- 1Department of Hematology, Huzhou Transportation Hospital, Huzhou, Zhejiang, China
- 2Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 3Clinical Laboratory, The Third People’s Hospital of Deqing, Deqing Hospital of Hangzhou Normal University, Huzhou, Zhejiang, China
Background: Prognostic nutritional index (PNI) has been extensively investigated for its effect on forecasting multiple myeloma (MM) survival; however, the conclusions are conflicting. This meta-analysis identified an accurate MM prognosis forecasting role for the PNI.
Methods: We systematically searched PubMed, Web of Science, Embase, Cochrane Library, and CNKI databases until July 2, 2025, and evaluated the overall survival (OS) and progression-free survival (PFS) forecasting ability of the PNI by determining pooled hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: This study included seven articles involving 1120 participants. From the pooled findings, a lower PNI exhibited a remarkable correlation with unfavorable OS (HR = 2.62, 95% CI = 1.76–3.89) and shorter PFS (HR = 1.52, 95% CI = 1.23–1.89, p<0.001) of MM. Additionally, lower PNI was significantly associated with ISS stage III (odds ratio [OR]=1.80, 95% CI = 1.19–2.73, p=0.005). However, PNI did not have a marked correlation with sex (OR = 1.02, 95% CI = 0.71–1.47, p=0.900), age (OR = 1.1, 95% CI = 0.70–1.93, p=0.558), and lactate dehydrogenase (OR = 0.98, 95% CI = 0.57–1.69, p=0.955) in MM. The meta-analysis had some limitations, such as retrospective design, small sample size, and inconsistent cut-off values of PNI.
Conclusion: Collectively, the present work including 1120 patients showed the relationship between a lower PNI and unfavorable MM OS and PFS. Furthermore, a lower PNI was significantly associated with an advanced ISS stage of MM. The PNI can be a creditable and cost-effective factor for forecasting MM prognosis.
Introduction
Multiple myeloma (MM) accounts for the bone marrow plasma cell cancer, occupying approximately 10% of hematologic malignancies (1). It is the second most prevalent blood cancer in adults, with an average age at diagnosis of 65 years (2). The incidence rate of MM is 1% among all tumors, affecting 4.5 – 6 individuals per 100,000 population (3). MM develops slowly and lacks clear symptoms initially, making it prone to misdiagnosis in clinical settings (4). Despite improvements in treatment, many patients with MM experience relapse and develop resistance to standard therapies, resulting in the disease being mostly incurable (5, 6). Identifying high-risk factors using risk stratification among MM cases can significantly help in planning treatment and assessing patient prognostic outcomes (7). Consequently, identification of novel and reliable markers for MM prognosis is important.
Current research highlights that nutritional status, which is subject to change, is a crucial element in cancer prognosis (8). Prognostic models based on data from physical examinations and laboratory results have been established to predict tumor progression and patient prognosis. The prognostic nutritional index (PNI) can comprehensively determine nutrition and immunity and was initially created to assess preoperative nutritional and immune conditions in patients with gastrointestinal cancer (9). PNI is computed as follows; PNI = 10 × serum albumin (ALB) level (g/dL) + 0.005 × lymphocyte quantity (number/mm³) (9). A low PNI is widely suggested to be of significant prognostic significance for different cancers, such as osteosarcoma (10), prostate cancer (11), renal cell carcinoma (12), lung cancer (13), and hepatocellular carcinoma (14). Previously, the prognostic value of PNI for MM has been analyzed; however, the findings are inconsistent (15–21). PNI has been shown to significantly predict MM prognosis (16–19). However, other studies have suggested no obvious association between them (15, 20). Therefore, we performed this meta-analysis to determine the precise prognostic effect of the PNI on MM. The correlations between PNI and MM clinicopathological features were investigated in this meta-analysis.
Materials and methods
Study guideline
This work was completed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (22).
Ethics statement
Experiments on humans or animals were not performed in this study, which waived ethical approval.
Literature search
We systemically searched PubMed, Web of Science, Embase, Cochrane Library, and CNKI until July 2, 2025, using keywords, such as prognostic nutritional index (PNI) and multiple myeloma or myeloma. No language restrictions were applied. References were manually retrieved to identify any literature that might have been missed in the initial search.
Study selection
The following studies were enrolled; (1) MM diagnosis based on pathology, (2) patients with MM were treated by any methods in adherence with the treatment guidelines, (3) PNI was derived before treatment, based on ALB level and lymphocyte counts. And ALB and lymphocyte counts were calculated at one time, (4) studies investigating the relationship between PNI and MM survival, (5) obtainable or computable hazard ratios (HRs) with 95% confidence intervals (CIs), (6) available PNI threshold, and (7) unrestricted language. Moreover, these studies excluded (1) reviews, case reports, meeting abstracts, letters, and comments, (2) studies that enrolled duplicate patients, and (3) animal studies.
Data acquisition and quality evaluation
Two researchers (Y.N. and Z.Z.) acquired the data from these studies. Disputes between them were settled by a third researcher (X.T.). We obtained information on first author’s name, country, year, sample size, sex, study period, age, study design, study center, International Staging System (ISS) stage, treatment, threshold PNI, threshold determination method, survival outcomes, survival analysis, follow-up, HRs, and 95% CIs. OS and PFS was considered the primary and secondary outcomes, respectively. We used the Newcastle–Ottawa Quality Assessment Scale (NOS) to assess the enrolled study quality (23), and studies with 7 – 9 points were of high quality.
Statistical analysis
We computed HRs with 95% CIs to assess the MM OS and PFS forecasting abilities of the PNI. Among-study heterogeneity was assessed using Cochran’s test and I2 statistics. I2 > 50% or p < 0.10 (Q-test) suggested distinct heterogeneity; therefore, a random-effects model must be used; otherwise, a fixed-effects model would be adopted. Subgroup analyses were conducted to investigate the prognostic value of the PNI in patient groups. The relationships between PNI and MM clinicopathological characteristics were explored using combined ORs with 95% CIs. Stability and creditability were assessed through sensitivity analysis, which involved eliminating every individual article and recalculating the HRs and 95% CIs. Possible publication bias was evaluated using funnel plot, Begg’s test, and Egger’s test. We employed Stata 12.0 software (Stata Corp., College Station, Texas, USA) for statistical analyses. Statistical significance was set at p<0.05.
Results
Literature screening
Ninety-seven studies were obtained from the primary search, of which 75 were retained after removing duplicates (Figure 1). We removed 56 articles after reading the title and abstract owing to irrelevance or animal studies. We assessed 19 articles through full-text reading, of which 12 were excluded because they were not on PNI (n=9), survival information was unavailable (n=2), and repeated participants were enrolled (n=1). Therefore, we enrolled seven studies with 1120 patients (15–21) in the meta-analysis (Figure 1).
Enrolled article features
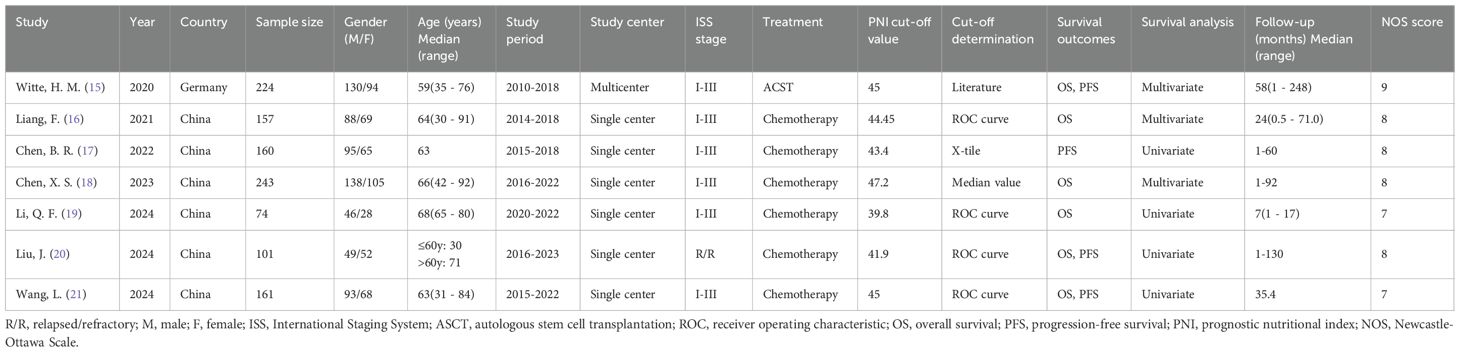
The retrospective fundamental article features (15–21) published between 2020 and 2024 are shown in Table 1. Six of them were conducted in China (16–21) and one in Germany (15). The sample size was 74 – 243 (median; 160). Six studies were published in Chinese (16–21) and one in English (15). Six studies enrolled MM cases of ISS stage I–III (15–19, 21) and one recruited relapsed/refractory (R/R) patient with MM (20). One study treated patients with autologous stem cell transplantation (ASCT) (15) and six studies applied chemotherapy (16–21). The chemotherapy regimens include Bortezomib + Dexamethasone, Bortezomib + Cyclophosphamide + Dexamethasone, and VAD ± T (18, 21). All included studies recorded the PNI value before treatment. The PNI thresholds ranged from 39.8 – 47.2 (median; 44.45). Four studies determined the threshold using the receiver operating characteristic (ROC) curve (16, 19–21) and one each applied literature (15), X-tile software (17), and the median value (18) Six articles mentioned PNI significance in predicting OS (15, 16, 18–21) and four mentioned the relationship between PNI and PFS (15, 17, 20, 21) in MM. Four articles derived HRs and 95% CIs on univariate regression (17, 19–21), whereas three applied multivariate regression (15, 16, 18). Our studies had NOS scores of 7 – 9 (median; 8), suggesting a high quality (Table 1). The detailed NOS scores of each included study were shown in Supplementary File 1.
PNI and OS
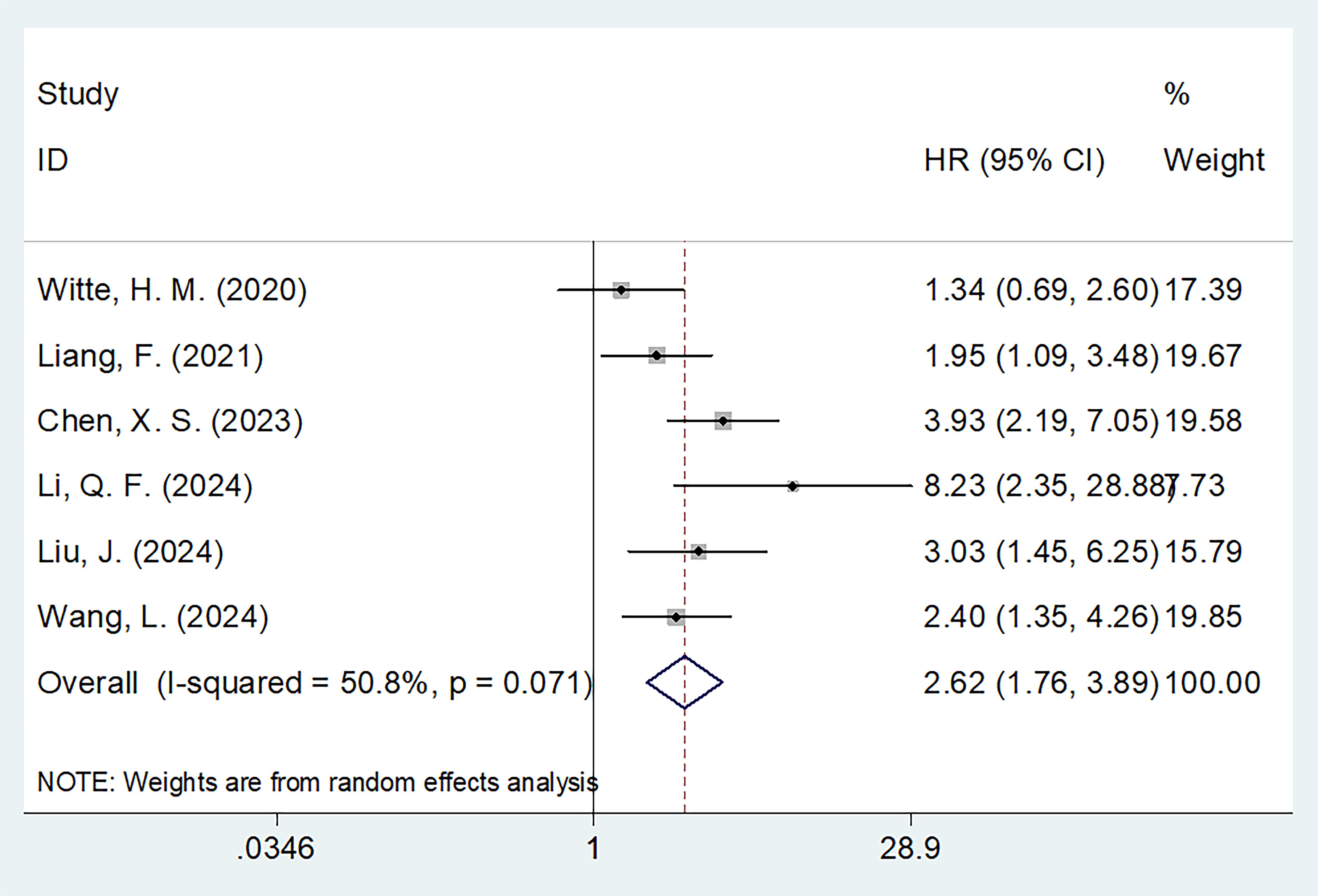
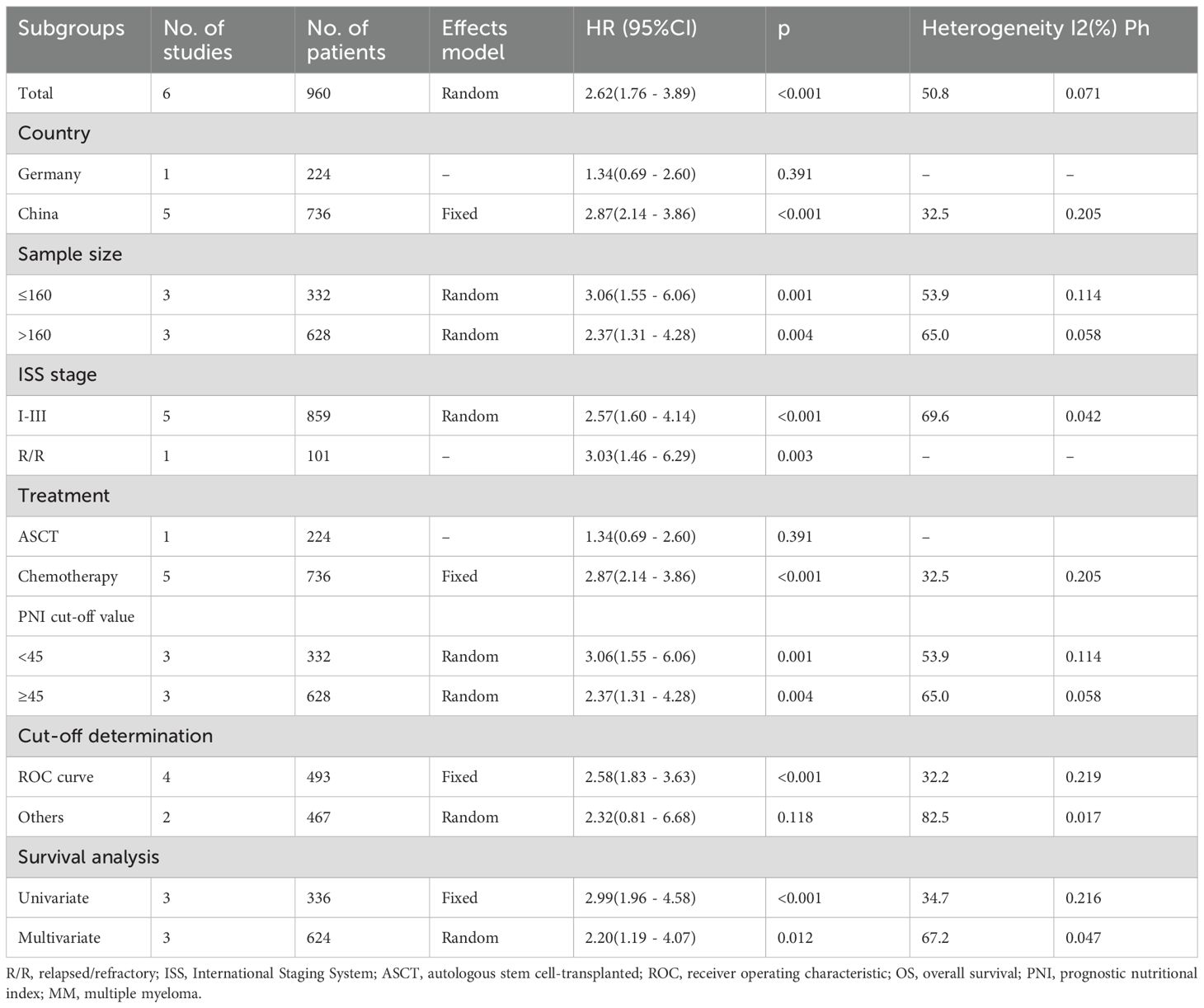
Six studies with 960 patients (15, 16, 18–21) mentioned the role of PNI in predicting OS. A random effects model was used because of obvious heterogeneity (I2 = 50.8%, p=0.071). Based on the pooled results, decreased PNI was significantly correlated with dismal OS in MM (HR = 2.62, 95% CI = 1.76–3.89, p<0.001; Figure 2; Table 2). Subgroup analyses revealed that a significant effect of PNI on forecasting OS is not influenced by sample size, ISS stage, cut-off value, or survival analysis type (Table 2). Additionally, the PNI remarkably forecast dismal OS in subgroups of studies in China, patients receiving chemotherapy, and threshold determination based on the ROC curve (p<0.05; Table 2).
PNI and PFS
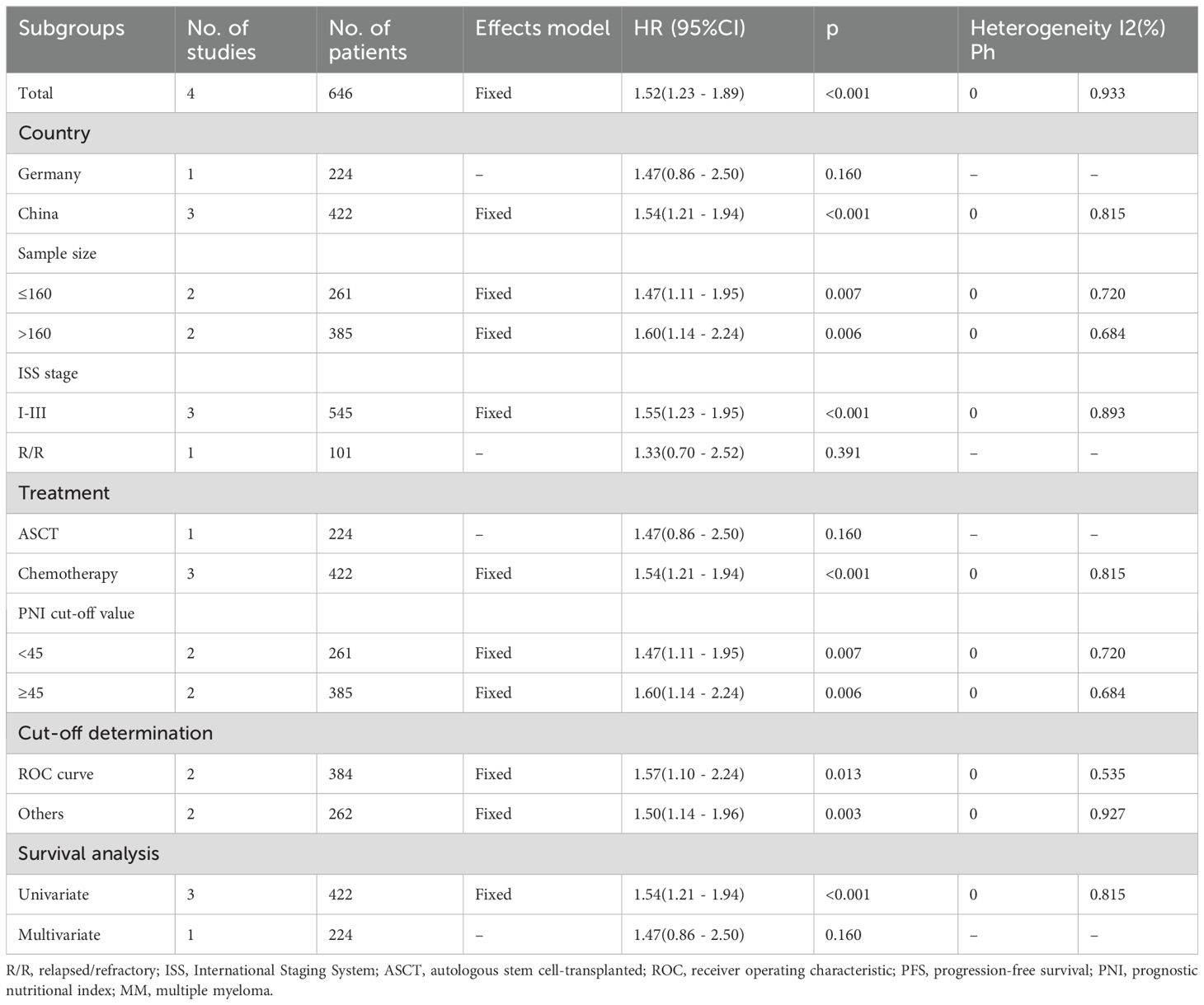
Four studies comprising 646 patients (15, 17, 20, 21) reported the relationship between PNI and PFS in MM. Because of non-significant heterogeneity (I2 = 0, p=0.933), we used the fixed-effects model. Consequently, decreased PNI markedly forecast unfavorable PFS of MM (HR = 1.52, 95% CI = 1.23-1.89, p<0.001; Figure 3; Table 3). In subgroup analyses, PNI significantly predicted PFS, despite the sample size, threshold, and threshold determination (Table 3). Additionally, decreased PNI was significantly associated with PFS in subgroups of Chinese studies (p<0.001), ISS stages I–III (p<0.001), patients treated with chemotherapy (p<0.001), and univariate survival analysis (p<0.001) (Table 3).
Correlations of PNI with MM clinicopathological features
Four studies comprising 492 patients (16, 17, 19, 20) reported connections between PNI and MM clinicopathological factors. The pooled data demonstrated that a low PNI was associated with ISS stage III (OR = 1.80, 95% CI = 1.19–2.73, p=0.005; Figure 4; Table 4). However, correlations between PNI and sex (OR = 1.02, 95% CI = 0.71–1.47, p=0.900), age (OR = 1.1, 95% CI = 0.70–1.93, p=0.558), and lactate dehydrogenase (OR = 0.98, 95% CI = 0.57–1.69, p=0.955) were not significant in MM (Figure 4; Table 4).

Figure 4. Meta-analyses of the association between PNI and clinicopathological features of MM. (A) Gender (male vs female); (B) Age (years) (>65 vs ≤65); (C) ISS stage (III vs I-II); and (D) LDH (U/L) (>220 vs ≤220).
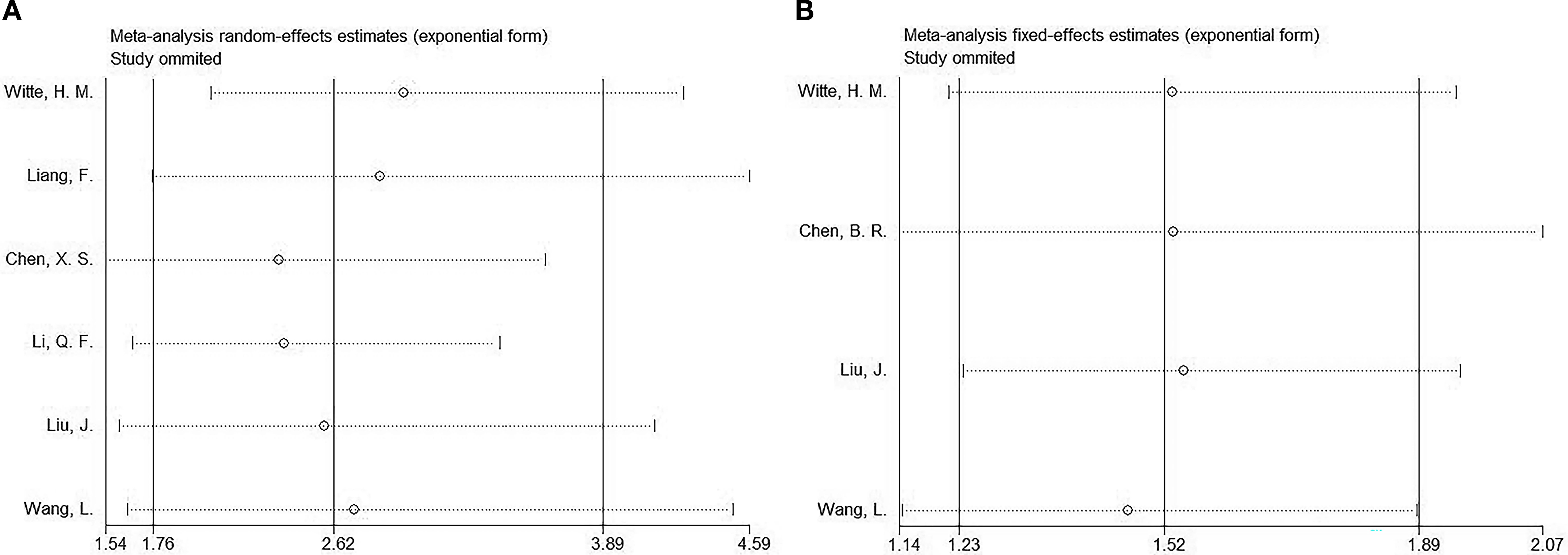
Sensitivity analysis
We performed a sensitivity analysis of the OS and PFS results to assess the effect of articles on the overall HR by removing them gradually. The combined HRs and 95% CIs showed no significant changes, indicating the robustness of the findings (Figure 5).
Publication bias
This study performed Begg’s and Egger’s tests to evaluate potential publication bias, which did not detect a publication bias for OS (p=0.452/0.311 from Begg’s/Egger’s tests) or PFS (p=0.308/0.578 from Begg’s/Egger’s tests) (Figure 6).
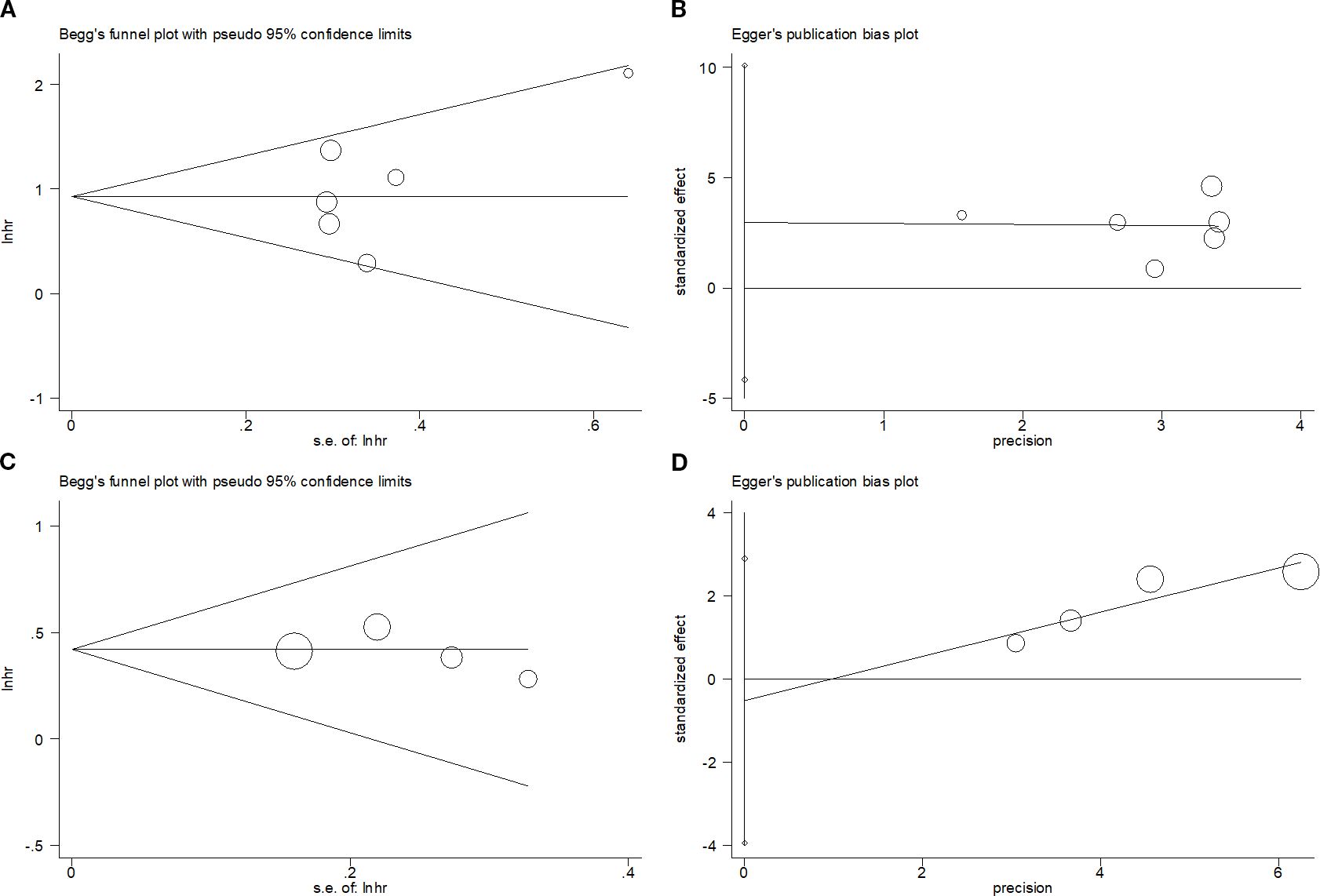
Figure 6. Publication bias test. (A) Begg’s test for OS, p=0.452; (B) Egger’s test for OS, p=0.311; (C) Begg’s test for PFS, p=0.308; and (D) Egger’s test for PFS, p=0.578.
Discussion
The role of PNI in predicting MM prognosis has been widely explored; however, the findings remain conflicting. Therefore, we aggregated data from seven studies with 1120 patients (15–21). A lower PNI was significantly related to poor OS and dismal PFS in patients with MM. The significant prognostic role of PNI for OS and PFS remained unaffected by sample size and threshold. Moreover, a lower PNI correlated with ISS stage III in MM. Based on the publication bias test and sensitivity analysis, the findings were stable. Collectively, the PNI remarkably predicts the long- and short-term prognoses of MM. The present work provides initial meta-analysis evidence for the significance of PNI in MM prognosis forecasting.
PNI includes ALB and lymphocyte count, and a low PNI can be due to these two elements. The mechanisms by which the PNI predicts MM prognosis remain unclear and are explained below. Individuals with advanced cancer frequently experience nutritional depletion, and ALB is a marker of nutritional status. Serum ALB is essential for plasma osmotic pressure and nutrition and exerts an important effect on regulating body fluid distribution and acid-based balance (24). There is a relationship between diminished ALB levels and elevated inflammation and tumor progression (25). Furthermore, albumin, which is the predominant component of plasma protein, is an essential biomarker for evaluating nutritional status and associated with the comorbidity and prognosis of various cancers (26). Cancer treatments, such as chemotherapy or radiotherapy, may cause decreased appetite, leading to less protein consumption and disrupted synthesis (27). If tissue damage and inflammation occur, they can accelerate this catabolic process, thereby reducing plasma ALB levels (28). Conversely, through cytokine-related cytotoxicity, lymphocytes aid in cellular immunity by inhibiting cancer cell growth and migration (29). The lymphocyte count serves as a basic parameter for assessing immune function, and its reduction can be attributed to malnutrition and weakened cellular immune function (30). Studies indicate that lymphocytes have an essential effect on tumor immune surveillance, limiting cancer growth and spread by causing cytotoxic death and releasing cytokines that suppress tumors (31). Several studies have shown that lymphocytopenia is associated with an impaired anti-tumor immune response, and lymphocyte levels can be used as an indicator to predict treatment outcomes in patients with cancer (32, 33). Therefore, a low PNI indicates a lack of proper nutrition and immune function in patients with cancer, which may lead to more aggressive tumor behavior.
Notably, this meta-analysis showed that a lower PNI correlated with ISS stage III in MM. As part of the ISS for MM, serum ALB has been demonstrated to be the most consistent prognostic factor across virtually all research on MM (34). Therefore, reduced PNI, which is associated with lower ALB levels, may correspond with a higher ISS stage, such as stage II or III. The subgroup analysis revealed that PNI remained a significant prognostic marker for OS and PFS in patients with MM treated with chemotherapy, but not for ASCT (Tables 2 and 3). Because only one study treated patients with ASCT. The prognostic role of PNI for patients with MM receiving ASCT should be verified in more studies.
The R-ISS (Revised International Staging System) incorporates the variables from the original ISS, such as serum beta-2 microglobulin and serum albumin, and adds prognostic data from serum LDH and high-risk chromosomal abnormalities identified through interphase fluorescent in situ hybridization (iFISH) following CD138 plasma cell purification (35). In this meta-analysis, only one study (20) included provide the information on correlation between PNI and R-ISS in MM. This study reported that there was no significant association between PNI and R-ISS in MM (p=0.893). The sample size was limited; therefore, more studies should investigate the correlation between PNI and R-ISS in the future. Moreover, ASCT is utilized in the consolidation treatment for all myeloma patients, regardless of their ISS-R stage, and most are considered candidates for this treatment.
Current evidences show that there are some nutritional indexes for cancer prognostication, such as controlling nutritional status (CONUT) score, nutritional risk index (NRI), and body mass index (BMI) (11). There are some similarities and differences between these nutritional parameters and PNI. The similarities are as follows. First, they are all indexes reflecting the nutritional status of patients. Second, they all can be applied as prognostic indicators in cancer. The differences are as follows. PNI is calculated based on two elements. Therefore, PNI is easier to be computed compared with CONUT and NRI, which are calculated according to three elements.
PNI has been widely investigated for its significance in predicting cancer prognosis by mete-analysis (36–40). Tobing et al. suggested in their meta-analysis of 2,229 cases that a low PNI predicted shorter OS and PFS in prostate cancer (36). Zhang et al. suggested a significant relationship between a lower PNI and poorer OS and PFS among patients with endometrial cancer from their meta-analysis of 10 articles (37). In a meta-analysis comprising 23,756 cases, a low PNI was significantly associated with shorter OS, (RFS, and cancer-specific survival (CSS) in patients with gastric cancer undergoing gastrectomy (38). Based on a recent meta-analysis of 3,130 cases, low pretreatment PNI predicted unfavorable OS and disease-free survival (DFS) for oral cancer (39). Xue et al. suggested from a meta-analysis containing 15 articles that a lower pretreatment PNI level predicted unfavorable OS, PFS, CSS, and DFS in patients with renal cell carcinoma (40).
This study has certain limitations. First, most of the enrolled articles were from China; therefore, our findings are suitable for Chinese MM cases. No regional or language restrictions were applied in the literature search and infiltration. Second, the sample size is relatively small. This study enrolled only 1,120 cases. Third, the thresholds were non-uniform among the eligible articles. Fourth, the absence of information on the induction regimen, cycle count, dosage schedule, and post-induction treatments, such as transplant or maintenance therapy, complicates the acceptance of these results. Fifth, only one eligible study used ASCT. ASCT is currently the standard consolidation treatment for every myeloma patient following successful induction, typically after 4 cycles, and can be offered to patients up to 70 years old or even beyond. Six studies did not use ASCT in this meta-analysis. Moreover, it would be useful to analyze larger databases, such as SEER. Therefore, large-scale, cross-regional prospective investigations are necessary to verify the findings of this meta-analysis.
Conclusion
In summary, a lower PNI evidently predicts unfavorable OS and PFS among MM cases and is significantly correlated with an advanced ISS stage of MM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. ZZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. XT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1545096/full#supplementary-material
Abbreviations
PNI, prognostic nutritional index; MM, multiple myeloma; HR,hazard ratio; CI,confidence interval; OS, overall survival; PFS, progression-free survival; LDH, lactate dehydrogenase; ALB, albumin; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ISS, International Staging System; NOS, Newcastle-Ottawa Quality Assessment Scale; OR,odds ratio; R/R, relapsed/refractory; ROC, receiver operating characteristic; CSS, cancer-specific survival; RFS, recurrence-free survival; CSS, cancer-specific survival; DFS, disease-free survival; SEER, Surveillance, Epidemiology, and End Results.
References
1. Garfall AL. New biological therapies for multiple myeloma. Annu Rev Med. (2024) 75:13–29. doi: 10.1146/annurev-med-050522-033815
2. Monteith BE, Sandhu I, and Lee AS. Management of multiple myeloma: A review for general practitioners in oncology. Curr Oncol (Toronto Ont). (2023) 30:4382–401. doi: 10.3390/curroncol30050334
3. Wang P and Jin SY. Meta-analysis of the efficacy and safety of daratumumab in the treatment of multiple myeloma. World J Clin cases. (2023) 11:7091–100. doi: 10.12998/wjcc.v11.i29.7091
4. van de Donk N, Pawlyn C, and Yong KL. Multiple myeloma. Lancet. (2021) 397:410–27. doi: 10.1016/s0140-6736(21)00135-5
5. Leong S, Lam HPJ, Kirkham Z, and Popat R. Antibody drug conjugates for the treatment of multiple myeloma. Am J Hematol. (2023) 98 Suppl 2:S22–s34. doi: 10.1002/ajh.26750
6. Elbezanti WO, Challagundla KB, Jonnalagadda SC, Budak-Alpdogan T, and Pandey MK. Past, present, and a glance into the future of multiple myeloma treatment. Pharm (Basel). (2023) 16(3):415. doi: 10.3390/ph16030415
7. Bryant A and Quach H. Biomarker-directed therapy in multiple myeloma. Curr Opin Oncol. (2024) 36:600–9. doi: 10.1097/cco.0000000000001091
8. Thrastardottir TO, Copeland VJ, and Constantinou C. The association between nutrition, obesity, inflammation, and endometrial cancer: A scoping review. Curr Nutr Rep. (2023) 12:98–121. doi: 10.1007/s13668-022-00447-8
9. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, and Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
10. Jiang J, Zhao T, Yao L, Zhang T, Ji L, Zhang W, et al. Preoperative prognostic nutritional index and systemic immune inflammation index for predicting the efficacy and survival time of patients with osteosarcoma undergoing neoadjuvant chemotherapy combined with surgery. Am J Cancer Res. (2024) 14:4946–55. doi: 10.62347/mhxs8480
11. Li SY, Wan LL, Liu YF, Li YW, Huang X, and Liu RJ. Prognostic value of three clinical nutrition scoring system (NRI, PNI, and CONUT) in elderly patients with prostate cancer. Front Nutr. (2024) 11:1436063. doi: 10.3389/fnut.2024.1436063
12. Ma W, Liu W, Dong Y, Zhang J, Hao L, Xia T, et al. Predicting the prognosis of patients with renal cell carcinoma based on the systemic immune inflammation index and prognostic nutritional index. Sci Rep. (2024) 14:25045. doi: 10.1038/s41598-024-76519-2
13. Mi HL, Wei WL, Zhang DH, Liang HY, Yue CF, and Xu JN. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and prognostic nutritional index as prognostic markers for lung carcinoma. Br J Hosp Med (Lond). (2024) 85:1–13. doi: 10.12968/hmed.2024.0270
14. Zhang N, Lin K, Qiao B, Yan L, Jin D, Yang D, et al. Machine learning model based on prognostic nutritional index for predicting long-term outcomes in patients with HCC undergoing ablation. Cancer Med. (2024) 13:e70344. doi: 10.1002/cam4.70344
15. Witte HM, Bonorden B, Riecke A, Biersack H, Steinestel K, Merz H, et al. The glasgow prognostic score at diagnosis is a predictor of clinical outcome in patients with multiple myeloma undergoing autologous. Cancers. (2020) 12:921. doi: 10.3390/cancers12040921
16. Liang F, Dong XY, Tang GF, Qi KM, Chen W, Sang W, et al. Influence of prognostic nutritional index and controlling nutritional status on the prognosis of patients with multiple myeloma. Chin J Hematol. (2021) 42:332–7. doi: 10.3760/cma.j.issn.0253-2727.2021.04.011
17. Chen BR, Shu WX, and Le J. The relationship between prognostic nutritional index and clinical pathological characteristics and progression free survival in patients with newly diagnosed symptomatic multiple myeloma. Modern Pract Med. (2022) 34:730–3. doi: 10.3969/j.issn.1671-0800.2022.06.011
18. Chen XS, Gao ZJ, Gao PK, Xing R, Han XL, and Li JD. Relationship between LMR, PNI, and chemotherapy response and prognosis in patients with multiple myeloma. Chin J Gen Pract. (2023) 21:2022–6. doi: 10.16766/j.cnki.issn.1674-4152.003281
19. Li QF, Zhang QK, Wei XF, Feng YF, Fu Y, Zhao YY, et al. Effects of different nutritional scoring systems on prognosis of elderly patients with multiple myeloma. J Exp Hematol. (2024) 32:499–504. doi: 10.19746/j.cnki.issn.1009-2137.2024.02.027
20. Liu J, Chen ML, Huang LP, Li YQ, Yin W, Lin XL, et al. Significance of prognostic nutritional index in patients with multiple myeloma in first relapse. Med J West China. (2024) 36:1486–90.
21. Wang L, Li N, Wang X, Liu X, Zhu F, Li ZY, et al. The role of inflammatory-nutritional indicators in predicting the prognosis and early mortality of newly diagnosed multiple myeloma patients. J Clin Hematol. (2024) 37:649–56. doi: 10.13201/j.issn.1004-2806.2024.09.010
22. Moher D, Liberati A, Tetzlaff J, and Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Zeeshan F, Madheswaran T, Panneerselvam J, Taliyan R, and Kesharwani P. Human serum albumin as multifunctional nanocarrier for cancer therapy. J Pharm Sci. (2021) 110:3111–7. doi: 10.1016/j.xphs.2021.05.001
25. Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, and Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. (2015) 36:243–8. doi: 10.1093/carcin/bgu247
26. Xiang M, Zhang H, Tian J, Yuan Y, Xu Z, and Chen J. Low serum albumin levels and high neutrophil counts are predictive of a poorer prognosis in patients with metastatic breast cancer. Oncol Lett. (2022) 24:432. doi: 10.3892/ol.2022.13552
27. Sarett SM, Werfel TA, Lee L, Jackson MA, Kilchrist KV, Brantley-Sieders D, et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci U.S.A. (2017) 114:E6490–e7. doi: 10.1073/pnas.1621240114
28. Setrerrahmane S and Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. (2017) 16:153. doi: 10.1186/s12943-017-0721-9
29. Fan Z and Zhang Q. Molecular mechanisms of lymphocyte-mediated cytotoxicity. Cell Mol Immunol. (2005) 2:259–64.
30. Grisaru-Tal S, Dulberg S, Beck L, Zhang C, Itan M, Hediyeh-Zadeh S, et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. (2021) 81:5555–71. doi: 10.1158/0008-5472.Can-21-0839
31. Dunn GP, Old LJ, and Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
32. Yu P and Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Investig J Tech Methods Pathol. (2006) 86:231–45. doi: 10.1038/labinvest.3700389
33. Kinoshita T, Muramatsu R, Fujita T, Nagumo H, Sakurai T, Noji S, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. (2016) 27:2117–23. doi: 10.1093/annonc/mdw319
34. Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol. (2005) 23:3412–20. doi: 10.1200/jco.2005.04.242
35. Zhong L, Hao P, Zhang Q, Jiang T, Li H, Xiao J, et al. Revised International Staging System (R-ISS) stage-dependent analysis uncovers oncogenes and potential immunotherapeutic targets in multiple myeloma (MM). Elife. (2022) 11e75340. doi: 10.7554/eLife.75340
36. Tobing E, Tansol C, Tania C, and Sihombing AT. Prognostic nutritional index (PNI) as independent predictor of poor survival in prostate cancer: A systematic review and meta-analysis. Clin Genitourin Cancer. (2024) 22:102142. doi: 10.1016/j.clgc.2024.102142
37. Zhang L, Wang F, Wan C, Tang J, and Qin J. Prognostic nutritional index and the survival of patients with endometrial cancer: A meta-analysis. Reprod Sci. (2024) 31:3779–94. doi: 10.1007/s43032-024-01686-6
38. Deng H, He Y, Huang G, Huang Y, Wu J, and Qin X. Predictive value of prognostic nutritional index in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. Med (Baltimore). (2024) 103:e39917. doi: 10.1097/md.0000000000039917
39. Dai M and Sun Q. Prognostic and clinicopathological significance of prognostic nutritional index (PNI) in patients with oral cancer: a meta-analysis. Aging. (2023) 15:1615–27. doi: 10.18632/aging.204576
Keywords: prognostic nutritional index, multiple myeloma, meta-analysis, prognosis, biomarker
Citation: Nie Y, Zhang Z and Tang X (2025) Prognostic and clinicopathological value of prognostic nutritional index in patients with multiple myeloma: a meta-analysis. Front. Oncol. 15:1545096. doi: 10.3389/fonc.2025.1545096
Received: 14 December 2024; Accepted: 01 September 2025;
Published: 19 September 2025.
Edited by:
Massimo Gentile, University of Calabria, ItalyReviewed by:
Anna Sicuranza, University of Siena, ItalyEnrica Antonia Martino, Cosenza Hospital, Italy
Copyright © 2025 Nie, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohuan Tang, dHR4aW5zaGkxMjNAMTYzLmNvbQ==
 Yanchen Nie1
Yanchen Nie1 Xiaohuan Tang
Xiaohuan Tang