- Department of Gastroenterology, Shapingba Hospital Affiliated to Chongqing University, Chongqing, China
Primary malignant melanoma of the esophagus (PMME) is a rare type of gastrointestinal melanoma characterized by its aggressive nature and poor prognosis, with a 5-year survival rate of less than 5%. This study reports a case of a male patient with PMME complicated by primary lung adenocarcinoma. The main symptom of the patient was progressive dysphagia. Endoscopically, a polypoid mass was observed protruding into the lumen of the lower esophagus, with melanin pigmentation on the tumor surface, part of which was smooth and part showed ulceration. Enhanced chest and abdominal CT, as well as PET-CT, were consistent with esophageal malignancy, the left lung was consistent with lung cancer, and the right was considered metastatic. CT-guided percutaneous lung biopsy and immunohistochemistry indicated left lung invasive adenocarcinoma. PMME is extremely rare, and the co-occurrence of lung adenocarcinoma as a double primary cancer is even rarer in clinical practice. The disease has a high degree of malignancy and poor prognosis, with diagnosis mainly relying on endoscopic examination, pathological histological morphology, and immunohistochemistry. Early detection and diagnosis are currently key to treating this disease.
1 Introduction
Primary malignant melanoma of the esophagus (PMME) is an exceedingly rare type of esophageal malignancy, accounting for less than 0.1%–0.2% of all primary malignancies of the esophagus (1, 2). It shows male predominance (3), with onset typically after 60 years of age, and frequently involves the middle to lower esophagus (4–6). Symptoms include dysphagia (7), weight loss, and chest pain (8), but preoperative diagnosis is challenging due to nonspecific presentations (9–11). PMME is highly aggressive with a poor prognosis, and the 5-year survival rate is less than 5% (12). Despite advancements in diagnostic techniques, current treatment strategies are largely based on case reports and small studies (11–14).
This report discusses a 77-year-old male with PMME and primary lung adenocarcinoma, diagnosed via gastroscopy, histopathology, and genetic testing. It explores diagnostic methods, targeted therapy, and clinical features, providing insights into PMME management.
2 Case description
A 77-year-old male patient with a history of hypertension and no family history of malignancy presented to the gastroenterology department on April 15, 2024, with a 2-month history of progressive dysphagia. Physical examination revealed no hyperpigmentation or macules on the skin, mucosa, sclera, or oral cavity. No palpable superficial lymphadenopathy was noted. The abdomen was soft without tenderness, rebound pain, or muscle rigidity. Laboratory tests, including complete blood count, liver and renal function panels, and tumor markers (CA15-3, CEA, AFP, CA19-9), showed no significant abnormalities.
Esophagogastroduodenoscopy revealed smooth mucosa in the upper and middle esophagus without abnormalities. At 34–36 cm from the incisors, a hemispherical lesion protruding into the lumen was observed, with well-defined borders, a smooth surface, and localized pigmentation. Additionally, at 36–39 cm from the incisors, an irregular mass with a rough, depressed surface was noted, accompanied by ulceration at the apex, covered with necrotic tissue and minimal white exudate. The lesion was friable and prone to bleeding (Figure 1).
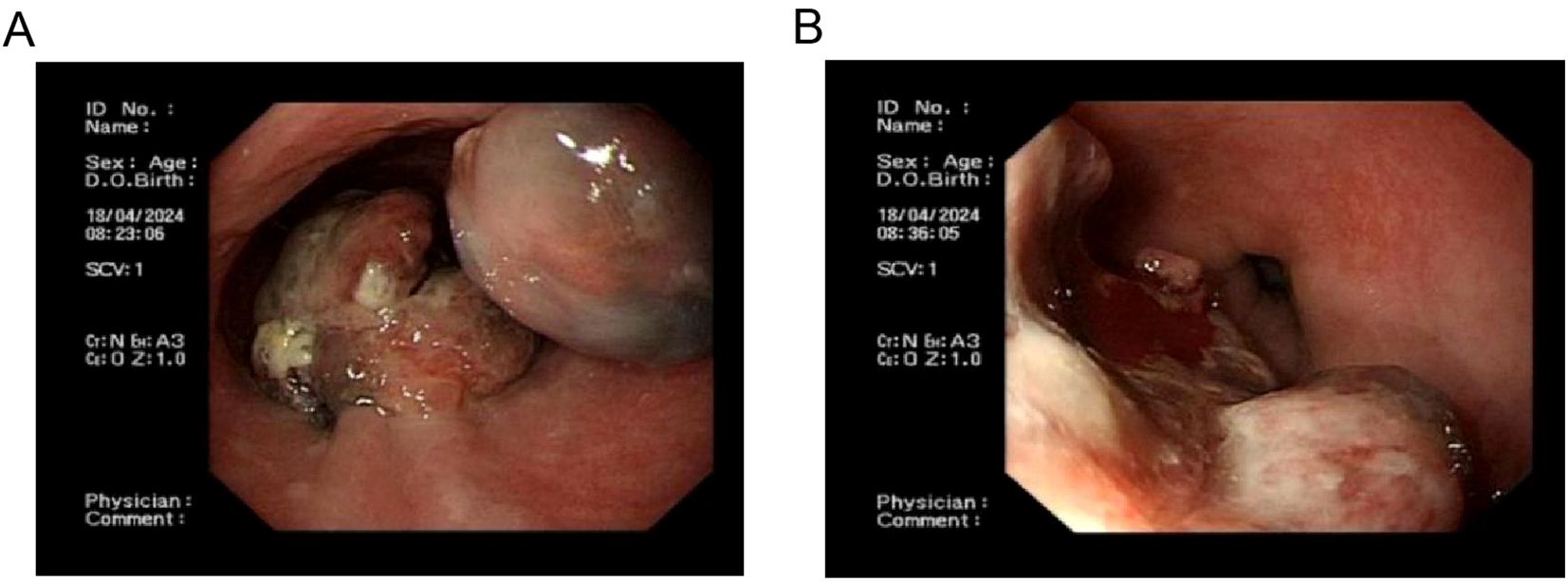
Figure 1. Endoscopic images of primary esophageal malignant melanoma. (A), Proximal aspect of primary esophageal malignant melanoma; (B) Distal aspect of primary esophageal malignant melanoma.
Biopsy specimens from the esophageal lesions were fixed in 10% neutral formalin, routinely dehydrated, embedded in paraffin, sectioned at a thickness of 4 μm, and stained with hematoxylin-eosin (HE) and immunohistochemical markers. Microscopic examination revealed tumor cells arranged in diffuse sheets or nests. The cells were large with oval, spindle-shaped, or polygonal nuclei, prominent eosinophilic nucleoli, and frequent mitotic figures. Abundant melanin pigment was observed within and outside the cytoplasm (Figure 2).
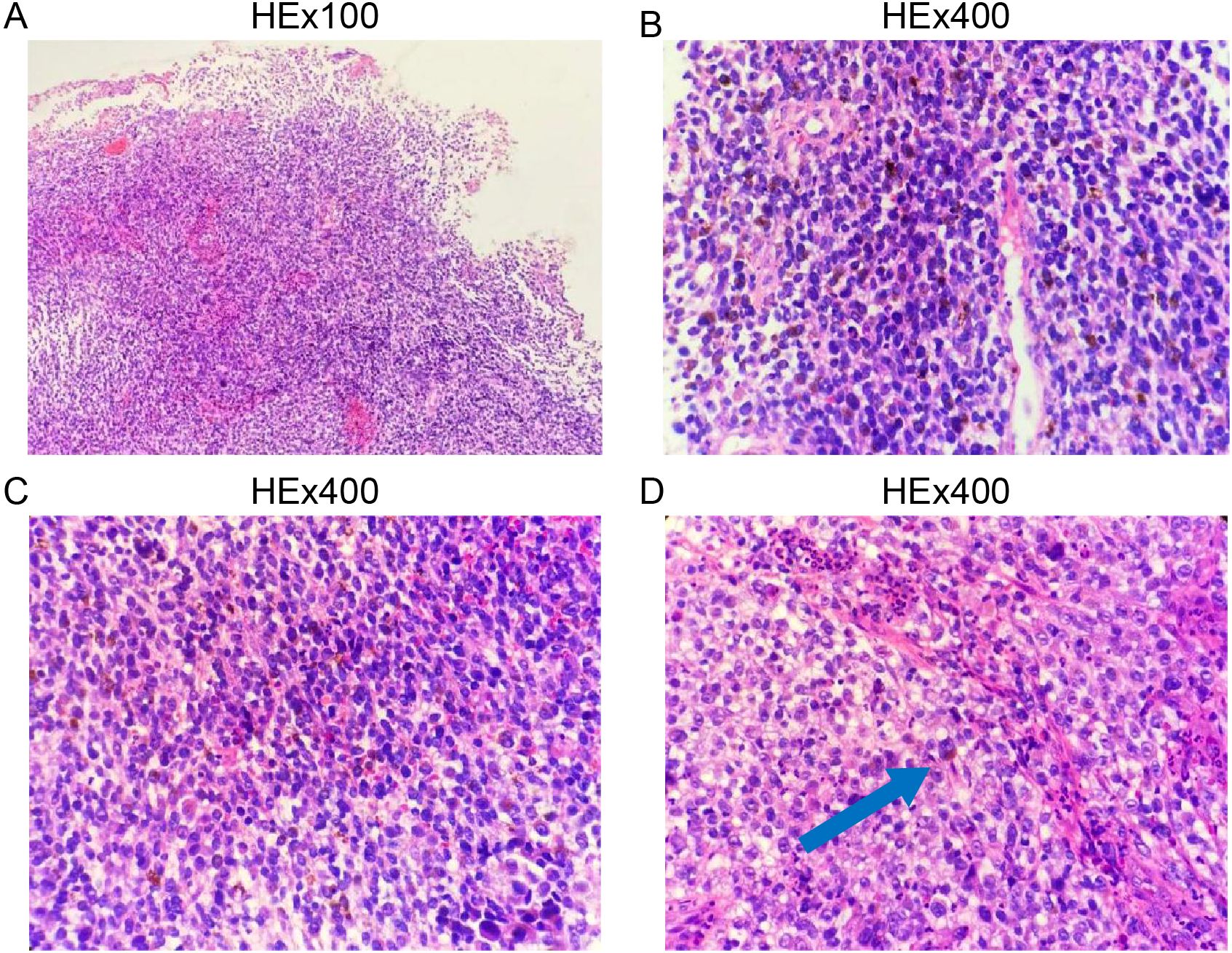
Figure 2. Histopathological features of primary esophageal malignant melanoma. (A), Low-power magnification; (B–D), High-power magnification.
Immunohistochemical staining demonstrated the following results: HMB-45 (+), SOX10 (+), Vimentin (+), Ki-67 (30–50%+), and focal positivity for P53 and S-100. Staining for CK, CK5/6, P40, P63, CgA, Syn, CD56, CEA, TTF-1, and CK7 was negative. CD34 highlighted vascular structures, and LCA was positive in lymphocytes (Figure 3). Based on histopathological and immunohistochemical findings, a diagnosis of PMME was established.
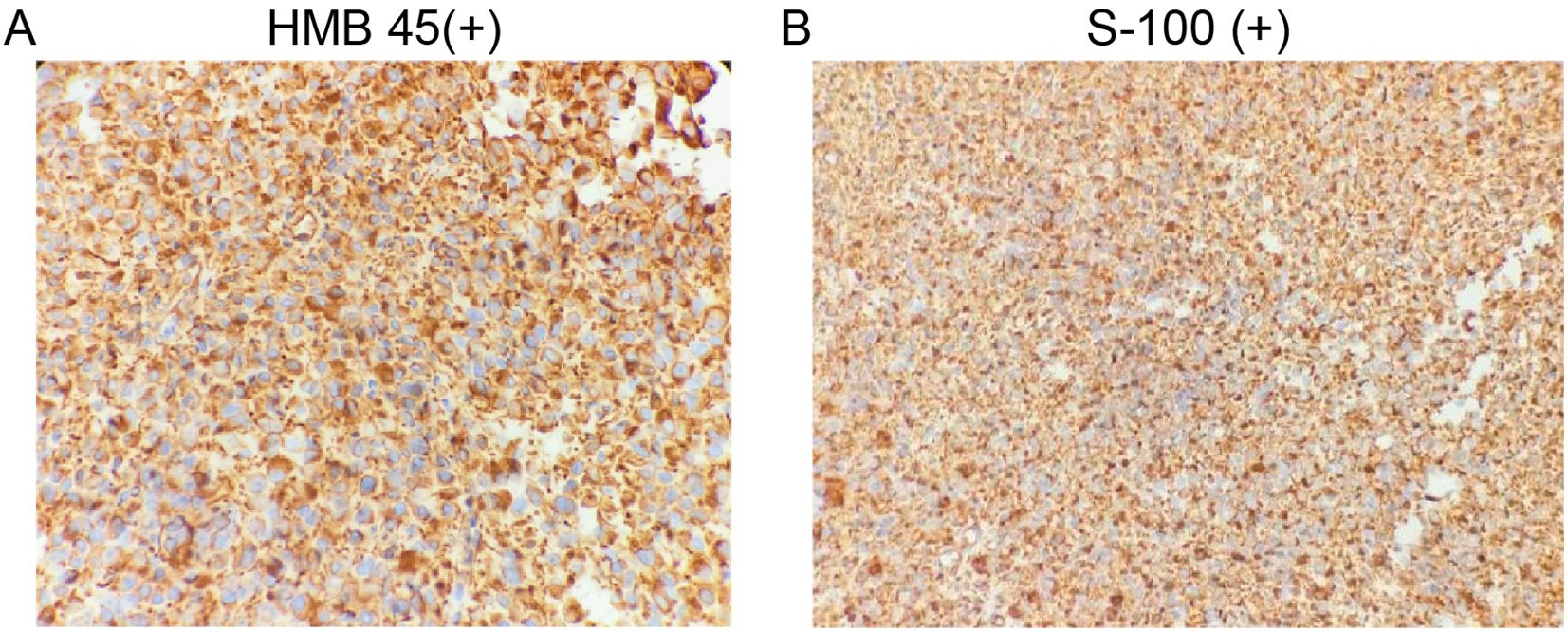
Figure 3. Immunohistochemical images of primary esophageal malignant melanoma. (A), HMB 45(+); (B), S-100(+).
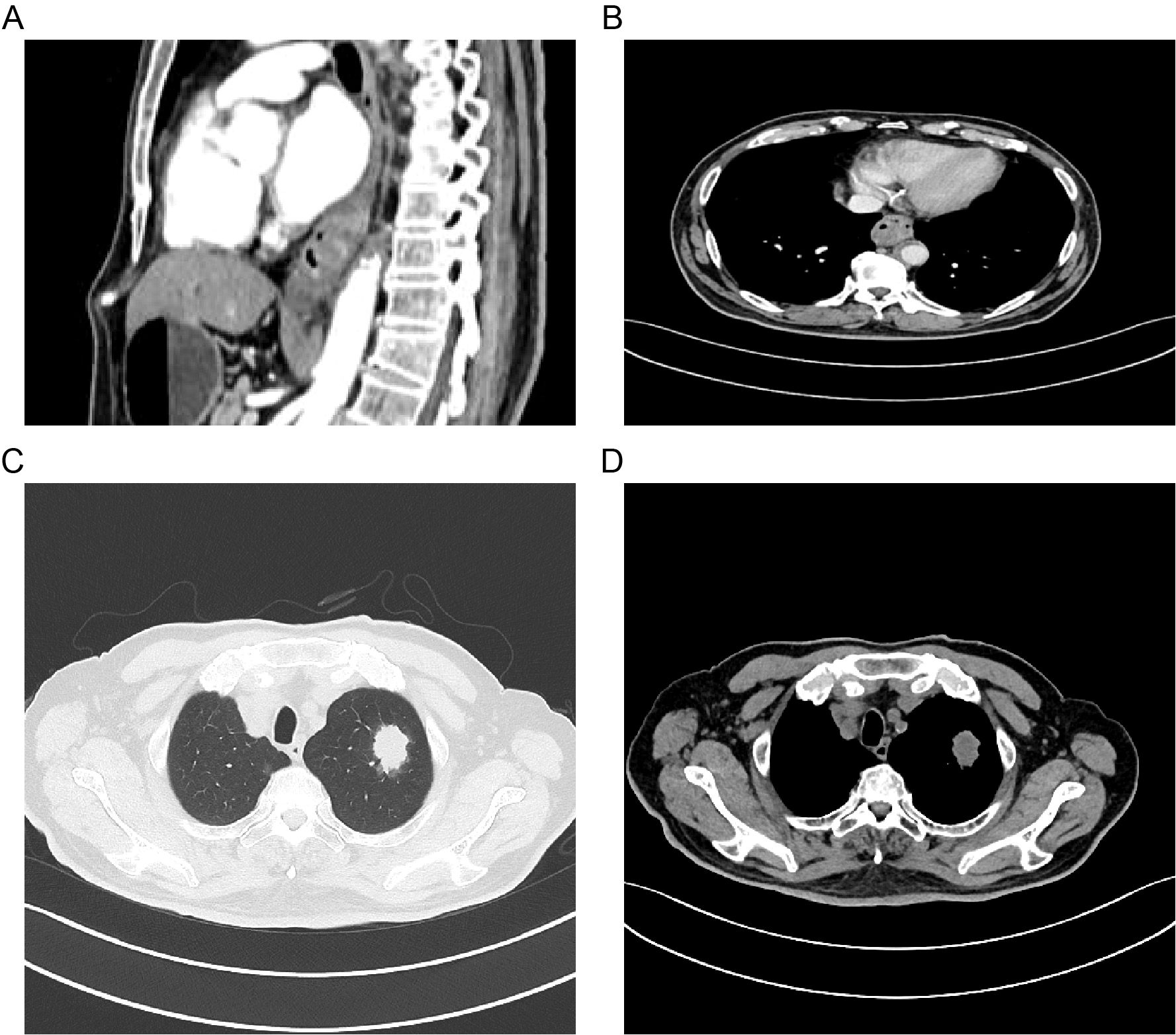
Further molecular testing using a targeted melanoma gene panel revealed a BRAF exon 15 missense mutation (c.1799T>A, p.V600E) with a mutation allele frequency of 1.19%. Imaging studies, including contrast-enhanced CT of the chest and abdomen and PET-CT, demonstrated a malignant tumor in the distal esophagus, findings consistent with primary esophageal melanoma, as well as a lesion in the left lung suggestive of primary lung cancer and a right lung lesion indicative of metastasis (Figure 4). CT-guided percutaneous lung biopsy of the left upper lobe revealed invasive adenocarcinoma. Immunohistochemistry and subsequent genetic analysis identified a KRAS exon 2 mutation (c.34G>T, p.G12C) with a mutation allele frequency of 15.04%. The final diagnosis was dual primary malignancies: PMME and left lung adenocarcinoma.
The patient was initiated on targeted therapy with dabrafenib and trametinib for melanoma and treated with oral vinorelbine capsules for lung adenocarcinoma.
3 Discussion
PMME is a rare and aggressive form of esophageal cancer, known for its rapid spread and poor prognosis (4). It typically occurs in the middle and lower parts of the esophagus (9–11), especially the lower segment, likely due to a higher concentration of melanocytes (15, 16). Esophageal melanocytosis is seen as a major precancerous condition for PMME (15, 16), with frequent reflux possibly causing abnormal growth and cancerous changes in melanocytes.
PMME symptoms are generally vague, with initial signs like swallowing discomfort and chest pain often mistaken for poorly differentiated carcinoma (4). Advanced symptoms such as worsening swallowing difficulties, painful swallowing, weight loss, and malnutrition suggest tumor progression. Diagnosis involves ruling out metastatic esophageal melanoma, poorly differentiated squamous cell carcinoma, and sarcomatoid carcinoma.
PMME predominantly depends on endoscopic evaluation, histopathological analysis, and immunohistochemical techniques. Endoscopically, PMME typically manifests as an intraluminal polyp, frequently exhibiting pigmentation (12). Pathological diagnosis involves identifying melanin granules and using melanoma markers such as S-100, SOX-10, and HMB-45 to improve diagnostic accuracy (17–19).
Esophagectomy is the main treatment for PMME, as some studies suggest it may prolong survival (3). However, because PMME is often diagnosed late or with metastases, surgery alone has limited effectiveness (20, 21). Adjuvant therapies like radiotherapy, chemotherapy, and immunotherapy can enhance local control, but their effectiveness varies by individual. Recently, the use of genetic testing and targeted therapies in PMME has grown. For patients with BRAF V600 mutations, the American Joint Committee advises treatment with BRAF inhibitors (dabrafenib, vemurafenib, encorafenib) and MEK inhibitors (trametinib, cobimetinib, binimetinib) (22).
In short, PMME is an aggressive esophageal tumor with a poor prognosis. Diagnosis involves endoscopy, pathology, and molecular tests. Surgery is the primary treatment, but combined therapies like chemoradiotherapy, immunotherapy, and targeted therapy can enhance survival. Early detection and precise diagnosis are crucial for better survival rates.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shapingba Hospital Affiliated to Chongqing University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Conceptualization, Supervision, Writing – review & editing. FY: Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li J, Guan W, Ren W, Liu Z, Wu H, Chen Y, et al. Longitudinal genomic alternations and clonal dynamics analysis of primary Malignant melanoma of the esophagus. Neoplasia. (2022) 30:100811. doi: 10.1016/j.neo.2022.100811
2. Dai J, Bai X, Gao X, Tang L, Chen Y, Sun L, et al. Molecular underpinnings of exceptional response in primary Malignant melanoma of the esophagus to anti-PD-1 monotherapy. J Immunother Cancer. (2023) 11:005937. doi: 10.1136/jitc-2022-005937
3. Kim TS, Min BH, Min YW, Lee H, Rhee PL, Kim JJ, et al. Clinical characteristics and treatment outcomes of primary Malignant melanoma of esophagus: a single center experience. BMC Gastroenterol. (2022) 22:157. doi: 10.1186/s12876-022-02235-8
4. Iwanuma Y, Tomita N, Amano T, Isayama F, Tsurumaru M, Hayashi T, et al. Current status of primary Malignant melanoma of the esophagus: clinical features, pathology, management and prognosis. J Gastroenterol. (2012) 47:21–8. doi: 10.1007/s00535-011-0490-y
5. Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. Clinicopathological characteristics and survival of primary Malignant melanoma of the esophagus. Oncol Letters. (2019) 18:1872–80. doi: 10.3892/ol.2019.10519
6. Bisceglia M, Perri F, Tucci A, Tardio M, Panniello G, Vita G, et al. Primary Malignant melanoma of the esophagus: a clinicopathologic study of a case with comprehensive literature review. Adv Anatom Pathol. (2011) 18:235–52. doi: 10.1097/PAP.0b013e318216b99b
7. Revilla Morato C, Mora Soler A, Prieto Bermejo AB, Piñero Pérez C, Pérez Corte D, Acosta Materán R, et al. Primary Malignant melanoma of the esophagus: A rare cause of dysphagia. Gastroenterol Hepatol. (2016) 39:47–8. doi: 10.1016/j.gastrohep.2015.04.001
8. Pun AH and Devitt PG. Primary Malignant melanoma of the oesophagus: two case reports. J Surg Case Rep. (2014) 2014:rju005. doi: 10.1093/jscr/rju005
9. Tang Y, Jiang M, Hu X, Chen C, and Huang Q. Difficulties encountered in the diagnosis of primary esophageal Malignant melanoma by 18F-fluorodeoxyglucose positron emission tomography/computed tomography: a case report. Ann Palliat Med. (2021) 10:4975–81. doi: 10.21037/apm-21-649
10. Ling C, Feng J, Li J, and Liu Q. Primary Malignant melanoma of the esophagus. Turk J Gastroenterol. (2018) 29:711–3. doi: 10.5152/tjg.2018.18065
11. Jin X, Li WZ, and Chen F. A case of primary Malignant melanoma of the esophagus. Radiol Case Rep. (2024) 19:5627–32. doi: 10.1016/j.radcr.2024.08.061
12. Cazzato G, Cascardi E, Colagrande A, Lettini T, Resta L, Bizzoca C, et al. The thousand faces of Malignant melanoma: A systematic review of the primary Malignant melanoma of the esophagus. Cancers. (2022) 14:3725. doi: 10.3390/cancers14153725
13. Gason R, Heieck F, and Felten M. Primary Malignant melanoma of the oesophagus. United Eur Gastroenterol J. (2024) 12:649–51. doi: 10.1002/ueg2.12574
14. Deng L, Wang HY, Hu CF, Liu XY, Jiang K, Yong JJ, et al. Comprehensive molecular findings in primary Malignant melanoma of the esophagus: A multicenter study. Pigment Cell Melanoma Res. (2024) 37:363–71. doi: 10.1111/pcmr.13157
15. DiCostanzo DP and Urmacher C. Primary Malignant melanoma of the esophagus. Am J Surg Pathol. (1987) 11:46–52. doi: 10.1097/00000478-198701000-00006
16. Morita FH, Ribeiro U Jr., Sallum RA, Tacconi MR, Takeda FR, da Rocha JR, et al. Primary Malignant melanoma of the esophagus: a rare and aggressive disease. World J Surg Oncol. (2013) 11:210. doi: 10.1186/1477-7819-11-210
17. Bonfrer JM, Korse CM, Nieweg OE, and Rankin EM. The luminescence immunoassay S-100: a sensitive test to measure circulating S-100B: its prognostic value in Malignant melanoma. Br J Cancer. (1998) 77:2210–4. doi: 10.1038/bjc.1998.368
18. Harris ML, Baxter LL, Loftus SK, and Pavan WJ. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res. (2010) 23:496–513. doi: 10.1111/j.1755-148X.2010.00711.x
19. Adema GJ, Bakker AB, de Boer AJ, Hohenstein P, and Figdor CG. pMel17 is recognised by monoclonal antibodies NKI-beteb, HMB-45 and HMB-50 and by anti-melanoma CTL. Br J Cancer. (1996) 73:1044–8. doi: 10.1038/bjc.1996.202
20. Chen H, Fu Q, and Sun K. Characteristics and prognosis of primary Malignant melanoma of the esophagus. Medicine. (2020) 99:e20957. doi: 10.1097/MD.0000000000020957
21. Wang L, Zong L, Nakazato H, Wang WY, Li CF, Shi YF, et al. Primary advanced esophago-gastric melanoma: A rare case. World J Gastroenterol. (2016) 22:3296–301. doi: 10.3748/wjg.v22.i11.3296
22. Waninger JJ, Ma VT, Journey S, Skvarce J, Chopra Z, Tezel A, et al. Validation of the american joint committee on cancer eighth edition staging of patients with metastatic cutaneous melanoma treated with immune checkpoint inhibitors. JAMA Netw Open. (2021) 4:e210980. doi: 10.1001/jamanetworkopen.2021.0980
Keywords: melanoma, lung adenocarcinoma, primary malignant melanoma of the esophagus, dual primary cancers, treatment
Citation: Yang L and Yang F (2025) Case Report: Esophageal malignant melanoma with lung adenocarcinoma: a rare case of dual primary cancers. Front. Oncol. 15:1546806. doi: 10.3389/fonc.2025.1546806
Received: 17 December 2024; Accepted: 27 May 2025;
Published: 16 June 2025.
Edited by:
Andee Dzulkarnaen Zakaria, Universiti Sains Malaysia, MalaysiaReviewed by:
Leonidas Papastavrou, Athens Medical Center, GreeceApurva Patel, Gujarat Cancer & Research Institute, India
Copyright © 2025 Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yang, eWxfMTAxQDE2My5jb20=
 Li Yang
Li Yang Fan Yang
Fan Yang