- 1Graduate School, Jiangxi University of Traditional Chinese Medicine Nanchang, Jiangxi, China
- 2Nanchang Normal University, Infirmary of the Logistics Support Department, Nanchang, Jiangxi, China
- 3The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, China
Background: JAK1, a member of the JAK kinase family, is involved in the signal transduction of multiple cytokine pathways and is crucial in the onset and progression of inflammation and tumours. Consequently, JAK1 has garnered significant attention in recent years.
Methods: We use bibliometric and visual analysis to evaluate the thematic trends and knowledge structure of TRPV1’s research papers on JAK1, sourced from the Web of Science core collection from 2003 to 2024. CiteSpace is used to analyze references and keywords of authors, institutions, countries, and commonly cited, and applies co-current and clustering functions to generate visual knowledge maps.
Results: A total of 3,686 articles were incorporated. The primary research domain of JAK1 is oncology; the United States leads in publication volume, with the University of Texas holding the most prominent central position. The keyword distribution indicates that the literature on JAK1 from 2003 to 2009 primarily concentrated on mechanistic studies, encompassing gene expression, activation, pathways, and cell apoptosis. From 2008 to 2018, research hotspots predominantly examined the association between JAK1 and various disease atlases. Beginning in 2012 and extending to 2024, the focus shifted towards the research and development of clinical pharmaceuticals, along with their safety and efficacy. Gene expression, signal transduction, atopic dermatitis, and JAK1-selective inhibitors have emerged as prominent research areas in recent years, exhibiting significant potential for development.
Conclusion: This study presents the contemporary status and prospective trends of JAK1 research over the last two decades. Current research focuses on skin inflammation, rheumatoid arthritis, and tumor-related diseases, while new signaling pathways are constantly being discovered. JAK1 inhibitors are gradually being used in clinical practice and have good development prospects, which will become the main trend of future research.
1 Introduction
Janus kinase (JAK) comprises a family of four enzymes. JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) transmit downstream signals of cytokines via the JAK/STAT pathway, modulate the expression of target genes, and engage in processes such as cell proliferation, differentiation, and immune regulation (1, 2), which is closely related to a variety of diseases (3). The JAK1 subtype is the common core of multiple pathways. Different JAK family members tend to control different STATs. After every 2–3 JAK molecules combine to form a polymer, they are jointly responsible for a pathway, producing specific biological effects. JAK1 is closely related to inflammation, cancer, immunity, and other diseases (4–6), JAK2 is primarily related to diseases of the blood system (7, 8), and JAK3 is related to a variety of autoimmune diseases (9, 10). Among the members of the JAK family, JAK1 is the only subtype that can form heterodimers with all three types of JAK, and JAK1 also participates in the information transduction of various signaling pathways. Currently, JAK1 has emerged as a therapeutic target for numerous immune and inflammatory disorders. Nevertheless, the thematic trends and knowledge structure of JAK1 have not been examined through bibliometric analysis. The bibliometric analysis method can objectively analyze the current situation and development trend of the discipline, devoid of personal subjective judgment, thereby accurately reflecting the present development status, research focal points, and prospective trends (11, 12). Therefore, this study adopts bibliometric analysis to perform a thorough review of JAK1 studies from 2003 to 2024 in order to understand the co-citation of literature, establish a research collaboration network, and evaluate research trends and emerging hotspots.
2 Materials and methods
2.1 Source of data and search strategy
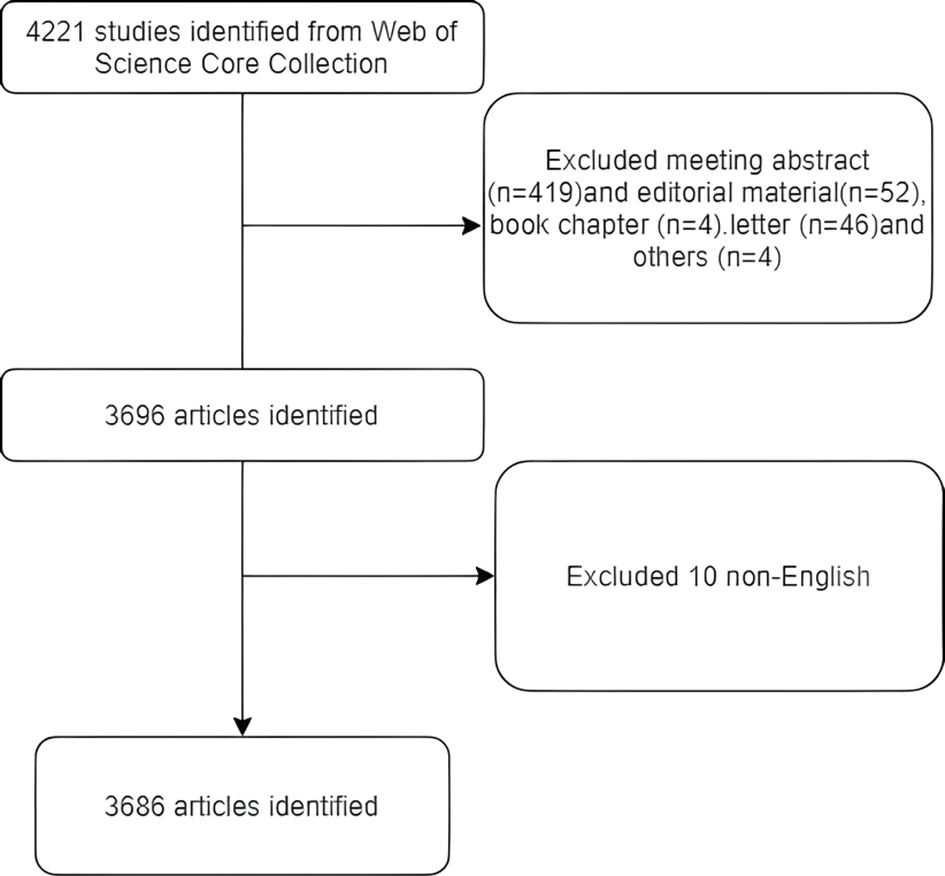
The literature retrieval was conducted online via the Web of Science core collection Science Citation Index-Expanded, on September 1, 2024. The data search strategy is as follows: Topic: JAK1; Date: 2003-01–01 to 2024-09-01; Article type: full text; Following the removal of duplicates, a total of 4,221 documents were ultimately incorporated into the literature measurement analysis. Excluding conference papers, editorial materials, book chapters, letters, and other unrelated documents, 3,696 articles remain. Excluding non-English literature, there remain 3,686 articles. Figure 1 illustrates the search flowchart.
2.2 Data analysis
The English literature is exported in”RefWorks” format, and the literature in WOS is exported in”Plain text” format and renamed download * *. txt. After converting data and creating a new project in the CiteSpace software, data analysis is carried out according to the selection. The CiteSpace parameter is as follows: The time span was from January 2003 to September 2024, and the time slices were “1 year per slice.”; Node selection type, one at a time, and other parameters in the configuration function area are set according to the default value of the system. The broader the range of nodes on the map, the higher the frequency (or reference frequency) of the analyzed research object; the color and thickness of the node’s inner circle denote the frequency of occurrence across various time periods; the connecting lines between nodes illustrate the co-occurrence (or co-citation) relationship, with thickness signifying the strength of this co-occurrence (or co-citation), and color indicating the initial time of co-occurrence (or co-citation) of the nodes. The purple circles signify mediating centrality, with nodes exhibiting high centrality deemed more significant (13).
2.3 Results
2.3.1 Analysis of publications
The Web of Science Core Collection included a total of 3,686 articles from 2002 to 2024, with the overall trend categorized into three phases. The initial phase spanned from 2003 to 2010, a stable period, and the annual publication volume remained basically at the same level. The second stage was from 2011 to 2020, a development period, and the overall trend of publication volume continued to rise. After 2020, the growth rate began to increase markedly and peaked in 2022 (399 articles), indicating that JAK1 research continues to attract the attention of researchers and has good development potential. See Figure 2A for details.
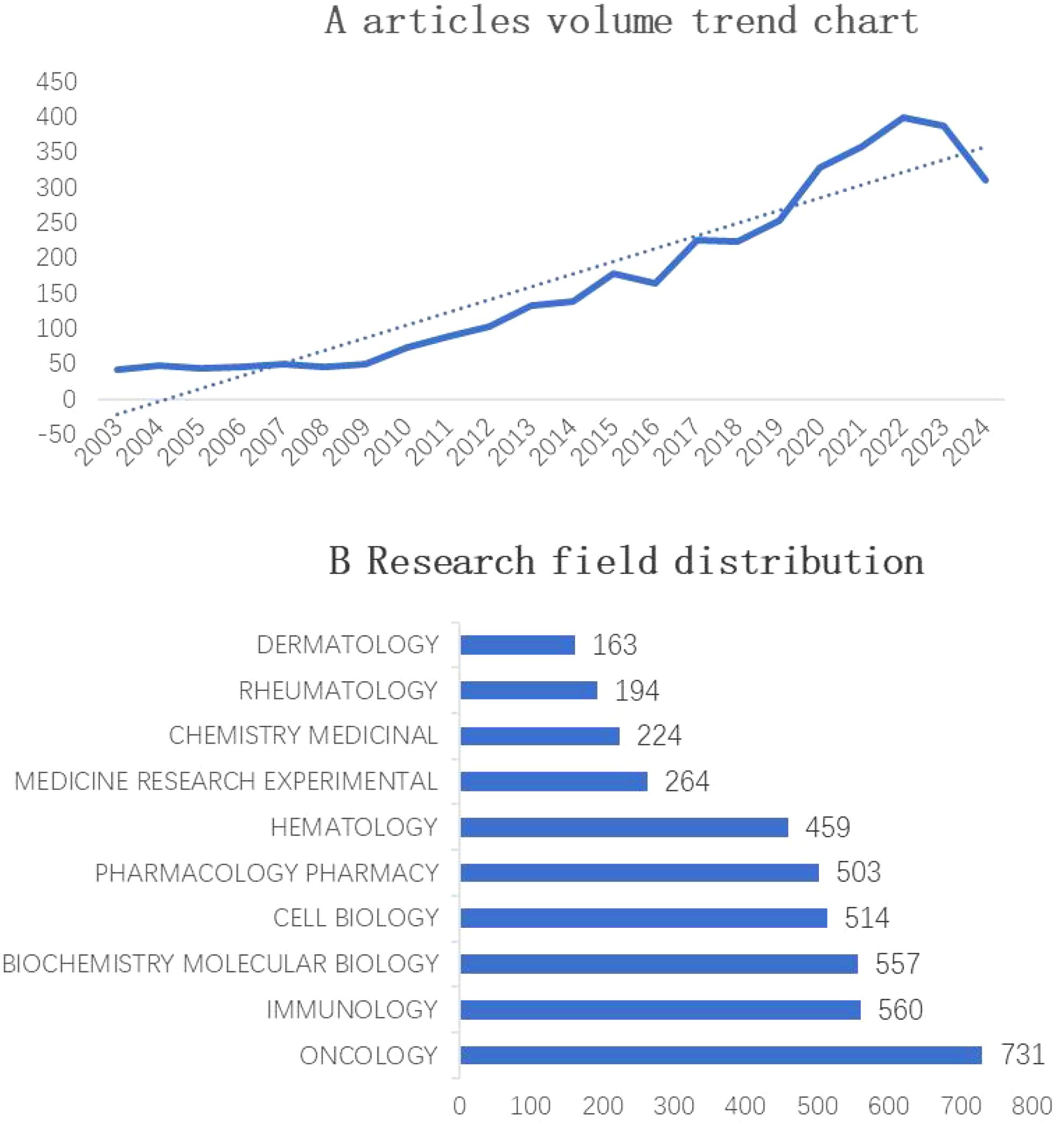
Figure 2. (A) Annual publication trends in JAK1 research (2003–2024). X-axis: Year (2003–2024); Y-axis: Number of publications. The dashed line represents a polynomial trendline illustrating three growth phases. (B) Top 10 research fields of JAK1 research (2003–2024).
2.3.2 Analysis of the research field
The top three research domains for JAK1 are oncology (642 articles), biochemistry (541 articles), and immunology (507 articles), collectively accounting for 46% of all JAK1-related publications. Figure 2B shows the top 10 research fields of JAK1 from 2002 to 2024: oncology, biochemistry, immunology, cell biology, pharmacology, hematology, medical research, medicine, chemistry, multidisciplinary science, and dermatology.
2.3.3 Analysis of countries/regions
In total, 86 countries or regions have disseminated research papers on JAK1. We utilized Citespace software to construct a collaborative network of countries, as depicted in Figure 3A. Every node signifies a country. In the cooperation network of the issuing country, a larger node signifies a greater volume of documents dispatched by the country; a denser connection indicates a closer collaboration among entities. The connection line in the network signifies the collaboration between countries, while the line’s thickness denotes the degree of cooperation. Figure 3A illustrates that the United States and China are the foremost nations in JAK1 research, signifying their substantial influence in this domain and extensive collaboration with other countries. Figure 3B illustrates the top 10 countries by publication volume, which collectively represent 74% of the total publications, highlighting the concentration of nations engaged in JAK1-related research.
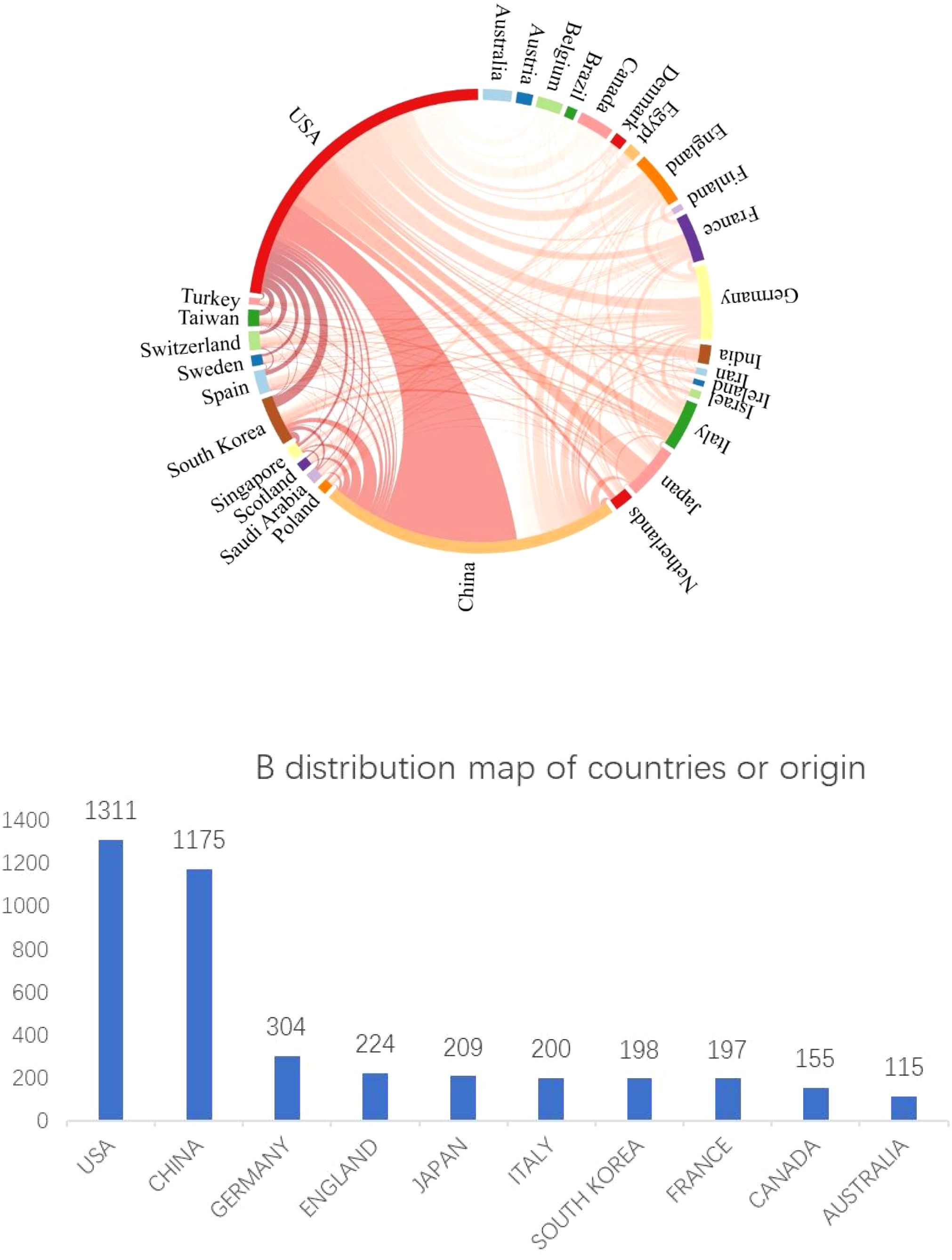
Figure 3. (A) Global collaboration network in JAK1 research (Citespace Analysis). (B) Top 10 countries by JAK1 publication volume (2003–2024).
2.3.4 Analysis of institutions
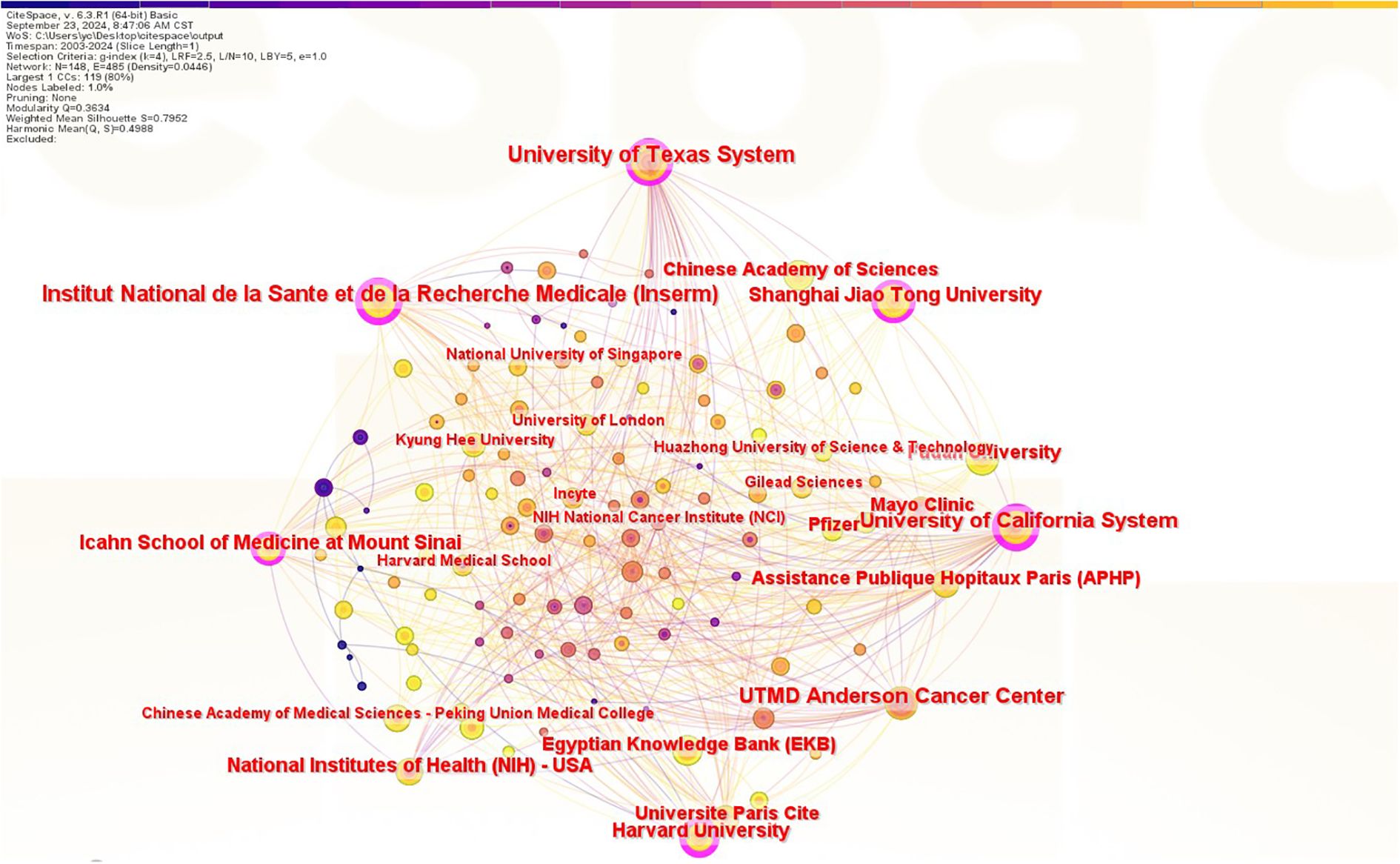
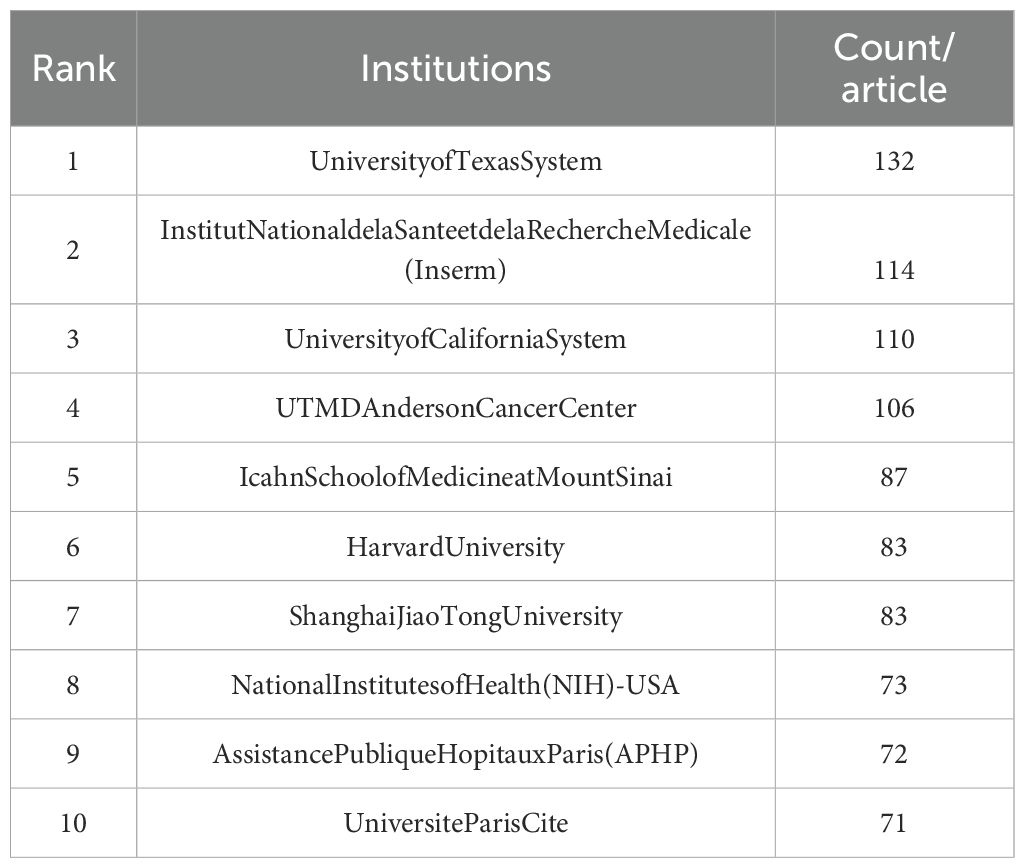
As shown in Figure 4, a total of 199 institutions engaged in JAK1 research, with 6 institutions exhibiting centrality values of ≥0.1, indicating that multiple institutions are publishing key or transformative research. The University of California has the highest centrality (centrality = 0.38) as shown in Table 1. The University of Texas has the most publications, followed by the French National Institute of Health and Medical Research, the University of California, the Icahn School of Medicine at Mount Sinai, Harvard University, Shanghai Jiao Tong University, the National Institutes of Health, the Paris Hospital Public Assistance (APHP), and the University of Paris. However, the collaboration between the main publishing institutions with the largest number of publications is weak.
2.3.5 Analysis of the author
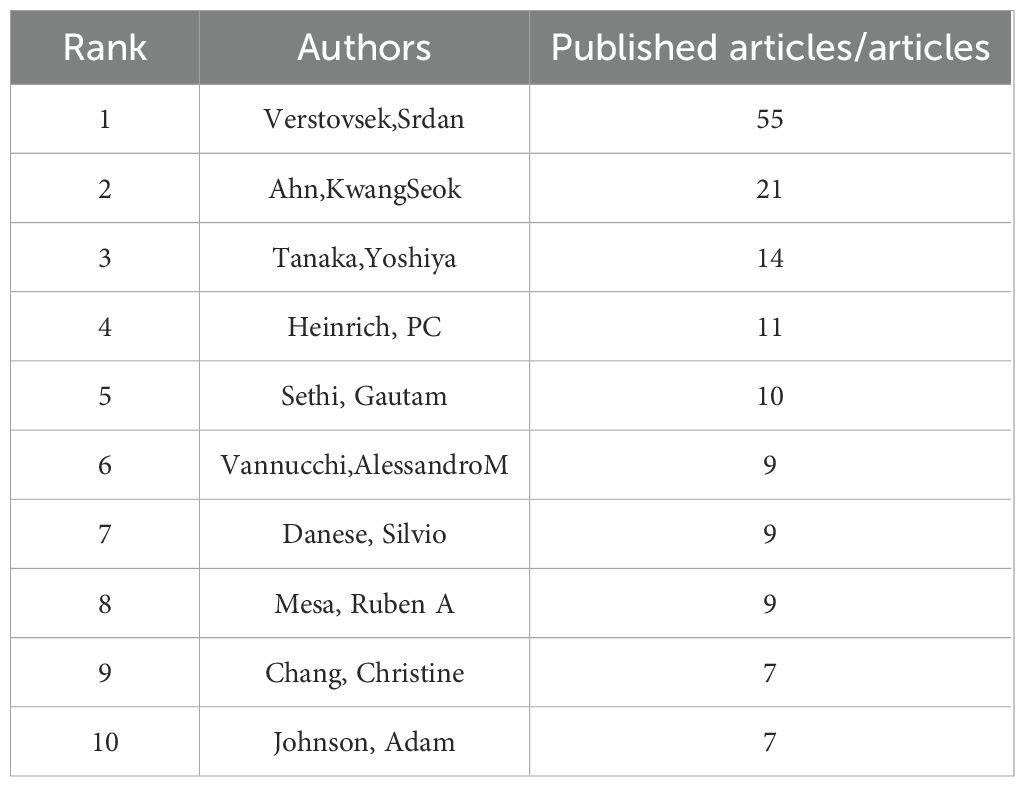
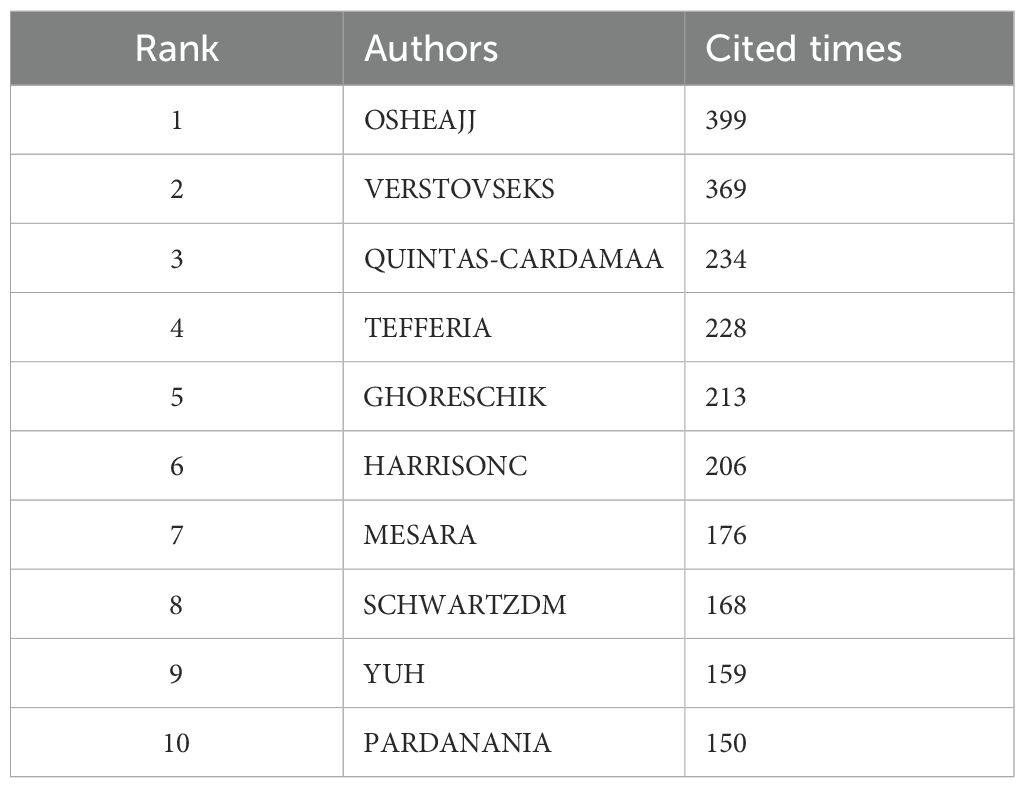
We identified core authors using Price’s law. The calculation formula is , where ηmax represents the highest number of publications by an author, and authors with a publication count ≥N are considered core authors. Calculations reveal that the core author’s volume is ≥6 (5.554) articles, totaling 35 authors; The number of articles and the top 10 authors cited are shown in Table 2A, including Verstovsek, Srdan (55 articles), Ahn, KwangSeok (21 articles), Tanaka, Yoshiya (14 articles), Heinrich, PC (11 articles), Set Hi, Gautam (10 articles) is the top five authors in the volume of articles. The number of cited articles is the total number of cited articles for all articles published in the JAK1 research area by each author, as shown in Table 2B. The top five authors cited are OSHEAJJ (399 TIMES), VERSTOVSEKS (369 TIMES), QUINTÁS-CARDAMAA (234 TIMES), TEFFERIA (228 TIMES), and GHORESCHIK (213 TIMES).
2.3.6 Keyword co-occurrence analysis
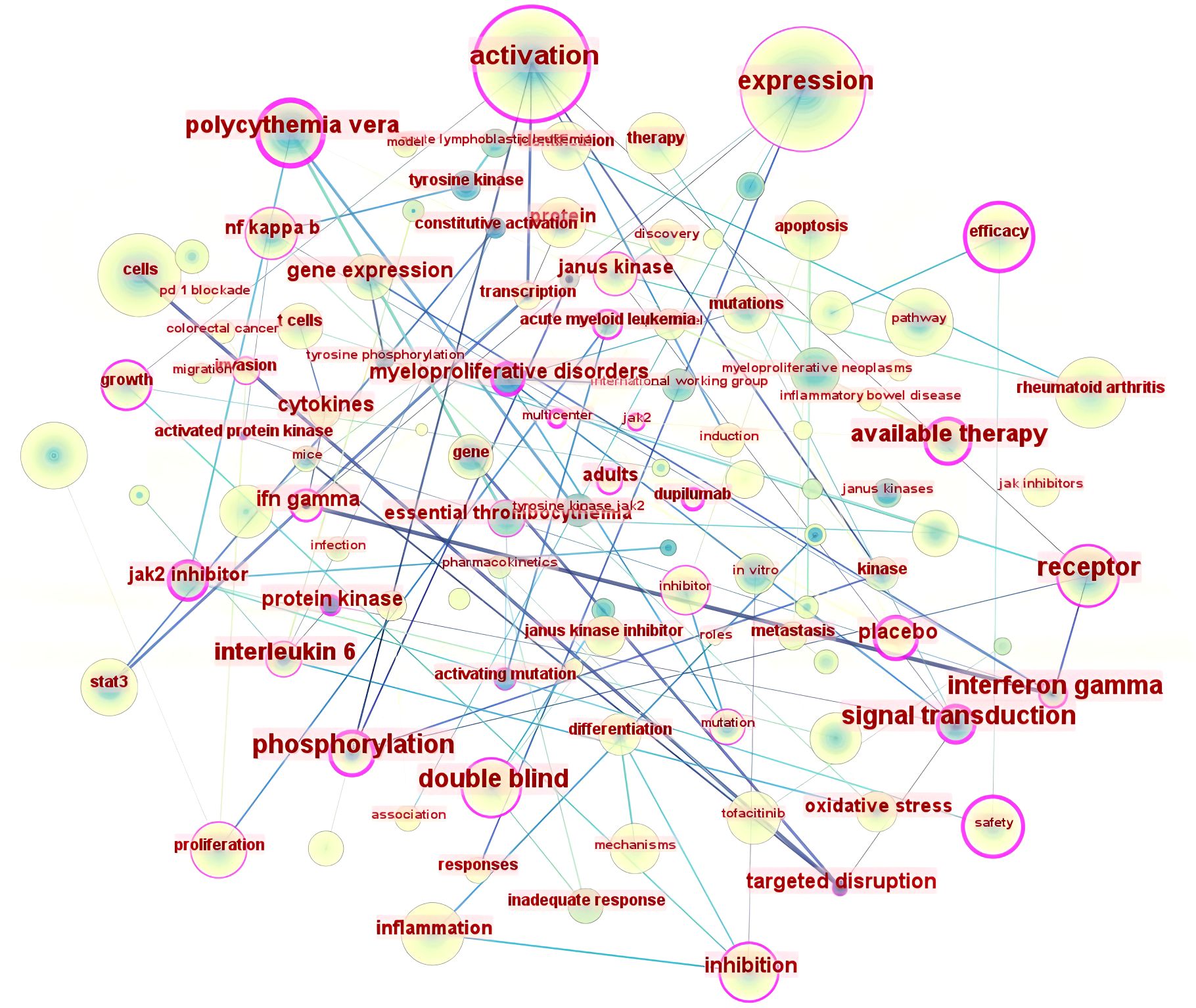
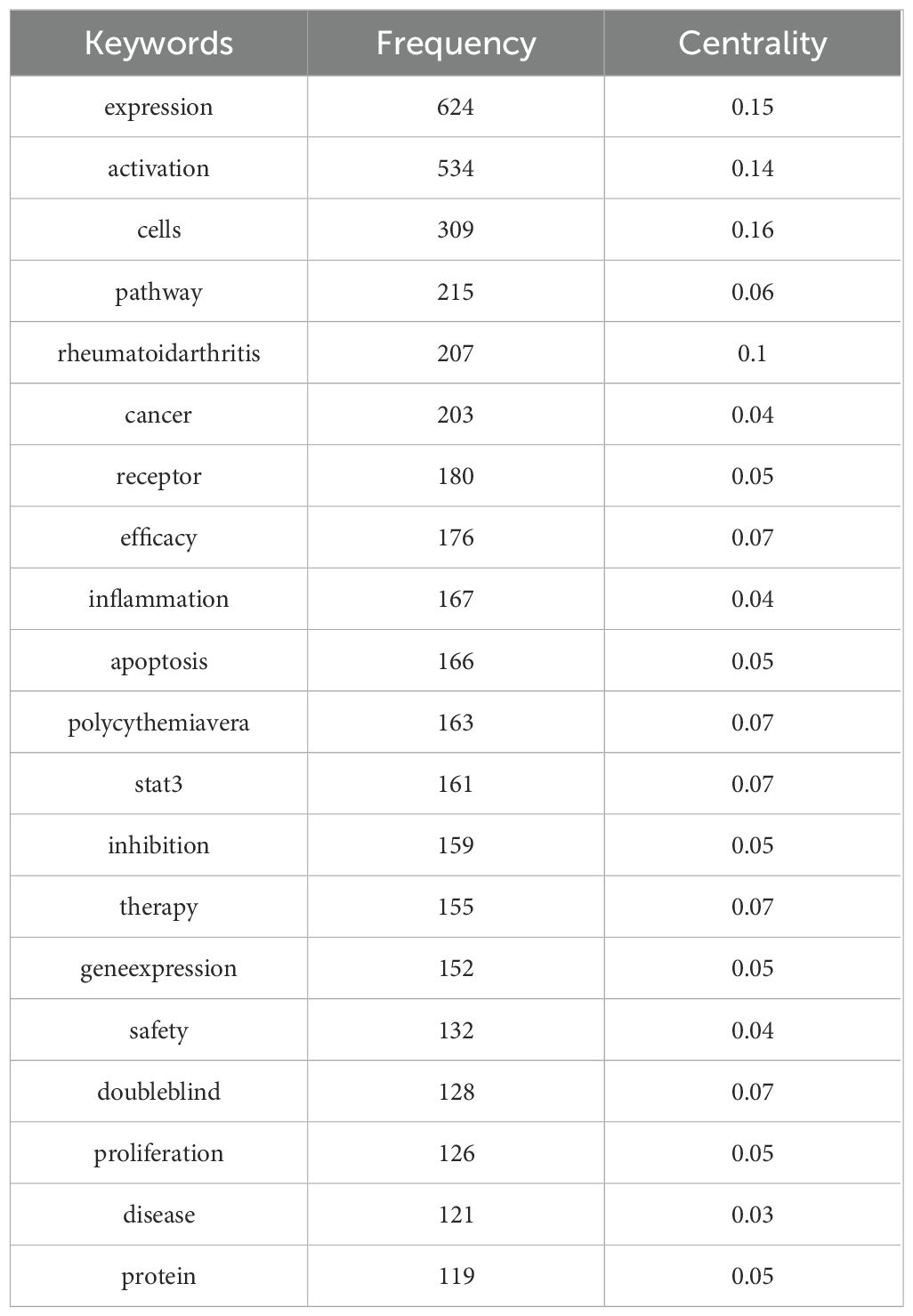
Keywords typically serve to summarize and condense, thereby enhancing comprehension of the article’s principal themes. The literature keyword co-occurrence map (Figure 5) comprises 174 nodes and 1461 links. The twenty most frequent keywords (Table 3) pertain to mechanisms including gene expression (14), activation (15), pathways (16), and apoptosis (17), and are closely associated with diseases such as rheumatoid arthritis and cancer. They also encompass the analysis of safety and efficacy in clinical treatment. The keywords exhibiting a centrality exceeding 0.1 in the keyword co-occurrence analysis are expression (18), activation (19), cells (20), and rheumatoid arthritis (21), indicating that mechanistic research and rheumatoid arthritis are significant aspects of JAK1 research.
2.3.7 Keyword cluster graph
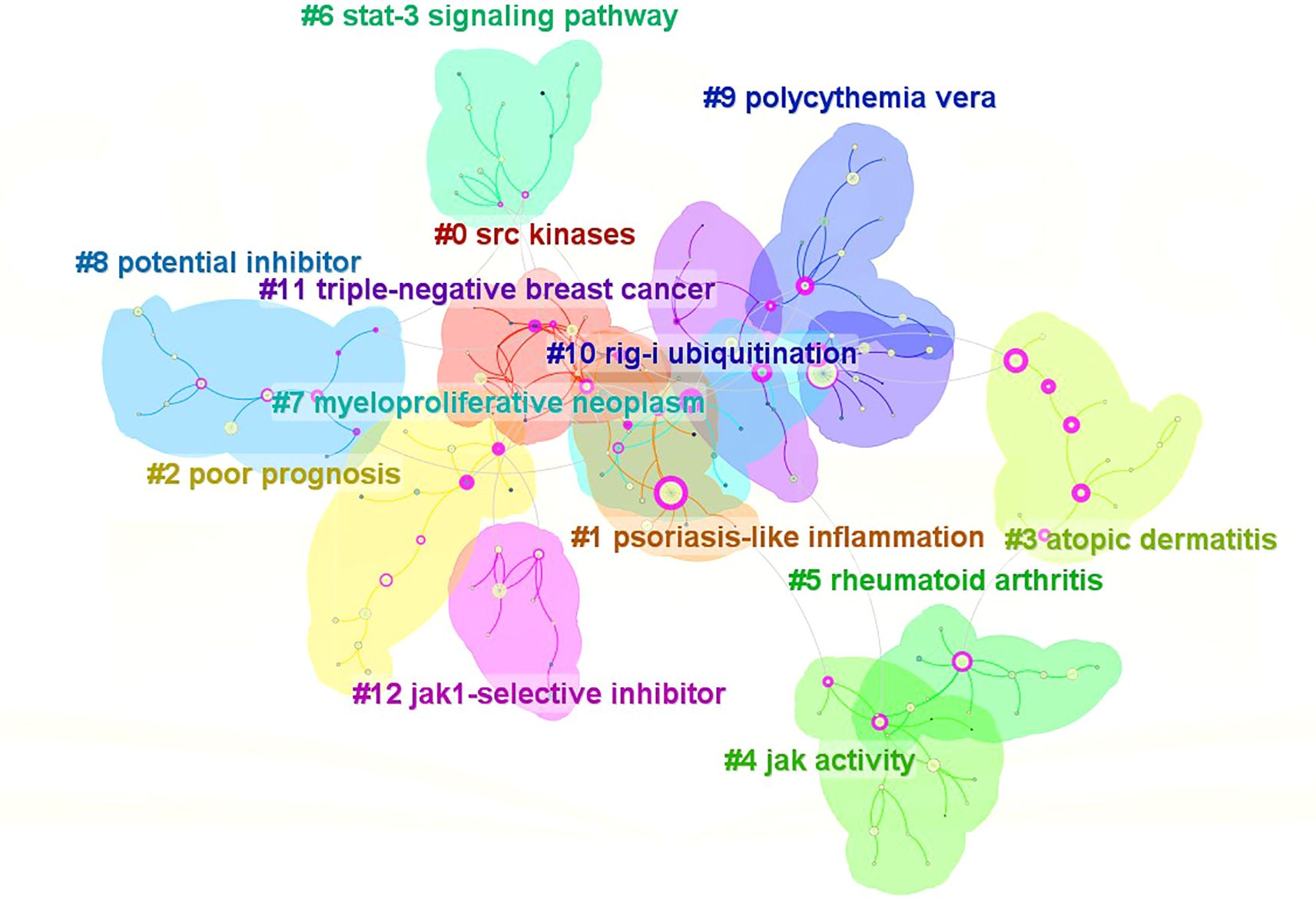
Utilizing the keyword co-occurrence map, the log-likelihood ratio (LLR) algorithm clusters the literature (Figure 6), resulting in 13 cluster labels. The cluster labels of the literature keywords are: #0(srckinasesSRC), #1(psoriasis-likeinflammation), #2(poorprognosis), #3(atopicdermatitis), #4(jakactivityjak), #5(rheumatoidarthritis), #6(stat-3signalingpathway), #7(myeloproliferativeneoplasm), #8(potentialinhibitor), #9(polycythemiavera), #10(rig-iubiquitination), #11(triple-negativebreastcancer), #12(jak1-selectiveinhibitor).Clusters #0, #4, #6, and #10 primarily investigate the mechanism of JAK1; clusters #1, #3, #5, #7, #9, and #11 predominantly examine the disease spectrum of JAK1; clusters #8 and #12 chiefly focus on the clinical application of JAK1.
The literature cluster module value (Q)=0.7927(>0.3) signifies that the network structure of the cluster results is exceptional. The average contour value (S) serves as a metric for assessing the homogeneity of the network. A value approaching 1 indicates greater homogeneity within the network. If the value exceeds 0.7, it indicates that the cluster results are reliable. This study reveals that the average outline value for clustering in Chinese and English literature is 0.9374 (>0.7), signifying the credibility of the clustering results. The keyword cluster map of the literature indicates that each cluster overlaps and intersects, demonstrating the strong interconnection among them. The literature primarily focuses on the mechanistic research of JAK1 and the clinical investigation of surgical and tumor-associated diseases linked to skin inflammation.
2.3.8 Keyword timeline chart and keywords with the strongest citation bursts analysis
A keyword timeline chart and highlight analysis facilitate comprehension of the research landscape in this domain and enable the identification of research trends. This study employs the keyword cluster map, utilizing the year as the horizontal axis and the cluster label as the vertical axis, to analyze the keyword timeline diagram using CiteSpace software (Figure 7A).
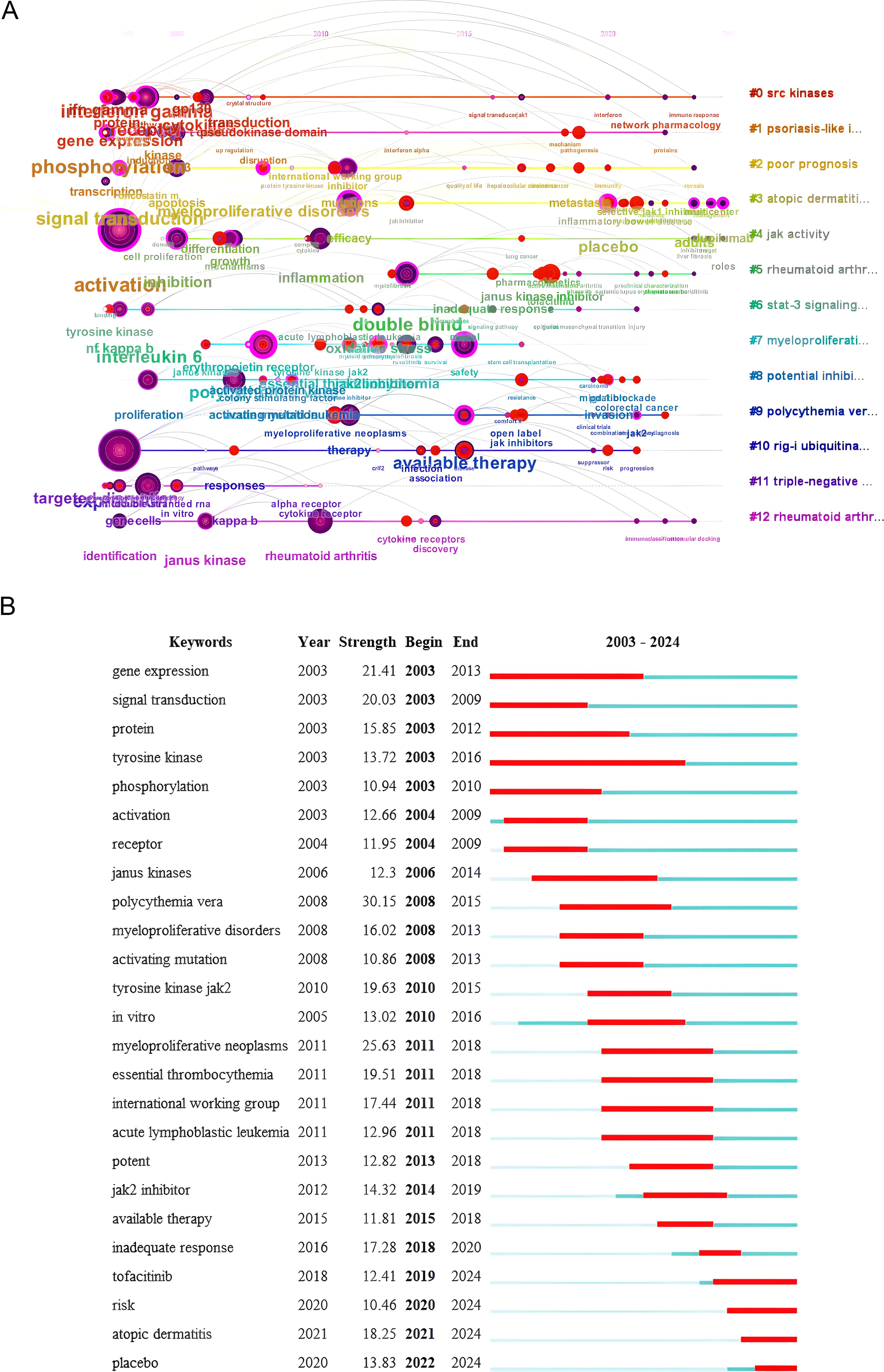
Figure 7. (A) Keyword timeline chart (2003–2024). (B) Keywords with the strongest citation bursts (2003–2024).
The keyword timeline diagram shows that the SRC kinase (cluster #0) has been widely studied since 2003. Atopic dermatitis (cluster #3) and JAK1-selective inhibitors (cluster #12) have become research hotspots in recent years. This implies that the main research focus and trend of JAK1 in the future will focus on clinical trials and mechanism research.
Keywords with the Strongest Citation Bursts refer to a significant increase in the frequency of a certain keyword in a short period, which indicates that research with high attention during this period can be used to determine hot spots and trends in the research field. “Begin” and “End” indicate the start and end time of Keywords with the strongest citation bursts, and “Strength” indicates the strength of Keywords with the strongest citation bursts. Increased strength correlates with enhanced influence.
Figure 7B shows the keywords with the strongest citation bursts analysis of JAK1 research literature. The top five keywords with the strongest citation bursts are polycythemiavera, myeloproliferativeneoplasms, geneexpression, signaltransduction, tyrosinekinasejak2. From the perspective of the length of highlight time, JAK1 literature research can be divided into three stages: from 2003 to 2009, the research focus was on the mechanism of JAK1; from 2008 to 2018, the main focus was on the combination of JAK1 and clinical diseases; from 2012 to 2024, the research focus began to shift to the development of JAK1 clinical drugs and their safety and efficacy.
3 Discussion
3.1 Advancements in understanding the mechanism of action of JAK1-associated signaling pathways
3.1.1 Inhibit the proliferation of cancer cells
Numerous studies have demonstrated that JAK1-related signaling pathways exhibit significant Anti-cancer efficacy (22, 23). The JAK/STAT signaling pathway suppresses the proliferation, differentiation, and apoptosis of tumor cells by inhibiting the activity of the suppressor of cytokine signaling (SOCS) family (24). In patients with colorectal cancer, JAK1 overexpression was significantly correlated with reduced survival, and the inhibition of JAK1 protein expression can impede cancer cell proliferation and progression, thereby effectively treating tumors (25). Moreover, JAK/STAT signaling pathways can combat cancer by modulating energy metabolism (26), influencing the immune microenvironment (27), and managing drug resistance (28), among other mechanisms.
3.1.2 Regulate cell autophagy
Autophagy is the process of sending abnormal cell components in the body, such as unfolded proteins, aging and damaged cells, and accumulated harmful substances, to lysosomes or vesicles for digestion, degradation, and recycling. The occurrence of autophagy provides cells with the energy and environment needed for survival, which is a self-protection mechanism for somatic cells (29). The lack or overactivation of autophagy will cause the destruction of the normal structure of cells and cell death. Therefore, the balance of the autophagy process plays an important role in maintaining the stability of the intracellular environment (30). The JAK/STAT signaling pathway is an important pathway involved in autophagy. Many studies have shown (31, 32) that by inhibiting the expression of JAK/STAT-related signaling pathways, cancer cell autophagy can be regulated, thereby inhibiting the proliferation of cancer cells and achieving the purpose of inhibiting the malignant progression of cancer. Simultaneously, it modulates cellular autophagy through JAK/STAT-associated signaling pathways, which is significantly critical in diseases such as rheumatoid arthritis and diabetes (33, 34).
3.1.3 Control the inflammatory reaction
The JAK-STAT pathway is among the most expedited pathways in inflammation and serves as an essential pathway for inflammatory signaling (35). By inhibiting the activation of JAK/STAT-associated signaling pathways and modulating the release of various inflammatory mediators, it can ameliorate airway inflammation, influence skin barrier integrity, and restore the pathological condition of the gastric mucosa, thereby yielding a favorable therapeutic outcome for pulmonary disorders, inflammatory dermatoses, gastrointestinal ailments, and more (36–38).
3.1.4 Inhibit oxidative stress reaction
Oxidative stress is a phenomenon of oxidation or imbalance in the body’s antioxidant system (39). When oxidation should be stimulated, the original redox dynamic balance in the cell is broken, resulting in the production of a large number of oxidation intermediate products, ROS, thus causing cell dysfunction and tissue organ damage. When the JAK1/STAT1 signaling pathway is inhibited, the oxidative stress response lobe is significantly inhibited, reducing brain tissue apoptosis, which reduces the apoptosis rate of neurons, plays a protective role in the brain, and is crucial in ischemia-reperfusion injury and many cardiovascular diseases (40).
3.2 Trend analysis of JAK1 clinical inhibitor research
(41–43) Since the first JAK inhibitor was discovered in the 1990s, many JAK1-targeting drugs have been approved globally. JAK1 has also become a prominent target for future drug development. To date, nine JAK1 drugs have been approved worldwide, which can be classified into two categories based on target selectivity: first-generation pan-JAK inhibitors (e.g., ruxolitinib, tofacitinib, and baricitinib) with lower selectivity and broader binding spectra, and second-generation agents with higher specificity. These first-generation inhibitors simultaneously bind to JAK1, JAK2, JAK3, TYK2, and other homologous targets, thereby blocking multiple downstream signaling pathways, thereby treating a variety of autoimmune diseases (41–43).
Second-generation inhibitors with high selectivity and specific binding to JAK1, including upadacitinib, abrocitinib, etc. For the first-generation pan-JAK inhibitors, safety has grown to be a major issue, though. For instance, improper inhibition of JAK2 might cause thrombocytopenia and anemia (44); improper inhibition of JAK3 might cause immunodeficiency and infection (45). Second-generation inhibitors have tremendously enhanced the safety of clinical drug use. They can specifically target particular JAK family members, so blocking particular disease-related signalling pathways without compromising the roles of other cytokines. Good clinical efficacy and safety of JAK1 inhibitors have been demonstrated by several studies (46–48). It is well known that JAK1 inhibitors help to treat atopic dermatitis and rheumatoid arthritis (49–51). Furthermore, the most recent development trend (52–54), and has great application possibilities is applying JAK1 inhibitors in gastrointestinal diseases, rheumatoid arthritis, and alopecia areata.
3.3 Clinical therapeutic effects of JAK1
JAK1 inhibitors have been demonstrated to possess multiple pharmacological effects and have become therapeutic agents for various diseases, including inflammatory bowel disease (55), rheumatoid arthritis (51), autoimmune diseases (56), and various cancers (57).
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract, with Crohn’s disease (CD) and ulcerative colitis being its two primary forms (58). JAK1 inhibitors modulate the innate and adaptive immune responses involved in IBD by blocking JAK-mediated inflammatory pathways, thereby reducing chronic gastrointestinal inflammation (59). In the treatment of rheumatoid arthritis (RA), Janus kinase inhibitors (JAK inhibitors) are a new class of targeted therapies in addition to biologics (60). Studies have shown that JAK1 inhibitors can alleviate RA pain caused by both inflammatory and non-inflammatory mechanisms, and various cytokines directly or indirectly regulated by the JAK/STAT pathway play a crucial role in the various mechanisms mediating RA pain (61). Cytokines play a central role in the pathophysiology of autoimmune diseases. JAK inhibitors are increasingly being used in the treatment of autoimmune diseases such as rheumatoid arthritis, systemic sclerosis, alopecia areata, vitiligo, and systemic lupus erythematosus (56, 62, 63). Currently, JAK1 inhibitors are being extensively explored in oncology. Certain drugs, such as Ruxolitinib, have undergone clinical trials to assess their antitumour efficacy in various solid tumours. These include pancreatic cancer (64), breast cancer (65), metastatic lung cancer (66), non-small cell lung cancer (67), liver cancer (68), and myeloproliferative tumours (69), among others, with promising clinical outcomes. In summary, JAK1 shows great potential in the treatment of the aforementioned diseases.
4 Conclusion
This study used VOSviewer and Citespace to comprehensively analyze the research on JAK1 over the past 20 years and systematically reviewed the development of this field. The results showed that JAK1 mechanism research, clinical drug research and related disease spectrum were the main research areas during this period. Importantly, JAK1-related research has shifted over time from initial mechanistic studies to drug development in combination with clinical disease. It has been proven to be effective and safe in the treatment of Rheumatoid Arthritis, atopic dermatitis, alopecia areata and gastrointestinal diseases; this emerging trend is expected to become an important research focus in the future. As a pivotal target in autoimmune therapeutics, JAK1 inhibitors demonstrate global potential for transformative drug development. Future research should emphasize international collaboration across academic institutions by leveraging shared expertise in JAK1 mechanisms and clinical validation to address global healthcare needs.
Author contributions
CY: Conceptualization, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JC: Methodology, Project administration, Validation, Writing – original draft. XX: Validation, Writing – original draft. WC: Software, Visualization, Writing – original draft. EH: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the NATCM's Project of High-level Construction of Key TCM Disciplines (No.zyyzdxk-2023111) and Jiangxi Traditional Chinese Medicine Administration Clinical Characteristics and Advantages of Traditional Chinese Medicine Project (No. YWB2021120703) and the Jiangxi Provincial Chinese Medicine Standard Committee (No. 2022B10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gadina M, Chisolm DA, Philips RL, McInness IB, Changelian PS, and O’Shea JJ. Translating JAKs to jakinibs. J Immunol. (2020) 204:2011–20. doi: 10.4049/jimmunol.1901477
2. Spinelli FR, Colbert RA, and Gadina M. JAK1: Number one in the family; number one in inflammation? Rheumatol (Oxford). (2021) 60:ii3–ii10. doi: 10.1093/rheumatology/keab024
3. Horesh ME, Martin-Fernandez M, Gruber C, Buta S, Le Voyer T, Puzenat E, et al. Individuals with JAK1 variants are affected by syndromic features encompassing autoimmunity, atopy, colitis, and dermatitis. J Exp Med. (2024) 221(6):e20232387. doi: 10.1084/jem.20232387
4. Li R, Liang Q, Yang Q, Dai W, Xiao Y, Pan H, et al. Hexahydrocurcumin from Zingiberis rhizoma attenuates lipopolysaccharide-induced acute pneumonia through JAK1/STAT3 signaling pathway. Phytomedicine. (2024) 122:155141. doi: 10.1016/j.phymed.2023.155141
5. Xu H, Zhang X, Wang X, Li B, Yu H, Quan Y, et al. Cellular spermine targets JAK signaling to restrain cytokine-mediated autoimmunity. Immunity. (2024) 57:1796–1811.e8. doi: 10.1016/j.immuni.2024.05.025
6. Mathew D, Marmarelis ME, Foley C, Bauml JM, Ye D, Ghinnagow R, et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. (2024) 384:eadf1329. doi: 10.1126/science.adf1329
7. Bader MS and Meyer SC. JAK2 in myeloproliferative neoplasms: still a protagonist. Pharm (Basel). (2022) 15(2):160. doi: 10.3390/ph15020160
8. Perner F, Perner C, Ernst T, and Heidel FH. Roles of JAK2 in aging, inflammation, hematopoiesis and Malignant transformation. Cells. (2019) 8(8):854. doi: 10.3390/cells8080854
9. O'Shea JJ, Husa M, Li D, Hofmann SR, Watford W, Roberts JL, et al. Jak3 and the pathogenesis of severe combined immunodeficiency. Mol Immunol. (2004) 41:727–37. doi: 10.1016/j.molimm.2004.04.014
10. Uckun FM, Pitt J, and Qazi S. JAK3 pathway is constitutively active in B-lineage acute lymphoblastic leukemia. Expert Rev Anticancer Ther. (2011) 11:37–48. doi: 10.1586/era.10.203
11. Ellegaard O and Wallin JA. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics. (2015) 105:1809–31. doi: 10.1007/s11192-015-1645-z
12. Ninkov A, Frank JR, and Maggio LA. Bibliometrics: Methods for studying academic publishing. Perspect Med Educ. (2022) 11:173–6. doi: 10.1007/s40037-021-00695-4
13. van Eck NJ and Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. (2017) 111:1053–70. doi: 10.1007/s11192-017-2300-7
14. Padmanabhan S, Gaire B, Zou Y, Uddin MM, and Vancurova I. IFNγ-induced PD-L1 expression in ovarian cancer cells is regulated by JAK1, STAT1 and IRF1 signaling. Cell Signal. (2022) 97:110400. doi: 10.1016/j.cellsig.2022.110400
15. Sun Y, Gong W, and Zhang S. METTL3 promotes colorectal cancer progression through activating JAK1/STAT3 signaling pathway. Cell Death Dis. (2023) 14:765. doi: 10.1038/s41419-023-06287-w
16. Zhang X, Hu B, Sun YF, Huang XW, Cheng JW, Huang A, et al. Arsenic trioxide induces differentiation of cancer stem cells in hepatocellular carcinoma through inhibition of LIF/JAK1/STAT3 and NF-kB signaling pathways synergistically. Clin Transl Med. (2021) 11:e335. doi: 10.1002/ctm2.335
17. Xu F, Chen R, Shen Y, Liu H, Hu L, and Zhu L. CircUBXN7 suppresses cell proliferation and facilitates cell apoptosis in lipopolysaccharide-induced cell injury by sponging miR-622 and regulating the IL6ST/JAK1/STAT3 axis. Int J Biochem Cell Biol. (2022) 153:106313. doi: 10.1016/j.biocel.2022.106313
18. Chen H, Li M, Sanchez E, Soof CM, Bujarski S, Ng N, et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br J Haematol. (2020) 188:283–94. doi: 10.1111/bjh.16158
19. Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. (2018) 10:117–27. doi: 10.1177/1759720X18776224
20. Frede N, Lorenzetti R, Hüppe JM, Janowska I, Troilo A, Schleyer MT, et al. JAK inhibitors differentially modulate B cell activation, maturation and function: A comparative analysis of five JAK inhibitors in an in-vitro B cell differentiation model and in patients with rheumatoid arthritis. Front Immunol. (2023) 14:1087986. doi: 10.3389/fimmu.2023.1087986
21. Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis. (2015) 74:1311–6. doi: 10.1136/annrheumdis-2014-206028
22. Jia S, Jia Y, Liang S, and Wu L. Research progress of multi-target HDAC inhibitors blocking the BRD4-LIFR-JAK1-STAT3 signaling pathway in the treatment of cancer. Bioorg Med Chem. (2024) 110:117827. doi: 10.1016/j.bmc.2024.117827
23. Yu Z, Zhang Q, Wei S, Zhang Y, Zhou T, Zhang Q, et al. CD146(+)CAFs promote progression of endometrial cancer by inducing angiogenesis and vasculogenic mimicry via IL-10/JAK1/STAT3 pathway. Cell Commun Signal. (2024) 22:170. doi: 10.1186/s12964-024-01550-9
24. Xia T, Zhang M, Lei W, Yang R, Fu S, Fan Z, et al. Advances in the role of STAT3 in macrophage polarization. Front Immunol. (2023) 14:1160719. doi: 10.3389/fimmu.2023.1160719
25. Patil N, Abdelrahim OG, Leupold JH, and Allgayer H. JAK1 is a novel target of tumor- and invasion-suppressive microRNA 494-5p in colorectal cancer. Cancers (Basel). (2023) 16(1):24. doi: 10.3390/cancers16010024
26. Huang WL, Abudureheman T, Xia J, Chu L, Zhou H, Zheng WW, et al. CDK9 inhibitor induces the apoptosis of B-cell acute lymphocytic leukemia by inhibiting c-myc-mediated glycolytic metabolism. Front Cell Dev Biol. (2021) 9:641271. doi: 10.3389/fcell.2021.641271
27. Liu L, Wang C, Li S, Qu Y, Xue P, Ma Z, et al. ERO1L is a novel and potential biomarker in lung adenocarcinoma and shapes the immune-suppressive tumor microenvironment. Front Immunol. (2021) 12:677169. doi: 10.3389/fimmu.2021.677169
28. Benabbou N, Mirshahi P, Cadillon M, Soria J, Therwath A, and Mirshahi M. Hospicells promote upregulation of the ATP-binding cassette genes by insulin-like growth factor-I via the JAK2/STAT3 signaling pathway in an ovarian cancer cell line. Int J Oncol. (2013) 43:685–94. doi: 10.3892/ijo.2013.2017
29. Liu S, Yao S, Yang H, Liu S, and Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. (2023) 14:648. doi: 10.1038/s41419-023-06154-8
30. Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. (2021) 54:437–53. doi: 10.1016/j.immuni.2021.01.018
31. Gao C, Yan Y, Chen G, Wang T, Luo C, Zhang M, et al. Autophagy activation represses pyroptosis through the IL-13 and JAK1/STAT1 pathways in a mouse model of moderate traumatic brain injury. ACS Chem Neurosci. (2020) 11:4231–9. doi: 10.1021/acschemneuro.0c00517
32. Dong G, You M, Fan H, Ding L, Sun L, and Hou Y. STS-1 promotes IFN-α induced autophagy by activating the JAK1-STAT1 signaling pathway in B cells. Eur J Immunol. (2015) 45:2377–88. doi: 10.1002/eji.201445349
33. Xiao T, Cheng X, Zhi Y, Tian F, Wu A, Huang F, et al. Ameliorative effect of Alangium chinense (Lour.) Harms on rheumatoid arthritis by reducing autophagy with targeting regulate JAK3-STAT3 and COX-2 pathways. J Ethnopharmacol. (2024) 319:117133. doi: 10.1016/j.jep.2023.117133
34. Chen D, Liu Y, Chen J, Lin H, Guo H, Wu Y, et al. JAK/STAT pathway promotes the progression of diabetic kidney disease via autophagy in podocytes. Eur J Pharmacol. (2021) 902:174121. doi: 10.1016/j.ejphar.2021.174121
35. Surbek M, Tse W, Moriggl R, and Han X. A centric view of JAK/STAT5 in intestinal homeostasis, infection, and inflammation. Cytokine. (2021) 139:155392. doi: 10.1016/j.cyto.2020.155392
36. Lv M, Shao J, Jiang F, and Liu J. Curcumol may alleviate psoriasis-like inflammation by inhibiting keratinocyte proliferation and inflammatory gene expression via JAK1/STAT3 signaling. Aging (Albany NY). (2021) 13:18392–403. doi: 10.18632/aging.203287
37. Xu F, Wang S, Wang Y, Hu L, and Zhu L. Inhibition of gp130 alleviates LPS-induced lung injury by attenuating apoptosis and inflammation through JAK1/STAT3 signaling pathway. Inflammation Res. (2023) 72:493–507. doi: 10.1007/s00011-022-01686-9
38. Cao L, Tan Q, Zhu R, Ye L, Shi G, and Yuan Z. LncRNA MIR4435-2HG suppression regulates macrophage M1/M2 polarization and reduces intestinal inflammation in mice with ulcerative colitis. Cytokine. (2023) 170:156338. doi: 10.1016/j.cyto.2023.156338
39. Anila PA, Sutha J, Nataraj D, and Ramesh M. In vivo evaluation of Nano-palladium toxicity on larval stages and adult of zebrafish (Danio rerio). Sci Total Environ. (2021) 765:144268. doi: 10.1016/j.scitotenv.2020.144268
40. Kurdi M and Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. (2009) 297:H1545–1556. doi: 10.1152/ajpheart.00032.2009
41. Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. (2012) 367:495–507. doi: 10.1056/NEJMoa1109071
42. Verstovsek S, Mesa RA, Gotlib J, Gupta V, DiPersio JF, Catalano JV, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. (2012) 366:799–807. doi: 10.1056/NEJMoa1110557
43. Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. (2017) 376:652–62. doi: 10.1056/NEJMoa1608345
44. Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: A randomized clinical trial. JAMA Oncol. (2018) 4:652–9. doi: 10.1001/jamaoncol.2017.5818
45. Genovese MC, van Vollenhoven RF, Pacheco-Tena C, Zhang Y, and Kinnman N. VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. (2016) 68:46–55. doi: 10.1002/art.39473
46. Kirby JS, Okun MM, Alavi A, Bechara FG, Zouboulis CC, Brown K, et al. Efficacy and safety of the oral Janus kinase 1 inhibitor povorcitinib (INCB054707) in patients with hidradenitis suppurativa in a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study. J Am Acad Dermatol. (2024) 90:521–9. doi: 10.1016/j.jaad.2023.10.034
47. He Q, Sun X, Niu J, Yang J, Wang Y, Huang C, et al. A novel JAK1 inhibitor SHR0302 combined with prednisone for first-line treatment of chronic graft-versus-host disease: A phase I clinical trial. Cell Transplant. (2024) 33:9636897241254678. doi: 10.1177/09636897241254678
48. King B, Senna MM, Mesinkovska NA, Lynde C, Zirwas M, Maari C, et al. Efficacy and safety of deuruxolitinib, an oral selective Janus kinase inhibitor, in adults with alopecia areata: Results from the Phase 3 randomized, controlled trial (THRIVE-AA1). J Am Acad Dermatol. (2024) 91(5):880–8. doi: 10.1016/j.jaad.2024.06.097
49. Blauvelt A, Silverberg JI, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. (2022) 86:104–12. doi: 10.1016/j.jaad.2021.05.075
50. Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. (2019) 71:1788–800. doi: 10.1002/art.41032
51. Singh JA. Filgotinib, a JAK1 inhibitor, for treatment-resistant rheumatoid arthritis. JAMA. (2019) 322:309–11. doi: 10.1001/jama.2019.9056
52. Sandborn WJ, Ghosh S, Panes J, Schreiber Serror, Tanida S, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. (2020) 158:2139–2149.e14. doi: 10.1053/j.gastro.2020.02.030
53. King B, Mesinkovska N, Mirmirani P, Bruce S, Kempers S, Guttman-Yassky E, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe alopecia areata. J Am Acad Dermatol. (2022) 87:306–13. doi: 10.1016/j.jaad.2022.03.045
54. Sandborn WJ, Danese S, Leszczyszyn J, Romatowski J, Altintas E, Peeva E, et al. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin Gastroenterol Hepatol. (2023) 21:2616–2628.e7. doi: 10.1016/j.cgh.2022.12.029
55. Harris C and Cummings JRF. JAK1 inhibition and inflammatory bowel disease. Rheumatol (Oxford). (2021) 60:ii45–51. doi: 10.1093/rheumatology/keaa896
56. Jamilloux Y, El Jammal T, Vuitton L, Gerfaud-Valentin M, Kerever S, and Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. (2019) 18:102390. doi: 10.1016/j.autrev.2019.102390
57. Wei XH and Liu YY. Potential applications of JAK inhibitors, clinically approved drugs against autoimmune diseases, in cancer therapy. Front Pharmacol. (2023) 14:1326281. doi: 10.3389/fphar.2023.1326281
58. Ordás I, Eckmann L, Talamini M, Baumgart DC, and Sandborn WJ. Ulcerative colitis. Lancet. (2012) 380:1606–19. doi: 10.1016/S0140-6736(12)60150-0
59. Coskun M, Salem M, Pedersen J, and Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. (2013) 76:1–8. doi: 10.1016/j.phrs.2013.06.007
60. Langbour C, Rene J, Goupille P, and Carvajal Alegria G. Efficacy of Janus kinase inhibitors in rheumatoid arthritis. Inflammation Res. (2023) 72:1121–32. doi: 10.1007/s00011-023-01717-z
61. Simon LS, Taylor PC, Choy EH, Sebba A, Quebe A, Knopp KL, et al. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin Arthritis Rheumatol. (2021) 51:278–84. doi: 10.1016/j.semarthrit.2020.10.008
62. Moriana C, Moulinet T, Jaussaud R, and Decker P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun Rev. (2022) 21:103168. doi: 10.1016/j.autrev.2022.103168
63. Muddebihal A, Khurana A, and Sardana K. JAK inhibitors in dermatology: the road travelled and path ahead, a narrative review. Expert Rev Clin Pharmacol. (2023) 16:279–95. doi: 10.1080/17512433.2023.2193682
64. Ruxolitinib benefits some with pancreatic cancer. Cancer Discov. (2015) 5:1231. doi: 10.1158/2159-8290.CD-NB2015-142
65. Schneider J, Jeon YW, Suh YJ, and Lim ST. Effects of ruxolitinib and calcitriol combination treatment on various molecular subtypes of breast cancer. Int J Mol Sci. (2022) 23(5):2535. doi: 10.3390/ijms23052535
66. Taverna JA, Hung CN, DeArmond DT, Chen M, Lin CL, Osmulski PA, et al. Single-cell proteomic profiling identifies combined AXL and JAK1 inhibition as a novel therapeutic strategy for lung cancer. Cancer Res. (2020) 80:1551–63. doi: 10.1158/0008-5472.CAN-19-3183
67. Patel MR, Dash A, Jacobson BA, Ji Y, Baumann D, Ismail K, et al. JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models. Cancer Gene Ther. (2019) 26:411–8. doi: 10.1038/s41417-018-0074-6
68. Wilson GS, Tian A, Hebbard L, Duan W, George J, Li X, et al. Tumoricidal effects of the JAK inhibitor Ruxolitinib (INC424) on hepatocellular carcinoma in vitro. Cancer Lett. (2013) 341:224–30. doi: 10.1016/j.canlet.2013.08.009
Keywords: jak1, bibliometric, visual analysis, research hotspots, citespace
Citation: Yu C, Cao J, Xie X, Chen W and Hong E (2025) A bibliometric and visual analysis of Jak1 to explore trends and frontiers. Front. Oncol. 15:1537508. doi: 10.3389/fonc.2025.1537508
Received: 01 December 2024; Accepted: 23 June 2025;
Published: 17 July 2025.
Edited by:
Zhenhua Chen, Jinzhou Medical University, ChinaReviewed by:
James K. Fields, Johns Hopkins University, United StatesAna Basquiera, Private University Hospital of Córdoba, Argentina
Copyright © 2025 Yu, Cao, Xie, Chen and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ensi Hong, MjIyNDkwMTk2NUBxcS5jb20=
†These authors have contributed equally to this work
 Cong Yu
Cong Yu Jiamin Cao2†
Jiamin Cao2† Wenguang Chen
Wenguang Chen