- 1The Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang, China
- 2Guangyuan Zhaohua District People’s Hospital, Guangyuan, China
Thalidomide, once discovered and used to treat pregnancy sickness, fell out of favor with teratogenicity scandals, and then re-entered the public eye with its amazing efficacy against leprosy and multiple myeloma, and its anti-tumor and anti-inflammatory effects were gradually discovered. In recent years, thalidomide has also begun to gain prominence for its role in digestive disorders, particularly for its anti-angiogenic effects, which are effective in the treatment of dysplastic gastrointestinal hemorrhage. The therapeutic effects of thalidomide in other digestive disorders, including radiation proctitis, inflammatory bowel disease, and malignant tumors of the digestive tract, such as liver, colorectal, gastric, esophageal, and pancreatic cancers, have been proposed in several clinical trials and case reports. Due to its potential adverse effects and controversial clinical utility, it has not been put into clinical use for digestive diseases in large numbers, but its unique therapeutic effects still warrant further study, so this paper analyses and summarizes the use, mechanism of action, and potential therapeutic uses of thalidomide in digestive diseases.
1 Introduction
Thalidomide was first synthesized in 1950 but was quickly withdrawn in 1962 due to reports of severe congenital abnormalities (1). Since 1965, thalidomide has been found to possess anti-inflammatory and anti-angiogenic properties and has been used under stringent conditions for the treatment of leprosy (2). In the 1980s and early 1990s, researchers discovered that thalidomide could treat certain autoimmune diseases (3, 4). In 1990, thalidomide was considered a first-in-class immunomodulatory drug (IMiD) due to its discovery in combination with other antineoplastic drugs for the treatment of multiple myeloma (1, 5), and was also considered a promising clinical ally for cancer immunotherapy (5). Thalidomide was approved by the US FDA for the treatment of Erythema nodosum leprosum (ENL) and myeloma in 1998 and 2006, respectively (6). A new generation of analogues of thalidomide was developed in the 2000s, including lenalidomide and pomalidomide, collectively referred to as immunomodulatory imide drugs (IMiD) because of their potent immunomodulatory effects (1, 7). Thalidomide has also appeared on the clinical scene more frequently due to the development of its analogues. In recent years, thalidomide has also been gradually found to be effective in many digestive disorders, including angiodysplastic gastrointestinal hemorrhage, radiation proctitis, inflammatory bowel disease, and gastrointestinal malignancies, such as hepatocellular carcinoma, colorectal carcinoma, gastric carcinoma, esophageal carcinoma, and pancreatic carcinoma. However, the current drug insert for thalidomide is only approved for tumor-type leprosy. Therefore the use of thalidomide in most diseases is not entered in the drug insert, such as it is an over-the-counter antiangiogenic therapy for patients with hereditary hemorrhagic telangiectasia, HHT (8), but thalidomide has been popularized in such patients, including epistaxis and visceral vascular malformation bleeding (9, 10) with excellent efficacy. Whereas thalidomide has been used in the treatment of malignant tumors, partly because of its direct antitumor effect and partly because of its ability to ameliorate the malignant state of the tumor.
There are many studies on thalidomide and its effects on digestive disorders, but it has not been put into clinical use for digestive disorders in large numbers due to its potential adverse effects and controversial clinical utility. However, its unique therapeutic effects still warrant further research. Therefore, this paper analyses and summarizes the use of thalidomide in digestive diseases, its mechanism of action and potential therapeutic uses, with a view to providing more strategic options for patient care.
2 Thalidomide mechanism
2.1 Chemical structure of thalidomide
The unpredictable toxicity of thalidomide is mainly due to its specific chirality, which is a racemic glutamate analogue consisting of S (–) and R(+) enantiomers, which are interconverted under physiological conditions and cannot be completely separated (11, 12). Although S (–) inhibits the release of tumor necrosis factor α and acts as an immunosuppressant, the presence of S (–) also contributes to the teratogenicity of thalidomide, whereas the R(+) form mainly acts as an antipregnant, antiemetic, and sedative (13).
2.2 Mechanism of action of thalidomide
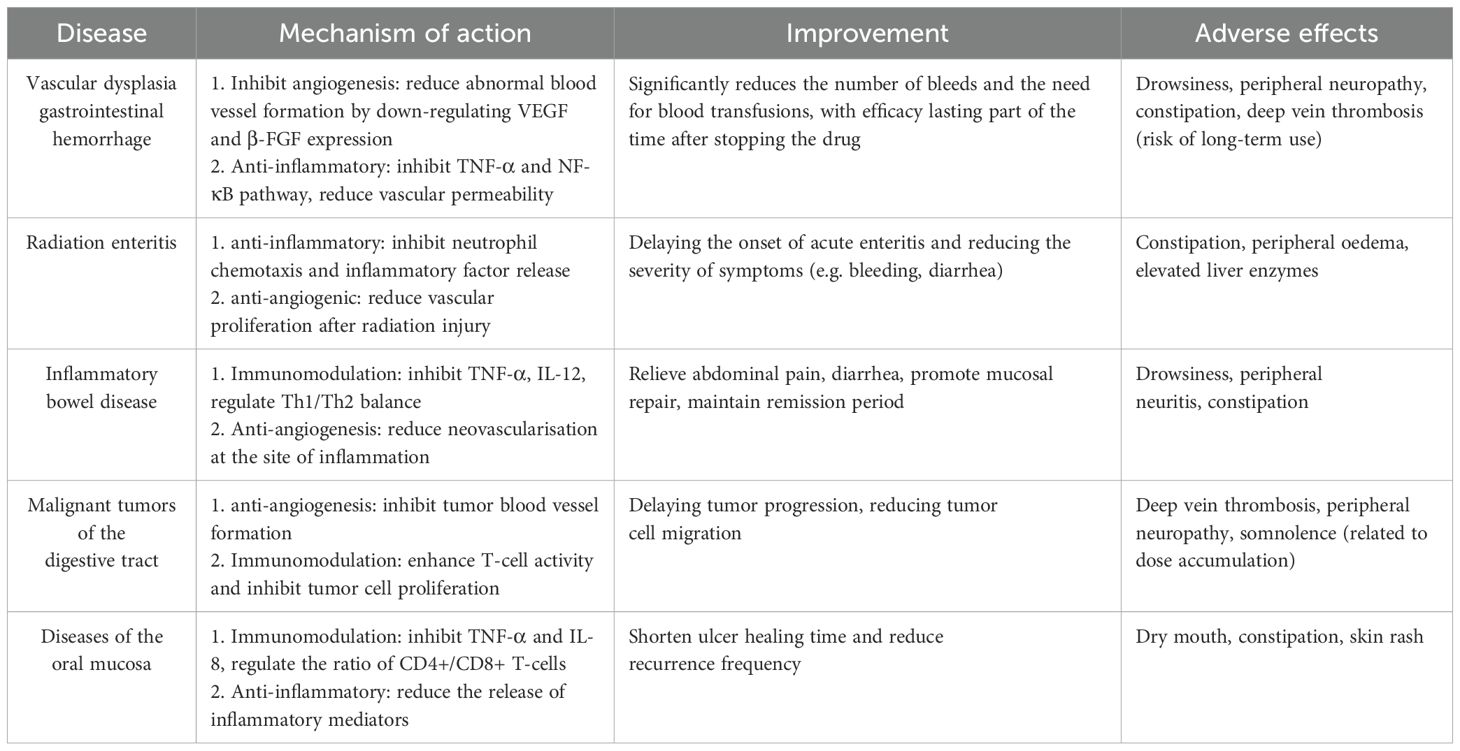
Thalidomide exerts its therapeutic properties primarily through the modulation of cytokines, particularly interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). 12, IL-12), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), interferon, cyclooxygenase 2, and nuclear factor κB (12). Thus, the main mechanisms of action of thalidomide include inhibition of angiogenesis, inhibition of cytokine-mediated pathways, inhibition of regulation of adhesion molecules, inhibition of cyclooxygenase-2 and inhibition of stimulation of immune responses. Its most prominent adverse drug reactions are drowsiness, constipation, dizziness and fatigue. As described in Table 1. The most important reason for limiting the use of thalidomide is its severe teratogenicity. Studies have indicated that thalidomide-binding protein (cereblon, CRBN) is the target of teratogenic effects, and that thalidomide exerts its teratogenic effects by binding to CRBN and inhibiting the inhibition of E3 ubiquitin ligase function (6). It has also been suggested that thalidomide-induced oxidative stress and antiangiogenic effects are possible and plausible reasons for the teratogenicity of thalidomide (14, 15).
3 Thalidomide and digestive disorders
3.1 Thalidomide for vascular dysplasia gastrointestinal hemorrhage
Gastrointestinal angiodysplasia (GIAD) can be found in any part of the gastrointestinal tract, and the most common cause of GI bleeding due to GI dysplasia is small bowel hemorrhage, however, small bowel hemorrhage has limitations in both endoscopic and surgical treatments, and ends up recurring leading to costly transfusion and treatment costs. As a result, thalidomide, a potent angiogenesis inhibitor, was discovered and Huimin Chen from Shanghai Jiaotong University found that thalidomide reduced the incidence of recurrent bleeding due to small bowel vascular dysplasia (16). After three months of treatment of patients with refractory severe recurrent intestinal bleeding, thalidomide strongly inhibited serum vascular endothelial growth factor levels compared with pre-treatment levels (17), thus concluding that thalidomide inhibits the growth of vascular endothelium, thus improving vascular dysplasia to achieve hemostasis. The ability of thalidomide to induce vascular maturation may also be a therapeutic strategy for the treatment of vascular dysplasia (16, 18). A review of capsule endoscopy after 3 months of thalidomide treatment showed a significant reduction in the number, size and color intensity of vascular dysplasia (19). Also, thalidomide is an effective and relatively safe treatment for patients with refractory bleeding due to vascular malformations of the gastrointestinal tract (20). Recent studies have found that thalidomide analogues have the same efficacy, and thalidomide analogues such as lenalidomide and pomalidomide have been found to be potentially effective in the treatment of HHT (9, 21) and, of course, visceral arteriovenous malformation (AVM) (9, 10). Currently available clinical studies confirm the major adverse events of thalidomide, specifically constipation, somnolence, limb numbness, peripheral oedema, dizziness and elevated liver enzyme levels (16). The main reason for discontinuation due to long-term use is because of neurotoxicity (22).
Thalidomide has also been reported in the treatment of recalcitrant bleeding due to portal hypertensive gastropathy (23). In addition recurrent bleeding from the watermelon stomach associated with cirrhosis cannot be controlled by argon knife coagulation therapy (APC), but can be successfully treated with thalidomide (24). Interestingly, GIAD is very common in patients with chronic renal failure (CRF) and its incidence increases further with the duration and severity of renal disease (25). Therefore thalidomide is also frequently used in patients with recurrent bleeding in renal failure.
3.2 Thalidomide for radiation enteritis
According to studies indicated that most cancer patients receive radiation therapy during the course of their disease, but radiation toxicity to normal tissues around the radiation range remains a major obstacle to disease control and quality of life in patients with localized tumors (26). Radiation enteritis is the most common complication of pelvic radiotherapy (27), and the treatment of radiation enteritis remains limited in the clinic, whether it is by medication, enemas, or the newest therapeutic modalities such as hyperbaric oxygen.
Pro-inflammatory factors such as TNF-α, IL-1 and IL-6 play an important role in the pathogenesis of the inflammatory response in acute radiation proctitis. Thalidomide is not only an angiogenesis inhibitor but also an anti-inflammatory agent (12). Studies have shown that thalidomide not only reduces the incidence and grade of acute radiation proctitis, but also improves the patient’s tolerated dose of radiotherapy and delays the onset of radiation proctitis, thus achieving effective prevention and treatment of acute radiation proctitis after postoperative intensity-modulated radiotherapy (IMRT) for cervical cancer (28).
3.3 Thalidomide for the treatment of inflammatory bowel disease
Thalidomide is used in the treatment of inflammatory bowel disease mainly because of its immunomodulatory effects. The potent immunomodulatory activity of thalidomide is mainly due to its ability to alter the secretion and activity of various cytokines, including IL-6, IL-10, IL-12, IL-1β, and TNF-α (29–31), suggesting that it may be effective against UC and CD.
TNF-α is a proinflammatory cytokine associated with CD (32), and thalidomide reduces the production of TNF-α (31). In 1999, thalidomide was shown to be effective in some patients with refractory Crohn’s disease. In this study, 22 patients with refractory Crohn’s disease were initiated on treatment with thalidomide, and 16 patients completed 4 weeks of treatment (12 clinical responses, 4 clinical remission), and 14 patients who completed 12 weeks of treatment met clinical response criteria (33). And low-dose thalidomide (50 mg) also appeared to be well-tolerated and effective at 12 weeks (34). thalidomide has also been shown to improve clinical remission rates in refractory Crohn’s disease in children and adolescents in a randomized controlled study in children and adolescents by Italian investigators in 2013 (35). Thalidomide treatment is equally effective in most patients with refractory active intestinal and/or perineal CD (36). In thalidomide in the treatment of refractory ulcerative colitis, complete clinical remission was achieved in 78.6% of patients treated with a 50 mg dose for 8 weeks (37). However, a high-quality randomized controlled study in 2015 concluded that thalidomide was only effective in inducing remission of CD in children, and current evidence is insufficient to support the use of thalidomide to induce remission of UC or adult CD (38). Thus the role of thalidomide in inflammatory bowel disease remains controversial, and the most recent study in 2022 by Peng Xiang et al. concluded that 100 mg of thalidomide taken continuously for 8 weeks can be used for induction and maintenance therapy of refractory CD in adults (39). However, its toxicity limits its use as maintenance therapy, and neuropathy is the main reason for discontinuation of long-term thalidomide use (35, 36). Interestingly, the powerful XGBoost algorithm of Chinese researchers accurately predicted thalidomide-induced peripheral neuropathy (TiPN) using 18 clinical features and 14 genetic variables. Its ability to identify high-risk patients using single nucleotide polymorphisms provides a viable option for improving thalidomide efficacy in patients with CD (40), but this algorithm is not currently in clinical use.
3.4 Thalidomide in the treatment of primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is a chronic progressive cholestatic disease and an autoimmune disorder. A double-blind randomized controlled trial of thalidomide for the treatment of PBC was reported only in 1994 by the British physician P A McCormick (41), which indicated that apart from ursodeoxycholic acid, which is the only approved drug, drugs such as thalidomide, methotrexate, penciclovir, or colchicine are unlikely to be effective in altering the natural course of primary biliary cirrhosis (41, 42).
3.5 Thalidomide for the treatment of digestive malignancies
Thalidomide is currently used as an antineoplastic therapeutic agent in multiple myeloma (43–46), melanoma (47), glioblastoma multiforme (48), and renal cell carcinoma (49, 50). Among malignant tumors of the digestive system, only studies have shown some anti-tumor effects on hepatocellular liver cancer and colorectal cancer (51–55). Among liver malignancies, antitumor effects have only been found against cirrhosis-associated hepatocellular carcinoma (HCC), probably because the rich vascular distribution of HCC provides an attractive target for anti-angiogenic therapy that may be tolerated by cirrhotic patients, and a phase II clinical study in 2005 indicated that thalidomide may render the stabilization in patients with HCC (51). Longer patient survival may be achieved with thalidomide in combination with other chemotherapeutic agents at different doses, or with thalidomide analogues (51). In colon cancer, studies have indicated that thalidomide is an effective adjuvant antineoplastic agent, and in a clinical study of 60 colon cancer cases, it was concluded that combining thalidomide had a significant therapeutic effect in high-risk patients undergoing surgery for stage II and III colon or rectal cancer who received downstaged, first-line, palliative oxaliplatin + capecitabine chemotherapy after surgical resection of metastatic colon cancer (56). Radioimmunotherapy (RIT) in combination with thalidomide antiangiogenic therapy produces a better tumor response than monotherapy, and antiangiogenic therapy may prolong the dormancy of cancer lesions (55). Irinotecan is the only accepted second-line treatment for colorectal cancer in the United States (57), however dosing is often limited by associated late-onset diarrhea, and studies have found that thalidomide virtually eliminates the dose-limiting gastrointestinal toxic effects of irinotecan, particularly diarrhea and nausea (58).
As for solid tumors of the digestive system other than hepatocellular carcinoma and colorectal cancer, thalidomide has been found to improve malignant tumor cachexia (59). A large number of patients with advanced cancer develop malignancy before death, and it is more common in malignant tumors of the digestive system, including pancreatic, gastric, esophageal, and colorectal cancers. However, there are no effective drugs for treating cancer cachexia, and promising pharmacological treatments are limited, including megestrol acetate, anamorelin, thalidomide, and delta-9-tetrahydrocannabinol (60). In contrast, thalidomide alters the cytokine triggers of the wasting response through its potent anti-TNF-alpha action, inhibiting the production of transcription factor (NkB), which limits downstream gene expression, which in turn affects the control of pro-inflammatory cytokines, cellular growth, and regulation, ultimately leading to an amelioration of the weight loss associated with malignant stroma (61).Gordon JN also found that thalidomide was effective in attenuating weight loss in patients with advanced weight and lean body mass loss in patients with pancreatic cancer cachexia (59). And Khan ZH’s study concluded that thalidomide also reversed weight loss in patients with advanced esophageal cancer within 2 weeks (62). However, a 2012 study analyzing large amounts of data ultimately did not provide a positive answer and there was insufficient evidence to recommend it for clinical practice (61).
3.6 Thalidomide for the treatment of oral mucosal diseases
Thalidomide has been widely used in oral mucosal diseases, such as oral lichen planus, recurrent aphthous ulcers, leucosis involving the oral mucosa, oral cancer, etc (63–65).
National guidelines for the treatment of oral lichen planus recommend a starting dose of 50–100 mg/d of thalidomide, which is gradually reduced to the lowest effective dose (65, 66). Thalidomide has shown some efficacy in controlling the frequency of recurrence of recurrent aphthous ulcers, and should be used mainly in the heavy or recalcitrant type, but national and international guidelines do not explicitly recommend the dose to be given (67, 68). To reduce adverse effects, some studies have used reduced dosing regimens, and although they have been shown to significantly prolong the ulcer interval in patients, none have compared the efficacy and safety of different doses of thalidomide (69, 70).
There is increasing evidence from in vitro and in vivo experiments that thalidomide is a promising anticancer agent for oral cancer, and therefore there is a strong need for more clinical trials with larger sample sizes to demonstrate the potential role of thalidomide in the routine clinical management of oral disease (63). With the expansion of thalidomide’s clinical scope of application, the adverse drug reaction (ADR) caused by thalidomide has been gradually taken into account, especially the damage to the nervous system, and the potential teratogenicity of thalidomide should be highly concerned for the population of childbearing age (71, 72).
Therefore, more high-quality clinical trials of thalidomide for the treatment of oral mucosal diseases are needed to determine the optimal dosing regimen and safe dosage to enhance the safety of the drug for patients.
4 Conclusions and outlook
Thalidomide was once restricted because of its teratogenicity, but as more and more of its effects continue to be discovered, it is now well used in clinical treatment. For example, multiple myeloma and some skin diseases. However, at present, the use of thalidomide in digestive system diseases is not popular, but its over-the-counter use in digestive system diseases is not uncommon. This includes the treatment of unexplained gastrointestinal hemorrhage, which is first considered to be due to vascular dysplasia and congenital capillary malformations. While its use in inflammatory bowel disease has been found to induce remission of CD and UC, a high-quality RCT conducted by Canadian pediatrician C Yang concluded that thalidomide was only effective in inducing remission of CD in children (38). The use of thalidomide for the treatment of radiation proctitis is similarly controversial, but there are fewer clinical studies in this area, and correspondingly fewer reports, but because of its controversial clinical role and greater susceptibility to adverse effects, it has not been put into clinical use in large numbers.
At present, the higher adverse reaction rate of thalidomide remains a pressing issue, but its utility in improving the malignant status of tumors remains promising, and its clinical application, including gastrointestinal tumors, still requires extensive clinical research and practice.
Author contributions
ZX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. WJ: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing. ZL: Supervision, Writing – review & editing. SS: Formal analysis, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Publisher’s note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
References
1. Holstein SA and McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. (2017) 77:505–20. doi: 10.1007/s40265-017-0689-1
2. Zhou S, Wang F, Hsieh TC, Wu JM, and Wu E. Thalidomide-a notorious sedative to a wonder anticancer drug. Curr Med Chem. (2013) 20:4102–8. doi: 10.2174/09298673113209990198
3. McCarthy DM, Kanfer EJ, and Barrett AJ. Thalidomide for the therapy of graft-versus-host disease following allogeneic bone marrow transplantation. BioMed Pharmacother. (1989) 43:693–7. doi: 10.1016/0753-3322(89)90089-9
4. Pelle MT and Werth VP. Thalidomide in cutaneous lupus erythematosus. Am J Clin Dermatol. (2003) 4:379–87. doi: 10.2165/00128071-200304060-00002
5. Colley A, Brauns T, Sluder AE, Poznansky MC, and Gemechu Y. Immunomodulatory drugs: a promising clinical ally for cancer immunotherapy. Trends Mol Med. (2024) 30:765–80. doi: 10.1016/j.molmed.2024.05.001
6. Ito T, Ando H, and Handa H. Teratogenic effects of thalidomide: molecular mechanisms. Cell Mol Life Sci. (2011) 68:1569–79. doi: 10.1007/s00018-010-0619-9
7. Yamamoto J, Ito T, Yamaguchi Y, and Handa H. Discovery of CRBN as a target of thalidomide: a breakthrough for progress in the development of protein degraders. Chem Soc Rev. (2022) 51:6234–50. doi: 10.1039/D2CS00116K
8. Al-Samkari H. Systemic antiangiogenic therapies for bleeding in hereditary hemorrhagic telangiectasia: A practical, evidence-based guide for clinicians. Semin Thromb Hemost. (2022) 48:514–28. doi: 10.1055/s-0042-1743467
9. Al-Samkari H. How I treat bleeding in hereditary hemorrhagic telangiectasia. Blood. (2024) 144:940–54. doi: 10.1182/blood.2023021765
10. Al-Samkari H, Kasthuri RS, Iyer VN, Pishko AM, Decker JE, Weiss CR, et al. Pomalidomide for epistaxis in hereditary hemorrhagic telangiectasia. N Engl J Med. (2024) 391:1015–27. doi: 10.1056/NEJMoa2312749
11. Teo SK, Colburn WA, Tracewell WG, Kook KA, Stirling DI, Jaworsky MS, et al. Clinical pharmacokinetics of thalidomide. Clin Pharmacokinet. (2004) 43:311–27. doi: 10.2165/00003088-200443050-00004
12. Franks ME, Macpherson GR, and Figg WD. Thalidomide. Lancet. (2004) 363:1802–11. doi: 10.1016/S0140-6736(04)16308-3
13. Wnendt S and Zwingenberger K. Thalidomide’s chirality. Nature. (1997) 385:303–4. doi: 10.1038/385303b0
14. Therapontos C, Erskine L, Gardner ER, Figg WD, and Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci U S A. (2009) 106:8573–8. doi: 10.1073/pnas.0901505106
15. Hansen JM and Harris C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid Redox Signal. (2004) 6:1–14. doi: 10.1089/152308604771978291
16. Chen H, Wu S, Tang M, Zhao R, Zhang Q, Dai Z, et al. Thalidomide for recurrent bleeding due to small-intestinal angiodysplasia. N Engl J Med. (2023) 389:1649–59. doi: 10.1056/NEJMoa2303706
17. Bauditz J, Schachschal G, Wedel S, and Lochs H. Thalidomide for treatment of severe intestinal bleeding. Gut. (2004) 53:609–12. doi: 10.1136/gut.2003.029710
18. Franchini M and Lippi G. Thalidomide for hereditary haemorrhagic telangiectasia. Lancet Haematol. (2015) 2:e457–8. doi: 10.1016/S2352-3026(15)00222-7
19. Bauditz J, Lochs H, and Voderholzer W. Macroscopic appearance of intestinal angiodysplasias under antiangiogenic treatment with thalidomide. Endoscopy. (2006) 38:1036–9. doi: 10.1055/s-2006-944829
20. Ge ZZ, Chen HM, Gao YJ, Liu WZ, Xu CH, Tan HH, et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. (2011) 141:1629–37.e1-4. doi: 10.1053/j.gastro.2011.07.018
21. Ugur MC, Baysal M, and Umit EG. The role of thalidomide and its analogs in the treatment of hereditary hemorrhagic telangiectasia: A systematic review. J Clin Med. (2024) 13(18):5404. doi: 10.3390/jcm13185404
22. Gerich ME, Yoon JL, Targan SR, Ippoliti AF, and Vasiliauskas EA. Long-term outcomes of thalidomide in refractory Crohn’s disease. Aliment Pharmacol Ther. (2015) 41:429–37. doi: 10.1111/apt.2015.41.issue-5
23. Karajeh MA, Hurlstone DP, Stephenson TJ, Ray-Chaudhuri D, and Gleeson DC. Refractory bleeding from portal hypertensive gastropathy: a further novel role for thalidomide therapy? Eur J Gastroenterol Hepatol. (2006) 18:545–8.
24. Moser S, Tischer A, Karpi A, Schleicher M, Stavjanik S, and Gschwantler M. Evidence that thalidomide is effective in recurrent bleeding from watermelon stomach associated with liver cirrhosis. Endoscopy. (2014) 46 Suppl 1 UCTN:E384. doi: 10.1055/s-0034-1377369
25. Chalasani N, Cotsonis G, and Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. (1996) 91:2329–32.
26. Hauer-Jensen M, Denham JW, and Andreyev HJ. Radiation enteropathy–pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. (2014) 11:470–9. doi: 10.1038/nrgastro.2014.46
27. Zhou Y, Bao L, Gong S, Dou G, Li Z, Wang Z, et al. T Cell-Derived Apoptotic Extracellular Vesicles Hydrolyze cGAMP to Alleviate Radiation Enteritis via Surface Enzyme ENPP1. Adv Sci (Weinh). (2024) 11:e2401634. doi: 10.1002/advs.202401634
28. Jiao Y, Yu L, Ronghui J, and Xin C. Clinical observation on prevention and treatment effect of thalidomide on acute radiation proctitis. Cancer Res Prev Treat. (2017) 44:5. doi: 10.3971/j.issn.1000-8578.2017.05.008
29. Corral LG and Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis. (1999) 58 Suppl 1:I107–13. doi: 10.1136/ard.58.2008.i107
30. Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, et al. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. (1999) 9:1625–30. doi: 10.1016/S0960-894X(99)00250-4
31. Bauditz J, Wedel S, and Lochs H. Thalidomide reduces tumour necrosis factor alpha and interleukin 12 production in patients with chronic active Crohn’s disease. Gut. (2002) 50:196–200. doi: 10.1136/gut.50.2.196
32. Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, Groessner B, et al. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn’s disease. Lancet. (1999) 353:459–61. doi: 10.1016/S0140-6736(98)03339-X
33. Ehrenpreis ED, Kane SV, Cohen LB, Cohen RD, and Hanauer SB. Thalidomide therapy for patients with refractory Crohn’s disease: an open-label trial. Gastroenterology. (1999) 117:1271–7. doi: 10.1016/S0016-5085(99)70276-3
34. Vasiliauskas EA, Kam LY, Abreu-Martin MT, Hassard PV, Papadakis KA, Yang H, et al. An open-label pilot study of low-dose thalidomide in chronically active, steroid-dependent Crohn’s disease. Gastroenterology. (1999) 117:1278–87. doi: 10.1016/S0016-5085(99)70277-5
35. Lazzerini M, Martelossi S, Magazzù G, Pellegrino S, Lucanto MC, Barabino A, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. Jama. (2013) 310:2164–73. doi: 10.1001/jama.2013.280777
36. Simon M, Pariente B, Lambert J, Cosnes J, Bouhnik Y, Marteau P, et al. Long-term outcomes of thalidomide therapy for adults with refractory crohn’s disease. Clin Gastroenterol Hepatol. (2016) 14:966–72.e2. doi: 10.1016/j.cgh.2015.10.034
37. Chen JR, Mai L, Sun JC, Peng X, Zhang M, and Zhi M. Efficacy and safety of low-dose thalidomide combined with mesalazine in the treatment of refractory ulcerative colitis in adults. Gastroenterol Rep (Oxf). (2022) 10:goac032. doi: 10.1093/gastro/goac032
38. Yang C, Singh P, Singh H, Le ML, and El-Matary W. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. (2015) 41:1079–93. doi: 10.1111/apt.2015.41.issue-11
39. Peng X, Lin ZW, Zhang M, Yao JY, Zhao JZ, Hu PJ, et al. The efficacy and safety of thalidomide in the treatment of refractory Crohn’s disease in adults: a double-center, double-blind, randomized-controlled trial. Gastroenterol Rep (Oxf). (2022) 10:goac052. doi: 10.1093/gastro/goac052
40. Mao J, Chao K, Jiang FL, Ye XP, Yang T, Li P, et al. Comparison and development of machine learning for thalidomide-induced peripheral neuropathy prediction of refractory Crohn’s disease in Chinese population. World J Gastroenterol. (2023) 29:3855–70. doi: 10.3748/wjg.v29.i24.3855
41. McCormick PA, Scott F, Epstein O, Burroughs AK, Scheuer PJ, and McIntyre N. Thalidomide as therapy for primary biliary cirrhosis: a double-blind placebo controlled pilot study. J Hepatol. (1994) 21:496–9. doi: 10.1016/S0168-8278(94)80092-8
42. Oo YH and Neuberger J. Options for treatment of primary biliary cirrhosis. Drugs. (2004) 64:2261–71. doi: 10.2165/00003495-200464200-00001
43. Malard F, Neri P, Bahlis NJ, Terpos E, Moukalled N, Hungria VTM, et al. Multiple myeloma. Nat Rev Dis Primers. (2024) 10:45. doi: 10.1038/s41572-024-00529-7
44. Palumbo A, Facon T, Sonneveld P, Bladè J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. (2008) 111:3968–77. doi: 10.1182/blood-2007-10-117457
45. Folkman J and Rogers MS. Thalidomide for multiple myeloma. N Engl J Med. (2006) 354:2389–90; author reply -90.
46. Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. (2006) 354:1021–30. doi: 10.1056/NEJMoa053583
47. Hwu WJ, Krown SE, Panageas KS, Menell JH, Chapman PB, Livingston PO, et al. Temozolomide plus thalidomide in patients with advanced melanoma: results of a dose-finding trial. J Clin Oncol. (2002) 20:2610–5. doi: 10.1200/JCO.2002.09.034
48. Puduvalli VK, Giglio P, Groves MD, Hess KR, Gilbert MR, Mahankali S, et al. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol. (2008) 10:216–22. doi: 10.1215/15228517-2007-060
49. Amato RJ. Renal cell carcinoma: review of novel single-agent therapeutics and combination regimens. Ann Oncol. (2005) 16:7–15. doi: 10.1093/annonc/mdi002
50. Hernberg M, Virkkunen P, Bono P, Ahtinen H, Mäenpää H, and joensuu H. Interferon alfa-2b three times daily and thalidomide in the treatment of metastatic renal cell carcinoma. J Clin Oncol. (2003) 21:3770–6. doi: 10.1200/JCO.2003.01.536
51. Patt YZ, Hassan MM, Lozano RD, Nooka AK, Schnirer II, Zeldis JB, et al. Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial. Cancer. (2005) 103:749–55. doi: 10.1002/cncr.v103:4
52. Chiou HE, Wang TE, Wang YY, and Liu HW. Efficacy and safety of thalidomide in patients with hepatocellular carcinoma. World J Gastroenterol. (2006) 12:6955–60. doi: 10.3748/wjg.v12.i43.6955
53. Shao YY, Chen BB, Ou DL, Lin ZZ, Hsu CH, Wang MJ, et al. Lenalidomide as second-line therapy for advanced hepatocellular carcinoma: exploration of biomarkers for treatment efficacy. Aliment Pharmacol Ther. (2017) 46:722–30. doi: 10.1111/apt.2017.46.issue-8
54. Lv J, Liu N, Liu KW, Ding AP, Wang H, and Qiu WS. A randomised controlled phase II trial of the combination of XELOX with thalidomide for the first-line treatment of metastatic colorectal cancer. Cancer Biol Med. (2012) 9:111–4.
55. Kinuya S, Kawashima A, Yokoyama K, Koshida K, Konishi S, Watanabe N, et al. Cooperative effect of radioimmunotherapy and antiangiogenic therapy with thalidomide in human cancer xenografts. J Nucl Med. (2002) 43:1084–9.
56. Zhong N, Yu Y, and Yang P. Effect of CAPEOX combined with thalidomide in the treatment of elderly patients with colon cancer: A single-center report. Asian J Surg. (2024) 47:3746–7. doi: 10.1016/j.asjsur.2024.04.108
57. Antoniotti C, Rossini D, Pietrantonio F, Salvatore L, Lonardi S, Tamberi S, et al. Upfront fluorouracil, leucovorin, oxaliplatin, and irinotecan plus bevacizumab with or without atezolizumab for patients with metastatic colorectal cancer: updated and overall survival results of the ATEZOTRIBE study. J Clin Oncol. (2024) 42:2637–44. doi: 10.1200/JCO.23.02728
58. Govindarajan R, Heaton KM, Broadwater R, Zeitlin A, Lang NP, and Hauer-Jensen M. Effect of thalidomide on gastrointestinal toxic effects of irinotecan. Lancet. (2000) 356:566–7. doi: 10.1016/S0140-6736(00)02586-1
59. Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, and Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. (2005) 54:540–5. doi: 10.1136/gut.2004.047563
60. Ispoglou T, McCullough D, Windle A, Nair S, Cox N, White H, et al. Addressing cancer anorexia-cachexia in older patients: Potential therapeutic strategies and molecular pathways. Clin Nutr. (2024) 43:552–66. doi: 10.1016/j.clnu.2024.01.009
61. Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, and Donnelly M. Thalidomide for managing cancer cachexia. Cochrane Database Syst Rev. (2012) 2012:Cd008664.
62. Khan ZH, Simpson EJ, Cole AT, Holt M, MacDonald I, Pye D, et al. Oesophageal cancer and cachexia: the effect of short-term treatment with thalidomide on weight loss and lean body mass. Aliment Pharmacol Ther. (2003) 17:677–82. doi: 10.1046/j.1365-2036.2003.01457.x
63. Jin X, Lu S, Xing X, Wang L, Mu D, He M, et al. Thalidomide: features and potential significance in oral precancerous conditions and oral cancer. J Pathol Med. (2013) 42:355–62. doi: 10.1111/jop.2013.42.issue-5
64. Shi LJ and Zhou ZT. Diagnosis and management of recurrent aphthous ulcer. Zhonghua Kou Qiang Yi Xue Za Zhi. (2022) 57:314–8. doi: 10.3760/cma.j.cn112144-20220111-00012
65. Society of Oral Medicine, Chinese Stomatological Association. Guideline for the diagnosis and treatment of oral lichen planus (revision). Zhonghua Kou Qiang Yi Xue Za Zhi. (2022) 57:115–21. doi: 10.3760/cma.j.cn112144-20211115-00505
66. Ioannides D, Vakirlis E, Kemeny L, Marinovic B, Massone C, Murphy R, et al. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. (2020) 34:1403–14. doi: 10.1111/jdv.16464
67. Tarakji B, Gazal G, Al-Maweri SA, Azzeghaiby SN, and Alaizari N. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Health. (2015) 7:74–80.
68. Milia E, Sotgiu MA, Spano G, Filigheddu E, Gallusi G, and Campanella V. Recurrent aphthous stomatitis (RAS): guideline for differential diagnosis and management. Eur J Paediatr Dent. (2022) 23:73–8.
69. Zeng Q, Shi X, Yang J, Yang M, Zhao W, Zhao X, et al. The efficacy and safety of thalidomide on the recurrence interval of continuous recurrent aphthous ulceration: A randomized controlled clinical trial. J Pathol Med. (2020) 49:357–64. doi: 10.1111/jop.12960
70. Lau CB and Smith GP. Recurrent aphthous stomatitis: A comprehensive review and recommendations on therapeutic options. Dermatol Ther. (2022) 35:e15500. doi: 10.1111/dth.15500
71. Xiaojuan X, Jing H, Bin F, Weigang W, and Jiao Y. Adverse drug reactions associated with thalidomide treatment for oral mucosal diseases: a report of 44 cases. Chin J Primary Med Pharmacy. (2024) 31:1457–62.
Keywords: thalidomide, digestive diseases, bleeding, inflammation, tumors
Citation: Xi Z, Jie W, Long Z and Shasha S (2025) A review of thalidomide and digestive system related diseases. Front. Oncol. 15:1543757. doi: 10.3389/fonc.2025.1543757
Received: 11 December 2024; Accepted: 09 May 2025;
Published: 29 May 2025; Corrected: 19 June 2025.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Aarti Nagayach, The Ohio State University, United StatesToshiyuki Matsui, Fukuoka University, Japan
Copyright © 2025 Xi, Jie, Long and Shasha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Shasha, emwwODE2MzY5N0AxNjMuY29t
 Zhao Xi
Zhao Xi Wang Jie2
Wang Jie2