- 1Gynecologic Oncology Center, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Oncology, The Sixth People’s Hospital of Chengdu, Chengdu, China
Objective: We aimed to evaluate the clinicopathological characteristics and survival outcomes of patients with second primary ovarian carcinomas after a breast cancer diagnosis.
Materials and methods: We reviewed the medical reports of 23 patients at Sichuan Cancer Hospital between May 2002 and October 2021. We analyzed demographic and clinicopathologic characteristics, the time interval between diagnoses, and survival time. Kaplan-Meier and Cox proportional hazards regression tests were used to determine survival outcomes.
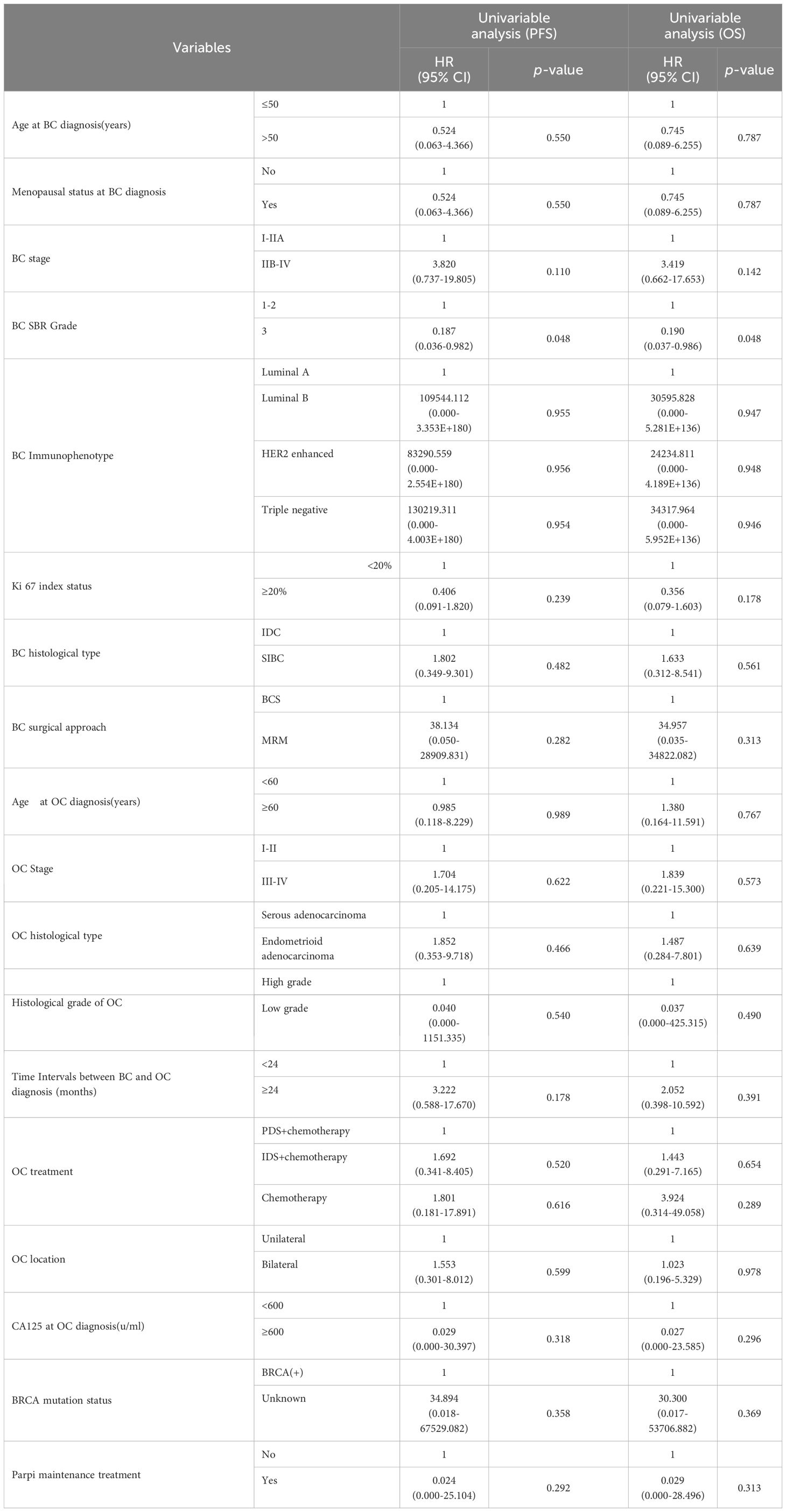
Results: The median age of patients at the time of diagnosis of breast cancer (BC) and ovarian cancer (OC) was 46 and 49 years, respectively. Among them were 6 cases of synchronous OC and 17 instances of metachronous OC. The average interval between diagnoses of the two cancers was 62.48 months. The median OS after the second primary OC diagnosis was 38 months. According to Kaplan-Meier’s analysis, the advanced stage at presentation of BC (p=0.023) resulted in a significantly shorter interval between BC and OC diagnosis. On univariate Cox regression analysis, only BC Scarff-Bloom-Richardson (SBR) 3 Grade resulted in a considerably worse PFS (HR 0.187, p=0.048) and OS (HR 0.190, p=0.048), respectively.
Conclusion: We should strengthen the follow-up management of breast cancer patients.The later the stage of breast cancer, the shorter the time interval of diagnosis of OC was. Early control of ovarian tumors and active comprehensive treatment for synchronous and metachronous breast and ovarian cancer can achieve good results.
Highlights
● We should strengthen the follow-up management of breast cancer patients.
● The later the stage of breast cancer, the shorter the time interval of diagnosis of OC was.
● Early control of ovarian tumors and management throughout the treatment of BC and OC can yield good result.
1 Introduction
Multiple primary cancer (MPC) refers to the simultaneous or sequential occurrence of two or more primary cancers in the same patient’s single or multiple organs, with the same or different tissue types but no subordinate relationship between the tumors. Synchronous tumors are defined as the diagnosis of both primary tumors at the same time or second primary cancers detected within six months of primary tumor detection, while metachronous tumors have been defined as the diagnosis of both primary tumors more than six months apart.
In recent years, more and more patients with breast cancer have ovarian cancer many years later. Still, the diagnosis and treatment of some patients are often delayed due to a lack of attention. Lee et al. reported that the incidence of recurrent breast cancer second tumors in breast cancer was 2.02%, of which 0.34% were patients with ovarian cancer (1). Young women with a history of breast cancer were at high risk for ovarian cancer even 20 years after diagnosis (2). It is known that both breast and ovary are target organs of the sex hormone regulation axis, and they also have some common regulatory genes (3, 4), which may be the disease basis of breast cancer combined with ovarian cancer. Hereditary breast cancer ovarian cancer syndrome (HBOCS) is the most common underlying cause of hereditary breast cancer, with an incidence of 2%~7% in all breast cancer (5). BC and OC presenting as a synchronous or metachronous tumour often pose a diagnostic dilemma.There are many similarities between the primary ovarian cancer and the ovarian metastases from breast cancer but the treatment methods and prognosis between the two groups are different. So we should be focused on the differential diagnosis of metastatic disease and primary ovarian neoplasms.
There is little known regarding the time intervals between initial BC and OC diagnosis, as well as the prognosis of people with both cancers. So our study looked at the clinicopathological characteristics, time intervals between these two primary tumors, and survival outcomes of patients with second primary ovarian carcinomas after a breast cancer diagnosis.
2 Materials and methods
2.1 Patients
We reviewed medical reports of patients with second primary ovarian carcinomas after breast cancer diagnosis between January 2002 and December 2021 at Sichuan Cancer Hospital. The information includes demographic data, tumor stage (BC staging according to the 8th edition American Joint Committee on Cancer staging system while OC staging according to the 2018 International Federation of Gynecology and Obstetrics staging system), histological type, tumor grade, hormonal/HER2 receptors status (BC), surgical and medical treatment, the time intervals between double primary breast and ovarian cancers and disease-related outcomes, et al. were collected. The Institutional Review Board of Sichuan Cancer Hospital approved this study. The requirement for informed consent was waived because we analyzed de-identified data secondarily.
2.2 Pathology
Synchronous and Metachronous Breast and Ovarian Cancer was confirmed using a panel of IHC markers, PAX8, WT1, P53, CA125, and P16 for OC, and estrogen receptor (ER), progesterone receptor (PR), HER2, mammaglobin, GCDFP-15, GATA3 for BC. Additional markers such as CK7, CK20, and Vimentin were performed to rule out metastatic disease from the gastrointestinal tract and when required. These markers were used to approximately classify patients into four breast cancer subtypes: luminal A (ER + or PR +, and HER2 -); luminal B (ER + or PR +, and HER2 +); HER2 enhanced (ER -, PR- and HER2 +); and triple negative (ER -, PR - and HER2-).
2.3 Statistical analysis
Statistical analysis was conducted using SPSS statistical software version 27 (IBM Corp.). The association between variables was evaluated using the χ2 or t-test, as appropriate. Overall survival (OS) was calculated as the interval between each cancer diagnosis and death or last follow-up. We also evaluated the progression-free survival (PFS) after the OC diagnosis, calculated from the time of OC diagnosis to progression or last follow-up. Survival curves were estimated using the Kaplan–Meier model and the hazard ratio (HR) and 95% confidence interval (95% CI) were calculated with the Cox regression model. Level of significance was set at 0.05. The data cut-off for the survival events was March 2024.
3 Results
3.1 Clinico-pathological characteristics
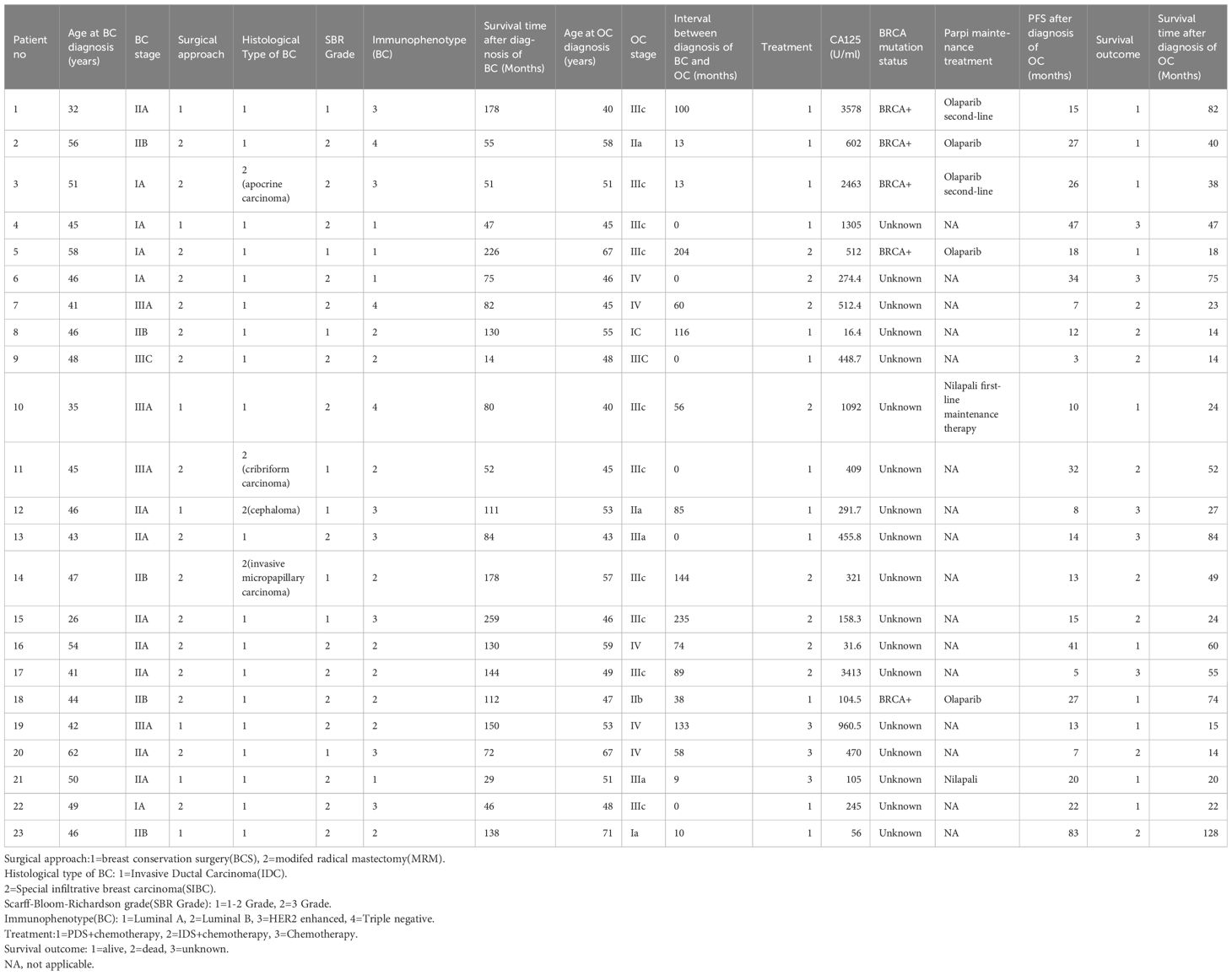
A total of 23 patients were included in the study, and the median age at BC or OC diagnosis was 46 years (32-62 years)and 49 years (40-71 years), respectively. Among them were 6 synchronous OC and 17 cases of metachronous OC. Only one patient has a family history of tumors; her mother suffers from rectal cancer, and her brother suffers from lung cancer. Approximately 56.5% of OC cases occurred during the first 60 months after the BC diagnosis. The most common histological subtype of OC was high-grade serous carcinoma (19/24, 82.6%), other 4 patients was endometrioid adenocarcinoma. At the time of diagnosis of OC, the median serum CA-125 levels were 448.7 U/mL (16.4-3578 U/mL) (Table 1).
All patients were treated following discussion in a multidisciplinary team. Surgery for BC comprised modified radical mastectomy (MRM) and breast conservation surgery (BCS), followed by chemo-endocrine therapy and radiotherapy as per institutional protocols depending on the stage and immunophenotype of breast cancer. For patients with OC, 3 patients received chemotherapy alone due to extensive lesions or refusal of surgery. Among 6 cases of synchronous OC who were all diagnosed at the same time with BC, five patients underwent PDS and one patient underwent IDS; considering that the prognosis of ovarian cancer is worse than that of breast cancer, all patients firstly underwent surgery for ovarian cancer and adjuvant chemotherapy then obtained breast cancer surgery (Table 2).
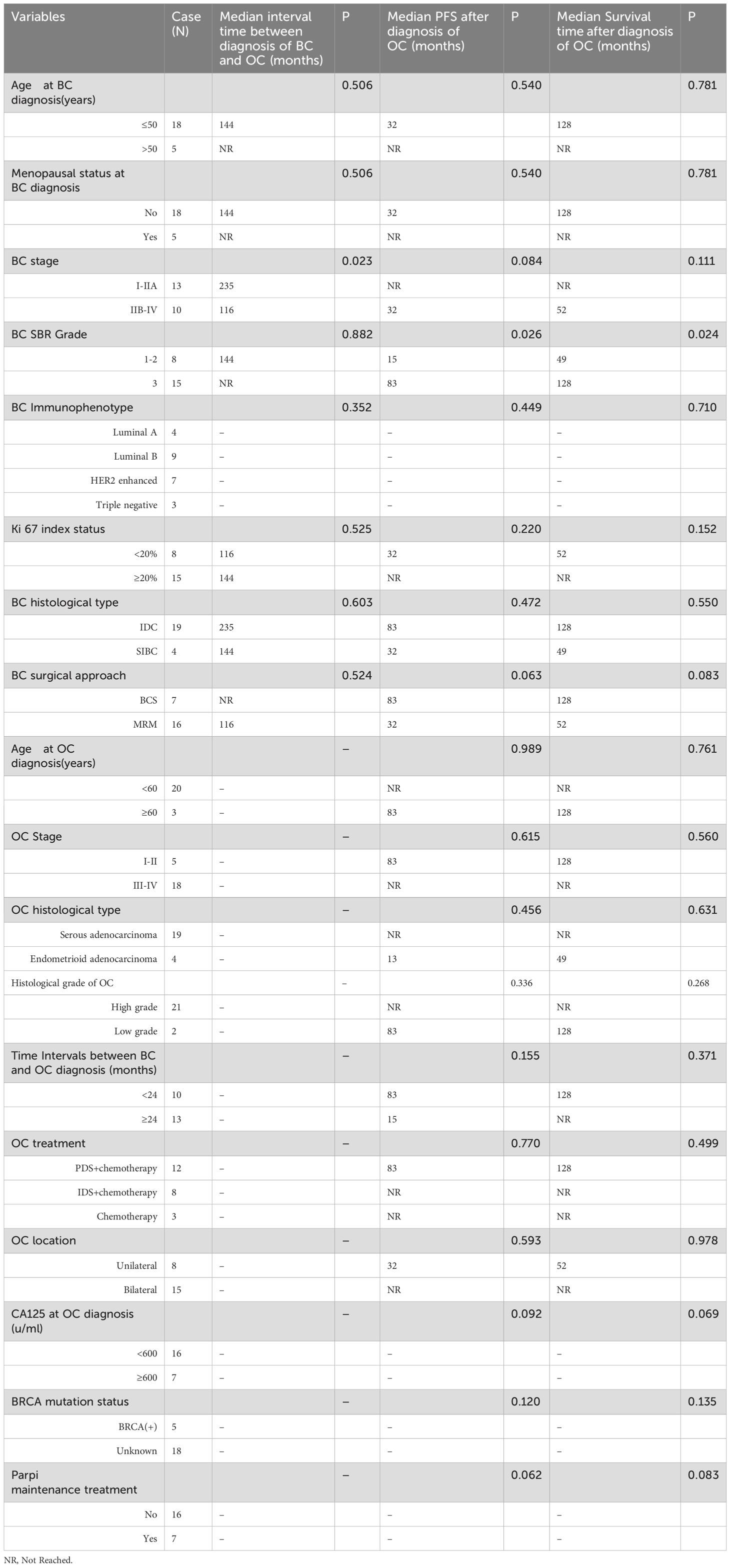
Table 2. Univariate analysis of interval time between diagnosis of BC and OC and Median PFS and OS of 23 patients.
3.2 Long-term outcome
Five patients (21.7%) was lost to follow-up, 8 patients (34.8%) died due to progression of OC. The average interval between BC and OC diagnosis was 62.48 months (Table 2). The median PFS and OS after OC diagnosis was 15 months and 38 months, respectively.
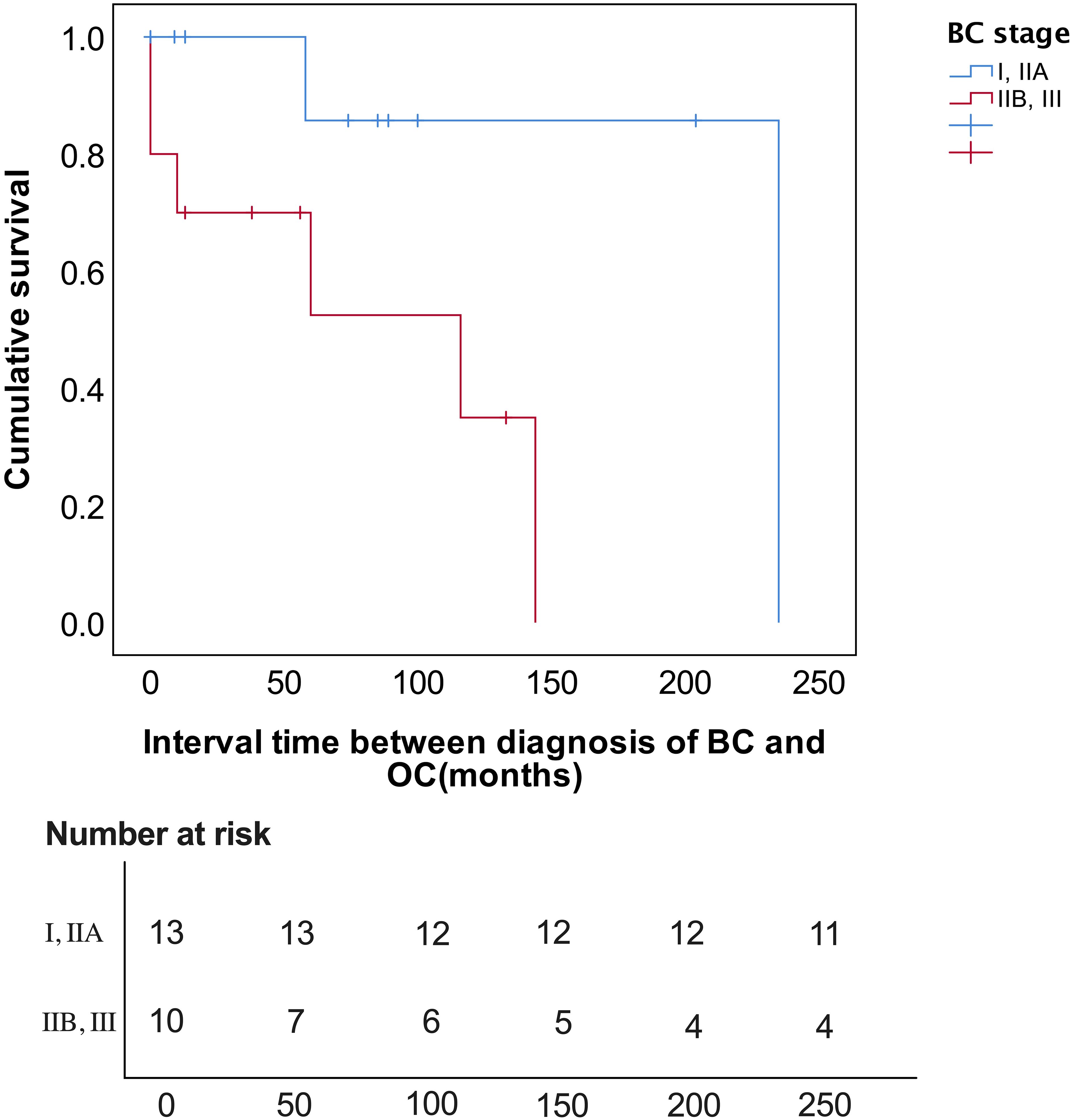
On Kaplan-Meier analysis, the advanced stage at presentation of BC (p=0.023) resulted in a significantly shorter interval time between BC and OC diagnosis (Figure 1). On the univariate Cox regression analysis, it was also found that the later the BC stage was, the shorter interval time between the diagnosis of BC and OC was (HR 8.047, p = 0.055), although it was not statistically significant (Table 3). On univariate Cox regression analysis, only BC SBR Grade 3 resulted in a significantly worse PFS (HR 0.187, p=0.048) and OS (HR 0.190, p=0.048), respectively (Table 4).
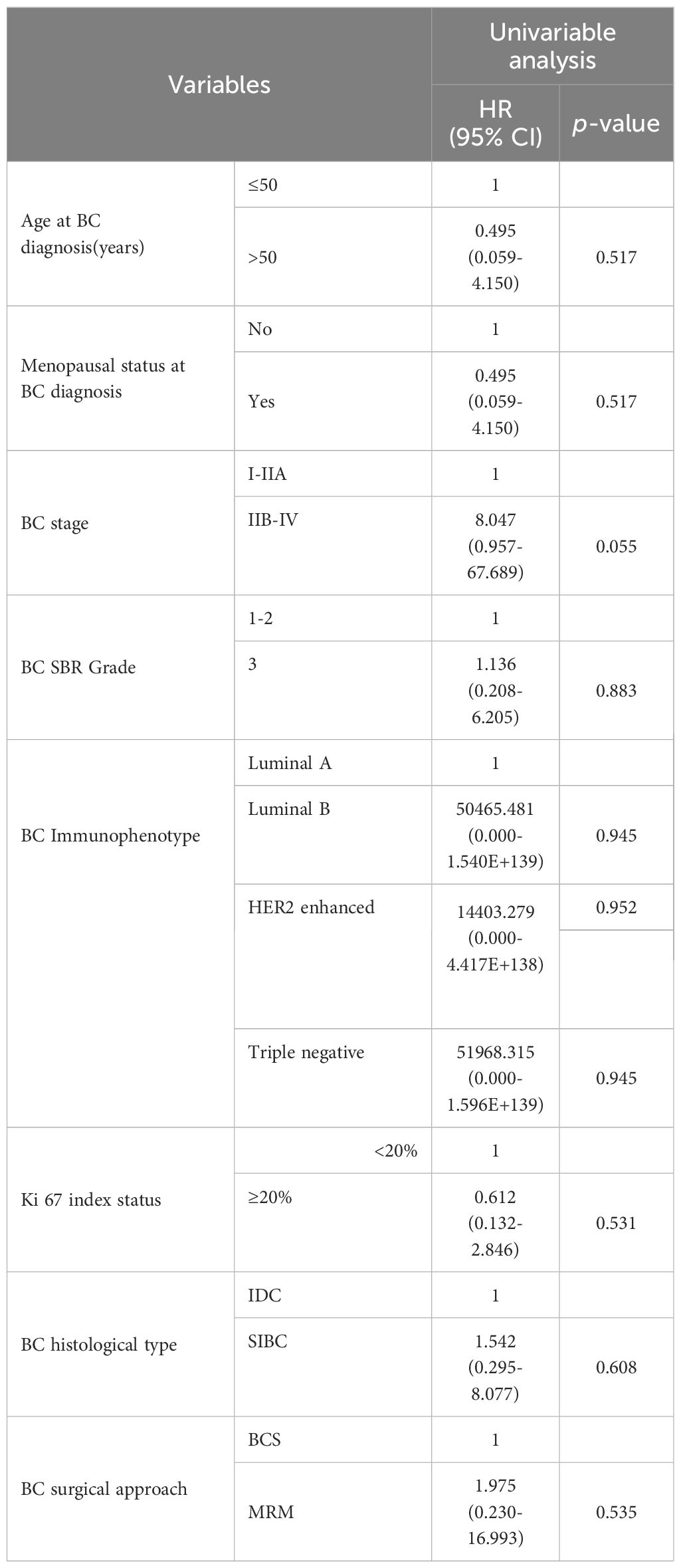
Table 3. Cox regression univariate of influence factors of interval time between BC and OC diagnosis.
4 Discussion
In this article, the average age of BC was 45.78 years and 73.9% of them were under 50 years old. 78.3% patients were in premenopausal. Tong et al. (6) reported that MPC patients onset 10-20 years earlier than primary epithelial ovarian cancer (average age 56-60 years) and are more common in premenopausal women. Berkowitz et al. (2) found that for women diagnosed with BC at an age younger than 50, the relative risk (RR) of OC diagnosed within two months to 5 years was 2.5 [95% confidence interval (CI): 2.1 -2.9]. Thus, it can be seen that the risk of postoperative OC in young patients with BC is significantly higher, which is similar to the results of this study.
Patients with double primary BC and OC are sporadic. Suppose women have a history of unmarried and childless, family inheritance, triple-negative breast cancer, postoperative oral selective estrogen antagonist, and BRCA1/BRCA2 gene mutation. In that case, they should be particularly alert to the possibility of the occurrence of OC after breast cancer surgery. The most important risk factors are the family history of the tumor (especially the medical history of first-degree relatives) and BRCA1/2 gene mutations (7). The estimated risk of developing ovarian cancer is increased approximately 2-fold for patients with a history of breast cancer (8). Metcalfe et al. reported a 10-year actuarial risk of developing EOC after BC of 12.7% for BRCA1 and 6.8% for BRCA2 mutation carriers (p=0.03) (9). In our study, due to the lack of widespread testing technology and high testing costs in earlier years, the vast majority of patients had not undergone BRCA gene mutation testing. Five patients had BRCA germline mutation. Thus, it can be seen that BRCA1/2 gene screening is essential for the prevention and early detection of hereditary breast cancer/ovarian cancer. By accurately stratifying patients’ risk and guiding targeted screening and preventative interventions, development of a novel prediction model for carriage of BRCA1/2 pathogenic variant in patients with breast cancer will contribute to improved management and outcomes of HBOC (10). In this article, only one patient has a family history of tumors, which suggesting that double primary breast and ovarian tumors can also appear in those without prior relevant clinical or family histories and other common etiological factors such as hormonal and reproductive aspects and mutation of different genes involved in tumor suppression may also induce cancer to occur (11–13).Other relevant genes are related to HBOC syndrome diagnosis, prognosis, and treatment, including TP53, PALB2, CHEK2, ATM, etc. Multi-gene testing implementation improves the detection of often overlooked genes related to HBOC pathogenesis and treatment (14). The majority of cases of breast cancer were initial (IA - IIA), luminals and treated with radical mastectomy in our study,Generally, this type of breast cancer has a good prognosis, but the simultaneous occurrence of ovarian cancer in this type of patients indirectly proves that genetic factors such as BRCA mutations may lead to secondary ovarian cancer rather than metastasis.In our study, 5 patients had BRCA mutations, and 18 patients had unknown BRCA detection status. The study showed that the mutation status of BRCA did not affect the survival of the patients.As several reports suggested (15–17), the initial survival advantage among EOC patients with BRCA mutations may reflect a higher initial sensitivity of BRCA carriers to chemotherapy and short-term survival but this response does not predict long-term survival. The strongest predictor of long-term survival is the status of no residual disease at resection. Though BRCA mutations appear to be associated with improved OS and PFS in patients with ovarian cancer, there has no difference in the surgical resection rate between participants in the two groups (18). Another study also proves that BRCA-status is not a prognostic factor in early ovarian cancer regarding PFS (19). It should emphasize the major impact of achieving complete surgery on the prognosis of HRD EOCs with or without BRCA1/2 mutation, whether in primary surgery or interval surgery, despite a better sensitivity to chemotherapy and maintenance treatments with PARP inhibitors (20).
In our study, the average interval between BC and OC diagnosis was 62.48 months, and approximately 43.5% of second primary OC cases occurred after the first 60 months of the BC diagnosis, suggesting that breast cancer patients who have achieved long-term survival should also be alert to the occurrence of ovarian cancer. Metcalfe et al. (9) found a mean time of 8.1 years (range 0.1–25.5 years) from BC to OC. In this article, the Kaplan-Meier approach revealed that the later the stage of breast cancer, the shorter the time interval of diagnosis of OC was. It reminds doctor to strengthen the follow-up management of breast cancer patients, especially the screening management of high-risk patients with ovarian cancer. During the follow-up of breast cancer, attention should be paid to gynecological examination and gynecological ultrasound examination. If the patient is taking tamoxifen and the uterus and ovary are not removed surgically, they should be examined every 3-6 months. If patients with BRCA mutations are recommended to undergo prophylactic bilateral adnexectomy to reduce the risk of ovarian cancer. One meta-analysis revealed that prophylactic interventions significantly reduced cancer risk and mortality. Risk-reducing surgeries (RRS) were more effective than chemoprevention, with RRS notably reducing cancer risk by 56% compared to 39% for chemoprevention (21). Joshi S suggested timing prophylactic oophorectomy 5–6 years after diagnosis of first BC based on the median time interval before development of second cancer to be 77 months after diagnosis of OC (22). Our study found that approximately 56.5% of OC cases occurred during the first 60 months after the BC diagnosis, therefore, prophylactic oophorectomy should be performed more earliar.
In our study, most of the patients were bilateral tumors (65.2%), ovarian tumors’ size was >5cm (73.9%), the pathological types of ovarian cancer were mostly serous papillary carcinoma and endometrioid carcinoma, and the ovarian tumor was located in the epithelium. The main emphasis should be to differentiate BC from primary OC and vice versa for subsequent best management of these patients to decide curative or palliative approach. The clinicopathological characteristics of breast cancer with ovarian metastasis are as follows: most of them have no obvious symptoms, and a few of them have symptoms, such as abdominal distention, ascites, abnormal vaginal bleeding, etc (14).Breast metastases to the ovary most frequently are bilateral solid masses at ultrasonographic (US) with small tumor size, multiple nodules and involvement of the surface (17, 23–26), while patients with primary OC had predominantly cystic images at US (27). Previous tumor history is an important factor to assist in the differential diagnosis of primary or metastatic ovarian cancer. It is reported that patients with BC are 3-7 times more likely to develop primary ovarian cancer (POC) than ovarian metastases (OM) (28). A single imaging or CA125 examination is not meaningful in distinguishing between primary and metastatic ovarian tumors. Many ovarian metastatic adenocarcinoma and primary ovarian cancer have extremely similar pathological morphology and clinical pathological types, requiring careful pathological identification. Immunohistochemistry has a pivotal role in differentiating primary and secondary ovarian adenocarcinoma. In our study, ovarian tumor cell immunohistochemistry showed positive expression of P53, WT-1, PAX8, Ck7, P16, ER, and CA125, while GCDFP-15, mammaglobin, GATA3, CK20, Vimentin expression were negative. WT1 and PAX8 appear to have utility in differentiating primary OC from metastatic BC due to their higher sensitivity and low potential for aberrant expression (29). GATA3 is a very specific marker for breast cancer (and urothelial carcinomas) (30). Wick et al. reported that the overall sensitivity of GCDFP 15 in the diagnosis of breast cancer is 74%, the specificity is 95%, and the negative predictive value is 95% (31). Monteagudo et al.found that GCDFP-15 was positive in 71% of breast cancer metastatic to the ovary, while none of the primary ovarian cancer was positive (32). Mammaglobin, GCDFP-15, CK7, and GATA-3 are the most commonly used markers to confirm breast origin. Because none of these markers is completely specific and/or sensitive, ovarian markers should be included to obtain the differential diagnosis (33).
In our study, there were 6 cases of synchronous OC. Among them, 5 patients underwent PDS and one patient underwent IDS. All of them firstly underwent surgery for ovarian cancer and adjuvant chemotherapy then obtained breast cancer surgery. Primary BC and OC in the same patient must be treated following the best evidence available for each tumor, considering disease stage, pathology findings and molecular characteristics. Currently, there is no standard treatment of patients with synchronous OC and BC. Neoadjuvant chemotherapy was effective in both breast and ovarian cancer. We used platinum drugs, taxanes, and anthracycline agents, which were active in both diseases. In the context of advanced disease, treatment may be tailored according to prognosis, patient´s performance status and preferences. According to research reports, early control of ovarian tumors with poorer prognosis can avoid distant metastasis and widespread implantation metastasis as early as possible (34–36). In our study, all of patients were died of OC progression. Studies reported that OS survival was dominated by the stage of disease (37, 38) and the most virulent of the synchronous tumors defined mortality rates, while the mortality rate of individuals with metachronous tumors was determined by second malignancies after the first neoplasm was cured (39).
As we known,PARP inhibitors (olaparib, niraparib) are effective targeted therapies in BRCAm ovarian cancer in first-line treatment as well as in maintenance therapy after platinum based chemotherapy for ovarian cancer recurrence (40–43). In our study,the vast majority of patients did not receive maintenance treatment with PARPi due to economic reasons and the fact that PARPi was only approved for ovarian cancer in China after 2018. Only 6 patients received PARP inhibitor maintenance treatment, among them, four patients receiving first-line maintenance therapy, including two BRCA mutation patients receiving olaparib maintenance therapy, two patients with unknown genetic testing status receiving nirapali maintenance therapy, and two second-line maintenance therapy patients with BRCA mutations receiving olaparib maintenance therapy. PARP inhibitors reduced the risk of disease progression and death (HR=0.024) and (HR= 0.029), respectively, although it was not statistically significant, which may be related to only a few patients in our study had received PARPi maintenance treatment.
To the best of our knowledge, this is one of the few study to investigate the time intervals and prognosis of individuals with both primary BC and OC. But it also has several shortcomings. Firstly, It was retrospective study and the sample size was relatively small which limits the generalizability of the conclusions. Secondly, there were a limited number of patients who underwent BRCA1/2 gene testing and maintenance therapy. Thirdly, the analysis did not include the assessment of treatment after OC recurrence. Future studies with larger sample sizes and more rigorous research designs are necessary to further elucidate the prognostic value of variables such as age, disease characteristics, mutation type, targeted therapy, intervention, surveillance and prophylactic surgery and to identify the appropriate management strategies for this specific population.
In conclusion, patients with breast cancer may have ovarian cancer later,it reminds doctor to strengthen the follow-up management of breast cancer patients, especially the screening management of high-risk patients with ovarian cancer. Early control of ovarian tumors and active comprehensive treatment for synchronous and metachronous breast and ovarian cancer can achieve good results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Sichuan Cancer Hospital Institutional Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HL: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal analysis, Writing – original draft. CP: Formal analysis, Funding acquisition, Investigation, Writing – original draft. XC: Funding acquisition, Investigation, Methodology, Writing – original draft. GL: Investigation, Methodology, Writing – original draft. DW: Investigation, Methodology, Writing – original draft. GZ: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Key Research and Development Project from Science & Technology Department of Sichuan Province (Grant No: 2019YFS0036).
Acknowledgments
We gratefully acknowledge Sichuan Cancer Hospital & Institute for the clinical data support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lee KD, Chen SC, Chan CH, Lu CH, Chen CC, Lin JT, et al. Increased risk for second primary Malignancies in women with breast cancer diagnosed at young age: a population-based study in Taiwan. Cancer Epidemiol Biomarkers Prev. (2008) 17:2647–55. doi: 10.1158/1055-9965
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Berkowitz Z, Rim SH, and Peipins LA. Characteristics and survival associated with ovarian cancer diagnosed as first cancer and ovarian cancer diagnosed subsequent to a previous cancer. Cancer Epidemiol. (2011) 35:112–9. doi: 10.1016/j.canep.2010.07.001
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Couch FJ, Nathanson KL, and Offit K. Two decades after BRCA: setting paradigmsin personalized cancer care and prevention. Science. (2014) 343:1466–70. doi: 10.1126/science.1251827
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Chen S and Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. (2007) 25:1329–33. doi: 10.1200/JCO.2006.09.1066
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstetrics Gynecology. (2010) 22:72–8. doi: 10.1097/GCO.0b013e328332dca3
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Tong SY, Lee YS, Park JS, Bae SN, Lee JM, and Namkoong SE. Clinical analysis of synchronous primary neoplasms of the female reproductive tract. Eur J Obstet Gynecol Reprod Biol. (2008) 136:78–82. doi: 10.1016/j.ejogrb.2006.09.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Moore EK, Roylance R, and Rosenthal AN. Breast cancer metastasising to the pelvis and abdomen: what the gynaecologist needs to know. BJOG. (2012) 119:788–94. doi: 10.1111/j.1471-0528.2012.03314.x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Bergfeldt K, Rydh B, Granath F, Grönberg H, Thalib L, Adami HO, et al. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: a population-based cohort study. Lancet. (2002) 360:891–4. doi: 10.1016/S0140-6736(02)11023-3
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Metcalfe KA, Lynch HT, Ghadirian P, Tung N, Olivotto IA, Foulkes WD, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. (2005) 96:222–6. doi: 10.1016/j.ygyno.2004.09.039
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Komatsu N, Chishima T, Watanabe C, Taruno K, Inuzuka M, Oshi M, et al. Development of a novel prediction model for carriage of BRCA1/2 pathogenic variant in Japanese patients with breast cancer based on Japanese organization of hereditary breast and ovarian cancer registry data. Breast Cancer Res Treat. (2025) 209:177–88. doi: 10.1007/s10549-024-07485-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. (1994) 344:1250–4. doi: 10.1016/s0140-6736(94)90749-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Lambe M, Hsieh CC, Chan HW, Ekbom A, and Trichopoulos D and Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat. (1996) 38:305–11. doi: 10.1007/BF01806150
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Riman T, Persson I, and Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol (Oxf). (1998) 49:695–707. doi: 10.1046/j.1365-2265.1998.00577.x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Aguilar D, Garza-Rodríguez ML, Muñiz-Garza CE, Nuñez FA, Villarreal-Garza CM, Vidal-Gutiérrez O, et al. Los olvidados: Non-BRCA variants associated with Hereditary breast cancer in Mexican population. Breast Cancer Res. (2025) 27:7. doi: 10.1186/s13058-024-01957-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Kotsopoulos J, Rosen B, Fan I, Moody J, McLaughlin JR, Risch H, et al. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol. (2016) 140:42–7. doi: 10.1016/j.ygyno.2015.11.009
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. De Jong D, Otify M, Chen I, Jackson D, Jayasinghe K, Nugent D, et al. Survival and Chemosensitivity in Advanced High Grade Serous Epithelial Ovarian Cancer Patients with and without a BRCA Germline Mutation: More Evidence for Shifting the Paradigm towards Complete Surgical Cytoreduction. Medicina (Kaunas). (2022) 58:1611. doi: 10.3390/medicina58111611
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Petrillo M, Marchetti C, De Leo R, Musella A, Capoluongo E, Paris I, et al. BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: a multicenter study. Am J Obstet Gynecol. (2017) 217:334.e1–9. doi: 10.1016/j.ajog.2017.05.036
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Wang Y, Li N, Ren Y, and Zhao J. Association of BRCA1/2 mutations with prognosis and surgical cytoreduction outcomes in ovarian cancer patients: An updated meta-analysis. J Obstet Gynaecol Res. (2022) 48:2270–84. doi: 10.1111/jog.15326
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Marchetti C, Ataseven B, Perrone AM, Cassani C, Fruscio R, Sassu CM, et al. Clinical characteristics and survival outcome of early-stage, high-grade, serous tubo-ovarian carcinoma according to BRCA mutational status. Gynecol Oncol. (2024) 187:170–7. doi: 10.1016/j.ygyno.2024.05.008
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Colombo PE, Taoum C, Fabbro M, Quesada S, Rouanet P, and Ray-Coquard I. Impact of molecular testing on the surgical management of advanced epithelial ovarian cancer. Crit Rev Oncol Hematol. (2024) 202:104469. doi: 10.1016/j.critrevonc
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Liu T, Yu J, Gao Y, Ma X, Jiang S, Gu Y, et al. Prophylactic interventions for hereditary breast and ovarian cancer risks and mortality in BRCA1/2 Carriers. Cancers (Basel). (2023) 16:103. doi: 10.3390/cancers16010103
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Joshi S, Murali-Nanavati S, Shylasree TS, Hawaldar R, Tripathi S, Sahay A, et al. Synchronous and metachronous breast and ovarian cancers: experience from a single tertiary care cancer centre in India. Indian J Surg Oncol. (2023) 14:809–21. doi: 10.1007/s13193-023-01749-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Bigorie V, Morice P, Duvillard P, Antoine M, Cortez A, Flejou JF, et al. Ovarian metastases from breast cancer: report of 29 cases. Cancer. (2010) 116:799–804. doi: 10.1002/cncr.24807
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian Malignancy: imaging findings of adnexal mass in the Radiology Diagnostic Oncology Group Study. Radiology. (2001) 219:213–8. doi: 10.1148/radiology.219.1.r01ap28213
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Ueland FR, DePriest PD, Pavlik EJ, Kryscio RJ, and van Nagell JR Jr. Preoperative differentiation of Malignant from benign ovarian tumors: the efficacy of morphology indexing and doppler flow sonography. Gynecol Oncol. (2003) 91:46–50. doi: 10.1016/s0090-8258(03)00414-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Abd El hafez A and Monir A. Diagnostic spectrum of ovarian masses in women with breast cancer; magnetic resonance imaging: histopathology correlation. Ann Diagn Pathol. (2013) 17:441–7. doi: 10.1016/j.anndiagpath.2013.06.003
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Hahn LE, Lui D, Shi W, Bach A, Selland D, and Castiel M. Adnexal masses in women with breast cancer: US findings with clinical and histopathologic correlation. Radiology. (2000) 216:242–7. doi: 10.1148/radiology.216.1.r00jl15242
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Tian W, Zhou Y, Wu M, Yao Y, and Deng Y. Ovarian metastasis from breast cancer: a comprehensive review. Clin Transl Oncol. (2019) 21:819–27. doi: 10.1007/s12094-018-02007-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Tornos C, Solslow R, Chen S, Akram M, Hummer AJ, Abu-Rustum N, et al. Expression of WT1, CA 125, and GCDFP-15 as useful markers in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol. (2005) 29:1482–9. doi: 10.1097/01.pas.0000176429.88702.36
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Kandalaft P and Gown A. Pratical applications of immunohistochemistry: carcinomas of unkown primary site. Arch Pathol Lab Med. (2016) 140:508–23. doi: 10.5858/arpa.2015-0173-CP
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, and Kiang DT. Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. (1989) 20:281–7. doi: 10.1016/0046-8177(89)90137-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Monteagudo C, Merino MJ, LaPorte N, and Neumann RD. Value of gross cystic disease fluid protein-15 in distinguishing metastatic breast carcinomas among poorly differentiated neoplasms involving the ovary. Hum Pathol. (1991) 22:368–72. doi: 10.1016/0046-8177(91)90084-3
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
33. Carboni F, Covello R, Carosi MA, and Valle M. The diagnostic challenge of solitary ovarian mass after breast cancer. Breast J. (2020) 26:1412–3. doi: 10.1111/tbj.13819
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Wolff EF, Martel M, Gwin K, and Lannin D. Synchronous primary breast and ovarian cancer with ovarian cancer metastases to a breast sentinel lymph node. Breast J. (2009) 15:203–5. doi: 10.1111/j.1524-4741.2009.00699.x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Sandor V, Reed E, Sarosy G, Middleton LP, Davis P, and Kohn E. Synchronous inflammatory breast cancer and advanced ovarian carcinoma: a case with prolonged disease-free survival. Ann Oncol. (1999) 10:585–8. doi: 10.1023/a:1008239124657
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Lesnock JL, Darcy KM, Tian C, Deloia JA, Thrall MM, Zahn C, et al. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a gynecologic oncology group study. Br J Cancer. (2013) 108:1231–7. doi: 10.1038/bjc.2013.70
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Tasca G, Dieci MV, Baretta Z, Faggioni G, Montagna M, Nicoletto MO, et al. Synchronous and metachronous breast and ovarian cancer: experience from two large cancer center. Front Oncol. (2020) 10:608783. doi: 10.3389/fonc.2020.608783
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Liou W-S, Hamilton CA, Cheung MK, Osann K, Longacre TA, Teng NN, et al. Outcomes of women with metachronous breast and ovarian carcinomas. Gynecol Oncol. (2006) 103:190–4. doi: 10.1016/j.ygyno.2006.02.022
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
39. Rose PG, Herterick EE, Boutselis JG, Moeshberger M, and Sachs L. Multiple primary gynecologic neoplasms. Am J Obstetrics Gynecology. (1987) 157:261–7. doi: 10.1016/s0002-9378(87)80148-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. (2018) 379:2495–505. doi: 10.1056/NEJMoa1810858
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. González Martín A, Pothuri B, Vergote IB, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. (2019) 381:2391–402. doi: 10.1056/NEJMoa1910962
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-ov21): A final analysis of a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Oncol. (2021) 22:620–31. doi: 10.1016/S1470-2045(21)00073-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
43. Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. (2019) 37:2968–73. doi: 10.1200/JCO.18.02238
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: double primary malignancy, breast cancer, ovarian cancer, interval between diagnoses, survival outcome
Citation: Liu H, Luo M, Peng C, Cheng X, Luo G, Wang D and Zhang G (2025) Title of report: second primary ovarian carcinomas after breast cancer diagnosis- an analysis of a single cancer centre in China. Front. Oncol. 15:1553366. doi: 10.3389/fonc.2025.1553366
Received: 30 December 2024; Accepted: 19 May 2025;
Published: 03 June 2025.
Edited by:
Jianrong Zhang, Cancer Council Victoria, AustraliaReviewed by:
Giuseppe D’Ermo, Sapienza University of Rome, ItalyYe Zhang, The University of Melbourne, Australia
Copyright © 2025 Liu, Luo, Peng, Cheng, Luo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guonan Zhang, emhhbmdnbkBob3RtYWlsLmNvbQ==
†Present address: Guonan Zhang, Gynecologic Oncology Center, Sichuan Cancer Hospital &
Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
‡ORCID: Hong Liu, orcid.org/0000-0002-2380-8286
Min Luo, orcid.org/0000-0001-8333-8995
Chunrong Peng, orcid.org/0000-0001-6691-4292
Xinghan Cheng, orcid.org/0000-0002-4572-9618
Gupo Luo, orcid.org/0009-0009-1806-8410
Dengfeng Wang, orcid.org/0000-0002-1832-1270
Guonan Zhang, orcid.org/0000-0002-2228-9197
 Hong Liu
Hong Liu Min Luo
Min Luo Chunrong Peng
Chunrong Peng Xinghan Cheng1‡
Xinghan Cheng1‡ Guonan Zhang
Guonan Zhang