- 1Department of Hepatobiliary Surgery, Qinghai Provincial People’s Hospital, Xining, China
- 2Department of Pathology, Qinghai Provincial People’s Hospital, Xining, China
Angiomyolipoma (AML) is a type of tumor of mesenchymal origin that is most commonly found in the kidney, although it has been found in the liver in rare cases. Herein, we document the first known instance of an AML identified within the liver’s round ligament. A 22-year-old woman presented with a 41-month history of a painless abdominal mass that gradually enlarged. She was misdiagnosed with primary hepatic carcinoma 34 months prior at another hospital and received transcatheter arterial chemoembolization (TACE). After failure of TACE, computed tomography (CT) indicated possible malignant neoplasms. In our hospital, an abdominal CT scan revealed a large mass occupying the right hypochondrium but no evidence of metastatic disease. Consequently, the decision was made to proceed with surgery. During laparotomy, a large, well-defined tumor was discovered, which, surprisingly, was attached to the liver’s round ligament and isolated from any other intra-abdominal structures. The tumor was excised with ease, and monobloc resection and macroscopically total removal were achieved. Histopathological examination confirmed that the tumor was an AML originating from the liver’s round ligament. Postoperative care did not include adjuvant therapy. The patient remains alive and free from any indications or symptoms of recurrence 46 months postsurgery. This case represents the first documented instance of an AML developing from the liver’s round ligament and underscores the diagnostic challenges posed by such tumors. Although uncommon, AML diagnoses should be considered to facilitate early detection and complete surgical excision, which are pivotal for optimal patient outcomes.
1 Introduction
Angiomyolipoma (AML) is a type of tumor of mesenchymal origin that is most commonly found in the kidney, but AMLs have been found in the liver in rare circumstances. Approximately 600 AML cases have been reported in the literature in the last 4 decades (1). Most patients with hepatic angiomyolipoma (HAML) lack typical clinical manifestations and specificity in laboratory examinations. Moreover, due to the different proportions of each component of HAMLs, preoperative imaging findings vary greatly, resulting in a high misdiagnosis rate and difficulty in preoperative diagnosis (2). As a rare liver tumor, HAML is generally considered a benign tumor, but studies have shown that it has a potential risk of recurrence and metastasis. A retrospective study by Klompenhouwer et al. (3) reported that the postoperative recurrence rate of HAML was 2.4% and the mortality rate was 0.8%. Given that HAMLs have a certain risk of malignancy and some HAMLs are difficult to distinguish from malignant tumors before surgery, simple conservative treatment may miss malignant tumors, and most scholars advocate early surgical treatment at present (4). This case represents the first documented instance in the medical literature of an AML originating from the round ligament of the liver.
2 Case presentation
A 22-year-old female patient with a right subcostal mass was admitted to the first hospital. An abdominal CT scan revealed that a large mass with an abnormal density shadow measuring 90×50 mm in the abdominal cavity below the liver was significantly enhanced, and necrosis was visible in the lesion, which highly suggested the possibility of malignant neoplasms in the abdomen (Figures 1A–C). The hospital staff considered the diagnosis of primary liver cancer and provided TACE treatment (Figure 1D). The patient was re-examined in the outpatient department of the hospital after discharge. The results of the third re-examination indicated that the mass was larger than before. Approximately three years later, the patient was transferred to our hospital due to the progressive growth of a mass located below the right costal margin. This mass had been observed for 41 months without any notable alterations in pain levels or tenderness. The patient’s overall health remained stable during this time. No relevant family or personal medical history had contributed to the condition. The patient had a World Health Organization performance status (PS) of 0, indicating no impairment in daily activities. The liver, pancreatic, and kidney functions of the patient were all within normal ranges. A biopsy guided by ultrasound was conducted, and the histological analysis revealed the presence of scattered lymphocytes within the cyst fluid. A subsequent CT scan revealed a solid and cystic mass measuring 120×100 mm and situated beneath the abdominal wall. This mass caused displacement of both the left and right lobes of the liver but remained separate from them; thus, the origin of the mass was likely mesenchymal neoplasms (Figures 2A–C). On the basis of these findings, the decision was made to proceed with surgical intervention.
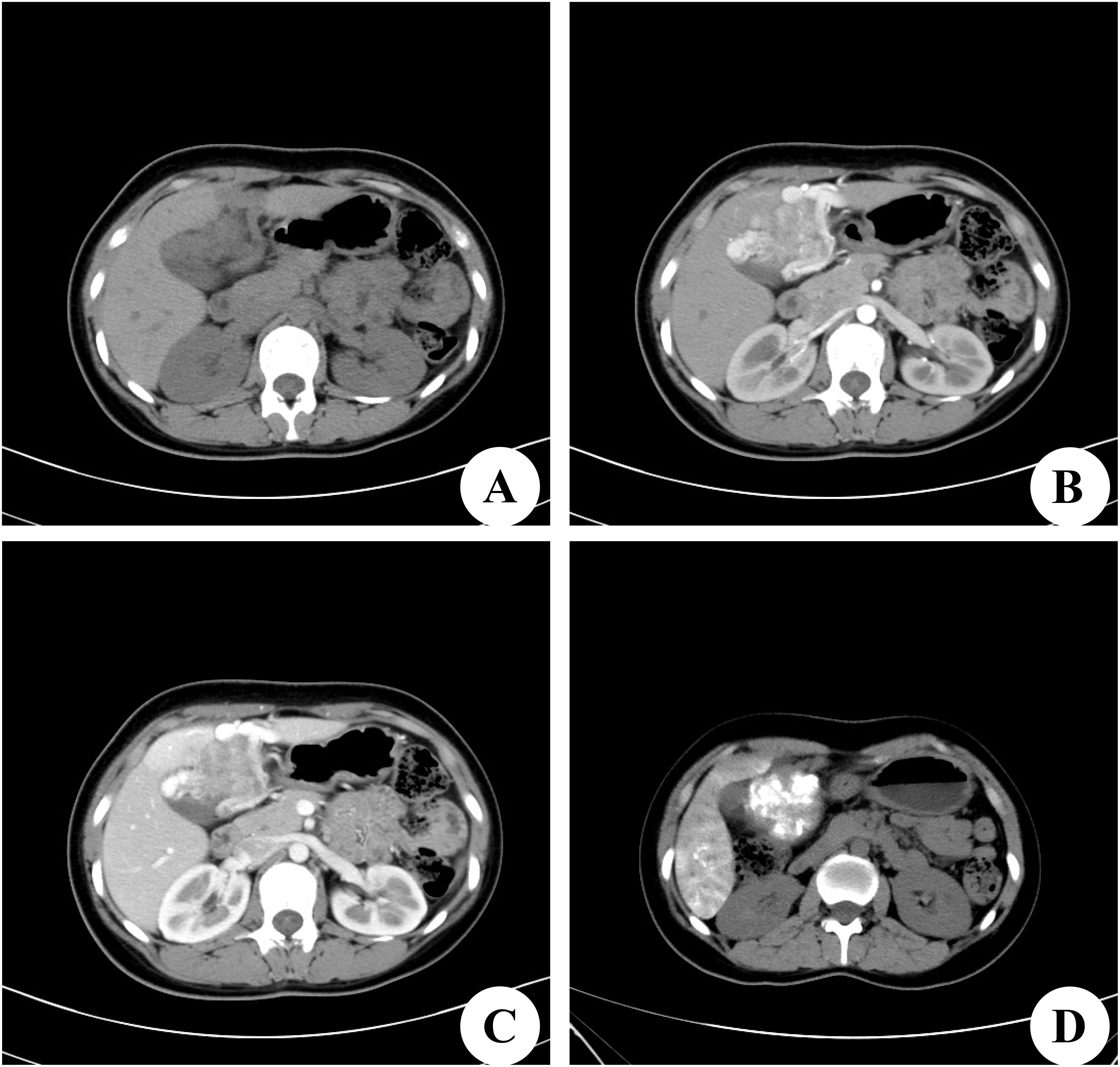
Figure 1. Computed tomography (CT) scan of the first hospital. (A) Preoperative CT plain scan. (B) On dynamic CT in the arterial phase, there were strongly enhancing areas in the tumor. (C) CT scan in the portal venous phase. (D) On postoperative CT scan, there is Iodine oil deposition in the center of the tumor.
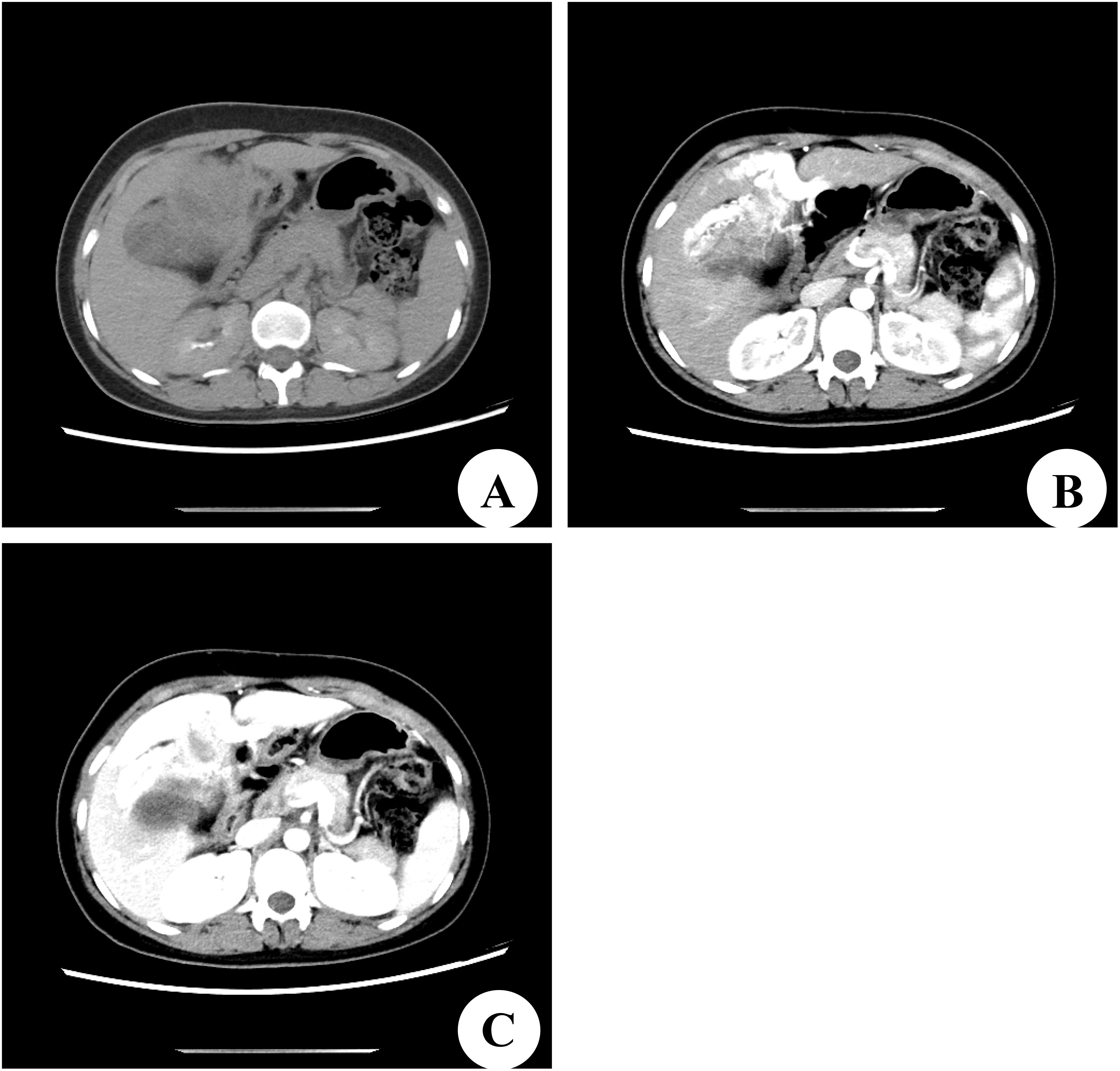
Figure 2. Computed tomography (CT) scan of our hospital. (A) Preoperative CT plain scan. (B) CT scan in the arterial phase. (C) CT scan in the portal venous phase.
During laparotomy, a large and well-defined tumor was discovered, which was unexpectedly attached to the round ligament of the liver (Figure 3A) and exhibited a ‘caput medusae’ pattern of vascularization. The tumor was isolated from other intra-abdominal structures, particularly the liver parenchyma (Figure 3B), and was easily excised, requiring no additional dissection. The removal was performed en bloc and was macroscopically complete. The excised tumor measured 120×90 mm and weighed 2,030 g (Figure 3C). Upon sectioning, the tumor presented a fleshy consistency with gray–white coloration (Figure 3D). Unlike the initial findings from the microbiopsy, the histological examination of the surgically removed specimen revealed that the cells were arranged in a cord-like arrangement, and most of the cells were epithelioid, with no clear nuclear mitosis or necrosis; blood sinusoid spaces or bleeding between the cells, hyalinoid degeneration of the vascular wall of the interstitium, and little fat around the mass were observed (Figures 4A–C). Immunohistochemistry (IHC) analysis confirmed the expression of Melan-A, HMB-45 and SMA by the tumor cells (Figures 4D–F). The final diagnosis confirmed an AML originating from the round ligament of the liver. The patient’s recovery after surgery was smooth, with no complications reported. Adjuvant therapy was not administered as part of the treatment plan. A schedule for regular clinical and radiological check-ups was established to monitor the patient’s condition. At the 46-month follow-up visit, the patient was alive, maintained an excellent performance status (PS), and showed no indications or symptoms of disease recurrence.
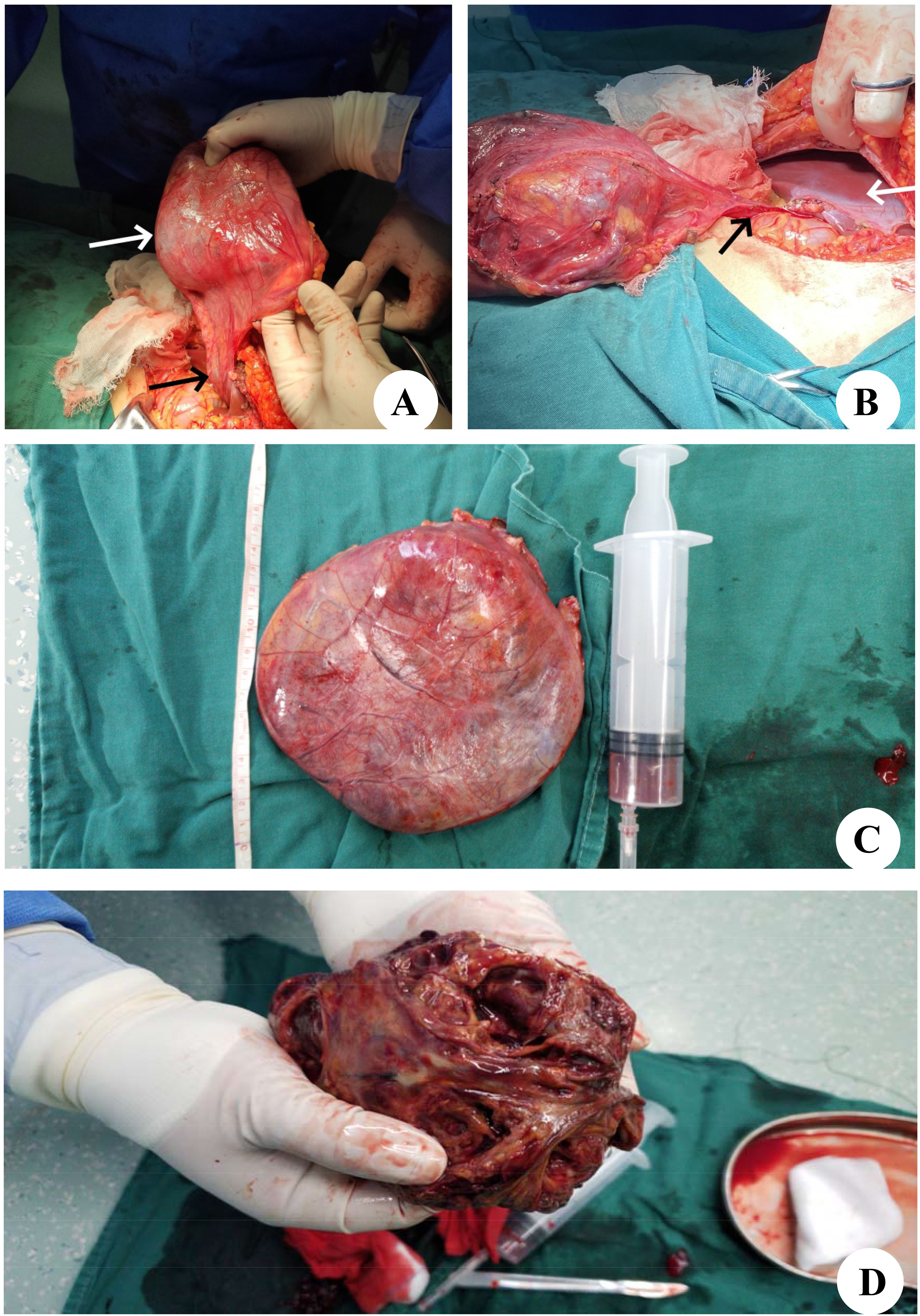
Figure 3. Intraoperative findings and histological aspects. (A) The tumor (white arrow) is appended to the round ligament of the liver (black arrow). (B) The tumor is free from any other intra-abdominal contact. The black arrow shows the round ligament of the liver, and the white arrow shows the liver parenchyma. (C) After complete resection of the tumor. (D) Macroscopic aspect on cut section showed a firm, gray-white tumor.
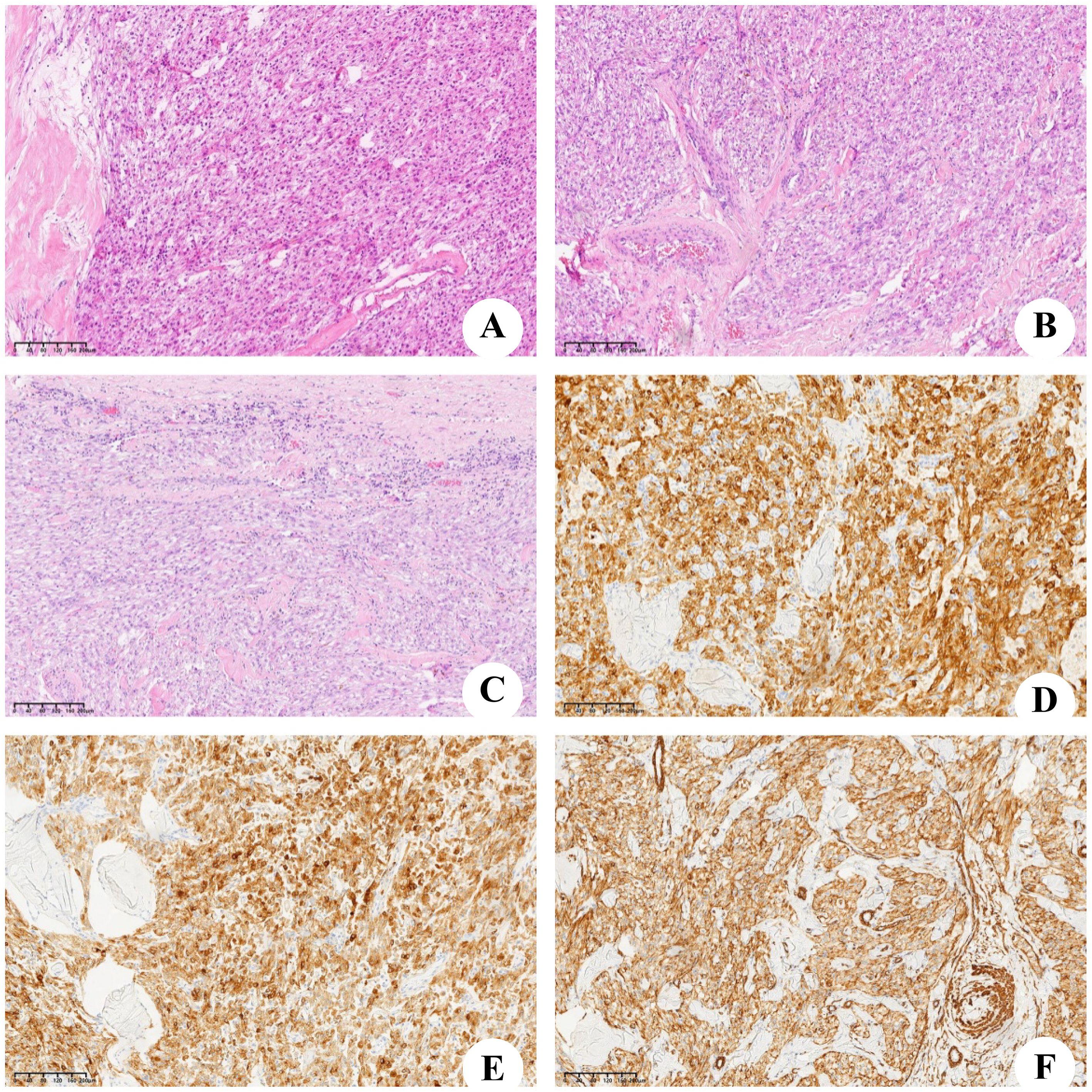
Figure 4. Microscopic and immunohistochemical findings. (A–C) Microscopic aspect, the tumor was consist of lots of spindle cells and little adipocyte. (D) Immunostain for Melan-A. (E) Immunostain for HMB45. (F) Immunostain for smooth muscle actin (SMA).
3 Discussion
The etiology and pathogenesis of AML are not entirely clear. Currently, the tumor cells are believed to originate from perivascular epithelioid cells (PECs) associated with perivascular epithelioid tumors (PEComas) (5) and have multidirectional differentiation potential. As a benign mesenchymal tumor, AML can differentiate into vascular smooth muscle and epithelial cells and expresses melanoma-related antibodies (6–8). HAML may be associated with tuberous sclerosis (TSC) gene mutations (9, 10).
The round ligament, also known as the ligamentum teres, is a fibrous structure resulting from the involution of the umbilical vein after birth. It serves as an anatomical divider, separating the left lobe of the liver into medial and lateral sections. Tumors originating in the round ligament are exceptionally rare, and the ligamentum teres hepatis (LTH) is one of the least common locations for primary tumor development. A review of the literature revealed that both benign and malignant tumors can develop in the LTH, and most of these tumors are metastases originating from gastrointestinal cancers, particularly mucinous appendiceal neoplasms, as well as peritoneal mesothelioma (11). In rarer cases, they may also arise from breast cancer or hepatocellular carcinoma (12, 13). Instances of primary mesenchymal tumors have also been documented, including sarcomas (malignant fibrous histiocytoma, leiomyosarcoma) (14), and benign tumors such as lipomas, lymphangiomas, stromal tumors (15) and SFTs (16). To the best of our knowledge, there are only 10 reported cases of PEComas of the falciform ligament/ligamentum teres (17–21). To date, there have been no reported cases of AML originating from the round ligament of the liver.
HAML is more common in young and middle-aged females. Klompenhouwer et al. (22) researched and analyzed 292 cases of HAML and reported that the proportion of males to females was approximately 1:3. HAML was first reported by Ishak (23) in 1976 and in China by Wen-Ming Cong (24) in 1992. Most patients had no obvious clinical symptoms, and a small number of them had atypical symptoms such as dull pain or abdominal distension with increasing tumor volume. Imaging examination lacks specificity, and distinguishing HAML from liver cancer or other benign tumors is difficult sometimes. The correct diagnosis depends on pathology and IHC. Under a microscope, a tumor is composed of three components: blood vessels, smooth muscle cells and fat cells. The initial histopathological examination suggested a diagnosis of either leiomyoma or fibroma, but these possibilities were later ruled out by our team. The positive expression of Melan-A, HMB-45 and SMA in tumor cells by IHC is reliable evidence for the diagnosis of HAML (25–27). Ki-67, an index of cell proliferation, is positively correlated with the degree of malignancy of the tumor. IHC of the tumor of this patient revealed a Ki-67 value of 5%, indicating that the tumor was in a state of low proliferation.
HAMLs are typically classified as benign tumors according to their prognosis. However, their clinical progression can be unpredictable, and malignant transformation of HAML is rare, especially in the epithelioid subtype (28). Many experts consider complete surgical resection of the tumor to be the most critical prognostic factor. The AML originating from the hepatic round ligament typically originates within the ligamentum teres and is characterized by relatively low fat content and a weaker association with tuberous sclerosis complex (TSC). In contrast, hepatic AMLs can occur in any location within the liver parenchyma, exhibits more variable fat content, and is associated with TSC in approximately 5%-10% of cases. In this particular case, the tumor’s “fortunate” location in the round ligament allowed straightforward and total removal, with the only adverse factor being its considerable size. Surgical removal is the preferred treatment for AMLs, and when the tumor is fully excised, the 5-year survival rate approaches 100%.
4 Conclusion
This case represents the first documented instance in the medical literature of an AML originating from the round ligament of the liver. This case highlights the diagnostic challenges associated with such tumors. Despite their rarity, this diagnosis of AML should be considered by physicians to allow for early diagnosis and complete surgical removal, which offer the highest likelihood of a successful recovery.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethics committee of Qinghai Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
H-BZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. X-QW: Conceptualization, Data curation, Formal analysis, Writing – original draft. X-NL: Resources, Writing – review & editing. X-MH: Conceptualization, Formal analysis, Writing – review & editing. H-HZ: Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by “KunLun talents High-end Innovation and Entrepreneurship Talent Program” of Qinghai Province(Youth Talent character (2021) No.
Acknowledgments
We are deeply grateful to the radiologist for his expert guidance on the manuscript. We also thank Dr. Xian-Ni Liu for the acquisition of data. Finally, we appreciate the constructive comments from the reviewers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AML, Angiomyolipoma; TACE, transcatheter arterial chemoembolization; CT, computed tomography; HAML, hepatic angiomyolipoma; PS, performance status; IHC, Immunohistochemistry; PECs, perivascular epithelioid cells; PEComa, perivascular epithelioid tumor; TSC, tuberous sclerosis; SFTs, Solitary fibrous tumors.
References
1. Camanni M, Bonino L, Delpiano EM, Ferrero B, Migliaretti G, and Deltetto F. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol. (2010) 17:59–65. doi: 10.1016/j.jmig.2009.10.013
2. Klompenhouwer AJ, Dwarkasing RS, Doukas M, Pellegrino S, Vilgrain V, Paradis V, et al. Hepatic angiomyolipoma: an international multicenter analysis on diagnosis, management and outcome. HPB (Oxford). (2020) 22:622–9. doi: 10.1016/j.hpb.2019.09.004
3. Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, et al. Management of hepatic angiomyolipoma: A systematic review. Liver Int. (2017) 37:1272–80. doi: 10.1111/liv.2017.37.issue-9
4. Ding GH, Liu Y, Wu MC, Yang GS, Yang JM, and Cong WM. Diagnosis and treatment of hepatic angiomyolipoma. J Surg Oncol. (2011) 103:807–12. doi: 10.1002/jso.v103.8
5. Folpe AL and Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. (2010) 41:1–15. doi: 10.1016/j.humpath.2009.05.011
6. Makhlouf HR, Remotti HE, and Ishak KG. Expression of KIT (CD117) in angiomyolipoma. Am J Surg Pathol. (2002) 26:493–7. doi: 10.1097/00000478-200204000-00012
7. Yang CY, Ho MC, Jeng YM, Hu RH, Wu YM, and Lee PH. Management of hepatic angiomyolipoma. J Gastrointest Surg. (2007) 11:452–7. doi: 10.1007/s11605-006-0037-3
8. Kamimura K, Nomoto M, and Aoyagi Y. Hepatic angiomyolipoma: diagnostic findings and management. Int J Hepatol. (2012) 2012:410781. doi: 10.1155/2012/410781
9. Jóźwiak S, Sadowski K, Borkowska J, Domańska Pakieła D, Chmielewski D, Jurkiewicz E, et al. Liver angiomyolipomas in tuberous sclerosis complex-their incidence and course. Pediatr Neurol. (2018) 78:20–6. doi: 10.1016/j.pediatrneurol.2017.09.012
10. Yan Z, Grenert JP, Joseph NM, Ren C, Chen X, Shafizadeh N, et al. Hepatic angiomyolipoma: mutation analysis and immunohistochemical pitfalls in diagnosis. Histopathology. (2018) 73:101–8. doi: 10.1111/his.2018.73.issue-1
11. Sugarbaker PH and Bijelic L. The porta hepatis as a site of recurrence of mucinous appendiceal neoplasms treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Tumori. (2008) 94:694–700. doi: 10.1177/030089160809400509
12. Kim SY and Lim JH. Extension of hepatoma to the rectus abdominis muscle via ligamentum teres hepatis. Gastrointest Radiol. (1985) 10:119–21. doi: 10.1007/BF01893084
13. Prete FP, Di Giorgio A, Alfieri S, and Doglietto GB. Breast-cancer metastasis in the round ligament of the liver. Lancet Oncol. (2006) 7:354. doi: 10.1016/S1470-2045(06)70663-5
14. Yamaguchi J, Azuma T, Fujioka H, Tanaka K, Furui J, Tomioka T, et al. Leiomyosarcoma occurring in the ligamentum teres of the liver: a case report and a review of seven reported cases. Hepatogastroenterology. (1996) 43:1051–6.
15. Poilblanc M, Coquaz S, Welschbillig K, Arnaud JP, and Hamy A. A case report of stromal tumor in the ligamentum teres hepatis (in French). Gastroenterol Clin Biol. (2006) 30:469–70. doi: 10.1016/S0399-8320(06)73204-3
16. Beyer L, Delpero JR, Chetaille B, Sarran A, Perrot D, Moureau-Zabotto L, et al. Solitary fibrous tumor in the round ligament of the liver: a fortunate intraoperative discovery. Case Rep Oncol. (2012) 5:187–94. doi: 10.1159/000338616
17. Sotiropoulos GC, Moskalenko V, and Lang H. Clinical challenges and images in GI. Image 2. Perivascular epithelioid cell (PEC) tumor of the ligamentum teres. Gastroenterology. (2009) 136:2065. doi: 10.1053/j.gastro.2009.01.045
18. Martignoni G, Pea M, Reghellin D, Zamboni G, and Bonetti F. PEComas: the past, the present and the future. Virchows Arch. (2008) 452:119–32. doi: 10.1007/s00428-007-0509-1
19. Tanaka Y, Ijiri R, Kato K, Kato Y, Misugi K, Nakatani Y, et al. HMB-45/melan-A and smooth muscle actin-positive clear-cell epithelioid tumor arising in the ligamentum teres hepatis: additional example of clear cell ‘sugar’ tumors. Am J Surg Pathol. (2000) 24:1295–9. doi: 10.1097/00000478-200009000-00015
20. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, and Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. (2005) 29:1558–75. doi: 10.1097/01.pas.0000173232.22117.37
21. Coker D, Kench J, Ansari N, Zhou R, and Sandroussi C. Education and imaging: hepatobiliary and pancreatic: clear cell myomelanotic tumor of ligamentum teres. J Gastroenterol Hepatol. (2013) 28:381. doi: 10.1111/jgh.2013.28.issue-2
22. Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, et al. Management of hepatic angiomyolipoma: A systematic review. Liver Int. (2017) 37:1272–80. doi: 10.1111/liv.2017.37.issue-9
23. Ishak KG. Mesenchymal tumors of the liver [M]. In: Okuda K and Peters RL, editors. Hepatocellular carcinoma. John Wiley and Sons, New York (1976). p. 247–304.
24. Cong WM, Wu MC, Chen H, and Shen F. A case of hepatic angiomyolipoma. Chin J Surg. (1992) 30:618.
25. Wang YR, Qiu FB, Li ZK, and Zhong X. The epidemiological characteristics, diagnosis and treatment of hepatic angiomyolipoma (HAML) in China in the past 23 years. J Hepatopancreatobiliary Surgery. (2012) 24:183–7.
26. Jung DH, Hwang S, Hong SM, Kim KH, Ahn CS, Moon DB, et al. Clinico-pathological correlation of hepatic angiomyolipoma: a series of 23 resection cases. ANZ J Surg. (2018) 88:E60–65. doi: 10.1111/ans.2018.88.issue-1-2
27. Li SL, Qian JZ, and Xu HM. Analysis of the imaging and clinicopathological features of hepatic angiomyolipoma. Chin J Gen Surgery. (2011) 20:696–9.
Keywords: angiomyolipoma, the round ligament of the liver, tumor, surgery, histological
Citation: Zheng H-B, Wang X-Q, Liu X-N, Han X-M and Zhu H-H (2025) A giant angiomyolipoma arising from the hepatic round ligament: a case report and literature review. Front. Oncol. 15:1551361. doi: 10.3389/fonc.2025.1551361
Received: 25 December 2024; Accepted: 02 June 2025;
Published: 26 June 2025.
Edited by:
Lovenish Bains, University of Delhi, IndiaReviewed by:
Naonori Kawakubo, Kyushu University, JapanPrashant Ramteke, All India Institute of Medical Sciences Nagpur, India
Copyright © 2025 Zheng, Wang, Liu, Han and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Hong Zhu, emh1aGFpaG9uZzEyMTRAMTI2LmNvbQ==; Xiu-Min Han, cWh4bjY2QDE2My5jb20=
†These authors share first authorship
 Hai-Bo Zheng
Hai-Bo Zheng Xiang-Qian Wang1†
Xiang-Qian Wang1† Hai-Hong Zhu
Hai-Hong Zhu