- 1Neurology Unit, Department of Medicine, College of Medicine, King Saud University, King Saud University Medical City, Riyadh, Saudi Arabia
- 2Department of Otolaryngology-Head & Neck, King Faisal Specialist Hospital and Research Centre, College of medicine, Alfaisal University, Riyadh, Saudi Arabia
- 3Department of Otorhinolaryngology, Imam Abdulrahman Bin Faisal University Hospital, Dammam, Saudi Arabia
- 4Department of Medicine and Surgery, College of Medicine, Imam Muhammad Ibn Saud Islamic University, Riyadh, Saudi Arabia
Background and objectives: Cranial nerve palsies occur in approximately 20% of nasopharyngeal carcinoma (NPC) cases, often correlating with tumor location and cranial extension. This report describes a rare case involving ten unilateral cranial nerves.
Methods: A 55-year-old female presented with right-sided cranial nerve palsies (II, III, IV, V, VI, VII, IX, X, XI, and XII). Imaging showed a locally invasive nasopharyngeal mass with anterior, posterior, and intracranial extension but without distant metastasis. Biopsy confirmed a poorly differentiated, non-keratinizing squamous cell carcinoma, staged as IV/A T4-N2-M0. Treatment involved concurrent chemoradiotherapy and multidisciplinary care.
Results: Six months post-treatment, there was complete recovery in cranial nerves XI and XII and near-complete recovery in nerves III, IV, and VI. Cranial nerves II, V, VII, IX, and X showed no improvement at interim follow-up.
Discussion: This case highlights an uncommon presentation of NPC with extensive cranial nerve involvement and no distant metastasis. Partial recovery of cranial nerve function following chemoradiotherapy emphasize the potential for neurological improvement in advanced NPC with comprehensive, multidisciplinary care.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy with a reported incidence of 1 in 100,000 and is two to three times more prevalent in males than in females (1). It commonly arises from the fossa of Rosenmüller. Owing to this anatomical location and the tumor’s ability to infiltrate, patients may exhibit a diverse array of symptoms, such as neck masses, nasal and visual disturbances, headaches, and cranial nerve (CN) impairments (2). The involvement of CN can occur in the first presentation of the disease; later in the disease progression, indicating a poor prognosis; or as an adverse effect resulting from radiation and chemotherapy treatments (3).
Case presentation
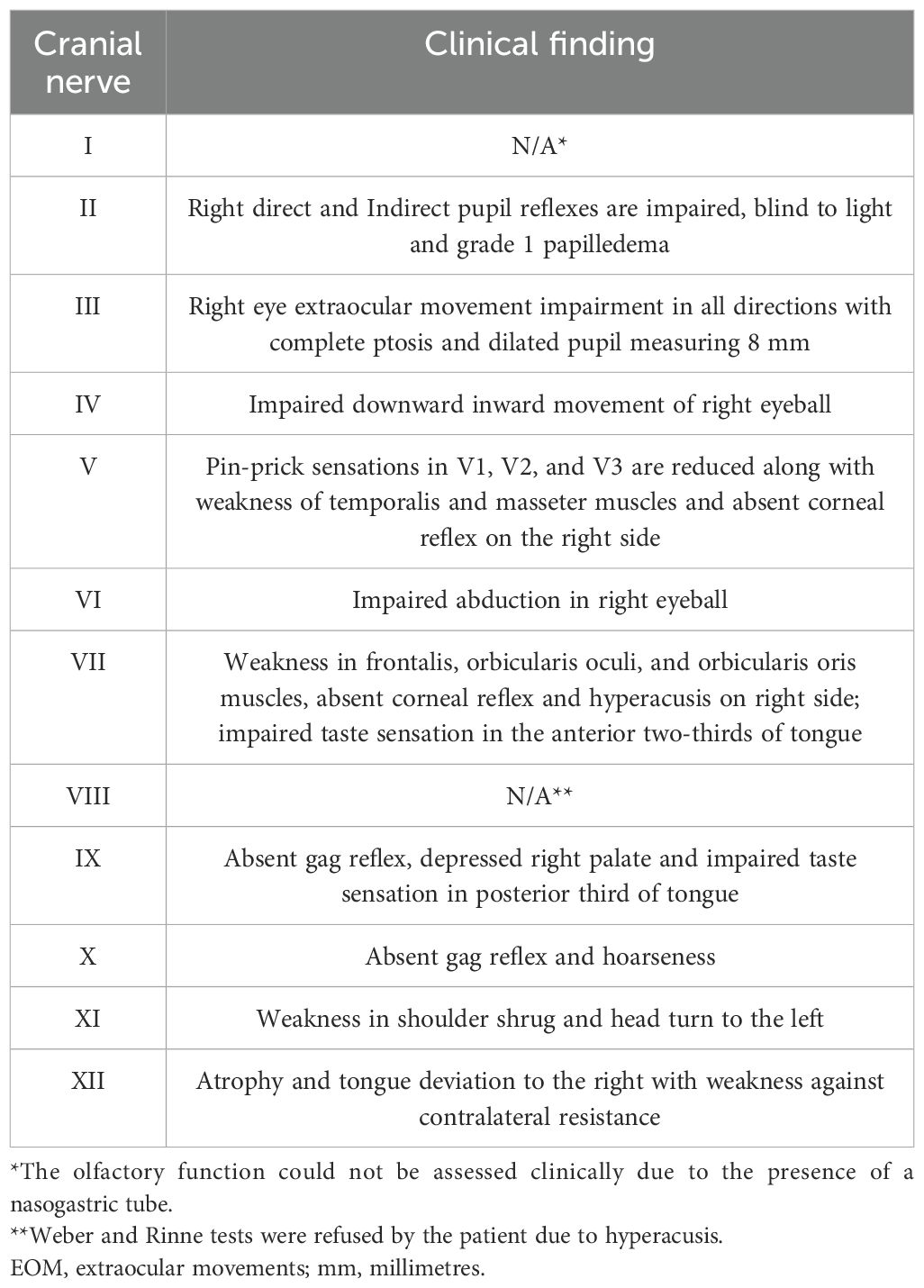
A 55-year-old female patient, previously healthy, was referred to us due to the rapid progression of a constellation of clinical symptoms that had occurred over 2 months. The sequence of symptoms was as follows: throbbing right facial pain for 2 days that was exacerbated by chewing, followed by right facial swelling, right eyelid ptosis, and right-side mouth droop for 1 week, as well as by progressive dysphonia, dysphagia affecting her oral intake, progressive blurriness of vision in the right eye, and right ear hyperacusis for 6 weeks. Negative family history for tumors. Neurological examination revealed the patient to be alert and oriented with intact language and memory. Multiple CN exams were abnormal (see Table 1, Figure 1). The rest of the neurological examination, including motor, sensory, coordination, and gait, was normal.
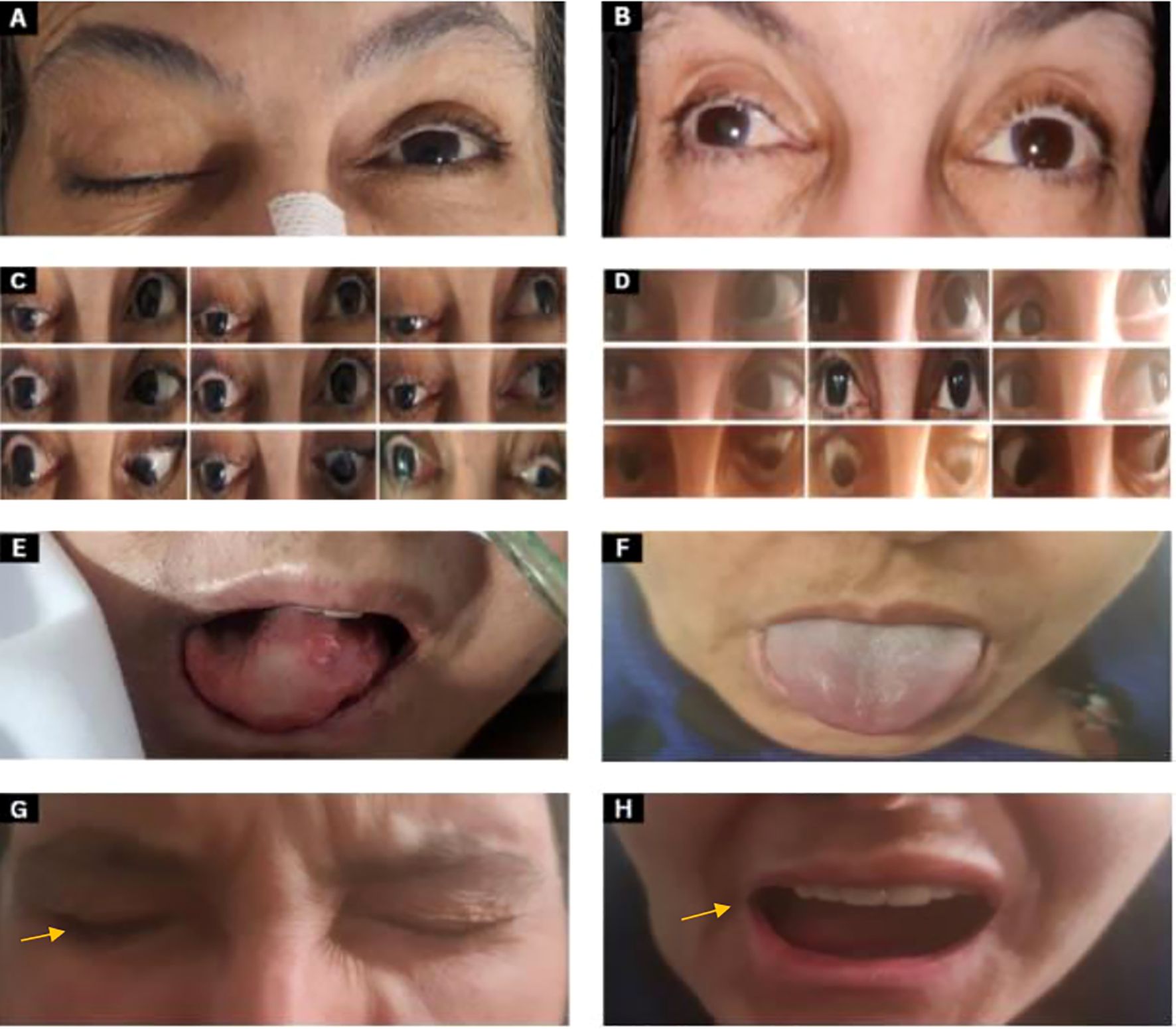
Figure 1. Clinical images pre and post-CRT (A) CN III examination shows complete ptosis of the right eye. (B) Anisocoria: the right eye pupil size is 5 mm while the left eye pupil size is 2 mm 6 months post-chemoradiation. (C) Two months after the onset of symptoms, extraocular movements show a frozen right eye (deficit of extraocular movements in all directions when asked to follow these arrows directions  ) with ptosis (eyes look towards the arrows). (D) Six months post-chemoradiation showed improvement of extraocular movements in all directions in the right eye and improved ptosis. (E) CN XII examination: tongue deviation to the right side with atrophy at the time of presentation. (F) The tongue shows improvement after 6 months post-chemoradiation. (G, H) CN VII examination shows right orbicularis oculi weakness during eye closure with incomplete burying of eyelashes and drooping of the right angle of the mouth as indicated by the arrow.
) with ptosis (eyes look towards the arrows). (D) Six months post-chemoradiation showed improvement of extraocular movements in all directions in the right eye and improved ptosis. (E) CN XII examination: tongue deviation to the right side with atrophy at the time of presentation. (F) The tongue shows improvement after 6 months post-chemoradiation. (G, H) CN VII examination shows right orbicularis oculi weakness during eye closure with incomplete burying of eyelashes and drooping of the right angle of the mouth as indicated by the arrow.
Radiological assessment by MRI of the nasopharynx revealed a nasopharyngeal mass with extensive involvement primarily on the right side, extending into the parapharyngeal region and central skull base with the destruction of the clivus, occipital condyle, and right petrous apex. The bilateral sphenoid sinuses, pterygopalatine fossa, and pterygoid plates are infiltrated with extension into the right orbital apex. Intracranial extension was present, with invasion of the cavernous sinus and right Meckel’s cave, along with extension into the right temporal fossa, accompanied by vasogenic edema in the right temporal lobe (Figure 2).
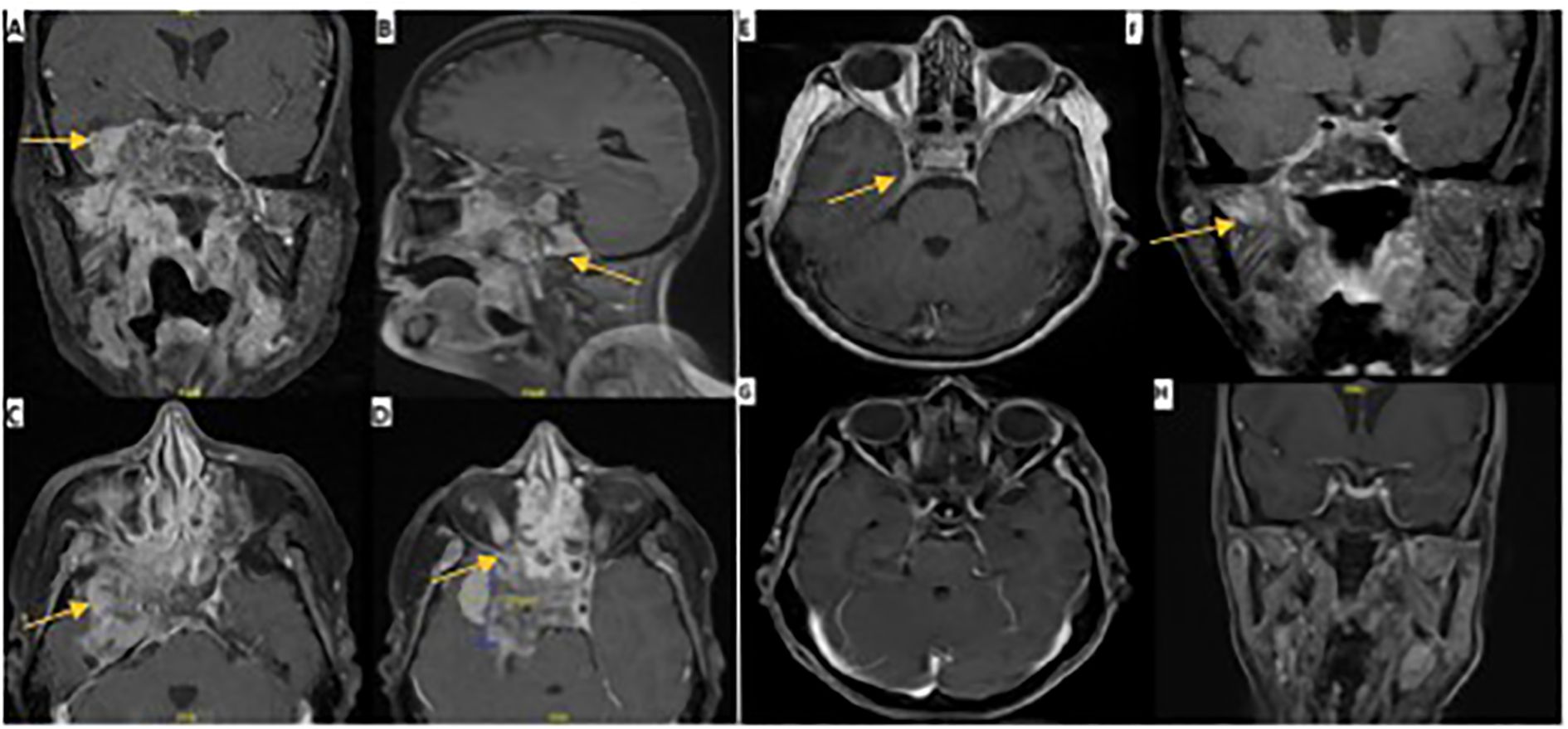
Figure 2. MRI images pre and post-CRT. Pre-ICT: (A) axial, coronal, and sagittal views of T1 with contrast MRI brain showing an aggressive mass invading the temporal lobe intracranially. (B) extension to the skull base. (C) extension to cavernous sinus. (D) extension to superior orbital fissure. One month post-ICT: (E, F) demonstrate axial and coronal views of T1 with contrast 4 months post-ICT with significant regression of tumor size and few remnants as indicated by the arrows. Eight months post-CRT: (G, H) demonstrate axial and coronal views of T1 with contrast with almost complete resolution of tumor.
A tissue biopsy was performed, and three specimens were collected. Histopathological examination revealed poorly differentiated, non-keratinizing squamous cell carcinoma. Immunohistochemistry was positive for P63 and CK5/6 and negative for CK-7, CK-20, and EBV. A CT of the chest, abdomen, and pelvis showed no distant metastasis, while a PET-CT scan showed multiple bilateral cervical lymph node uptake. Imaging and biopsy-based staging revealed a locally advanced nasopharyngeal squamous cell carcinoma without metastasis stage IV/A T4-N2-M0.
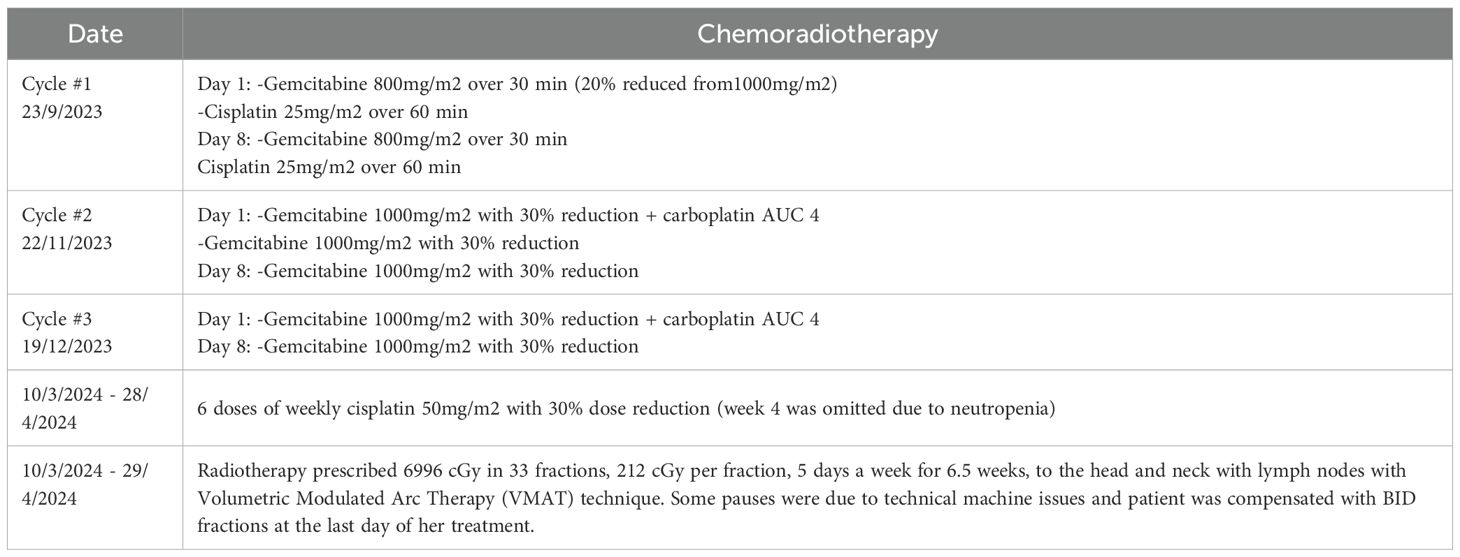
The case was discussed on the tumor board, and a decision for concurrent chemoradiotherapy (CRT) was made. The patient received three cycles of induction chemotherapy. The initial cycle included induction chemotherapy (ICT) with gemcitabine and cisplatin, followed by two subsequent cycles of gemcitabine and carboplatin. A cumulative radiation dose of 6996 centigray (cGy) dispersed across 33 fractions was prescribed which were tolerable with no reported toxicity. We contoured the CT image obtained for simulation in our radio-oncology department (after chemotherapy), then we fused it with MRI and PET scans pre-chemotherapy. Rather than contouring individual cranial nerves, which are not well visualized on imaging, we contoured anatomical structures based on their anatomical relationship with neural pathways. These include foramina and bony landmarks (4). The patient was concurrently undergoing maintenance therapy with weekly cisplatin. The regimen of CRT is detailed in (Table 2).
Follow-up at 6 months showed significant improvement in right eyelid ptosis. All extraocular movements of the right eye almost completely recovered, and there was complete recovery in shoulder shrugging and head turning. Oral examination revealed normal tongue power, without deviation or atrophy. These findings indicate near-complete recovery of CN III, IV, and VI and complete recovery of CN XI and XII (Figure 1). Although depression and anxiety scales were not used during the follow up visit, patient did confirm that she feels much better compared when she was seen first six months ago, as she mentioned during her last visit “I feel a lot better now”. Two follow-up MRI images were done. The first one was one month post-ICT which revealed significant tumor size regression with few remnants and the second was 8 months post-CRT with almost complete resolution of tumor (Figure 2).
Discussion
We present a rare case of locally advanced NPC stage IV/A T4-N2-M0 with no distant metastasis involving 10 unilateral CN palsies (II, III, IV, V, VI, VII, IX, X, XI, and XII), indicative of extensive anterior and posterior skull base and intracranial involvement. To the best of our knowledge, this report is the first of a case of NPC involving 10 CN with recovery of 5 CN within 6 months post-chemoradiation.
Nasopharyngeal carcinoma most commonly affects CN VI (56.4%) and V2 (47.9%) and V3 (29.2%) branches of CN V, and it rarely affects CN II (8.6%) or VII (2.3%) (5). The CN deficits from NPC depends mainly on the anatomical extension patterns of the tumor. For example, an extension of the tumor into the orbital apex and cavernous sinus affects CN II, III, IV, V, and VI and leads to impaired vision, ophthalmoplegia, and facial sensory dysfunction (2). An extension to the cerebellopontine angle, the middle ear, or the parotid can involve CN VII (6). The presentation of CN VII palsy may differ depending on which nerve segment is affected during its anatomical course (7). Tumor extension into the jugular foramen affects CN IX, X, and XI, leading to dysphagia, vocal cord palsy, and weakness of the trapezius and sternocleidomastoid muscles (2). Involvement of the hypoglossal canal affects CN XII and presents with ipsilateral tongue deviation (8). One previous case reported involvement of six CN (III, V, VI, VII, IX, and XI) (7). Another study reported the involvement of nine unilateral CN (III, IV, V, VI, VII, VIII, IX, X, XII) as a Garcin’s Syndrome secondary to NPC with improvement post-CRT. However, the extent of clinical and radiological improvement was not detailed (9).
In our case, the involvement of CN II, III, IV, V, and VI was explained by the anterior tumor extension to the orbital apex and cavernous sinus as described by MRI. The patients’ optic neuropathy, ptosis, complete ophthalmoplegia, and hypoesthesia of the ophthalmic distribution of V1 on the right side are consistent with orbital apex syndrome that has been described in the literature (10). In addition, the involvement of the lower divisions of the right CN V in this case was represented by the lower facial pain and hypoesthesia across the distribution of V2 and V3 along with weakness of the masseter and temporalis muscles on the right side. These symptoms are indicative of tumor infiltration into the superior orbital fissure, foramen rotundum, and foramen ovale (5). The posterior extension of the tumor resulted in the involvement of CN VII, IX, X, XI, and XII by compressing the internal auditory meatus, jugular foramen, and hypoglossal canal (2, 8).
A multidisciplinary team should discuss the appropriate treatment approach for patients with advanced NPC (11). Concurrent chemoradiation is the cornerstone treatment protocol for patients diagnosed with advanced, non-metastatic NPC, whereas radiotherapy plays a major role in locoregional therapy (11). According to the European Society of Medical Oncology, Stages III and IV/A are commonly managed with CRT with cisplatin as the agent of choice. At such stages, induction chemotherapy with cisplatin and gemcitabine followed by CRT has been shown to result in better overall survival, recurrence-free survival (RFS), and distant RFS (12). Our patient was not a candidate for surgical resection due to the complex anatomy of this region. However, a multidisciplinary team, including various specialties, participates in the management of different systems. In addition, the patient received three cycles of induction chemotherapy followed by CRT as described in the case presentation.
The recovery of CN in advanced NPC post-CRT was evaluated, and it was concluded that the oculomotor, trochlear, and abducens nerves have the highest 5-year full recovery rate. In addition, involvement of the optic nerve, smoking, and more than 2 months of neuropathy are all negative predictors of recovery (5). Our patient exhibited a near-complete recovery of CN III, IV, and VI, and a complete recovery of CN XI and XII at the 6-month follow-up due to the significant response to the therapy and regression of the tumor extensions. Regarding psychological complications from NPC, it has been reported that up to a third of patients with NPC have anxiety and depression (13). When our patient was asked about her neurological and depressive symptoms post-CRT, she replied “I feel a lot better now”. To our knowledge, complete recovery, or near-complete recovery of a total of five CN in advanced NPC has not been described previously in the literature. In addition, our case report supports that the current recommended chemo-radiation regimen is capable of treating extensive NPCs with complete or near-complete recovery of some the impaired CN.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the King Saud University Instiutional Review Board Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AAlb: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NA: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AAlf: Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. KA: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. AAlh: Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chang ET, Ye W, Zeng YX, and Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. (2021) 30:1035–47. doi: 10.1158/1055-9965.EPI-20-1702, PMID: 33849968
2. Adham M, Mat Lazim N, and Carlos R. Clinical presentation of nasopharyngeal carcinoma. In: An evidence-based approach to the management of nasopharyngeal cancer. Cambridge, MA, USA: Elsevier (2020). p. 93–109. doi: 10.1016/B978-0-12-814403-9.00006-9
3. Sham JS, Cheung YK, Choy D, Chan FL, and Leong L. Cranial nerve involvement and base of the skull erosion in nasopharyngeal carcinoma. Cancer. (1991) 68:422–6. doi: 10.1002/1097-0142(19910715)68:2<422::aid-cncr2820680235>3.0.co;2-f
4. Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiotherapy Oncol. (2017) 126:25–36. doi: 10.1016/j.radonc.2017.10.032, PMID: 29153464
5. Chow JCH, Lee A, Bao KKH, Cheung KM, Chan JCH, Tam AHP, et al. Cranial neuropathies in advanced nasopharyngeal carcinoma: Neurological recovery after modern radiotherapy and systemic chemotherapy. Radiotherapy Oncol. (2021) 163:221–8. doi: 10.1016/j.radonc.2021.08.022, PMID: 34506830
6. Low WK. Facial palsy from metastatic nasopharyngeal carcinoma at various sites: Three reports. Ear Nose Throat J. (2002) 81:99–101. doi: 10.1177/014556130208100214, PMID: 11868483
7. Denton AJ, Khunger A, and Reyes-Corcho A. Case of nasopharyngeal carcinoma presenting with rare combination of multiple cranial nerve palsies. Cureus. (2021) 13(12):e20357. doi: 10.7759/cureus.20357, PMID: 35028234
8. Huang CC, Fang FM, Chen HC, Hsu HC, Huang TL, Su YL, et al. Therapeutic outcome of nasopharyngeal carcinoma with cranial nerve palsy: a single institution experience of 104 patients. Onco Targets Ther. (2017) 10:2069–75. doi: 10.2147/OTT.S129653, PMID: 28435298
9. Patel S, Patel A, Majmundar M, Madabhavi I, Shah R, and Soni J. Nasopharyngeal carcinoma presenting as Garcin’s syndrome: A rare case report. Iran: Iranian Journal of Neurology. (2015).
10. Badakere A and Patil-Chhablani P. Orbital apex syndrome: A review. Eye Brain. (2019) 11:63. doi: 10.2147/EB.S180190, PMID: 31849556
11. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, and Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0, PMID: 31178151
12. Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2021) 32:452–65. doi: 10.1016/j.annonc.2020.12.007, PMID: 33358989
13. Zhan Z, Huang H, Xiao Y, Zhao Y, Cao X, Cai Z, et al. Anxiety and depression in nasopharyngeal carcinoma patients and network analysis to identify central symptoms: A cross-sectional study from a high-incidence area. Radiotherapy Oncol. (2024) 197:110324. doi: 10.1016/j.radonc.2024.110324, PMID: 38735537
Keywords: nasopharyngeal carcinoma, cranial nerve palsies, chemoradiotherapy invasive nasopharyngeal squamous cell carcinoma, 10 cranial nerve palsies, chemoradiotherapy, case report
Citation: Albilali A, Alotaibi NH, Alfadley A, Alqarni KM and Alhajlah A (2025) Extensive locally invasive nasopharyngeal carcinoma involving 10 cranial nerves palsies: an interesting case report. Front. Oncol. 15:1545838. doi: 10.3389/fonc.2025.1545838
Received: 18 December 2024; Accepted: 14 July 2025;
Published: 31 July 2025.
Edited by:
Saba Battelino, University Medical Centre Ljubljana, SloveniaReviewed by:
Carla Pisani, Azienda Ospedaliero Universitaria Maggiore della Carità, ItalyJongmyung Kim, Moffitt Cancer Center, United States
Copyright © 2025 Albilali, Alotaibi, Alfadley, Alqarni and Alhajlah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulrazaq Albilali, YWFsYmlsYWxpQGtzdS5lZHUuc2E=
 Abdulrazaq Albilali
Abdulrazaq Albilali Naif H. Alotaibi
Naif H. Alotaibi Abdulaziz Alfadley
Abdulaziz Alfadley Khalid M. Alqarni
Khalid M. Alqarni Abdullah Alhajlah
Abdullah Alhajlah