- 1Department of Gastrointestinal Surgery, The People’s Hospital of Leshan, Leshan, China
- 2Department of Scientific Research and Teaching, The People’s Hospital of Leshan, Leshan, China
Background: Long-course neoadjuvant chemoradiotherapy (Lc-NCRT) is the conventional treatment for locally advanced rectal cancer (LARC). It improves R0 resection rate and reduces local recurrence rate, but it cannot improve long-term oncological outcomes. It also causes several radiotherapy-related side effects. In recent years, some studies have shown that neoadjuvant chemotherapy (NCT) may be noninferior to Lc-NCRT. Therefore, we systematically evaluated the efficacy and safety of NCT and Lc-NCRT for LARC.
Methods: Cochrane Library, Embase, PubMed, WanFang Data, and CNKI were systematically searched as the relevant literature. The literature was screened independently by two groups, and data were extracted and evaluated for bias. A meta-analysis was performed using Revman5.4 software. The primary outcomes were tumor response to neoadjuvant therapy and long-term oncological outcomes.
Results: A total of 17 studies with 5,168 cases (1,957 cases in NCT and 3,211 cases in Lc-NCRT) were included in our meta-analysis. Compared with the Lc-NCRT group, although the NCT group had a lower pCR rate [RR = 0.65, 95% CI (0.56–0.75), P < 0.0001], less downstaging [RR = 1.11, 95%CI(1.03–1.19), P = 0.06] and more adverse events of neoadjuvant therapy [RR = 1.11, 95% CI (1.03–1.19); P = 0.06], it had no difference in long-term survival outcome [3-year overall survival: HR = 1.13, 95% CI (0.70–1.83), P = 0.62; 3-year disease-free survival: HR = 1.16, 95% CI (0.96–1.39), P = 0.12; 3-year local recurrence-free survival: HR = 1.36, 95% CI (0.9–2.08), P = 0.15] and serious adverse events [RR = 0.84, 95% CI (0.45–1.57), P = 0.58] from the Lc-NCRT group. Moreover, the incidence of anastomotic leakage [RR = 0.48, 95% CI (0.34–0.45)] and permanent stoma rate [RR = 0.7, 95% CI (0.58–0.84), P < 0.0001] after operation was lower in the NCT group.
Conclusion: NCT is a potential option for the treatment of LARC as it is beneficial for improving the sphincter preservation rate and reducing anastomotic leakage, the long-term oncological outcome is considerable, and the safety is controllable. Larger randomized controlled trials (RCT) with longer follow-up data are needed to clarify the specific regimens of NCT and the risk stratification of rectal cancer.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/home identifier, CRD42024579586.
1 Introduction
For nearly two decades, the standard treatment for LARC has been a model that included fluorouracil-based Lc-NCRT, total mesorectal excision (TME), and adjuvant chemotherapy (1). Compared with postoperative adjuvant therapy alone, Lc-NCRT has improved the quality of TME, increased the R0 resection rate, and reduced the local recurrence rate to less than 10% (2, 3), but it seems hard to improve the long-term survival outcome (2, 4). Although it can reduce the toxicity of chemoradiotherapy compared to postoperative adjuvant therapy, the uninterrupted course of Lc-NCRT for 28 days also makes it difficult for the patients to comply fully. Neoadjuvant radiotherapy causes pelvic tissue fibrosis, and autonomic nerve damage is associated with both short- and long-term morbidities. It may increase the difficulty of surgery and the incidence of postoperative anastomotic leakage, leading to a high stoma rate, and is a risk factor for low anterior resection syndrome. At the same time, it also affects fertility and sexual function and weakens pelvic bone marrow hematopoietic function (5–8). Moreover, the early use of systemic chemotherapy may reduce tumor micrometastases, and Lc-NCRT may delay the initiation of systemic therapy and provide a time window for the development of micrometastases, which may make distant recurrence a major cause of death in LARC (9). Therefore, to improve patient compliance, reduce the adverse events of neoadjuvant therapy, and improve long-term survival, some centers have explored the role of NCT in LARC, and it seems that NCT alone may result in an acceptable tumor response and disease-free survival (10–17), while others have yielded mixed results (18, 19). Many of these studies were small-sample, retrospective, and single-arm trials. Considering the inconsistent results of the published studies, we hope to compare the clinical efficacy of NAC and Lc-NCRT in the treatment of LARC through a systematic review and meta-analysis in order to obtain a higher level of evidence.
2 Methods
This study was performed according to the current Preferred Reporting Program for Systematic Reviews and Meta-analyses (PRISMA) (20) checklist and guidelines for methodological quality of systematic reviews AMSTER. It was registered at PROSPERO (registration number CRD42024579586).
21 Literature search strategy
Studies were searched by computer in public databases, including Cochrane Library, Embase, PubMed, WanFang Data, and CNKI. The search protocol used subject words in combination with free words to search the original studies of NCT compared to Lc-NCRT in the treatment of LARC, without limit in the search language. The search terms were “rectal neoplasm”, “chemotherapy”, “neoadjuvant”, and “chemoradiotherapy”, and the list of references of the retrieved studies was screened to identify citations that may be relevant to the analysis. The last literature search was conducted on September 9, 2024. Two researchers independently screened the retrieved literature and assessed the eligibility of each study included in the meta-analysis. Differences should be resolved through consensus and, if necessary, through meetings.
2.2 Inclusion and exclusion criteria of literature
The inclusion criteria were as follows: (1) biopsy specimen diagnosed as rectal adenocarcinoma, including mucinous adenocarcinoma and signet ring cell carcinoma, (2) staging at a locally advanced stage, TanyN+M0 and T3/4NanyM0 included, (3) intervention measures including NCT and Lc-NCRT, and (4) source data study with results including at least one of oncology, safety, and surgery-related indicators. The exclusion criteria were as follows: (1) rectal tumors of other pathological types, including stromal tumors and neuroendocrine tumors, (2) uncontrolled studies, (3) stage I or IV medical records, (4) data not derived from the original study, and (5) Newcastle–Ottawa Scale (NOS) score less than 6 for cohort studies. The most recent study was selected for inclusion if duplicate or overlapping articles were published by the same institution and researcher.
2.3 Data extraction
Two researchers independently extracted the literature data. When there were differences, they were verified by a third one, and the final analysis data were discussed and determined. The data we extracted were as follows:(1) the general data of the literature included the first author’s name, years of publication, region, study type, propensity score or not, number of study centers, enrollment time, and sample size enrolled in each group, (2) basic information of patients, including age, body mass index (BMI), sex, Eastern Cooperative Oncology Group (ECOG), tumor location, pathological type, clinical and pathological stage, American Society of Anesthesiologists (ASA) score, etc., (3) treatment regimen, neoadjuvant treatment regimen, cycle, postoperative adjuvant regimen, adverse events of adjuvant therapy, etc., (4) basic information of surgery, including operation type, sphincter preservation rate, stoma rate, R0 resection rate, pathological results, and postoperative complications, and (5) oncology outcomes included pathological complete response (pCR), downstaging, disease control rate (DCR), objective response rate (ORR), overall survival (OS), disease-free survival (DFS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS). The primary outcomes of this study were oncological outcomes, and the secondary outcomes were adverse events of chemoradiotherapy, R0 resection rate, sphincter preservation rate, and surgical complications. If only K-M plots are provided without HR data, HR is converted using the method of Jayne F. Tierney (21), and valid data that could not be extracted were not included in the meta-analysis. For studies with multiple chemoradiotherapy groups, only fluorouracil-based Lc-NCRT was included. For those with multiple NCT regimens, the results were combined for meta-analysis and separately included in the subgroup analysis. If the PSM study had similar data before and after matching, then the matched data were included in the meta-analysis.
2.4 Quality assessment
Two researchers independently evaluated the quality of the literature, and all disagreements were resolved by consensus. For RCT studies, the Cochrane Risk Bias Assessment Tool was implemented using the Revman 5.4 software. For cohort studies, the Newcastle–Ottawa Quality Assessment Tool (NOS scale) was used, which rates the quality of eight items in three domains: selectivity (up to four points), comparability (up to two points), and outcome (up to three points). The higher the score, the higher the quality is.
2.5 Statistical analysis
Meta-analysis was performed using Revman5.4 software. Relative risk (RR), mean difference (MD), hazard ratio (HR), and their corresponding 95% confidence intervals (95% CI) were calculated for dichotomous data and continuous and survival data, respectively. Q test and I2 test analyses were used to examine heterogeneity in the literature. If heterogeneity was low (I2 < 50%), the pooled estimate was calculated using the fixed-effects method. Otherwise, a random-effects model was used. For moderate to high heterogeneity, one-way sensitivity and subgroup analyses were used to explore the source of heterogeneity. Subgroup analysis according to study type (RCT or not, PSM or not), study publication time (before median publication time vs. later than median publication time), NCT regimens (FOLFOX vs. CAPOX, two-drug vs. three-drug, etc.), study center (multicenter vs. single-center), sample size (≥median sample size vs. <median sample size), and population characteristics (phase II vs. III, T4 vs. T1–3, N- vs. N+, EMVI+ vs. EMVI-, etc.). A funnel plot was used to test the stability of the meta-analysis for publication bias in studies that included more than 10 studies. A p-value of <0.05 for the combined data was significant.
3 Results
3.1 Study characteristics
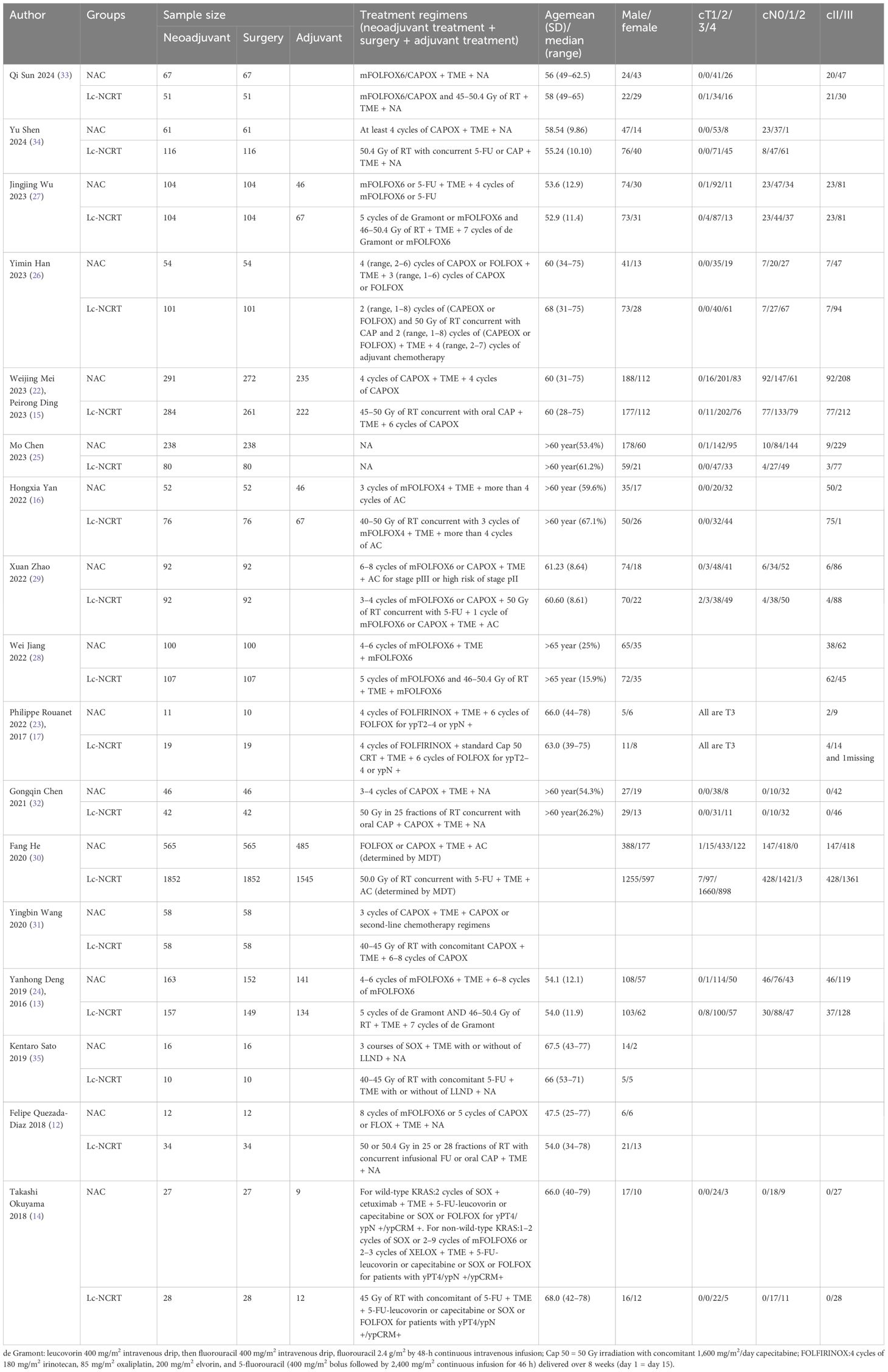
A total of 13,717 articles were retrieved according to the initial search strategy, and 20 articles meeting the literature inclusion criteria were meta-analyzed. The literature screening process is illustrated in Figure 1. A total of 20 articles from 17 studies were included in this meta-analysis (three RCTs reported initial (13, 17, 22) and final results (15, 23, 24) in two separate studies). A total of 5,168 neoadjuvant patients were included in the analysis (NCT, 1,957 cases; Lc-NCRT, 3,211 cases), among which 5,106 cases received surgery (NCT, 1,926 cases; Lc-NCRT, 3,180 cases).
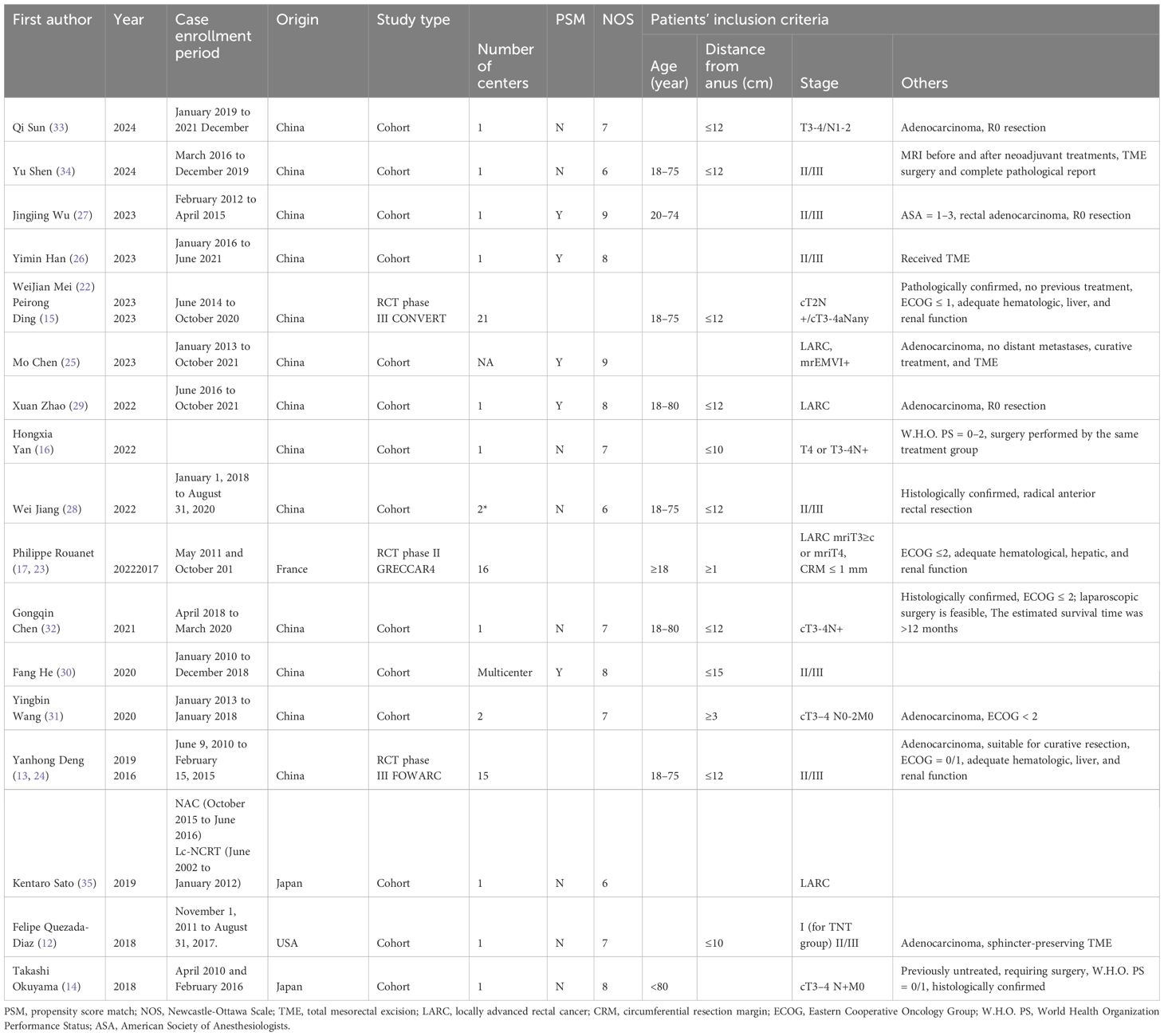
There were three RCTs (13, 15, 17, 22–24); the rest were cohort studies, five of which were propensity-score-matched (25–30). Only two studies were from the USA and Europe (12, 17), and the rest were from Asia. Seven were multi-center results (13, 15, 17, 28, 30, 31), nine studies have mid- or long-term oncology outcomes (14, 15, 23–27, 29, 30), and five studies were multi-arm studies (including surgery alone, short-course radiotherapy group, chemotherapy with different regimens, etc.) (12, 13, 25, 31, 32), only long-course cases of radiotherapy concurrent with fluorouracil-based chemotherapy were included as controls.
As shown in Table 1, CAPOX and FOLFOX (including mFOLFOX6 and FOLFOX4) were the most common NCT regimens, and SOX (14) and FOLFIRINOX (17) were included in some earlier studies. In cohort studies, all patients underwent surgery, and patients from Japan underwent selective lateral lymph node dissection(LLND) (14, 35), while RCT studies had slightly fewer surgical samples than neoadjuvant therapy samples. Most of the studies performed postoperative chemotherapy, but only seven provided data (13, 14, 22, 25, 26, 28, 30). There seems to be no significant difference in preoperative T stage, N stage, clinical stage, age, and gender between groups. Some studies also performed subgroup analysis according to distance from the anus (24, 27) and clinical stage (30), and the patients were all EMVI+ of LARC in one study (25). One study adopted different neoadjuvant regimens according to the stratum. This meta-analysis extracted data from the stratum of favorable response for the investigator and only compared NCT and Lc-NCRT in this stratification (17). The characteristics of the study, including population, are presented in Table 1.
3.2 Quality evaluation of the included literature
Of the 20 included studies, 14 were cohort studies. The quality of the literature was using the NOS scale from eight points in three aspects of selectivity, control line, and result. The scores in the literature are listed in Table 2. The higher the score is, the higher the quality is, and the NOS scores of all studies are more than 5. The Cochrane bias risk assessment tool was used to assess the article quality of the three RCTs (Figure 2), and there was no significant bias except for the blinding method. Therefore, the overall quality of the 20 articles from these 17 studies was considered to be good.
3.3 Meta-analysis results
We systematically evaluated the effects of neoadjuvant therapy on LARC in terms of tumor response to neoadjuvant therapy, short- and long-term survival outcomes, safety of neoadjuvant therapy, surgical complications, and anal retention.
3.3.1 Tumor response
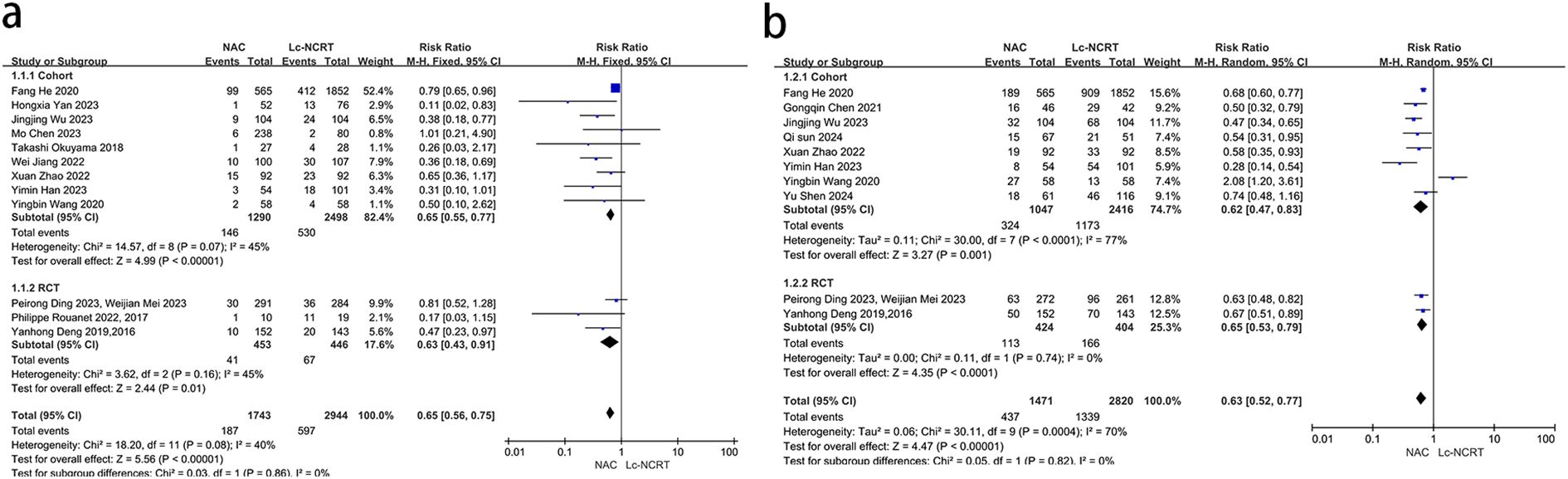
pCR results were reported in 12 studies (Figure 3a). There was mild heterogeneity among the studies (I2 = 40%, chi-square test, P = 0.08). A fixed-effect model was used. The results showed that the probability of achieving pCR in the NCT group was less than that in the Lc-NCRT group, and the difference was statistically significant [RR = 0.65, 95% CI (0.56–0.75), P < 0.0001]. The results of the subgroup analyses were consistent.
The results of pTRG0–1 were reported in 10 studies (Figure 3b). There was moderate heterogeneity among the studies (I2 = 70%, chi-square test, P = 0.0004). A random-effect model was used. The results showed that the probability of achieving pTRG0–1 in the NCT group was less than that in the Lc-NCRT group, and the difference was statistically significant [RR = 0.63, 95% CI (0.52–0.77), P < 0.0001]. There was no heterogeneity in the RCT subgroup, while there was still high heterogeneity in the cohort subgroup. However, the results did not change.
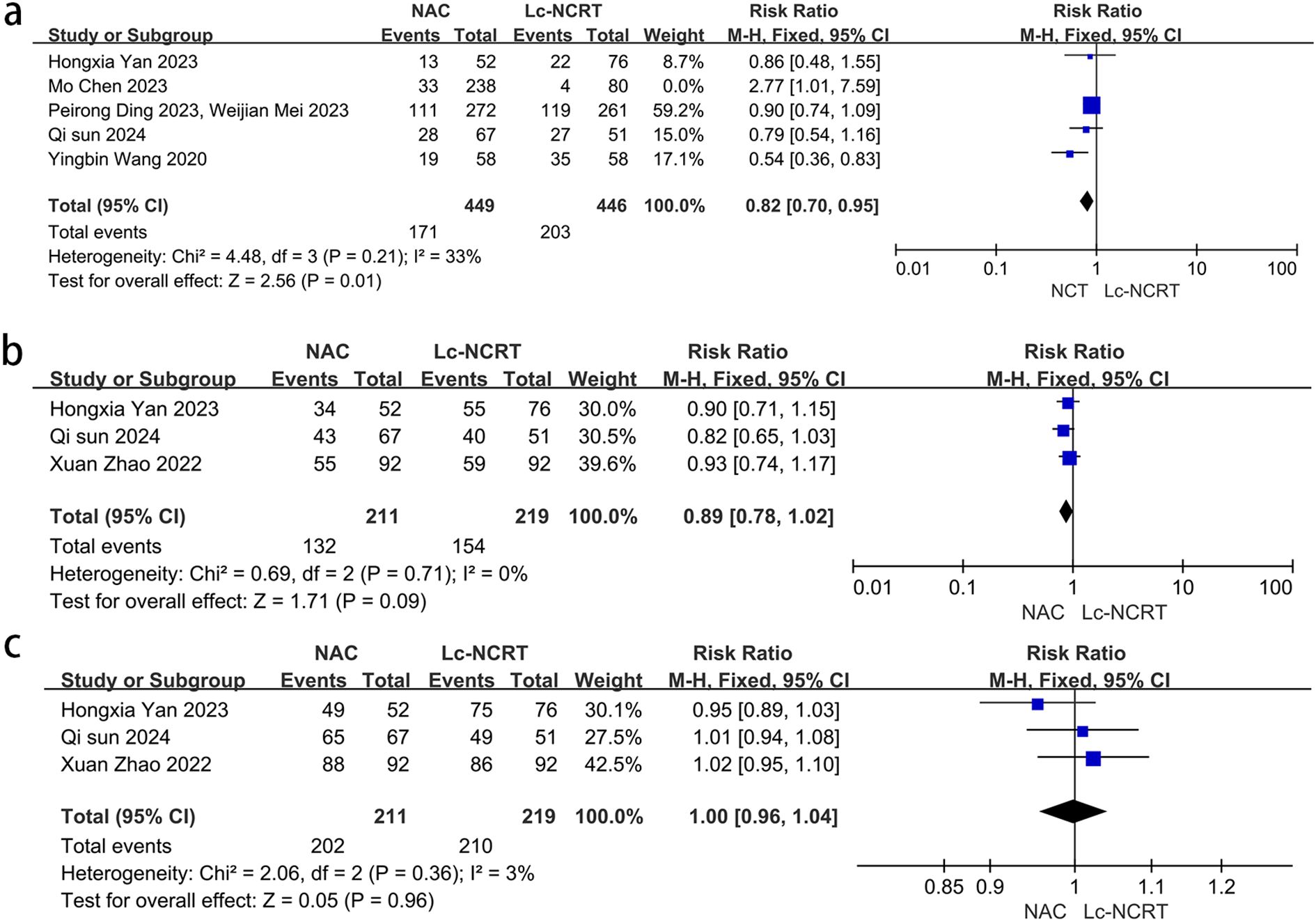
Five studies reported the outcome of downstaging (Figure 4a), and there was moderate heterogeneity among the studies (I2 = 61%, chi-square test, P = 0.61). The sensitivity analysis suggested that the study by Mo Chen (11) may be the source of heterogeneity (I2 = 33%, chi-square test, P = 0.21). Considering that the cases in this study were all EMVI-positive, which was different from other studies, they were excluded. A fixed-effect model was adopted, and the results showed that the downstaging rate in the NCT group was lower than that in the Lc-NCRT group [RR = 0.82, 95% CI (0.70–0.95); P = 0.01].
Three studies reported the outcomes of ORR and DCR (Figures 4b, c). There was no heterogeneity among these studies (ORR: I2 = 0%, chi-square test, P = 0.71 and DCR: I2 = 3%, chi-square test, P = 0.36). The results showed that there was no significant difference in direct ORR and DCR between the NCT and the Lc-NCRT groups using a fixed-effects model [ORR: RR = 0.89, 95% CI (0.78–1.02), P = 0.09; DCR: RR = 1.0, 95% CI (0.96–1.04), P = 0.96].
3.3.4 Survival outcomes
Nine papers reported long-term oncological outcomes, including 3 years of OS, DFS, and LRFS (Figure 5). Only one or two studies reported survival outcomes at 2 and 5 years, respectively; therefore, no meta-analysis was performed.
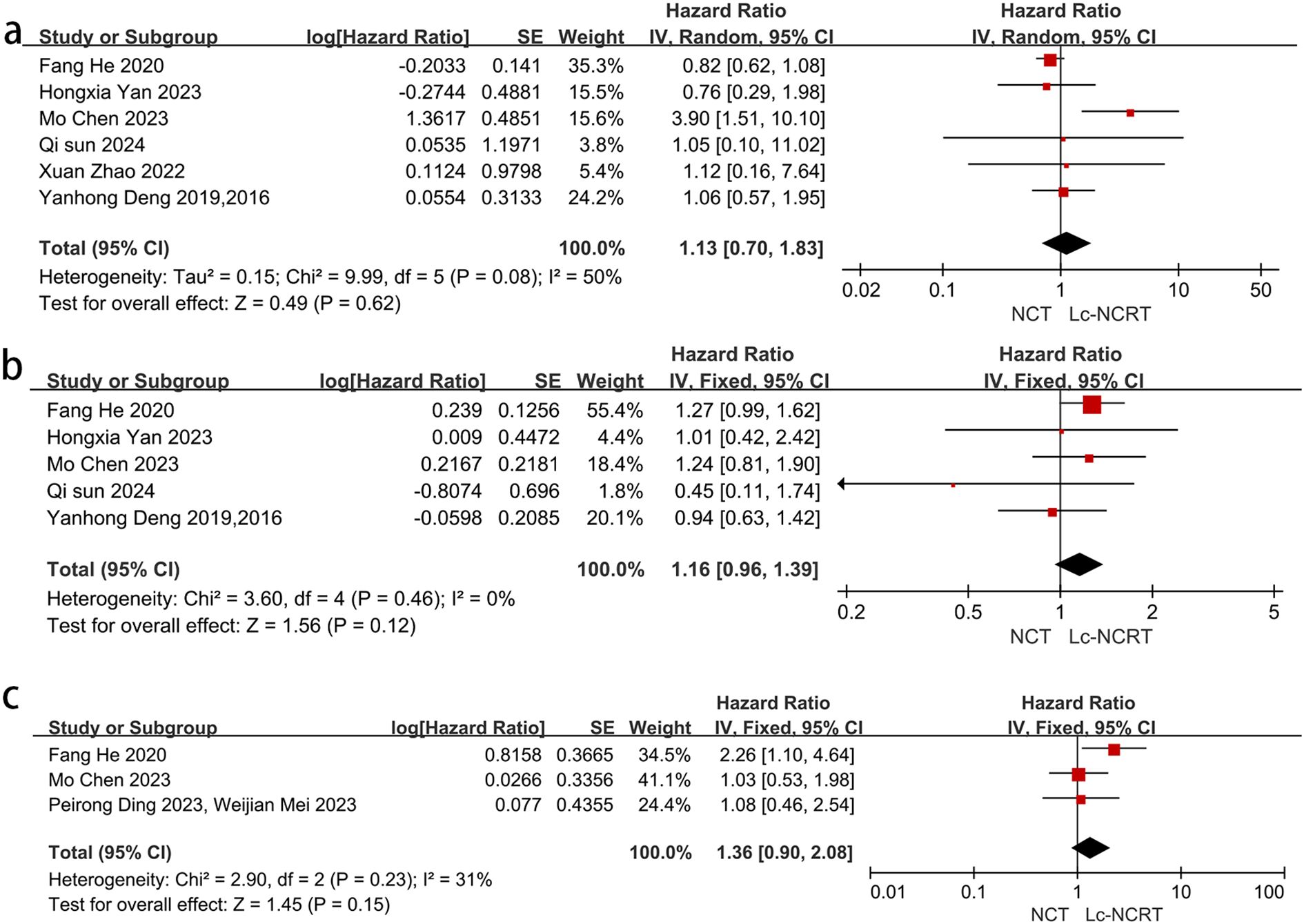
Figure 5. Forest plots of (a) 3-year OS and (b) 3-year DFS. (c) 3-year LRFS between groups of NCT and Lc-NCRT.
Six studies reported a 3-year OS. The meta-analysis showed moderate heterogeneity among the studies (I2 = 50%, chi-square test, P = 0.08). No significant difference in 3-year OS was observed between the two groups using a random-effects model [HR = 1.13, 95% CI (0.70–1.83), P = 0.62]. In the sensitivity analysis, Mo Chen (11) was considered as a heterogeneous source because all of the patients included in Mo Chen (11) were EMVI-positive. Heterogeneousness was reduced after the exclusion of Mo Chen’s study (I2 = 0%, chi-square test, P = 0.95), and there was still no significant difference between the two groups using the fixed-effects model [HR = 0.85, 95% CI (0.67–1.08), P = 0.19].
Five studies reported 3-year DFS, and the meta-analysis showed no significant difference between the two groups in 3-year DFS [HR = 1.16, 95% CI (0.96–1.39), P = 0.12]. There was no heterogeneity among the studies (I2 = 0%, chi-square test, P = 0.46).
Only three studies reported 3-year LRFS. There was mild heterogeneity among these studies (I2 = 31%, chi-square P = 0.23), and the meta-analysis showed no significant difference between the two groups [HR = 1.36, 95% CI (0.90–2.08), P = 0.15].
3.3.3 Adverse events of neoadjuvant therapy
There was no heterogeneity among all of the five studies that reported adverse events of neoadjuvant therapy (I2 = 0%, chi-square test, P = 0.47), and a fixed-effect model was used (Figure 6a). The results showed that there were more adverse events in the NCT group, with a statistically significant difference [RR = 1.11, 95% CI (1.03–1.19), P = 0.06]. However, the subgroup analysis suggested that there may be no significant difference between the two groups in the RCT subgroup [RR = 1.08, 95% CI (1.00–1.17), P = 0.006].
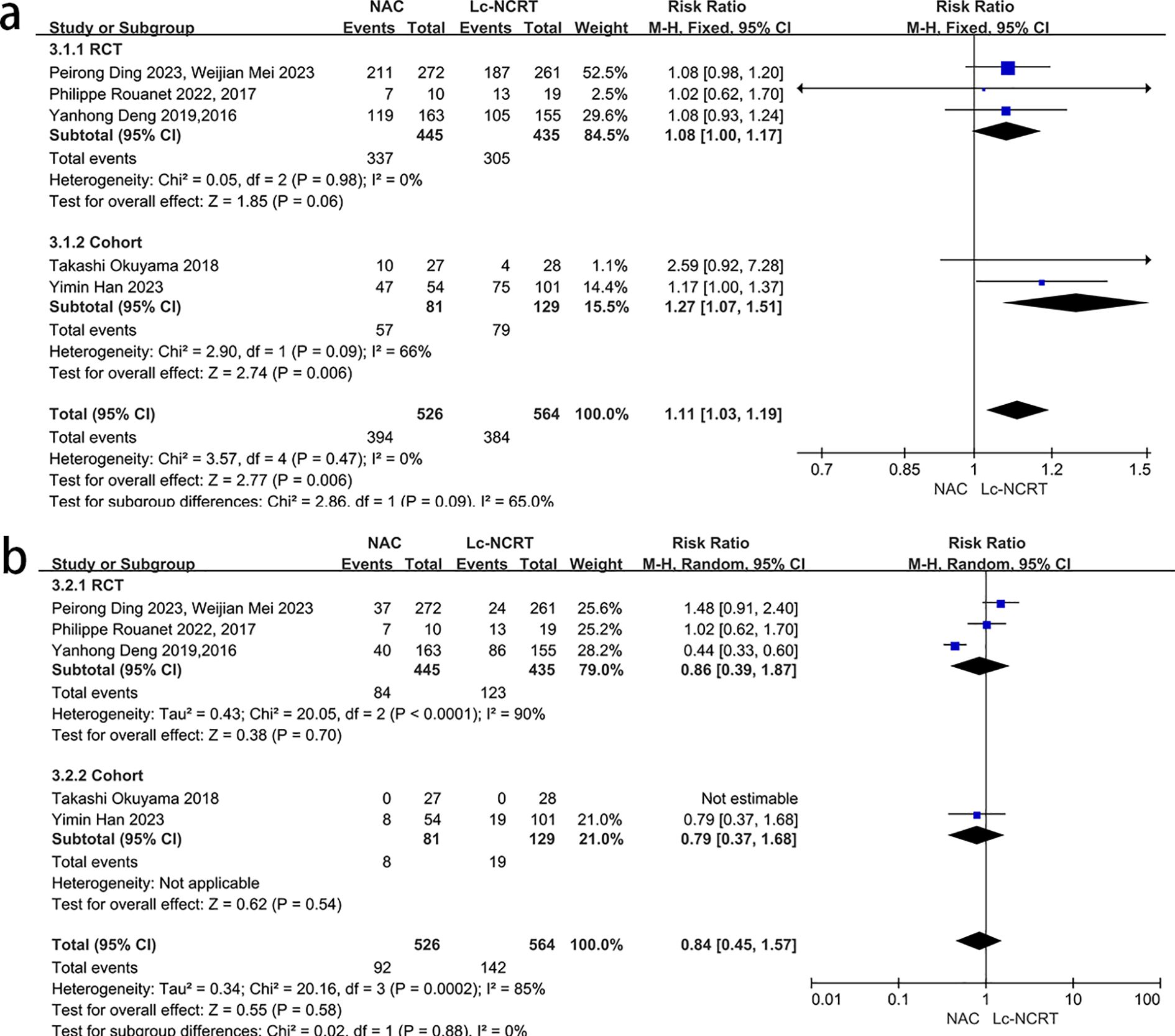
Figure 6. Forest plots of (a) adverse events and (b) severe adverse events of neoadjuvant therapy between groups of NCT and Lc-NCRT.
Five studies reported severe adverse events of neoadjuvant therapy, and there was high heterogeneity among the studies (I2 = 85%, chi-square test, P = 0.0002) (Figure 6b). The subgroup analysis failed to identify the source of heterogeneity. The sensitivity analysis suggested that Yanhong Deng (2, 7) may be the source of heterogeneity, but there was no definite clinical indication to exclude it. Therefore, the random-effects model was used, and the results showed that there was no statistically significant difference between the two groups [RR = 0.84, 95% CI (0.45–1.57), P = 0.58]. The subgroup analysis results did not change.
3.3.4 Surgical complications and anal retention
Six articles reported R0 resection rates (Figure 7a). The meta-analysis showed that there was no significant heterogeneity among the studies (I2 = 0%, chi-square test, P = 0.94), and no significant difference was found between the two groups [RR = 1.0, 95% CI (0.98–1.03), P = 0.74].
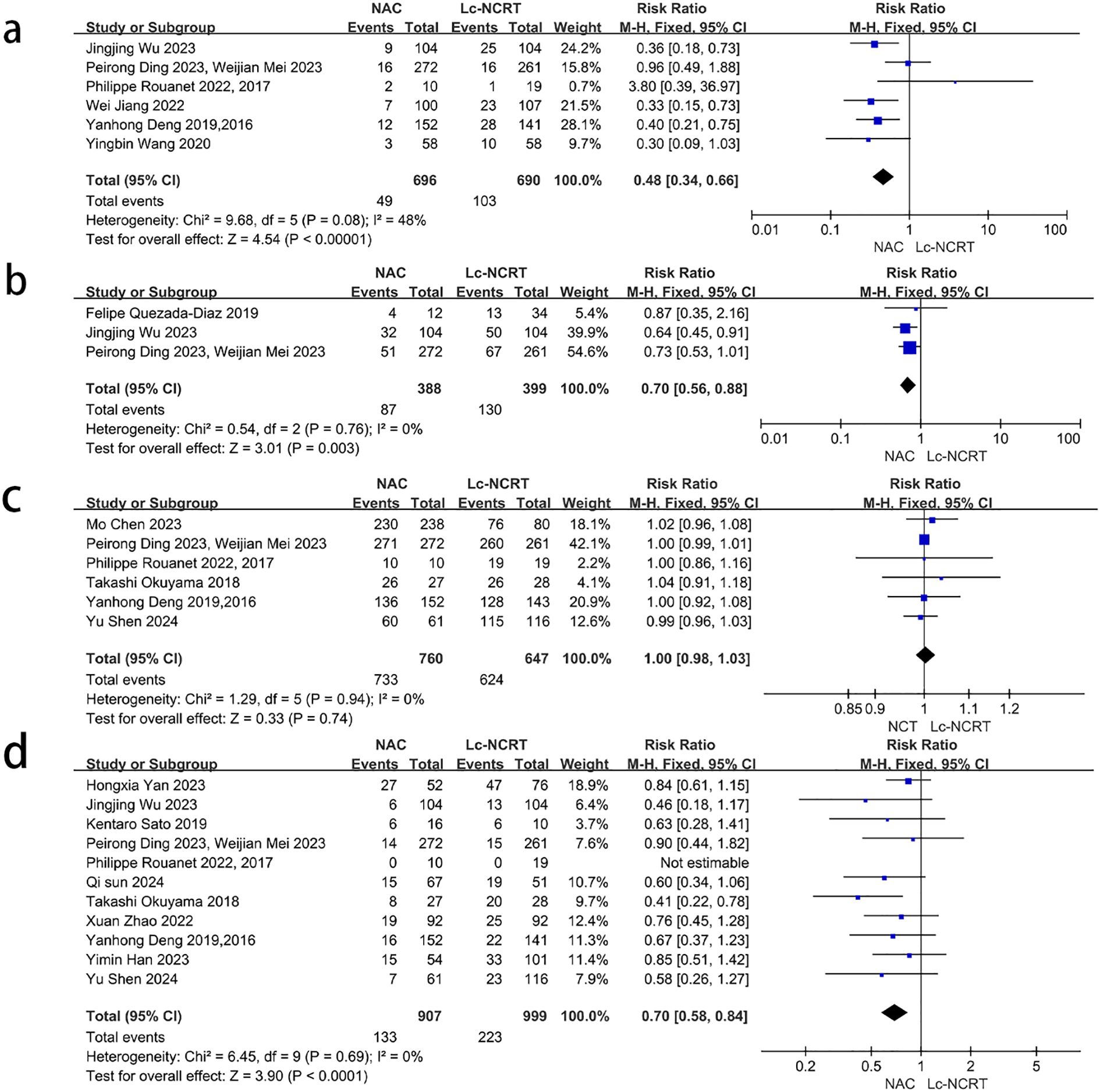
Figure 7. Forest plots of (a) R0 rate, (b) postoperative complications, (c) postoperative anastomotic leakage, and (d) permanent stoma rate between groups of NCT and Lc-NCRT.
Three articles reported the total number of postoperative complications (Figure 7b), and there was no significant heterogeneity among studies (I2 = 0%, chi-square test, P = 0.76). The postoperative complications in the NCT group were less than that in the Lc-NCRT group [RR = 0.70, 95% CI (0.56–0.88), P = 0.003]. Only Jingjing Wu reported that there were more grade III–IV complications in the Lc-NCRT group than in the NCT group. The incidence of postoperative anastomotic leakage was significantly lower in the NCT group (RR = 0.48, 95% CI (0.34–0.66), P < 0.00001), and there was slight heterogeneity (I2 = 48%, chi-square test, P = 0.08) (Figure 7c). The sensitivity analyses suggested that Wei-Jian Mei (4) may be a source of heterogeneity, but there is no clinical evidence for its exclusion, and even its exclusion does not change the result.
A meta-analysis of 11 studies showed that the permanent stoma rate in the NCT group was significantly lower than that in the Lc-NCRT group [RR = 0.7, 95% CI (0.58–0.84), P < 0.0001], and there was no heterogeneity among these studies (I2 = 0%, chi-square test, P = 0.69) (Figure 7d).
3.4 Bias analysis
We performed funnel plot analysis for the meta-analyses of more than 10 studies, and it can be seen that the scatter points fall within the funnel plot. However, the scatter points are not very symmetrical, indicating that there may be some publication bias (Figure 8).
4 Discussion
TME surgical techniques significantly reduce the risk of postoperative local recurrences (36). The primary purpose of preoperative chemoradiotherapy is tumor downstaging to obtain better R0 resection and TME quality in LRAC. However, in the past decade, laparoscopic and robotic technologies have developed rapidly, enabling surgeons to perform TME better (37–39). Various systemic treatment methods such as immunotherapy have also made great progress (40, 41). Therefore, overemphasis on high-intensity neoadjuvant radiotherapy to achieve pCR may increase adverse events, delay the timing of systemic therapy, and lead to distant metastasis (42). These therapeutic advances and conceptual changes pose a challenge to the Lc-NCRT model. Whether NCT alone can replace Lc-NCRT to create the same surgical conditions, achieve similar oncological outcomes, and avoid the side effects of radiotherapy has become a research hotspot.
Our meta-analysis comprehensively evaluated the differences between NCT and Lc-NCRT groups from four aspects—tumor response, long-term oncological outcomes, safety, and surgical-related indicators—and is the one with the highest quality of literature, the most reports of medium- and long-term oncology outcomes, and the most detailed subgroup analysis. Two similar meta-analyses were published in 2021 (43, 44), focusing on differences in short-term outcomes, and the conclusion was partly consistent with our study. However, the included literature was highly variable. Some of their studies included patients with stage I and IV disease (45–48), and some used surgery alone or other chemotherapy regimens as controls (47–49). When conducting a meta-analysis of anastomotic leak versus sphincter preservation, they repeatedly included data from the FOWARC study (5, 8, 13), which may lead to a bias. Our results showed that, although the pCR rate of NCT was lower than that of Lc-NCRT and the total adverse event rate of NCT was higher, there was no statistical difference in R0 resection rate and mid- and long-term oncological outcomes and neoadjuvant treatment toxicity above grade III. The incidence of surgical complications such as anastomotic leakage and the rate of permanent stoma were lower in the NCT than that in the Lc-NCRT group. There is still some controversy in short-course and non-FU-based chemoradiotherapy (50–52). Therefore, we included only fluorouracil-based neoadjuvant chemoradiotherapy as a control group.
In terms of tumor response, although there was no significant difference between the two groups in terms of DRR and ORR, postoperative pathological results such as pCR, TRG0-1, and pathological downstaging showed that NCT was inferior to Lc-NCRT, which differ from previous studies (43, 44). This difference may be due to the difference in sample sizes or because the imaging diagnosis could not fully reflect the pathological results and even for the heterogeneity among the cases in each study. As we can see, the meta-analysis results of tumor downstaging changed after excluding Mo Chen’s study (25). It may be because all of the patients included in the study were EMVI-positive, which was different from that of others. Although the patients in the NCT group had less pathological downgrading than those in the Lc-NCRT group, the pathological response does not reflect long-course survival outcomes. In this study, we found no difference between the two groups in 3-year OS, DFS, and LRFS. Unfortunately, only two articles involved distant metastasis-free survival (25, 30) and 5-year survival outcome (23, 27). Thus, it is difficult to perform a meta-analysis. Therefore, the effect of the NCT scheme on long-term survival of LRAC should be considered cautiously. Some conference abstracts indicated that the use of Lc-NCRT was independently associated with better OS than NCT alone (18). However, such database studies with non-original data did not meet the inclusion criteria of our study.
Regarding safety, we found more adverse events of neoadjuvant therapy in the NCT group than in the Lc-NCRT group, which may be related to the generally longer duration of chemotherapy in the NCT group but was balanced between the two groups in the RCT subgroups. These adverse events of neoadjuvant therapy were mainly of grades I–II, for there was no significant difference between the two groups in events greater than grade III. Overall, although more neoadjuvant adverse events occurred in the NCT group, they were manageable, and the safety was completely acceptable. Although the tumor response in the NCT group was inferior to that of Lc-NCRT, it did not reduce the R0 resection rate in our meta-analysis. On the contrary, the rates of permanent stoma, anastomotic leak, and surgical complications were significantly lower. Tissue damage caused by radiotherapy significantly increases the difficulty of the operation and thus affects the quality of the anastomosis (53). In addition, given the high incidence of anastomotic leakage and severe impairment of defecation control after radiotherapy, surgeons may prefer stoma, at least protective stoma, especially for patients with low rectal cancer (54).
There are obvious limitations in our studies. First, the included studies were mainly from Asia, with only two studies from Europe and America. Second, there were only three RCT studies, which was less than half of the total number of studies. Third, although patients of stage II–III were strictly included, the enrollment criteria of these studies are still slightly different. Fourth, the regimens and cycles of NCT are not completely unified. The chemotherapy regimens in the three RCTS were different. Fifth, the literature included in the analyses of long-term oncologic outcomes remains modest. Sixth, the scatter points in the funnel plot of this study were not very symmetrical, which may suggest the existence of publication bias. There may be multiple sources of bias. First, some articles with negative results may not have been published. Second, the database that we searched may not have covered all relevant literature, and no literature other than English and Chinese was retrieved. Third, the regimen and sequence of chemotherapy were inconsistent, and some studies of consolidation chemotherapy after radiotherapy may have resulted in favorable results for the radiotherapy group (9, 16, 26). Although we attempted to eliminate some studies, the results did not change, and several additional studies are ongoing and we look forward to their inclusion in the meta-analysis when available.
Based on the above-mentioned limitations, the results of this study should be interpreted with caution. In particular, the efficacy of NCT cannot be emphasized for all LARC (19). According to the ESMO Guideline Opinion (36), different treatment regimens should be adopted according to the risk stratification of stage II/III rectal cancer. We believe that the heterogeneity of the studies in this meta-analysis was partly due to the lack of risk stratification of the samples. Therefore, a separate study of neoadjuvant chemotherapy according to EMVI, CRM, anal distance, and T stage should be considered in the future. Although several studies have analyzed these risk strata (13, 25, 27, 28, 30, 55), a meta-analysis was not possible due to lack of data. The results of the PROSPECT series (56–58) published recently are very important. However, of the 585 patients in the NCT group, 53 (9.1%) were converted to Lc-NCRT because of dissatisfaction with NCT. This selective NCT study did not provide results for patients with NCT alone. Therefore, it was not included in this meta-analysis. It is undeniable that the design of selective NCT has, to some extent, prevented some patients who do not respond well to NCT from becoming victims of clinical trials. Therefore, the study design of selective radiotherapy is more ethical and worthy of reference for future clinical research. Recently, neoadjuvant chemoradiotherapy combined with immunotherapy has become popular. Radiotherapy may induce the aggregation of tumor-infiltrating lymphocytes and the expression of PDL-1 in tumor tissues, change the tumor microenvironment, and increase the sensitivity of tumors to immunotherapy in rectal cancer patients with proficient mismatch repair (pMMR) (59, 60). In the TORCH study, immunotherapy combined with total neoadjuvant therapy (iTNT) increased the CR rate to more than 50%, which was significantly higher than that of neoadjuvant therapy alone. Among them, the CR rate of the population that received radiotherapy first was higher than that of the population that received immunotherapy first, which may be due to the increased sensitivity of radiotherapy to immunotherapy (61). More abscopal effects have been observed in clinical studies of radiotherapy combined with immunotherapy, which is considered as strong evidence that radiotherapy stimulates anti-tumor immune responses (62, 63). Therefore, even if some low-risk patients benefit from NCT, some patients with lower tumor location still need additional radiotherapy or even total neoadjuvant therapy combined with immunotherapy to achieve anal preservation with a watch-and-wait approach (64).
In conclusion, compared with Lc-NCRT, NCT has comparable long-term oncological outcomes and controllable safety and is superior to Lc-NCRT in terms of surgical complications and sphincter preservation. Due to limited data, the results still need to be treated with caution and the strategy of selective radiotherapy should be adopted in clinical practice, especially for patients with an unsatisfactory tumor response to NCT. How to accurately select risk subgroups suitable for NCT is one of the future research directions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing, Data curation. XW: Conceptualization, Investigation, Supervision, Writing – review & editing, Project administration. JD: Formal Analysis, Software, Supervision, Project administration, Writing – review & editing. MZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. QL: Data curation, Formal Analysis, Funding acquisition, Methodology, Validation, Writing – review & editing. KL: Investigation, Methodology, Software, Supervision, Writing – review & editing, Data curation, Formal Analysis, Validation. JL: Data curation, Formal Analysis, Writing – review & editing. XJ: Data curation, Formal Analysis, Writing – review & editing. YW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Science and Technology Project of Sichuan Provincial Health Commission (Project Number: 24WSXT062).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
2. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, and Gunnarsson U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. (2005) 23:5644–50. doi: 10.1200/JCO.2005.08.144
3. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. (2004) 351:1731–40. doi: 10.1056/NEJMoa040694
4. Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. (2007) 246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce
5. Qin Q, Huang B, Cao W, Zhou J, Ma T, Zhou Z, et al. Bowel dysfunction after low anterior resection with neoadjuvant chemoradiotherapy or chemotherapy alone for rectal cancer: A cross-sectional study from China. Dis Colon Rectum. (2017) 60:697–705. doi: 10.1097/DCR.0000000000000801
6. Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan L, et al. Impact of Long-Course neoadjuvant radiation on postoperative low anterior resection syndrome and quality of life in rectal cancer: Post hoc analysis of a randomized controlled trial. Ann Surg Oncol. (2019) 26:746–55. doi: 10.1245/s10434-018-07096-8
7. van der Sande ME, Hupkens B, Berbée M, van Kuijk S, Maas M, Melenhorst J, et al. Impact of radiotherapy on anorectal function in patients with rectal cancer following a watch and wait programme. Radiother Oncol. (2019) 132:79–84. doi: 10.1016/j.radonc.2018.11.017
8. Huang M, Lin J, Yu X, Chen S, Kang L, Deng Y, et al. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: A randomized trial report. Int J Colorectal Dis. (2016) 31:1349–57. doi: 10.1007/s00384-016-2605-7
9. Bedrikovetski S, Traeger L, Seow W, Dudi-Venkata NN, Selva-Nayagam S, Penniment M, et al. Oncological outcomes and response rate after total neoadjuvant therapy for locally advanced rectal cancer: A network Meta-Analysis comparing induction vs. Consolidation chemotherapy vs. Standard chemoradiation. Clin Colorectal Cancer. (2024) 23:326–36. doi: 10.1016/j.clcc.2024.06.001
10. Shen Y, Wu Q, Meng W, Wei M, Deng X, and Wang Z. Neoadjuvant chemotherapy (CAPOX) alone for low- and intermediate-risk stage II/III rectal cancer: Long-term follow-up of a prospective single-arm study. Eur J Surg Oncol. (2023) 49:107115. doi: 10.1016/j.ejso.2023.107115
11. Ke TW, Chang SC, Liao YM, Lin CH, Chen WTL, Liang JA, et al. Neoadjuvant chemotherapy With/Without radiotherapy for locally advanced rectal cancer: A nationwide retrospective cohort study. Anticancer Res. (2023) 43:5713–22. doi: 10.21873/anticanres.16777
12. Quezada-Diaz F, Jimenez-Rodriguez RM, Pappou EP, Joshua Smith J, Patil S, Wei I, et al. Effect of neoadjuvant systemic chemotherapy with or without chemoradiation on bowel function in rectal cancer patients treated with total mesorectal excision. J gastrointestinal Surg. (2018) 23:800–7. doi: 10.1007/s11605-018-4003-7
13. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: Initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. (2016) 34:3300–7. doi: 10.1200/JCO.2016.66.6198
14. Okuyama T, Sameshima S, Takeshita E, Yoshioka R, Yamagata Y, Ono Y, et al. Therapeutic effects of oxaliplatin-based neoadjuvant chemotherapy and chemoradiotherapy in patients with locally advanced rectal cancer: A single-center, retrospective cohort study. World J Surg Oncol. (2018) 16:105. doi: 10.1186/s12957-018-1403-9
15. Ding P, Wang X, Li Y, Sun Y, Yang C, Wu Z, et al. LBA26 Neoadjuvant chemotherapy with CAPOX versus chemoradiation for locally advanced rectal cancer with uninvolved mesorectal fascia (CONVERT): Final results of a phase III trial. Ann Oncol. (2023) 34:S1267–8. doi: 10.1016/j.annonc.2023.10.018
16. Yan H, Zhang Y, Hao Z, Lu Y, and Liu H. MFOLFOX4 with or without radiation in neoadjuvant treatment of locally advanced middle and low rectal cancer. J Cancer Res Ther. (2022) 18:2027–32. doi: 10.4103/jcrt.jcrt_1207_22
17. Rouanet P, Rullier E, Lelong B, Maingon P, Tuech JJ, Pezet D, et al. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: Preliminary results of the french phase II multicenter GRECCAR4 trial. Dis Colon Rectum. (2017) 60:653–63. doi: 10.1097/DCR.0000000000000849
18. IIICassidy RJSS, Liu YSS, Zhong JSS, Gillespie TWSS, and Landry JCSS. Survival impact of neoadjuvant chemotherapy alone versus neoadjuvant chemoradiation therapy for patients with T2N1, T3N0, and T3N1 rectal adenocarcinomas. Int J Radiat Oncol Biol Phys. (2016) 96:E148. doi: 10.1016/j.ijrobp.2016.06.963
19. Ogura A, Uehara K, Aiba T, Sando M, Tanaka A, Ohara N, et al. Indications for neoadjuvant treatment based on risk factors for poor prognosis before and after neoadjuvant chemotherapy alone in patients with locally advanced rectal cancer. Eur J Surg Oncol. (2021) 47:1005–11. doi: 10.1016/j.ejso.2020.10.038
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, and Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Mei WJ, Wang XZ, Li YF, Sun YM, Yang CK, Lin JZ, et al. Neoadjuvant chemotherapy with CAPOX versus chemoradiation for locally advanced rectal cancer with uninvolved mesorectal fascia (CONVERT): Initial results of a phase III trial. Ann Surg. (2023) 277:557–64. doi: 10.1097/SLA.0000000000005780
23. Rouanet P, Rullier E, Lelong B, Maingon P, Tuech JJ, Pezet D, et al. Tailored strategy for locally advanced rectal carcinoma (GRECCAR 4): Long-term results from a multicenter, randomized, Open-Label, phase II trial. Dis Colon Rectum. (2022) 65:986–95. doi: 10.1097/DCR.0000000000002153
24. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: Final results of the Chinese FOWARC trial. J Clin Oncol. (2019) 37:3223–33. doi: 10.1200/JCO.18.02309
25. Chen M, Ma Y, Song YW, Huang J, Gao YH, Zheng J, et al. Survival outcomes of different neoadjuvant treatment regimens in patients with locally advanced rectal cancer and MRI-detected extramural venous invasion. Cancer Med-Us. (2023) 12:20523–37. doi: 10.1002/cam4.6625
26. Han YM, Qi WX, Wang SB, Cao WG, Chen JY, and Cai G. Identification of patients with locally advanced rectal cancer eligible for neoadjuvant chemotherapy alone: Results of a retrospective study. Cancer Med. (2023) 12:13309–18. doi: 10.1002/cam4.6029
27. Wu J, Huang M, Wu Y, Hong Y, Cai L, He R, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy alone for patients with locally advanced rectal cancer: A propensity-score-matched analysis combined with SEER validation. J Cancer Res Clin. (2023) 149:8897–912. doi: 10.1007/s00432-023-04779-y
28. Jiang W, Wang H, Zheng J, Zhao Y, Xu S, Zhuo S, et al. Post-operative anastomotic leakage and collagen changes in patients with rectal cancer undergoing neoadjuvant chemotherapy vs chemoradiotherapy. Gastroenterol Rep. (2022) 10:1–10. doi: 10.1093/gastro/goac058
29. Zhao X, Han P, Zhang L, Ma J, Dong F, Zang L, et al. Prolonged neoadjuvant chemotherapy without radiation versus total neoadjuvant therapy for locally advanced rectal cancer: A propensity score matched study. Front Oncol. (2022) 12:953790. doi: 10.3389/fonc.2022.953790
30. He F, Yu L, Ding Y, Li ZH, Wang J, Zheng J, et al. Effects of neoadjuvant chemotherapy with or without intensity-modulated radiotherapy for patients with rectal cancer. Cancer Sci. (2020) 111:4205–17. doi: 10.1111/cas.14636
31. Wang Yingbin’, Xu X, Shao Q, Li B, Wang Y, and Pengming W. Preliminary study for neoadjuvant chemotherapy on locally advanced rectal cancer. Chin J Gen Surg. (2020) 35:768–72. doi: 10.3760/cma.j.cnl13855-20200227-00132
32. Chen G, Zhang Y, GUO H, and Yang O. Effect of neoadjuvant treatment on TRG, surgical complications and LARS in patients with rectal cancer. Cancer Res Prev Treat. (2021) 48:1108–12. doi: 10.3971/j.issn.1000-8578.2021.21.0421
33. Sun Q, Liu C, Ye J, Huang W, Xu Y, Yao C, et al. Comparison of the efficacy of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy in locally advanced mid-low rectal cancer. J Xi’an Jiaotong Univ (Medical Sciences). (2024) 45:278–83. doi: 10.7652/jdyxb202402016
34. Shen Y, Wen Y, Bi L, Yang X, Gong X, Deng X, et al. Do treated rectal tumors appear differently on MRI after chemotherapy versus chemoradiotherapy? Abdom Radiol. (2024) 49:774–82. doi: 10.1007/s00261-023-04115-5
35. Sato K, Miura T, Morohashi S, Sakamoto Y, Morohashi H, Yoshida T, et al. Comparable regional therapeutic effects between neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy for locally advanced lower rectal cancer in terms of histopathological analysis. Mol Clin Oncol. (2019) 10:619–24. doi: 10.3892/mco.2019.1835
36. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:v22–40. doi: 10.1093/annonc/mdx224
37. Bianchi PP, Petz W, Luca F, Biffi R, Spinoglio G, and Montorsi M. Laparoscopic and robotic total mesorectal excision in the treatment of rectal cancer. Brief review and personal remarks. Front Oncol. (2014) 4:98. doi: 10.3389/fonc.2014.00098
38. Seow W, Dudi-Venkata NN, Bedrikovetski S, Kroon HM, and Sammour T. Outcomes of open vs laparoscopic vs robotic vs transanal total mesorectal excision (TME) for rectal cancer: A network meta-analysis. Tech Coloproctol. (2023) 27:345–60. doi: 10.1007/s10151-022-02739-1
39. Chen J, Zhang Z, Chang W, Yi T, Feng Q, Zhu D, et al. Short-Term and Long-Term outcomes in mid and low rectal cancer with robotic surgery. Front Oncol. (2021) 11:603073. doi: 10.3389/fonc.2021.603073
40. Nguyen NP, Mohammadianpanah M, SunMyint A, Page BR, Vinh-Hung V, Gorobets O, et al. Immunotherapy and radiotherapy for older patients with locally advanced rectal cancer unfit for surgery or decline surgery: A practical proposal by the International Geriatric Radiotherapy Group. Front Oncol. (2024) 14:1325610. doi: 10.3389/fonc.2024.1325610
41. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, et al. Neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer: A new era for anal preservation. Front Immunol. (2022) 13:1067036. doi: 10.3389/fimmu.2022.1067036
42. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen C, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:29–42. doi: 10.1016/S1470-2045(20)30555-6
43. Lin H, Wang L, Zhong X, Zhang X, Shao L, and Wu J. Meta-analysis of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for locally advanced rectal cancer. World J Surg Oncol. (2021) 19:141. doi: 10.1186/s12957-021-02251-0
44. Wu P, Xu HM, and Zhu Z. Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer: A meta-analysis. World J Gastrointest Oncol. (2021) 13:1196–209. doi: 10.4251/wjgo.v13.i9.1196
45. Suárez J, Amat I, Vera R, Balén E, Gómez M, and Lera JM. Pathologic response of primary rectal cancer to oxaliplatin-based chemotherapy. Clin Colon Rectal Surg. (2011) 24:119–24. doi: 10.1055/s-0031-1278409
46. Kim SH, Kim JH, and Jung SH. Comparison of oncologic outcomes of metastatic rectal cancer patients with or without neoadjuvant chemoradiotherapy. Int J Colorectal Dis. (2015) 30:1193–9. doi: 10.1007/s00384-015-2272-0
47. Sakuyama N, Kojima M, Kawano S, Akimoto T, Saito N, Ito M, et al. Histological differences between preoperative chemoradiotherapy and chemotherapy for rectal cancer: A clinicopathological study. Pathol Int. (2016) 66:273–80. doi: 10.1111/pin.12409
48. Ikematsu H, Fukuoka S, Ito M, Kojima M, Imajoh M, Osera S, et al. Mo1920 the investigation regarding endoscopic and pathological response for Neo-Adjuvant chemotherapy or chemoradiotherapy in patients with low rectal cancer. Gastroenterology. (2014) 146:S691–2. doi: 10.1016/S0016-5085(14)62510-5
49. Zhang JZJS, Deng YDYS, Wu ZWZS, Hu HHHS, Cai YCYS, and Ling JLJS. MFOLFOXIRI versus mFOLFOX6 as neoadjuvant chemotherapy in locally advanced rectal cancer: A propensity score analysis from two prospective trials. Ann Oncol. (2018) 29:x28. doi: 10.1016/j.clcc.2021.11.009
50. Ma B, Gao P, Song Y, Huang X, Wang H, Xu Q, et al. Short-Course radiotherapy in neoadjuvant treatment for rectal cancer: A systematic review and meta-analysis. Clin Colorectal Cancer. (2018) 17:320–30. doi: 10.1016/j.clcc.2018.07.014
51. Azria D, Doyen J, Jarlier M, Martel-Lafay I, Hennequin C, Etienne P, et al. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405-PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate-risk rectal cancer. Ann Oncol. (2017) 28:2436–42. doi: 10.1093/annonc/mdx351
52. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2015) 16:979–89. doi: 10.1016/S1470-2045(15)00159-X
53. Hassan I, Larson DW, Wolff BG, Cima RR, Chua HK, Hahnloser D, et al. Impact of pelvic radiotherapy on morbidity and durability of sphincter preservation after coloanal anastomosis for rectal cancers. Dis Colon Rectum. (2008) 51:32–7. doi: 10.1007/s10350-007-9099-x
54. Garg PK, Goel A, Sharma S, Chishi N, and Gaur MK. Protective diversion stoma in low anterior resection for rectal cancer: A Meta-Analysis of randomized controlled trials. Visc Med. (2019) 35:156–60. doi: 10.1159/000497168
55. Cai J, Lin K, Luo T, Weng J, Liu H, Yuan Z, et al. Neoadjuvant chemotherapy is noninferior to chemoradiotherapy for early-onset locally advanced rectal cancer in the FOWARC trial. Br J Cancer (2024) 130:1434–40. doi: 10.1038/s41416-024-02652-4
56. Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, et al. PROSPECT: A randomized phase III trial of neoadjuvant chemoradiation versus neoadjuvant FOLFOX chemotherapy with selective use of chemoradiation, followed by total mesorectal excision (TME) for treatment of locally advanced rectal cancer (LARC) (Alliance N1048). J Clin Oncol. (2023) 41. doi: 10.1200/JCO.2023.41.17_suppl.LBA2
57. Schrag D, Weiser MR, Goodman KA, Gonën M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: A pilot trial. J Clin Oncol. (2014) 32:513–8. doi: 10.1200/JCO.2013.51.7904
58. Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, et al. Preoperative treatment of locally advanced rectal cancer. N Engl J Med. (2023) 389:322–34. doi: 10.1056/NEJMoa2303269
59. Dovedi SJ and Illidge TM. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. (2015) 4:e1016709. doi: 10.1080/2162402X.2015.1016709
60. Brix N, Tiefenthaller A, Anders H, Belka C, and Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. (2017) 280:249–79. doi: 10.1111/imr.12573
61. Xia F, Wang Y, Wang H, Shen L, Xiang Z, Zhao Y, et al. Randomized phase II trial of Immunotherapy-Based total neoadjuvant therapy for proficient mismatch repair or microsatellite stable locally advanced rectal cancer (TORCH). J Clin Oncol. (2024) 42:3308–18. doi: 10.1200/JCO.23.02261
62. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
63. Twyman-Saint VC, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
64. Thompson HM, Omer DM, Lin S, Kim JK, Yuval JB, Verheij FS, et al. Organ preservation and survival by clinical response grade in patients with rectal cancer treated with total neoadjuvant therapy: A secondary analysis of the OPRA randomized clinical trial. JAMA Netw Open. (2024) 7:e2350903. doi: 10.1001/jamanetworkopen.2023.50903
Keywords: locally advanced rectal cancer, neoadjuvant, chemotherapy, chemoradiotherapy, systematic review, meta-analysis
Citation: Zhou W, Wang X, Dan J, Zhu M, Liao Q, Liu K, Li J, Jiang X and Wang Y (2025) Neoadjuvant chemotherapy versus long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis of 5,168 cases. Front. Oncol. 15:1542885. doi: 10.3389/fonc.2025.1542885
Received: 10 December 2024; Accepted: 06 June 2025;
Published: 26 June 2025.
Edited by:
Nobu Oshima, Kyoto University Graduate School of Medicine, JapanCopyright © 2025 Zhou, Wang, Dan, Zhu, Liao, Liu, Li, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjie Zhou, endqXzg5MDlAMTYzLmNvbQ==; Yonghong Wang, MjcxNTc4MjY0QHFxLmNvbQ==
 Wenjie Zhou
Wenjie Zhou Xueting Wang
Xueting Wang Jie Dan1
Jie Dan1 Ke Liu
Ke Liu Jiangpeng Li
Jiangpeng Li Yonghong Wang
Yonghong Wang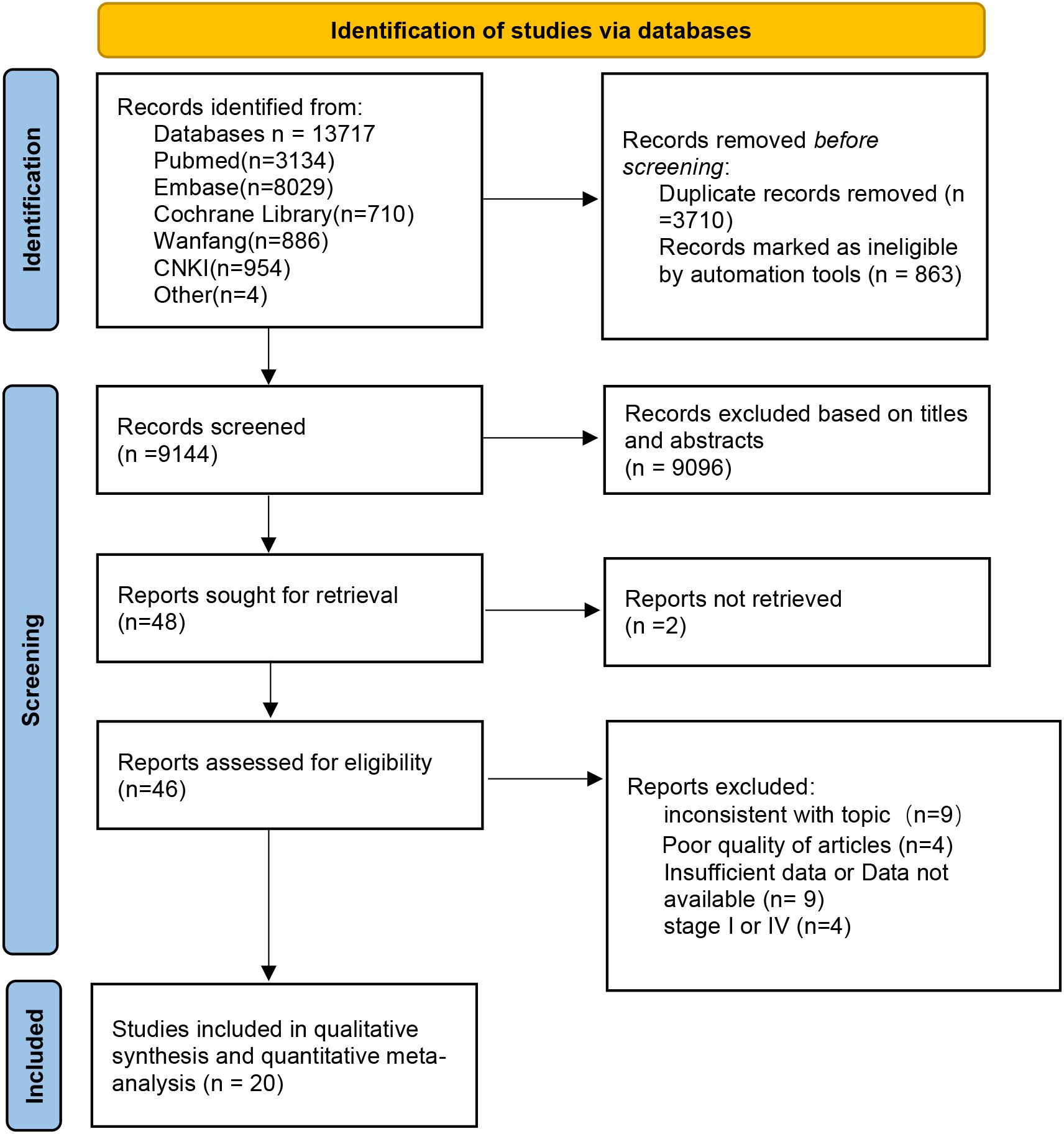
![Two funnel plots labeled “a” and “b” show the standard error of the logarithm of the relative risk (SE(log[RR])) on the y-axis against the relative risk (RR) on the x-axis. Plot “a” features points distributed with some asymmetry, while plot “b” shows more symmetry, both within dashed lines.](https://www.frontiersin.org/files/Articles/1542885/fonc-15-1542885-HTML-r1/image_m/fonc-15-1542885-g008.jpg)