- 1Department of General Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Medical College, Nankai University, Tianjin, China
Background: The incidence of early onset gastric cancer(EOGC) is increasing. However, few studies have focused on early onset early stage gastric cancer(EEGC). The aim of this study was to determine the threshold age of patients with EOGC, identify the clinicopathological characteristics associated with lymph node metastasis(LNM) in EEGC, and develop a predictive model for LNM in EEGC.
Methods: A retrospective cohort study was conducted, including 1765 patients with early-stage gastric cancer. Logistic inflection point and stratified analysis were used to determine the threshold age. 266 patients met the criteria for EEGC and were included for further analysis. The patients were divided into two groups for the purposes of the study: a training dataset and an external validation dataset. The division of patients into these two groups was conducted in accordance with the time of surgery, with the ratio of patients in each group being approximately 7:3.Univariate and multivariate logistic regression analysis were used to identify LNM risk factors. A predictive nomogram was developed and validated using calibration plots and the area under the curve (AUC).The constructed logistic regression model was then validated using the external validation dataset.
Results: The threshold age for EOGC was determined to be 45 years. Of the 266 patients with EEGC, 20.7% had LNM. Tumor maximum diameter and lymphovascular invasion were identified as independent risk factors for LNM. The nomogram demonstrated high predictive accuracy, with an AUC of 0.809.
Conclusions: This study demonstrated that tumor maximum diameter and lymphovascular invasion were independent risk factors for LNM in EEGC. The predictive nomogram showed promising accuracy and might assist in identifying patients at higher risk of LNM, potentially informing treatment strategies. Given the relatively high LNM rate, endoscopic submucosal dissection may not be suitable for EEGC patients. Further large-scale multicenter studies are needed to deepen the understanding of this population and to confirm these findings.
1 Introduction
Early-onset gastric cancer (EOGC) refers to gastric cancer that occurs at a younger age. Currently, there is no universally defined onset age. Some studies used a threshold of 40 or 50 years old (1–4), but the academic community at large considers the threshold age for EOGC to be 45 years (5, 6). The incidence of early onset gastric cancer is increasing (7). Numerous studies have highlighted the distinct characteristics of EOGC compared to late-onset cases, emphasizing the significance of recognizing it as a separate pathological entity (8)- (9). Several studies showed that EOGC is more aggressive than traditional gastric cancer (10).
Early stage GC is limited to mucosal (T1a) or submucosal (T1b), regardless of lymph node metastasis (LNM) (11). According to the sixth edition of the Japanese Gastric Cancer Treatment Guidelines, patients with early gastric cancer may be eligible for endoscopic submucosal dissection (ESD) in addition to radical gastrectomy (12). ESD is particularly indicated for tumors with a minimal likelihood of LNM and that are amenable to an bloc resection (13, 14),which prioritizes organ preservation and quality of life (15). Previous studies have suggested that EOGC tends to exhibit lower differentiation and more advanced staging at diagnosed compared to the older patients (16, 17). Consequently, EEGC might be more prone to LNM due to the more aggressive biological behaviour and advanced pathological features observed in EOGC. Therefore, understanding the incidence and risk factors of LNM in EEGC is crucial for guiding treatment strategies. However, to date few studies focus on EEGC patients. Further investigation is needed to better define the optimal management strategies for EEGC, especially in terms of LNM risk and therapeutic approaches.
Consequently, we conducted a retrospective analysis aimed at exploring the appropriate definition of EOGC and the risk factors associated with LNM in EEGC. Furthermore, we attempted to construct a nomogram to predict the occurrence of LNM to identify the appropriate candidates for ESD among these patients.
2 Materials and methods
2.1 Patients
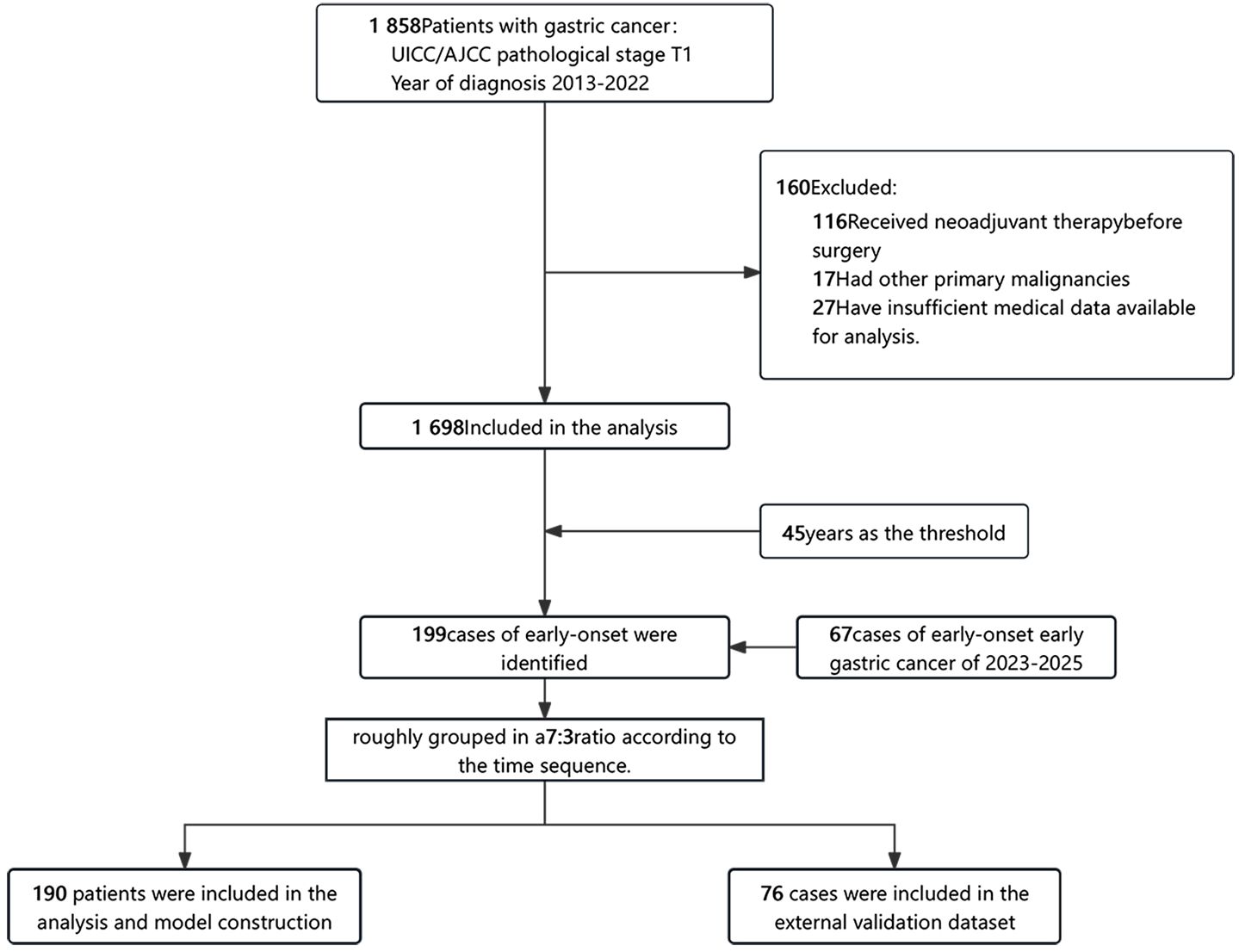
This study included patients who underwent a radical resection for primary gastric cancer and were histologically proven to have pT1 gastric adenocarcinoma, as defined by the 8th edition of the AJCC TNM staging system, at the First Medical Center of the PLA General Hospital in Beijing, China, from 2013 to 2025. All patients were of Asian ethnicity. For inclusion in this analysis, patients had to meet the following criteria: (1) they were diagnosed with pT1 stage gastric cancer based on histological examination; (2) they possessed comprehensive medical information relevant to the study; (3) they underwent surgery without any neoadjuvant therapy; (4) they had no other concurrent malignant tumors. In total. This study was approved by the Ethics Committee of Chinese PLA General Hospital. (No. S2023-275-01). Due to the study’s retrospective design, the requirement for informed consent was waived. Finally, a total of 1,765 patients were included in the study, of whom 266 had early-stage gastric cancer (Figure 1).
2.2 Patient characteristics and clinical data
This article analyzed various clinical characteristics, including age, gender, family history of gastric cancer, body mass index (BMI), and pathological characteristics. The pathological characteristics considered for analysis included tumor location, tumor maximum diameter, invasion depth, histological type, lymphovascular invasion, perineural invasion and lymph node status. Additionally, laboratory indicators such as Serum Markers (CEA, AFP, CA19-9, CA15-3, CA125, CA724) and immune cell indicators (neutrophils, lymphocytes, monocytes) were included. The histological types were reviewed and categorized into four groups: adenocarcinoma, signet ring cell adenocarcinoma, mixed adenocarcinoma with signet ring cell carcinoma and mixed adenocarcinoma with neuroendocrine carcinoma. Two independent and experienced pathologists reviewed hematoxylin-eosin (H&E)-stained slides from each case, and in instances of inconsistent diagnoses between the two pathologists, a third pathologist was consulted.
2.3 Statistical analysis
Categorical variables were compared using Chi-square or Fisher’s exact test, and continuous variables were compared using analysis of variance (ANOVA) or Kruskal-Wallis test. Statistical analyses were performed using the R software package (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.8. Two-tailed tests were performed, and a significance level of P value < 0.05 was used to determine statistical significance.
3 Results
3.1 Optimal age threshold for EOGC
A logistic inflection point analysis was conducted to evaluate the relationship between age and the probability of LNM in the patients from 2013 to 2022. The analysis identified a significant turning point at 51.8 years (95% CI: 51.1–52.5), indicating a distinct change in the effect of age on the probability of LNM. Prior to the turning point, the slope of the relationship between age and LNM was significantly negative (OR: 0.957, 95% CI: 0.918–0.997, P = 0.035), indicating a decreasing probability of LNM with increasing age. However, beyond the turning point, the slope flattened and became statistically nonsignificant (OR: 0.997, 95% CI: 0.974–1.020, P = 0.769), indicating a plateau in the effect of age on LNM probability in older patients (Figure 2).
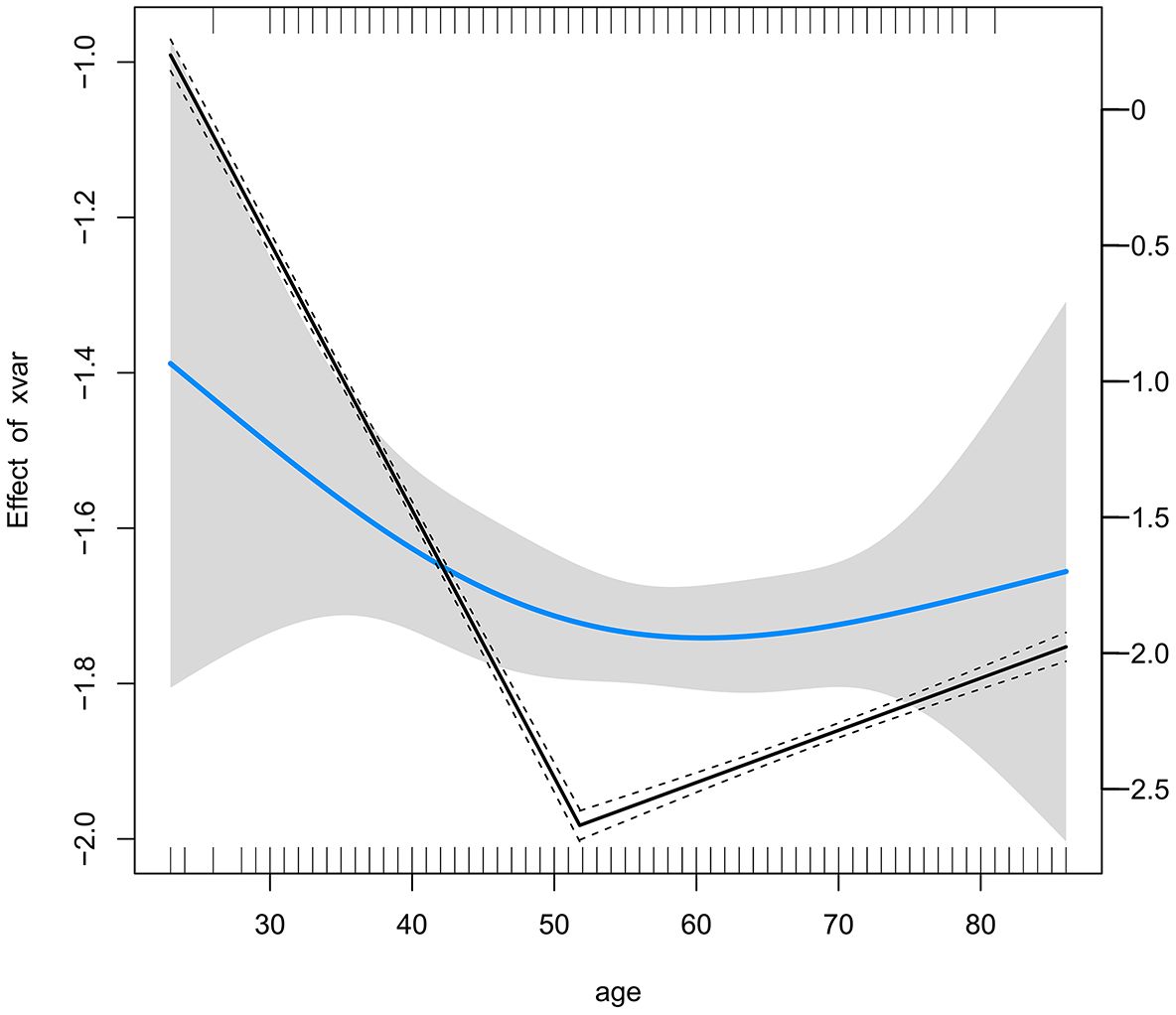
Figure 2. Threshold Effect of Age on Lymph Node Metastasis Risk Identified by Logistic Inflection Point Analysis.
To further determine the optimal age threshold for defining EOGC, we conducted a stratified analysis of patients aged between 40 and 51 years. For each age cutoff (e.g., ≤40 vs. >40, ≤41 vs. >41), a contingency table was constructed to compare the LNM rates between the two groups. The results demonstrated that cutoffs at 42 years (OR: 1.72, 95% CI: 1.09–2.71, P = 0.027), 43 years (OR: 1.59, 95% CI: 1.03–2.47, P = 0.048), 45 years (OR: 1.54, 95% CI: 1.04–2.19, P = 0.036), and 46 years (OR: 1.51, 95% CI: 1.04–2.19, P = 0.036) showed statistical significance (Supplementary Table S1). The results indicated that defining EOGC at 46 years is also reasonable and practical. However, to align with the widely accepted definition within the academic community, our study adopted 45 years as the threshold to ensure consistency and comparability with existing research, and included 199 cases of early-onset gastric cancer.
3.2 Clinicopathological characteristics
Among the 190 cases of EEGC patients of training dataset, the age of the patients ranged from 24 to 45 years, with a mean age of 39 ± 5 years. The median tumor size was 1.8 cm. Among these cases, 37 patients exhibited LNM, resulting in a metastasis rate of 19.5%. The influence of clinical factors—including age, sex, BMI, tumor maximum diameter, location, tumor marker levels, and pathological characteristics—on LNM was assessed. Patients with LNM tended to have a larger median tumor maximum diameter (2.0 cm vs. 1.5 cm, P = 0.001) and a significantly higher incidence of lymphovascular invasion (27.0% vs. 5.2%, P < 0.001) compared to those without metastasis. Submucosal invasion was also more common in the LNM+ group (62.2% vs. 34.6%, P = 0.002). No significant differences were observed between the two groups for gender, age, family history, BMI, tumor markers, or neural invasion. The demographic and clinicopathological data for both cohorts are summarized in Table 1.
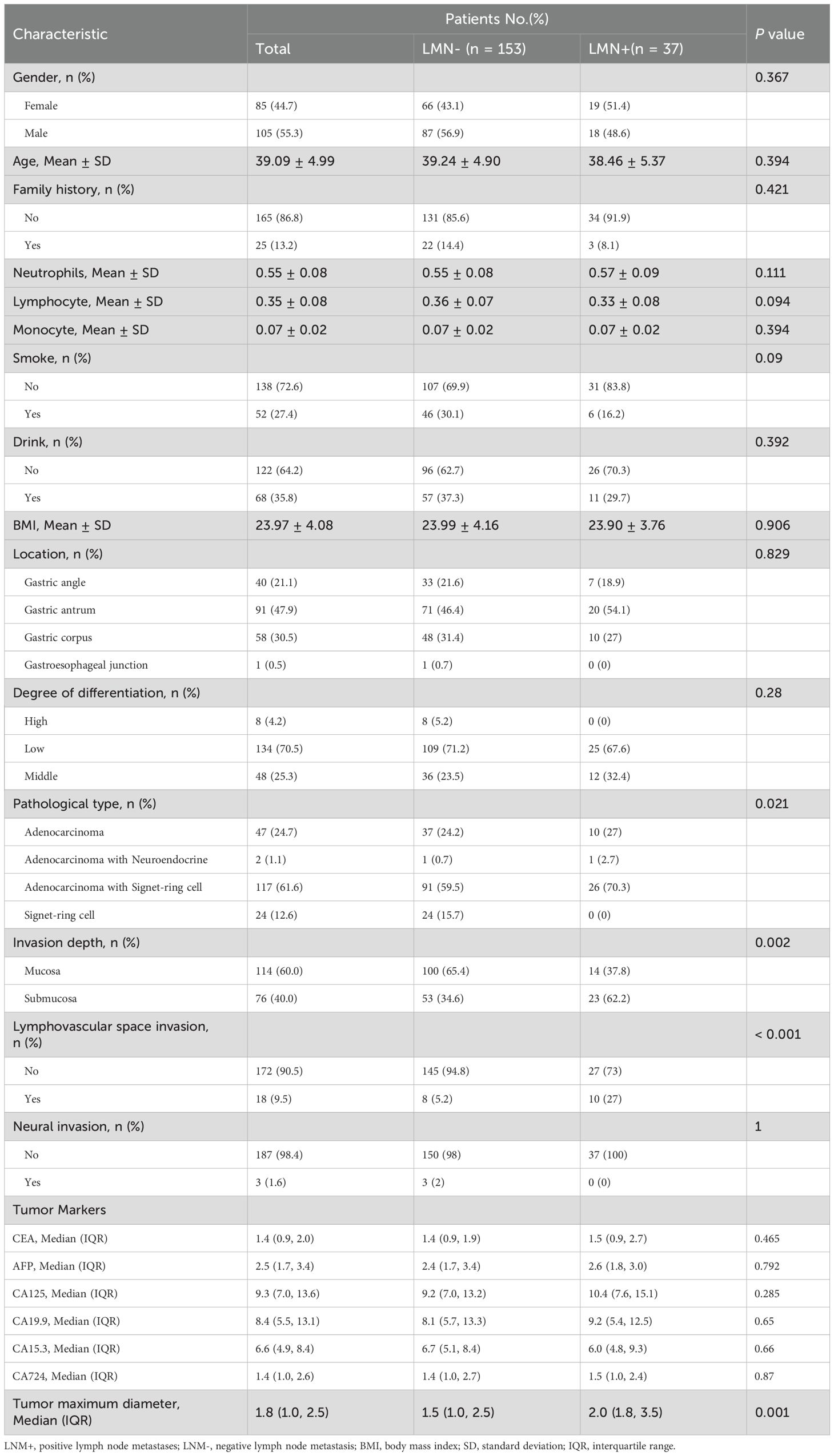
Table 1. Characteristics of early onset early stage gastric cancer with and without lymph node metastasis.
3.3 Risk factors of lymph node metastasis
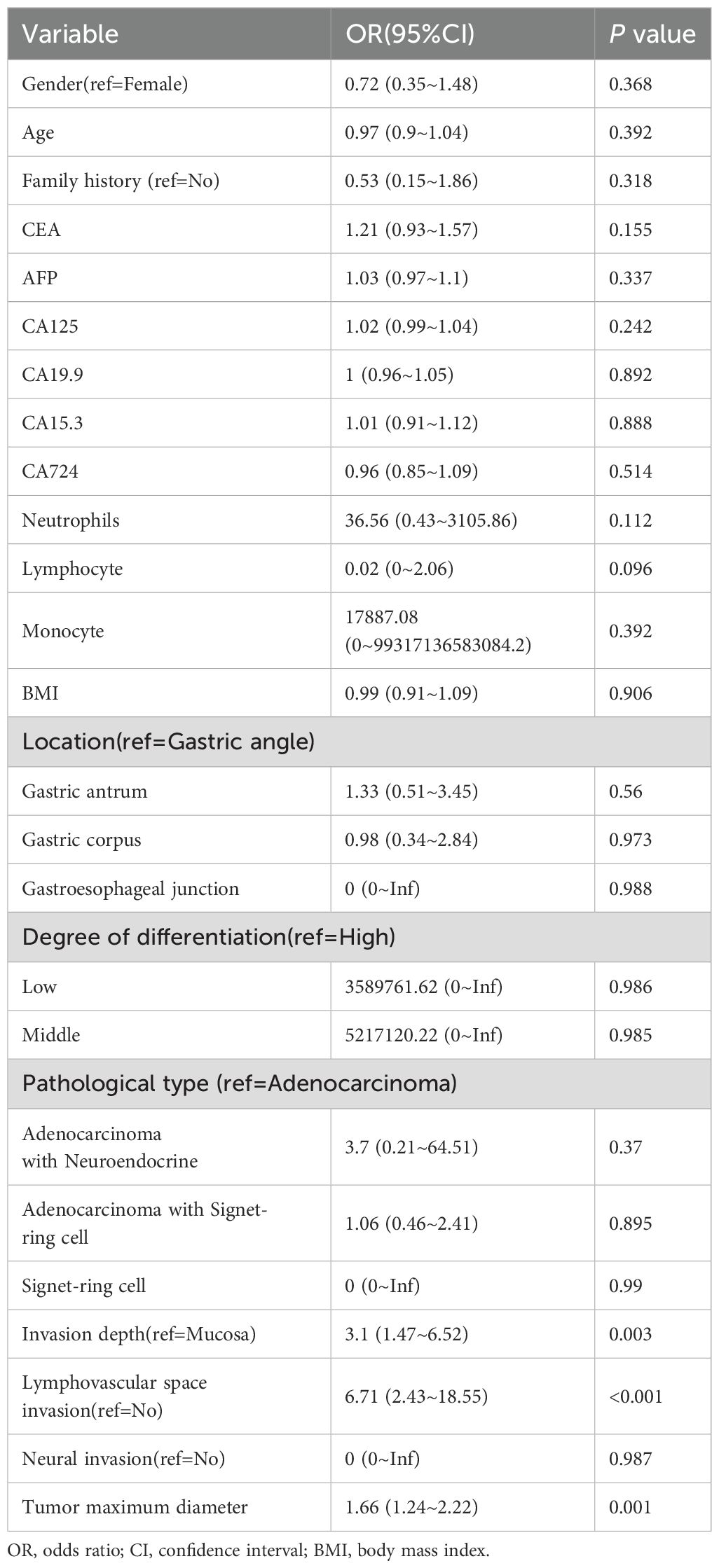
To identify the risk factors associated with LNM, we performed univariate and multivariate logistic regression analyses in training dataset. In the univariate analysis, lymphovascular invasion (OR: 6.71; 95% CI: 2.43–18.55; P < 0.001), invasion depth (OR: 3.11; 95% CI: 1.47–6.52; P = 0.003), and maximum tumor diameter (OR: 1.66; 95% CI: 1.24–2.22; P = 0.001) were identified as significant risk factors for LNM. No other variables were found to be significantly associated with LNM. The results of the univariate logistic regression analysis are presented in Table 2. In the multivariate logistic regression analysis, lymphovascular invasion (OR: 5.28; 95% CI: 1.52–18.35; P = 0.009) and maximum tumor diameter (OR: 1.45; 95% CI: 1.06–1.99; P = 0.021) remained independent risk factors for LNM. The results of the multivariate logistic regression analysis are presented in Table 3. Subsequently, we constructed a nomogram based on the multivariate analysis results. The accuracy of this nomogram was evaluated using the Harrell C-index and the area under the curve (AUC).
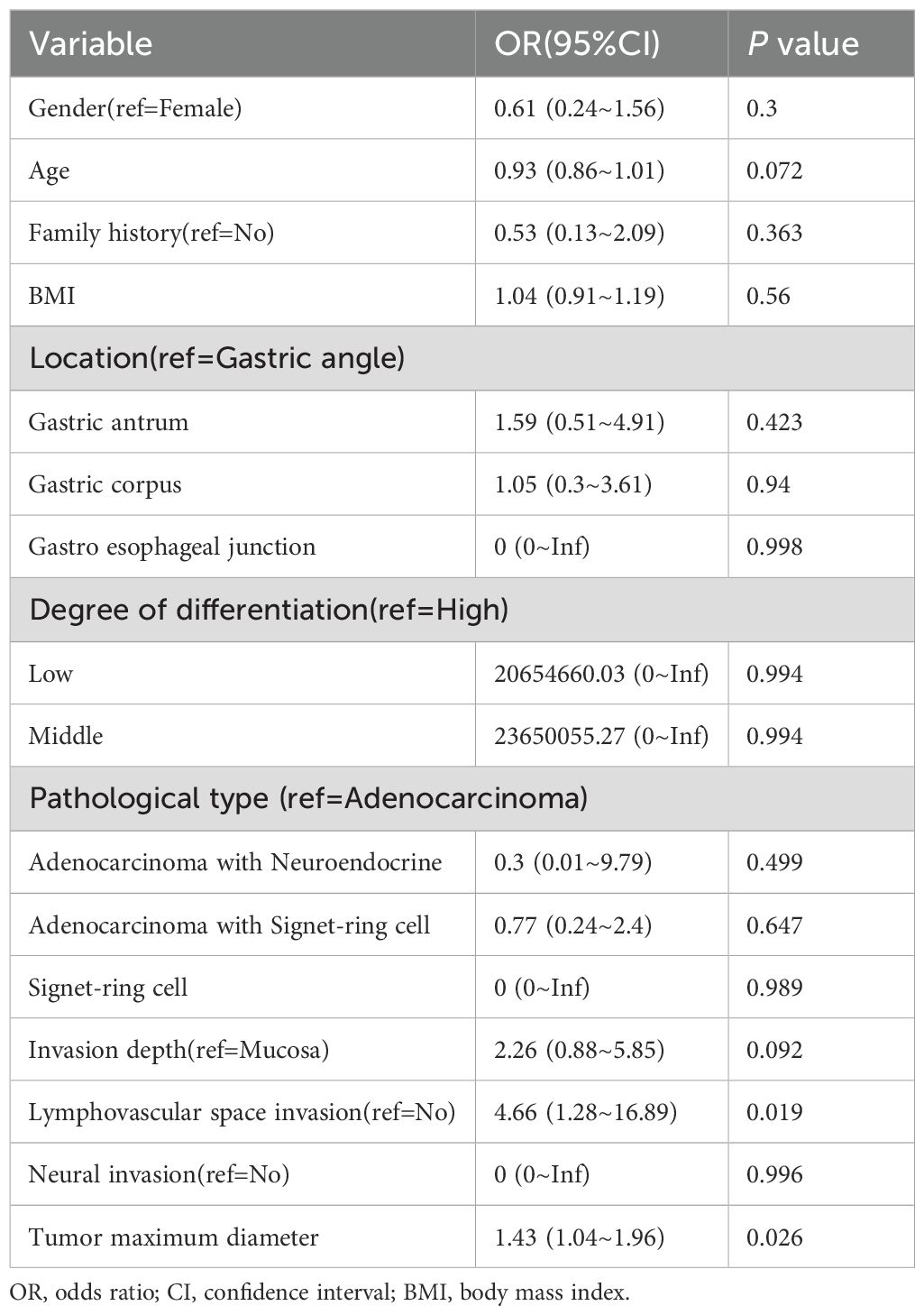
Table 3. Multivariate logistic regression analysis of independent risk factors for lymph node metastasis.
3.4 Development and validation of predictive models for LNM risk
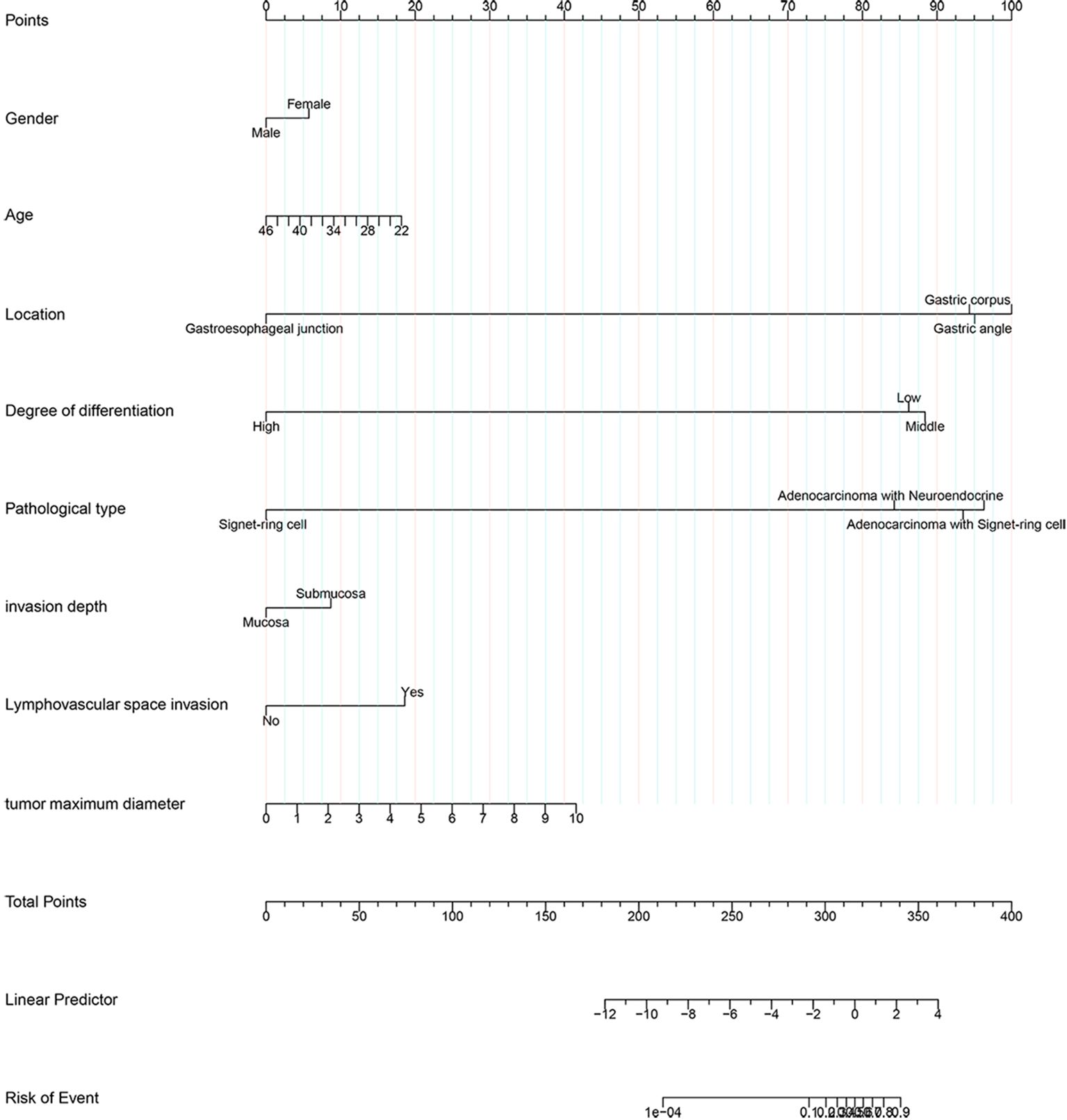
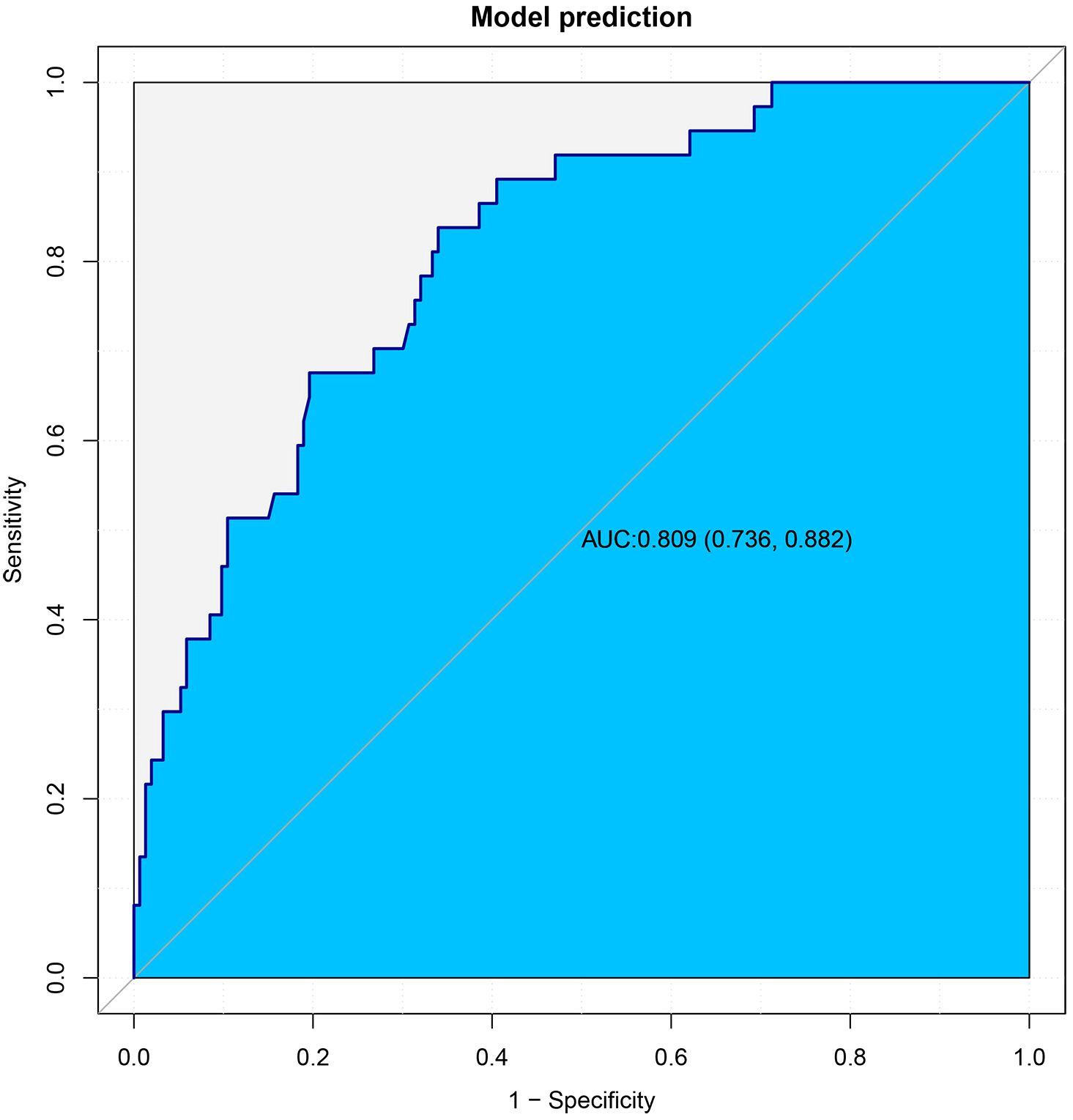
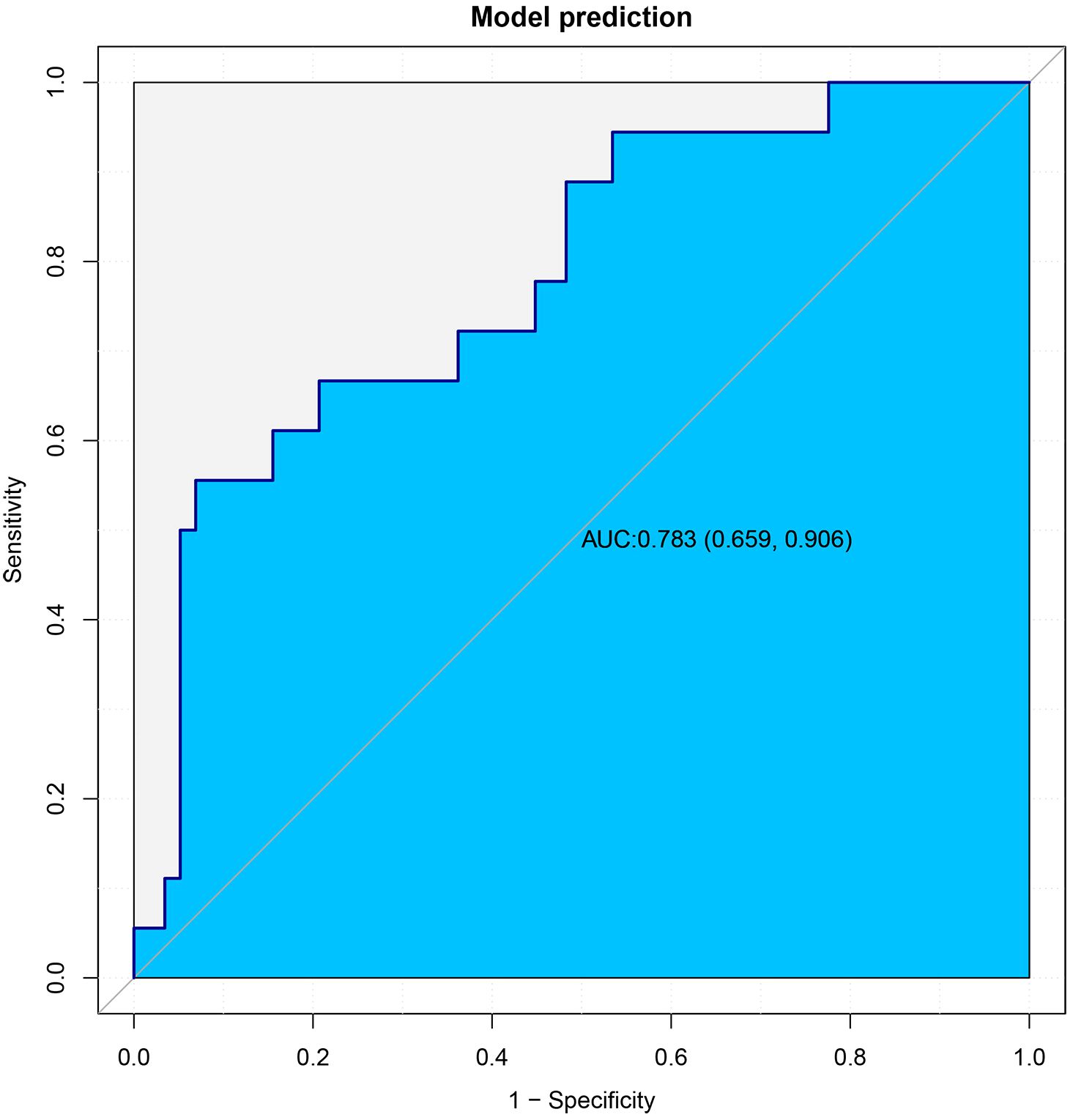
This article presented a predictive model and visualized it by constructing nomogram (Figure 3). The model incorporated clinical laboratory indicators and pathological data that can be obtained for ESD, including gender, age, tumour size, tumour location, degree of tumour differentiation, tumour pathological type, invasion depth and lymphovascular invasion. ROC subject working characteristic curves were constructed to evaluate the effectiveness of the model. The area AUC of the model was 0.809 (95%CI: 0.736-0.882), and the C index for predicting LNM was 0.739 (Figure 4). Supplementary Figure S1 showed the calibration curve for predicting LNM in EEGC patients which demonstrated a strong correlation between nomogram predictions and actual outcomes.
In the subsequent stage of the study, the 76 cases that had been retained were used as an external validation dataset, with the objective of validating the model that had been constructed. The demographic and clinicopathological data of the patients in the external validation dataset are summarized in Supplementary Table S2. The results demonstrated that the area under the curve (AUC) of the model in the external validation dataset was 0.783, which indicates that the model that was built has good discriminative ability in the external validation dataset (Figure 5).
4 Discussion
The present study included 1,765 patients with early gastric cancer. Through inflection point analysis and stratified analysis, it was determined that the definition of EOGC as <46 years was also reasonable. Furthermore, the results provided additional support for the academic definition of EOGC. In accordance with the widely accepted definition in academia, 190 patients with EOGC were included for further analysis. Notable differences in maximum tumor diameter, lymphovascular invasion and invasion depth were identified in relation to the presence of LNM in this study. Univariate analysis indicated a correlation among them, while multivariate analysis confirmed that tumor maximum diameter and lymphovascular invasion were independent risk factors for LNM in EEGC patients, consistent with previous research findings (18).
A multicenter study revealed that the incidence of LNM in patients with early gastric cancer was significantly higher among young women, particularly those with tumors located in the lower third of the stomach, greater than 2 cm in size, of the depressed type, poorly differentiated or nondifferentiated, and exhibiting lymphovascular invasion, nerve invasion, and submucosal infiltration (19). Another study demonstrated that multivariate analyses identified lymphovascular emboli, CA19–9 levels, ulceration, tumor size, tumor infiltration, and histological grade as independent risk factors for LNM (20). Additionally, factors significantly associated with LNM included age, sex, lymphatic invasion, depth of invasion, anatomical site, circumferential location, gross type, differentiation, and tumor size (21). Mixed histologic type was an independent risk factor for LNM in early gastric cancer patients (22). These findings were generally consistent with our experimental results. Discrepancies in experimental outcomes may arise from variations in the studied populations. However, there were relatively few articles exploring the factors and predictive models of LNM in patients with early-onset gastric cancer.
Our study focused on the clinical pathological characteristics and risk factors of LNM in EEGC for the first time. However, when exploring the risk factors for LNM, the correlation between differentiation degree and lymph node status appeared to be weak. Among the 37 patients with LNM from 2018 to 2022, no cases were observed in patients with upper gastric cancer. According to the absolute indications for ESD surgery (12), of the 135 patients with a tumor maximum diameter ≤ 2cm and infiltration limited to the mucosa, 20 (14.8%) exhibited LNM. The risk of LNM is relatively high compared to general early gastric cancer (14), which suggested that ESD is not suitable for EEGC (13). Studies showed that percentage of monocytes, hematocrit (HCT), and lymphocyte-monocyte ratio (LMR) might predict the metastasis of lymph node19. But our study showed that there was no difference between two groups. These findings indicated a need for further investigation into the assessment of tumour markers in patients prior to treatment, with a view to determining any potential impact. Preoperative diagnostic methods for LNM included endoscopic ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). However, the perigastric lymph nodes are situated within the perigastric omentum tissue in the abdominal cavity, increasing the difficulty and risk associated with imaging, localization, and puncture procedures. Endoscopic ultrasonography is effective for evaluating tumor size and depth of invasion prior to surgery (23). Among the 37 patients with LNM, 31 underwent preoperative enhanced CT scans, but only 5 cases indicated LNM (Supplementary Table S3). It proved that for patients with early-onset gastric cancer, enhanced CT examine may have weaker detection efficacy for LNM in early gastric cancer. Therefore, the screening of LNM remained an area deserving further investigation.
Our study highlighted that maximum tumor diameter and lymphovascular invasion were independent risk factors associated with LNM in EEGC patients. More importantly, we constructed a predictive model based on available data based on ESD to assess the risk of lymph node metastasis. The model offered a considerable clinical insight into assessing the necessity of additional surgery following ESD, providing partial data support and scientific rationale for doctor’s decision-making. While we have refined our experimental design as much as possible, our research still leaves some limitations. (1) It was a single-center retrospective analysis, which may introduce potential selection bias; (2) The considerable time span of patient enrollment could introduce variability due to advancements in diagnostic and treatment modalities for gastric cancer. Factors such as the extent of resection, the scope of intraoperative lymph node dissection, the pathological detection method, and the experience of postoperative pathologists may influence LNM detection in early gastric cancer and lead to false negatives; and (3) Some missing data were imputed using multiple imputation, which may introduce bias.
In the future, we will combine the predictive model in this article and conduct proteomic analysis on this disease to explore the biomarkers associated with LNM in patients with early-onset gastric cancer, so as to make predictions in preoperative diagnosis.
5 Conclusion
This study supports the existing academic consensus that 45 years is a reasonable threshold age for defining EEGC. Tumor maximum diameter and lymphovascular invasion were identified as significant risk factors for LNM in EEGC. Given the relatively high rate of LNM in this population, ESD might not be appropriate for many EEGC patients. The developed predictive nomogram showed potential to facilitate risk stratification and inform the necessity of additional surgery following ESD. Further large-scale multicenter studies are essential to validate these findings and to refine predictive models for better clinical application.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Chinese PLA General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the study’s retrospective design.
Author contributions
BZ: Data curation, Visualization, Writing – original draft, Writing – review & editing. MG: Data curation, Visualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Writing – review & editing. JL: Data curation, Writing – original draft. MW: Data curation, Writing – original draft. DW: Conceptualization, Methodology, Writing – review & editing. SL: Conceptualization, Methodology, Writing – review & editing. LL: Conceptualization, Methodology, Visualization, Writing – review & editing. XW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1544758/full#supplementary-material
Supplementary Figure 1 | The calibration curve for predicting LNM in EEGC patients.
References
1. Zhang C, Tang R, Zhu H, Ge X, Wang Y, Wang X, et al. Comparison of treatment strategies and survival of early-onset gastric cancer: a population-based study. Sci Rep. (2022) 12:6288.
2. Niu P, Huang H, Zhao L, Wang T, Zhang X, Wang W, et al. Clinicopathological characteristics, survival outcomes, and genetic alterations of younger patients with gastric cancer: Results from the China National Cancer Center and cBioPortal datasets. Cancer Med. (2022) 11:3057.
3. De B, Rhome R, Jairam V, Özbek U, Holcombe RF, Buckstein M, et al. Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer. (2018) 21:889–99.
4. Jiang Y, Xie J, Huang W, Chen H, Xi S, Li T, et al. Chemotherapy use and survival among young and middle-aged patients with gastric cancer. Clin Transl Gastroenterol. 2020;11(10):e00253.
5. Puhr HC, Karner A, Taghizadeh H, Jomrich G, Schoppmann SF, Preusser M, et al. Clinical characteristics and comparison of the outcome in young versus older patients with upper gastrointestinal carcinoma. J Cancer Res Clin Oncol. (2020) 146:3313.
6. Ma Z, Liu X, Paul ME, Chen M, Zheng P, Chen H. Comparative investigation of early-onset gastric cancer. Oncol Lett. (2021) 21:374.
7. Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. (2020) 69:823–9.
8. Pocurull A, Herrera-Pariente C, Carballal S, Llach J, Sánchez A, Carot L, et al. Clinical, molecular and genetic characteristics of early onset gastric cancer: analysis of a large multicenter study. Cancers (Basel). (2021) 13:3132.
9. Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Białek A, Kulig J, et al. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol. (2020) 55:62–6.
10. Rompen IF, Nienhüser H, Crnovrsanin N, Musa J, Haag GM, Longerich T, et al. Clinical characteristics and oncological outcomes of surgically treated early-onset gastric adenocarcinoma - a retrospective cohort study. J Cancer. (2023) 14:1470–8.
11. In H, Ravetch E, Langdon-Embry M, Palis B, Ajani JA, Hofstetter WL, et al. The newly proposed clinical and post-neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer. (2018) 21(1):1–9. doi: 10.1007/978-1-4757-3656-4
12. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25.
13. Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. (2009) 12:148–52.
14. Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. (2000) 3:219–25.
15. Choi JH, Kim ES, Lee YJ, Cho KB, Park KS, Jang BK, et al. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointestinal Endosc. (2015) 82:299–307.
16. Niu P, Huang H, Zhao L, Wang T, Zhang X, Wang W, et al. Clinicopathological characteristics, survival outcomes, and genetic alterations of younger patients with gastric cancer: Results from the China National Cancer Center and cBioPortal datasets. Cancer Med. (2022) 11:3057–73.
17. Qu X, Zhao X, Liu Y, Wang N, Zhang L, Zhu X, et al. The clinicopathological characteristics of early-onset gastric cancer and its evolutionary trends: a retrospective study. Am J Cancer Res. (2022) 12:2757–69.
18. Tian H, Ning Z, Zong Z, Liu J, Hu C, Ying H, et al. Application of machine learning algorithms to predict lymph node metastasis in early gastric cancer. Front Med (Lausanne). (2021) 8:759013.
19. Zhu H, Wang G, Zheng J, Zhu H, Huang J, Luo E, et al. Preoperative prediction for lymph node metastasis in early gastric cancer by interpretable machine learning models: A multicenter study. Surgery. (2022) 171:1543–51.
20. Zhang M, Ding C, Xu L, Feng S, Ling Y, Guo J, et al. A nomogram to predict risk of lymph node metastasis in early gastric cancer. Sci Rep. (2021) 11:22873.
21. Kim SM, Lee H, Min BH, Kim JJ, An JY, Choi MG, et al. A prediction model for lymph node metastasis in early-stage gastric cancer: Toward tailored lymphadenectomy. J Surg Oncol. (2019) 120:670–5.
22. Zhao B, Huang R, Lu H, Mei D, Bao S, Xu H, et al. Risk of lymph node metastasis and prognostic outcome in early gastric cancer patients with mixed histologic type. Curr Probl Cancer. (2020) 44:100579.
Keywords: early onset gastric cancer, early onset early stage gastric cancer, lymph node metastasis, nomogram, endoscopic submucosal dissection
Citation: Zhao B, Gu M, Wang Z, Li J, Wen M, Wu D, Li S, Liu L and Wang X (2025) Risk factors and nomogram development for lymph node metastasis in early-onset early-stage gastric cancer: a retrospective cohort study. Front. Oncol. 15:1544758. doi: 10.3389/fonc.2025.1544758
Received: 13 December 2024; Accepted: 07 April 2025;
Published: 30 April 2025.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaReviewed by:
Michela Giulii Capponi, Santo Spirito in Sassia Hospital, ItalyMeng Zhuo, Shanghai Jiao Tong University, China
Copyright © 2025 Zhao, Gu, Wang, Li, Wen, Wu, Li, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Wang, d2FuZ3h4MzAxQDE2My5jb20=; MzAxd3h4QHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Binghe Zhao
Binghe Zhao Mingyu Gu
Mingyu Gu Zijian Wang1†
Zijian Wang1† Di Wu
Di Wu Shuo Li
Shuo Li Lu Liu
Lu Liu Xinxin Wang
Xinxin Wang