databases was conducted by two authors for studies published from the inception to August 18, 2024. The systematic search in Medical Subject Headings (MeSH) was conducted on the basis of the identified research questions to ensure the accuracy of the final search. The chosen key terms are: “Colorectal cancer”, and “Prehabilition”. The search strategy was customized basis of the characteristics of each database (Supplementary Appendix 1 shows an example of the search strategy for the PubMed database). All findings were imported into EndNote (version X9.2) and duplicates were removed.
2.2 Eligibility criteria
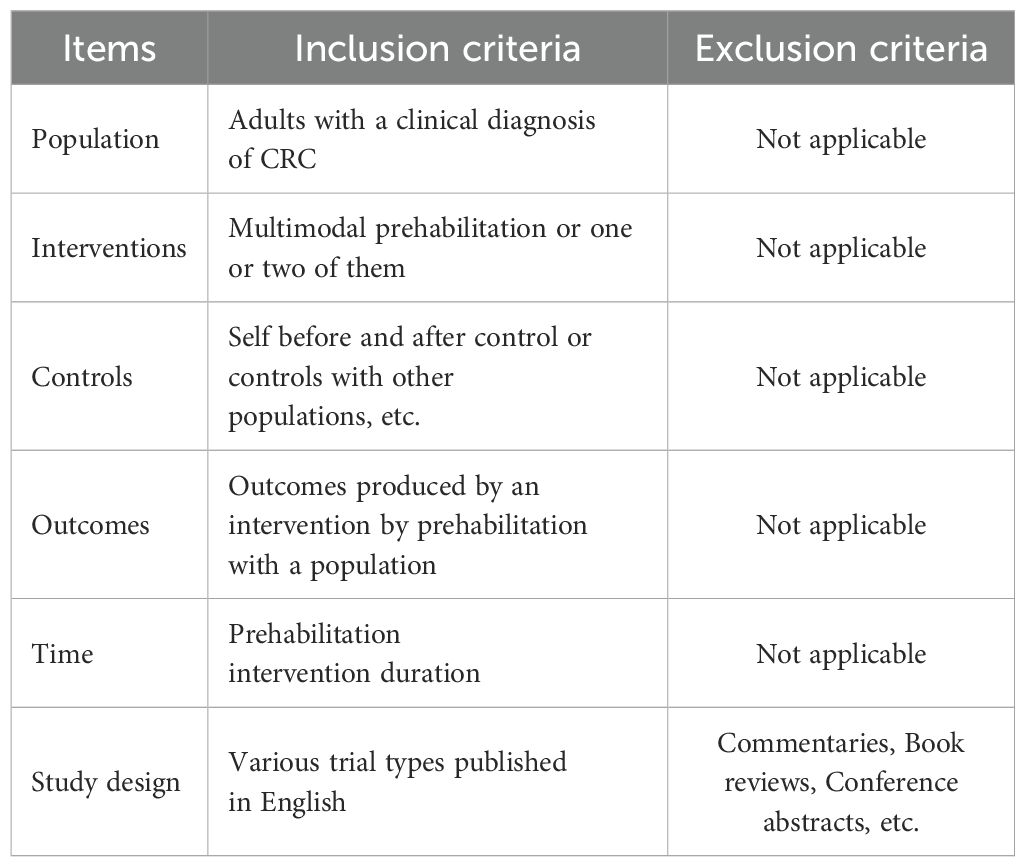
The PICOTS model (10) is widely used in evidence-based healthcare as a guideline for developing search strategies and characterizing clinical studies. The following inclusion and exclusion criteria were developed for our study on the basis of the PICOTS model (Table 1).
Two authors performed an initial analysis of the study to minimize bias by screening the title and abstract independently, and a third author was responsible for resolving any discrepancies.
2.3 Data screening and extraction
Following the completion of the selection process, two authors extracted the characteristics of the studies including author, year, and country of origin, study design, participants, sample size, prehabilitation protocol and outcome measures, via a predesigned standardized data extraction form. In addition, one of the authors extracted outcome measures from the results and counted their frequency.
3 Results
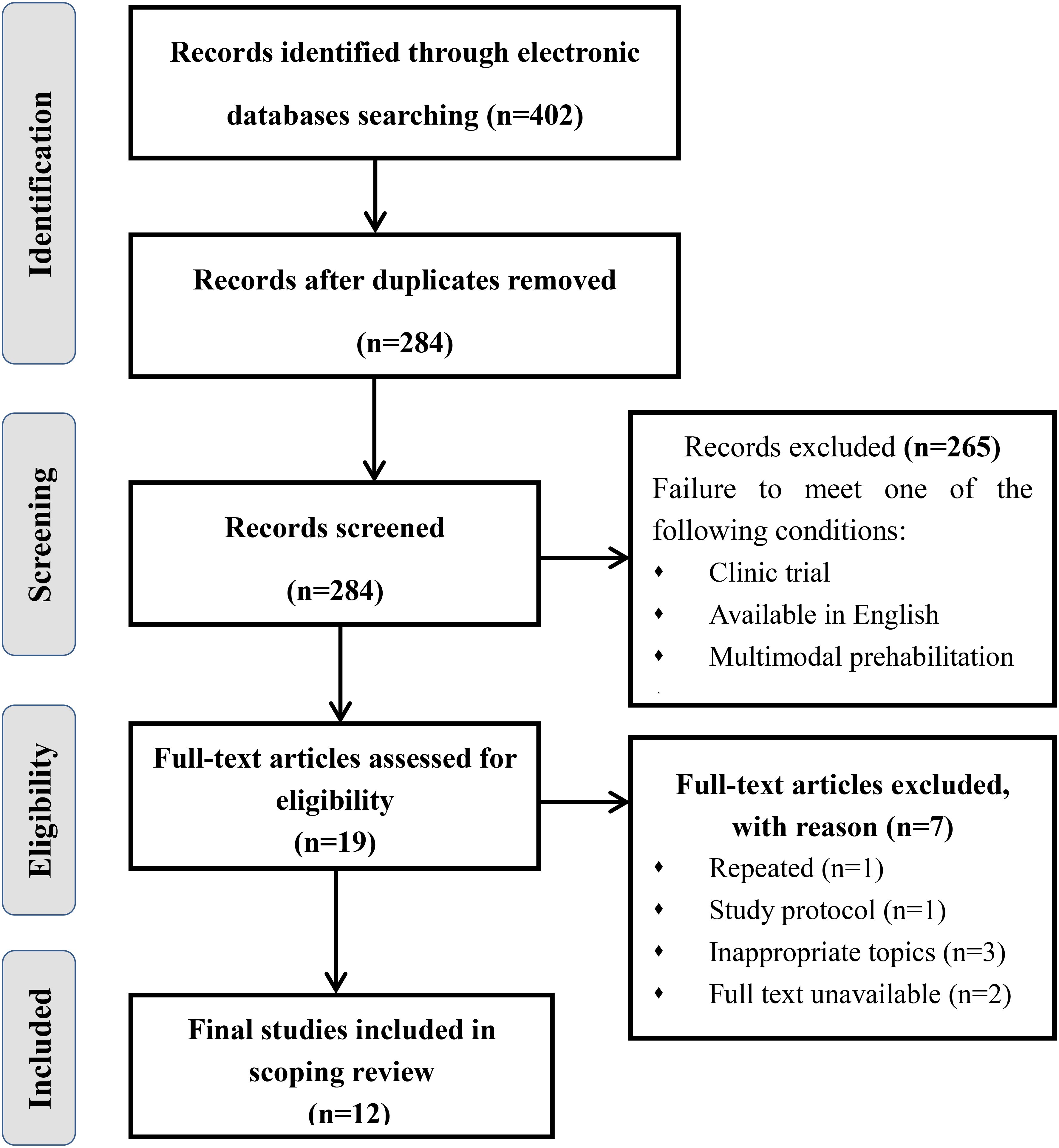
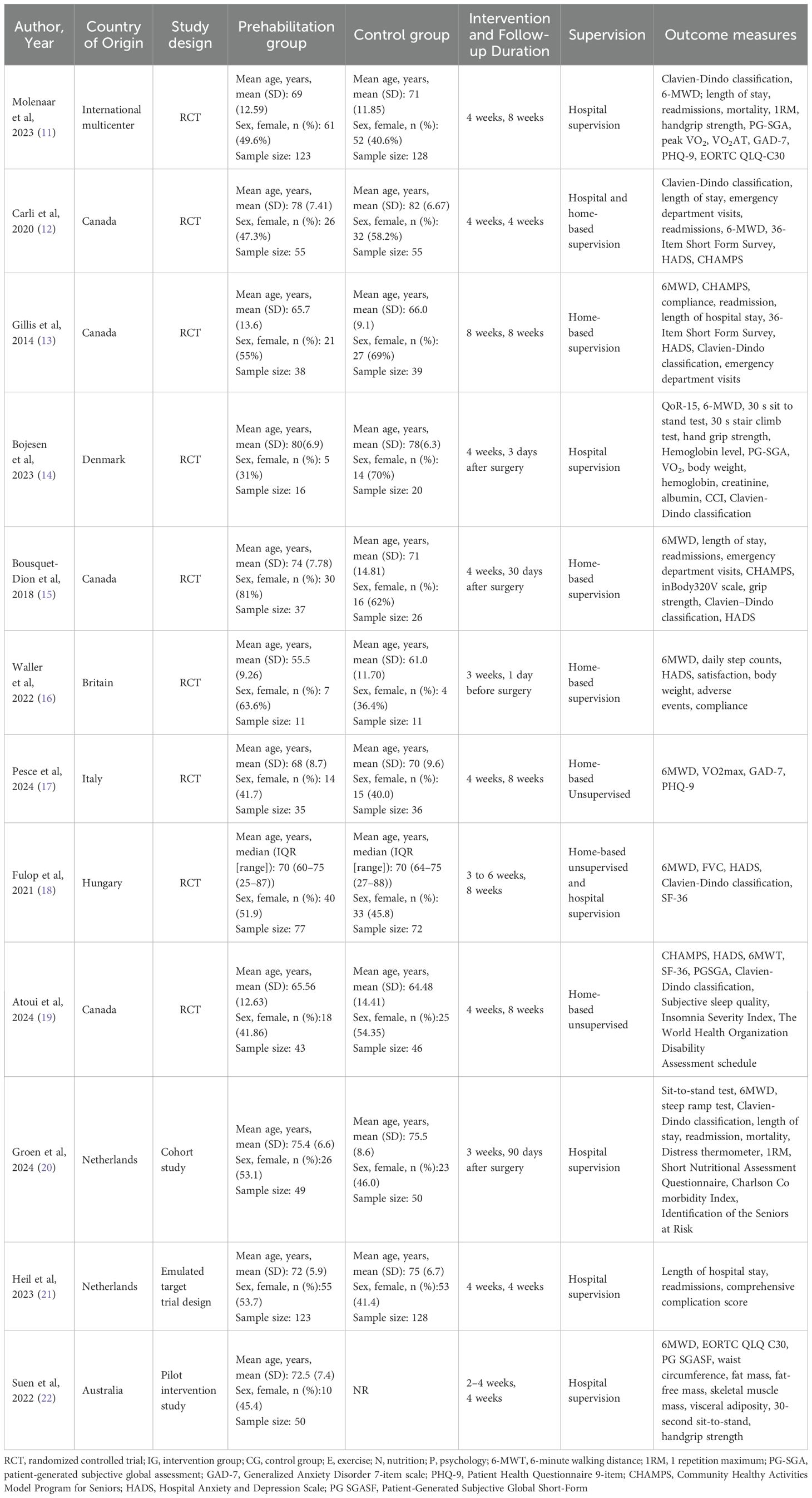
The initial search yielded a total of 402 documents. After removing duplicates, 284 papers remained. Of which 265 papers were excluded because their abstracts did not satisfy the inclusion criteria of “clinic trial”, “available in English”, and “multimodal prehabilitation”. After that, 19 articles were subjected to full-text review because the abstracts could not be ascertained to be eligible. Subsequently, 9 articles were omitted for the following reasons: repeated, study protocol, full text unavailable and inappropriate topics. Ultimately, a collection of 10 studies was included in this review. Figure 1 displays the literature review process. All of them were published between 2017 and 2024. Most of the studies were originally from Canada (n = 4), Denmark (n = 1), the Netherlands (n = 2), Australia (n =1) or International multicenter (n=2). In addition, there are seven RCTs, a pilot intervention study, a cohort study and an emulated target trial design. Table 2 shows the characteristics of the studies included in this review.
3.1 Multimodal prehabilitation program
3.1.1 Physical exercise intervention
The exercise interventions included in the study mainly included aerobic training, resistance training, breathing, balance and flexibility training. Intervention programs usually follow the “FITT” principle, which includes exercise frequency, exercise intensity, exercise duration, exercise type, and precautions (23). Common exercises include running, cycling, climbing stairs and swimming. When a program is implemented, patients are generally asked to mix aerobic exercise, anaerobic exercise and resistance exercise to allocate time reasonably (24).
3.1.2 Nutritional intervention
This part of the intervention program focuses on instructing patients to take protein and vitamins according to existing guidelines (25).The guidelines recommended that surgical patients consume at least 1.2-2.0 g/kg of protein per day (26). The group of the European Society of Nutrition and Metabolism recommends that healthy adults consume at least 1.0-1.2 g/kg protein per day through a normal diet, and elderly people who are malnourished or at risk of malnutrition due to acute or chronic diseases should consume at least 1.2 - 1.5 g/kg of protein per day (27, 28). Moreover, 20–30 grams of whey protein are supplemented after exercise, and vitamins and minerals are supplemented if necessary to maximize the stimulation of the synthesis of muscle protein networks in healthy individuals (7).
3.1.3 Psychological intervention
The psychological intervention included in the study generally began with the start of exercise and nutrition intervention, which generally uses relevant scales for initial assessment, and then, almost every weekly, psychological performed 1–2 times; each time, patients with psychological risk receive a higher level of intervention (8, 29). Psychological interventions commonly used in studies include teaching relaxation techniques (deep breathing, progressive muscle relaxation, and meditation), guided visualization (patients are asked to imagine the stages before and after surgery to control anxiety, or to imagine being in a safe, comfortable place to reduce stress), problem solving, and guidance on coping strategies.
3.1.4 Others
In the interventions included in the present study, adverse lifestyle habits such as alcohol abuse and smoking were corrected before surgery, as these adverse health behaviors can affect patient prognosis and health-related outcomes (30, 31). Similarly, anemia increases the incidence of perioperative complications and mortality in patients and increases the likelihood of perioperative red blood cell transfusions. Patients with anemia are also targeted for intervention, with oral iron or even intravenous iron therapy if necessary (32). Supplementary Appendix 2 showed the multimodal prehabilitation protocols for colorectal cancer.
3.2 Outcome measures
The use of prehabilitation measures before surgery in patients with CRC brings a range of benefits. We included the indicators in the article for statistics, and used a tool to represent the most frequent keywords in a larger font, and vice versa. As shown in Figures 2–6. The detailed frequency data are shown in Supplementary Appendix 4.
3.3 Duration, supervision, compliance and methods taken to improve compliance
3.3.1 Duration and supervision
As shown in Supplementary Appendix 3, the duration in the studies ranged from 2–8 weeks, mostly 4 weeks. Patients receive prehabilitation at the hospital (11, 14), the community (20) and the home (13, 33), or in conjunction with the hospital and the home (12, 21, 22). The form of supervision can be divided into supervision and non-supervision. The choice depends on the patient and medical resources.
3.3.2 Compliance and methods taken to improve compliance
The compliance outcomes for prehabilitation among CRC patients reported in the included studies varied widely, between 50% and 100%, which may be related to intervention site, observation time, and observation content. Only three studies did not report methods to improve patients’ adherence to prehabilitation programs (11, 14, 21). The main methods used to improve compliance are telephone supervision and having patients report their progress regularly.
4 Discussion
The 10 studies included in this review described multimodal prehabilitation programs for patients undergoing elective CRC surgery, including exercise, nutrition and psychological intervention, which demonstrated that implementation of multimodal prehabilitation, has clinical benefits for CRC patients, such as reducing the incidence of postoperative complications and improving prognosis. However, due to the heterogeneity of multimodal prehabilitation programs in each study, this interferes with further standardized assessment of outcomes (34).
Initial preoperative rehabilitation is dominated by a single preoperative exercise intervention, typically consisting of aerobic, resistance and respiratory training aimed at improving patients’ preoperative functional reserve (35–37). Preoperative aerobic exercise is beneficial in reducing the risk of postoperative complications in patients undergoing major abdominal surgery, and patients with low fitness have a higher rate of postoperative complication morbidity and mortality, with increased cardiorespiratory-related complications being particularly pronounced (38, 39). Currently, the World Health Organization recommendations for the adult population are 150–300 min of moderate-intensity physical activity per week, 75–150 min of vigorous-intensity physical activity, or an equivalent combination of moderate-intensity and vigorous-intensity aerobic exercise (40). Exercise interventions should be presented in the form of an exercise prescription, which usually follows the “FITT” principle, i.e., it should include frequency, intensity, duration, type of exercise and precautions (23). However, the exercise interventions currently implemented do not satisfy the “FITT” principle, and the amount of exercise needs to be standardized, individualized for each patient, and captured and analyzed via lightweight e-health technology to facilitate the achievement of daily goals (41).
The role of nutritional interventions in the multimodal prehabilitation of CRC patients is not only to correct long-standing nutritional problems but also to alleviate the catabolic damage associated with the patient’s perioperative trauma (42). Additionally, adequate protein supplementation in an exercise intervention program is a goal of nutritional intervention, which facilitates muscle strength and improves functional reserve, improving clinical outcomes in patients with CRC (43). Patients who undergo major abdominal surgery are often malnourished because of reduced food intake and metabolic disruption caused by the cancer or tumor itself. Malnutrition, in turn, can lead to weakened physical function, which in turn increases complication rates, and readmission rates, prolongs hospitalization, and surgical recovery time, and decreases quality of life (44). The American Association for Accelerated Rehabilitation and the Perioperative Quality Advancement Consortium recommend that all patients undergoing elective abdominal surgery should be considered for preoperative immunonutritional intervention and that immunonutritional preparations should be administered 5 to 7 days before surgery (25). Nutritional interventions require periodic measurement of outcomes or indicators by the interventionist to assess the appropriateness of the nutritional prescription, so that adjustments can be made if the nutritional prescription does not adequately meet or exceeds the patient’s needs.
Psychological prehabilitation plays an important role in the preoperative multimodal prehabilitation of patients with CRC, alleviating preoperative anxiety while enhancing patients’ intrinsic drive for exercise and nutritional interventions, and improving adherence to regimens (45). Notably, although psychological interventions are recognized in the definition of the multimodal prehabilitation concept, this component is lacking in most current clinical intervention studies (46). Studies that have included psychological interventions in multimodal prehabilitation also suffer from inadequate descriptions, poor reporting of adherence, and substantial heterogeneity in duration and related outcome measures. In addition, with only a few studies risk-stratifying psychology, individualized coping strategies are missing, which contributes to heterogeneity (8).
There is heterogeneity in the evaluation indicators of the effects of multimodal prehabilitation between studies, which affects our further analysis, but also provides us with a direction of thinking. In the functional assessment of the included studies, the 6MWD was one of the most commonly used indicators. This index can simply reflect the basic functional reserve of the patient, the operation is simple, and it is also a method of exercise in the evaluation process. In addition, weekly aerobic and anaerobic exercise is an important part of multimodal prehabilitation, and the assessment of lung function should be increased, which is also the concept of pulmonary rehabilitation and may be associated with postoperative lung infection (47). The PG-SGA is relatively widely used in nutritional assessment, whereas the other indicators are related mainly to blood biochemistry and body composition measurements, however, these indicators have been used in only a few studies (14, 22). Future studies may use them to quantify nutrition-related outcomes, which is especially important for CRC patients.
Patient compliance in multimodal prehabilitation is a concern, especially in the unsupervised setting (48). In general, patients with CRC have 4 to 8 weeks of preoperative time to receive multimodal prehabilitation interventions at healthcare facilities, in the community, or at home. Prehabilitation is usually implemented under the supervision of healthcare professionals, and face-to-face and supervised forms of prehabilitation are considered the gold standard for implementing prehabilitation (49). Home-based prehabilitation is considered to have better implementation outcomes and higher levels of participation. The advantages of home-based prehabilitation programs include convenience and low cost, whereas the disadvantages include that compliance may be compromised for more severely ill patients and those who lack in-hospital supervision. The place of implementation depends on the patient’s preference and the availability of health resources, and regardless of the place of implementation of the program, it is necessary to assess patient adherence and provide reinforcement through regular feedback. With the advancement of technology and the widespread use of e-health technology in healthcare, patients can be supervised for home training through the use of tele-rehabilitation modalities such as video conferencing (16). Wearable devices can also be used to enable mutual understanding and feedback between patients and healthcare providers, which is a promising approach to improving adherence (50).
5 Conclusion
This review demonstrates the current evidence of multimodal prehabilitation in patients undergoing colorectal cancer surgery, showing a positive impact and beneficial trend on patient clinical outcomes. However, owing to the heterogeneity of multimodal prehabilitation in terms of specific content, duration, and evaluation metrics, many high-quality trials by researchers are still needed to explore the optimal model of multimodal prehabilitation and promote its standardization.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
SG: Methodology, Writing – original draft. YH: Writing – original draft. LJ: Writing – review & editing. JY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1532624/full#supplementary-material
References
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
2. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from globocan. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (Concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, europe, and northern america. Cancer Lett. (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
5. Bojesen RD, Grube C, Buzquurz F, Miedzianogora REG, Eriksen JR, Gögenur I, et al. Effect of modifying high-risk factors and prehabilitation on the outcomes of colorectal cancer surgery: controlled before and after study. Bjs Open. (2022) 6. doi: 10.1093/bjsopen/zrac029
6. Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol. (2018) 44:919–26. doi: 10.1016/j.ejso.2018.04.016
7. Smeuninx B, Elhassan YS, Sapey E, Rushton AB, Morgan PT, Korzepa M, et al. A single bout of prior resistance exercise attenuates muscle atrophy and declines in myofibrillar protein synthesis during bed-rest in older men. J Physiol. (2023) 603(1):87–105. doi: 10.1113/JP285130
8. Hirst N, Mcbride K, Steffens D. Psychological interventions in prehabilitation randomized controlled trials for patients undergoing cancer surgery: sufficient or suboptimal? Ann Surg Oncol. (2024) 31:2183–6. doi: 10.1245/s10434-023-14853-x
9. Tricco AC, Lillie E, Zarin W, KK, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (Prisma-scr): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
10. Samson D, Schoelles KM. Chapter 2: medical tests guidance (2) developing the topic and structuring systematic reviews of medical tests: utility of picots, analytic frameworks, decision trees, and other frameworks. J Gen Intern Med. (2012) 27 Suppl 1:S11–19. doi: 10.1007/s11606-012-2007-7
11. Molenaar CJL, Minnella EM, Coca-Martinez M, Cate Ten DWG, Regis M, Awasthi R, et al. Effect of multimodal prehabilitation on reducing postoperative complications and enhancing functional capacity following colorectal cancer surgery: the prehab randomized clinical trial. JAMA Surg. (2023) 158:572–81. doi: 10.1001/jamasurg.2023.0198
12. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: A randomized clinical trial. JAMA Surg. (2020) 155:233–42. doi: 10.1001/jamasurg.2019.5474
13. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: A randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. (2014) 121:937–47. doi: 10.1097/ALN.0000000000000393
14. Bojesen RD, Dalton SO, Skou ST, Jørgensen LB, Walker LR, Eriksen JR, et al. Preoperative multimodal prehabilitation before elective colorectal cancer surgery in patients with who performance status I or ii: randomized clinical trial. Bjs Open. (2023) 7. doi: 10.1093/bjsopen/zrad134
15. Bousquet-Dion G, Awasthi R, Loiselle S, Minnella EM, Agnihotram RV, Bergdahl A, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncol. (2018) 57:849–59. doi: 10.1080/0284186X.2017.1423180
16. Waller E, Sutton P, Rahman S, Allen J, Saxton J, Aziz O. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: A randomised controlled pilot study (Trial registration: nct04047524). Surg Endosc. (2022) 36:1008–17. doi: 10.1007/s00464-021-08365-6
17. Pesce A, Fabbri N, Colombari S, Uccellatori L, Grazzi G, Lordi R, et al. A randomized controlled clinical trial on multimodal prehabilitation in colorectal cancer patients to improve functional capacity: preliminary results. Surg Endosc. (2024) 38(12):7440–50. doi: 10.1007/s00464-024-11198-8
18. Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: A randomised clinical trial. Anaesthesia. (2021) 76:82–90. doi: 10.1111/anae.15215
19. Atoui S, Carli F, Bernard P, Lee L, Stein B, Charlebois P, et al. Does A multimodal prehabilitation program improve sleep quality and duration in patients undergoing colorectal resection for cancer? Pilot randomized control trial. J Behav Med. (2024) 47:43–61. doi: 10.1007/s10865-023-00437-3
20. Groen LC, Van Gestel T, Daams F, van den Heuvel B, Taveirne A, Bruns ER, et al. Community-based prehabilitation in older patients and high-risk patients undergoing colorectal cancer surgery. Eur J Surg Oncol. (2024) 50:107293. doi: 10.1016/j.ejso.2023.107293
21. Heil TC, Verdaasdonk EGG, Maas H, van Munster BC, Rikkert M, de Wilt JHW, et al. Improved postoperative outcomes after prehabilitation for colorectal cancer surgery in older patients: an emulated target trial. Ann Surg Oncol. (2023) 30:244–54. doi: 10.1245/s10434-022-12623-9
22. Suen M, Liew A, Turner JD, Khatri S, Lin Y, Raso KL, et al. Short-term multimodal prehabilitation improves functional capacity for colorectal cancer patients prior to surgery. Asia Pac J Clin Oncol. (2022) 18:E103–10. doi: 10.1111/ajco.13564
23. Scheede-Bergdahl C, Minnella EM, Carli F. Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia. (2019) 74 Suppl 1:20–6. doi: 10.1111/anae.14505
24. Franssen RFW, Janssen-Heijnen MLG, Barberan-Garcia A, Vogelaar FJ, van Meeteren NLU, Bongers BC. Moderate-intensity exercise training or high-intensity interval training to improve aerobic fitness during exercise prehabilitation in patients planned for elective abdominal cancer surgery? Eur J Surg Oncol. (2022) 48:3–13. doi: 10.1016/j.ejso.2021.08.026
25. Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia. (2019) 74 Suppl 1:27–35. doi: 10.1111/anae.2019.74.issue-S1
26. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. Espen guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
27. Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within A surgical enhanced recovery pathway. . Anesth Analg. (2018) 126:1883–95. doi: 10.1213/ANE.0000000000002743
28. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the espen expert group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
29. Fritz BA, Holzer KJ. Identifying the blue patient: preoperative screening for depression. Br J Anaesth. (2024) 133:7–10. doi: 10.1016/j.bja.2024.04.012
30. Yoshida N, Eto K, Horinouchi T, Harada K, Sawayama H, Ogawa K, et al. Preoperative smoking cessation and prognosis after curative esophagectomy for esophageal cancer: A cross-sectional study. Ann Surg Oncol. (2022) 29:8172–80. doi: 10.1245/s10434-022-12433-z
31. Eliasen M, Grønkjær M, Skov-Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS, et al. Preoperative alcohol consumption and postoperative complications: A systematic review and meta-analysis. Ann Surg. (2013) 258:930–42. doi: 10.1097/SLA.0b013e3182988d59
32. Mosieri C, Chandler D, Reed DS, Craig MK, Hyatali F, Kallurkar A, et al. Managing preoperative anemia: evolving concepts and strategies for improving patient outcomes. Best Pract Res Clin Anaesthesiol. (2020) 34:183–97. doi: 10.1016/j.bpa.2020.04.005
33. Van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. (2019) 19:98. doi: 10.1186/s12885-018-5232-6
34. Steffens D, Nott F, Koh C, Jiang W, Hirst N, Cole R, et al. Effectiveness of prehabilitation modalities on postoperative outcomes following colorectal cancer surgery: A systematic review of randomised controlled trials. Ann Surg Oncol. (2024) 31(12):7822–49. doi: 10.1245/s10434-024-15593-2
35. West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br J Anaesth. (2015) 114:244–51. doi: 10.1093/bja/aeu318
36. Onerup A, Li Y, Afshari K, Angenete E, De La Croix H, Ehrencrona C, et al. Long-term results of A short-term home-based pre- and postoperative exercise intervention on physical recovery after colorectal cancer surgery (Physsurg-C): A randomized clinical trial. Colorectal Dis. (2024) 26:545–53. doi: 10.1111/codi.16860
37. Van Rooijen SJ, Engelen MA, Scheede-Bergdahl C, Carli F, Roumen RMH, Slooter GD, et al. Systematic review of exercise training in colorectal cancer patients during treatment. Scand J Med Sci Sports. (2018) 28:360–70. doi: 10.1111/sms.2018.28.issue-2
38. Berkel AEM, Bongers BC, Kotte H, Weltevreden P, De Jongh FHC, Eijsvogel MMM, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of A randomized clinical trial. Ann Surg. (2022) 275:E299–306. doi: 10.1097/SLA.0000000000004702
39. Peng LH, Wang WJ, Chen J, Jin JY, Min S, Qin PP. Implementation of the pre-operative rehabilitation recovery protocol and its effect on the quality of recovery after colorectal surgeries. Chin Med J (Engl). (2021) 134:2865–73. doi: 10.1097/CM9.0000000000001709
40. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
41. Steffens D, Denehy L, Solomon M, Koh C, Ansari N, McBride K, et al. Consumer perspectives on the adoption of A prehabilitation multimodal online program for patients undergoing cancer surgery. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15205039
42. Gillis C, Loiselle SE, Fiore JF Jr., Awasthi R, Wykes L, Liberman AS, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: A pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. (2016) 116:802–12. doi: 10.1016/j.jand.2015.06.007
43. Van Exter SH, Drager LD, Van Asseldonk M, Strijker D, Van der Schoot ND, Van den Heuvel B, et al. Adherence to and efficacy of the nutritional intervention in multimodal prehabilitation in colorectal and esophageal cancer patients. Nutrients. (2023) 15. doi: 10.3390/nu15092133
44. Looijaard S, Slee-Valentijn MS, Otten RHJ, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: A systematic review. J Geriatr Phys Ther. (2018) 41:236–44. doi: 10.1519/JPT.0000000000000125
45. Manou-Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: A narrative review. Br J Anaesth. (2019) 123:570–83. doi: 10.1016/j.bja.2019.08.011
46. Raichurkar P, Koh C, Steffens D. Aso author reflections: future directions in prehabilitation research. Ann Surg Oncol. (2023) 30:8755–6. doi: 10.1245/s10434-023-14304-7
47. Kahn PA, Mathis WS. Accessibility of pulmonary rehabilitation in the us. JAMA Netw Open. (2024) 7:E2354867. doi: 10.1001/jamanetworkopen.2023.54867
48. Bruns ER, Van Den Heuvel B, Buskens CJ, Van Duijvendijk P, Festen S, Wassenaar EB, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: A systematic review. Colorectal Dis. (2016) 18:O267–277. doi: 10.1111/codi.2016.18.issue-8
49. Coderre D, Brahmbhatt P, Hunter TL, Baima J. Cancer prehabilitation in practice: the current evidence. Curr Oncol Rep. (2022) 24:1569–77. doi: 10.1007/s11912-022-01304-1
Keywords: multimodal prehabilitation, preoperative, colorectal cancer, review, surgy
Citation: Gao S, He Y, Jiang L and Yang J (2025) Multimodal prehabilitation program for patients undergoing elective surgery for colorectal cancer: a scoping review. Front. Oncol. 15:1532624. doi: 10.3389/fonc.2025.1532624
Received: 26 November 2024; Accepted: 07 April 2025;
Published: 30 April 2025.
Edited by:
Pasquale Cianci, Azienda Sanitaria Localedella Provincia di Barletta Andri Trani (ASL BT), ItalyReviewed by:
Filippo Banchini, Guglielmo da Saliceto Hospital, ItalyVinicio Rizza, Azienda Usl Teramo, Italy
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, Italy
Copyright © 2025 Gao, He, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yang, bXlqYW1pZUAxMjYuY29t
 Shilin Gao
Shilin Gao Yuhua He
Yuhua He