Abstract
Background:
Ovarian cancer complicated with chylothorax is extremely rare, and only eight papers can be searched in PubMed. We report a case of advanced ovarian cancer with extensive lymphatic metastasis that led to chylothorax and then chylous ascites. Combined with the literature review, we try to identify its clinical characteristics.
Case description:
A 64-year-old patient with advanced ovarian cancer relapsed, presenting with extensive lymphatic metastasis and a right pleural effusion. Thoracentesis was performed. The drainage fluid had a milky white appearance with triglyceride levels >1.24 mmol/L (110 mg/dL), a cholesterol level <5.18 mmol/L (200 mg/dL), and a positive chyle test. A multi-disciplinary team recommended anti-tumor therapy for ovarian cancer and conservative treatment of chylothorax, including a strict diet with low-fat medium–long-chain triglycerides and chest tube insertion with interleukin-2 intrapleural perfusion as the pleurodesis agent. Chylothorax improved significantly with progressively reduced and transparent pleural effusion. However, strict diet control affected her quality of life negatively, and the patient was not able to adhere to the diet plan. Therefore, chylothorax recurred, but chyle was produced slowly; thoracentesis was performed repeatedly with IL-2 or cisplatin pleural perfusion at intervals of 2–5 months (a total of six times) in March 2021–June 2023. In June 2023, the patient was not able to tolerate systemic chemotherapy because of severe myelosuppression after fourth-line chemotherapy. The patient developed bilateral chylothorax and then chylous ascites in December 2023. Finally, the patient died in March 2024.
Conclusion:
Ovarian cancer complicated with chylothorax is a rare condition with a relatively poor prognosis. Pleural fluid analysis can identify this condition when clinical suspicion exists. Anti-tumor treatment of ovarian cancer and conservative management of chylothorax were first recommended instead of surgery, which have been proven to be effective in treating OCC.
1 Introduction
Pleural effusion is common in advanced ovarian cancer, but ovarian cancer with chylothorax (OCC) is extremely rare; only seven papers can be searched in PubMed (1–7). Ovarian cancer with chylothorax and chylous ascites was reported only in one paper. Different from pleural effusion, OCC has its own particularity in clinical features and treatment. We report a case of advanced ovarian cancer with extensive lymphatic metastasis that led to chylothorax and then chylous ascites. Combined with the literature review, we try to identify the characteristics of the disease.
2 Case description
A 64-year-old woman presenting with abdominal distension and loss of appetite for 1 month was referred to our hospital in May 2018 (Table 1). Physical examination revealed massive abdominal distension with free fluid in the abdominal cavity. Contrast-enhanced computed tomography (CT) showed multiple cystic solid mass lesions with rich blood supply in the pelvis, the largest approximately 18 × 15 cm; multiple enlarged retroperitoneal lymph nodes (up to 5 cm in diameter); enlarged mediastinal lymph nodes; massive ascites; and bilateral pleural effusion (9.2 and 3.9 cm in depth in the left and right, respectively). The serum carbohydrate antigen 125 (CA-125) was 1,539 U/mL. Abdominal puncture and thoracentesis were performed. Both ascites and pleural fluid were serous and yellow in appearance. Adenocarcinoma cells were found on cytology from both ascites and pleural fluid. Ovarian epithelial cancer was diagnosed in stage IV. Neoadjuvant chemotherapy with paclitaxel and carboplatin was given for three cycles with interleukin-2 left-sided chest perfusion once (from May 2018 to October 2018). Both ascites and pleural fluid disappeared after one course of chemotherapy. An optimal intermediate cytoreductive procedure was performed with residual lesions <1 cm (R1) on November 13, 2018. Histopathology result showed moderately differentiated (G2) serous papillary adenocarcinoma in both ovaries, diffuse metastatic adenocarcinoma nodules in the omentum, and multiple metastases existing in the para-aortic lymph nodes and bilateral pelvic lymph nodes (4/6, 13/22, and 14/15, respectively). Four courses of chemotherapy were given after the operation (from December 2018 to February 2019).
Table 1
| Event | Date |
|---|---|
| Initial diagnosis | May 2018 |
| Initial treatment | May 2018 to February 2019 |
| The first recurrence (lymph node enlargement) | May 2020 |
| Recurrence treatment | August 2020 to December 2020 |
| Chylothorax (the second recurrence) | March 2021 |
| The second recurrence treatment | March 2021 to October 2021 |
| Maintenance treatment | November 2021 to May 2022 |
| Third-line/fourth-line treatment (increased CA-125, lymph node enlargement, and chylothorax) | June 2022 to June 2023 |
| Chylous ascites | December 2023 |
| Death | March 2024 |
The timeline from the initial diagnosis to death.
CA-125, carbohydrate antigen 125.
In May 2020 (15 months after the last chemotherapy), an enlarged lymph node (8 cm in diameter) was palpable in the left neck, and biopsy confirmed metastatic serous carcinoma. Ultrasound result showed disseminated multiple enlarged lymph nodes. The patient was diagnosed with platinum-resistant recurrence of ovarian cancer. CA-125 was 1,038 U/mL. After five cycles (from August 2020 to December 2020) of bevacizumab and second-line chemotherapy (paclitaxel + carboplatin), the lymph nodes in the left neck reduced to 0.8 cm, and CA-125 was normal.
In March 2021 (3 months after the last chemotherapy), a hard mass that was approximately 5 cm in diameter on the left lower jaw was palpable. CA-125 was 1,863 U/mL. Ultrasound result showed a right pleural effusion (9.8 cm in depth, Figure 1). Thoracentesis was performed, and the drainage fluid had a milky white appearance (Figure 2). The laboratory analysis of the pleural fluid revealed exudative chylous fluid with triglyceride levels >1.24 mmol/L (110 mg/dL), a cholesterol level <5.18 mmol/L (200 mg/dL), and a positive chyle test. Cytological pathology revealed adenocarcinoma cells. The patient was diagnosed with platinum-resistant recurrence of ovarian cancer combined with right-sided chylothorax. Conservative treatment of chylothorax and chemotherapy for recurrent ovarian cancer were recommended by a multi-disciplinary team, including nutriology, respiratory oncology, thoracic surgery, and intervention departments. Surgery was not feasible because of disseminated multiple metastatic enlarged lymph nodes of ovarian cancer. Chylothorax was managed conservatively, including a strict diet with low-fat medium–long-chain triglycerides and chest tube insertion with interleukin-2 intrapleural perfusion (3 million units). Chylothorax improved significantly with progressively reduced and transparent pleural effusion (Figure 2). The drainage fluid was reduced to 50 mL/day on the fifth day, and then the drainage tube was removed. However, long-term intake of medium–long-chain triglycerides affected her quality of life negatively, and the patient was not able to adhere to the diet plan and gradually resumed the regular diet. Therefore, chylothorax recurred, and thoracentesis was performed repeatedly with IL-2 or cisplatinum pleural perfusion at intervals of 2–5 months (a total of six times). Chyle was produced slowly and did not affect the quality of life of the patient. Oral etoposide and bevacizumab were maintained from November 2021 to May 2022 (the patient refused PARP inhibitors due to economic reasons). The patient achieved a partial response (CA-125 91 U/L, lymph node reduction, and pleural fluid maintained at 3.1–5.8 cm).
Figure 1
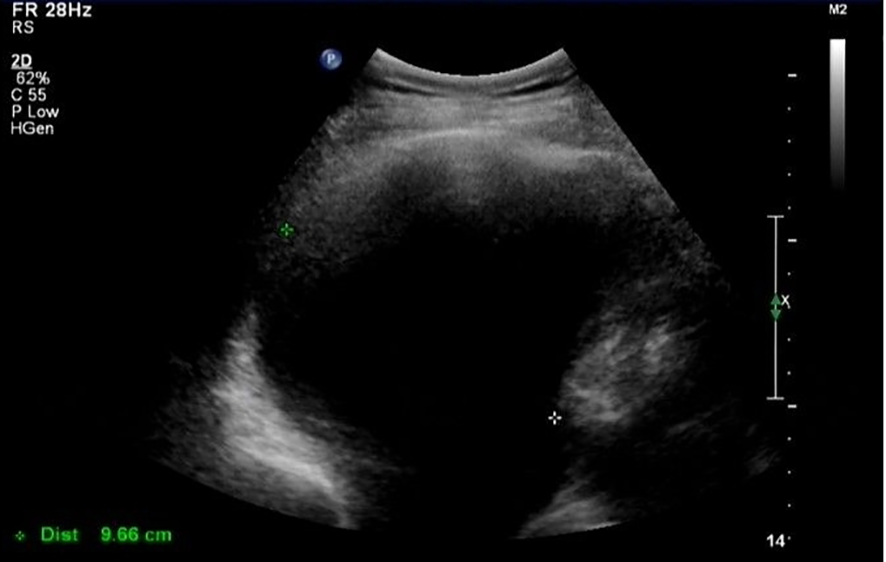
Ultrasound image of pleural effusion.
Figure 2

Chylous pleural fluid on day 1, day 3, and day 5.
The patient had repeated tumor recurrence in June 2022, which mainly manifested as increased CA-125 and distant lymph node metastasis (maximum diameter approximately 2–3 cm). Partial remission was achieved after third-line (gemcitabine) and fourth-line (albumin-bound paclitaxel) chemotherapy, targeted therapy, and immunotherapy. The chyme volume in the right thoracic cavity was stable, the patient had no chest congestion or dyspnea, and her nutritional status was fine. The treatment was discontinued due to severe myelosuppression.
In June 2023, the patient complained of chest tightness and dyspnea. Bilateral pleural effusion was found. The pleural drainage fluid was still chylous. She could not tolerate systemic chemotherapy because of severe myelosuppression. Symptomatic treatments for chylothorax were performed, including thoracic puncture drainage and injection of bevacizumab and IL-2 into the pleural cavity. A large increase in abdominal fluid appeared in December 2023, and the drainage fluid was also chylous (Figure 3). Symptoms were partially relieved after perfusion of cisplatin and bevacizumab into the abdomen. The patient died in March 2024.
Figure 3
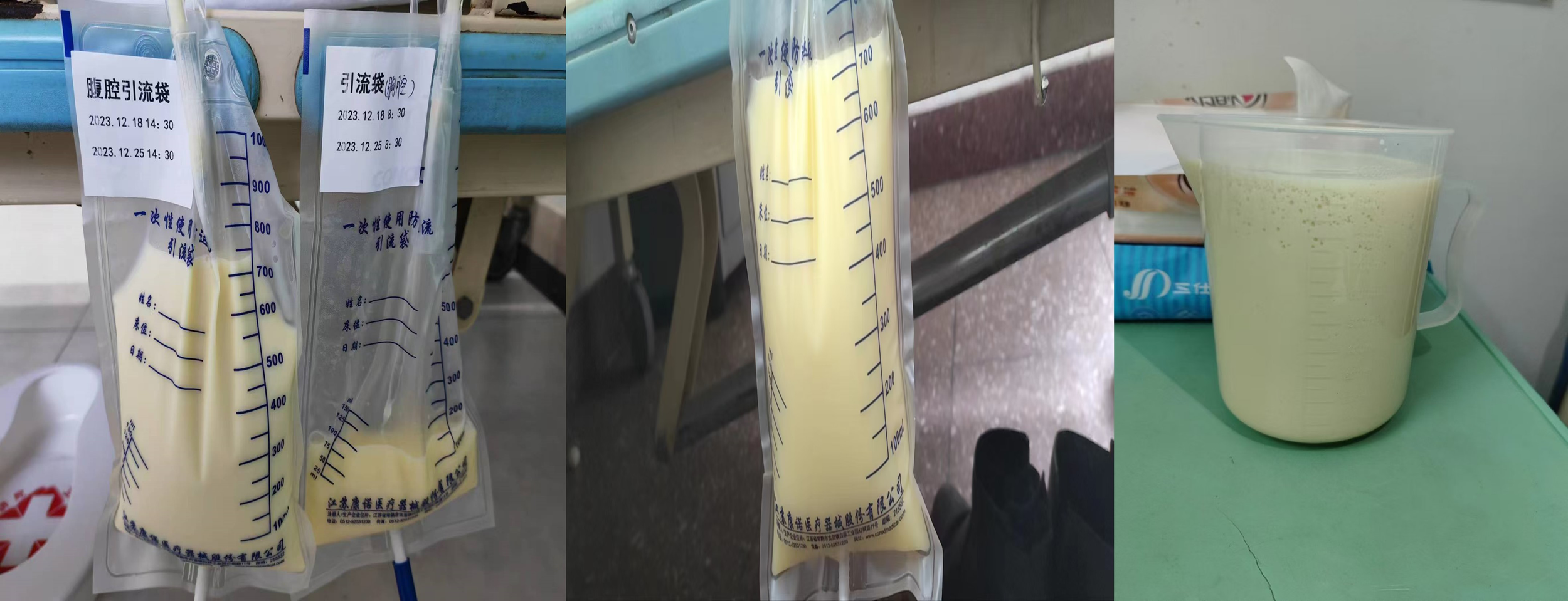
Chylous ascites.
3 Discussion
Chylothorax is a rare condition that results from thoracic duct damage or blockage with chyle leakage from the lymphatic system into the pleural space. Ovarian cancer complicated with chylothorax is rarely reported. Because of its rarity, the characteristics of the disease are not well established in the published works. We reported a case of OCC with a long-term follow-up of 6 years and tried to identify its clinical characteristics.
3.1 Etiology of ovarian cancer complicated with chylothorax
The thoracic duct, the largest lymph vessel in the human body, is approximately 36–45 cm long and 2–3 mm wide; it starts from the chylous pool in front of the second lumbar vertebra, enters the thoracic cavity through the aortic hiatus of the diaphragm, courses upward behind the esophagus in the mediastinum, and terminates in the subclavian vein. The primary role of the thoracic duct is to carry 60%–70% of ingested fat from the intestine to the circulatory system. Chyle contains large amounts of cholesterol, triglycerides, chylomicrons, and fat-soluble vitamins. Approximately 1.5–2.5 L of chyle is transported through the lymphatic system every day. Chyle transportation is maximal after a high-fat meal and minimal with starvation (8). Damage to or blockage of the thoracic duct can cause chylothorax.
Tumors and trauma are the most common etiologies of chylothorax (9). 1) Thoracic duct obstruction due to malignant tumor is the most common cause, of which lymphoma accounts for 70%. Rarely can a metastatic lymph node give rise to duct obstruction. In this paper, the patient relapsed mainly with extensive systemic lymphatic metastasis, including mediastinal lymphatic metastasis, which invaded and compressed the thoracic duct and its branches, resulting in chylothorax. Ruchi Gupta reported a similar case of ovarian epithelial malignancy with chylothorax (1). 2) Trauma is the second leading cause, accounting for 23% of chylothorax, which can be further sub-classified as iatrogenic or non-iatrogenic. The incidence of chylothorax caused by esophageal surgery was 4%. However, in ovarian cancer, only two patients were reported to have presented with chylothorax due to a thoracic duct injury after the resection of cardiophrenic lymph nodes through a transdiaphragmatic approach (2, 3). Therefore, the etiology of chylothorax in ovarian cancer includes two aspects: mediastinal lymphatic metastasis invading the thoracic duct and a surgical injury of the thoracic duct during cytoreductive surgery.
3.2 Clinical features and diagnosis
A clinical feature of chylothorax is a lack of specificity, depending on the rate of chyle loss. Rapid loss is associated with respiratory distress, such as dyspnea, chest pain, and cough. Patients may experience malnutrition, immunosuppression, and other internal environmental disorders in severe long-term chylothorax.
Chylothorax may be unilateral, either right-sided (50%) or left-sided (33.3%) or bilateral (16.66%), and is dependent on the location of the leak (8). Damage to the duct above the fifth thoracic vertebra results in a left-sided effusion, whereas damage to the duct below this level leads to a right-sided effusion. An injury of the duct between the third and sixth thoracic vertebrae may cause bilateral chylothorax.
The color of chylothorax shows the classical milky white appearance in most cases, but may be serous, yellow, or bloody in appearance in some cases, which may lead to misdiagnosis. Therefore, the exact diagnosis of chylothorax is conducted via thoracentesis and laboratory analysis of the pleural fluid. The diagnostic criteria include the following (10, 11): 1) the presence of chylomicrons in the pleural fluid, 2) a positive chyle test (Sudan III staining method, the presence of red fat globules under the microscope indicates a positive result), and 3) pleural fluid triglyceride levels >1.24 mmol/L (110 mg/dL) with a cholesterol level <5.18 mmol/L (200 mg/dL). Pseudochylothorax presenting a milky white appearance should be differentiated (8, 11, 12). A triglyceride level <0.56 mmol/L (50 mg/dL) with a cholesterol level >5.18 mmol/L (200 mg/dL) can be found in pseudochylothorax. Cholesterol crystals are also often seen in pseudochylothorax but are not present in chylothorax. Chylothorax can be differentiated from pseudochylothorax by adding 1–2 mL of ethyl ether. The milky appearance disappears in pseudochylothorax. Maldonado et al. (13) analyzed protein in pleural fluid and found that pleural effusions were exudative in 86% of cases and transudative in 14%. Exudative chylothoraces were found in patients with malignancy, while transudative chylothoraces were found in patients with cirrhosis, surgery, and pancreatic cancer.
CT of the thorax and abdomen should be performed to identify the amount of chylothorax and its relation to malignant tumors. In this case, multiple mediastinal enlarged lymph nodes could be clearly seen on CT, leading to chylous pleural fluid formation. Lymphangiography may be used to demonstrate the site of leakage or blockage. Matsumoto et al. (14) performed lymphangiography on nine patients who were unlikely to respond to conservative measures. They found that lymphangiography not only identified the site of the leak but also led to the leak resolving in all cases. They recommend early lymphangiography only in cases unlikely to be treated by conservative methods.
3.3 Treatment and prognosis
Compared to pleural fluid in ovarian cancer, it is difficult to treat chylothorax. The treatment of OCC includes three aspects: anti-tumor treatment of ovarian cancer and its metastasis, conservative management, and surgical management of chylothorax.
Anti-tumor treatment plays an important role in treating OCC. The patient in this case relapsed with extensive systemic lymph node metastasis of ovarian cancer, and chylothorax was formed due to an enlarged metastatic mediastinal lymph node and invasion of the thoracic duct. Therefore, the patient had to accept chemotherapy. Multi-line chemotherapy plus bevacizumab brought benefits for controlling ovarian cancer overall, reducing the metastatic lymph nodes, as well as leading to an improvement in chylothorax.
Conservative treatment of chylothorax involves the following: 1) medium- and long-chain triglycerides are directly absorbed into the portal system, bypassing the intestinal lymph system and thoracic duct. The diet of low-fat medium–long-chain triglycerides can reduce the flow of chyle in the thoracic duct and resolve approximately 50% of chylothorax (11). If a chyle leak does not reduce yet, then total parenteral feeding should be considered. 2) Somatostatin and octreotide, which can reduce intestinal chyle production, have been proven to be useful in the conservative treatment of chylothorax (15). 3) Draining pleural effusion using thoracentesis should be conducted to resolve respiratory distress and ensure complete lung expansion. At the same time, pleurodesis agents including talc, tetracycline, bleomycin, povidone, and elemene have been successfully used to produce chemical pleurodesis in the majority of patients (16, 17).
In this paper, we adopted a combined treatment plan consisting of anti-tumor therapy and conservative treatment of chylothorax. In more than 2 years, the patient had no or slight chest tightness or dyspnea, right-sided chylothorax was stable, and her nutritional status was fine. Nevertheless, chyle did not disappear completely, which is consistent with literature reports (16). However, Cowan and Helene (2, 3) reported two cases of chylothorax caused by damage to the thoracic duct during ovarian cancer surgery, which were treated after conservative management with fat-free enteral nutrition for 6 weeks. Based on this, we believed that, unlike chylothorax caused by trauma, conservative treatment is effective but has a low success rate in treating chylothorax caused by tumor blockage of the thoracic duct. Due to severe myelosuppression, the antineoplastic treatment was stopped, and the disease progressed with bilateral chylothorax and chylous ascites, which eventually led to the patient’s death.
Surgery is recommended if the drainage fluid exceeds 1.5 L/day or more than 1.0 L/day for five consecutive days or continues to drain for more than 2 weeks after conservative treatment (15). Surgical options include thoracic duct percutaneous embolization, surgical ligation of the thoracic duct, and pleuroperitoneal shunt (18). Thoracic duct ligation is advocated when chylothoraces are caused by an operation, especially esophageal surgery. The key point of thoracic duct ligation is identifying the origin of a chyle leak. However, extensive dissection to find the origin is discouraged in order to reduce further trauma. In the two cases of OCC, the authors failed to identify the origin of the chyle leak and were not able to perform thoracic duct ligation during thoracotomy (2, 3). Therefore, we do not recommend thoracic duct ligation first when chylothorax is caused by metastatic enlarged lymph nodes in patients with OCC.
The mortality rate for cases of chylothorax is approximately 10%; chylothoraces caused by thoracic duct obstruction due to mediastinal malignancies have the worst prognosis (19). Because of the rarity of OCC, the prognosis is unclear yet. Gupta R reported a case of ovarian epithelial malignancy with chylothorax in which the patient ultimately succumbed to the illness (1). The patient in this case died after 6 years of comprehensive treatment. Due to the rarity of ovarian cancer with chylothorax, the prognosis is unknown.
Taken together, OCC is a rare condition with a relatively poor prognosis. Pleural fluid analysis can identify this condition when clinical suspicion exists. Anti-tumor treatment of ovarian cancer and conservative management of chylothorax are first recommended, which have been proven to be effective in treating OCC.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. XZ: Data curation, Investigation, Methodology, Resources, Writing – original draft. KN: Data curation, Investigation, Methodology, Resources, Writing – original draft. WS: Conceptualization, Methodology, Resources, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Gupta R Bal A Das A Kumar Marwah R . Serous cystadenocarcinoma of the ovary in an 11-year-old girl with extensive dissemination and chylothorax: a case report and a brief review of literature. J Pediatr Hematol Oncol. (2009) 31:533–5. doi: 10.1097/MPH.0b013e3181984dd3
2
Leray H Brouchet L Tanguy Le Gac Y Bouharaoua S Otal P Ferron G et al . Postoperative chest liver herniation after cardiophrenic lymph node resection by a transdiaphragmatic approach following primary cytoreductive surgery for advanced endometrioid ovarian cancer: A case report. Gynecol Oncol Rep. (2021) 36:100727. doi: 10.1016/j.gore.2021.100727
3
Cowan RA Tseng J Murthy V Srivastava R Long Roche KC Zivanovic O et al . Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol Oncol. (2017) 147:262–6. doi: 10.1016/j.ygyno.2017.09.001
4
Gupta PR Taly A Joshi N Gupta S Meena RC Jain S . Chylothorax in a patient with ovarian tumour. Indian J Chest Dis Allied Sci. (2005) 47:43–6.
5
Lawton F Blackledge G Johnson R . Co-existent chylous and serous pleural effusions associated with ovarian cancer: a case report of Contarini’s syndrome. Eur J Surg Oncol. (1985) 11(2):177–8. doi: 10.1055/s-2007-1018512
6
Davidsen JR Laursen CB Madsen PH . Måling af pH i pleuravæske er af værdi ved udredning af årsagen til purulent pleuraeffusion [Measuring pH in pleural fluid is valuable when identifying the cause of purulent pleural effusion. Ugeskr Laeger. (2012) 174(21):1469–70.
7
Vavilis D Papadopoulos N Agorastos T et al . Primary ovarian angiosarcoma–review of the literature and report of a case with coexisting chylothorax. Eur J Gynaecol Oncol. (2007) 28(4):287–9. doi: 10.1016/j.earlhumdev.2006.05.007
8
McGrath EE Blades Z Anderson PB . Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. (2010) 104:1–8. doi: 10.1016/j.rmed.2009.08.010
9
Nair SK Petko M Hayward MP . Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. (2007) 32:362–9. doi: 10.1016/j.ejcts.2007.04.024
10
Seitelman E Arellano JJ Takabe K Barrett L Faust G Angus LD . Chylothorax after blunt trauma. J Thorac Dis. (2012) 4(3):327–30. doi: 10.3978/j.issn.2072-1439.2011.09.03
11
de Beer HG Mol MJ Janssen JP . Chylothorax. Neth J Med. (2000) 56:25–31. doi: 10.1016/S0300-2977(99)00114-X
12
Hillerdal G . Chylothorax and pseudochylothorax. Eur Respir J. (1997) 10:1157–62. doi: 10.1183/09031936.97.10051157
13
Maldonado F Hawkins FJ Daniels CE Doerr CH Decker PA Ryu JH et al . Pleural fluid characteristics of chylothorax. Mayo Clin Proc. (2009) 84:129–33. doi: 10.4065/84.2.129
14
Matsumoto T Yamagami T Kato T Hirota T Yoshimatsu R Masunami T et al . The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. (2009) 82:286–90. doi: 10.1259/bjr/64849421
15
Al-Zubairy SA Al-Jazairi AS . Octreotide as a therapeutic option for management of chylothorax. Ann Pharmacother. (2003) 37(5):679–82. doi: 10.1345/aph.1C265
16
Mares DC Mathur PN . Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest. (1998) 114(3):731–5. doi: 10.1378/chest.114.3.731
17
Rizzardi G Loy M Marulli G Rea F . Persistent chylothorax in lymphangioleiomyomatosis treated by intrapleural instillation of povidone. Eur J Cardiothorac Surg. (2008) 34:214–5. doi: 10.1016/j.ejcts.2008.03.050
18
Martucci N Tracey M Rocco G . Postoperative chylothorax. Thorac Surg Clin. (2015) 25:523–8. doi: 10.1016/j.thorsurg.2015.07.014
19
Shah RD Luketich JD Schuchert MJ Christie NA Pennathur A Landreneau RJ et al . Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg. (2012) 93:897–903; discussion 903-4. doi: 10.1016/j.athoracsur.2011.10.060
Summary
Keywords
chylothorax, chylous ascites, ovarian cancer, diagnosis, treatment
Citation
Wang Q, Zhang X, Niu K and Shen W (2025) Chylothorax in a patient with advanced ovarian cancer: a case report and literature review. Front. Oncol. 15:1555038. doi: 10.3389/fonc.2025.1555038
Received
03 January 2025
Accepted
09 September 2025
Published
30 September 2025
Volume
15 - 2025
Edited by
Robert Fruscio, University of Milano Bicocca, Italy
Reviewed by
Federica Perelli, Azienda Ospedaliera Universitaria Meyer IRCCS - Firenze, Italy
Elsa María Vasquez-Trespalacios, CES University, Colombia
Updates
Copyright
© 2025 Wang, Zhang, Niu and Shen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjie Shen, 13552929646@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.