- 1First Division of Hepatobiliary and Pancreatic Surgery, Affiliated Hospital of North China University of Science and Technology, Tangshan, Hebei, China
- 2Graduate School, North China University of Science and Technology, Tangshan, Hebei, China
- 3Hepatobiliary Surgery, Affiliated Kailuan General Hospital of North China University of Science and Technology, Tangshan, Hebei, China
- 4Health Care Center, Affiliated Kailuan General Hospital of North China University of Science and Technology, Tangshan, Hebei, China
Background: Using the Kailuan Cohort, we investigated the association between cumulative exposure to the Chinese visceral adiposity index (CVAI) and the risk of developing gastrointestinal (GI) cancers.
Methods: A prospective cohort study was conducted among participants who underwent three consecutive health examinations in the Kailuan Cohort from 2006 to 2010. Participants were categorized into quartiles based on their cumulative CVAI (cumCVAI). The cumulative incidence of GI cancers was estimated using Kaplan-Meier curves. The dose-response relationship between CVAI and the risk of developing GI cancers was examined using restricted cubic spline (RCS) in multivariable adjusted models. Multivariate Cox proportional hazards regression analysis was performed to assess the association between cumCVAI quartiles and the incidence of GI cancers. Furthermore, subgroup analyses and sensitivity analyses were conducted.
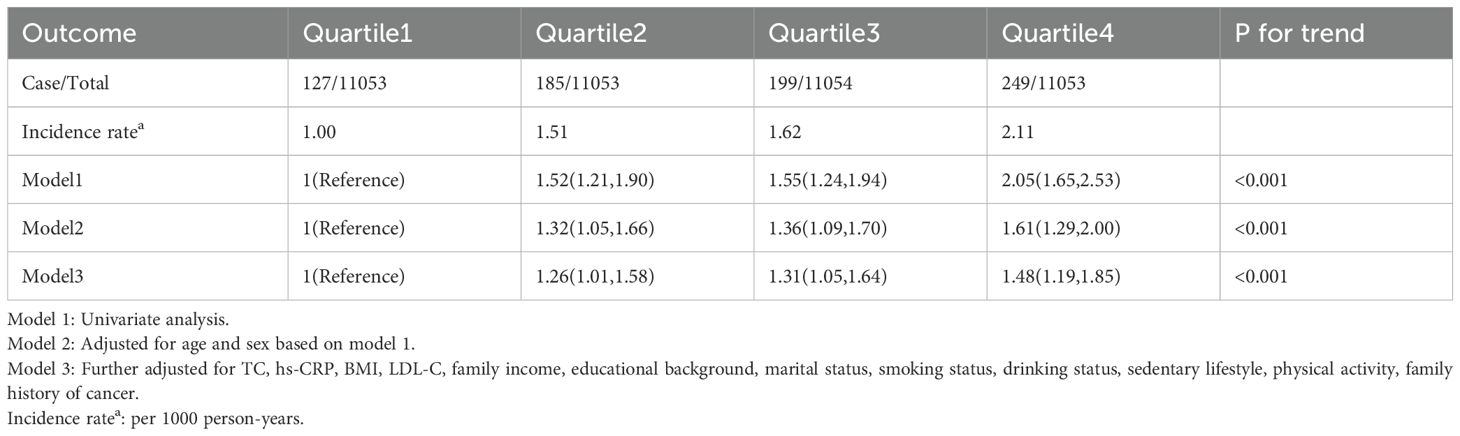
Results: A total of 44,213 individuals were included in this study. The incidence rates of GI cancers per 1,000 person-years for the Q1 to Q4 groups were 1.00,1.45,1.62 and 2.11, respectively. The RCS curve demonstrated a significant dose-response relationship between cumCVAI and the occurrence of GI cancers events (P for overall trend < 0.001; P for nonlinear trend < 0.001). After adjusting for potential confounders, compared with the Q1 group, the risk of GI cancers was significantly elevated in the Q2 group (HR 1.26; 95% CI 1.01, 1.58), Q3 group (HR 1.31; 95% CI 1.05, 1.64), and Q4 group (HR 1.48; 95% CI 1.19, 1.85). This association was particularly evident in older individuals, females, those with a BMI ≥ 24 kg/m2, non-smokers, and non-drinkers.
Conclusion: Our findings demonstrate a significant association between high cumCVAI and an increased incidence of GI cancers. Prolonged maintenance of CVAI within optimal levels may serve as a potential preventive strategy for GI cancers.
1 Introduction
Gastrointestinal (GI) cancers account for approximately one-quarter of global cancer incidence and one-third of global cancer-related deaths (1). Despite continuous improvements in screening and treatment for GI cancers in recent years, their incidence and mortality rates remain high, and patients often have poor prognoses. As a populous country, China has the highest number of new cases and deaths from GI cancers worldwide (2). Therefore, it is crucial to investigate the risk factors for GI cancers and provide preventive strategies.
Obesity has been established as a risk factor for GI cancers (3),Commonly used surrogate measures of obesity, including body mass index (BMI) (4),waist circumference(WC), waist‐to‐hip ratio (WHpR), waist‐to‐height ratio (WHtR) have been shown to increase cancer risk (5, 6).In recent years, studies have found that visceral obesity is a significant risk factor for cancer development (7),and increases the risk of GI cancers (8, 9).Visceral adipose tissue (VAT), the most direct manifestation of visceral obesity, can more accurately describe fat distribution compared to traditional obesity indicators (10). Clinical assessment of VAT primarily relies on magnetic resonance imaging (MRI) and computed tomography (CT), whose high costs significantly limit their use in epidemiological studies (11). In 2010, a study suggested using the visceral adiposity index (VAI) as a surrogate for VAT, and it showed high concordance with MRI and CT measurements (12). However, the VAI was developed for Caucasian populations and may have limitations in assessing visceral fat distribution in Chinese populations. The Chinese visceral adiposity index (CVAI) was proposed based on the metabolic characteristics of the Chinese population and is a good surrogate indicator of visceral fat distribution in Chinese people (13). Multiple studies have demonstrated that CVAI is significantly associated with diabetes and cardiovascular diseases (14, 15), but its association with GI cancers has been rarely reported.
Previous studies have been limited to single time-point measurements of CVAI, neglecting the longitudinal impact of cumCVAI over time. In this study, using the Kailuan Cohort, we prospectively investigated the association between cumulative exposure to CVAI and the risk of developing GI cancers.
2 Methods
2.1 Study population
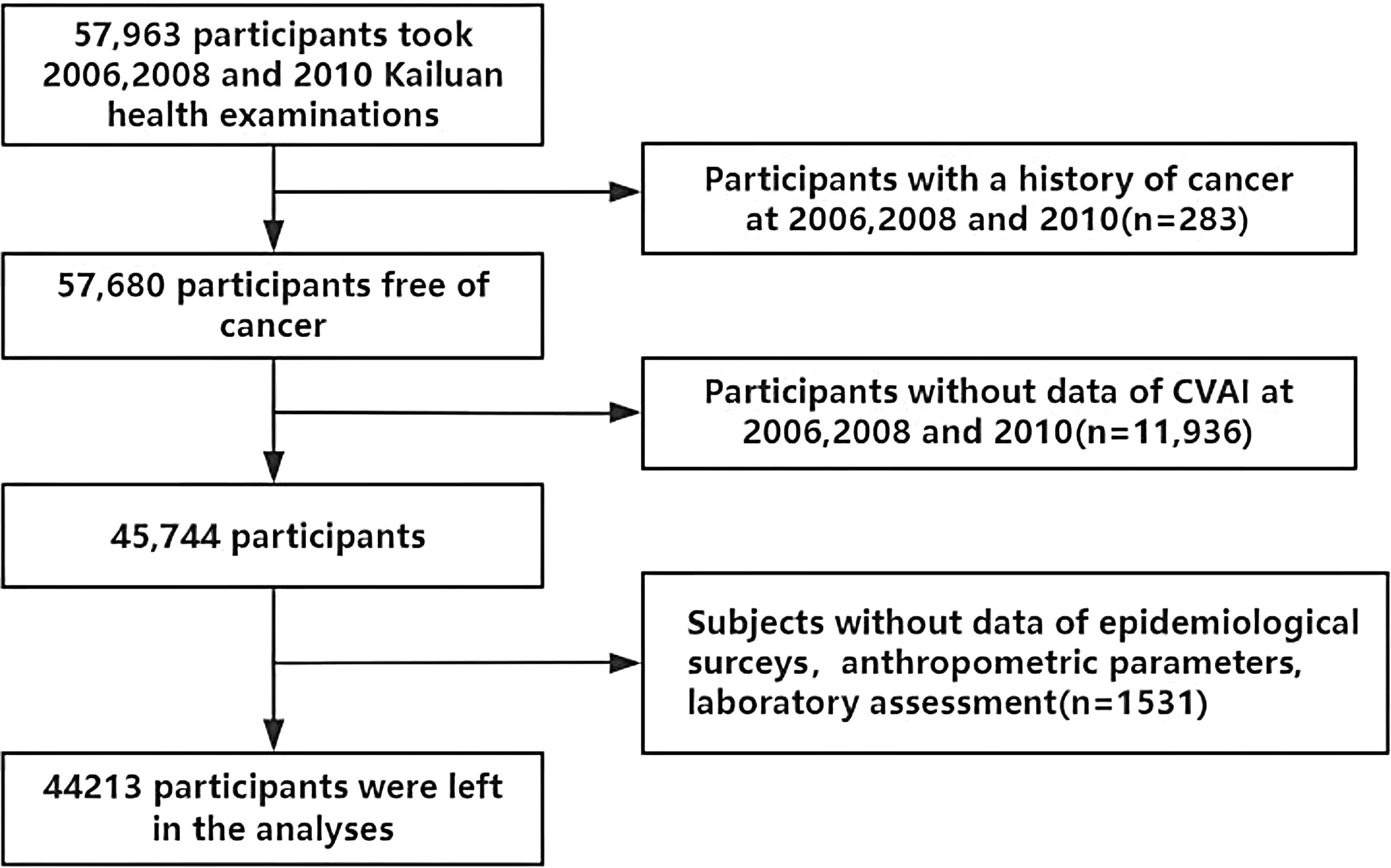
Our study population was derived from the Kailuan Cohort (registration number:ChiCTR-TNC-11001489; https://www.chictr.org.cn/showprojEN.html?proj=48316), which consists of employees (including retirees) of the Kailuan Group. Detailed information about the Kailuan Cohort has been previously reported (16). In summary, the Kailuan Cohort was established in 2006 by recruiting 101,510 employees (aged 18–98 years) from the Kailuan Group. Participants underwent a series of physical examinations, biochemical tests, and questionnaires at 11 affiliated hospitals of the Kailuan Group. Subsequently, these assessments were repeated every two years, resulting in eight waves of data collection to date. Participants were excluded if they met any of the following criteria: (1) did not complete three consecutive health examinations between 2006 and 2010; (2) had missing data for any of the CVAI-related variables, including age, BMI, WC, triglyceride (TG), and high-density lipoprotein-cholesterol (HDL-C) in the three examinations; (3) had a history of cancer at baseline in 2006 or developed cancer between 2006 and 2010; (4) had missing data for any of the following covariates: age, sex, reported income, education level, marital status, smoking status, drinking status, physical activity, family history of malignancy, total cholesterol (TC), high-sensitivity C-reactive protein (hs-CRP), and low-density lipoprotein-cholesterol (LDL-C). Finally, a total of 44,213 participants were included in this study (Figure 1).
2.2 Collection and definitions of covariates
Participants underwent physical examinations conducted by trained medical staff, including measurements of height, weight, and WC. BMI was calculated as weight (kg) divided by height squared (m²). After fasting for at least 8 hours, each participant underwent an ultrasound examination of the liver, gallbladder, and other organs by a trained sonographer. Diagnosis of cirrhosis, fatty liver, gallstones, and gallbladder polyps was made according to pre-established clinical criteria (17, 18).
Blood samples were collected from all participants in the morning after an overnight fast. A total of 5 mL of peripheral venous blood was drawn by trained medical staff and analyzed on a Hitachi 747 automatic analyzer (Hitachi, Tokyo, Japan) to determine serum concentrations of TG, TC, HDL-C, LDL-C, and hs-CRP. Serum TC and TG were measured using enzymatic colorimetric methods, while hs-CRP was measured using a high-sensitivity turbidimetric assay.
Participants completed a standardized questionnaire to collect data on age, gender, reported household income, education level, marital status, smoking status, drinking status, physical activity, and family history of malignancy. Smokers were defined as those who reported smoking at least one cigarette per day, on average, for at least 6 months in the past year. Drinkers were defined as those who reported consuming at least 100 ml of alcohol per day, on average, for at least 6 months in the past year. Physical activity was defined as engaging in at least 30 minutes of exercise per session, at least 3 times per week.
2.3 Assessment of Chinese visceral adiposity index
The CVAI calculation formula is (13):
The cumCVAI calculation formula is (19):[(CVAI2006+CVAI2008)/2×time1-2]+ [(CVAI2008+CVAI2010)/2×time2-3], That is the weighted sum of the mean CVAI obtained at each examination, where CVAI2006, CVAI2008, and CVAI2010 represent the CVAI obtained at the examinations in 2006, 2008, and 2010, respectively, and time1–2 and time2–3 represent the time intervals between consecutive examinations.
2.4 Outcome ascertainment
The follow-up period started from the physical examination in 2010 and ended with the occurrence of new-onset GI cancers. For participants who did not develop GI cancers, the follow-up ended on December 31, 2021. For those who died without developing GI cancers, the follow-up ended at the time of death. According to the International Classification of Diseases, Tenth Revision (ICD-10), this study included the following GI cancers: liver cancer (C22), gallbladder or extrahepatic bile duct cancer (C23 and C24), stomach cancer (C16), pancreatic cancer (C25), small bowel cancer (C17), esophageal cancer (C15), and colorectal cancer (C18-C21). Diagnosis was confirmed through the following methods: (1) conducting a physical examination and questionnaire survey for participants every two years, with a cutoff date of December 31, 2021; (2) annually querying relevant medical records from the municipal healthcare system and the Social Security Information System of Kailuan General Hospital; (3) reviewing death certificates from the provincial vital statistics office to obtain additional missing information. These three methods maximize the collection of all cancer cases and minimize omissions. All cancer cases required a definitive pathological diagnosis for re-confirmation. In the absence of a pathological diagnosis, the cases were further evaluated by two or more specialized oncologists. Only when the physicians reached a consensus on the cancer diagnosis would the case be confirmed and included in the cohort.
2.5 Statistical analysis
Data analysis was performed using SAS 9.4 statistical software, with a two-sided P-value of <0.05 considered statistically significant. Normally distributed continuous data are presented as mean ± standard deviation, and comparisons between groups were conducted using analysis of variance (ANOVA). Skewed continuous data are presented as median and interquartile range, with comparisons between groups performed using the non-parametric Kruskal-Wallis test. Categorical data are expressed as frequencies and percentages, with intergroup comparisons conducted using the chi-square (χ²) test.
The incidence of GI cancers is expressed per 1,000 person-years, and the cumulative incidence of GI cancers was estimated using Kaplan-Meier curves and log-rank tests. A multivariable-adjusted restricted cubic spline (RCS) analysis was conducted to examine the dose-response relationship between CVAI and the risk of GI cancer occurrence. A multivariable Cox proportional hazards regression analysis was conducted to calculate the hazard ratios (HR) and 95% confidence intervals (CI) for the association between cumCVAI quartiles and the incidence of GI cancers. Model 1 represents the univariate analysis; Model 2 adjusts for age and gender based on Model 1; Model 3 further adjusts for TC, hs-CRP, BMI, LDL-C, physical activity, reported income, educational background, marital status, smoking status, drinking status, and family. In multivariate analysis of specific cancer sites, adjustments were made for fatty liver and liver cirrhosis in liver cancer cases, and for gallstones and gallbladder polyps in gallbladder or extrahepatic bile duct cancer cases. Subgroup analyses were conducted stratified by age (<60 years vs. ≥60 years), sex (male vs. female), BMI (<24 kg/m² vs. ≥24 kg/m²), as well as smoking and alcohol consumption status. Finally, several sensitivity analyses were performed to validate the robustness of the study results. We excluded individuals diagnosed with GI cancers within one year of follow-up to eliminate the influence of reverse causation on the findings. Additionally, we excluded participants who were taking statin medications to mitigate the effects of drugs on CVAI levels.
3 Results
3.1 Baseline characteristics of the participants
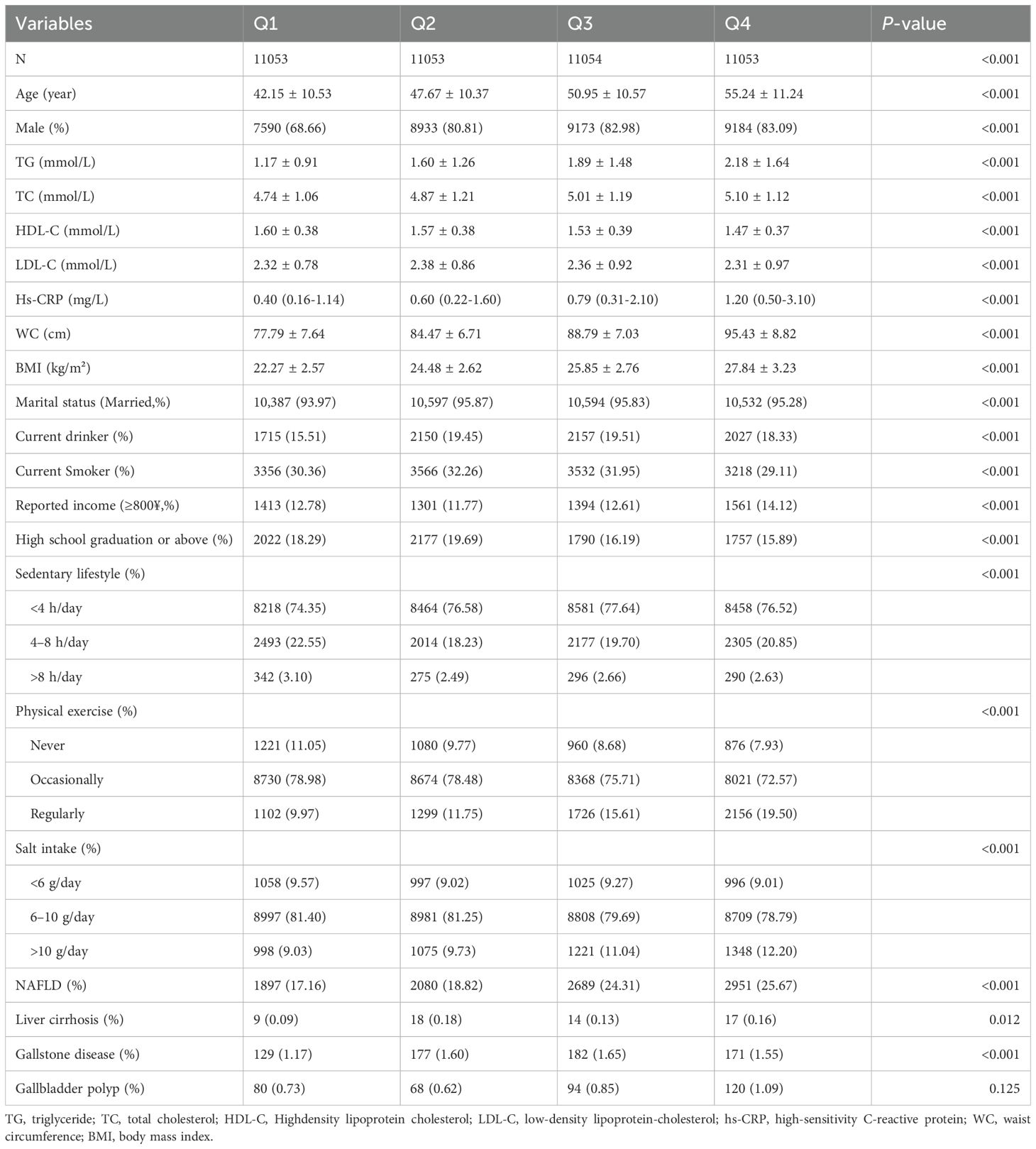
This study included a total of 44,213 participants, comprising 34,880 males and 9,333 females, with a mean age of 49.00 ± 11.70 years. Participants were categorized into four groups based on their cumCVAI quartiles. The baseline characteristics of the four groups are presented in Table 1, which reveals significant differences in age, sex, TG, TC, HDL-C, LDL-C, WC, BMI, marital status, current drinking status, current smoking status, reported income, educational background (high school graduation or above), sedentary lifestyle, physical exercise, salt intake, non-alcoholic fatty liver disease (NAFLD), liver cirrhosis, and gallstone disease (P <0.05). However, no significant differences were observed in the prevalence of gallbladder polyps (P >0.05).
3.2 Association between cumulative CVAI and the risk of GI cancers
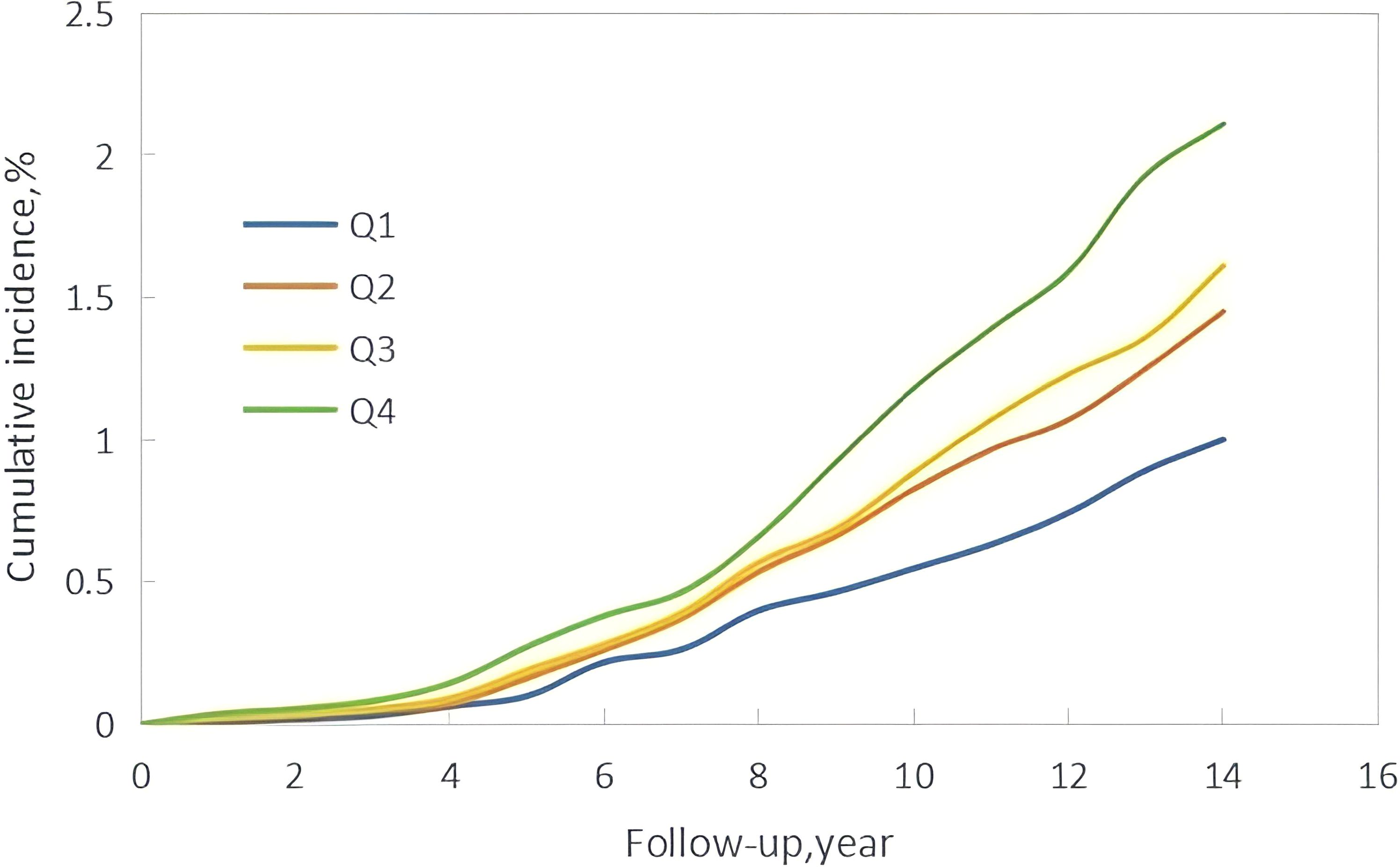
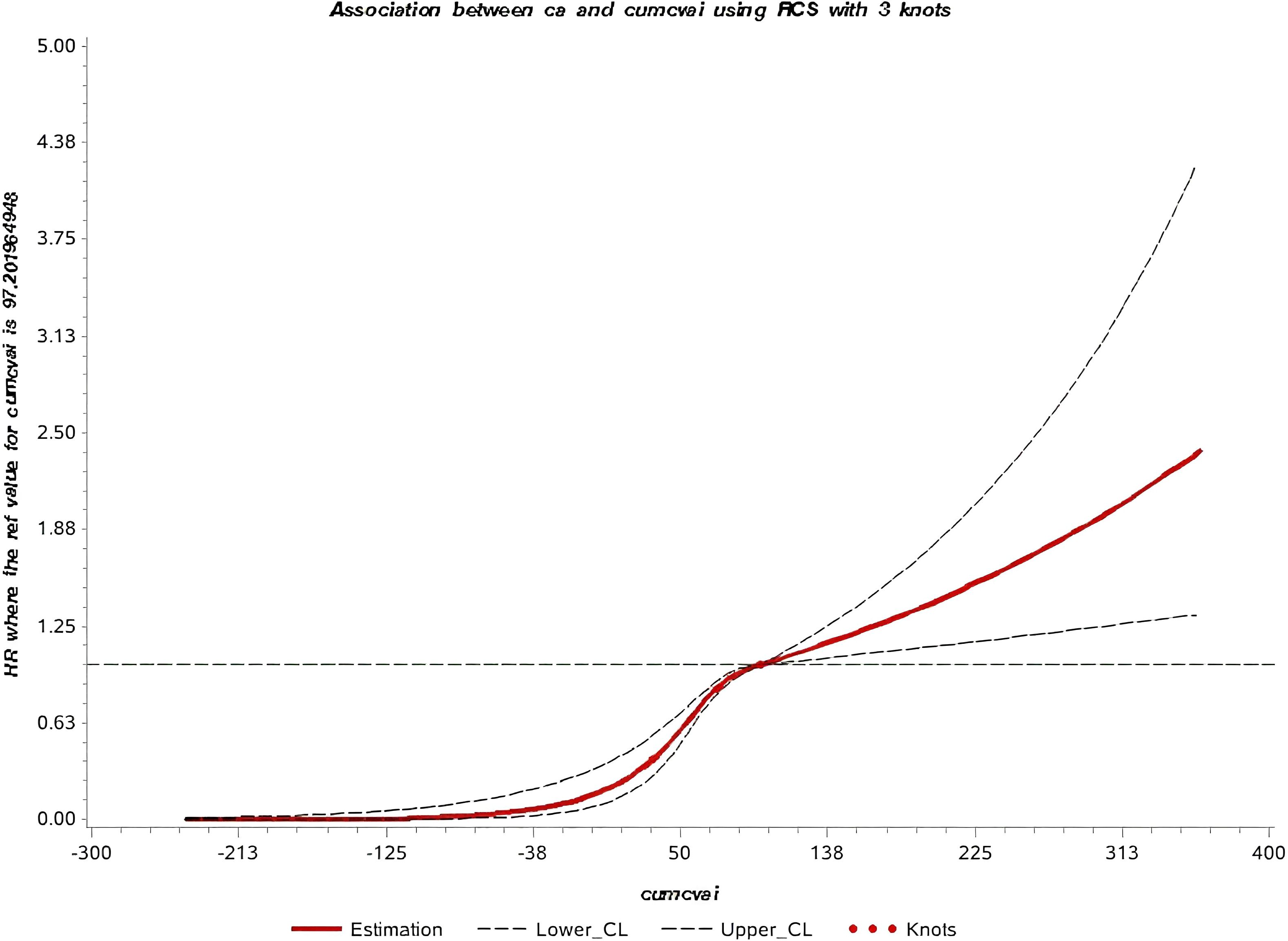
The median follow-up time in this study was 10.57 ± 1.72 years, during which 760 new cases of GI cancers were identified. These included 74 cases of esophageal cancer, 156 of gastric cancer, 20 of small intestine cancer, 281 of colorectal cancer, 158 of liver cancer, 21 of gallbladder or extrahepatic bile duct cancer, and 50 of pancreatic cancer. Kaplan-Meier curves showed a progressive increase in GI cancers incidence from Q1 to Q4, with statistically significant differences in cumulative incidence across the groups (P < 0.001) (Figure 2). Additionally, the restricted cubic spline (RCS) curve indicated a nonlinear relationship between cumCVAI and GI cancers events (P for overall trend < 0.001; P for nonlinear trend < 0.001) (Figure 3).
The multivariable analysis of the association between cumCVAI quartiles and the risk of GI cancers, along with incidence rates, is presented in Table 2. The incidence rates for GI cancers in the Q1 to Q4 groups were 1.00, 1.45, 1.62, and 2.11 per 1,000 person-years. Compared to the Q1 group, the risk of GI cancers was significantly elevated in the Q2 group (HR 1.26; 95% CI 1.01, 1.58), Q3 group (HR 1.31; 95% CI 1.05, 1.64), and Q4 group (HR 1.48; 95% CI 1.19, 1.85) in Model 3. In the multivariable analysis for specific cancer sites, the risk of cancer was significantly elevated in the gastric cancer Q4 group (HR 1.86; 95% CI 1.16, 2.99), colorectal cancer Q2 group (HR 1.29; 95% CI 1.09, 1.66), Q3 group (HR 1.34; 95% CI 1.02, 1.96), and Q4 group (HR 1.44; 95% CI 1.07, 2.13), as well as in the liver cancer Q3 group (HR 1.49; 95% CI 1.01, 2.51) and Q4 group (HR 1.61; 95% CI 1.05, 2.72). However, no significant associations were found for esophageal cancer, small intestine cancer, gallbladder or extrahepatic bile duct cancer, or pancreatic cancer (Supplementary Table S1).
3.3 Association between baseline CVAI and the risk of GI cancers
Based on baseline CVAI quartiles, participants were divided into four groups. The Kaplan-Meier curve indicated that the incidence of GI cancers progressively increased from the Q1 to Q4 groups, with statistically significant differences in cumulative incidence rates between the groups (p < 0.001) (Supplementary Figure S1). After adjusting for confounding factors, the risk of GI cancers was significantly higher in the Q2 group (HR 1.29; 95% CI 1.03, 1.62), Q3 group (HR 1.36; 95% CI 1.09, 1.70), and Q4 group (HR 1.59; 95% CI 1.28, 1.97) compared to the Q1 group (Supplementary Table S2).
3.4 Results of the subgroup and sensitivity analyses
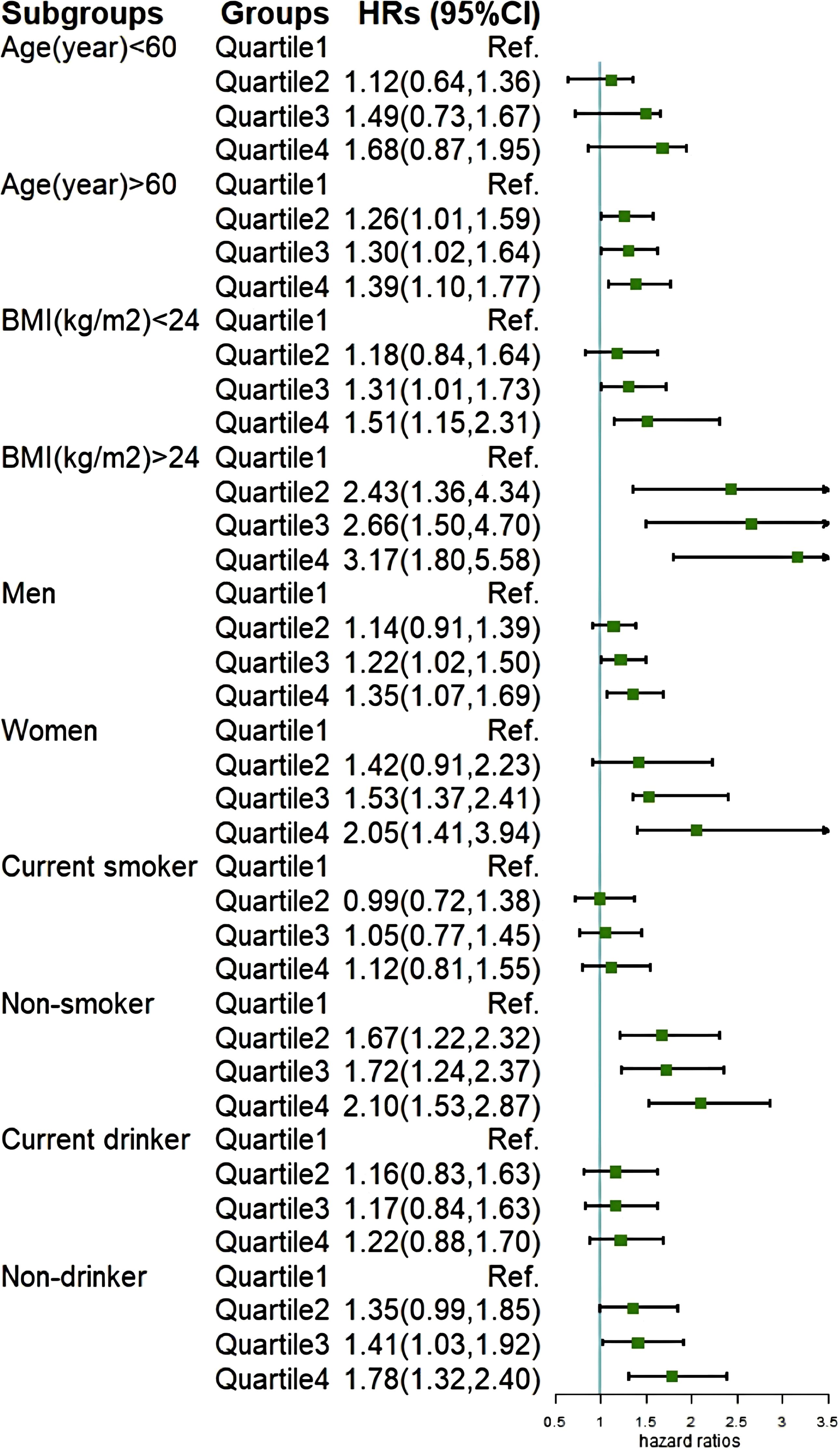
We conducted stratification based on participants’ age, gender, BMI, smoking status, and drinking status (Figure 4), and found no significant interactions with cumCVAI (p > 0.05). Except for younger individuals, current smokers, and current drinkers, all other subgroups demonstrated a significant increase in the risk of GI cancers with higher quartiles of cumCVAI. In sensitivity analyses, we excluded 117 participants who developed GI cancers within one year of follow-up (Supplementary Table S3) and 372 participants taking lipid-lowering medications (Supplementary Table S4). The results showed no significant changes, and the association between cumCVAI and the risk of GI cancers remained significant.
4 Discussion
In this prospective cohort study based on the Kailuan cohort, we found that cumCVAI is a risk factor for new-onset GI cancers, with the risk of GI cancers gradually increasing as cumCVAI quartiles rise. Furthermore, the RCS curve indicates a clear dose-response relationship between cumCVAI and the incidence of GI cancers. In the multivariable analysis of specific cancer sites, high cumCVAI was associated with an increased risk of gastric cancer, colorectal cancer, and liver cancer. Subgroup analyses and sensitivity analyses did not alter the original conclusions, further validating the robustness of the findings in this study.
Globally, the number of obese individuals exceeds 2 billion, with a significant increase in obesity rates across Asia. As early as 2014 (20), China became the country with the highest number of obese individuals in the world, accounting for 14% of the global obese population (21). BMI is the most commonly used surrogate measure for obesity; however, it is more applicable to overall obesity and does not reflect the relationship between fat and lean body mass, nor does it provide insights into fat distribution. Compared to fat in other areas, VAT poses greater harm to the human body (22). Excessive VAT can lead to metabolic disorders and an inflammatory state, promoting the onset and progression of cancer (23). Increasing evidence suggests that VAT is a risk factor for various types of cancer (24).
Due to its ease of acquisition, CVAI serves as a good surrogate marker for VAT in the Chinese population. Previous studies have not reached a definitive conclusion regarding the relationship between CVAI and overall gastrointestinal cancers. Our research indicates that high cumCVAI significantly increases the incidence of gastrointestinal cancers, with VAT being a critical factor in this association. A cohort study from the United States demonstrated that, after adjusting for confounding factors, VAT is a risk factor for cancer, a finding consistent with our results, particularly in the male subgroup. However, no association was observed in the female population (25). A similar study from the United States, which included both White and Black participants, found a positive association between VAT and cancer incidence, with conclusions independent of racial differences (26). The relationship between CVAI and GI cancers can be attributed to several factors. Firstly, adipose tissue releases inflammatory factors, leading to a chronic inflammatory state in the body (27). There is evidence suggesting that VAT exerts a stronger pro-inflammatory effect than subcutaneous adipose tissue (SAT), thereby leading to enhanced metabolic activity in the body (28), the release of a greater number of cytokines leads to DNA damage (29), impacting DNA synthesis and repair (30), which increases the mutation rate and facilitates the transformation of normal cells into cancerous cells. What’s more, obesity is directly associated with insulin resistance, which stimulates compensatory insulin secretion, leading to hyperinsulinemia (31). Insulin activates intracellular signaling pathways, thereby promoting accelerated growth and increased invasiveness of tumor cells (32). A prospective cohort study conducted in China similarly indicated a significant positive correlation between elevated CVAI and the risk of developing diabetes (33), Diabetes is a known risk factor for GI cancers (34, 35), further corroborating the findings of our study.
We found that high cumCVAI is associated with an increased risk of gastric cancer, colorectal cancer, and liver cancer. Previous studies on the impact of visceral fat on colorectal cancer have yielded inconsistent conclusions. A cross-sectional study conducted among East Asian populations indicated that participants with a visceral fat area exceeding 136.6 cm² had a significantly elevated risk of colorectal cancer (OR = 4.07, 95% CI 1.01–16.43, P = 0.03) (36). Similarly, a dose-response meta-analysis indicated that for every 25 cm² increase in VAT, the risk of developing colorectal adenomas increased by 13% (37), Colorectal adenomas are precursors to colorectal cancer (38). However, a case-control study by Elife Erarslan et al. found that visceral fat accumulation does not increase the risk of colorectal cancer (39). Previous studies on the impact of visceral fat on gastric and liver cancers have yielded results that are generally consistent with ours. A case-control study from Japan indicated a positive correlation between visceral fat area and the incidence of gastric cancer, while BMI showed a negative correlation with gastric cancer incidence. Furthermore, when the visceral fat area was ≥100 cm², the risk of developing gastric cancer significantly increased, with an OR (95% CI) of 1.96 (1.02-3.76) (40). A Mendelian randomization study based on the UK Biobank and the Adult Health and Aging Genetic Epidemiology Study cohort found that liver fat and VAT are associated with an increased risk of primary liver cancer, with VAT showing a higher predictive value than traditional obesity measures such as BMI and WC (41). However, we did not find that high cumCVAI increases the risk of esophageal cancer, small intestine cancer, gallbladder and extrahepatic bile duct cancer, or pancreatic cancer, indicating the need for further research to explore this issue.
Subgroup analysis further validated our findings. In the gender-specific analysis, we found that high cumCVAI was more strongly associated with gastrointestinal cancers in females compared to males. Research has indicated that women are more prone to central obesity, and there are differences in visceral fat distribution between genders (42). At the same time, G. Boden et al. found that elevated plasma free fatty acid (FFA) lead to insulin resistance (43), and the clearance rate of FFA in females is 64% higher than that in males (44). Therefore, an increase in CVAI among women can be regarded as a warning sign, prompting timely intervention measures. We also observed that the predictive capability of CVAI is stronger in non-smoking and non-drinking populations. This may be attributed to the fact that both smoking and drinking are recognized carcinogenic factors (45–47), which could potentially obscure the impact of CVAI on gastrointestinal malignancies.
Our study has several strengths: (1) it offers a unique perspective on the risk factors for GI cancers; (2) it is based on the Kailuan cohort, which has a large sample size, relatively long follow-up duration, a wide age range among participants, and an almost zero loss to follow-up rate; (3) it extensively evaluated potential confounding factors, including lifestyle habits and family history of cancer. However, certain limitations should also be noted: (1) information on smoking, drinking, physical activity, and sedentary habits was self-reported by participants, which may introduce recall bias; (2) while endoscopy is the gold standard for diagnosing gastric and colorectal cancers, participants were not subjected to this procedure due to budget constraints within the Kailuan cohort, potentially leading to missed asymptomatic cancer cases; (3) the cohort exhibits an uneven gender distribution, with a higher proportion of males, given the industrial nature of the Kailuan Group; (4) the Kailuan cohort is primarily based on an urban population in northern China, which may not be representative of the entire Chinese population.
5 Conclusions
This study demonstrates a positive correlation between high cumCVAI and the incidence of GI cancers, particularly among participants with older adults, women, BMI ≥ 24, non-smoker and non-drinker. Therefore, monitoring dynamic changes in CVAI may provide a theoretical basis for the prevention of GI cancers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Kailuan General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Ethics Committee of Kailuan General Hospital. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. WWa: Supervision, Writing – original draft, Writing – review & editing. CM: Writing – review & editing. KL: Methodology, Supervision, Writing – review & editing. SC: Investigation, Methodology, Supervision, Writing – review & editing. YT: Investigation, Methodology, Supervision, Writing – review & editing. ZD: Methodology, Supervision, Writing – review & editing. WWe: Methodology, Supervision, Writing – review & editing. SW: Methodology, Resources, Supervision, Visualization, Writing – review & editing. SL: Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank all the staff and participants of the Kailuan Cohort Study for their significant contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1534682/full#supplementary-material
Abbreviations
CVAI, Chinese visceral adiposity index; GI, Gastrointestinal; cumCVAI, Cumulative CVAI; RCS, Restricted cubic spline; BMI, Body mass index; WC, Waist circumference; WHpR, Waist‐to‐hip ratio; WHtR, Waist‐to‐height ratio; VAT, Visceral adipose tissue; CT, Computed tomography; MRI, Magnetic resonance imaging; VAI, Visceral adiposity index; TG, Triglyceride; HDL-C, High-density lipoprotein-cholesterol; TC, Total cholesterol; Hs-CRP, High-sensitivity C-reactive protein; LDL-C, Low-density lipoprotein-cholesterol; ICD-10, International Classification of Diseases, Tenth Revision; HR, Hazard ratios; CI, Confidence intervals; SAT, Subcutaneous adipose tissue; FFA, Free fatty acid.
References
1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. (2020) 159:335–349.e315. doi: 10.1053/j.gastro.2020.02.068
2. Cao W, Chen HD, Yu YW, Li N, and Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
3. Murphy N, Jenab M, and Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. (2018) 15:659–70. doi: 10.1038/s41575-018-0038-1
4. Belladelli F, Montorsi F, and Martini A. Metabolic syndrome, obesity and cancer risk. Curr Opin Urol. (2022) 32:594–7. doi: 10.1097/MOU.0000000000001041
5. Jayedi A, Rashidy-Pour A, Khorshidi M, and Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev. (2018) 19:654–67. doi: 10.1111/obr.12656
6. Hashemi Madani N, Etemadi A, Nalini M, Poustchi H, Khajavi A, Mirzazade E, et al. Obesity and incident gastrointestinal cancers: overall body size or central obesity measures, which factor matters? Eur J Cancer Prev. (2021) 30:267–74. doi: 10.1097/CEJ.0000000000000657
7. Crudele L, Piccinin E, and Moschetta A. Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients. (2021) 13:2101. doi: 10.3390/nu13062101
8. Li X, Lian Y, Ping W, Wang K, Jiang L, Li S, et al. Abdominal obesity and digestive system cancer: a systematic review and meta-analysis of prospective studies. BMC Public Health. (2023) 23:2343. doi: 10.1186/s12889-023-17275-2
9. Zou Y and Pitchumoni CS. Obesity, obesities and gastrointestinal cancers. Dis mon. (2023) 69:101592. doi: 10.1016/j.disamonth.2023.101592
10. Aparecida Silveira E, Vaseghi G, de Carvalho Santos AS, Kliemann N, Masoudkabir F, Noll M, et al. Visceral obesity and its shared role in cancer and cardiovascular disease: A scoping review of the pathophysiology and pharmacological treatments. Int J Mol Sci. (2020) 21:9042. doi: 10.3390/ijms21239042
11. Wang L, Liu Y, Li K, Zhang W, Yuan Y, Ma K, et al. Age and BMI have different effects on subcutaneous, visceral, liver, bone marrow, and muscle adiposity, as measured by CT and MRI. Obes (Silver Spring). (2024) 32:1339–48. doi: 10.1002/oby.24040
12. Yu J, Yi Q, Chen G, Hou L, Liu Q, Xu Y, et al. The visceral adiposity index and risk of type 2 diabetes mellitus in China: A national cohort analysis. Diabetes Metab Res Rev. (2022) 38:e3507. doi: 10.1002/dmrr.v38.3
13. Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. (2016) 6:38214. doi: 10.1038/srep38214
14. Wei J, Liu X, Xue H, Wang Y, and Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. (2019) 11:1580. doi: 10.3390/nu11071580
15. Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. (2022) 21:225. doi: 10.1186/s12933-022-01670-x
16. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. (2012) 5:487–93. doi: 10.1161/CIRCOUTCOMES.111.963694
17. Esterson YB and Grimaldi GM. Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. (2018) 22:93–108. doi: 10.1016/j.cld.2017.08.005
18. Seneviratne N, Fang C, and Sidhu PS. Ultrasound-based hepatic fat quantification: current status and future directions. Clin Radiol. (2023) 78:187–200. doi: 10.1016/j.crad.2022.10.003
19. Wang X, Feng B, Huang Z, Cai Z, Yu X, Chen Z, et al. Relationship of cumulative exposure to the triglyceride-glucose index with ischemic stroke: a 9-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. (2022) 21:66. doi: 10.1186/s12933-022-01510-y
20. Agius R, Pace NP, and Fava S. Phenotyping obesity: A focus on metabolically healthy obesity and metabolically unhealthy normal weight. Diabetes Metab Res Rev. (2024) 40:e3725. doi: 10.1002/dmrr.v40.2
21. NCD Risk Factor Collaboration (NCD-RisC)M. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. (2016) 387:1377–96. doi: 10.1016/S0140-6736(16)30054-X
22. Lu Y, Zhao YC, Liu K, Bever A, Zhou Z, Wang K, et al. A validated estimate of visceral adipose tissue volume in relation to cancer risk. J Natl Cancer Inst. (2024) 116:1942–51. doi: 10.1093/jnci/djae193
23. Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. (2022) 20:494. doi: 10.1186/s12916-022-02672-y
24. Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, and de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes Rev. (2021) 22:e13088. doi: 10.1111/obr.13088
25. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. (2013) 62:921–5. doi: 10.1016/j.jacc.2013.06.027
26. Katzmarzyk PT, Brown JC, Yang S, Mire EF, Wu XC, Miele L, et al. Association of abdominal visceral adiposity and total fat mass with cancer incidence and mortality in white and black adults. Cancer Epidemiol Biomarkers Prev. (2022) 31:1532–8. doi: 10.1158/1055-9965.EPI-22-0207
27. Spallanzani RG. Visceral adipose tissue mesenchymal stromal cells in the intersection of immunology and metabolism. Am J Physiol Endocrinol Metab. (2021) 320:E512–e519. doi: 10.1152/ajpendo.00341.2020
28. Osorio-Conles Ó, Vega-Beyhart A, Ibarzabal A, Balibrea JM, Vidal J, de Hollanda A, et al. Biological determinants of metabolic syndrome in visceral and subcutaneous adipose tissue from severely obese women. Int J Mol Sci. (2022) 23:2394. doi: 10.3390/ijms23042394
29. Turgeon MO, Perry NJS, and Poulogiannis G. DNA damage, repair, and cancer metabolism. Front Oncol. (2018) 8:15. doi: 10.3389/fonc.2018.00015
30. Patra KC and Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. (2014) 39:347–54. doi: 10.1016/j.tibs.2014.06.005
31. Barberio AM, Alareeki A, Viner B, Pader J, Vena JE, Arora P, et al. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat Commun. (2019) 10:383. doi: 10.1038/s41467-018-08159-w
32. Chiefari E, Mirabelli M, La Vignera S, Tanyolaç S, Foti DP, Aversa A, et al. Insulin resistance and cancer: in search for a causal link. Int J Mol Sci. (2021) 22:11137. doi: 10.3390/ijms222011137
33. Pan L, Xu Q, Liu J, Gao Y, Li J, Peng H, et al. Dose-response relationship between Chinese visceral adiposity index and type 2 diabetes mellitus among middle-aged and elderly Chinese. Front Endocrinol (Lausanne). (2022) 13:959860. doi: 10.3389/fendo.2022.959860
34. Lawler T, Walts ZL, Steinwandel M, Lipworth L, Murff HJ, Zheng W, et al. Type 2 diabetes and colorectal cancer risk. JAMA Netw Open. (2023) 6:e2343333. doi: 10.1001/jamanetworkopen.2023.43333
35. Cao L, Wang T, Li H, El Hafa F, Zhu X, Yu Y, et al. Type 2 diabetes, glycemic traits and upper gastrointestinal cancer risk: a prospective cohort study. J Epidemiol Community Health. (2024) 1:jech-2023-221351. doi: 10.1136/jech-2023-221351
36. Oh TH, Byeon JS, Myung SJ, Yang SK, Choi KS, Chung JW, et al. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol. (2008) 23:411–7. doi: 10.1111/j.1440-1746.2007.05125.x
37. Keum N, Lee DH, Kim R, Greenwood DC, and Giovannucci EL. Visceral adiposity and colorectal adenomas: dose-response meta-analysis of observational studies. Ann Oncol. (2015) 26:1101–9. doi: 10.1093/annonc/mdu563
38. Sun Y, Zhang X, Hang D, Lau HC, Du J, Liu C, et al. Integrative plasma and fecal metabolomics identify functional metabolites in adenoma-colorectal cancer progression and as early diagnostic biomarkers. Cancer Cell. (2024) 42:1386–1400.e1388. doi: 10.1016/j.ccell.2024.07.005
39. Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N, et al. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. (2009) 54:862–8. doi: 10.1007/s10620-008-0440-6
40. Watanabe K, Koizumi S, Shirane K, Tsuda H, Watanabe H, Tsuji T, et al. Visceral obesity is associated with an increased risk of developing esophago-gastric junctional adenocarcinoma in Japan: a population-based case-control study in Akita Prefecture. Esophagus. (2022) 19:477–85. doi: 10.1007/s10388-021-00906-1
41. Xu FQ, Xu QY, Zhu ZJ, Jin L, Ye TW, Du CF, et al. Visceral and ectopic fat are more predictively associated with primary liver cancer than overall obesity from genetic sights: A Mendelian randomization study. Int J Cancer. (2024) 154:530–7. doi: 10.1002/ijc.v154.3
42. Sorimachi H, Omote K, Omar M, Popovic D, Verbrugge FH, Reddy YNV, et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail. (2022) 24:1359–70. doi: 10.1002/ejhf.v24.8
43. Dragano NRV, Milbank E, Haddad-Tóvolli R, Garrido-Gil P, Nóvoa E, Fondevilla MF, et al. Hypothalamic free fatty acid receptor-1 regulates whole-body energy balance. Mol Metab. (2024) 79:101840. doi: 10.1016/j.molmet.2023.101840
44. Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD, et al. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab. (2011) 96:541–7. doi: 10.1210/jc.2010-1651
45. Warren GW, Sobus S, and Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. (2014) 15:e568–580. doi: 10.1016/S1470-2045(14)70266-9
46. Yuan S, Chen J, Ruan X, Sun Y, Zhang K, Wang X, et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife. (2023) 12:e84051. doi: 10.7554/eLife.84051
Keywords: Chinese visceral adiposity index, gastrointestinal cancer, visceral fat, visceral adipose tissue, cohort study
Citation: Li J, Ma C, Liu K, Wang W, Cui S, Tian Y, Dong Z, Wei W, Wu S and Liu S (2025) Association of cumulative exposure to Chinese visceral adiposity index and gastrointestinal cancer: a prospective cohort study. Front. Oncol. 15:1534682. doi: 10.3389/fonc.2025.1534682
Received: 14 February 2025; Accepted: 16 June 2025;
Published: 03 July 2025.
Edited by:
Irene Lidoriki, Harvard University, United StatesReviewed by:
Dafeng Liu, Public Health and Clinical Center of Chengdu, ChinaKexun Li, Sichuan Cancer Hospital, China
Copyright © 2025 Li, Ma, Liu, Wang, Cui, Tian, Dong, Wei, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siqing Liu, c2lxaW5nbGl1QDE2My5jb20=
 Jiaxing Li
Jiaxing Li Chao Ma1,2
Chao Ma1,2 Siqing Liu
Siqing Liu