- 1Department of Breast Surgery, The First Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Breast Cancer, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 3Department of Emergency Surgery, The First Hospital of Jilin University, Changchun, Jilin, China
- 4Clinical Medicine, Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, China
Purpose: To clarify the risk factors of phyllodes tumor (PT) for local recurrence (LR).
Methods: Data from 829 patients with pathologically confirmed benign, borderline and malignant PT of the breast, diagnosed from between 2011 to 2023, were retrieved from the electronic databases of the First Hospital of Jilin University and the First Affiliated Hospital of Xi’an Jiaotong University. Kaplan-Meier curves and Cox proportional-hazards model were conducted to determine the independent risk factors for LR in each group.
Results: Of 829 PT patients, 634 (76.5%), 142 (17.1%), and 53 (6.4%) were diagnosed with benign, borderline, and malignant PT, respectively. The LR rates were 5.4%, 9.9%, 13.2%, respectively. The median patient age was 38 years and the median follow-up time was 2.8 (range, 0.2-12.1 years). Of these patients, 13 (2.1%) were diagnosed with benign bilateral PT. Multivariate analysis identified bilateral involvement as a risk factor for LR of benign PT (p=0.010). Also, univariate analysis identified young age (≤35 years, p=0.046) as an independent risk factors for LR of borderline PT. Of the patients with malignant PT, univariate analysis found that breast-conserving surgery (BCS) (p=0.008) were associated with an increased risk for LR of malignant PT.
Conclusions: Bilateral PT was a risk factor for LR of benign PT, young age (≤35 years) was associated with poor prognosis of borderline PT, BCS were high risk factors for LR of malignant PT. This study identifies LR risk factors based on tumor grading, which contributes to individualized clinical risk assessment. Future research could further explore how to incorporate these factors into clinical decision-making models for PT and other soft tissue tumors.
1 Introduction
Phyllodes tumor (PT) of the breast is a rare and complex growth, accounting for 2%–3% of all fibroepithelial neoplasms and 0.3%–1.0% of all breast tumors (1). PT most commonly occurs in women aged 45–49 years, while comparatively infrequent in men (2). The World Health Organization classifies PT into three categories based on histological features: benign, borderline and malignant (as shown in Table 1).
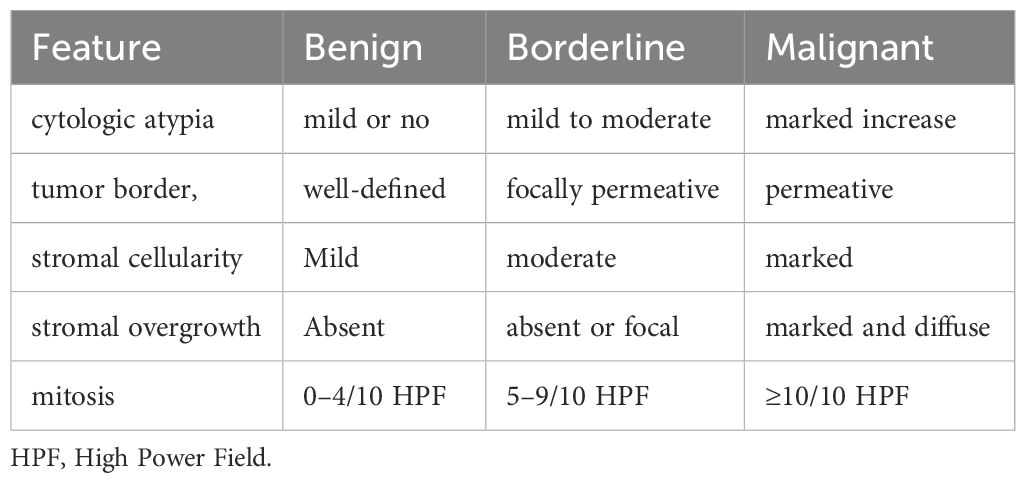
Table 1. Histological characteristics of benign, borderline, and malignant PT (3).
Of all breast phyllodes tumors, the prevalence of benign PT is notably higher than malignant PT (60% vs. 25%–30%, respectively) (4, 5).
Clinical presentation in many phyllodes tumor is a palpable breast mass, but benign PT is ofen misdiagnosed as fibroadenoma. Meanwhile, differentiation of borderline and malignant PTs from breast carcinoma can be challenging. An accurate diagnosis relies on pathological examination following complete tumor excision. However, there is currently no consensus regarding the most appropriate surgical type.
PT have a high risk of recurrence, but the factors affecting their recurrence remain unclear. Therefore, the aim of this study was to clarify the clinical and pathological characteristics in addition to risk factors associated with LR of PT to provide evidence-based guidance for clinical diagnosis and treatment.
2 Materials and methods
2.1 Study approval and patient consent
The study protocol was approved by the Ethics Committees of the First Hospital of Jilin University(approval no. 2024–627) and the First Affiliated Hospital of Xi’an Jiaotong University (approval no. XJTU1AF2021LSK-238) and conducted in accordance with the ethical principles for medical research involving human subjects described in the Declaration of Helsinki.
2.2 Patients and variables
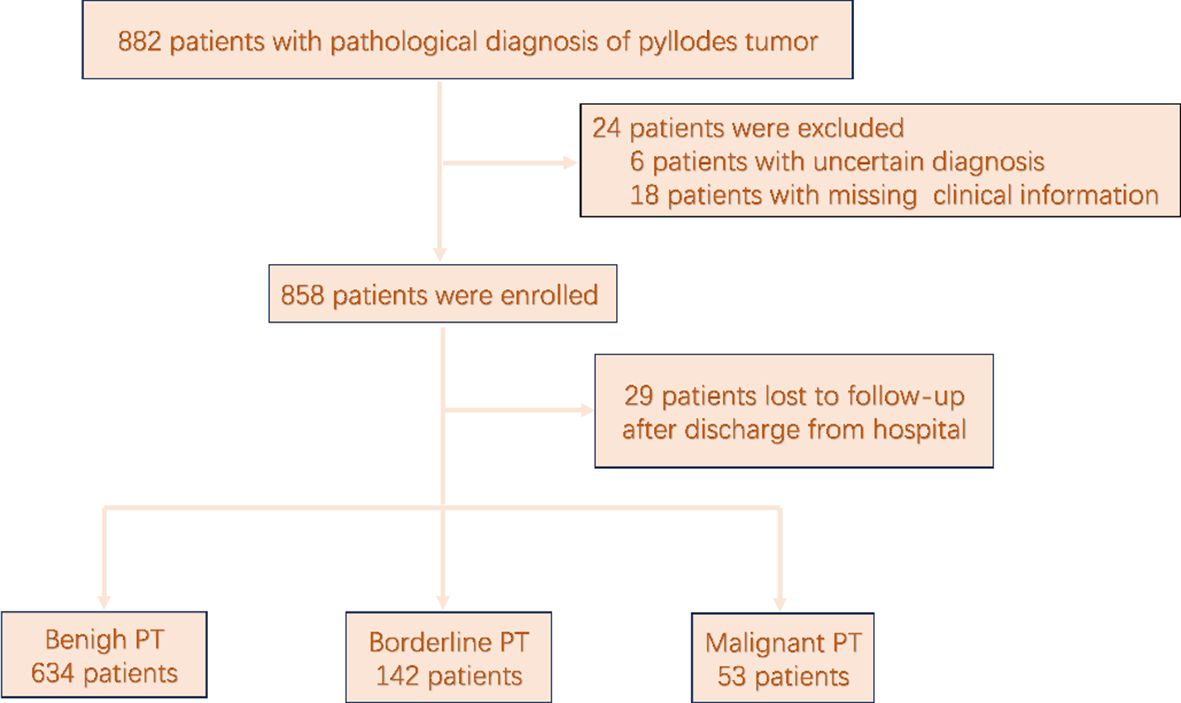
The medical records of 882 patients with pathologically confirmed PT following resection from August 2011 to October 2023 were retrieved from the electronic databases of the Breast Surgery Departments of the First Hospital of Jilin University and the First Affiliated Hospital of Xi’an Jiaotong University. A flowchart of the patient selection process in presented in Figure 1. Of the 882 patients with pathologically confirmed PT, 829 (94.0%) who had undergone surgical resection and had a pathologically confirmed diagnosis of PT, with complete follow-up data available met the inclusion criteria and were included for analysis. In accordance with the classification criteria proposed by the World Health Organization, PT was classified as benign, borderline, or malignant.
The patient baseline characteristics included age at initial diagnosis, body mass index (BMI), tumor size, laterality, estimated blood flow by ultrasonography, and type of surgery.
Groups were established based on age at initial diagnosis (≤ 35vs.>35 years) (6–8) and tumor size (≤ 50vs.> 50 mm) (2). BMI was calculated as weight (kg)/height (m)². Based on the calculated BMI, patients were classified as overweight (BMI>24) or normal weight (BMI≤ 24) (9). Vacuum-assisted excision (VAE) is a minimally invasive therapeutic technique derived from vacuum-assisted biopsy (VAB). As the technology has advanced, VAE has seen increasingly widespread use in treating benign breast lesions (10). Therefore, in our study, the surgical approaches included vacuum-assisted breast excision (VAE), breast-conserving surgery (BCS), and total mastectomy (TM).
The outcome variable was LR, defined as cytologically or histologically confirmed LR of PT involving the ipsilateral breast or chest wall (including the ipsilateral axilla, internal breast, subclavian or supraclavian lymph nodes) at ≥60 days after initial surgery. Local recurrence-free survival (LRFS) was defined as the survival time from the date of surgery to the first determination of LR. Distant metastasis (DM) is defined as the spread of neoplastic components to distant organs. For all patients with confirmed LR of malignant PT, the pathological records were scrutinized to ascertain disease status.
2.3 Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 26.0. (IBM Corporation, Armonk, NY, USA and Prism 10.0 software (GraphPad Software, Inc., San Diego, CA, USA). Descriptive analysis of the three patient groups included categorical data (frequency and percentage) and continuous data (median, interquartile range [IQR]). The Kaplan-Meier method was used to estimate the 5-year LRFS, and the log-rank test was performed to compare differences between groups.
Univariate analysis with the Cox proportional-hazards model was conducted to prevent the omission of variables with statistical significance for LR due to confounding factors. Variables with a p-value<0.1 (11)were included in the multivariable Cox regression analysis to identify independent risk factors for LR in each patient group. A two-sided p-value < 0.05 was considered statistically significant.
2.4 Follow-up
Patients diagnosed with PT were recommended to undergo breast ultrasonography every six months after surgery. For those with borderline or malignant PT, annual mammography and systemic examinations were additionally advised. Patients were followed up by telephone or the outpatient electronic medical record system. Baseline characteristics, imaging results, surgical records, postoperative pathological results, and postoperative recurrence were recorded. Based on the longest observed time to LR, the maximum follow-up period was 12.1 years after initial surgery.
3 Results
3.1 Baseline characteristics
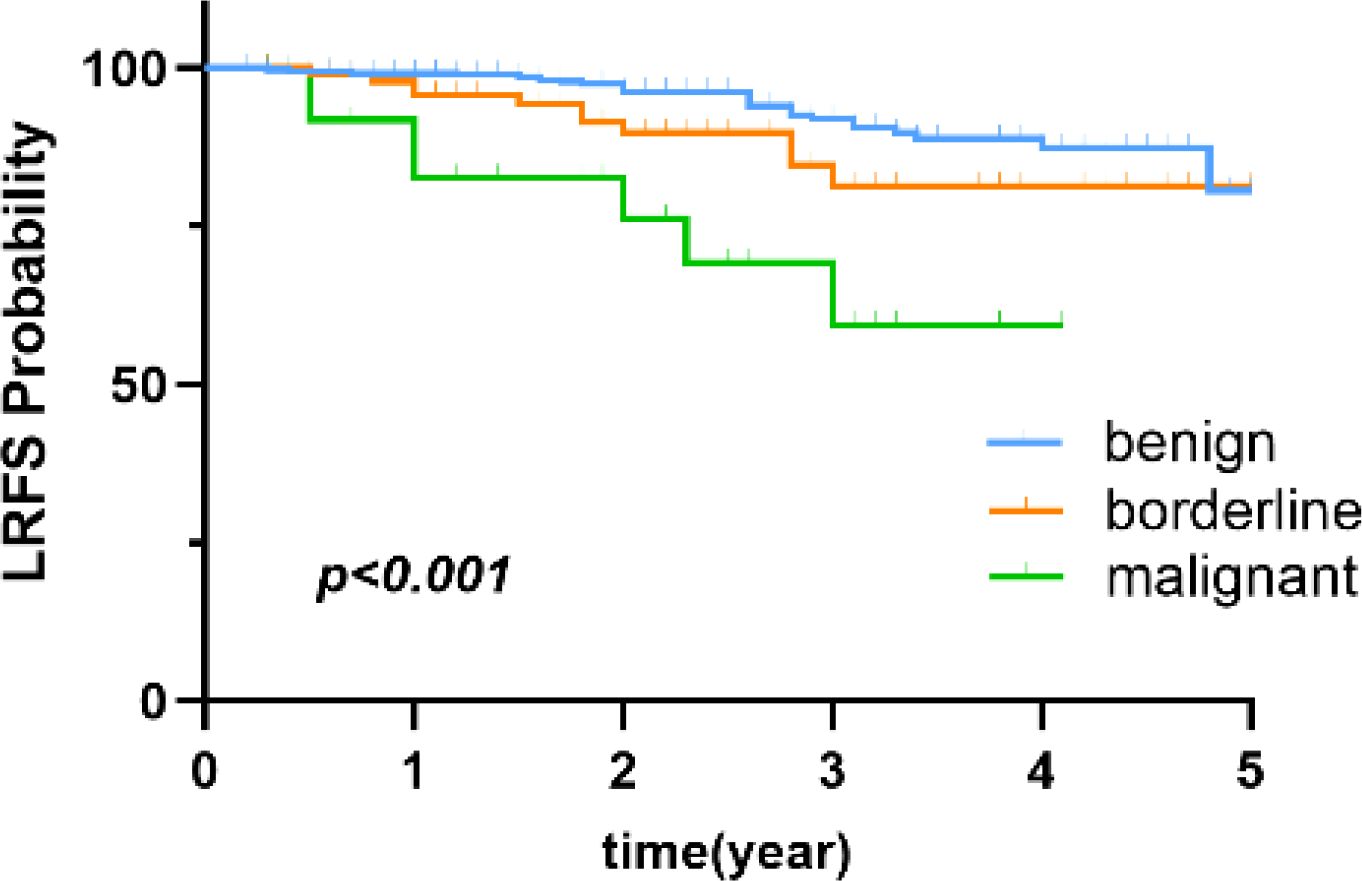
The clinicopathological characteristics of the 829 patients are summarized in Table 2. All 829 patients were female. Of these patients, 634 (76.5%), 142 (17.1%), and 53 (6.4%) were diagnosed with benign, borderline, and malignant PT, respectively. The median follow-up time was 2.8 (range, 0.2-12.1) years. Comparisons of the 5-year LRFS for benign, borderline, and malignant PT are shown in Figure 2.
3.2 Benign PT
For patients with benign PT, the median time to LR was 2.6 (IQR, 1.7-3.3) years, with the majority occurring within 5 years of initial diagnosis, the 5-year local recurrence rate (LRR) was 4.7%, the LRR of benign PT was 5.4%. the median age at initial diagnosis was 36 (IQR, 26-44) years, and the median tumor size was 27.6 (IQR, 20.0–39.8) mm.
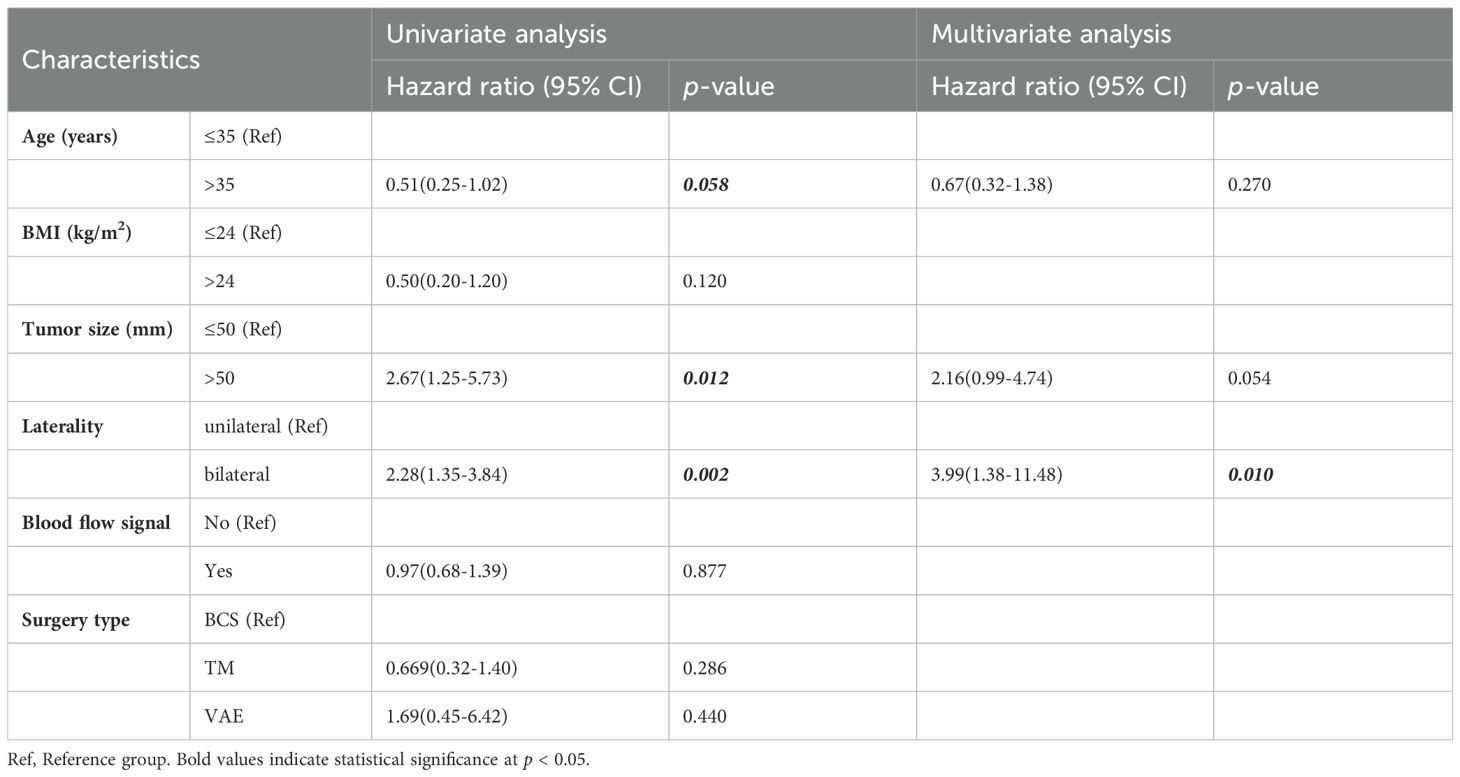
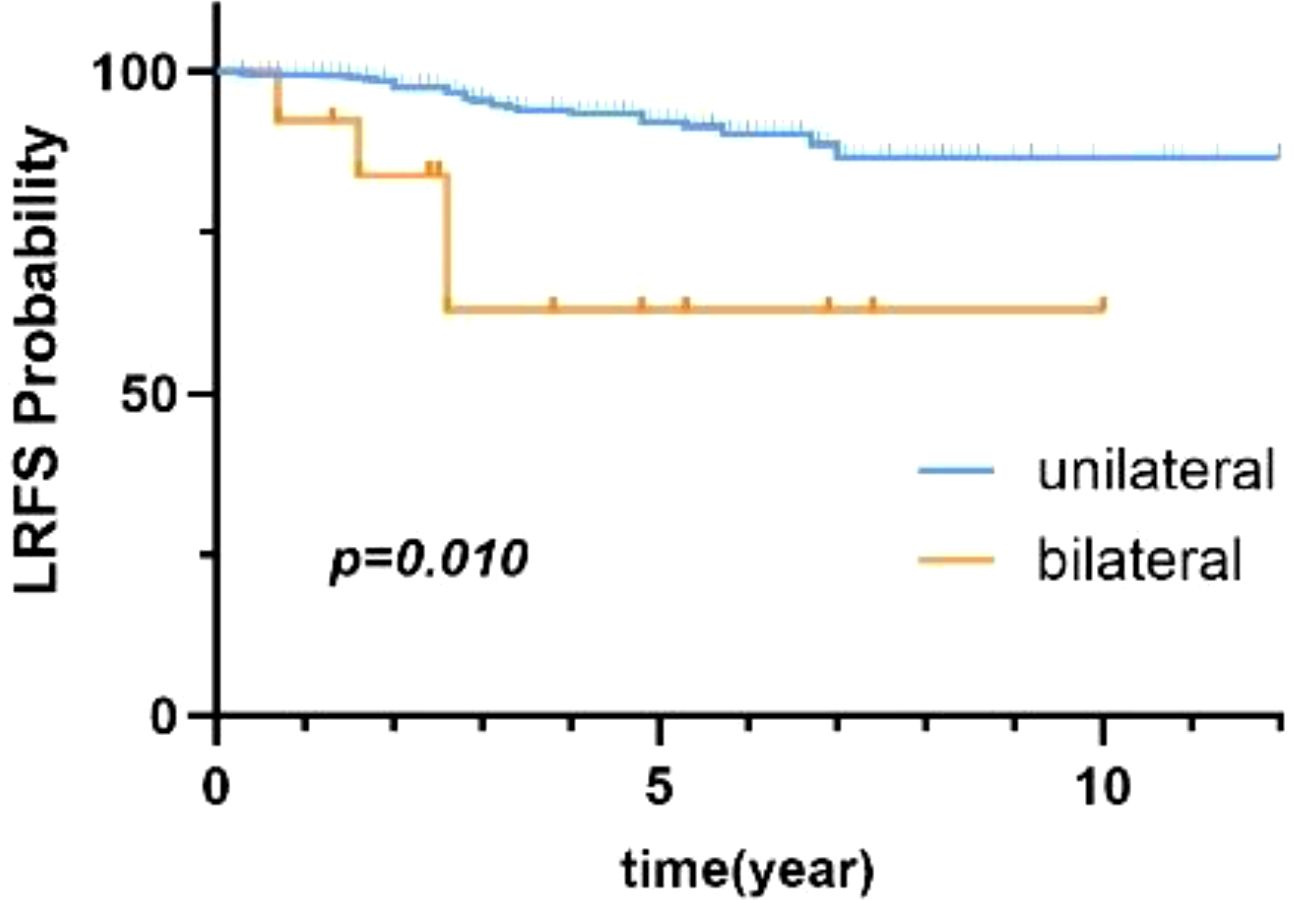
Univariate analysis with the Cox proportional-hazards model revealed that age, BMI, tumor size, and laterality were prognostic factors for patients with benign PT (p < 0.1). Thus, these four factors were included in multivariate analysis. As shown in Table 3, laterality was the only factor influencing local control in patients with benign PT. Additionally, compared to unilateral PT, bilateral breast PT significantly increased the LRR of PT (HR=3.99, 95% CI:1.38-11.48),. There was a statistically significant difference in 5-year LRFS between patients with unilateral and bilateral PT (p=0.010) (Figure 3).
3.3 Borderline PT
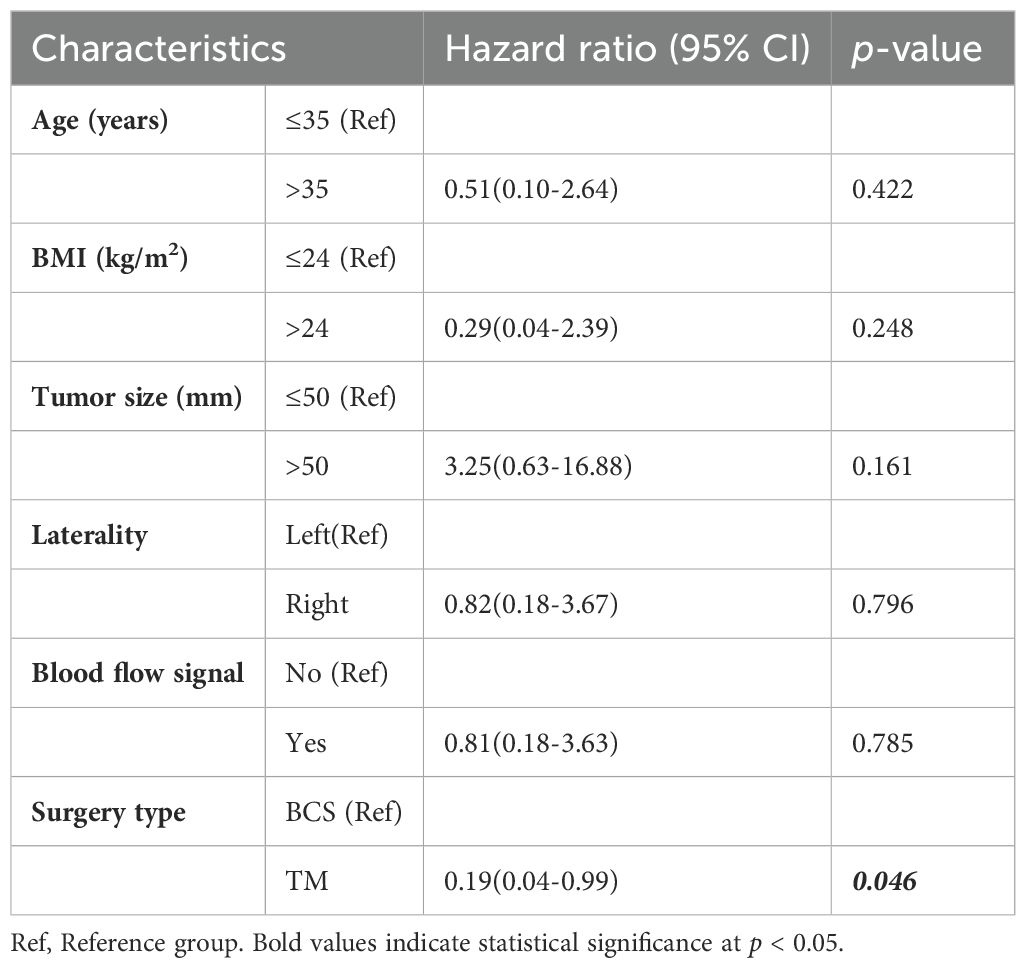
For patients with borderline PT, the median time to LR was 1.9 (IQR, 1.7–6.0) years, the 5-year LRR was 7.7%, the LRR of borderline PT was 9.9%. The median age at initial diagnosis was 47 (IQR, 37–54) years, and the median tumor size was 44.9 (IQR, 28.9–65.0) mm. Table 4 shows the results of univariate analysis revealed that younger age (≤35 years) was the only factor influencing LR of borderline PT. Compared to those aged ≤35, individuals aged >35 had a significantly lower risk of LR (HR=0.24, 95% CI:0.08-0.68). Figure 4 shows the comparison of LRFS in borderline PT between patients aged ≤35 and those aged>35.
3.4 Malignant PT
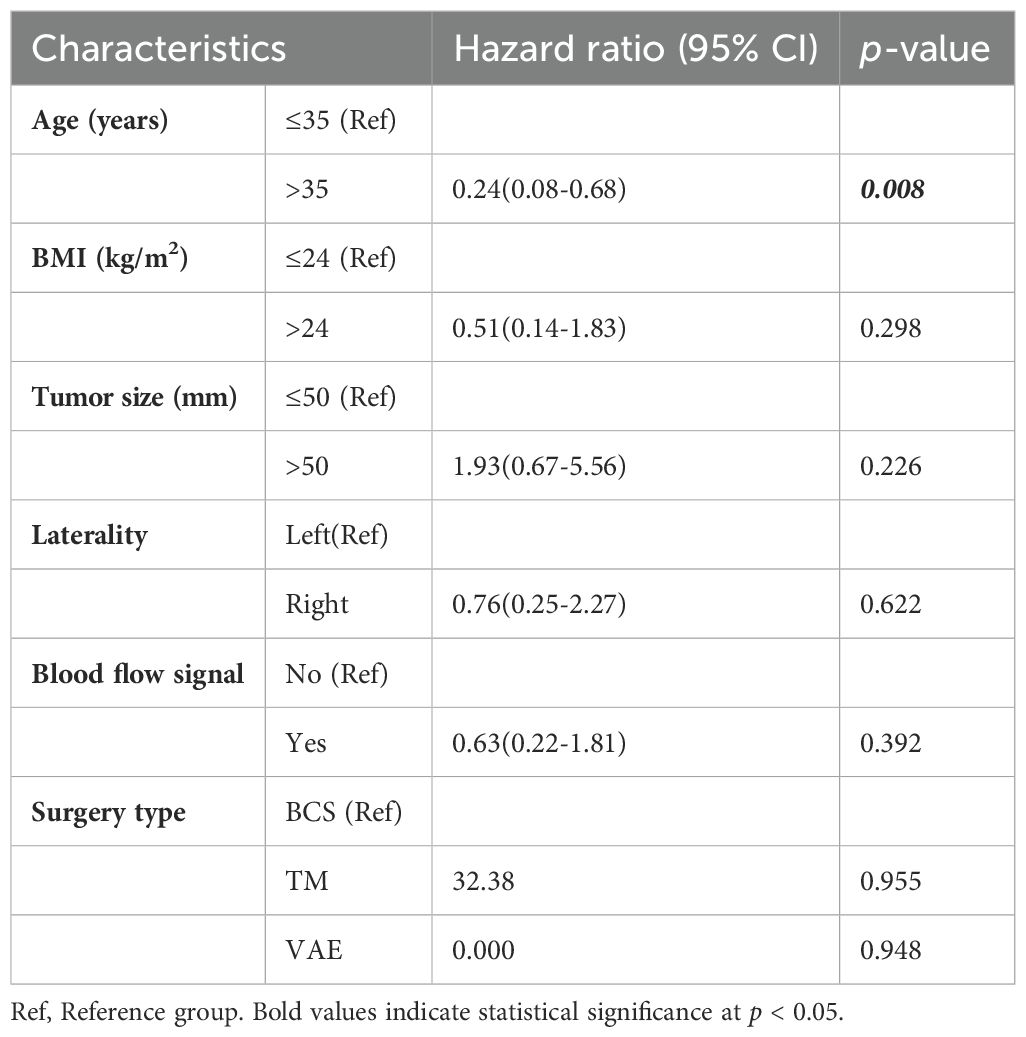
For patients with malignant PT, the median time to LR was 1 (IQR, 0.5–2.3) year, the 5-year LRR was 13.2%, the distant metastasis rate for malignant PT was 14.2%. The median age at initial diagnosis was 49 (IQR, 36.5–56) years, and the median tumor size was 48.3 (IQR, 32-87.6) mm.
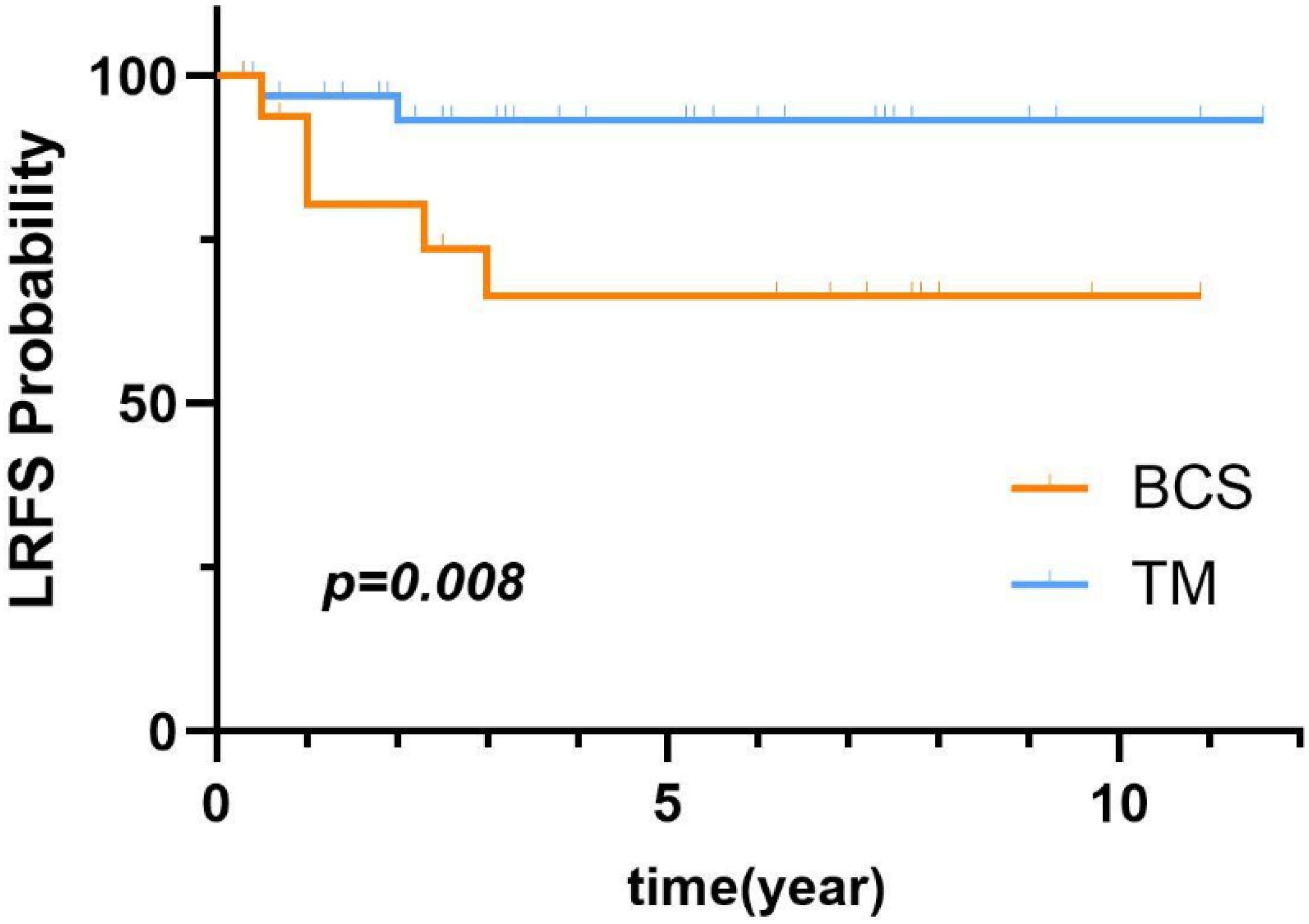
COX univariate analysis showed that the surgery type was a prognostic factor for patients with malignant PT. Figure 5 illustrates the comparison of LRFS between malignant PT patients who underwent BCS and those who underwent TM. As shown in Table 5, univariate analysis revealed that BCS (p=0.046) were risk factors for LR of malignant PT. Additionally, compared to BCS, TM significantly reduced the LRR (HR=0.19, 95% CI:0.04-0.99).
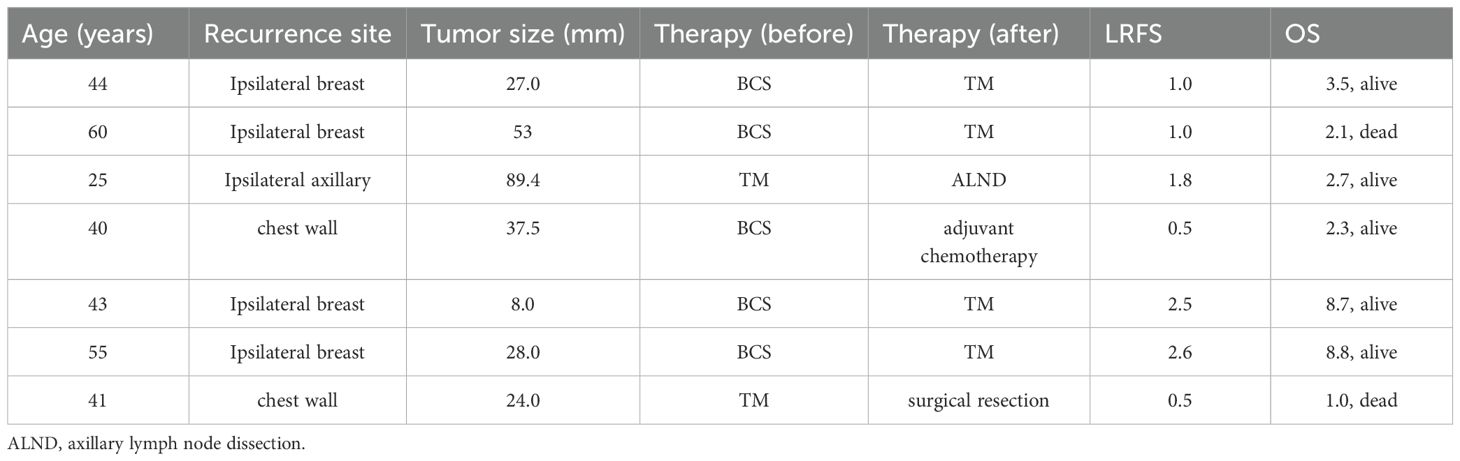
The clinical characteristics of 7 patients with LR of malignant PT are presented in Table 6.
4 Discussion
This is the retrospective study conducted in China to investigate the clinical and pathological characteristics, as well as the risk factors, for LR of PT in two representative hospitals.
The LRR of benign PT was 5.4%, which is consistent with recent studies reporting between 5% and 10% (12). Among patients with benign PT, 13 (2.1%) were diagnosed with bilateral benign PT, which included 3 (23.1%) with LR. Univariate analysis with the Cox proportional-hazards model identified bilateral PT as a possible risk factor for LR of benign PT. A large retrospective study conducted in 2023 confirmed LR in 7 (24.1%) of 29 patients with bilateral benign PT and suggested that this relatively high LRR might be attributed to genetic (4). In the present study, 71.1% patients with benign PT chose BCS, while only 0.8% chose TM, and 28.1% chose VAE. However, as compared to BCS, patients with tumors < 20 mm in the last 5 years tended to prefer VAE because the cost of ultrasound-guided VAE is approximately 80% less than surgery (13), is less time-consuming, and yields better aesthetics. Most importantly, ultrasound-guided VAE is safe and effective for resection of lesions < 20 mm (14). Therefore, VAE is increasingly preferred for resection of benign tumors.
The LRR of borderline PT was 9.9%. Younger age (≤35 years) was the only factor significantly associated with LR. As compared to younger patients (≤35 years), 5-year LRFS was significantly increased for older patients (>35 years), in agreement with a prior report (15). BSC is most commonly employed for borderline PT. In the present study, there was no significant difference in local control of borderline PT among the three types of surgery.
In this study, 66% of patients selected TM for resection of malignant tumors. Moreover, as a favorable prognostic factor for malignant PT, TM significantly improved LRFS, in agreement with the results of a previous study (16). However, some studies suggest that as compared to TM, BCS did not improve the LRR (17–19). There was no significant difference in oncological outcomes between BCS and TM for early stage malignant PT, thus BCS is preferable (20). Numerous studies have demonstrated an association between TM and reduced risk of LR in patients with borderline and malignant PT. However, no specific surgery type has been found to directly impact cancer-specific survival rates (2).
Large tumor size (>50 mm) is also considered a possible risk factor for LR of malignant PT in previous studies (6, 11, 21, 22). A study conducted in 2013 suggested that small tumor size (<4.0 cm) was associated with increased LRR (23). We attribute these disparate findings to small sample sizes and the possibility of rapid tumor growth within a short period of time, which often occurs with high-grade malignant tumors, indicating more rapid progression of the tumor and a relatively poor prognosis. However, there is currently no reliable data demonstrating a correlation between the tumor growth rate and prognosis (6).
The LRR of malignant PT was 13.2%, with a high metastasis rate of up to 20% (24), indicating a poor prognosis. In this study, 2 (28.6%) of 7 patients with malignant PT experienced LR of the ipsilateral chest wall. One of these patients underwent surgical resection of the chest wall lesion but died of lung metastasis one year later, while the second received chemotherapy. Patients with LR of malignant PT to the chest post-TM reportedly respond well to radiotherapy (22). The National Comprehensive Cancer Network guidelines also recommend adjuvant radiotherapy (25). However, the efficacy of chemotherapy for treatment of malignant PT remains unclear (26). In this study, as most PT patients did not receive radiotherapy or chemotherapy, and due to incomplete treatment records, this study did not include these variables in the analysis. Therefore, large-scale prospective clinical trials are still needed to evaluate the role of adjuvant chemotherapy or radiotherapy in malignant PT.
The relationship between surgical margin status and the risk of local recurrence has garnered significant attention in recent years. Ditsatham et al. reported that surgical margins < 1 cm have no significant impact on local recurrence rates or 5-year disease-free survival in PT patients (27). A recent study revealed that patients with positive surgical margins have over 10 times the risk of local recurrence compared to those with wide margins (>10 mm) and over 6 times the risk compared to those with narrow margins (<10 mm) (28). For benign PT, complete mass resection is sufficient. Multiple studies have demonstrated no correlation between surgical margin status and recurrence risk in benign PT (4), and postoperative adjuvant therapy is unnecessary for these patients. For borderline and malignant PT, narrow surgical margins are linked to a higher risk of local recurrence. Consequently, wide local excision with a surgical margin of ≥1 cm is typically recommended (29). Unfortunately, this study does not address the correlation between surgical margins and recurrence risk due to the lack of accurate margin data from two representative hospitals. Therefore, future studies are encouraged to clearly margin width in order to better elucidate the potential association between surgical margins and the risk of LR, thereby providing stronger evidence to optimize surgical strategies.
In this study, prognosis was better for benign and borderline PT, probably because some patients with small tumors chose observation rather than resection. The lack of pathological evidence for LR has resulted in underestimation of the recurrence rate.
This study has several limitations. Firstly, the median follow-up of 2.8 years is relatively short, longer follow-up is warranted in future studies to further validate our findings. Secondly, the low incidence of the disease resulted in a small sample size, with only 14 cases of borderline PT recurrence and 7 cases of malignant PT recurrence, and 13 (2.1%) were diagnosed with bilateral benign PT, which included 3 (23.1%) with LR introducing potential statistical bias. Unfortunately, among the 7 patients with recurrent malignant PT, some were unable to provide detailed information about their recurrence during follow-up, limiting the ability to perform a comprehensive comparison before and after recurrence. Thirdly, descriptions and investigations of certain pathological features of PT are lacking. In future research, we plan to expand the sample size and incorporate additional pathological features to provide more comprehensive and compelling results.
These findings, particularly the identification of LR risk factors stratified by tumor grade, may contribute to more personalized risk assessment in clinical practice. Future studies could explore how these factors might be incorporated into decision-making algorithms for patients with PT and other soft tissue tumors.
5 Conclusion
The results of this retrospective dual-center study found that bilaterality was a risk factor for LR of benign PT, younger age (≤35 years) is an unfavorable prognostic factor for borderline PT, and BCS is a risk factor for LR of malignant PT.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committees of the First Hospital of Jilin University and the First Affiliated Hospital of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Formal Analysis, Investigation, Methodology, Writing – review & editing. HC: Investigation, Methodology, Writing – original draft. FQ: Methodology, Writing – original draft. XQ: Investigation, Writing – original draft. QZ: Investigation, Writing – original draft. KF: Investigation, Writing – original draft. XL: Investigation, Writing – original draft. HZ: Methodology, Supervision, Writing – review & editing. ZF: Formal Analysis, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou ZR, Wang CC, Yang ZZ, Yu XL, and Guo XM. Phyllodes tumors of the breast: diagnosis, treatment and prognostic factors related to recurrence. J Thorac Dis. (2016) 8:3361–8. doi: 10.21037/jtd.2016.11.03
2. Bogach J, Shakeel S, Wright FC, and Hong NJL. Phyllodes tumors: A scoping review of the literature. Ann Surg Oncol. (2021) 29:446–59. doi: 10.1245/s10434-021-10468-2
3. Zhang Y and Kleer CG. Phyllodes tumor of the breast: histopathologic features, differential diagnosis, and molecular/genetic updates. Arch Pathol Lab Med. (2016) 140:665–71. doi: 10.5858/arpa.2016-0042-RA
4. Van Olmen JP, Beerthuizen AWJ, Bekers EM, Viegen I, Drukker CA, Vrancken Peeters MTFD, et al. Management of benign phyllodes tumors: A dutch population-based retrospective cohort between 1989 and 2022. Ann Surg Oncol. (2023) 30:8344–52. doi: 10.1245/s10434-023-14128-5
5. Onkendi EO, Jimenez RE, Spears GM, Harmsen WS, Ballman KV, and Hieken TJ. Surgical treatment of borderline and Malignant phyllodes tumors: the effect of the extent of resection and tumor characteristics on patient outcome. Ann Surg Oncol. (2014) 21:3304–9. doi: 10.1245/s10434-014-3909-x
6. Liu J, Li F, Liu X, Lang R, Liang R, and Lu H. Malignant phyllodes tumors of the breast: the Malignancy grading and associations with prognosis. Breast Cancer Res Treat. (2023) 199:435–44. doi: 10.1007/s10549-023-06933-z
7. Wei J, Tan Y-T, Cai Y-C, Yuan ZY, Yang D, Wang SS, et al. Predictive factors for the local recurrence and distant metastasis of phyllodes tumors of the breast: a retrospective analysis of 192 cases at a single center. Chin J Cancer. (2014) 33(10):492–500. doi: 10.5732/cjc.014.10048
8. Choi N, Kim K, Shin KH, Kim Y, Moon HG, Park W, et al. The characteristics of local recurrence after breast-conserving surgery alone for Malignant and borderline phyllodes tumors of the breast (KROG 16-08). Clin Breast Cancer. (2019) 19:345–53.e2. doi: 10.1016/j.clbc.2019.04.003
9. Tu WJ, Zhao Z, Yan F, Zeng X, and Li J. Geographic and ethnicity variation in the prevalence of middle-aged and elderly adult obesity in China in 2020. Diabetes Obes Metab. (2024) 26:1897–907. doi: 10.1111/dom.15506
10. Bicchierai G, Pugliese F, Amato F, De Benedetto D, Boeri C, Vanzi E, et al. Percutaneous vacuum-assisted excision (VAE) of breast lesions of uncertain Malignant potential (B3 lesions): a preliminary single-centre Italian experience. Radiol Med. (2023) 128:528–36. doi: 10.1007/s11547-023-01626-5
11. Kapiris I, Nasiri N, A’Hern R, Healy V, and Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of High-grade Malignant phyllodes tumours of the Breast. Eur J Surg Oncol (EJSO). (2001) 27:723–30. doi: 10.1053/ejso.2001.1207
12. Ranjbar A, Zangouri V, and Shokripour M. Margin status impact on recurrence of phyllodes tumors in high-risk groups: a retrospective observational study. BMC Cancer. (2024) 24(1). doi: 10.1186/s12885-023-11805-2
13. Bennett IC and Saboo A. The evolving role of vacuum assisted biopsy of the breast: A progression from fine-needle aspiration biopsy. World J Surg. (2019) 43:1054–61. doi: 10.1007/s00268-018-04892-x
14. Bennett I, de Viana D, Law M, and Saboo A. Surgeon-performed vacuum-assisted biopsy of the breast: results from a multicentre Australian study. World J Surg. (2019) 44:819–24. doi: 10.1007/s00268-019-05266-7
15. Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, et al. Breast phyllodes tumor: A review of literature and a single center retrospective series analysis. Crit Rev Oncol/Hematol. (2013) 88:427–36. doi: 10.1016/j.critrevonc.2013.06.005
16. Neron M, Sajous C, Thezenas S, Piperno-Neumann S, Reyal F, Laé M, et al. Surgical margins and adjuvant therapies in Malignant phyllodes tumors of the breast: A multicenter retrospective study. Ann Surg Oncol. (2020) 27:1818–27. doi: 10.1245/s10434-020-08217-y
17. Jang JH, Choi M-Y, Lee SK, Kim S, Kim J, Lee J, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol. (2012) 19:2612–7. doi: 10.1245/s10434-012-2307-5
18. Macdonald OK, Lee CM, Tward JD, Chappel CD, and Gaffney DK. Malignant phyllodes tumor of the female breast. Cancer. (2006) 107:2127–33. doi: 10.1002/cncr.v107:9
19. Shin E and Koo JS. Prognostic factors of breast phyllodes tumors. Histol Histopathol. (2023) 38:865–78. doi: 10.14670/HH-18-600
20. Chen C-Y and Ya C. Better survival was found in patients treated with breast-conserving surgery compared with mastectomy in Malignant phyllodes tumor of the breast. Updates Surg. (2023) 76:265–70. doi: 10.1007/s13304-023-01547-y
21. Fou A, Schnabel FR, Hamele-Bena D, Wei XJ, Cheng B, El Tamer M, et al. Long-term outcomes of Malignant phyllodes tumors patients: an institutional experience. Am J Surg. (2006) 192:492–5. doi: 10.1016/j.amjsurg.2006.06.017
22. Bartels SAL, van Olmen JP, Scholten AN, Bekers EM, Drukker CA, Vrancken Peeters MTFD, et al. Real-world data on Malignant and borderline phyllodes tumors of the breast: A population-based study of all 921 cases in the Netherlands (1989 –2020). Eur J Cancer. (2024) 201:113924. doi: 10.1016/j.ejca.2024.113924
23. Kim S, Kim J-Y, Kim DH, Jung WH, and Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. (2013) 141:353–63. doi: 10.1007/s10549-013-2684-x
24. Strode M, Khoury T, Mangieri C, and Takabe K. Update on the diagnosis and management of Malignant phyllodes tumors of the breast. Breast. (2017) 33:91–6. doi: 10.1016/j.breast.2017.03.001
25. Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, Alhilli Z, et al. NCCN guidelines® Insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2024. J Natl Compr Canc Netw. (2023) 21:1000–10. doi: 10.6004/jnccn.2023.0051
26. Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, and Souba WW. Surgical management of phyllodes tumors. Arch Surg. (1999) 134:487–93. doi: 10.1001/archsurg.134.5.487
27. Ditsatham C and Chongruksut W. Phyllodes tumor of the breast: diagnosis, management and outcome during a 10-year experience. Cancer Manag Res. (2019) 11:7805–11. doi: 10.2147/CMAR.S215039
28. Del Calvo H, Wu Y, Lin HY, Nassif EF, Zarzour MA, Guadagnolo BA, et al. Margin width and local recurrence in patients with phyllodes tumors of the breast. Ann Surg Oncol. (2024) 31:8048–56. doi: 10.1245/s10434-024-15892-8
Keywords: breast phyllodes tumor, fibroepithelial tumor, rare breast tumor, breast surgery, local recurrence (LR)
Citation: Li Y, Chen H, Qu F, Qu X, Zhang Q, Feng K, Li X, Zhang H and Fan Z (2025) Clinicopathological features and risk factors for local recurrence of breast phyllodes tumors: a dual-center, 12-year, retrospective study. Front. Oncol. 15:1538523. doi: 10.3389/fonc.2025.1538523
Received: 03 December 2024; Accepted: 28 April 2025;
Published: 26 May 2025.
Edited by:
Shuhei Suzuki, Yamagata Prefectural Shinjo Hospital, JapanReviewed by:
Yi Zhang, Sichuan University, ChinaHideharu Yamada, National Hospital Organization (NHO), Japan
Copyright © 2025 Li, Chen, Qu, Qu, Zhang, Feng, Li, Zhang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Zhang, emhtb2JpbGVAMTI2LmNvbQ==; Zhimin Fan, ZmFuem1Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yiwen Li
Yiwen Li Heyan Chen2
Heyan Chen2 Fengjiang Qu
Fengjiang Qu Huimin Zhang
Huimin Zhang Zhimin Fan
Zhimin Fan