- 1Department of Urology, West China Xiamen Hospital, Sichuan University, Xiamen, Fujian, China
- 2Department of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
This article provides a comprehensive review of studies and case analyses on ectopic splenic tissue, with a particular focus on renosplenic disease. Ectopic splenic tissue refers to the abnormal non-physiological localization of spleen tissue, commonly resulting from splenic tissue implantation or hematogenous metastasis following splenectomy. Renosplenic disease is rare and often misdiagnosed as a renal tumor or tumor recurrence, which can lead to unnecessary surgical interventions. By discussing two cases of postoperative renosplenic disease in detail and combining them with a literature review, this article explores the pathogenesis, clinical presentation, imaging characteristics, and diagnostic methods of the condition. Analysis of 39 previously reported cases of nephrosplenopathy revealed that it predominantly affects male patients, typically occurs on the left side, and is often associated with a history of splenectomy, with lesions identified on average 20 years post-splenectomy. The clinical manifestations of nephroplenic disease are nonspecific and are mostly incidental findings during imaging examinations. Hybrid SPECT/CT and SPIO-enhanced MRI are considered the gold standards for diagnosing ectopic splenic tissue. However, the majority of cases are still confirmed through needle biopsy or surgical resection. While surgical diagnosis allows for lesion removal, it also carries risks of postoperative complications, such as intestinal fistula, as reported in one of the cases in this study. Research indicates that ectopic splenic tissue is generally benign but can cause symptoms by compressing adjacent structures. For asymptomatic patients, conservative management or active surveillance is a viable approach. However, in cases of large lesions, the decision between conservative treatment and surgical intervention should be carefully weighed. By summarizing 48 years of nephroplenic disease case data, this article aims to provide a clinical reference for the diagnosis and management of the condition. It emphasizes the critical role of imaging examinations and the potential for conservative treatment, aiming to reduce surgical risks and recovery times while improving diagnostic accuracy, treatment outcomes, and patients’ quality of life.
Introduction
Ectopic splenic tissue, also known as accessory spleen or splenic heterotopia, is the presence of splenic tissue outside its normal anatomical location. It is characterized by nonphysiological displacement of the spleen and can be classified as either congenital or acquired. Acquired splenic heterotopia, also called splenic disease, is typically caused by spleen injury in which the splenic tissue is locally implanted or hematogenously disseminated to other body parts, and in such regions, it is often misdiagnosed as a tumor (1). Ectopic splenic tissue is uncommon in clinical practice and is usually asymptomatic, often discovered incidentally. Reports frequently describe ectopic splenic tissue in the liver, pancreas, gastric fundus, or abdominal cavity (2). Moreover, ectopic splenic tissue located in the kidney or renal fossa is easily mistaken for renal or recurrent tumor, presenting a significant challenge for urologists. This arises from the difficulty in distinguishing ectopic splenic tissue from renal cell carcinoma or other benign renal tumors (3). Accurate characterization of such tissue masses can help avoid unnecessary partial or total nephrectomy, including its associated risks and complications, in asymptomatic patients. Herein, we report on two patients with a history of tumor and splenectomy in whom incidental findings of tumor recurrence led to reoperation, ultimately resulting in a diagnosis of splenic disease.
Case history
Case 1
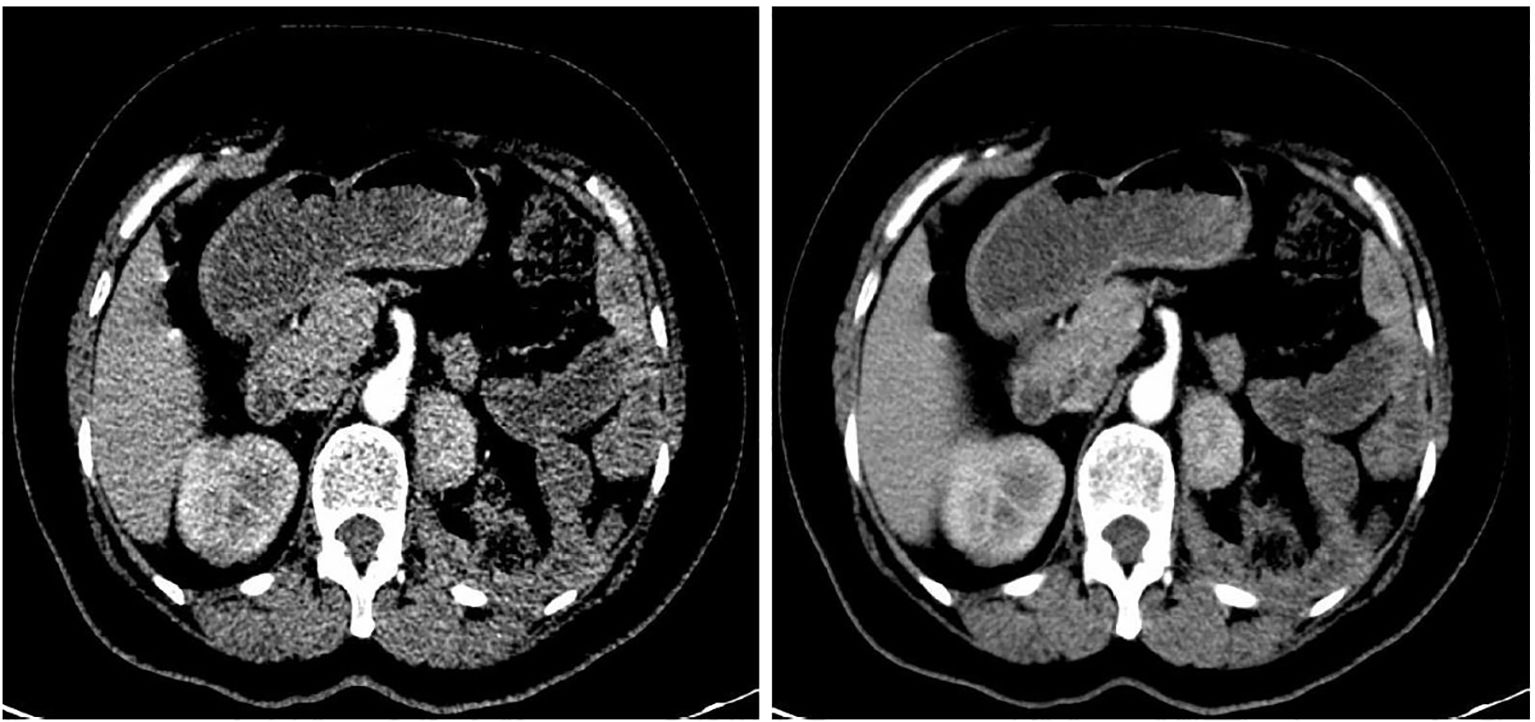
A 50-year-old woman underwent a computed tomography (CT) scan on October 25, 2023, which revealed a left renal mass. The lesion exhibited an abnormal enhancement, raising the suspicion of a neoplastic lesion located at the middle–upper pole of the left kidney, measuring approximately 3.1 × 2.6 cm (Figure 1). She reported no significant symptoms. She had experienced hypertension for the past 4 months, which was well controlled with oral medication. Additionally, she underwent open surgery for bilateral adrenal pheochromocytomas and splenectomy 18 years ago. After further evaluation, pheochromocytoma recurrence was ruled out, and laparoscopic left partial nephrectomy was performed. The postoperative pathology confirmed the presence of splenic tissue. She was followed up for 12 months, with stable disease and no new findings of splenic disease.
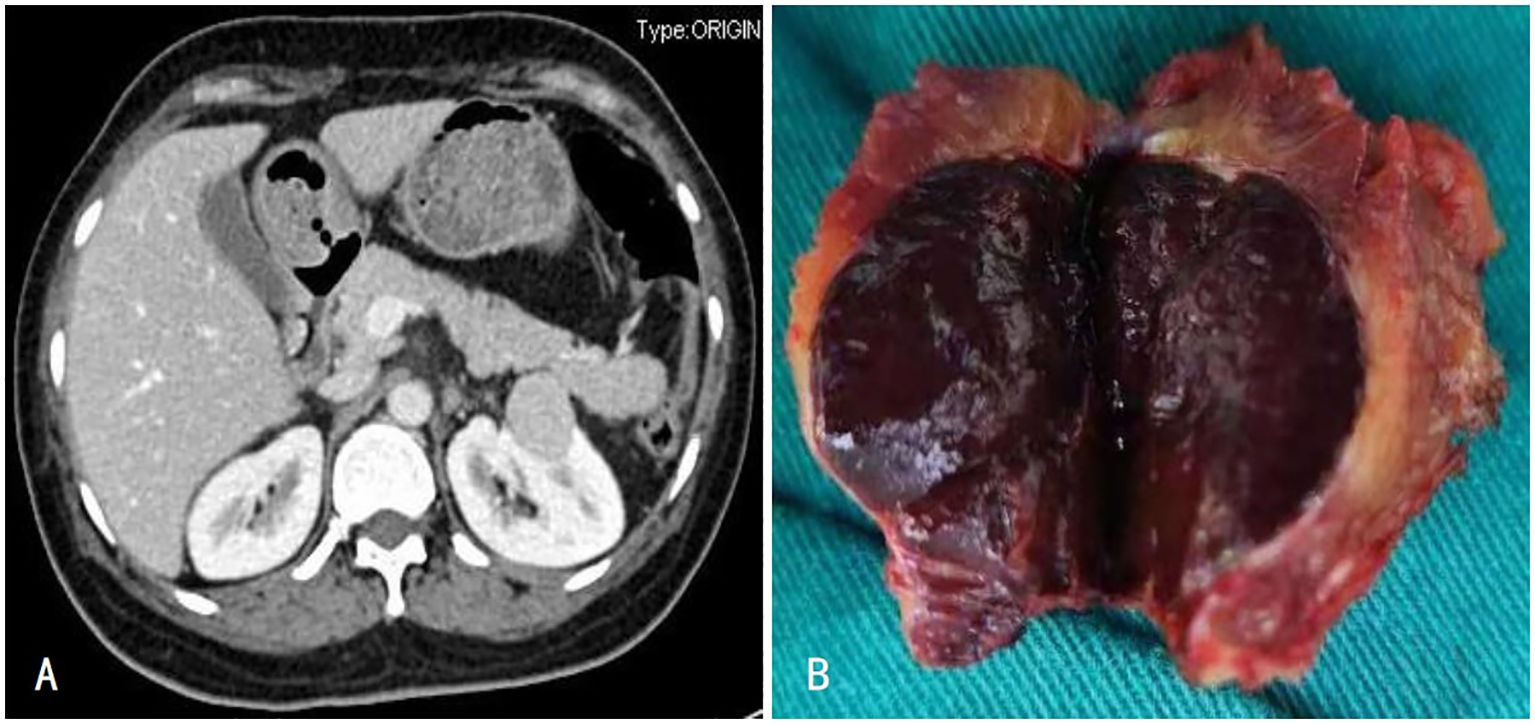
Figure 1. (A) Enhanced CT showed enhancement of the left renal tumor with capsule; (B) The left renal tumor was a dark red mass of tissue.
Case 2
A 62-year-old woman underwent a CT scan on October 26, 2023; the findings revealed a homogeneously enhancing mass measuring approximately 3.6 × 2.8 cm (Figure 2) in the left renal fossa, which was suspected to be a neoplastic lesion. The patient reported no significant symptoms and had no underlying medical conditions. She had undergone left radical nephrectomy and splenectomy for a large renal tumor 13 years ago. Suspecting tumor recurrence, she underwent tumor resection of the left renal fossa under general anesthesia. The postoperative pathology confirmed the presence of splenic tissue. She developed an intestinal fistula postoperatively, which was successfully treated conservatively over 6 months. She was followed up for 12 months, with stable disease and no new findings of splenic disease.
Discussion
The diagnosis of splenic tissue implantation or hematogenous metastasis to other parts of the body, often misdiagnosed as tumors in other locations, is referred to as splenic disease. It can manifest in any body cavity, except the spleen itself, including the abdominal cavity, retroperitoneum, and pancreas (4). Its association with urinary system diseases is relatively rare, as the latter primarily involve the kidneys, urinary tract, and surrounding tissues. The occurrence of ectopic splenic tissue in the kidney or renal fossa is termed renal-splenic disease. In clinical practice, differentiating renal-splenic disease from renal cancer or other benign renal tumors is difficult, often resulting in misdiagnosis as renal or recurrent tumor. This misdiagnosis can result in unnecessary surgical interventions.
Herein, we report two cases of renal-splenic disease that developed after surgical treatment. Furthermore, we provide a literature review based on a MEDLINE (PubMed) database search, identifying 37 cases of renal-splenic disease (Table 1). The earliest case was reported by Rao AK in 1976 (5), whereas the most recent one was published by Oyebola T in BJU International (6).
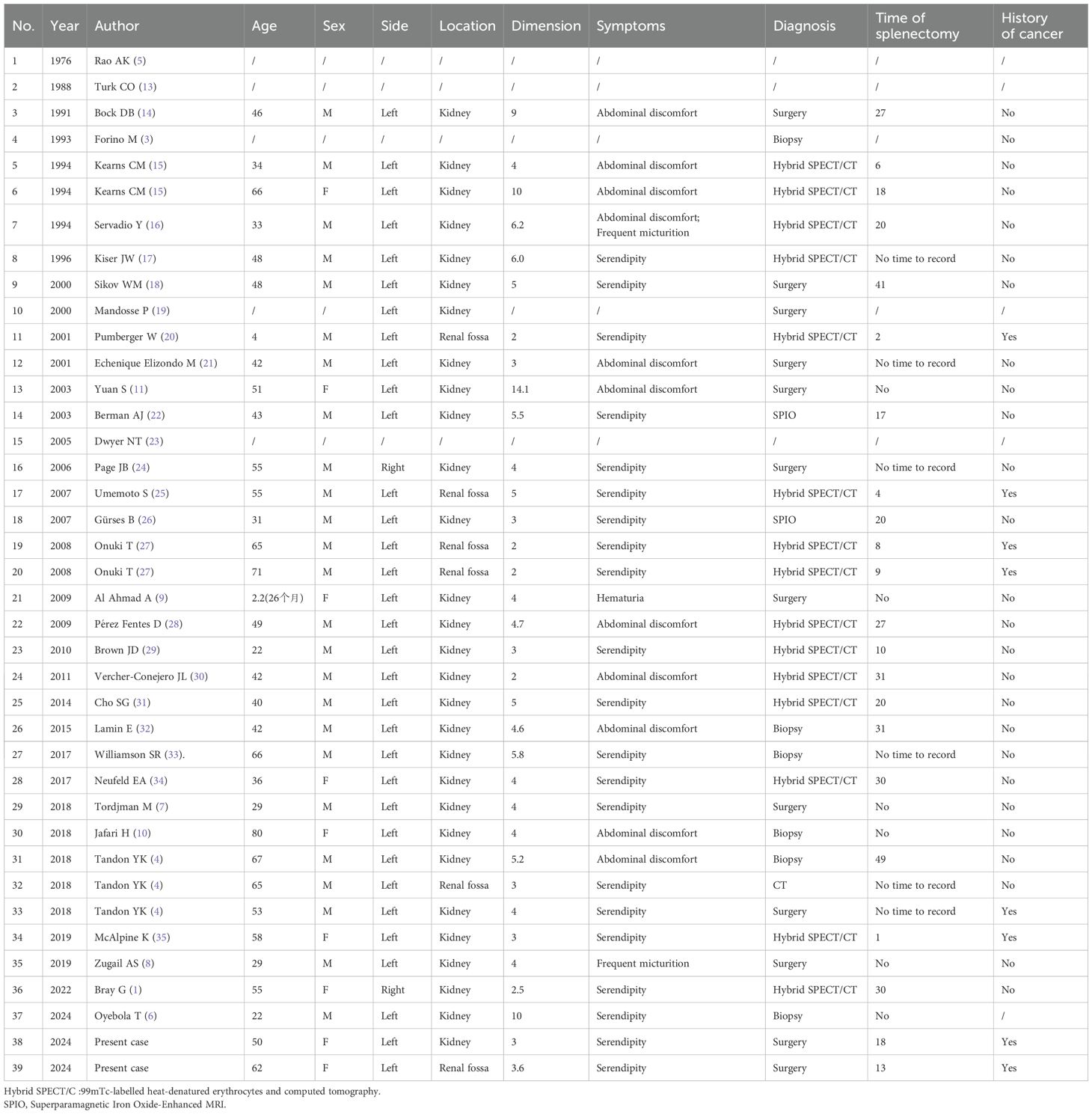
Table 1. Epidemiologic and clinical characteristics of 39 confirmed cases with concurrent renal and splenic pathologies.
Among the 39 patients with renal-splenic disease, 25 and 9 were men and women, respectively, whereas the sex of the remaining 5 patients could not be determined from the literature. The disease occurred on the left side in 33 cases and on the right side in 2, whereas in 4 cases, the involved side was unknown. Furthermore, the disease affected the kidneys in 29 cases and renal fossa in 6, whereas in 4 cases, the location was unspecified.
In the 39 cases, the diagnosis was made using various imaging and diagnostic methods: 15 cases using 99mTc-labeled heat-denatured erythrocytes and CT; 2 using superparamagnetic iron oxide-enhanced magnetic resonance imaging (MRI); 1 using enhanced CT; 6 via biopsy, and 12 after surgical resection. In three cases, the diagnostic method was not specified. The majority of diagnoses were incidental (n = 21), with 11 cases discovered during investigations on abdominal discomfort, 2 cases with a symptom of frequent urination, 1 case with hematuria, and 5 cases with unspecified symptoms. The patients’ ages ranged from 2.2 to 80 years, with an average age of 45.91 years. Of the 28 patients with a history of splenectomy, the time from splenectomy to diagnosis ranged from 1 to 49 years, with an average of approximately 20 years. Imaging showed lesions measuring 2 to 14.1 cm, with an average diameter of 4.71 cm. Of the 39 patients, 8 had a history of malignant tumors whereas 26 had none.
The primary cause of splenic disease is the transfer of splenic tissue to the kidneys or renal fossa following spleen injury (7). Our analysis revealed that renal-splenic disease was more common in men (25:9), which could be attributed to the higher incidence of spleen injury in this population. Splenic tissue metastasis may occur through implantation or hematogenous spread, with the left kidney more frequently involved. This supports the hypothesis that splenic tissue implantation is the primary route of metastasis, consistent with current literature reports (8). Most patients with renal-splenic disease experience nonspecific symptoms and are incidentally diagnosed. However, when ectopic splenic tissue compresses surrounding structures, such as the renal pelvis or ureter, it can lead to urinary obstruction, hydronephrosis, or urinary tract infection, presenting as nonspecific abdominal discomfort, frequent urination, or hematuria (6, 9).
Renal-splenic disease affects all age groups, without specific age predilection. In our cohort, the youngest patient was 2.2 years old (9) and the oldest was 80 years old (10). Among the 28 patients with a history of splenectomy, the time from splenectomy to diagnosis ranged from 1 to 49 years, with an average of approximately 20 years. This long latency can reduce clinicians’ awareness of the need to screen for renal-splenic disease, thereby increasing the risk of misdiagnosis.
Interestingly, nearly half of the cases were diagnosed via imaging, such as Hybrid SPECT/CT (99mTc-labeled heat-denatured erythrocytes and computed tomography) and SPIO (Superparamagnetic Iron Oxide-Enhanced MRI) (1, 15–17, 20, 22, 25–31, 34, 35), and six were diagnosed via biopsy (3, 4, 6, 10, 32, 33). However, as many patients with renal-splenic disease have a history of abdominal surgery, which disrupts normal anatomical structures, performing a biopsy may be difficult. Furthermore, owing to the rich vascular supply of splenic tissue, biopsy may cause bleeding or injury to surrounding organs, although these complications were seldom reported in the literature.
There were 12 cases who were diagnosed after surgical resection, including partial nephrectomy, radical nephrectomy, or renal fossa tumor excision. Surgical resection has certain advantages, particularly in confirming the diagnosis and removal of the lesion. However, it is also associated with surgical complications, prolonged recovery, and potential functional impairment. Therefore, the decision to proceed with surgery should be based on the patient’s specific condition, disease characteristics, and overall health status. Although surgical complications were not reported in the literature, one patient in our cohort developed postoperative enterocutaneous fistula after a prior laparoscopic left nephrectomy.
In conclusion, for asymptomatic patients with renal-splenic disease, conservative treatment does not generally lead to severe disease progression. This is particularly true for patients with a history of splenectomy as the regenerated splenic tissue may perform functions similar to those of the original spleen, such as 1) participant in immune function, i.e., the removal of pathogens and aging red blood cells; 2) blood filtration, i.e., the removal of impurities and aging cells from the blood; 3) blood storage, i.e., the storage of red blood cells and platelets; and 4) hematopoiesis, i.e., the resumption of blood cell production under certain conditions (e.g., bone marrow failure) as they are ectopic splenic tissue (11). While ectopic splenic tissue is typically benign, it may grow large enough to compress adjacent structures, which may prompt surgical removal to prevent potential complications. Interestingly, previous reports have indicated that retroperitoneal ectopic splenic tissue rarely exceeds 4 cm in size (12). However, in our cohort, the average diameter of the lesions was 4.7 cm, which raises questions regarding whether conservative treatment is truly appropriate in these cases or if it delays the optimal time for surgery.
Additionally, we observed that approximately 70% of the patients had a history of splenectomy, with an average of 20 years between splenectomy and the onset of renal-splenic disease. This indicates that splenic tissue slowly generates and that conservative management or active monitoring could be reasonable options.
Although pathological examination remains the gold standard for diagnosis, Hybrid SPECT/CT (99mTc-labeled heat-denatured erythrocytes and computed tomography) and SPIO offer comparable diagnostic value, helping patients avoid invasive procedures or treatments (7).
In the reviewed cases where biopsy or surgical resection was performed, neither Hybrid SPECT/CT nor SPIO were employed, highlighting the diagnostic challenges of renal-splenic disease in the context of urinary system diseases. These challenges include preoperative diagnosis difficulties, increased surgical risks, postoperative complications, and potential impacts on the patient’s overall condition.
Conclusion
Herein, we report 48 years of case data on renal-splenic disease and offer a comprehensive review to inform clinical practice. Correct identification of renal-splenic disease is crucial for patient safety and improve treatment outcomes. Providing rational diagnostic and therapeutic strategies is essential to enhance clinical practice, minimize recovery time and complications, and ultimately improving patients’ quality of life.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LX: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. GS: Conceptualization, Resources, Supervision, Writing – original draft. HZ: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray G, Bahadori A, Teng R, and McClintock DS. Right renal mass diagnosed as focal splenosis; a rare differential for a small renal mass highlighting utility of heat damaged Tc-99m RBC scintigraphy to avoid unnecessary surgery. Urol Case Rep. (2022) 43:102101. doi: 10.1016/j.eucr.2022.102101
2. Pereira Arias JG, Ullate Jaime V, Carral Tellítu I, Ateca Díaz-Obregón R, Gutiérrez Díaz JM, and Berreteaga Gallastegui JR. Pseudomasa renal izquierda infrecuente: ectopia esplénica [Infrequent left renal pseudomass: ectopic spleen. Actas Urol Esp. (2002) 26:574–8. doi: 10.1016/s0210-4806(02)72830-1
3. Forino M, Davis GL, and Zins JH. Renal splenic heterotopia, a rare mimic of renal neoplasia: case report of imaging and fine-needle aspiration biopsy. Diagn Cytopathol. (1993) 9:565–9. doi: 10.1002/dc.2840090520
4. Tandon YK, Coppa CP, and Purysko AS. Splenosis: a great mimicker of neoplastic disease. Abdom Radiol (NY). (2018) 43:3054. doi: 10.1007/s00261-018-1601-5
5. Rao AK and Silver TM. Normal pancreas and splenic variants simulating suprarenal and renal tumors. AJR Am J Roentgenol. (1976) 126:530–7. doi: 10.2214/ajr.126.3.530
6. Oyebola T, Shetty A, Bochinski A, Onicha A, Sudhakar V, Tanabalan C, et al. Case of the Month from Northampton General Hospital, Northampton, UK: renal accessory spleen. BJU Int. (2025) 135:586–590. doi: 10.1111/bju.16435
7. Tordjman M, Eiss D, Dbjay J, Crosnier A, Comperat E, Correas JM, et al. Renal pseudo-tumor related to renal splenosis: imaging features. Urology. (2018) 114:e11–e15. doi: 10.1016/j.urology.2018.01.017
8. Zugail AS, Ahallal Y, Comperat EM, and Guillonneau B. Splenorenal fusion mimicking renal cancer: One case report and literature review. Urol Ann. (2019) 11:211–3. doi: 10.4103/UA.UA_47_18
9. Al Ahmad A, Jourabian M, and Pipelzadeh M. Splenorenal fusion in a 26-month-old girl. Pediatr Radiol. (2009) 39:735–8. doi: 10.1007/s00247-009-1201-1
10. Jafari H, Iranpour P, and Haseli S. Renal pseudomass: be aware of splenorenal fusion. BMJ Case Rep. (2018) 2018:bcr2018226652. doi: 10.1136/bcr-2018-226652
11. Yuan S, Vaughan M, and Agoff SN. Left-sided splenorenal fusion with marked extramedullary hematopoiesis and concurrent lithium toxicity. A case report and review of the literature. Arch Pathol Lab Med. (2003) 127:e1–3. doi: 10.5858/2003-127-e1-LSSFWM
12. Palumbo V, Mannino M, Teodoro M, Menconi G, Schembari E, Corsale G, et al. An extremely rare case of an oversized accessory spleen: case report and review of the literature. BMC Surg. (2019) 19:45. doi: 10.1186/s12893-019-0510-z
13. Turk CO, Lipson SB, and Brandt TD. Splenosis mimicking a renal mass. Urology. (1988) 31:248–50. doi: 10.1016/0090-4295(88)90152-5
14. Bock DB, King BF, Hezmall HP, and Oesterling JE. Splenosis presenting as a left renal mass indistinguishable from renal cell carcinoma. J Urol. (1991) 146:152–4. doi: 10.1016/s0022-5347(17)37737-6
15. Kearns CM, Liu HY, Wollin M, and Lepor H. Splenosis presenting as a left renal mass: a report of two cases. Eur Urol. (1994) 26:264–6. doi: 10.1159/000475392
16. Servadio Y, Leibovitch I, Apter S, Mor Y, and Goldwasser B. Symptomatic heterotopic splenic tissue in the left renal fossa. Eur Urol. (1994) 25:174–6. doi: 10.1159/000475275
17. Kiser JW, Fagien M, and Clore FF. Splenosis mimicking a left renal mass. AJR Am J Roentgenol. (1996) 167:1508–9. doi: 10.2214/ajr.167.6.8956586
18. Sikov WM, Schiffman FJ, Weaver M, Dyckman J, Shulman R, and Torgan P. Splenosis presenting as occult gastrointestinal bleeding. Am J Hematol. (2000) 65:56–61. doi: 10.1002/1096-8652(200009)65:1<56::aid-ajh10>3.0.co;2-1
19. Mandosse P, Bourg S, Paulhac P, Dumas JP, and Colombeau P. Lobulation splénique et pseudo-cancer du rein gauche [Splenic lobulation and pseudo-cancer of the left kidney. Prog Urol. (2000) 10:291–4.
20. Pumberger W, Wiesbauer P, and Leitha T. Splenosis mimicking tumor recurrence in renal cell carcinoma: detection on selective spleen scintigraphy. J Pediatr Surg. (2001) 36:1089–91. doi: 10.1053/jpsu.2001.24765
21. Echenique Elizondo M, Arrosagarav J, and Sanz Jaka JP. Esplenosis: una entidad infradiagnosticada [Splenosis: underdiagnosed entity. Arch Esp Urol. (2001) 54:1133–5. doi: 10.1016/S0009-739X(01)71812-X
22. Berman AJ, Zahalsky MP, Okon SA, and Wagner JR. Distinguishing splenosis from renal masses using ferumoxide-enhanced magnetic resonance imaging. Urology. (2003) 62:748. doi: 10.1016/s0090-4295(03)00509-0
23. Dwyer NT and Whelan TF. Renal splenosis presenting as a renal mass. Can J Urol. (2005) 12:2710–2.
24. Page JB, Lenz DL, and Wong C. Right-sided intrarenal splenosis mimicking a renal carcinoma. ScientificWorldJournal. (2006) 6:2442–4. doi: 10.1100/tsw.2006.380
25. Umemoto S, Miyoshi Y, Nakaigawa N, Yao M, Takebayashi S, and Kubota Y. Distinguishing splenosis from local recurrence of renal cell carcinoma using a technetium sulfur colloid scan. Int J Urol. (2007) 14:245–7. doi: 10.1111/j.1442-2042.2007.01677.x
26. Gürses B, Kabakçi N, Akşit HZ, Yencilek F, Kovanlikaya A, and Kovanlikaya I. Cystic splenosis mimicking a renal mass: a case report and review of the literature. Australas Radiol. (2007) 51:B52–5. doi: 10.1111/j.1440-1673.2007.01783.x
27. Onuki T, Terao H, Muraoka K, Nakamura M, Kobayashi K, Oogo Y, et al. Splenosis mimicking local recurrence after radical nephrectomy: a report of two cases. Hinyokika Kiyo. (2008) 54:353–6.
28. Pérez Fentes D, Pazos González G, Blanco Parra M, Pubul Núñez V, Toucedo Caamaño V, Puñal Pereira A, et al. Esplenosis simulando una masa renal izquierda [Splenosis simulating a renal mass. Arch Esp Urol. (2009) 62:396–9. doi: 10.4321/S0004-06142009000500010
29. Brown JD and Kwee S. Pararenal splenosis encountered during the evaluation of a suspected pheochromocytoma. Am J Med Sci. (2010) 340:424–6. doi: 10.1097/MAJ.0b013e3181ee9599
30. Vercher-Conejero JL, Bello-Arqués P, Pelegrí-Martínez L, Hervás-Benito I, Loaiza-Góngora JL, Falgas-Lacueva M, et al. Esplenosis abdominal: una entidad frecuentemente infradiagnosticada [Abdominal splenosis: an often underdiagnosed entity. Rev Esp Med Nucl. (2011) 30:97–100. doi: 10.1016/j.remn.2010.04.009
31. Cho SG, Yi JH, Kim MY, Kim YH, Han SW, and Kim HJ. Identification of perirenal or renal splenosis from undetermined masses: case report and review of the literature. Clin Nephrol. (2014) 82:263–7. doi: 10.5414/CN107890
32. Lamin E, Smith ZL, Ramchandani P, Coronel M, and Mucksavage P. Intrarenal splenosis diagnosed in an incidentally found left renal mass. Urol Case Rep. (2015) 3:132–4. doi: 10.1016/j.eucr.2015.06.005
33. Williamson SR. Renal splenosis: renal mass biopsy diagnosis of a tumor clinically mimicking renal cell carcinoma. Appl Immunohistochem Mol Morphol. (2017) 25:e27–9. doi: 10.1097/PAI.0000000000000362
34. Neufeld EA, Sekhon S, and Gerscovich EO. Intra-renal splenosis mimicking a solid renal mass. Ultrasound. (2017)25:173–6. doi: 10.1177/1742271X17697752
Keywords: renal splenosis, diagnostic imaging, surgery, metastatic cancer, RCC (renal cell carcinoma)
Citation: Xu L, Wang J, Sun G and Zeng H (2025) Two case reports of renal-splenic disease presenting as renal tumors or metastases, with a literature review. Front. Oncol. 15:1551601. doi: 10.3389/fonc.2025.1551601
Received: 26 December 2024; Accepted: 11 August 2025;
Published: 27 August 2025.
Edited by:
Mihai Dorin Vartolomei, University of Bern, SwitzerlandReviewed by:
Daniel Oberlin, Golden Gate Urology, United StatesTao Wu, Affiliated Hospital of North Sichuan Medical College, China
Copyright © 2025 Xu, Wang, Sun and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijing Xu, NTk3MTM3MDcxQHFxLmNvbQ==; Hao Zeng, a3VjYWl6ZW5nQDE2My5jb20=
 Lijing Xu
Lijing Xu Jialin Wang1
Jialin Wang1 Hao Zeng
Hao Zeng