- 1Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Tianjin Lung Cancer Institute, Tianjin Medical University General Hospital, Tianjin, China
Phyllodes tumo (PT) of the breast are classified into benign, borderline, and malignant types. Malignant phyllodes tumor (MPT) with metastasis, particularly those containing sarcomatous components, have a notably poor prognosis. The most common sites of metastasis are the lungs, although metastases can also occur in the pleura and other areas. Metastatic PT is typically treated according to NCCN guidelines for soft tissue sarcomas. The prognosis for patients is extremely poor, with survival typically not exceeding five years. Therefore, the treatment of metastatic MPT presents significant challenges. A 67-year-old female with a history of PT surgery was hospitalized due to acute chest tightness and shortness of breath. MRI revealed a large mass in the left thoracic region, measuring 7.9 × 10.8 × 11.4 cm. A biopsy conducted prior to hospitalization indicated spindle cell soft tissue sarcoma. Due to critical vital signs, she underwent an emergency thoracotomy. Postoperative analysis confirmed the diagnosis of thoracic metastasis from MPT with sarcomatous components. Genetic analysis of the tumor tissue post-surgery revealed a KDM6A gene mutation. Unfortunately, subsequent imaging showed a recurring mass in the left thoracic space, approximately 8 cm in size. Considering the side effects of NCCN-recommended treatments (doxorubicin and ifosfamide) and the high cost of targeted therapies, the patient and her family chose tislelizumab. After six cycles of treatment, the patient’s progression-free survival reached 15 weeks. Due to unsatisfactory treatment effects, the patient and her family decided to discontinue therapy, and the patient passed away in July 2024. Although the combination of surgery and postoperative immune checkpoint inhibitors remains to be validated, this case provides valuable insights into the management of thoracic metastasis from MPT. It offers potential new options for personalized immunotherapy in metastatic MPT.
Introduction
Phyllodes tumor (PT), also known as cystosarcoma phyllodes, is a rare fibroepithelial tumor composed of stromal and epithelial components. These tumors are classified into three types: benign, borderline, and malignant (1, 2). The first documented case of malignant phyllodes tumor (MPT) with lung metastasis was recorded in 1931, highlighting the possibility of these tumors progressing to malignancy (3). Phyllodes tumors are relatively rare in females. A literature review reports that the overall recurrence rate for PT is approximately 12.6%, with recurrence rates for benign, borderline, and malignant PT at 7.1%, 16.7%, and 25.1%, respectively (4). When distant recurrence occurs, it typically presents as solid nodules or thin-walled cavities in the lungs. Fine Needle Aspiration (FNA) or core needle biopsy is usually insufficient to differentiate PT from fibroadenomas, making surgical excision necessary for an accurate pathological diagnosis (5). Histologically, PT are characterized by their unique leaf-like structures, consisting of dual-layered epithelium with both internal and external myoepithelial components. These tumors may also show pseudoangiomatous stromal hyperplasia and various metaplastic changes, including chondroid, osseous, lipomatous, and stromal giant cell formations. Squamous or apocrine metaplasia within the epithelium is less commonly observed. If features of liposarcoma, osteosarcoma, chondrosarcoma, or rhabdomyosarcoma are present, a diagnosis of MPT can be made (6). Surgical excision with a margin of at least 1 cm is the gold standard for treating local PT. The recurrence rate for benign PT is low, while borderline or malignant PT may have a recurrence rate of 10%-40%. Once MPT metastasizes, the median survival for patients is typically between 5 and 30 months. Adjuvant radiotherapy for MPT has not reached a consensus due to a lack of substantial data; however, it may be beneficial for local recurrence (7). Metastatic or recurrent PT should be treated according to the National Comprehensive Cancer Network (NCCN) guidelines for metastatic soft tissue sarcomas (8). Although immune checkpoint inhibitors (ICIs) have been studied in metastatic soft tissue sarcomas, such studies remain limited, and standard treatment guidelines still primarily recommend chemotherapy (9). Previous studies using doxorubicin alone or in combination with cyclophosphamide showed that only 10-20% of patients with metastatic sarcoma achieved a progression-free survival (PFS) of 4 to 6 months and an overall survival (OS) of 16 to 24 months, with significant side effects (10).
In this case report, we describe a female patient with MPT who experienced recurrence after thoracic surgery and was treated with tislelizumab, achieving a progression-free survival (PFS) of 3.5 months. This case provides new insights into the exploration of immunotherapy and targeted therapy for distant metastasis of MPT.
The ethical aspects of this case report were reviewed and approved by the Ethics Committee of Tianjin Medical University General Hospital. The patient provided written consent for the release of their detailed case information and associated images.
Case presentation
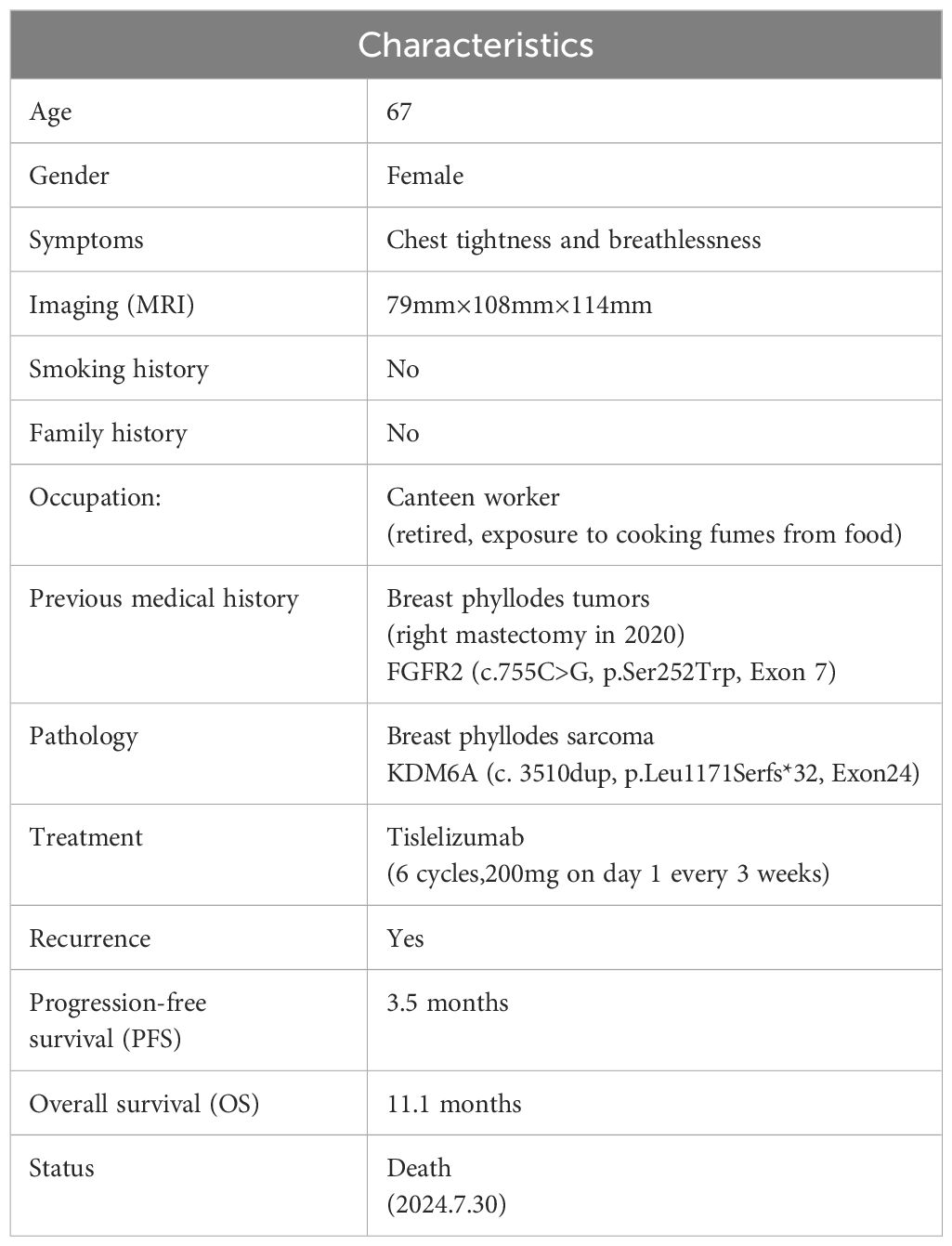
On August 30, 2023, a 67-year-old female patient was admitted to the Thoracic Surgery Department of Tianjin Medical University General Hospital due to chest tightness and breathlessness. The complete patient information is presented in Table 1, and the entire treatment process is shown in Figure 1A. Prior to admission, the patient underwent a CT scan at another hospital, which suggested a lung mass. A thoracic biopsy indicated a spindle cell soft tissue sarcoma, considered low-grade malignant, though a phyllodes tumor could not be excluded. The patient exhibited no symptoms of hemoptysis, fever, or chest pain. Upon admission, the patient underwent a thoracic enhanced MRI, which revealed a large mass in the left chest, approximately 79mm×108mm×114mm, showing uniform enhancement (Figure 1B). Prior to the biopsy results, specific laboratory tests, including lung tumor markers (CEA, CYFRA21-A, SCC, and ProGRP), were all negative. The patient had no history of smoking or alcohol consumption, worked in a canteen for many years with exposure to food oil fumes, and had been retired for three years at the time of diagnosis. There was no family history of similar tumors in first-degree relatives. The patient had a history of right mastectomy, and post-operative immunohistochemistry on July 20, 2020, showed positivity for E-cad, CK5/6, and VIM, with a Ki-67 proliferation index of approximately 20%, suggestive of a breast phyllodes tumor (Supplementary Figure S1A). Genetic testing revealed an FGFR2 mutation (c.755C>G, p.Ser252Trp, Exon 7; Geno-Truth Dx Lab). The patient had not received chemotherapy or radiation therapy after surgery.
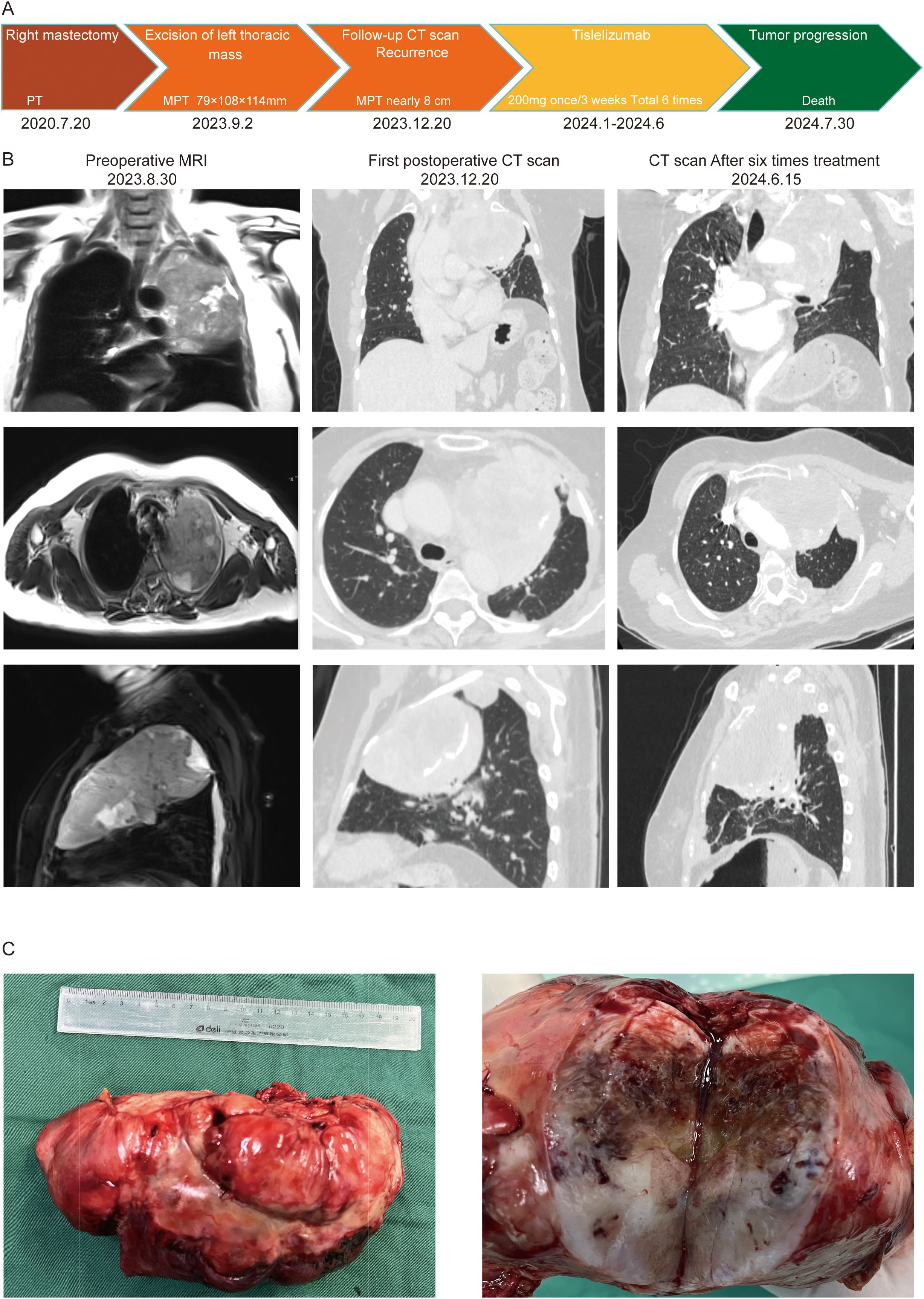
Figure 1. (A) Clinical treatment history; (B) Imaging data of the patient; (C) Tumor size and nature.
On September 2, 2023, after further examinations, an emergency thoracotomy was performed. During the surgery, it was found that the tumor had filled the upper and middle sections of the left lung, compressing the lower lobe and adhering to the chest wall. The left thoracic mass was excised, chest adhesions were loosened, and part of the left rib was removed. The postoperative mass measured 12.5×3.5×3.3 cm, with a gray-white and gray-yellow appearance and firm texture (Figure 1C). Pathological examination indicated a phyllodes tumor with sarcomatous elements, consistent with breast origin. Immunohistochemistry showed intramembranous SMA, Caldesmon, focal Desmin, and β-catenin positivity, with a Ki-67 hotspot area of approximately 40% (Figure 2). Histological sections revealed a sarcoma rich in blood vessels, with foam cells, multinucleated giant cell reactions, extensive necrosis, calcification, and ossification. Postoperatively, the patient’s symptoms of chest tightness significantly improved. Given the patient’s physical condition, follow-up and further treatment were recommended after three months. During this period, genetic testing of the patient’s tissue sections and blood samples revealed a KDM6A gene mutation (c.3510dup, p.Leu1171Serfs*32, Exon 24; Geno-Truth Dx Lab). The two gene mutations and their locations are shown in Supplementary Figure S1B.
Figure 2. Pathological Examination Report (10X and 20X magnification): (A) Hematoxyin and eosin(H&E) Staining; (B) Ki-67 Immunohistochemistry.
On December 20, 2023, during follow-up, tumor recurrence was observed, with the mass reaching nearly 8 cm (Figure 1B), and the recurrence was rapid, although the patient had no obvious symptoms. We then conducted a multidisciplinary team (MDT) discussion regarding the patient’s treatment plan, inviting specialists from oncology, radiation oncology, and interventional radiology. The expert consensus was that tumor embolization posed a high risk of chest bleeding, and the recommendation was to proceed with systemic chemotherapy combined with targeted therapy based on genetic testing. Therefore, we provided the NCCN-recommended first-line chemotherapy regimen, or the use of Pembrolizumab or KDM6A-related targeted therapies. However, the most common and severe side effects of Doxorubicin are myelosuppression and cardiotoxicity, and the cost of targeted therapies is 2-3 times higher than that of immunotherapy, with the local government not covering the cost of targeted drugs. Considering the side effects and financial burden, the patient and their family decided to forgo chemotherapy and targeted therapy. However, we did not give up on this patient. Upon reviewing the indications for Tislelizumab, a drug commonly used in lung cancer immunotherapy, we found that it meets the condition for “patients with other advanced solid tumors who have progressed after prior treatments and have no satisfactory alternative treatment options,” which aligns with the patient’s current condition. Additionally, the local government’s medical insurance covers the cost of immunotherapy. Therefore, we presented this treatment option to the patient and their family, and they ultimately accepted this regimen. The reason for not testing for PD-L1 expression is that before surgery, the patient had no intention of refusing postoperative adjuvant chemotherapy, so PD-L1 immunohistochemical testing was not included.
After discussion with the patient and her family, the patient decided to undergo six cycles of tislelizumab monotherapy (200 mg every 3 weeks). From January 2024 to June 2024, six treatments were completed, during which the only adverse reaction experienced by the patient was constipation. A follow-up enhanced CT report in June 2024 showed that the tumor had grown to approximately 9.1 cm, and a bone scan suggested local rib metastasis (with rib pain). Dissatisfied with the outcome, the patient and her family decided to discontinue treatment after completing the six cycles. In the subsequent follow-up, it was learned that the patient passed away on July 30, 2024, due to respiratory failure.
Discussion
The following are the highlights of this case. First, the patient underwent a complete resection of a large-scale PT. However, only three months after surgery, the tumor recurred, and its growth rate exceeded expectations, almost returning to its original size. Secondly, we identified rare mutations in both the previous in situ PT and the current metastatic MPT using next-generation sequencing (NGS), respectively FGFR2 and KDM6A mutations. Due to the patient’s inability to afford targeted therapy and the side effects of systemic chemotherapy, the domestic drug tislelizumab was ultimately chosen for treatment. Tislelizumab is indicated for advanced solid tumors, and it is covered by local government health insurance. The patient received six cycles of immunotherapy, with PFS of 3.5 months. To date, there have been no studies reporting the use of tislelizumab in the treatment of metastatic MPT, and this case provides new reference and hope for PD-L1 immune checkpoint inhibitors in the treatment of advanced MPT.
The diagnosis of breast PT is challenging. On ultrasound, benign PT typically presents with characteristics such as filled gaps, no microcalcifications, leaf-like splitting, and heterogeneous internal echo patterns (6). MPT is characterized by a large, irregular mass on ultrasound. For metastatic MPT, CT is selected as the primary imaging modality, with contrast-enhanced CT providing detailed information about the tumor’s relationship with surrounding tissues and organs, as well as its blood supply. CT-guided biopsy can confirm the pathological diagnosis, but due to the tumor’s rich blood supply, there is a risk of bleeding, requiring caution during the procedure (11). On MRI, PT usually appears as a well-defined, oval-shaped mass with isosignal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images. MPT may exhibit irregular cystic wall changes, and on T2-weighted images, the signal intensity is lower than that of normal glandular tissue. MRI is also important for evaluating distant metastasis of MPT to the spine and brain (12). Fine needle aspiration (FNA) is difficult to distinguish breast fibroadenomas from PT, and postoperative biopsy is often relied upon. Histologically, benign phyllodes tumors display abundant intercellular material, spindle cells, and low mitotic rates (less than 5 mitoses per 10 high-power fields). Borderline phyllodes tumors exhibit some but not all malignant features. Malignant phyllodes tumors are defined by significant nuclear pleomorphism in stromal cells, excessive growth of stromal components (without epithelial components in high-power fields), diffuse stromal infiltration, increased mitotic activity (greater than 10 mitoses per 10 high-power fields), and invasive borders (13). Immunohistochemically, phyllodes tumors express p53, Ki-67, CD117, EGFR, p16, and VEGF, with the lowest positivity rates in benign tumors and the highest in malignant ones (6). When phyllodes tumors metastasize to the chest, imaging may resemble isolated fibroadenomas, while cytopathology typically reveals spindle cell soft tissue tumors. Therefore, a combination of immunohistochemistry and patient history is crucial for accurate diagnosis. The incidence of distant metastasis in malignant phyllodes tumors can reach 10%, with almost all organs potentially affected, particularly the lungs and bones (14, 15). A large cohort study found that the metastasis rates for benign, borderline, and malignant phyllodes tumors were 0%, less than 2%, and 16%, respectively (16, 17). The risk of metastasis can be assessed based on factors such as necrosis, tumor size greater than 7 cm, invasive borders, significant stromal cell density, marked stromal overgrowth, and more than 5 mitoses per 10 high-power fields (18, 19).
Benign PT and fibroadenomas have overlapping histological features, making the diagnosis challenging. PT typically contains more cellular stroma and leaf-like structures, and when differentiation is unclear, it is best diagnosed as fibroadenoma to avoid overtreatment of the patient (6). For MPT, misdiagnosis as primary sarcoma can also occur, and more often, immunohistochemistry is used to assess the degree of epithelial differentiation (6, 7).
A large study on the genomic sequencing of advanced or metastatic MPT suggests that in 135 MPT patients (20), the most common gene mutation with a frequency ≥5% is the TERT promoter mutation (69.7%). Two different assays revealed that 36.8% of the samples were PD-L1 positive (≥1% immune cells) and 21.4% were PD-L1 positive (TPS ≥1). Interestingly, one patient exhibited an FGFR3-TACC3 fusion. This study highlights the significant potential for immunotherapy or targeted therapy for MPT.
The tumor gene mutation in this patient was identified through NGS sequencing. NGS can sequence large amounts of nucleotides within a short period and at a cost affordable to individual patients (21). Compared to other biomarker detection techniques such as fluorescence in situ hybridization (FISH), PCR, CRISPR, and immunohistochemistry (IHC), high-throughput NGS sequencing can detect more genes and unknown mutations, making it more suitable for developing targeted drug and immunotherapy regimens for advanced or metastatic cancer patients (22). In the ESMO report on the use of NGS in advanced solid tumors in Europe (21), experts pointed out that in countries offering “tumor-agnostic targeted therapies” and in patients with advanced non-squamous NSCLC, prostate cancer, ovarian cancer, and cholangiocarcinoma, multi-gene NGS testing should be routinely conducted. Although MPT is not specifically included in this ESMO report, it is clearly stated that NGS is an important tool for identifying histological subtypes of soft tissue sarcomas, particularly in metastatic soft tissue sarcomas, making the use of NGS more reasonable. However, not all situations require NGS; for instance, in cases of NTRK mutations, where TRK inhibitors have shown efficacy, other more cost-effective testing methods are recommended as alternatives to NGS.
Fibroblast growth factor receptors (FGFRs) have increasingly been recognized as important therapeutic targets in patients with advanced refractory tumors (23), with approximately 7% of tumors carrying FGFR mutations. FGFR signaling regulates cell differentiation and proliferation, while also promoting anti-apoptotic pathways that contribute to chemotherapy resistance. FGFR mutations are most commonly found in urothelial carcinoma. Futibatinib, a next-generation irreversible FGFR1-4 inhibitor, has shown antitumor effects in FGFR-mutant tumors, particularly in cholangiocarcinoma. Goyal reported a 42% response rate to futibatinib in cholangiocarcinoma patients, with one case achieving complete remission (24). In a clinical trial (NCT02052778) involving futibatinib treatment for advanced solid tumors with FGFR2 mutations (25), the overall response rate (ORR) was 44.1%, with a median progression-free survival (mPFS) of 9.0 months. Although there is currently no study proving the efficacy of this targeted drug for MPT, we believe futibatinib holds significant potential for treating MPT or metastatic MPT with FGFR2 mutations.
KDM6A is an X-linked histone lysine demethylase, and its precise role remains unclear. A few patients with Kabuki syndrome also exhibit KDM6A mutations, leading to abnormal facial features, skeletal malformations, and cardiac and cognitive impairments. KDM6A mutations are more common in bladder cancer and breast cancer, with some evidence suggesting that KDM6A loss may promote tumor progression through the TGF-β pathway (26). Currently, no effective targeted drugs for KDM6A have been thoroughly researched. To date, fewer than 30 KDM6A inhibitors have shown limited efficacy and selectivity in vitro and in cell models, with most not yet entering clinical trials (27). Specific inhibitors such as GSK-J1 and GSK-J4 have been developed to target the KDM6 family. Dr. Cregan reported that preliminary testing of GSK-J1 on NCI-H226 cell proliferation showed that it could inhibit the growth of malignant pleural mesothelioma (MPM) cells with KDM6A mutations (28).
PT is relatively rare, accounting for about 0.3%-1% of all breast tumors. Of these, 10%-20% are malignant, and 9%-27% experience distant metastasis (29). The lungs are the most common site of metastasis (30, 31), although there are rare cases involving the pleura and heart (32, 33). The prognosis for metastatic malignant phyllodes tumors is generally poor, with a median survival of 5 to 30 months. Due to the rarity of distant metastasis in PT, there is currently no standardized treatment protocol for managing postoperative metastasis in PT. The NCCN guidelines recommend treating distant metastasis of phyllodes tumors according to sarcoma treatment protocols (34). Currently, there is no evidence to suggest that radiotherapy provides significant long-term survival benefits for patients with MPT or metastatic MPT. According to the 2022 NCCN Soft Tissue Sarcoma Guidelines (35), the treatment for postoperative metastasis of breast tumors should resemble that for sarcomas. Recommended chemotherapy regimens include doxorubicin, ifosfamide, and mesna, or ifosfamide, epirubicin, and mesna. Previous reports suggest that adjuvant chemotherapy has not improved metastasis-free survival and both drugs can cause bone marrow suppression, and long-term use or high doses may lead to cardiotoxicity (6). A study by Moon et al. (36) indicated that despite the lack of standardized treatment protocols for PT, adjuvant chemotherapy with doxorubicin and ifosfamide could effectively achieve complete remission of pulmonary metastasis. For large tumors, surgical resection may be necessary to alleviate symptoms and prevent complications, as in this case. To ensure complete tumor removal and reduce the risk of tumor cell spread, a thoracotomy was performed. During this procedure, part of the fourth rib, the upper lobe of the left lung, and the pericardium were excised. The tumor capsule was intact, and the surgery was completed with minimal bleeding and no major complications.
Traditionally, immune checkpoint inhibitors (ICIs) are used in combination with chemotherapy, not only converting “cold” tumors to “hot” tumors but also reducing suppressive immune cells, enhancing tumor antigen presentation, and thereby improving tumor-killing activity (37, 38). Therefore, the combined application of immunotherapy and chemotherapy holds promise for future treatments of metastatic MPT. According to Pollack et al. (39), the combination of doxorubicin and pembrolizumab resulted in a median PFS of 8.1 months and a significantly higher median OS of 27.6 months. This study fills a gap in the treatment of breast phyllodes tumor metastasis to the chest and continues to explore the combination of Tislelizumab with various chemotherapy agents as a new direction for treating distant metastasis in MPT.
Conclusion
Given the potential for tumor recurrence following complete surgical excision, it remains essential to monitor patients consistently. The rarity of PT metastasizing to the lungs enhances our comprehension of their progression, pathology, and imaging characteristics, thereby enriching our treatment strategies. Furthermore, the limited availability of KDM6A-targeted therapies underscores the necessity for ongoing research in this area.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Ethics Committee of Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YHL: Conceptualization, Data curation, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. ZD: Data curation, Software, Supervision, Writing – review & editing. ML: Data curation, Software, Writing – review & editing. YWL: Software, Writing – review & editing. MW: Supervision, Writing – review & editing. JC: Funding acquisition, Software, Writing – review & editing. HZ: Data curation, Funding acquisition, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Tianjin Municipal Health Commission and the Tianjin Key Medical Discipline Sub-project (TJLCMS2021-06), the Tianjin Municipal Education Commission through the General Project of the Natural Science Foundation (2020KJ162), and the Wu Jieping Medical Foundation (320.6750.2022-11-43).
Acknowledgments
We thank all the staff involved in this research, especially DZX, LMH, LYW, WM, CJ and ZHL for their hard work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1535653/full#supplementary-material
Supplementary Figure 1 | (A) Histological sections of previous PT; (B) The location of mutated genes on the chromosomes.
References
1. Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw. (2007) 5:324–30. doi: 10.6004/jnccn.2007.0027
2. Anderson BO, Lawton TJ, Lehman CD, Moe RE. Phyllodes tumors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast, 3rd. Lippincott Williams & Wilkins, Philadelphia (2004).
3. Lee BJ, Pack GT. Giant intracanalicular myxoma of the breast:the so-called cystosarcoma phyllodes mammae of Johannes Muller. Ann Surg. (1931) 93:250–68. doi: 10.1097/00000658-193101000-00034
4. Yu CY, Huang TW, Tam KW. Management of phyllodes tumor: A systematic review and meta-analysis of real-world evidence. Int J Surg. (2022) 107:106969. doi: 10.1016/j.ijsu.2022.106969
5. Salvadori B, Cusumano F, Del Bo R, Delledonne V, Grassi M, Rovini D, et al. Surgical treatment of phyllodes tumors of the breast. Cancer. (1989) 63:2532–6. doi: 10.1002/1097-0142(19890615)63:12<2532::AID-CNCR2820631229>3.0.CO;2-Q
6. Lissidini G, Mule A, Santoro A, Papa G, Nicosia L, Cassano E, et al. Malignant phyllodes tumor of the breast: a systematic review. Pathologica. (2022) 114:111–20. doi: 10.32074/1591-951X-754
7. Goodwin B, Oyinlola AF, Palhang M, Lehman D, Platoff R, Atabek U, et al. Metastatic and Malignant phyllodes tumors of the breast: an update for current management. Am Surg. (2023) 89:6190–6. doi: 10.1177/00031348231198114
8. Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. (2011) 196:487–95. doi: 10.2214/AJR.10.4948
9. Yang H, Qin Z, He X, Xue Q, Zhou H, Sun J, et al. Tislelizumab immunotherapy combined with chemotherapy in the treatment of a patient with primary anterior mediastinal undifferentiated pleomorphic sarcoma with high PD-L1 expression: A case report and literature review. Front Oncol. (2023) 13:1110997. doi: 10.3389/fonc.2023.1110997
10. Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. (2017) 18:1397–410. doi: 10.1016/S1470-2045(17)30622-8
11. Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, et al. Solitary fibrous tumors of the pleura: Clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg. (2002) 21:1087–93. doi: 10.1016/S1010-7940(02)00099-4
12. Tay CK, Teoh HL, Su S. A common problem in the elderly with an uncommon cause: Hypoglycaemia secondary to the doege-potter syndrome. BMJ Case Rep. (2015), bcr2014207995. doi: 10.1136/bcr-2014-207995
13. Tan PH. Fibroepithelial tumors and hamartomas of the breast. In: Breast tumors. WHO classification of tumors, 5th. IARC Press, Lyone (2019). p. 165–76.
14. Liang MI, Ramaswamy B, Patterson CC, McKelvey MT, Gordillo G, Nuovo GJ, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol. (2008) 6:117. doi: 10.1186/1477-7819-6-117
15. Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. (1993) 53:4071–4.
16. Tse GM, Soon Lee C, Kung FYL, Scolyer RA, Law BKB, Lau T, et al. Hormonal receptors expression in epithelial cells of mammary phyllodes tumors correlates with pathologic grade of the tumor: a multicenter study of 143 cases. Am J Clin Pathol. (2002) 118:522–6. doi: 10.1309/D206-DLF8-WDNC-XJ8K
17. Rosen PP, Oberman HA. Tumors of the Mammary Gland. Washington, DC: Armed Forces Institute of Pathology (1993).
18. Papas Y, El Asmar A, Ghandour F, Hajj I. Malignant phyllodes tumors of the breast: A comprehensive literature review. Breast J. (2020) 26:240–4. doi: 10.1111/tbj.13523
19. National Comprehensive Cancer Network. Phyllodes tumor, version 1.2018. Plymouth Meeting (PA: NCCN (2018).
20. Rosenberger LH. Genomic landscape of Malignant phyllodes tumors reveals multiple targetable opportunities. Oncologist. (2024) 29:1024–31. doi: 10.1093/oncolo/oyae218
21. Mosele MF, Westphalen CB, Stenzinger A, Barlesi F, Bayle A, Bièche I, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol. (2024) 35:588–606. doi: 10.1016/j.annonc.2024.04.005
22. Loong HH. Recommendations for the use of next-generation sequencing in patients with metastatic cancer in the Asia-Pacific region: a report from the APODDC working group. ESMO Open. (2023) 8:101586. doi: 10.1016/j.esmoop.2023.101586
23. Touat M, Ileana E, Postel-Vinay S, André F, Soria JC. Targeting FGFR signaling in cancer. Clin Cancer Res. (2015) 21:2684–94. doi: 10.1158/1078-0432.CCR-14-2329
24. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. (2023) 388:228–39. doi: 10.1056/NEJMoa2206834
25. Javle M, King G, Spencer K, Borad MJ. Futibatinib, an irreversible FGFR1-4 inhibitor for the treatment of FGFR-aberrant tumors. Oncologist. (2023) 28:928–43. doi: 10.1093/oncolo/oyad149
26. Xiao JF, Kua LF, Ding LW, Sun QY, Myint KN, Chia XR, et al. KDM6A depletion in breast epithelial cells leads to reduced sensitivity to anticancer agents and increased TGFbeta activity. Mol Cancer Res. (2022) 20:637–49. doi: 10.1158/1541-7786.MCR-21-0402
27. Chen LJ, Xu X-Y, Zhong X-D, Liu Y-J, Zhu M-H, Tao F, et al. The role of lysine-specific demethylase 6A (KDM6A) in tumorigenesis and its therapeutic potentials in cancer therapy. Bioorg Chem. (2023) 133:106409. doi: 10.1016/j.bioorg.2023.106409
28. Cregan S, Breslin M, Roche G, Wennstedt S, MacDonagh L, Albadri C, et al. Kdm6a and Kdm6b: Altered expression in Malignant pleural mesothelioma. Int J Oncol. (2017) 50:1044–52. doi: 10.3892/ijo.2017.3870
29. Zhang Y, Kleer CG. Phyllodes tumor of the breast:histopathologic features, differential diagnosis, and molecular/genetic updates. Arch Pathol Lab Med. (2016) 140:665–71. doi: 10.5858/arpa.2016-0042-RA
30. Al-Zoubaidi M, Qiu S, Bonnen M, Joyner M, Roehl K, Silva C, et al. Malignant phyllodes tumor of the breast: a case report. Open Breast Cancer J. (2011) 3:45–8.
31. Khanal S, Singh YP, Bhandari A, Sharma R. Malignant phyllodes tumor with metastases to lung, adrenal and brain:a rare case report. Ann Med Surg. (2018) 36:113–17. doi: 10.1016/j.amsu.2018.10.030
32. Suriyan S, Sharma R, Narasimhan M, Shanmuganathan A, Rajendran A. A case of pleuroparenchymal metastasis: rare aetiology. J Clin Diagn Res. (2016) 10:OD03–5. doi: 10.7860/JCDR/2016/16532.7590
33. Yoshidaya F, Hayashi N, Takahashi K, Suzuki K, Akiyama F, Ishiyama M, et al. Malignant phyllodes tumor metastasized to the right ventricle: a case report. Surg Case Rep. (2015) 1:1–121. doi: 10.1186/s40792-015-0121-6
34. Ostapenko E, Burneckis A, Ostapenko A, Skaisgirytė A, Ostapenko V. Malignant phyllodes tumor of the breast with metastases to the lungs: A case report and literature review. Radiol Case Rep. (2022) 17:4006–12.
35. NCCN. Recently Updated Guidelines. Breast cancer version 3 (2022). Available online at: https://www.nccn.org/guidelines/recently-published-guidelines (Accessed May 31, 2022).
36. Moon SH, Jung JH, Lee J, Kim WW, Park HY, Lee JW, et al. Complete remission of giant Malignant phyllodes tumor with lung metastasis. Med (Baltimore). (2019) 98:e15762. doi: 10.1097/MD.0000000000015762
37. Lynch MM, Alexiev BA, Schroeder BA, Pollack SM. Combinations of chemotherapy and PD-1/PD-L1 inhibitors in sarcoma. Curr Treat Options Oncol. (2022) 23:1861–76. doi: 10.1007/s11864-022-01036-1
38. Meng WJ, Guo J-M, Huang L, Zhang Y-Y, Zhu Y-T, Tang L-S, et al. Anoikis-related long non-coding RNA signatures to predict prognosis and immune infiltration of gastric cancer. Bioengineering (Basel). (2024) 11(9):893. doi: 10.3390/bioengineering11090893
Keywords: phyllodes tumors, malignant phyllodes tumors, tislelizumab, immunotherapy, soft tissue sarcoma, FGFR2, KDM6A
Citation: Liu Y, Duan Z, Liu M, Li Y, Wang M, Chen J and Zhao H (2025) Unexpected outcomes of tislelizumab treatment in thoracic metastasis of malignant phyllodes tumors: a case report and literature review. Front. Oncol. 15:1535653. doi: 10.3389/fonc.2025.1535653
Received: 27 November 2024; Accepted: 20 March 2025;
Published: 14 April 2025.
Edited by:
Dan Liu, Sichuan University, ChinaReviewed by:
Yiping Zou, University of Chinese Academy of Sciences, ChinaZhenbin Qiu, Guangdong Provincial People’s Hospital Lung Cancer Institute, China
Zhao Wang, The First Affiliated Hospital of Sun Yat-sen University, China
Shuhei Suzuki, Yamagata Prefectural Shinjo Hospital, Japan
Copyright © 2025 Liu, Duan, Liu, Li, Wang, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen, aHVudGVyY2oyMDA0QHFxLmNvbQ==; Honglin Zhao, bmF2eXpoYW9AYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
 Yihao Liu
Yihao Liu Zhixuan Duan1,2†
Zhixuan Duan1,2† Minghui Liu
Minghui Liu Yongwen Li
Yongwen Li Jun Chen
Jun Chen Honglin Zhao
Honglin Zhao