- 1Department of Oncology, The Second Affiliated Hospital, Guizhou Medical University, Kaili, China
- 2Department of Pathology, Shengli Oilfield Central Hospital, Dong ying, China
- 3Department of Pathology, The Second Affiliated Hospital, Guizhou Medical University, Kaili, China
- 4Department of Oncology, Yibin First People's Hospital, Yibin, China
- 5Xinzhou Town Center Health Center Internal Medicine, Huangping, China
Purpose: This study investigates the feasibility of utilizing a combination of tumor-infiltrating lymphocytes (TILs) and residual cancer burden (RCB) to predict the prognosis of breast cancer (BC) individuals post-neoadjuvant chemotherapy (NAC).
Methods: Patients with BC who underwent surgery following NAC were recruited from three medical centers for this research. RCB and TIL levels were determined using established guidelines, and the integration of RCB and TIL assessments was termed “RCB-TILs”. The relationship between RCB-TILs and patients’ clinicopathological variables was analyzed, alongside the link between RCB-TILs and disease-free survival (DFS).
Results: The study comprised 242 BC patients who underwent NAC, among whom 98 were identified as RCB-TILs (+), while 144 were classified as RCB-TILs (-). Multivariate analysis demonstrated that RCB-TILs (+) served as an independent factor impacting recurrence following NAC across all BC patients (hazard ratio [HR] = 0.225, 95% confidence interval [CI]: 0.099 – 0.508, P < 0.001), including hormone receptor-positive patients (HR = 0.213, 95%CI: 0.067 – 0.682, P = 0.009), HER2-positive patients (HR = 0.216, 95%CI: 0.048 – 0.968, P = 0.045), and those with triple-negative BC (HR = 0.220, 95%CI: 0.049 – 0.989, P = 0.048).
Conclusions: RCB-TILs (+) are correlated with extended DFS in BC patients who have undergone surgery post-NAC. In these individuals, RCB-TILs may provide a more sensitive predictor of DFS than RCB or TILs individually.
1 Introduction
Breast cancer (BC) represents a prevalent malignant tumor among women, with both incidence and mortality rates ranking prominently in the World Cancer Spectrum. The majority of patients receive a diagnosis of lymph node metastasis, posing a significant threat to women’s health (1). Neoadjuvant chemotherapy (NAC) serves as a vital treatment modality for BC, capable of modifying the tumor microenvironment and impacting cancer cell viability (2). NAC is intended to downstage tumors, enhance surgical options, mitigate the risk of postoperative recurrence, and furnish data on drug sensitivity to inform subsequent treatment strategies (3). Nonetheless, due to the aggressive nature of tumor cells and their propensity for recurrence and metastasis, some patients experience unfavorable prognoses (4). Thus, monitoring the prognosis of BC patients undergoing NAC treatment holds considerable scientific and clinical value.
Prior research has indicated (5) that the residual cancer burden (RCB) index, which incorporates various elements such as the proportion of residual tumor cells and lymph node metastasis, serves as a tool to evaluate residual disease in BC patients post-surgery. Yau C et al. (6), through meta-analysis, have demonstrated that RCB is a significant determinant impacting the prognosis of BC patients. Nevertheless, the assessment of RCB focuses solely on residual disease status, neglecting the host immune response, potentially introducing bias into the accuracy of prognostic analysis. The adaptive immune response mediated by tumor-infiltrating lymphocytes (TILs) is crucial for effective and sustained anti-tumor activity. Within the tumor microenvironment, TILs are believed to play significant roles in immune response and regulation of tumor immune mechanisms (7). TILs correlate with treatment response and survival outcomes in various solid tumors and can predict disease-free survival (DFS) in cancer patients (8–10). Thus, the integration of RCB and TILs might provide more valuable prognostic insights. In this investigation, a novel “RCB-TILs” metric was established by integrating RCB and TILs, and its feasibility in predicting the prognosis of BC patients following NAC was assessed.
2 Materials and methods
2.1 Patients
A cohort of 242 individuals diagnosed with BC who received NAC prior to surgical procedures between January 2015 and December 2019 were incorporated in this investigation. These subjects were treated at three medical centers: the Second Affiliated Hospital of Guizhou Medical University, Shengli Oilfield Central Hospital, and the First People’s Hospital of Yibin. Diagnoses of stage II-III BC were made for all participants on the basis of the 8th edition of the American Joint Committee on Cancer TNM staging manual (11). Comprehensive clinical and pathological data were collected, encompassing age, histological characteristics, lymph node metastasis, and molecular subtypes. Prior to NAC, invasive BC was confirmed in the subjects through pathological biopsy. Surgical treatment was conducted following standard NAC regimens, and postoperative adjuvant therapy was tailored to each BC subtype. This study complied with the Declaration of Helsinki, with ethical approval obtained from the ethics committees of the three medical centers (approval No. 2023-Ethical Review-229). Informed consent was also secured from all participants involved in the study.
2.2 Molecular subtypes of BC
BC molecular subtypes were classified utilizing the immunohistochemical expression profiles of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67 (12). These subtypes are defined as follows: Luminal A, characterized by positivity for ER and PR with PR positivity of ≥ 20%, negative HER2, and Ki67 < 14%; Luminal B, which includes ER-positive, HER2-negative cases with any PR and Ki67 expression or those with ER positivity, PR negativity or PR < 20%, HER2 negativity, and Ki67 ≥ 14%; HER2-positive breast cancer (HER2BC), defined by HER2 positivity and ER/PR negativity; and triple-negative breast cancer (TNBC), marked by the absence of ER, PR, and HER2. For this study, Luminal A and Luminal B subtypes were grouped under hormone receptor-positive breast cancer (HRBC).
2.3 Histopathological evaluation of TILs
The histopathological evaluation of TILs was carried out in accordance with the International Immuno-Oncology Biomarker Working Group report (7) on sections of core needle biopsy specimens that were stained with hematoxylin and eosin (H&E) and obtained at diagnosis. Two pathologists independently conducted the assessment. Following the established criteria, the extent of mononuclear inflammatory cell infiltration surrounding the invasive tumor cell nests relative to the stromal area was categorized as ≥ 50%, 10% – 50%, or ≤ 10%. Cases exhibiting ≤ 10% infiltration were deemed TILs-negative, whereas those with greater infiltration were classified as TILs-positive.
2.4 Histopathological evaluation of RCB
As per the guidelines of the MD Anderson Cancer Center (13), the RCB is computed using the formula: RCB = 1.4 (proportion of invasive cancer × primary tumor diameter) 0.17 + [4 (1−0.75 number of positive lymph nodes) × largest metastasis diameter]0.17. The outcomes are divided into three distinct categories: minimal residual disease (RCB-I), moderate residual disease (RCB-II), and extensive residual disease (RCB-III). Given the more favorable prognosis linked with RCB-I in comparison to RCB-II and RCB-III, RCB-I is regarded as RCB-positive, whereas the latter are categorized as RCB-negative.
2.5 RCB-TILs assessment
RCB and TILs were combined as “RCB-TILs”. Cases exhibiting both positive RCB and TILs are classified as RCB-TILs positive [RCB-TILs (+)], whereas cases in which either RCB or TILs are negative are deemed RCB-TILs negative [RCB-TILs (-)].
2.6 Response assessment
The main outcome measure of the investigation was DFS, defined as the interval from surgical intervention to the recurrence of the disease (whether local or distant), death due to any cause, or the final follow-up.
2.7 Statistical analysis
The statistical analyses were executed utilizing SPSS 22.0 (IBM Corp., Armonk, USA). The relationships between various RCB-TIL levels and clinicopathological parameters were evaluated via the chi-square test. Survival outcomes were assessed employing Kaplan-Meier curves and contrasted utilizing the log-rank test. For the Cox regression analysis, an initial univariate analysis of the variables was conducted, followed by a multivariate analysis. The assessment metrics included the hazard ratio (HR) and the 95% confidence interval (CI). Statistical significance was established utilizing a threshold of P < 0.05.
3 Results
3.1 Relationship between RCB-TILs and clinicopathological characteristics of NAC BC patients
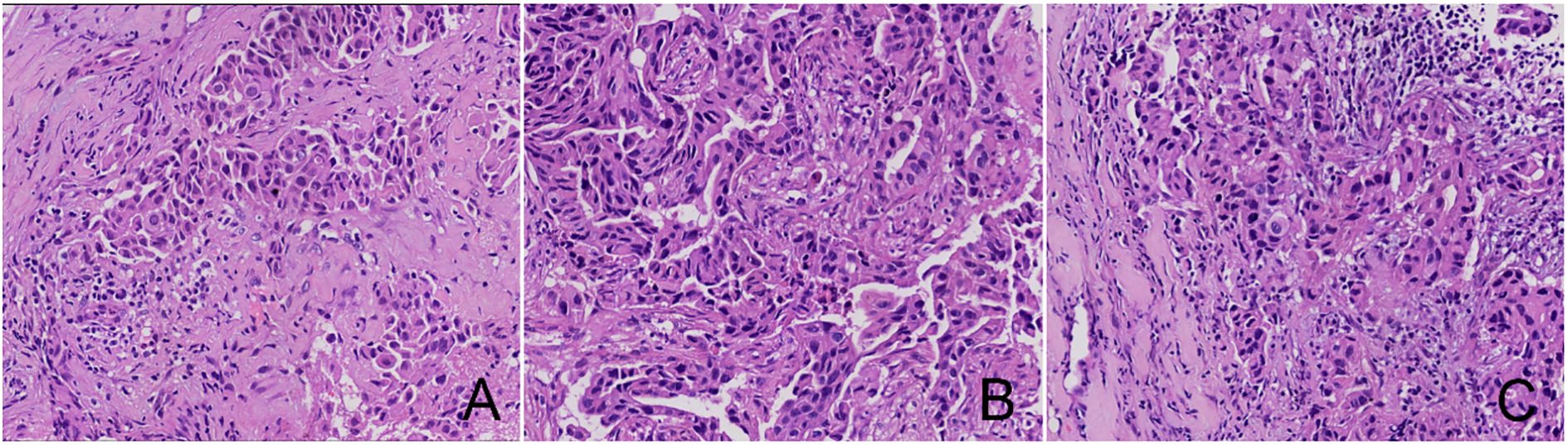
Table 1 displays the baseline characteristics of 242 participants (Figure 1 illustrates the HE staining of TILs). Among these participants, 98 (40.5%) were identified as RCB-TILs (+), whereas 144 (59.5%) were classified as RCB-TILs (-). RCB-TILs (+) was correlated with reduced vascular invasion (P = 0.011), a decreased number of lymph node metastases (P = 0.008), a smaller proportion of HER2BC (P = 0.024), and an elevated pathological complete response (PCR) rate (P = 0.014) compared to RCB-TILs (-). Moreover, analyses were conducted separately for each subtype. Within the HRBC subgroup, individuals with RCB-TILs (+) showed a diminished risk of lymph node metastasis (P = 0.019) and an increased PCR rate (P = 0.002), while in the HER2BC subgroup, RCB-TILs (+) was linked to reduced vascular invasion (P = 0.021) (Table 2).
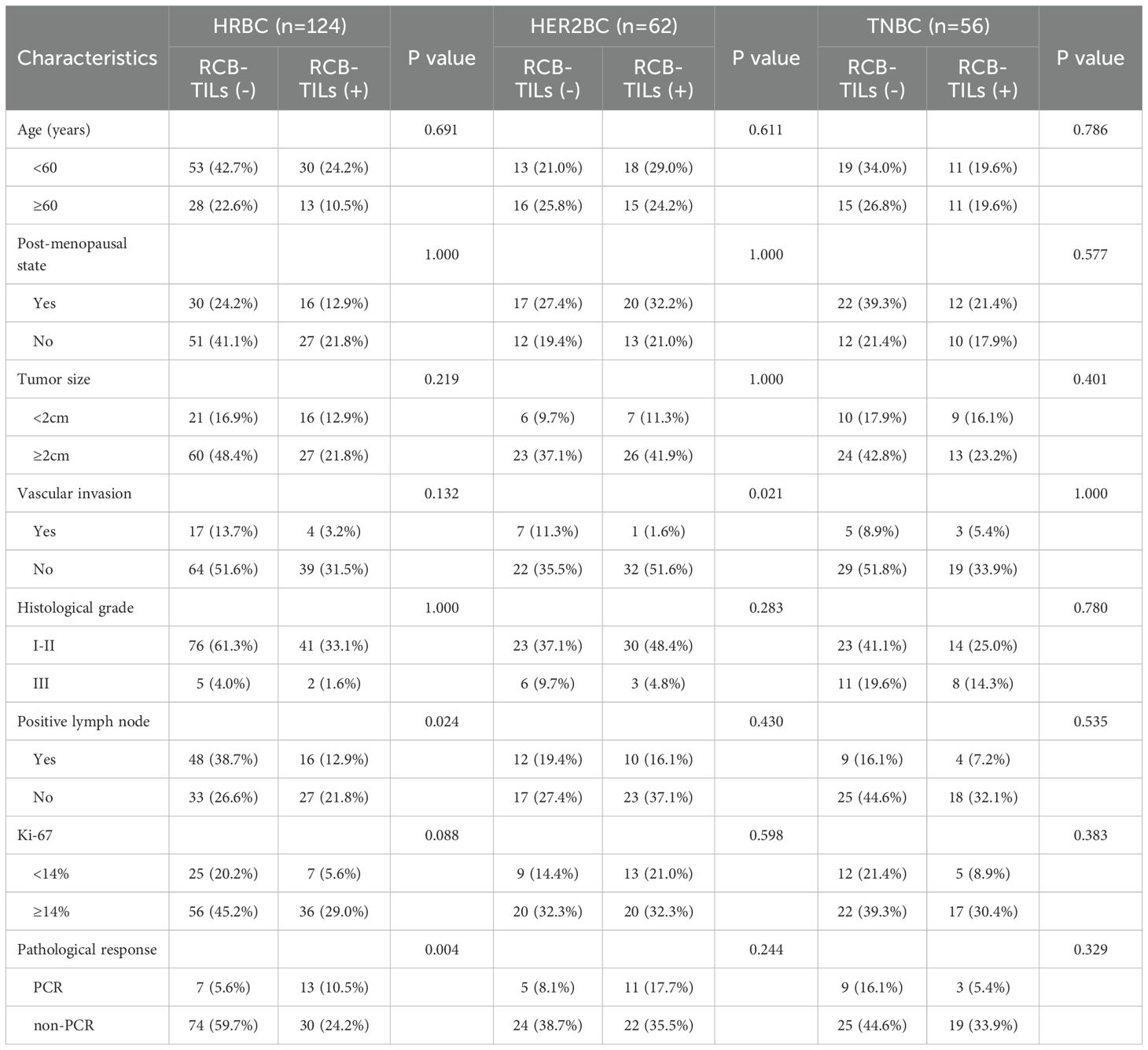
Table 2. Relationship between RCB-TILs and clinicopathological characteristics of NAC BC patients with different subtypes.
3.2 Prognostic analysis of NAC BC patients based on RCB-TILs
To comprehensively examine the prognostic significance of RCB-TILs in individuals with NAC BC, the Kaplan-Meier survival analysis was utilized to evaluate DFS. The findings revealed that the presence of RCB-TILs (+) was associated with a significant extension in DFS among all BC patients (P < 0.001), as well as within the subgroups of HRBC (P = 0.012), HER2BC (P = 0.003), and TNBC patients (P = 0.024) (Figures 2A-D).

Figure 2. Kaplan–Meier analysis of disease-free survival in patients with breast cancer according to RCB-TILs (A) RCB-TILs in all breast cancer (B) RCB-TILs in HRBC (C) RCB-TILs in HER2BC (D) RCB-TILs in TNBC.
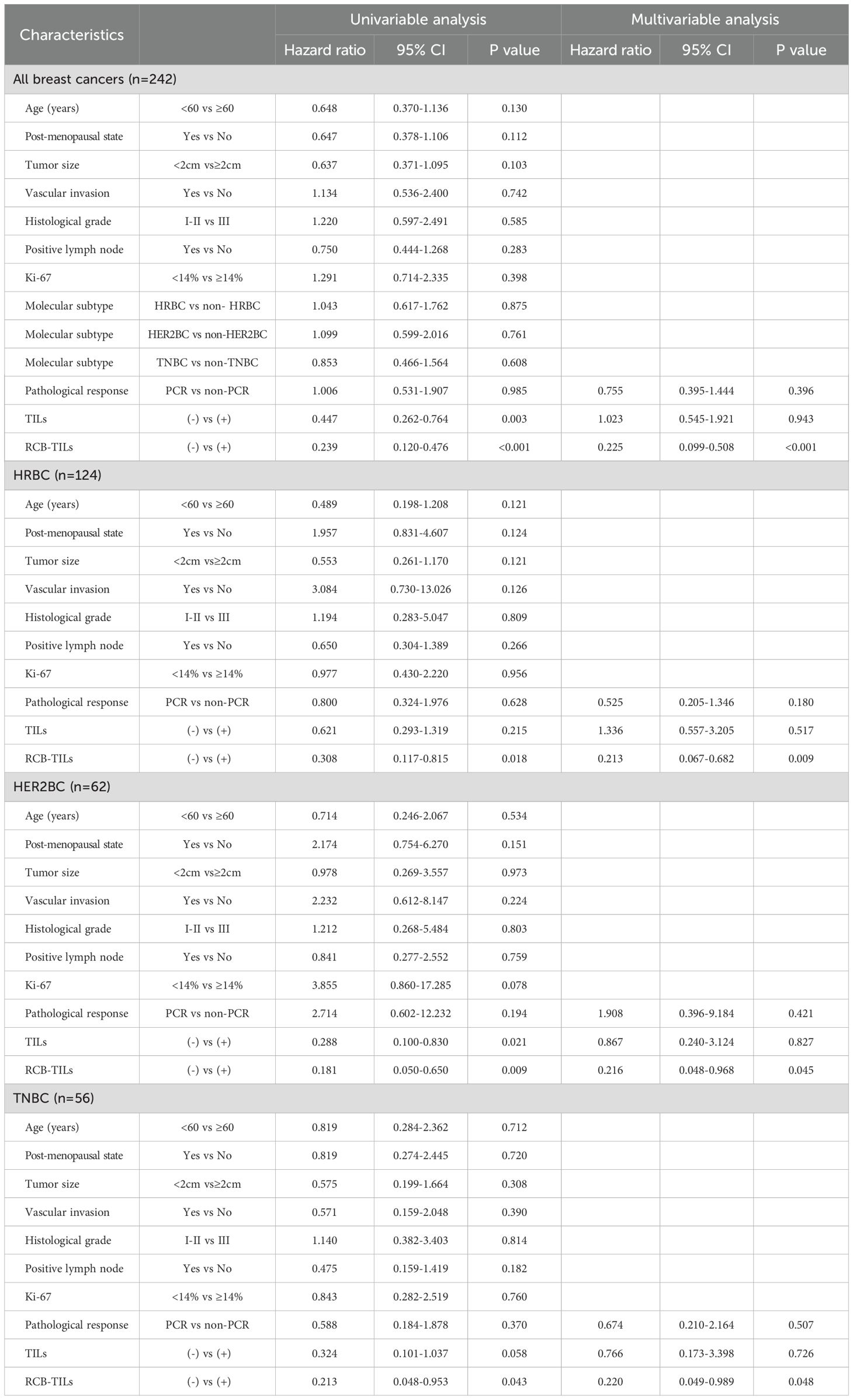
Subsequently, both univariate and multivariate Cox analyses were conducted. The univariate Cox analysis indicated that TILs (+) were associated with an extension in DFS among all BC patients (HR = 0.447, P = 0.003) and HER2BC patients (HR = 0.288, P = 0.021), although no marked impact on survival was observed in HRBC patients (HR = 0.621, P = 0.215) or TNBC patients (HR = 0.324, P = 0.058). Conversely, RCB-TILs (+) were found to significantly contribute to extended DFS across all BC patients (HR = 0.239, P < 0.001), as well as within the HRBC (HR = 0.308, P = 0.018), HER2BC (HR = 0.181, P = 0.009), and TNBC subgroups (HR = 0.213, P = 0.043) (Table 3). The multivariate Cox analysis verified that RCB-TILs (+) functioned as an independent prognostic factor influencing recurrence following NAC in the entire cohort of BC patients (HR = 0.225, P < 0.001), and in the HRBC (HR = 0.213, P = 0.009), HER2BC (HR = 0.216, P = 0.045), and TNBC (HR = 0.220, P = 0.048) subgroups.
Finally, a receiver operating characteristic (ROC) analysis was executed. The results indicated that RCB-TILs (area under the curve [AUC]: 0.647) surpassed both RCB (AUC: 0.537) and TILs (AUC: 0.596) in predicting outcomes for all BC patients (Figures 3A-C). Additional analyses by subtype showed consistent results in HRBC patients (AUC: RCB-TILs = 0.609, RCB = 0.554, TILs = 0.541) (Figures 3D-F), HER2BC patients (AUC: RCB-TILs = 0.705, RCB = 0.579, TILs = 0.646) (Figures 3G-I), and TNBC patients (AUC: RCB-TILs = 0.667, RCB = 0.536, TILs = 0.655) (Figures 3J-L).
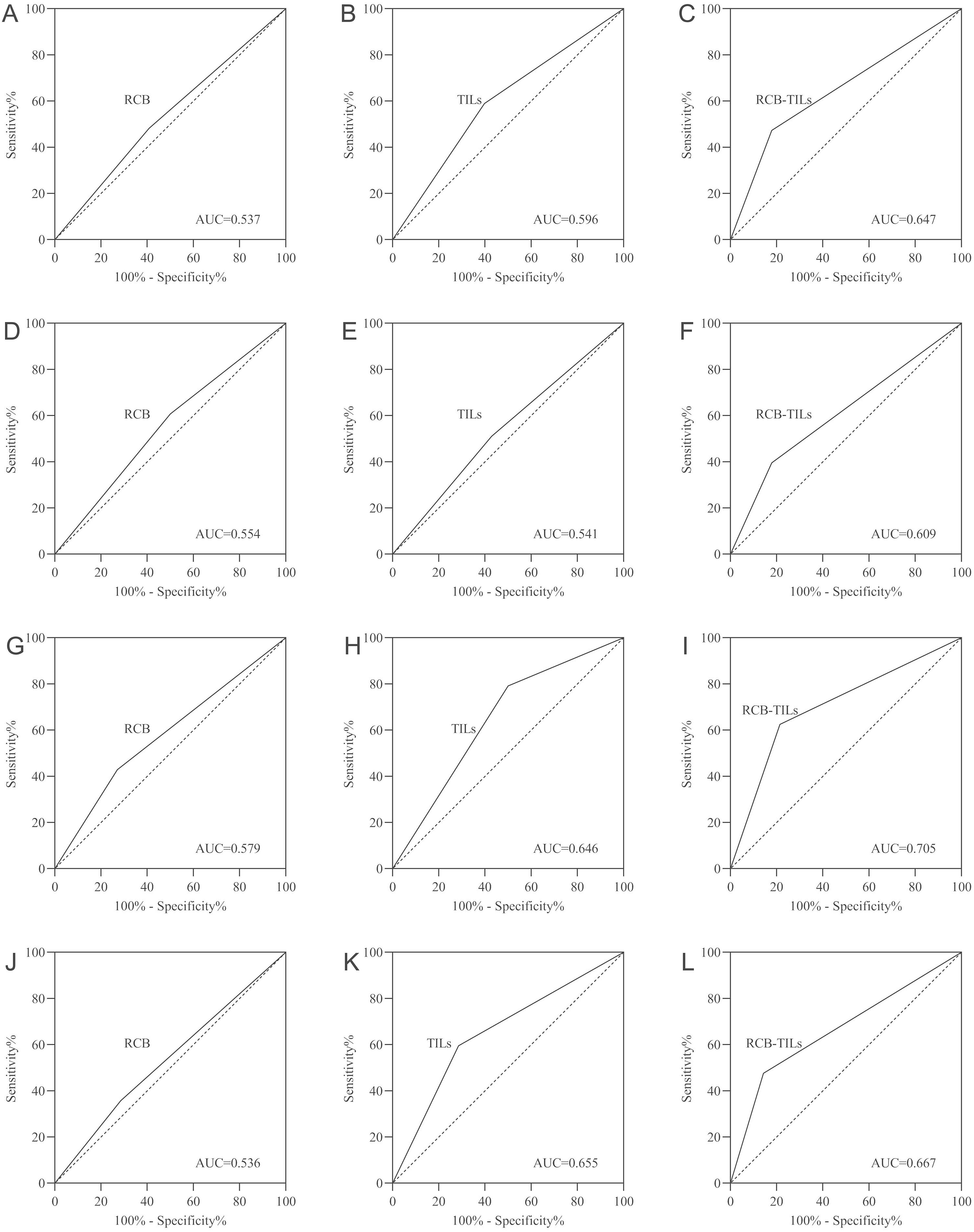
Figure 3. ROC curve analysis for all BC patients (A-C), HRBC patients (D-F), HER2BC patients (G-I), and TNBC patients (J-L).
4 Discussion
The utilization of NAC in BC treatment has become increasingly prevalent. By decreasing the staging of primary breast tumors and axillary lymph node metastases, NAC can effectively control disease progression, thereby enhancing patient survival rates and prognosis (14). Consequently, an accurate and effective prognostic indicator is vital for the diagnostic and therapeutic evaluation of BC patients undergoing NAC. PCR, characterized by the lack of invasive and in situ residual disease in both breast and lymph nodes, assists in identifying patients with favorable and unfavorable outcomes (15). Although PCR is correlated with a positive prognosis in HER2BC and TNBC subtypes, it is not suitable for prognostic assessment in HRBC subtype patients (15). Previous studies have demonstrated (16) that TILs can serve as an evaluation indicator for predicting the efficacy of TCHP regimen treatment in HER2BC patients. Additionally, the work of Hou Z et al. (8) confirmed that elevated levels of TIL infiltration in tumor tissue prolonged DFS and overall survival in non-small cell lung cancer patients receiving NAC treatment. Although TIL evaluation has shown good efficacy in predicting NAC treatment response for TNBC and HER2BC patients, satisfactory results are often challenging to obtain for predicting treatment response in the most common HRBC subtype (17). Furthermore, a multicenter analysis involving 5,161 patients indicated that post-NAC RCB assessment could be employed to predict survival in HRBC patients (6). Sano Y et al. (17) discovered that combining TIL assessment with RCB scoring could effectively enhance the predictive performance of the RCB assessment system. Therefore, this study integrated RCB with TILs to evaluate the RCB-TILs status of NAC BC patients diagnosed and treated at three medical centers, aiming to assess its effectiveness as a survival predictor for these patients.
TILs exert specific cytotoxic effects on tumor cells and are considered markers of highly immunogenic subtypes (18). In this study, the RCB-TILs (+) BC group exhibited reduced rates of vascular invasion and lymph node metastasis in comparison to the RCB-TILs (-) group, suggesting that elevated levels of TILs might exert a substantial influence on suppressing tumor cell proliferation and metastasis. Furthermore, a higher PCR rate was noted in the RCB-TILs (+) group, suggesting that patients with RCB-TILs (+) status were more likely to achieve PCR compared to those with RCB-TILs (-), potentially implying improved survival outcomes for RCB-TILs (+) patients. Some studies have proposed that RCB-TILs serve as a critical predictor of recurrence for all invasive BCs following NAC and could function as an effective indicator of NAC efficacy. It has also been observed that the TNBC subtype contains a higher proportion of RCB-TILs (+) cases compared to other subtypes (17). However, in this investigation, a higher percentage of RCB-TILs (+) was identified in the HER2BC subtype, which might be attributable to the relatively larger number of HER2BC subtype patients included or variations in the genetic backgrounds of the study subjects. This finding warrants further verification in future research. Multivariate Cox analysis was employed to evaluate survival across all BC subtypes, revealing that RCB-TILs (+) constitute a favorable factor for extended DFS in BC patients post-NAC. Moreover, ROC analysis demonstrated that RCB-TILs are a more sensitive predictor of survival compared to using RCB or TILs independently. Consequently, RCB-TILs hold promise as a predictor of post-NAC survival for patients with various BC subtypes. When contemplating additional treatment following NAC, RCB-TILs assessment may aid in formulating more suitable treatment strategies. Despite expressing ER or PR, some BC patients do not respond to endocrine therapy, while others develop resistance during treatment (19). In this study, all HRBC subtype patients who underwent NAC also received subsequent endocrine therapy. RCB-TILs (+) patients exhibited lower recurrence rates, suggesting that RCB-TILs could potentially serve as an alternative indicator for predicting endocrine therapy response in HRBC subtype patients. In light of this, some researchers have advocated a new treatment strategy where HRBC subtype patients with RCB-TILs (-) status could be considered for additional chemotherapy alongside standard endocrine therapy (17). Masuda N et al. (20) reported on a clinical trial applying capecitabine to HER2-negative BC patients after NAC and surgery. It is anticipated that future similar studies will also examine the correlation between RCB-TILs and prognosis in BC patients post-NAC. Although the effectiveness of RCB-TILs in predicting survival for BC patients after NAC has been evaluated through a three-center study, further research is necessary to ascertain whether RCB-TILs are equally applicable in other ethnic groups.
5 Conclusion
This study illustrates that RCB-TILs are linked to survival outcomes in BC patients undergoing NAC, potentially serving as a more sensitive predictor of recurrence than using RCB or TILs independently. Furthermore, RCB-TILs exhibit promise as a potential biomarker for identifying DFS in BC patients treated with NAC, offering valuable guidance for subsequent clinical diagnosis, treatment, and evaluation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Guizhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZH: Writing – original draft. XA: Conceptualization, Data curation, Writing – original draft. GM: Conceptualization, Data curation, Methodology, Writing – original draft. HZ: Conceptualization, Data curation, Investigation, Writing – original draft. SL: Data curation, Formal Analysis, Investigation, Writing – review & editing. XL: Data curation, Methodology, Resources, Writing – original draft. LZ: Conceptualization, Data curation, Methodology, Writing – original draft. WW: Conceptualization, Investigation, Project administration, Software, Writing – original draft. LF: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. GL: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Guizhou Provincial Health Commission Project (gzwkj2024-314) and Qiandongnan Prefecture Science and Technology Bureau Project (Qiandongnan Kehe J character (2023) 94).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. de Visser KE and Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. (2023) 41:374–403. doi: 10.1016/j.ccell.2023.02.016
3. See SHC and Siziopikou KP. Pathologic evaluation of specimens after neoadjuvant chemotherapy in breast cancer: Current recommendations and challenges. Pathol Res Pract. (2022) 230:153753. doi: 10.1016/j.prp.2021.153753
4. Izdebska M, Zielińska W, Krajewski A, and Grzanka A. Fascin in migration and metastasis of breast cancer cells - A review. Adv Med Sci. (2023) 68:290–7. doi: 10.1016/j.advms.2023.08.003
5. Gomes da Cunha JP, Goncalves R, Silva F, Aguiar FN, Mota BS, Chequim BB, et al. Validation of the Residual Cancer Burden Index as a prognostic tool in women with locally advanced breast cancer treated with neoadjuvant chemotherapy. J Clin Pathol. (2023) 76:239–43. doi: 10.1136/jclinpath-2021-207771
6. Yau C, Osdoit M, van der Noordaa M, Shad S, Wei J, de Croze D, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. (2022) 23:149–60. doi: 10.1016/S1470-2045(21)00589-1
7. El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer. (2021) 7:150. doi: 10.1038/s41523-021-00346-1
8. Hou Z, Zhao L, Zou L, and Li B. The prognostic value of tumor-infiltrating lymphocytes in non-small cell lung cancer patients who received neoadjuvant chemotherapy followed by surgery. Adv Clin Exp Med. (2023) 32:847–53. doi: 10.17219/acem/159242
9. Wu R, Oshi M, Asaoka M, Yan L, Benesch MGK, Khoury T, et al. Intratumoral tumor infiltrating lymphocytes (TILs) are associated with cell proliferation and better survival but not always with chemotherapy response in breast cancer. Ann Surg. (2023) 278:587–97. doi: 10.1097/SLA.0000000000005954
10. Wankhede D, Yuan T, Kloor M, Halama N, Brenner H, and Hoffmeister M. Clinical significance of combined tumour-infiltrating lymphocytes and microsatellite instability status in colorectal cancer: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. (2024) 9:609–19. doi: 10.1016/S2468-1253(24)00091-8
11. Zhu H and Doğan BE. American joint committee on cancer’s staging system for breast cancer, eighth edition: summary for clinicians. Eur J Breast Health. (2021) 17:234–8. doi: 10.4274/ejbh.galenos.2021.2021-4-3
12. Pan QH, Zhang ZP, Yan LY, Jia NR, Ren XY, Wu BK, et al. Association between ultrasound BI-RADS signs and molecular typing of invasive breast cancer. Front Oncol. (2023) 13:1110796. doi: 10.3389/fonc.2023.1110796
13. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. (2007) 25:4414–22. doi: 10.1200/JCO.2007.10.6823
14. Wang H and Mao X. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des Devel Ther. (2020) 14:2423–33. doi: 10.2147/DDDT.S253961
15. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. (2012) 30:1796–804. doi: 10.1200/JCO.2011.38.8595
16. Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. (2019) 173:255–66. doi: 10.1007/s10549-018-4981-x
17. Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer. (2017) 17:888. doi: 10.1186/s12885-017-3927-8
18. Lin B, Du L, Li H, Zhu X, Cui L, and Li X. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully. BioMed Pharmacother. (2020) 132:110873. doi: 10.1016/j.biopha.2020.110873
19. Mohla S, Stearns V, Sathyamoorthy N, Rosenfeld MG, and Nelson P. The biology of hormone refractory breast and prostate cancer: An NCI workshop report. Cancer Biol Ther. (2009) 8:1975–85. doi: 10.4161/cbt.8.21.9918
Keywords: breast cancer, tumor-infiltrating lymphocytes, residual cancer burden, disease-free survival, neoadjuvant chemotherapy
Citation: Hou Z, An X, Meng G, Zhao H, Liao S, Long X, Zou L, Wu W, Feng L and Liao G (2025) Correlation study of tumor-infiltrating lymphocytes combined with residual cancer burden and prognosis in breast cancer patients receiving neoadjuvant chemotherapy. Front. Oncol. 15:1538326. doi: 10.3389/fonc.2025.1538326
Received: 02 December 2024; Accepted: 22 August 2025;
Published: 09 September 2025.
Edited by:
Parth Malik, Ministry of Science and Technology, IndiaReviewed by:
Sanjay Goel, The State University of New Jersey, United StatesSumanta Goswami, Yeshiva University, United States
Copyright © 2025 Hou, An, Meng, Zhao, Liao, Long, Zou, Wu, Feng and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghui Liao, OTUzNTQyNjU0QHFxLmNvbQ==
 Zexin Hou
Zexin Hou Xueyuan An2
Xueyuan An2