- Department of Hematology and Oncology, Ningbo No.2 Hospital, Ningbo, China
There are very few reports of gastric cancer (GC) and gastrointestinal stromal tumor (GIST) occurring at the same time in the literature. In particular, the collision of GIST and human epidermal growth factor receptor-2 (HER-2)-positive gastric carcinoma in a patient with Waldenström macroglobulinemia (WM) has never been reported. We report the case of an 80-year-old male who initially presented with dizziness with fatigue for more than 1 month. He underwent total gastrectomy. Pathology revealed GC (moderately to poorly differentiated tubular adenocarcinoma), GIST (low-risk category) and low-grade small B-cell lymphoma with plasmacytoid differentiation. Furthermore, the diagnosis of WM was made after cytomorphologic and immunohistochemical analysis of the patient’s bone marrow revealed the presence of lymphoplasma cells along with the MYD88 L265P mutation and an increased level of serum monoclonal immunoglobulin M (IgM). To our knowledge, this is the first case of such an association where WM occurred with concomitant HER2-positive gastric adenocarcinoma and gastric GIST. A review of the literature concerning the incredibly uncommon simultaneous triple incidence of malignant tumors with distinct histogenesis is presented below.
Introduction
GC is a prevalent malignant tumor originating from epithelial tissue. Adenocarcinoma is the most common type of GC. Globally, the reported HER-2-positive rate in GC ranges from 7.3% to 20.2% (1). GIST, accounting for 1%~2% of gastrointestinal tumors, is the most common mesenchymal tumors and are most commonly observed in the stomach (50~60%) (2). However, the prevalence of both GC and GIST is between 0.29% and 0.53% (3). WM is an uncommon type of B-cell lymphoma in which the IgM protein is the hallmark of the disease. There are few reports of synchronous WM and second cancers. However, there has never been a report of GIST and HER-2-positive stomach cancer colliding in a WM patient. Herein, we present an extremely unwonted case of these three lesions.
Case presentation
An 80-year-old male was admitted to the Department of Gastrointestinal Surgery at our hospital on 8 September 2023 due to dizziness accompanied by fatigue and a poor appetite for more than a month. He had a history of cerebral infarction, hypertension, and prostatic hyperplasia. One month before hospitalization, the patient visited the local hospital for gastroscopy examination, and gastroscopy revealed space-occupying lesions in the fundus and body of the stomach. Pathologic examination revealed poorly differentiated adenocarcinoma. He was referred to our hospital for further treatment. Additionally, uneven thickening and augmentation of the gastric fundus wall were identified via abdominal contrast-enhanced computed tomography (Figure 1A). Positron emission tomography-computed tomography (PET-CT) confirmed thickening of the gastric wall on the side of the gastric cardia and lesser curvature with increased metabolism of FDG and multiple lymph node metastases beside the lesser curvature of the stomach, hepatogastric space, portal of the liver, retroperitoneum, superior diaphragm, and lower esophagus (Figures 1B, C).
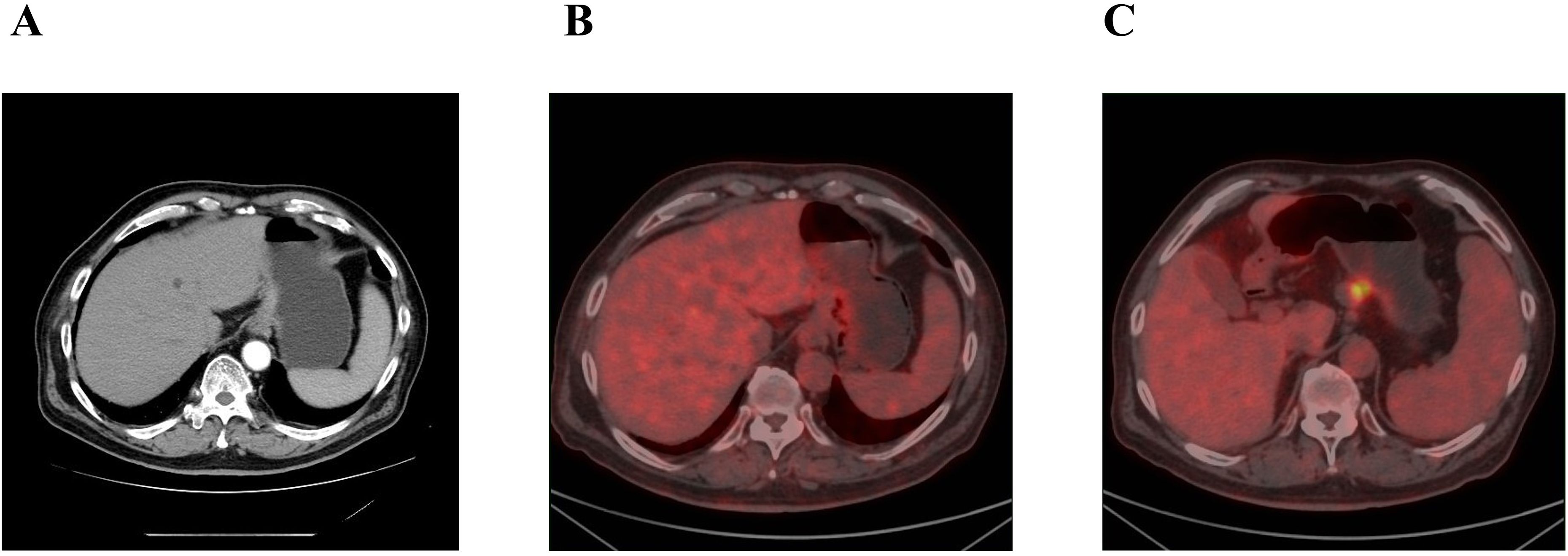
Figure 1. (A) Abdominal enhanved CT: uneven thickening augmentation of the gastric fundus. (B, C) PET-CT: (B) thickening of the gastric wall. (C) lymph node with increased metabolism of FDG.
He underwent laparoscopy-assisted total gastrectomy with Roux-en-Y esophagojejunostomy and D2 lymph node dissection on 9 September 2023. The operation was successful, with uneventful outcomes. The postoperative pathological examination of the stomach revealed a moderately to poorly differentiated tubular adenocarcinoma that had not spread to the visceral peritoneum but had infiltrated the connective tissue of the subserous membrane. The tumor was located in the lesser curvature of the body of the stomach and measured 40mm*33mm*12mm in size. Ten of the 25 resected lymph nodes were found to contain metastases, and all margins were negative (pT3N2aM0, Stage IIIB). Immunohistochemical findings included CK7 (-), CEA (focal +), CK20 (focal +), CDX-2 (+), E-cadherin (+), Her-2 (2+), Ki-67 (+80%), P53 (overexpress), and EBER (-). Mismatch repair (MMR) protein expression was intact (Figure 2A). Programmed death-ligand 1 (PD-L1) combined positive score (CPS) 50 (CPS < 1 is negative, and CPS≥1 is positive) (Figure 3A). The histopathology of the gastric cardia revealed GIST characteristics, including spindle-shaped cells, a mitotic ratio of 1/50HPF, neither substantial atypia nor visible necrosis. Immunostaining for CD34, vimentin (+) and c-kit was positive, whereas S-100, SMA and desmin were negative. GISTs were classified as very low risk according to the NIH2008 modified version (Figure 2B; Supplementary Table 1A).
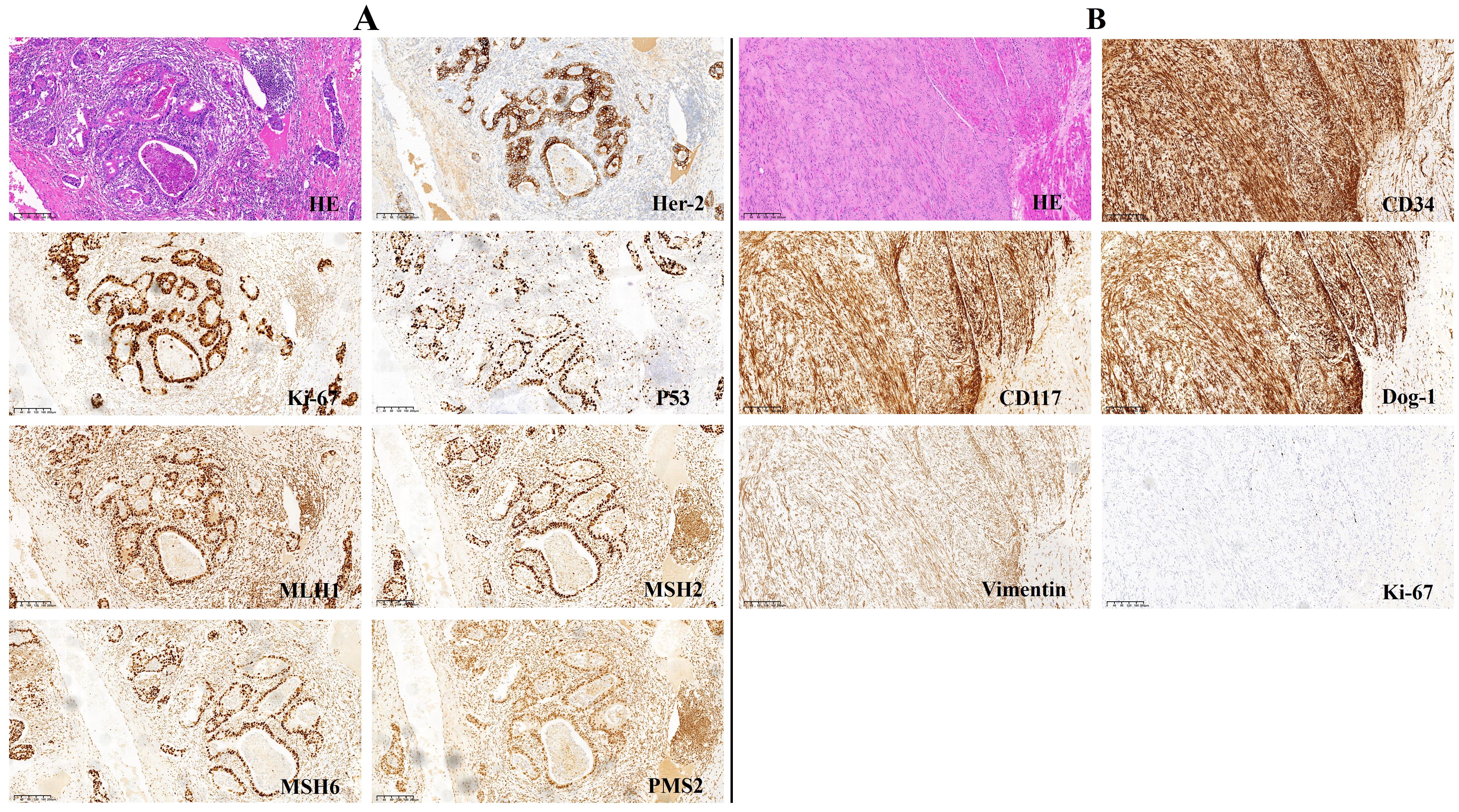
Figure 2. Immunohistochemistry analysis (magnification, x 100). (A) gastric adenocarcinoma. (B) GIST.
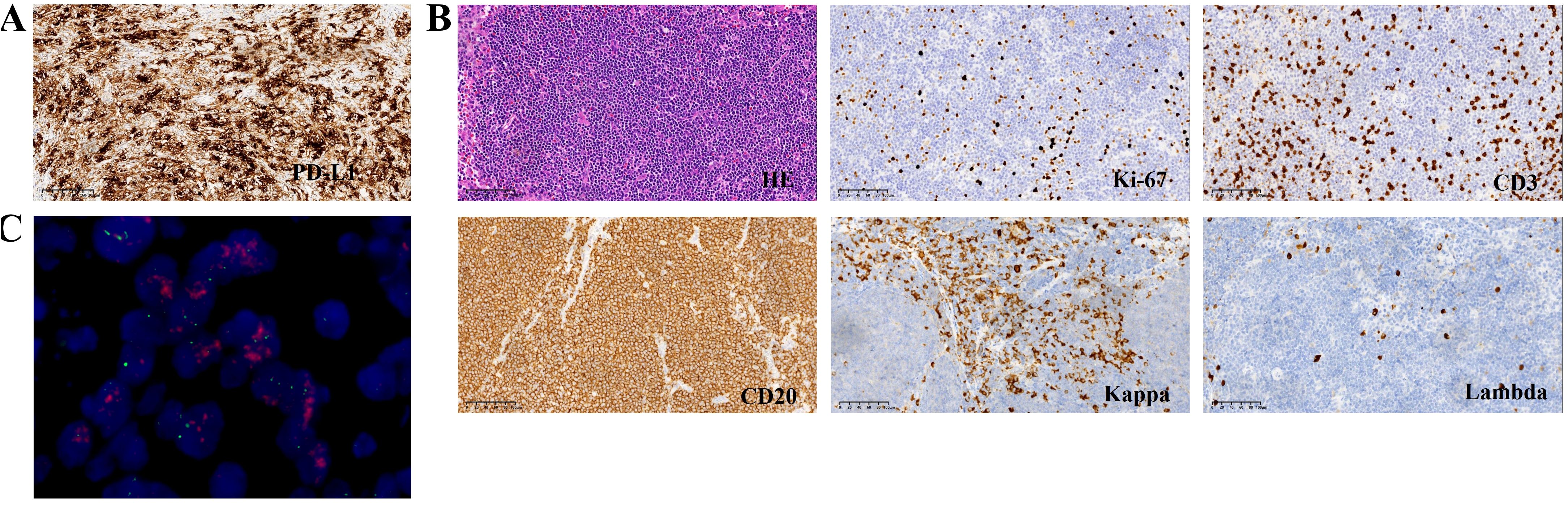
Figure 3. (A) Immunochemistry analysis showing expression of PD-L1. (B) Immunochemistry analysis (magnification, x 200) showing CD20+/CD3-/Kappa+/Lambda-/Ki-67 low phenotype of the No.8 Lymph node. (C) FISH showing HER-2 positive.
Furthermore, No.8 Lymph node analysis revealed low-grade small B-cell lymphoma with plasmacytoid differentiation. Immunohistochemistry revealed the following: kappa (+), lambda (-), PAX-5 (+), Ki-67 (+10%), Bcl-2 (+), Bcl-6 (-), CyclinD1 (-), SOX-11 (-), CD3 (-), CD5 (-), CD10 (-), CD20 (+), CD21 (+), CD23 (+), CD43 (-), and CD79a (+)(Figure 3B; Supplementary Table 1A).
He was subsequently hospitalized in our wards in November 2023 for further diagnosis and treatment. A laboratory test revealed mild anemia (hemoglobin of 94 g/L) together with low white blood cell (2.9×109/L) and platelet (111×109/L) counts. Biochemical tests revealed hyperphosphatemia (5.11 mmol/L), normal lactate dehydrogenase (120 U/L), and increased IgM (24.3 g/L). Serum beta-2-microglobulin (3.49 mg/L) was also above normal. The monoclonal immunoglobulin was identified as IgM-κ by serum immunofixation electrophoresis analysis, and immunoglobulin quantification was carried out (Figure 4A). Subsequently, bone marrow aspiration and biopsy were performed. The patient’s bone marrow smears revealed a small number of lymphoplasma cells (Figure 4B). Immunotyping revealed that CD45+CD19+ cells accounted for 11.4% of nuclear cells and expressed HLA-DR, CD19, CD22 and sKappa without CD5, CD10, CD11c, CD23, CD38, CD103, CD200 and Lambda. Cytogenetics revealed a normal karyotype. A mutation in MYD88 L265P, was detected in the patient via molecular biological testing (Figure 4C).
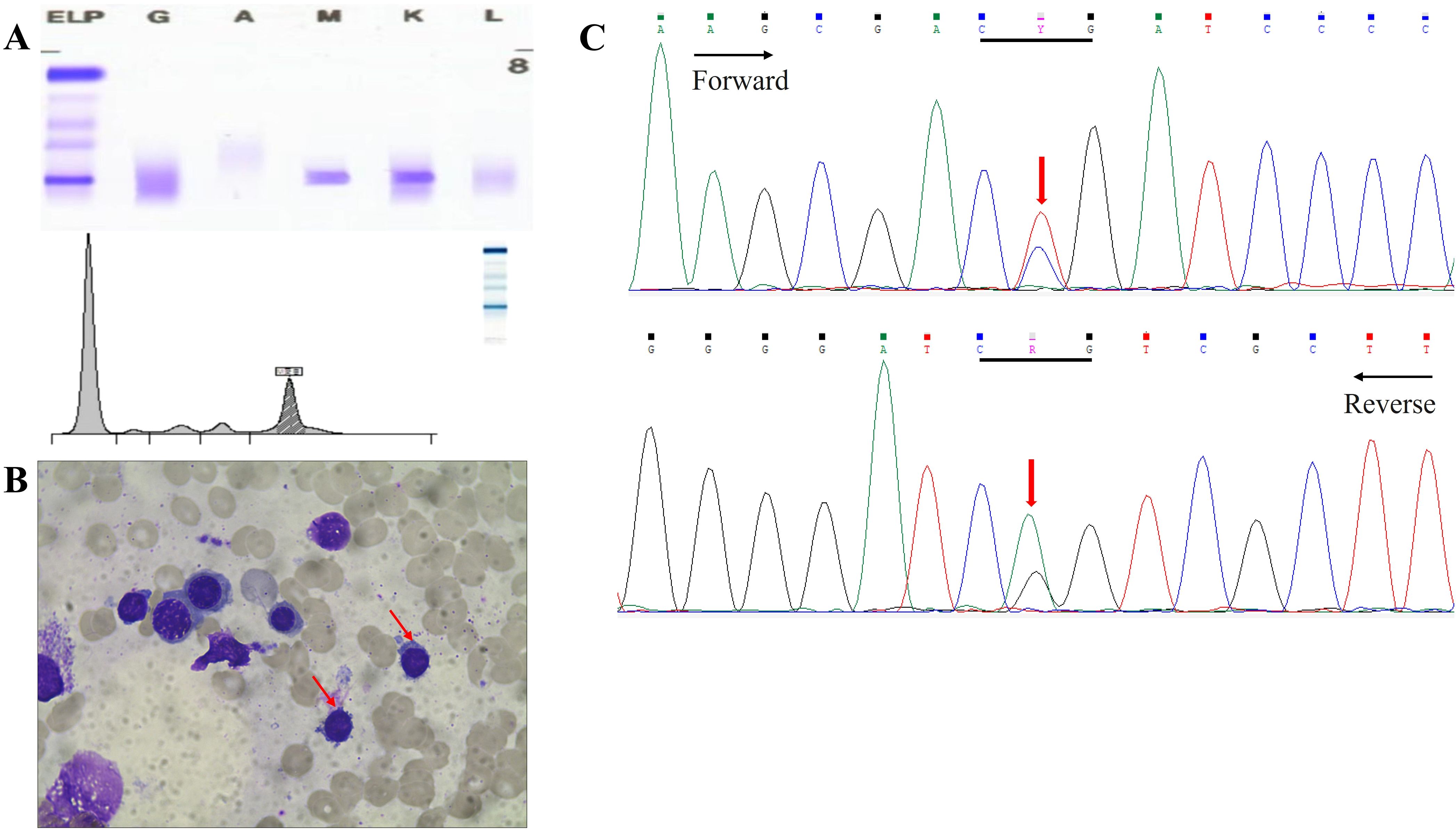
Figure 4. (A) Serum electrophoresis and immunofixation electrophoresis revealed IgM κ. (B) Bone marrow puncture showed lymphoplasma cells. (C) Perform MYD88L265P mutational analysis on bone marrow by Sanger sequencing. Y:C/T R:A/G.
BM aspiration confirmed the diagnosis of a mature B-cell neoplasm. No osteolytic bone lesions were found in association with multiple myeloma. Calcium and creatinine levels were normal. Eventually, WM was diagnosed. The patient had a high risk score according to the International Prognostic System for WM. Owing to his advanced age and poor performance, the patient and his family declined chemotherapy. Zanubrutinib (160 mg orally twice a day) and supportive care were given to the patient because of exhaustion, anemia, and dizziness. Four months later, the patient was hospitalized in our department due to dysphagia, vomiting after eating, and defecation difficulties. The tumor markers were elevated (Supplementary Table 1B), and gastroscopy revealed extensive hyperemic erosion of the esophageal mucosa with bleeding. We considered the possibility of gastric cancer recurrence, and zanubrutinib was suspended. HER-2 was positive on the basis of the combined interpretation of the results of the IHC assays (2+) and FISH (+) (Figure 3C). Given that his PD-L1 CPS was 50, he was administered immune checkpoint inhibitors plus anti-HER-2 treatment on April 4, 2024. Unfortunately, the patient refused further immunotherapy and targeted therapy because of poor performance status and was treated with traditional Chinese medicine without any outpatient follow-up for personal reasons.
Discussion
GCs are currently the fifth most common cancer worldwide, with approximately 75% of cases occurring in Asia, whereas GISTs represent the most common mesenchymal neoplasms of the digestive tract, with an incidence of 50~60% in the stomach (2, 4). However, the coexistence of GCs and GISTs is uncommon and is often detected incidentally during surgery, as in our case. It has been reported that the synchronous occurrence of GC and GIST is 0.29%~0.53% (3). Globally, there are very few case reports on GC with GIST. Notably, in our patient, HER-2 was positive on the basis of the combined interpretation of the IHC (2+) and FISH (+) results (5). Surprisingly, to the best of our knowledge, no cases of HER-2-positive GC or GIST have been reported thus far, and the prognostic role of HER-2 overexpression in GC is still controversial (6, 7). Typically, GISTs have low malignant potential, whereas GCs are usually advanced. However, the etiology of GC occurring simultaneously with GIST is still unclear. The co-occurrence of GC and GIST is thought by some researchers to be accidental, but other research has suggested that it may be linked to common carcinogens such as nitrite and Helicobacter pylori (8–10). However, there is currently no evidence linking GIST to H. pylori infection or clue of H. pylori infection in the patient. Gene mutations may cause two neighboring tissues to interact, disrupting the regulation of mesothelial and epithelial cell development and resulting in distinct cancers in two tissues of the same organ.
WM is a type of lymphoplasmacytic lymphoma with IgM monoclonal protein. It accounts for approximately 1~2% of hematologic malignancies. More than 90% of WM patients have the MYD88 L265P mutation (11, 12). The coexistence of gastric adenocarcinoma with lymphoma has been documented in a few cases. Most patients have GC diagnosed alongside non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL) and mucosa-associated lymphoid tissue (MALT) lymphoma (13–16). Feng et al. (16) reported that stomach cancer and precancerous abnormalities were present in 5.1% and 14.6% of patients with primary gastric lymphoma, respectively. However, according to our literature review, we rarely observe two cases of synchronous triple occurrence: adenocarcinoma, lymphoma, and GIST in the stomach. One patient had MALT lymphoma, and the other had DLBCL (10, 17). There are few reports of synchronous WM and second cancers. A total of 225 (24%) of the 924 patients with WM developed secondary malignancy (SM), although more than 60% of the cases were seen before the diagnosis of WM was made. The most frequent secondary cancers in that study were those of the breast, skin, hematologic, melanoma, lung, and thyroid (18). A retrospective analysis of 230 consecutive WM patients was conducted by Varettoni et al. (19). At the time of the WM diagnosis, 17 patients (7%) had a history of one (n = 15) or two (n = 2) solid malignancies. In another study, patients who were exposed to nucleoside analogs seemed to have a greater risk of developing MDS or AML (20). In a follow-up study, patients with WM who received treatment had a fivefold increased chance of developing secondary cancers (21). According to an analysis of the Surveillance, Epidemiology, and End Results (SEER) database (1992–2011), 681 SMs (14.56%) were found in 4676 WM patients, and the SM risk was 49% greater in WM patients than in the general population. At 5 and 10 years, the cumulative incidence of SMs was 10% and 16%, respectively (22). Our search of the literature revealed that there were only 3 cases of synchronous macroglobulinemia with stomach cancer (23–25). Coincidentally, 2 patients were reported in Japanese articles without available abstracts. Given the high incidence of stomach cancer in East Asian nations, the co-occurrence of macroglobulinemia and gastric cancer appears to be random from a clinical standpoint. The etiology of WM with second cancer is unknown. The occurrence of second tumors in WM patients may be associated with immune defects such as B-cell dysfunction, low gamma globulin levels and T-cell subgroup dysfunction, as well as treatment and genetic susceptibility (20).
The lack of concurrent treatment approaches makes managing multiple synchronous primary tumors challenging. MDT (multidisciplinary team) discussion is considered an essential component of care for patients with multiple malignant tumors. The therapeutic approach should be chosen on the basis of the experience of the physician in the absence of recommendations for the management of such disorders. Personalized treatment plans are suggested on the basis of the patient’s condition. For patients with HER2-positive disease, the recommended first-line regimen is trastuzumab (anti-HER2) in combination with platinum and fluoropyrimidine-based chemotherapy. However, the goal of individualized therapy is to avoid unnecessary therapeutic interventions for patients who are unlikely to respond to therapy. In our case, the frail elderly patient had poor nutritional status after total gastrectomy and was bed-ridden. Unfortunately, the patient refused further immunotherapy and targeted therapy and was treated with traditional Chinese medicine without any outpatient follow-up for personal reasons.
The limitations of our case are as follows: the patient refused next-generation sequencing (NGS) because of his advanced age and poor physical status. Hence, the genetic mutation was unknown. Additionally, owing to advanced age and poor tolerance, treatment cannot benefit from immunotherapy or targeted therapy.
This case is unique, as it demonstrates the rare coexistence of WM, gastric adenocarcinoma, and gastric GIST. Considering that enlarged lymph nodes often occur in solid tumors, clinicians and pathologists tend to overlook the possibility of secondary tumors such as lymphoma. Therefore, such cases highlight the importance of postoperative pathology in lymph node dissection. Additional cases, therefore, need to be investigated to further clarify the key diagnostic and therapeutic characteristics of synchronous neoplasms. Therefore, more cases need to be investigated to better understand the essential diagnostic and treatment features of synchronous malignancies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of Ningbo No.2 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YJ: Conceptualization, Funding acquisition, Writing – original draft. ZS: Data curation, Investigation, Validation, Writing – review & editing. KL: Data curation, Investigation, Validation, Writing – review & editing. TL: Data curation, Formal Analysis, Writing – review & editing. ZF: Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2024KY1562) and Clinical Medicine Special Fund Project of Zhejiang Medical Association (Grant No. 2022ZYC-A162).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1554206/full#supplementary-material
References
1. Abrahao-MaChado LF and Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World J Gastroenterol. (2016) 22:4619–25. doi: 10.3748/wjg.v22.i19.4619
2. Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the Preimatinib Mesylate Era. Cancer. (2005) 103:821–29. doi: 10.1002/cncr.20862
3. Liu J, Huang BJ, Ding FF, Tang FT, and Li YM. Synchronous occurrence of gastric cancer and gastrointestinal stromal tumor: A case report and review of the literature. World J Gastrointest Oncol. (2023) 15:1807–22. doi: 10.4251/wjgo.v15.i10.1807
4. Li Y, Jiang F, Wu CY, and Leung WK. Prevalence and temporal trend of gastric preneoplastic lesions in Asia: A systematic review with meta-analysis. United Eur Gastroenterol J. (2024) 12:139–51. doi: 10.1002/ueg2.12507
5. Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the college of american pathologists, american society for clinical pathology, and the american society of clinical oncology. J Clin Oncol. (2017) 35:446–64. doi: 10.1200/JCO.2016.69.4836
6. Kato S, Okamura R, Baumgartner JM, Patel H, Leichman L, Kelly K, et al. Analysis of circulating tumor DNA and clinical correlates in patients with esophageal, gastroesophageal junction, and gastric adenocarcinoma. Clin Cancer Res. (2018) 24:6248–56. doi: 10.1158/1078-0432.CCR-18-1128
7. Shitara K, Yatabe Y, Matsuo K, Sugano M, Kondo C, Takahari D, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer. (2013) 16:261–7. doi: 10.1007/s10120-012-0179-9
8. Sugimura T, Fujimura S, and Baba T. Tumor production in the glandular stomach and alimentary tract of the rat by N-methyl-N’-nitro-N-nitrosoguanidine. Cancer Res. (1970) 30:2455–65.
9. Cohen A, Geller SA, Horowitz I, Toth LS, and Werther JL. Experimental models for gastric leiomyosarcoma. The effects of N-methyl-N’-nitro-N-nitrosoguanidine in combination with stress, aspirin, or sodium taurocholate. Cancer. (1984) 53:1088–92. doi: 10.1002/1097-0142(19840301)53:5<1088:aid-cncr2820530512>3.0.co;2-y
10. Kaffes A, Hughes L, Hollinshead J, and Katelaris P. Synchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomach. J Gastroenterol Hepatol. (2002) 17:1033–6. doi: 10.1046/j.1440-1746.2002.02649.x
11. Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. (2012) 367:826–33. doi: 10.1056/NEJMoa1200710
12. Varettoni M, Arcaini L, Zibellini S, Boveri E, Rattotti S, Riboni R, et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom’s macroglobulinemia and related lymphoid neoplasms. Blood. (2013) 121:2522–8. doi: 10.1182/blood-2012-09-457101
13. Chan AO, Chu KM, Yuen ST, Leung SY, Lam SK, and Wong J. Synchronous gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma in associatio with Helicobacter pylori infection: comparing reported cases between the East and West. Am J Gastroenterol. (2001) 96:1922–4. doi: 10.1111/j.1572-0241.2001.03895.x
14. Phuong VT, Lieu DQ, Hanh BTM, Thu Hien HT, Khanh NV, Hang NT, et al. Synchronous occurrence of gastric adenocarcinoma and MALT-type lymphoma: A case report and literature review. Radiol Case Rep. (2023) 18:2730–4. doi: 10.1016/j.radcr.2023.05.035
15. Hao M, Zhao Y, Liu H, Shen X, and Li C. Gastric collision tumor with diffuse large B-cell lymphoma and adenocarcinoma: A rare case report and literature review. Indian J Pathol Microbiol. (2025) 68:151–4. doi: 10.4103/ijpm.ijpm_157_23
16. Feng Y, Duan TJ, Huang Q, Li ZY, Liu YP, Luo MS, et al. The clinicopathological characteristics of gastric cancer and precancerous conditions in gastric DLBCL and MALT lymphoma patients: a multi-center retrospective study. Ann Med. (2023) 55:2193423. doi: 10.1080/07853890.2023.2193423
17. Ojima T, Tabata H, and Yamaue H. Laparoscopic distal gastrectomy for synchronous adenocarcinoma, diffuse large B cell lymphoma and gastrointestinal stromal tumor in the stomach: a case report. Surg Case Rep. (2022) 8:89. doi: 10.1186/s40792-022-01446-1
18. Hanzis C, Ojha RP, Hunter Z, Manning R, Lewicki M, Brodsky P, et al. Associated Malignancies in patients with Waldenstrom’s macroglobulinemia and their kin. Clin Lymphoma Myeloma Leuk. (2011) 11:88–92. doi: 10.3816/CLML.2011.n.016
19. Varettoni M, Tedeschi A, Arcaini L, Pascutto C, Vismara E, Orlandi E, et al. Risk of second cancers in Waldenstrom macroglobulinemia. Ann Oncol. (2012) 23:411–5. doi: 10.1093/annonc/mdr119
20. Leleu X, Soumerai J, Roccaro A, Hatjiharissi E, Hunter ZR, Manning R, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenstrom macroglobulinemia treated with nucleoside analogs. J Clin Oncol. (2009) 27:250–5. doi: 10.1200/JCO.2007.15.1530
21. Morra E, Varettoni M, Tedeschi A, Arcaini L, Ricci F, Pascutto C, et al. Associated cancers in Waldenstrom macroglobulinemia: clues for common genetic predisposition. Clin Lymphoma Myeloma Leuk. (2013) 13:700–3. doi: 10.1016/j.clml.2013.05.008
22. Castillo JJ, Olszewski AJ, Hunter ZR, Kanan S, Meid K, Treon SP, et al. Incidence of secondary Malignancies among patients with Waldenstrom macroglobulinemia: an analysis of the SEER database. Cancer. (2015) 121:2230–6. doi: 10.1002/cncr.29334
23. Coppola A, Yermakov V, and Caggiano V. Pleomorphic lymphoma and gastric adenocarcinoma (collision neoplasm) associated with monoclonal macroglobulinemia and amyloidosis. A case report. Cancer. (1969) 23:576–85. doi: 10.1002/1097-0142(196903)23
24. Tanaka S, Nagao M, Ito T, Takizawa K, Kita K, Adachi M, et al. An autopsy case of macroglobulinemia with gastric cancer. Rinsho Ketsueki. (1985) 26:532–6.
Keywords: Waldenström macroglobulinemia, gastric adenocarcinoma, gastrointestinal stromal tumor (GIST), human epidermal growth factor receptor-2 (HER-2), synchronous occurrence
Citation: Jin Y, Shi Z, Li K, Liu T and Fang Z (2025) Synchronous occurrence of Waldenström macroglobulinemia and HER2-positive gastric adenocarcinoma with gastrointestinal stromal tumor: a rare case report. Front. Oncol. 15:1554206. doi: 10.3389/fonc.2025.1554206
Received: 01 January 2025; Accepted: 21 April 2025;
Published: 16 May 2025.
Edited by:
Andee Dzulkarnaen Zakaria, Universiti Sains Malaysia, MalaysiaReviewed by:
Mehdi Montazer, Mashhad University of Medical Sciences, IranAhmad Fardi Bin Sulaiman, Sultan Zainal Abidin University, Malaysia
Copyright © 2025 Jin, Shi, Li, Liu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Fang, ZmFuZ3poaTA1NzRAMTYzLmNvbQ==
 Yingming Jin
Yingming Jin Zhilong Shi
Zhilong Shi