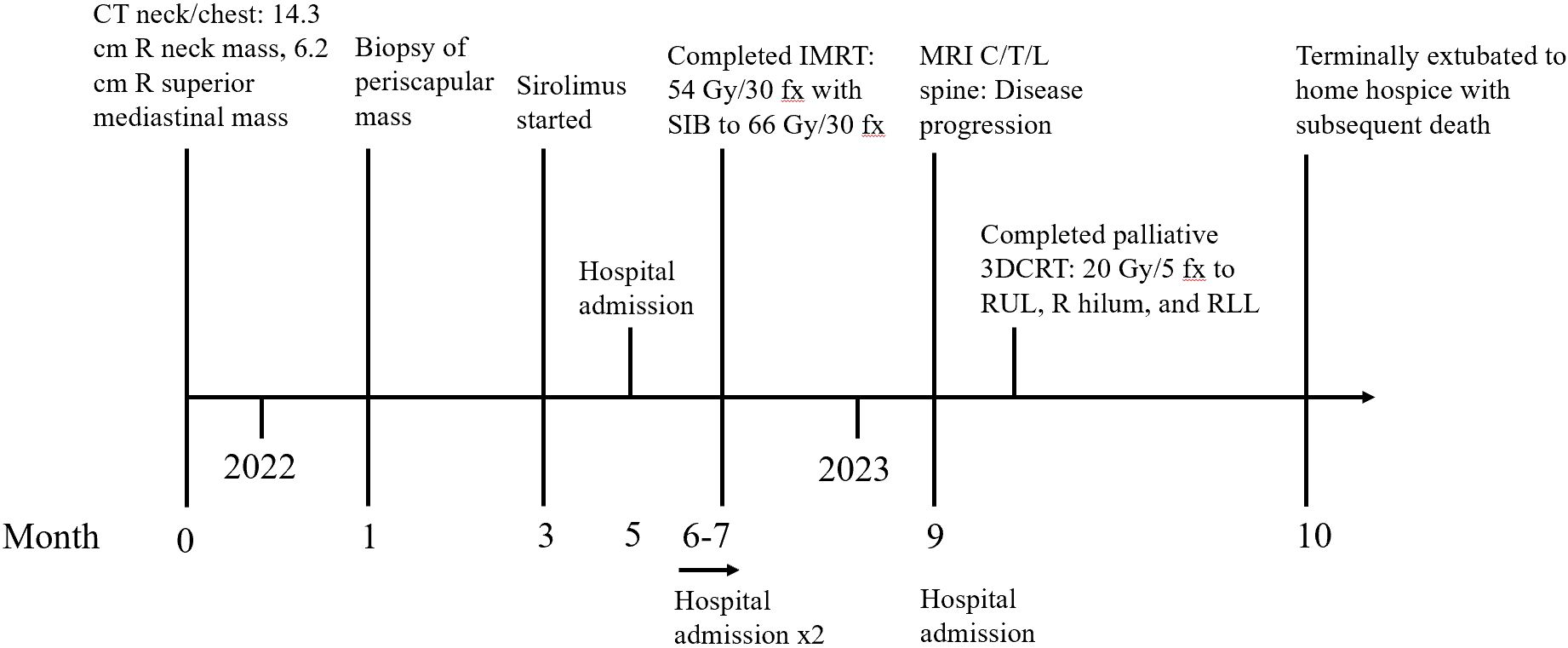
- 1Department of Radiation Oncology, The Ohio State University, Columbus, OH, United States
- 2Family Medicine, Ventura County Medical Center, Ventura, CA, United States
- 3Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, United States
- 4Radiation Oncology, Southeast Health, Dothan, AL, United States
Perivascular epithelioid cell tumors (PEComas) are rare, typically benign soft tissue tumors that can develop at various anatomic sites. Malignant PEComas are rarer entities but may present aggressively with metastasis to the lungs or local recurrence years after initial presentation. In unresectable or metastatic cases, treatment options are limited due to the resistance of PEComas to chemotherapy and radiotherapy. The present case describes a 59-year-old man with a highly aggressive malignant PEComa, which ultimately invaded the mediastinum and replaced the right middle and lower lobes of the lung despite systemic therapy with oral sirolimus and definitive radiotherapy. As only three prior cases have described malignant PEComas invading the mediastinum, we highlight the clinical course of such an aggressive cancer and review current treatment paradigms.
Introduction
Perivascular epithelioid cell tumors represent a group of rare, typically benign soft tissue tumors of mesenchymal origin characterized by a particular constellation of features on histopathology: epithelioid to spindle morphology, clear to granular cytoplasm, mild nuclear atypia with prominent nucleoli, co-expression of melanocytic markers (i.e., HMB-45, melan-A) and smooth muscle markers (i.e., smooth muscle actin, desmin), and perivascular distribution often with vascular smooth muscle infiltration (1–5). Recent reviews and a retrospective cohort study have identified a preponderance of perivascular epithelioid cell tumor (PEComa) cases among female patients with a relatively low median age in the forties (6, 7). Primary tumor sites vary by source but appear to be the kidney (8, 9), gastrointestinal tract (10), retroperitoneum (11, 12), uterus (13), and pelvic soft tissues (14). However, PEComas can arise from any organ, corroborated by the prevalence of case reports describing various primary tumor locations (4, 15) including the head and neck (16), lungs (17–19), pancreas (20, 21), liver (22, 23), genitourinary tract (24), adrenal glands (25), reproductive tract (26), skin (27), and bone (28).
The PEComa group is comprised of angiomyolipomas, pulmonary/extrapulmonary clear cell tumors, lymphangioleiomyomatosis, clear cell myomelanocytic tumor of the falciform ligament or ligamentum teres, soft tissue clear cell myomelanocytic tumors, abdominopelvic perivascular cell epithelioid sarcomas, renal capsular leiomyomas per some authors, and other tumors with similar characteristics at various anatomic sites broadly termed PEComa-not otherwise specified (PEComa-NOS) per the 2020 WHO classification of soft tissue tumors (1, 29, 30). PEComas have an association with tuberous sclerosis (TS), displaying similar genetic mutations and alterations (1–3) with clinical linkage described by several case reports (31, 32). However, in most cases, PEComas are diagnosed incidentally by diagnostic imaging.
Historically, PEComas have been classified as benign, uncertain malignant potential, malignant potential, and malignant, a scheme initially described by Folpe et al. Non-gynecologic histopathologic criteria include tumor size ≥5 cm, infiltrative pattern, high nuclear grade and cellularity, high mitotic rate (≥1/50 high-power field), necrosis, and lymphovascular invasion with ≥2 features required for malignant classification (33). However, Bleeker et al. showed in a retrospective review of 234 cases that tumor size >5 cm and high mitotic rate were the only pathologic features significantly associated with recurrence after surgical resection, proposing a modified risk classification scheme that removed malignant potential (34). To date, the 2020 WHO classification of soft tissue tumors divides PEComas into benign and malignant types and employs the above non-gynecologic-specific criteria as well as modified gynecologic-specific criteria whereby ≥3 histopathologic features are required for malignant classification (30).
Malignant PEComas can present aggressively with rapid growth, metastasis, and death rates similar to high-grade sarcomas (3, 35). Metastatic patterns commonly occur in the lungs (36, 37), followed by the liver, peritoneum, and bone (12, 38). Additionally, a few cases have reported synchronous presentations with malignant cancers (9, 10, 13, 19). Malignant PEComas with aggressive pulmonary invasion or metastasis are very rare, with a few cases described (39, 40), and may precipitate acute complications such as pneumothorax (41). Overall management of such locally advanced, unresectable, or multiply metastatic cases is unclear, as surgical resection with negative margins has been established as the cornerstone of treatment for malignant PEComas (6).
We report a unique presentation of an unresectable malignant PEComa in a 59-year-old man involving the neck and chest wall who developed mediastinal invasion and replacement of the right middle/lower lobes of the lung despite receiving systemic therapy with sirolimus and definitive hypofractionated radiotherapy (RT). Salvage re-irradiation and pazopanib were planned due to disease progression but could not be carried out due to subsequent acute hypoxic respiratory failure requiring intubation. To our knowledge, only three reports have described the clinical course of malignant PEComas invading the mediastinum (42–44). The present case highlights how aggressive malignant PEComas can behave and the limitations of non-invasive treatment.
Case presentation
A 59-year-old man presented to an outpatient clinic with a progressively enlarged posterior neck/upper back mass. He reported right upper extremity radiculopathy and a 30-lb weight loss over a period of 6 months. Physical exam revealed a soft, non-tender, 15 cm × 15 cm mass extending from the right clavicle up 8 cm along the lateral neck to the superior aspect of the right scapula and medial upper thoracic spine. Cranial nerves were intact. Upper extremity strength was equal and symmetric with a full range of motion bilaterally.
Comprehensive metabolic panel was unremarkable. Complete blood count showed microcytic anemia with a hemoglobin level of 10 g/dL and a mean corpuscular volume of 71 fL without clinical blood loss. Platelets were elevated at 563,000/µL favoring iron deficiency anemia, but iron studies were more consistent with anemia of chronic disease. Computed tomography (CT) chest showed a 6.2-cm × 5.5-cm right superior mediastinal mass with a partially visualized supraclavicular mass. CT neck showed a 14.3-cm × 13.3-cm heterogeneously enhancing mass at the base of the right neck extending along the posterior chest wall. Ultrasound-guided biopsy of the periscapular mass showed epithelioid cells in nests and sheets surrounding fragile blood vessels with accompanying necrosis. Immunohistochemistry (IHC) was positive for HMB-45, desmin, cathepsin K, and E-cadherin; TFE3 staining was not reported. Due to tumor size, presence of necrosis, and hallmark IHC findings, the biopsy was interpreted as a multifocal T3N0M0G3, clinical stage IIIB, malignant PEComa by the American Joint Committee on Cancer’s staging system. Positron emission tomography (PET)/CT showed a 12.8-cm mass extending inferiorly from the right posterior lower neck to the right scapula and a 6.4-cm right anterior mediastinal mass extending into the right upper/middle lobes of the lung. Both masses were intensely 18F-2-deoxy-D-glucose (FDG) avid. Of note, there were three pulmonary nodules, raising concern for pulmonary metastasis, especially due to enlargement on subsequent imaging.
As the mass intimately involved structures of the neck and mediastinum, it was deemed unresectable, so the patient was started on sirolimus 4 mg daily. However, the sirolimus level became supratherapeutic at 30.6 ng/mL, so the sirolimus dose was decreased to 2 mg daily.
The patient was briefly hospitalized after his initial diagnosis for acute on chronic anemia requiring transfusion. Hemoccult was positive for which he was started on intravenous pantoprazole. Esophagogastroduodenoscopy and colonoscopy showed no active source of gastrointestinal bleeding. CT chest did not reveal intrathoracic hemorrhage, only showing modest enlargement of the right upper posterior chest wall mass (now 13.2 cm × 7 cm) and the right anterior mediastinal mass (now 6.7 cm × 8.4 cm). Stable left lung nodules were also noted. Consequently, he was discharged on oral pantoprazole with supportive transfusions to maintain a hemoglobin level greater than 8 g/dL. A capsule endoscopy was recommended but ultimately not performed.
Definitive hypofractionated intensity-modulated radiation therapy (IMRT) to the right low posterior neck and right posterior chest wall was initiated with a dose limit of 54 Gy in 30 fractions at the field periphery, due to close proximity to the spinal cord, and a simultaneous integrated boost to 66 Gy in 30 fractions to reach an EQD2 of 70 Gy. The spinal cord received a maximum dose of 39 Gy. The treatment course was complicated by right plexopathy manifesting as right-hand weakness, and two additional admissions for acute on chronic anemia requiring transfusion, although no definite source of blood loss could be identified.
Subsequent CT neck was concerning for disease progression, showing enlargement of the heterogeneous, right posterior cervical neck/chest wall mass (now 16.4 cm × 17.1 cm) with associated osseous erosion of C7-T2 and epidural extension via the right neural foramina with dural sac displacement along C6-T3. Additionally, CT chest, abdomen, and pelvis showed increased size of the right mediastinal/pericardiophrenic mass (now 12.6 cm × 9.1 cm) with a large right pleural effusion and resultant mass effect on the right middle lobe, right atrium, and right mediastinal/hilar vascular structures.
Follow-up magnetic resonance imaging (MRI) of the cervical, thoracic, and lumbar spine redemonstrated the multilobulated, heterogeneous, right lower neck/posterior upper chest mass now with central necrosis extending inferiorly along the right posterior thorax to the level of the T7 vertebral body. Moreover, there was epidural extension through bilateral neural foramina from C6-T4, circumferential tumor involvement causing severe cord compression from T1-T4, and extension of the tumor into the C6-T1 vertebrae and right-sided ribs. The patient was started on dexamethasone to reduce spinal cord edema and compression. Neurosurgical intervention was deemed to entail excessive risk due to the tumor vascularity, aggressive nature, and likelihood of recurrence. Palliative radiotherapy was also considered to relieve cord compression, but the risk of radiation-induced myelopathy was judged to outweigh the potential benefit of tumor size reduction. Thoracic surgical intervention was deemed inappropriate due to the perceived lack of curative or palliative benefit justifying a procedure.
As such, a 6-week course of pazopanib and re-irradiation with hypofractionated IMRT to 60 Gy in 20 fractions to the chest were planned to relieve the mediastinal compression, but the patient developed acute hypoxic respiratory failure requiring intubation as well as circulatory shock, necessitating continuous vasopressor support. CT chest, abdomen, and pelvis revealed the etiology as secondary to near-total collapse of the right lung with moderate right malignant pleural effusion, collapse of the right heart chambers with leftward mediastinal shift, and numerous pulmonary metastases due to the large, heterogeneous, soft tissue tumor (now 15 cm × 16 cm) in the right intrathoracic chest. The right dorsal back component of the tumor measured 14 cm × 13 cm at that time, causing displacement of the right scapula (Figure 1).
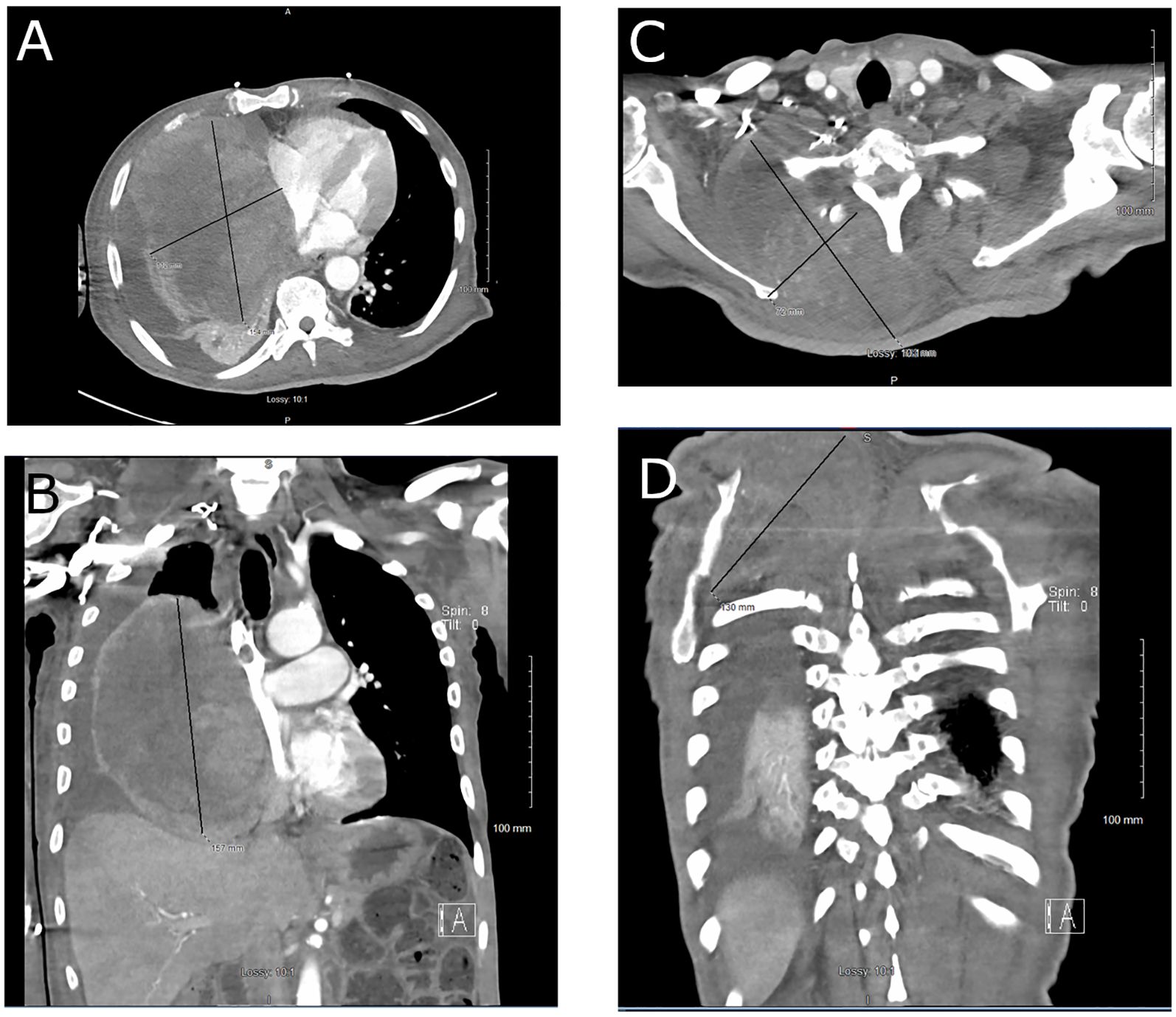
Figure 1. CT chest: (A) axial view and (B) coronal view, demonstrating replacement of the right middle and lower lobes of the lung by a large malignant PEComa. CT neck: (C) axial view and (D) coronal view, showing the right dorsal component of the tumor displacing the right scapula.
With the patient intubated, oral administration of pazopanib was not feasible. As such, palliative 3D conformal radiotherapy to 20 Gy in 5 fractions was initiated to the thoracic component of the tumor to relieve the respiratory symptoms caused by mediastinal invasion (Figure 2). Upon extubation, the patient failed a fiberoptic endoscopic evaluation of swallowing and thus was evaluated for percutaneous endoscopic gastrostomy (PEG) tube placement to permit administration of pazopanib. However, review of prior CT findings revealed significant esophageal compression secondary to mediastinal mass effect, so PEG tube placement could not be performed. The patient was reintubated shortly afterward due to worsening hypoxemic and hypercapnic respiratory failure. Given the poor prognosis and inability to proceed with pazopanib therapy, the patient and his family opted for hospice. He was terminally extubated to bilevel positive airway pressure and discharged to home hospice, ultimately passing away (Figure 3).

Figure 2. Palliative 3D conformal radiation treatment plan to the right upper lobe, right lower lobe, and right hilum. Color wash depicts the calculated 3D dose distribution: (A) axial view, (B) coronal view, (C) sagittal view, and (D) beam’s eye view, showing a treatment volume encompassing the majority of the right lung to treat progressive gross disease. Three prescription dose levels are depicted: blue areas received 100% of the prescription dose, 20 Gy; green areas received 102%–105% of the prescription dose; and red areas received 109% of the prescription dose with a global point maximum (Dmax) of 109.2% landing in an inferior right intercostal space.
Discussion
On a molecular level, PEComa pathogenesis has two potential major pathways. The first is linked to mutations in TSC1 and TSC2, genes also involved in the genesis of TS. Despite the lack of cases reporting TSC information, existing evidence shows that TSC genes were frequently, consistently, and significantly associated with PEComa pathogenesis, likely through an induction of cell proliferation by activation of the mammalian target of rapamycin (mTOR) pathway (45). The second major pathway is the TFE3 gene translocation; TFE3 is a gene encoding the broadly expressed transcription factor E3. Aberrant immunoreactivity for TFE3 protein has been previously observed in the vast majority of PEComas; subsequent analysis of the gene status revealed a distinct subset of PEComa cases with TFE3 gene fusions. Overexpression of TFE3 mediates expression of cathepsin K, which may represent an IHC marker useful in the identification of TFE3-altered PEComas (4). While cathepsin K was positive in our patient’s biopsy specimen, TFE3 expression or translocation was not reported. Two cases with malignant TFE3-rearranged PEComa showed treatment response with the antivascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor apatinib, suggesting that VEGF signaling may also be implicated in TFE3-associated PEComas (46, 47). With respect to TFE3 expression, a retrospective review of 29 patients showed that TFE3 overexpression correlated with more aggressive disease course, higher risk of death, and shorter median overall survival (OS) (48).
On a histopathologic level, several classification schemes have been utilized to predict prognosis and thus guide clinical management, including Folpe (33), Bleeker (34), modified Folpe, Bennet, Schoolmaster (49), and WHO (30), but entail variability in delineating benign/non-malignant versus malignant PEComas (4). A systematic review of uterine PEComas by Garzon et al. identified the modified Folpe classification as the most accurate in predicting the behavior of PEComas, but they proposed changing the requisite cutoffs for tumor size from ≥5 to ≥8 cm and mitoses from ≥1/50 hpf to ≥5/50 hpf to improve accuracy (49). Based on retrospective clinicopathologic analysis, Gantzer et al. successfully developed and validated a PEComa prognostic score (PEC-PRO) to reliably predict event-free survival after surgical resection; the PEC-PRO score also directly correlated with the Folpe classification (50).
Although PEComas are typically diagnosed incidentally, they can present with pain or discomfort associated with the tumor and weight loss (38), similar to our patient’s presentation. Radiographic findings of PEComas are variable, but CT can show intense post-contrast enhancement due to abundant vascular stroma, a finding present in our patient’s initial CT neck and chest (51). Contrast-enhanced Doppler ultrasonography can show a heterogeneous, hypoechoic lesion with peripheral vascularization (21, 23). On MRI, common features include well-circumscribed tumors with no infiltration or local invasion, calcification and/or hemorrhage, hypo- to isointensity relative to skeletal muscle on T1-weighted imaging, heterogeneous hyperintensity on T2-weighted imaging, and significant post-gadolinium enhancement (12, 52). While PEComas, especially those of gastrointestinal origin, tend to exhibit intratumoral hemorrhage on imaging due to their vascularity (53), our patient developed severe anemia without such signs of clinical blood loss, a phenomenon described by other case reports (12). It is likely that his acute on chronic anemia was secondary to exacerbation of anemia of chronic disease due to malignant disease progression, with contribution to anemia from sirolimus therapy (54). Interestingly, PEComas can display FDG avidity on PET/CT, which may not be interpreted as malignant due to their typically benign nature, masking a subset of PEComas with malignant potential (12, 51, 55). Given the heterogeneity in imaging findings, there is no consensus on posttreatment imaging surveillance. However, patients at greater risk have been surveilled with serial PET/CT over the years (56).
Malignant PEComas are primarily treated with radical surgical resection due to resistance to chemotherapy and RT (12, 20, 36, 38). Due to the rarity of malignant PEComas, there are limited data on surgical technique, but two factors have been identified as negative prognosticators by a retrospective cohort study: 1) an intraoperative period between primary tumor and first pulmonary metastasis of less than 30 months and 2) high histologic grade (57). Additional negative prognosticators include the presence of metastasis on diagnosis, grouped-Bleeker’s risk category, and, within the metastatic patient population, the presence of lymph node metastasis (4) due to the importance of surgical resection in treatment. In several cases, metastasectomy of metastatic foci (i.e., lungs, liver, retroperitoneum) permitted durable long-term disease control, with Dudek et al. showing a 5-year OS of 40.4% after the first pulmonary metastasis (57). As such, surgery should always be considered for countable and resectable metastases, similar to other sarcomas (36, 58). However, one case report described a good response to neoadjuvant chemoradiation for an upper extremity PEComa with six cycles of doxorubicin plus ifosfamide followed by preoperative RT to 50 Gy, causing 20% tumor necrosis prior to limb-sparing wide local excision (59). Additionally, neoadjuvant epirubicin with cisplatin and ifosfamide decreased tumor size and improved resectability in a patient with a uterine PEComa (60). Nonetheless, the benefit of neoadjuvant chemotherapy with ifosfamide, vincristine, and dactinomycin has been described as limited to devascularizing the tumor without decreasing tumor size (61); other cytotoxic chemotherapeutics exhibit a small objective response (38). While the role of RT in treating malignant PEComas appears limited due to the paucity of literature, preoperative ultrahypofractionated stereotactic body RT (SBRT) to 60 Gy in 8 fractions using 4D CT and MRI planning permitted a margin-negative resection of a liver PEComa initially invading the inferior vena cava with local control close to 2 years (62). In the adjuvant setting, surgical resection of an adrenal PEComa with unclear margin status followed by conventionally fractionated IMRT to 46.8 Gy in 26 fractions to the tumor bed also resulted in local control close to 2 years (63). In another case, two courses of SBRT up to 30 Gy in 6 fractions coupled with the programmed death (PD)-1 inhibitor tislelizumab provided disease control for a metastatic pelvic PEComa with positive PD-L1 expression (64); utilization of the SBRT technique in conjunction with SIBs appears to permit dose escalation to overcome the inherent radioresistance of malignant PEComas while sparing normal tissues (65). In a case described by McBride et al., pembrolizumab provided a complete response for recurrent PD-L1-positive PEComa metastatic to the lungs status post-lobectomy (66). Thus, the coordination of dose-escalated RT, surgery in operable patients, and immunotherapy may represent the clinical amalgam needed to improve both local and distant control as there appears to be limited overall benefit with cytotoxic chemotherapies, but such determinations require prospective randomized controlled trials.
Due to aberrant mTOR signaling, mTOR inhibitors have demonstrated efficacy in treating malignant PEComas with the phase II AMPECT trial showing median OS of 40.8 months and progression-free survival (PFS) of 10.6 months (67). Consequently, the National Comprehensive Cancer Network (NCCN) guidelines recommend albumin-bound sirolimus for unresectable locally advanced disease or metastatic disease; the intravenous administration of nanoparticle protein-bound sirolimus showed higher intratumoral accumulation, mTOR inhibition, and tumor growth inhibition compared to oral mTOR inhibitors (67, 68). A subsequent retrospective cohort study showed a 5-year OS of 65% over 55.7 months with albumin-bound sirolimus in patients with metastatic PEComa ineligible for surgery (69). However, sirolimus, everolimus, and temsirolimus remain the NCCN guideline-recommended systemic therapies, with sirolimus employed in the present case. Hypofractionated palliative RT to 24 Gy in 8 fractions delivered with concurrent sirolimus provided excellent intrathoracic disease control in a patient with metastatic PEComa thought to be of pulmonary primary, although there was a mixed response to sirolimus at distant metastatic sites (70). Everolimus was able to stabilize disease in a patient with recurrent metastatic PEComa (71). Temsirolimus utilized as adjuvant therapy after lobectomy for metastatic PEComa resulted in durable disease-free survival in a patient with a malignant uterine PEComa initially treated with surgical resection (72). Unfortunately, mTOR inhibitors do not appear to improve PFS relative to standard chemotherapy regimens or provide OS benefit in TSC1/TSC2 mutated malignant PEComas (38, 73). Thus, refractory cases have been treated with the VEGF inhibitors pazopanib and apatinib as salvage or combination therapy (46, 47, 74, 75) but with overall poor objective response and PFS typically less than 6 months.
Conclusions
In the present case, the patient developed mediastinal invasion and replacement of the right upper and middle lungs despite oral sirolimus and definitive hypofractionated RT, an aggressive presentation not previously described in the literature to our knowledge. While cases of progression on mTOR inhibitors have been documented, this case highlights how aggressive malignant PEComas invading the mediastinum can behave and the need for more population-based studies to guide treatment paradigms in inoperable patients. Although successful in other case reports, ultrahypofractionated SBRT would have been difficult to employ over such large treatment volumes in the mediastinum due to the proximity of dose-limiting structures such as the lungs, esophagus, and spinal cord. As our patient’s biopsy specimen was not tested for PD-L1 expression, it is unclear if immunotherapy would have represented a viable alternative salvage treatment, but, nonetheless, salvage treatment with pazopanib could not be implemented to determine the patient’s relative response to VEGF inhibitors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
DL: Conceptualization, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. MF: Investigation, Writing – review & editing. JA: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Dr. Yhana C. Chavis, DO, MSEd, Temple University/Fox Chase Cancer Center, Department of Radiation Oncology and Dr. Mohamad N. Jajeh, MD, Southeast Health, Internal Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
PEComa: perivascular epithelioid cell tumor
PEComa-NOS: Perivascular epithelioid cell tumor-not otherwise specified
RT: radiotherapy
WHO: World Health Organization
TS: tuberous sclerosis
HPF: High-power field
IHC: immunohistochemistry
HMB-45: human melanoma black 45
Melan-A: melanoma antigen
CT: computed tomography
PET: positron emission tomography
FDG: 18F-2-deoxy-D-glucose
IMRT: intensity-modulated radiotherapy
Gy: Gray
SIB: simultaneous integrated boost
EQD2: equivalent dose in 2 Gy fractions
MRI: magnetic resonance imaging
PEG: percutaneous endoscopic gastrostomy
mTOR: mammalian target of rapamycin
VEGF: vascular endothelial growth factor
OS: overall survival
SBRT: stereotactic body radiotherapy
PD-L1: programmed death ligand
PFS: progression-free survival
NCCN: National Comprehensive Cancer Network
References
1. Thway K and Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. (2015) 19:359–68. doi: 10.1016/j.anndiagpath.2015.06.003
2. Utpatel K, Calvisi DF, Köhler G, Kühnel T, Niesel A, Verloh N, et al. Complexity of PEComas: Diagnostic approach, molecular background, clinical management. Pathol. (2020) 41:9–19. doi: 10.1007/s00292-019-0612-5
3. Martignoni G, Pea M, Reghellin D, Zamboni G, and Bonetti F. PEComas: the past, the present and the future. Virchows Arch Int J Pathol. (2008) 452:119–32. doi: 10.1007/s00428-007-0509-1
4. Bourgmayer A, Nannini S, Bonjean P, Kurtz JE, Malouf GG, and Gantzer J. Natural history and treatment strategies of advanced PEComas: A systematic review. Cancers. (2021) 13:5227. doi: 10.3390/cancers13205227
5. Izubuchi Y, Hamada S, Tanzawa Y, Fujita I, Imanishi J, Koyanagi H, et al. Perivascular epithelioid cell tumors (PEComas) of the bone and soft tissue: a Japanese Musculoskeletal Oncology Group (JMOG) multi-institutional study. J Cancer Res Clin Oncol. (2023) 149:13065–75. doi: 10.1007/s00432-023-05114-1
6. Musella A, De Felice F, Kyriacou AK, Barletta F, Di Matteo FM, Marchetti C, et al. Perivascular epithelioid cell neoplasm (PEComa) of the uterus: A systematic review. Int J Surg Lond Engl. (2015) 19:1–5. doi: 10.1016/j.ijsu.2015.05.002
7. Nie P, Wu J, Wang H, Zhou R, Sun L, Chen J, et al. Primary hepatic perivascular epithelioid cell tumors: imaging findings with histopathological correlation. Cancer Imag. (2019) 19:32. doi: 10.1186/s40644-019-0212-x
8. Martignoni G, Pea M, Zampini C, Brunelli M, Segala D, Zamboni G, et al. PEComas of the kidney and of the genitourinary tract. Semin Diagn Pathol. (2015) 32:140–59. doi: 10.1053/j.semdp.2015.02.006
9. Danilewicz M, Strzelczyk JM, and Wagrowska-Danilewicz M. Perirenal perivascular epithelioid cell tumor (PEComa) coexisting with other Malignancies: a case report. Pol J Pathol Off J Pol Soc Pathol. (2017) 68:92–5. doi: 10.5114/pjp.2017.67623
10. Yamada S, Nabeshima A, Noguchi H, Nawata A, Nishii H, Guo X, et al. Coincidence between Malignant perivascular epithelioid cell tumor arising in the gastric serosa and lung adenocarcinoma. World J Gastroenterol. (2015) 21:1349–56. doi: 10.3748/wjg.v21.i4.1349
11. Marinho BM, Canha AG, Silva DS, and Silva JDP. Primary retroperitoneal PEComa: an incidental finding. BMJ Case Rep. (2022) 15:e250466. doi: 10.1136/bcr-2022-250466
12. Dhaliwal K and Marslender S. Malignant retroperitoneal PEComa: A case report with emphasis on radiological findings. Radiol Case Rep. (2023) 18:1358–63. doi: 10.1016/j.radcr.2022.11.042
13. Choi YJ, Hong JH, Kim A, Kim H, and Chang H. A case of Malignant PEComa of the uterus associated with intramural leiomyoma and endometrial carcinoma. J Pathol Transl Med. (2016) 50:469–73. doi: 10.4132/jptm.2016.04.20
14. D’Andrea D, Hanspeter E, D’Elia C, Martini T, and Pycha A. Malignant perivascular epithelioid cell neoplasm (PEComa) of the pelvis: A case report. Urol Case Rep. (2016) 6:36–8. doi: 10.1016/j.eucr.2016.02.004
15. Sobiborowicz A, Świtaj T, Teterycz P, Spałek MJ, Szumera-Ciećkiewicz A, Wągrodzki M, et al. Feasibility and long-term efficacy of PEComa treatment—20 years of experience. J Clin Med. (2021) 10. doi: 10.3390/jcm10102200
16. Bandhlish A, Leon Barnes E, Rabban JT, and McHugh JB. Perivascular epithelioid cell tumors (PEComas) of the head and neck: report of three cases and review of the literature. Head Neck Pathol. (2011) 5:233–40. doi: 10.1007/s12105-011-0268-9
17. Donato UM and Ferguson K. Perivascular epithelioid cell tumor (PEComa) of the lung in a 56-year-old female patient: A case report. Cureus. (2022) 14:e29246. doi: 10.7759/cureus.29246
18. Na HR, Suh JH, and Lee J. Primary perivascular epithelioid cell tumor of the lung: A case report. J Chest Surg. (2023) 56(5):367–70. doi: 10.5090/jcs.22.139
19. Zhao J, Teng H, Zhao R, Ding W, Yu K, Zhu L, et al. Malignant perivascular epithelioid cell tumor of the lung synchronous with a primary adenocarcinoma: one case report and review of the literature. BMC Cancer. (2019) 19:235. doi: 10.1186/s12885-019-5383-0
20. Zizzo M, Ugoletti L, Tumiati D, Castro Ruiz C, Bonacini S, Panebianco M, et al. Primary pancreatic perivascular epithelioid cell tumor (PEComa): A surgical enigma. A systematic review of the literature. Pancreatol Off J Int Assoc Pancreatol IAP Al. (2018) 18:238–45. doi: 10.1016/j.pan.2018.02.007
21. Okuwaki K, Kida M, Masutani H, Yamauchi H, Katagiri H, Mikami T, et al. A resected perivascular epithelioid cell tumor (PEComa) of the pancreas diagnosed using endoscopic ultrasound-guided fine-needle aspiration. Intern Med Tokyo Jpn. (2013) 52:2061–6. doi: 10.2169/internalmedicine.52.0746
22. Jafari A, Fischer HP, von Websky M, Hong GS, Kalff JC, and Manekeller S. Primary perivascular epitheloid cell tumour (PEComa) of the liver: case report and review of the literature. Z Gastroenterol. (2013) 51:1096–100. doi: 10.1055/s-0033-1350123
23. Matrood S, Görg C, Safai Zadeh E, and Alhyari A. Hepatic perivascular epithelioid cell tumor (PEComa): contrast-enhanced ultrasound (CEUS) characteristics-a case report and literature review. Clin J Gastroenterol. (2023) 16:444–9. doi: 10.1007/s12328-023-01779-w
24. Sukov WR, Cheville JC, Amin MB, Gupta R, and Folpe AL. Perivascular epithelioid cell tumor (PEComa) of the urinary bladder: report of 3 cases and review of the literature. Am J Surg Pathol. (2009) 33:304–8. doi: 10.1097/PAS.0b013e3181854929
25. Lau SK. Malignant PEComa of the adrenal gland. Pathol Res Pract. (2012) 208:113–7. doi: 10.1016/j.prp.2011.11.002
26. Yang W, Li G, and Wei-qiang Z. Multifocal PEComa (PEComatosis) of the female genital tract and pelvis: a case report and review of the literature. Diagn Pathol. (2012) 7:23. doi: 10.1186/1746-1596-7-23
27. Stuart LN, Tipton RG, DeWall MR, Parker DC, Stelton CD, Morrison AO, et al. Primary cutaneous perivascular epithelioid cell tumor (PEComa): Five new cases and review of the literature. J Cutan Pathol. (2017) 44:713–21. doi: 10.1111/cup.12972
28. Lao IW, Yu L, and Wang J. Malignant perivascular epithelioid cell tumor (PEComa) of the femur: a case report and literature review. Diagn Pathol. (2015) 10:54. doi: 10.1186/s13000-015-0292-2
29. Bao L, Shi Y, Zhong J, Zhao M, Wu J, Hai L, et al. Histopathologic characteristics and immunotypes of perivascular epithelioid cell tumors (PEComa). Int J Clin Exp Pathol. (2019) 12:4380–9.
30. Sbaraglia M, Bellan E, and Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. (2021) 113:70–84. doi: 10.32074/1591-951X-213
31. Torres Luna N, Mosquera JE, Comba IY, Kinaan M, and Otoya J. A primary adrenal epithelioid angiomyolipoma (PEComa) in a patient with tuberous sclerosis complex: report of a case and review of the literature. Case Rep Med. (2020) 2020:5131736. doi: 10.1155/2020/5131736
32. Larque AB, Kradin RL, Chebib I, Nielsen GP, Selig MK, Thiele EA, et al. Fibroma-like PEComa: A tuberous sclerosis complex-related lesion. Am J Surg Pathol. (2018) 42:500–5. doi: 10.1097/PAS.0000000000001024
33. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, and Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. (2005) 29:1558–75. doi: 10.1097/01.pas.0000173232.22117.37
34. Bleeker JS, Quevedo JF, and Folpe AL. Malignant” Perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Pollock R Editor Sarcoma. (2012) 2012:541626. doi: 10.1155/2012/541626
35. Zhong J, Hu Y, Si L, Xing Y, Geng J, Jiao Q, et al. Primary perivascular epithelioid cell tumor (PEComa) in bone: A review of the literature and a case arising in the humerus with multiple metastases. J Bone Oncol. (2021) 26:100336. doi: 10.1016/j.jbo.2020.100336
36. Fuse Y, Mori S, Sato S, Kato D, Shibazaki T, Nakada T, et al. A successful case of complete surgical resection via left upper and right lower lobectomy for bilateral lung metastases of a perivascular epithelioid cell tumor in the colon: a case report. Surg Case Rep. (2021) 7:233. doi: 10.1186/s40792-021-01314-4
37. Ascione A, Martignoni G, d’Amati G, Della Rocca C, Graziano P, and Pernazza A. Extremely late-onset pulmonary metastasis from uterine PEComa. Pathologica. (2022) 114:312–5. doi: 10.32074/1591-951X-762
38. Sobiborowicz A, Czarnecka AM, Szumera-Ciećkiewicz A, Rutkowski P, and Świtaj T. Diagnosis and treatment of Malignant PEComa tumours. Oncol Clin Pract. (2020) 16:22–33. doi: 10.5603/OCP.2020.0003
39. Tang SH, Liu YC, Hsiao HH, Cho SF, Tsai YF, Wang HC, et al. Huge soft tissue PEComa with aggressive lung and bone metastases. J Cancer Res Pract. (2017) 4:115–8. doi: 10.1016/j.jcrpr.2017.04.001
40. Harris GC, McCulloch TA, Perks G, and Fisher C. Malignant perivascular epithelioid cell tumour (“PEComa”) of soft tissue: a unique case. Am J Surg Pathol. (2004) 28:1655–8. doi: 10.1097/00000478-200412000-00017
41. Okamoto S, Komura M, Terao Y, Kurisaki-Arakawa A, Hayashi T, Saito T, et al. Pneumothorax caused by cystic and nodular lung metastases from a Malignant uterine perivascular epithelioid cell tumor (PEComa). Respir Med Case Rep. (2017) 22:77–82. doi: 10.1016/j.rmcr.2017.06.011
42. Kinskey JC, Schwartz MR, Guo CC, and Ro JY. Perivascular epithelioid cell tumor in the mediastinum: Metastasis or multiple primaries? Hum Pathol Rep. (2022) :29:300658. doi: 10.1016/j.hpr.2022.300658
43. Liang W, Xu S, and Chen F. Malignant perivascular epithelioid cell neoplasm of the mediastinum and the lung: one case report. Med (Baltimore). (2015) 94:e904. doi: 10.1097/MD.0000000000000904
44. Attia KH, Al Boukai AA, Mohammed MH, and Arafah M. Renal and mediastinal perivascular epithelioid cell tumors (PEComas) in a young child with tuberous sclerosis; a rare case report. BJR Case Rep. (2022) 9:20220105. doi: 10.1259/bjrcr.20220105
45. Pan CC, Jong YJ, Chai CY, Huang SH, and Chen YJ. Comparative genomic hybridization study of perivascular epithelioid cell tumor: molecular genetic evidence of perivascular epithelioid cell tumor as a distinctive neoplasm. Hum Pathol. (2006) 37:606–12. doi: 10.1016/j.humpath.2006.01.008
46. Xu J, Gong XL, Wu H, and Zhao L. Case report: gastrointestinal PEComa with TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. (2020) 10:582087/full. doi: 10.3389/fonc.2020.582087/full
47. Zhang N, Ren Y, Zan L, and Wang Y. Case report: Kidney perivascular epithelioid cell tumor treated with anti-VEGFR tyrosine kinase inhibitor and MTOR inhibitor. Front Oncol. (2022) 12:966818/full. doi: 10.3389/fonc.2022.966818/full
48. Testa S, Bui NQ, and Ganjoo KN. Systemic treatments and molecular biomarkers for perivascular epithelioid cell tumors: A single-institution retrospective analysis. Cancer Res Commun. (2023) 3:1212–23. doi: 10.1158/2767-9764.CRC-23-0139
49. Garzon S, Caliò A, Ferrari FA, Iannicello CQ, Zorzato PC, Bosco M, et al. Uterine perivascular epithelioid cell tumors (PEComa) and the accuracy of proposed classification systems in predicting the Malignant versus non-malignant behavior. Gynecol Oncol. (2024) :188:35–43. doi: 10.1016/j.ygyno.2024.06.007
50. Gantzer J, Toulmonde M, Severac F, Chamseddine AN, Charon-Barra C, Vinson C, et al. PEC-PRO: A new prognostic score from a series of 87 patients with localized perivascular epithelioid cell neoplasms (PEComas) treated with curative intent. Cancer. (2024) 130:2304–14. doi: 10.1002/cncr.35277
51. Zarbis N, Barth TFE, Blumstein NM, and Schelzig H. Pecoma of the lung: a benign tumor with extensive 18F-2-deoxy-D-glucose uptake. Interact Cardiovasc Thorac Surg. (2007) 6:676–8. doi: 10.1510/icvts.2007.154039
52. Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, Wagner AJ, et al. Imaging features of primary and metastatic Malignant perivascular epithelioid cell tumors. Am J Roentgenol. (2014) 202:252–8. doi: 10.2214/AJR.13.10909
53. Meredith L, Chao T, Nevler A, Basu Mallick A, Singla RK, McCue PA, et al. A rare metastatic mesenteric Malignant PEComa with TSC2 mutation treated with palliative surgical resection and nab-sirolimus: a case report. Diagn Pathol. (2023) 18:45. doi: 10.1186/s13000-023-01323-x
54. Wagner AJ, Ravi V, Ganjoo KN, Van Tine BA, Riedel RF, Chugh R, et al. ABI-009 (nab-sirolimus) in advanced Malignant perivascular epithelioid cell tumors (PEComa): Preliminary efficacy, safety, and mutational status from AMPECT, an open label phase II registration trial. J Clin Oncol. (2019) 37:11005–5. doi: 10.1200/JCO.2019.37.15_suppl.11005
55. Wu J, Jiang L, Zhang F, Huang Y, and Wang H. Malignant perivascular epithelioid cell tumor of lung on FDG PET/CT. Clin Nucl Med. (2019) 44:469–71. doi: 10.1097/RLU.0000000000002505
56. Ciarallo A, Makis W, Hickeson M, and Derbekyan V. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus: serial imaging with F-18 FDG PET/CT for surveillance of recurrence and evaluation of response to therapy. Clin Nucl Med. (2011) 36:e16. doi: 10.1097/RLU.0b013e31820ae032
57. Dudek W, Schreiner W, Mykoliuk I, Higaze M, and Sirbu H. Pulmonary metastasectomy for sarcoma-survival and prognostic analysis. J Thorac Dis. (2019) 11:3369–76. doi: 10.21037/jtd.2019.08.10
58. Czarnecka AM, Skoczylas J, Bartnik E, Świtaj T, and Rutkowski P. Management strategies for adults with locally advanced, unresectavle or metastatic Malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manag Res. (2023) 15:615–23. doi: 10.2147/CMAR.S351284
59. Osei DA, Alvandi F, Brooks JS, and Ogilvie CM. PEComa of the upper extremity: A unique case and description of an initial response to neoadjuvant chemotherapy. Sarcoma. (2007) 2007:53056. doi: 10.1155/2007/53056
60. Liu JL, Lin YM, Lin MC, Yeh KT, Hsu JC, and Chin CJ. Perivascular epithelioid cell tumor (PEComa) of the uterus with aggressive behavior at presentation. Hematol Oncol Stem Cell Ther. (2009) 2:426–30. doi: 10.1016/S1658-3876(09)50013-1
61. Armah HB and Parwani AV. Perivascular epithelioid cell tumor. Arch Pathol Lab Med. (2009) 133:648–54. doi: 10.5858/133.4.648
62. Kirste S, Kayser G, Zipfel A, Grosu AL, and Brunner T. Unresectavle hepatic PEComa: a rare Malignancy treated with stereotactic body radiation therapy (SBRT) followed by complete resection. Radiat Oncol Lond Engl. (2018) 13:28. doi: 10.1186/s13014-018-0974-5
63. Akay SU, Kesen O, Küçük D, and Yener E. Adrenal PEComa treated by surgical resection and postoperative radiotherapy: A case report. Am J Case Rep. (2024) 25:e945177. doi: 10.12659/AJCR.945177
64. Wang Y, Li W, Zuo X, Min K, Tang Y, Chen H, et al. Anti-PD-1 immunotherapy combined with stereotactic body radiation therapy and GM-CSF for the treatment of advanced Malignant PEComa: A case report. Front Oncol. (2023) 13:1045119. doi: 10.3389/fonc.2023.1045119
65. Zagardo V and Ferini G. Is there any role for radiotherapy in the management of uterine perivascular epithelioid cell tumors (PEComas)? Arch Gynecol Obstet. (2024) 310:1789–91. doi: 10.1007/s00404-024-07578-z
66. McBride A, Garcia AJ, Sanders LJ, Yiu K, Cranmer LD, Kuo PH, et al. Sustained response to pembrolizumab in recurrent perivascular epithelioid cell tumor with elevated expression of programmed death ligand: a case report. J Med Case Rep. (2021) 15:400. doi: 10.1186/s13256-021-02997-x
67. Wagner AJ, Ravi V, Riedel RF, Ganjoo K, Van Tine BA, Chugh R, et al. nab-sirolimus for patients with Malignant perivascular epithelioid cell tumors. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:3660–70. doi: 10.1200/JCO.21.01728
68. AMPECT. Long-term follow-up for duration of response (DoR) after weekly nab-sirolimus in patients with advanced Malignant perivascular epithelioid cell tumors (PEComa): Results from a registrational open-label phase II trial. J Clin Oncol. (2020). doi: 10.1200/JCO.2020.38.15_suppl.11516
69. Świtaj T, Sobiborowicz A, Teterycz P, Klimczak A, Makuła D, Wągrodzki M, et al. Efficacy of sirolimus treatment in PEComa–10 years of practice perspective. J Clin Med. (2021) 10:3705. doi: 10.3390/jcm10163705
70. Bajaj A and Abazeed ME. Molecularly targeted radiation therapy using mTOR inhibition for the management of Malignant perivascular epithelioid cell tumor (PEComa): A case report and review. Adv Radiat Oncol. (2021) 6. doi: 10.1016/j.adro.2021.100657
71. Cihan YB, Kut E, and Koç A. Recurrence of retroperitoneal localized perivascular epithelioid cell tumor two years after initial diagnosis: case report. Sao Paulo Med J Rev Paul Med. (2019) 137:206–8. doi: 10.1590/1516-3180.2017.0120050717
72. Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, et al. Treatment with the mTOR inhibitor temsirolimus in patients with Malignant PEComa. Ann Oncol. (2010) 21:1135–7. doi: 10.1093/annonc/mdq044
73. Liu L, Dehner C, Grandhi N, Lyu Y, Borcherding DC, Chrisinger JSA, et al. The impact of TSC-1 and -2 mutations on response to therapy in Malignant PEComa: A multicenter retrospective analysis. Genes. (2022) 13:1932. doi: 10.3390/genes13111932
74. Liapi A, Mathevet P, Herrera FG, Hastir D, and Sarivalasis A. VEGFR inhibitors for uterine metastatic perivascular epithelioid tumors (PEComa) resistant to mTOR inhibitors. A case report and review of literature. Front Oncol. (2021) 11:641376. doi: 10.3389/fonc.2021.641376
Keywords: perivascular epithelioid cell tumors, intensity-modulated radiotherapy, 3D conformal radiotherapy, palliative radiotherapy, mTOR inhibitors, VEGF inhibitors
Citation: Leach DF III, Margam S S, Foster M and Adkison JB (2025) Case Report: Malignant perivascular epithelioid cell tumor with aggressive mediastinal invasion and pulmonary metastasis. Front. Oncol. 15:1551663. doi: 10.3389/fonc.2025.1551663
Received: 26 December 2024; Accepted: 23 July 2025;
Published: 18 August 2025.
Edited by:
Eric Chi-ching Ko, Beth Israel Deaconess Medical Center Cancer Center, United StatesReviewed by:
Vikram R. Lele, Jaslok Hospital, IndiaZuheir Alshehabi, Tishreen University, Syria
Ioannis S Pateras, National and Kapodistrian University of Athens, Greece
Copyright © 2025 Leach, Margam S, Foster and Adkison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel F. Leach III, ZGZsMjlAY29ybmVsbC5lZHU=
 Daniel F. Leach III
Daniel F. Leach III Srivikram Margam S
Srivikram Margam S Marissa Foster3
Marissa Foster3