- 1Shandong Probincial Key Medical and Health Laboratory of Geriatric Gastrointestinal Tumor Pathology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, China
- 2Department of Pathology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, China
- 3Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Pathology, Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, China
- 5Department of Pathology, Ruijin-Hainan Hospital, Shanghai Jiao Tong University School of Medicine, Boao Research Hospital, Hainan, Qionghai, China
Objective: To investigate the clinicopathological characteristics, differential diagnosis and potential therapeutic targets of SMARCA4-deficient large cell neuroendocrine carcinoma (SD-LCNEC) in the lung.
Methods: We analyzed the clinicopathological features of 3 cases of SD-LCNEC and reviewed relevant literature. Differential diagnoses were conducted using a panel of immunohistochemical antibodies.
Results: The patients, aged 57 to 73 years, had tumors located in the right upper lobe, left hilum, and left upper lobe of the lung, respectively. All patients presented with lung masses. The tumors exhibited neuroendocrine carcinoma morphology, characterized by large tumor cells (nuclear diameter >3 lymphocytes), frequent mitoses, and prominent nucleoli. Tumor cells tested negative for SMARCA4 but were positive for Chromogranin-A(CgA), Synaptophysin (SYN), Insm-1, TTF-1 (partial), and Ki67 (90%). They were also negative for NapsinA, P63, P40, CK5/6, CK7, and NUT. Postoperative follow-up revealed one death, one case of Progressive Disease (PD), and one case of Stable Disease (SD).
Conclusion: SD-LCNEC is a rare and clinically aggressive carcinoma, often presenting with lymph node metastasis at the initial stage. Its morphology is equivalent to LCNEC; however, immunohistochemical staining indicates the absence of SMARCA4 and a reduction in neuroendocrine markers and TTF-1. There is no standardized treatment, but SMARCA4 may serve as a potential therapeutic target. Accurate identification of the molecular subtype of SD-LCNEC is crucial.
1 Introduction
Large cell neuroendocrine carcinoma (LCNEC) of the lung is a rare yet highly aggressive neuroendocrine tumor, accounting for 1-3% of all primary lung cancers (1). The SWI/SNF family, a major ATP-dependent chromatin remodeling complex, encompasses the BAF and PBAF complexes. Core subunits of the BAF complex, SMARCA4 (encoding BRG1) and SMARCA2 (encoding BRM), are highly homologous, with mutations or deletions closely associated with various tumors (2), including 10% of non-small cell lung cancers. SMARCA4-deficient large cell neuroendocrine carcinoma (SD-LCNEC) is particularly rare. This study examines the clinicopathological features and differential diagnosis of three cases of lung SD-LCNEC to provide reference for diagnosis and treatment.
2 Materials and methods
2.1 Case information
Three cases of lung SD-LCNEC were diagnosed at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, from September 2021 to May 2024 were retrospectively reviewed. All slides were re-evaluated by two experienced pathologists, and the diagnoses were verified according to the WHO’s fifth edition of lung tumor classification. This was done in conjunction with patient clinical history and imaging data, along with follow-up information.
2.2 Methods
Specimens from three cases of SD-LCNEC were fixed in 4% neutral formalin, routinely dehydrated, embedded in paraffin, and sectioned into 4 μm thick sections for HE staining and light microscopy. Immunohistochemical analysis was performed using Roche Ventana BenchMark GX automated immunohistochemistry staining system. Primary antibodies, including CKpan, CK7, TTF-1, CgA, SYN, CD56, Insm-1, POU2F3, BRG1, BRM, NapsinA, P63, P40, Claudin4, NUT, CD117, SSTR2A, SALL4, SOX2, PD-L1, and Ki67 were procured from Fuzhou Maixin Biotechnology Development Co. PD-L1 immunohistochemistry performed using 22C3 pharmDx kit (DAKO) on Autostainer Link 48. The specific procedures adhered to the kit instructions, incorporating both positive and negative controls. BRG1 and BRM deficiency was determined by complete loss of nuclear expression in tumor cells, while background inflammatory and stromal cells exhibited positive nuclear expression. TTF-1, NUT, P63, P40, SALL4, SOX2, POU2F3, Insm-1 and Ki67 were located in the nucleus of the cells. In contrast, CgA, SYN, CK7, NapsinA, CD117 and SSTR2A were located in the cytoplasm, while Claudin4, CKpan, CD56, PD-L1(22C3) were located in the cell membrane.
2.3 Multiplex fluorescent PCR
Multiplex fluorescent PCR was employed to detect EGFR, EML4-ALK, ROS1, RET, NTRK1/2/3, BRAF V600E, KRAS, HER2, and MET using a gene mutation detection kit for human lung cancer.
3 Results
3.1 Clinical features
3.1.1 Sex and age
Two males, one female, aged 57–73 years, with an average of 65 years and a median age of 66 years.
3.1.2 Largest tumor diameter
Ranging from 2.0 to 4.5 cm, with an average of 3.0 cm.
3.1.3 Smoking status
Two smokers and one non-smoker.
3.1.4 Location
All tumors were located in the lung. One patient underwent a upper lobe of the left lung, which resulted in a diagnosis of poorly differentiated carcinoma and later developed neck lymph node metastasis.
3.1.5 Clinical symptoms
All cases were detected during routine check-ups. One patient experienced systemic pain, while the others exhibited no obvious symptoms.
3.1.6 Imaging
In case 1, PET/CT revealed an irregular mass in the apical posterior segment of the left upper lobe, along with multiple enlarged lymph nodes observed in the mediastinal aortic window and the left hilum. In case 2, PET/CT indicated metabolic abnormalities in the apical segment of the right upper lobe and the presence of soft tissue nodules; multiple metabolically enlarged lymph nodes were noted in the mediastinum (4R) and both lung hilums. In Case 3, PET/CT showed increased metabolic activity in the soft tissue of the right middle lobe, characterized by lobulation, a burr-like appearance, and pleural retraction; multiple hypermetabolic lymph nodes were observed in the mediastinum region (10L) and both lung hilums. The clinicopathological data of the three cases are summarized in Table 1.
3.2 Pathological histological features
SD-LCNEC may not be morphologically specific; the morphology observed in the three cases presented in this paper aligns with that of common LCNEC, displaying neuroendocrine carcinoma characteristics. Case 1, at low power (10×), resembling small cell lung carcinoma (SCLC) with nest and beam formations, demonstrated a solid flake pattern accompanied by extensive necrosis (Figure 1A). On high-power examination (40×), the tumor cells ranged from medium to large in size, displaying irregular nuclear contours, finely granular chromatin, and occasional nucleoli (Figure 1B). Cases 2 and 3, at low power (10×), exhibiting classical neuroendocrine tumor morphology, were characterized by nested, organoid, and trabecular architectures (Figures 2A, 3A). On high-power examination (40×), the tumor cells displayed large size, abundant eosinophilic cytoplasm, coarsely granular chromatin, and prominent nucleoli (Figures 2B, 3B). All three cases displayed large tumor cells, with nuclear diameter >3 quiescent small lymphocytes, and mitotic images >10/2mm2. It can be differentiated from small cell carcinoma in terms of histomorphology.
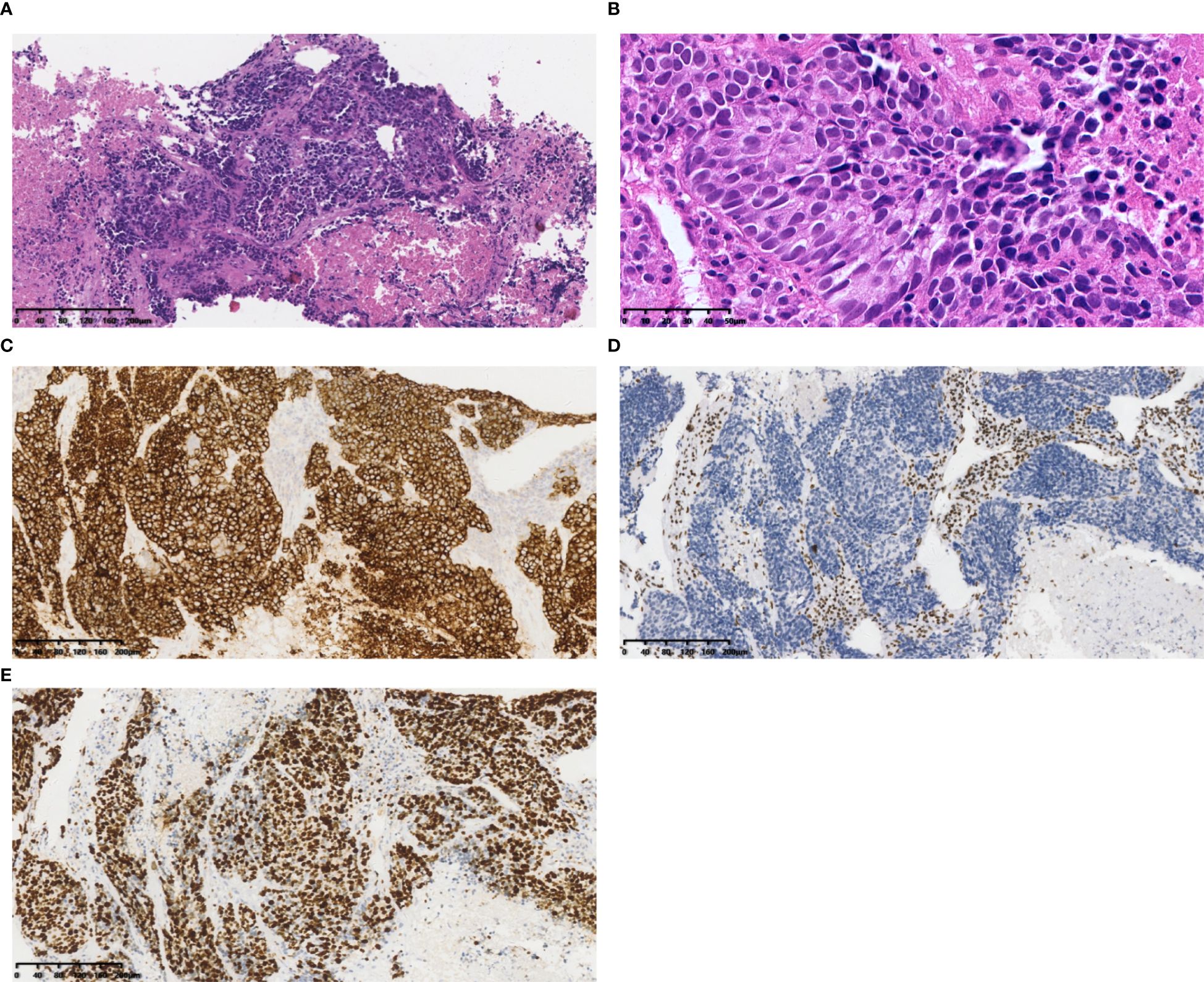
Figure 1. Case 1 (A) Tumor cells resembled small cell lung carcinoma (SCLC) with nest and beam formations, demonstrated a solid flake pattern accompanied by extensive necrosis. Hematoxylin and Eosin(HE), 10×objective, total magnification×100. (B) The tumor cells ranged from medium to large in size, displaying irregular nuclear contours, finely granular chromatin, and occasional nucleoli. HE 40× objective, total magnification ×400. (C) SYN positive. (D) BRG1 negative. (E) Ki-67 positivity rate: 80%. (C-E) Immunohistochemistry 10×objective, total magnification×100.
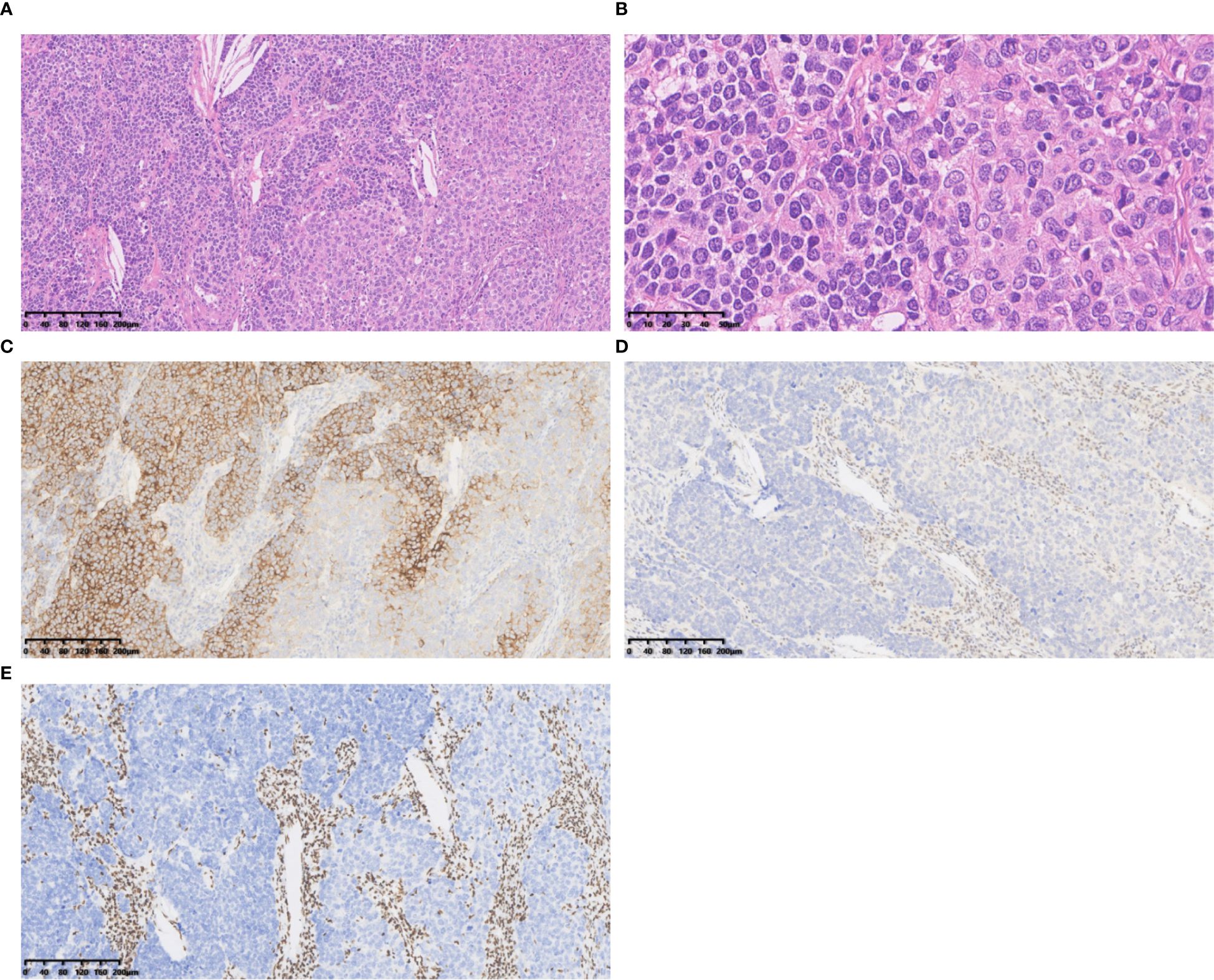
Figure 2. Case 2 (A) The tumour cells exhibited classical neuroendocrine tumour morphology, were characterized by nested, organoid, and trabecular architectures. Hematoxylin and Eosin(HE), 10×objective, total magnification×100. (B) The tumour cells displayed large size, abundant eosinophilic cytoplasm, coarsely granular chromatin, and prominent nucleoli. HE 40× objective, total magnification ×400. (C) SYN positive. (D) BRG1 negative. (E) BRM negative. (C-E) Immunohistochemistry 10×objective, total magnification×100.
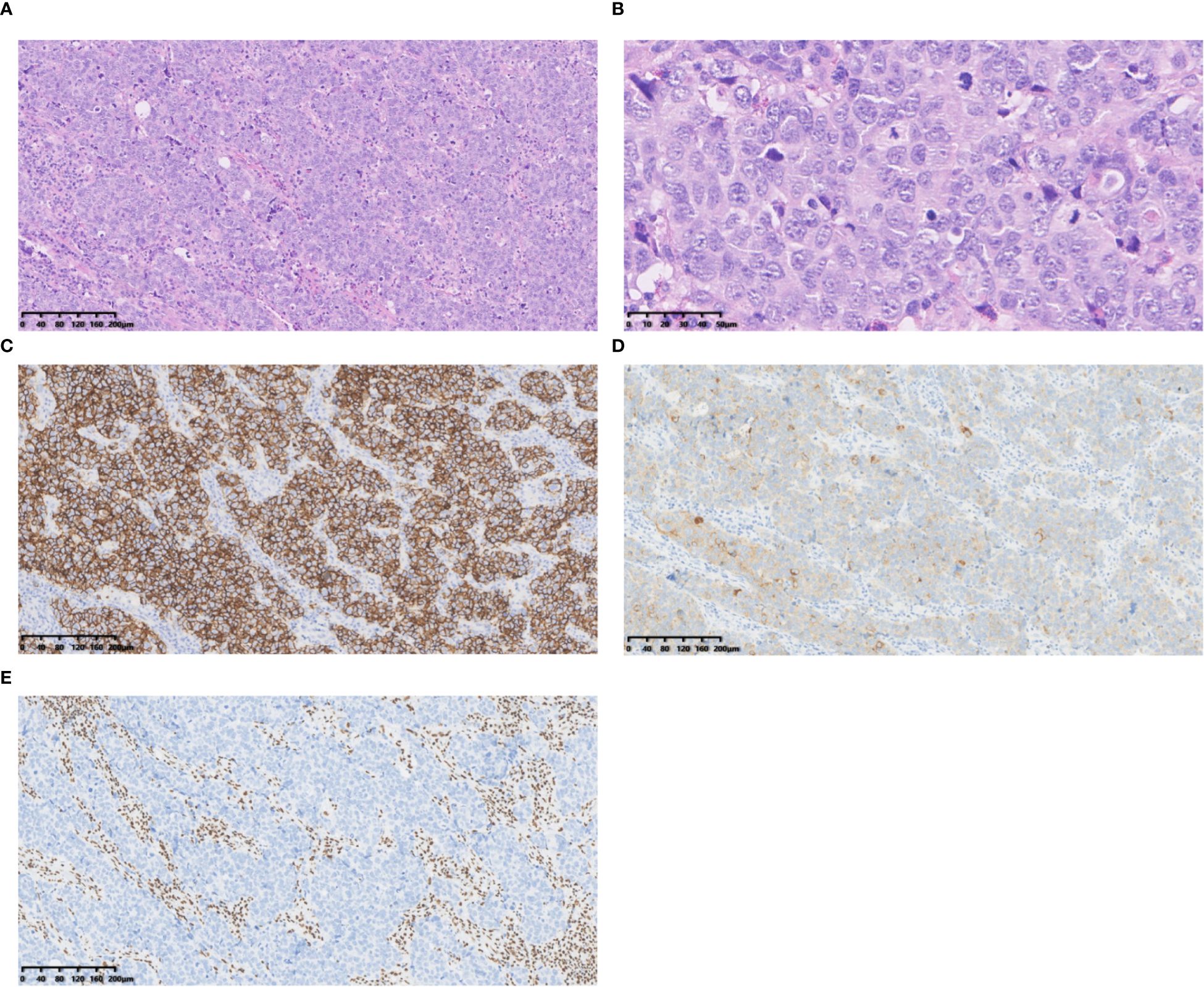
Figure 3. Case 3 (A) Tumour cells with large cytoplasmic bodies, nested clusters of trabecular pattern. Hematoxylin and Eosin(HE), 10×objective, total magnification×100. (B) Tumour cells with abundant cytoplasm, rough chromatin, distinct nucleoli and numerous nuclear divisions. HE 40× objective, total magnification ×400. (C) CD56 positive. (D) Syn positive. (E) BRG1 negative. C-E Immunohistochemistry 10×objective, total magnification×100.
3.3 Immunohistochemistry
All three tumors demonstrated deficient expression of the BRG1 protein (Figures 1D, 2D, 3E), with one also exhibiting deficient expression of the BRM protein (Figure 2E); Additionally, all tumors expressed the neuroendocrine markers CgA, CD56 (Figure 3C) and SYN (Figures 1C, 2C, 3D); two tumors expressed Insm-1and TTF-1. Combining these immunohistochemical results with the neuroendocrine morphology allowed us to make the diagnosis of LCNEC. The proliferation index of Ki67 exceeded 80% (Figures 1E). The markers CKpan, P40, P63, Claudin4, CK5/6, CK7, NUT, and NapsinA effectively ruled out lung adenocarcinoma, squamous cell carcinoma, NUT carcinoma and Thoracic SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) (see Table 1).
3.4 Multiplex fluorescent PCR
One case tested for 11 genes revealed no mutations.
3.5 Treatment and follow-up
In Case 1, treatment commenced with irinotecan and carboplatin for one course at the initial facility, followed by oral Anlotinib, which was discontinued after three days due to severe myelosuppression. Subsequent ultrasonography indicated cervical lymph node metastasis. After treatment with a low-dose EP regimen (etoposide and cisplatin), the cervical mass gradually increased. The patient then presented to our hospital for a lymph node biopsy, received three courses of chemotherapy with irinotecan and cisplatin, underwent particle implantation radiotherapy, and died 13 months after disease onset. In Case 2, the patient underwent right upper lobectomy with lymph node dissection and thoracoscopic pleural adhesion release. Postoperative pathological staging was IIIA (pT2bN2M0). Etoposide and carboplatin chemotherapy were administered, followed by radiotherapy targeting the focal lung areas and positive lymph node regions. A CT review 16 months post-operation revealed new foci in both lungs, with disease progression observed at 16-month follow-up (PD). In Case 3, the patient underwent pleural adhesion release and thoracoscopic mediastinal lymph node dissection, followed by immuno-EP chemotherapy (Serplulimab, cisplatin, and etoposide) after surgery. The disease remained stable (SD) at the 9-month follow-up post-surgery. The treatment and follow-up data for the three patients are shown in (Table 1).
4 Discussion
Lung SD-LCNEC, although rare, has gained attention as the SWI/SNF complex family becomes a focal point in research, as reported by Dagogo-Jack et al (3). The study of SMARCA4 deletion in a large number of lung cancer samples using second-generation sequencing technology revealed the presence of SMARCA4 deletion in neuroendocrine carcinomas, as previously reported by Gandhi et al (4). In this study, we report three cases diagnosed with SD-LCNEC at Ruijin Hospital of Shanghai Jiaotong University. All three cases presented with lymph node metastasis at the initial visit and were classified as locally advanced stage. The overall survival time of patients with advanced LCNEC was significantly shorter than that of patients with stage-matched lung adenocarcinoma (5). SD-LCNEC may not be morphologically specific compared to common LCNEC, the diagnosis is based on typical neuroendocrine morphology, neuroendocrine marker expression, and SMARCA4-deficient expression. The markers CKpan, P40, P63, Claudin4, CK5/6, CK7, NUT, and NapsinA effectively ruled out lung adenocarcinoma, squamous cell carcinoma, and NUT carcinoma. SD-LCNEC is mainly differentiated from SMARCA4-UT. According to the 2021 WHO classification of thoracic tumors, SMARCA4-UT is classified under “other tumors of epithelial origin of the lung”. It primarily affects young male smokers and is characterized by histological features such as solid sheets, islands, or nodules of cells, frequently accompanied by necrosis. The similarity of histomorphology poses challenges to diagnosis. Immunohistochemistry plays an important role in differential diagnosis. Immunohistochemistry demonstrates positivity for CD34, SOX2, and SALL4, with CKpan negativity or focal paranuclear punctate positivity, Claudin-4 negativity, and frequent double deletion of SMARCA4 and SMARCA2. While Case 2 demonstrated dual SMARCA4/SMARCA2 loss—a hallmark of SMARCA4-UT, the expression of CgA, SYN, CKpan, and Claudin4 aligned with LCNEC criteria, distinguishing it from SMARCA4-UT (typically CD34+/SOX2+/SALL4+ with epithelial marker negativity.
The literature indicates that approximately 3%-7% of lung neuroendocrine tumors harbor SMARCA2 and SMARCA4 mutations (6, 7). Notably, neuroendocrine markers and TTF-1 expression were found to decrease in the absence of SMARCA4, predominantly by SYN, but also by Insm-1, CgA or CD56 (8). In this study, all three cases exhibited weak positivity for CgA. Two cases were negative for CD56, and one case showed focal weak staining for Syn. Recent studies have demonstrated that SMARCA4 controls REST, a known suppressor of the NE phenotype, by regulating SRRM4-dependent REST transcript splicing. Moreover, inhibition of SMARCA4 activates of the ERBB family receptors ERBB2, ERBB3 and ERBB4 in SCLC, rendering these tumors sensitive to afatinib (9). Therefore, we hypothesized that this may explain the reduced expression of neuroendocrine markers observed in cases of SMARCA4 deletion.
Recent molecular genetics studies have identified three primary molecular subtypes of lung LCNEC: 1. A Small Cell Lung Cancer (SCLC)-like subtype characterized by RB1 and TP53 genomic alterations; 2. A carcinoid subtype lacking TP53 genomic mutations but exhibiting alterations in neuroendocrine tumor genes; 3. A carcinoid subtype in which SMARCA4, KRAS, FGF3/4/19, STK11, CDKN2A/B, and other genomic alterations predominate in non-small cell lung cancer-like subtypes (10–12). However, there are currently few therapeutic targets for the treatment of LCNEC molecular subtypes, and so far only everolimus (an mTOR inhibitor) has been identified as a targeted drug (1). In 1 of our 3 cases, no driver mutations were detected through 11 genetic tests related to lung cancer treatment. Emerging evidence highlights the role of SMARCA4 in lung cancer maintenance, carcinogenesis, and treatment (13). Inactivation of the SMARCA4 gene, which encodes BRG1 protein, increases tumor invasiveness and affects sensitivity to conventional platinum-based chemotherapeutic agents but may increase tumor sensitivity to EZH2 inhibitors, CDK4/6 inhibitors (3). All our 3 cases were treated with platinum-based chemotherapy and additional therapies, resulting in one death and one case of disease progression. In case 1, the patient received a low-dose EP chemotherapy regimen combined with particle implantation radiotherapy. Unfortunately, the patient succumbed to the disease 13 months after diagnosis. I case 2, the patient underwent surgical resection followed by adjuvant platinum-based chemotherapy and radiotherapy targeting the lung and lymph node regions. Despite multimodal treatment, disease progression was observed during the 16-month follow-up, with the emergence of new pulmonary lesions. Several small retrospective studies (1, 14, 15) suggest that immunotherapy demonstrates greater efficacy compared to drug toxicity in patients with LCNEC. Additionally, positive expression of PD-L1 (TPS ≥1%) was associated with SMARCA4 deficiency (16), which may predict response to immunotherapy. This is supported by Case 3. In case 3, the patient received platinum-based chemotherapy and immunotherapy, and at the 9-month postoperative follow-up, the disease remained stable (SD). As studies increasingly correlate SMARCA4 with lung cancer treatment and prognosis, the accurate identification of SMARCA4-deficient tumor subtype becomes critical and may necessitate specific therapeutic strategies. we will expect to explore and develop novel targeted therapies for SMARCA4-deficient tumors, including CDK4/6 inhibitors and EZH2 inhibitors. Further clinical trials are critical to evaluate targeted therapies and optimize therapeutic option for this rare subtype.
5 Summary
In this study, we retrospectively analyzed the clinicopathological data of three cases of SMARCA4-deficient large cell neuroendocrine carcinoma (SD-LCNEC), reporting for the first time the distinct features of this rare and aggressive subtype characterized by early metastasis and reduced expression of neuroendocrine markers. Our findings emphasize the critical role of SMARCA4 immunohistochemical analysis in accurately identifying SD-LCNEC, particularly in cases with low neuroendocrine marker and TTF-1 expression. Moving forward, expanding clinical case collections and exploring targeted therapies, such as CDK4/6 and EZH2 inhibitors, will be essential to improve patient outcomes and advance our understanding of SD-LCNEC’s biological behavior, classification, and treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JJ: Investigation, Methodology, Writing – review & editing, Formal analysis. YW: Investigation, Methodology, Formal analysis, Writing – review & editing. XF: Formal analysis, Methodology, Writing – review & editing. CW: Supervision, Writing – review & editing. XC: Formal analysis, Funding acquisition, Investigation, Supervision, Writing – review & editing, Project administration, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Scientific Research Project of Health Industry in Hainan Province (22A200173).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Andrini E, Marchese PV, De Biase D, Mosconi C, Siepe G, Panzuto F, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. (2022) 11:1461. doi: 10.3390/jcm11051461
2. Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human Malignancy. Nat Genet. (2013) 45:592–601. doi: 10.1038/ng.2628
3. Dagogo-Jack I, Schrock AB, Kem M, Jessop N, Lee J, Ali SM, et al. Clinicopathologic characteristics of BRG1-deficient NSCLC. J Thorac Oncol. (2020) 15:766–76. doi: 10.1016/j.jtho.2020.01.002
4. Gandhi JS, Alnoor F, Sadiq Q, Solares J, and Gradowski JF. SMARCA4 (BRG1) and SMARCB1 (INI1) expression in TTF-1 negative neuroendocrine carcinomas including merkel cell carcinoma. Pathol Res Pract. (2021) 219:153341. doi: 10.1016/j.prp.2021.153341
5. Yang Y, Shen C, Shao J, Wang Y, Wang G, and Shen A. Based on the development and verification of a risk stratification nomogram: predicting the risk of lung cancer-specific mortality in stage IIIA-N2 unresectable large cell lung neuroendocrine cancer compared with lung squamous cell cancer and lung adenocarcinoma. Front Oncol. (2022) 12:825598. doi: 10.3389/fonc.2022.825598
6. Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol. (2017) 241:488–500. doi: 10.1002/path.2017.241.issue-4
7. Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. (2020) 15:231–47. doi: 10.1016/j.jtho.2019.10.023
8. Metovic J, Bianchi F, Barella M, Papotti M, and Pelosi G. SMARCA2 deficiency while preserving SMARCA4 and SMARCB1 in lung neuroendocrine carcinomas. J Thorac Oncol. (2021) 16:e32–5. doi: 10.1016/j.jtho.2021.01.1613
9. Redin E, Sridhar H, Zhan YA, Mello BP, Zhong H, Durani V, et al. SMARCA4 controls state plasticity in small cell lung cancer through regulation of neuroendocrine transcription factors and REST splicing. J Hematol Oncol. (2024) 17:58. doi: 10.1186/s13045-024-01572-3
10. Rekhtman N. Lung neuroendocrine neoplasms: recent progress and persistent challenges. Mod Pathol. (2022) 35:36–50. doi: 10.1038/s41379-021-00943-2
11. Wu X, Yin J, Deng Y, and Zu Y. Whole-genome characterization of large-cell lung carcinoma: A comparative analysis based on the histological classification. Front Genet. (2022) 13:1070048. doi: 10.3389/fgene.2022.1070048
12. Burns L, Tukachinsky H, Raskina K, Huang RP, Schrock AB, Sands J, et al. Real-World comprehensive genomic profiling data for diagnostic clarity in pulmonary Large-Cell neuroendocrine carcinoma. Lung Cancer. (2024) 188:107454. doi: 10.1016/j.lungcan.2023.107454
13. Schoenfeld AJ, Bandlamudi C, Lavery JA, Montecalvo J, Namakydoust A, Rizvi H, et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin Cancer Res. (2020) 26:5701–8. doi: 10.1158/1078-0432.CCR-20-1825
14. Shi Z, Wei J, Xu M, and Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. (2023) 15:4172–81. doi: 10.21037/jtd-23-348
15. Yan N, Guo S, Zhang Z, Shen S, and Li X. Efficacy of immune checkpoint inhibitors in advanced large cell neuroendocrine carcinoma of the lung: A single−institution experience. Oncol Lett. (2024) 27:135. doi: 10.3892/ol.2024.14268
Keywords: lung neoplasms, SMARCA4-deficient, large cell neuroendocrine carcinoma, immunohistochemistry, differential diagnosis
Citation: Zou F, Jia J, Wang Y, Fei X, Wang C and Chen X (2025) Case Report: Three cases of lung large cell neuroendocrine carcinoma with clinicopathological features of SMARCA4 (BRG1) deficiency. Front. Oncol. 15:1538548. doi: 10.3389/fonc.2025.1538548
Received: 03 December 2024; Accepted: 10 June 2025;
Published: 30 June 2025.
Edited by:
Helmut H. Popper, Medical University of Graz, AustriaReviewed by:
Giuseppe Angelico, Kore University of Enna, ItalyMehmet Ali Bedirhan, Yedikule Teaching Hospital, Türkiye
Hiroyuki Kyoyama, Saitama Medical University, Japan
Copyright © 2025 Zou, Jia, Wang, Fei, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Chen, Y3h5MTE4MzJAcmpoLmNvbS5jbg==
 Fangfang Zou
Fangfang Zou Jingdan Jia3
Jingdan Jia3 Chaofu Wang
Chaofu Wang