- 1Department of Oncology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 2The First School of Clinical Medicine, Nanjing Medical University, Nanjing, China
Gastric cancer remains one of the most prevalent gastrointestinal malignancies, with certain subtypes, such as poorly cohesive carcinoma—including signet ring cell carcinoma (SRCC)—exhibiting aggressive progression and poor prognosis. Mesenchymal epithelial transition (MET) amplification, a relatively rare oncogenic driver in gastric cancer (~2–10.2% of cases), has been associated with resistance to conventional therapies and dismal survival (median <6 months in metastatic cases). While MET inhibitors such as crizotinib have shown efficacy in MET-altered non-small cell lung cancer (NSCLC), their role in gastric cancer remains uncertain due to tumor heterogeneity and the lack of robust clinical evidence. We report a case of a female patient with MET-amplified metastatic gastric cancer and systemic bone marrow involvement. Despite eventual disease progression, the initial response to crizotinib was remarkable, with rapid hematologic recovery (platelets: 7→216×109/L) and significant tumor regression. Although disease progression occurred after 5 months, characterized by pulmonary metastasis, biliary obstruction and multiple infections, the substantial initial benefits of crizotinib cannot be overlooked. The patient survived 8 months from diagnosis, highlighting the transient efficacy of MET inhibition and the impact of clonal evolution. This case underscores the potential and limitations of MET inhibitors in gastric cancer. Biomarker-driven selection, early resistance detection, and trials exploring crizotinib-chemotherapy/immunotherapy combinations are urgently needed to improve outcomes in this aggressive subtype.
1 Introduction
Gastric cancer is a global health concern, often diagnosed at an advanced stage due to subtle early symptoms. In 2018, it accounted for 784,000 deaths, making it the third leading cause of cancer-related mortality worldwide (1). Among its subtypes, signet ring cell carcinoma (SRCC) is a highly malignant and poorly differentiated form of poorly cohesive carcinoma, characterized by diffuse infiltrative growth, high metastatic potential, and a late-stage diagnosis (2).
The prognosis of poorly cohesive carcinomas, including SRCC, remains controversial, largely due to variations in its definition. While some studies question whether SRCC has a worse prognosis compared to other gastric cancer types, its aggressive nature, chemoresistance, and high metastatic potential pose significant treatment challenges. Surgery is often only feasible in localized disease, and effective treatments for advanced SRCC remain limited. Molecularly targeted therapies, particularly those addressing oncogenic drivers, may provide new therapeutic avenues (3).
One emerging molecular target is the mesenchymal epithelial transition (MET) gene, which is located on the long arm of human chromosome 7. It encodes the cellular-mesenchymal epithelial transition (c-Met) protein, a receptor tyrosine kinase that normally regulates essential cellular processes including tumor proliferation, invasion, and poor prognosis in gastric cancer (4). However, MET amplification is relatively rare, occurring in only 2–10.2% of gastric cancers (5). MET protein overexpression is observed in approximately 50% of advanced cases (6), but only a subset of these patients exhibit true MET-driven oncogenesis, which is essential for response to targeted therapy. MET inhibitors like crizotinib have demonstrated significant clinical benefits in non-small cell lung cancer (NSCLC), particularly in patients with MET exon 14 skipping mutations(METΔ14) and high-level MET amplification (7). Early studies demonstrated that crizotinib has potential efficacy in MET-amplified gastric cancer (8). However, clinical outcomes have been inconsistent, likely due to tumor heterogeneity, clonal evolution, and microenvironmental influences. These factors highlight both the therapeutic potential of crizotinib and the need for further investigation to optimize its use in this patient population.
Here, we report a case of MET-amplified gastric cancer with systemic bone marrow metastases, a rare and aggressive disease presentation. The patient developed severe thrombocytopenia, a complication of disseminated carcinomatosis of the bone marrow (DCBM), and was treated with crizotinib. This case highlights the potential role of MET-targeted therapy in gastric cancer, while also underscoring the challenges of resistance and disease progression in this setting.
2 Case report
2.1 Patient information
This case report describes a 51-year-old female patient with no significant prior medical history. In July 2023, she presented with neck and upper back pain, along with left shoulder pain and restricted range of motion. She also reported occasional chest tightness, palpitations, fatigue, and scattered bruising on her limbs.
2.2 Clinical progression
Upon admission, laboratory tests revealed significantly elevated levels of carcinoembryonic antigen (CEA), CA153, CA125, CA724, and cytokeratin fragment 19. CT imaging indicated a suspected primary malignant pancreatic lesion with lymph node metastasis, raising concerns about both pancreatic malignancy and possible lymphoma. Additionally, diffuse thickening of the gastric wall was noted (Figure 1A), prompting recommendations for further endoscopic evaluation. PET-CT confirmed diffuse thickening of the gastric wall with irregularly increased Fluorodeoxyglucose (FDG) uptake, pancreatic swelling with diffuse FDG uptake, and multiple enlarged lymph nodes (Figure 1B) showing increased FDG metabolism in the bilateral cervical, supraclavicular and many other individual regions. Focal areas of increased FDG uptake were also observed throughout the bone marrow (Figure 1C), with several regions demonstrating osteolytic bone destruction.
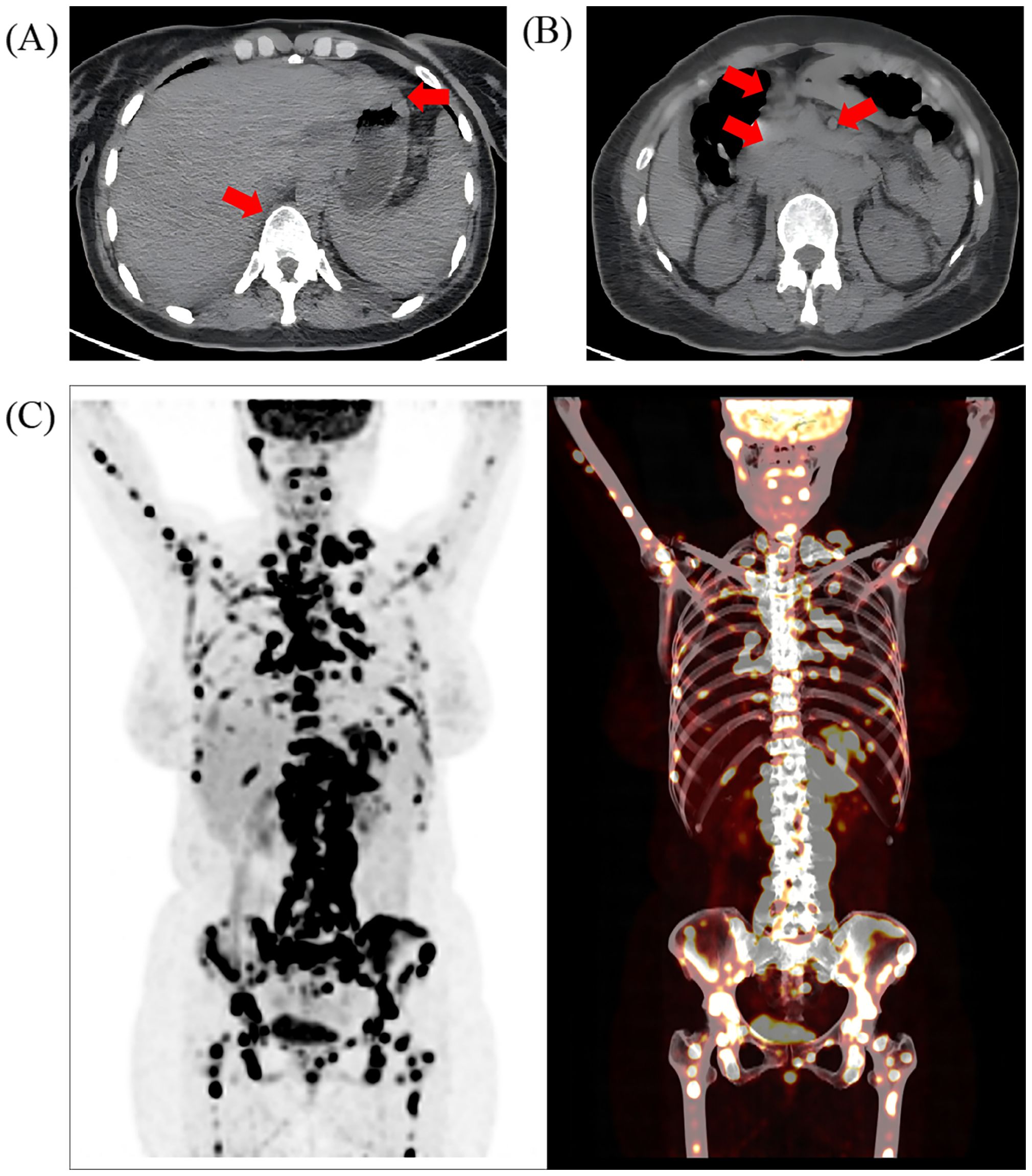
Figure 1. The imaging evaluation of the patient at admission. Positron emission computed tomography (PET-CT) showed (A) thickening of gastric wall as well as mixed metastasis of the vertebras and (B) multiple enlarged abdominal lymph nodes; the red arrow represents the location of the lesion; (C) increased FDG uptake in multiple areas of the bone marrow, including the skull, right mandible, several vertebrae and their attachments, sternum, multiple ribs, pelvic bones, clavicles, scapulae, humeri, and femurs.
These findings raised the possibility of a malignancy with multi-system involvement, particularly lymphoma, necessitating further pathological investigation. During the hospitalization, the patient developed progressive thrombocytopenia reaching a critically low platelet count of 7×109/L, alongside elevated D-dimer levels, hypofibrinogenemia and prolonged prothrombin time. Persistent platelet transfusions and thrombopoietin therapy failed to restore platelet counts, with peak levels remaining subnormal at 39×109/L. This indicated rapid progression of the malignancy with escalating tumor burden, placing the patient at significant bleeding risk and making it difficult to identify the primary lesion site.
2.3 Diagnosis and treatment
The patient underwent a bone marrow biopsy (Figure 2A) and histological examination (Figure 2B), which ruled out lymphoma and confirmed metastatic poorly differentiated adenocarcinoma. Further investigation was recommended to locate the primary tumor, which was suspected to be in the stomach. Given the patient’s high bleeding risk from severe thrombocytopenia and coagulopathy, the oncology team suggested genetic testing. The next-generation sequencing (NGS) analysis of peripheral blood and malignant bone marrow effusion revealed focal amplifications of the MET gene on chromosome 7, with copy numbers exceeding 10 (Figure 2E). Moreover, no mutations were detected in other oncogenic drivers, including HER2, RAS/MAPK and PI3K/AKT pathways. Following multidisciplinary consultation with external specialists, the patient was initiated on crizotinib (1 tablet once daily). Remarkably, after treatment with this MET inhibitor, the patient’s hematopoietic function showed significant improvement. Her platelet count substantially increased to 93×109/L within one week and normalized to 216×109/L after two weeks of therapy (Figures 2C, D). Additionally, her coagulation function also improved rapidly.
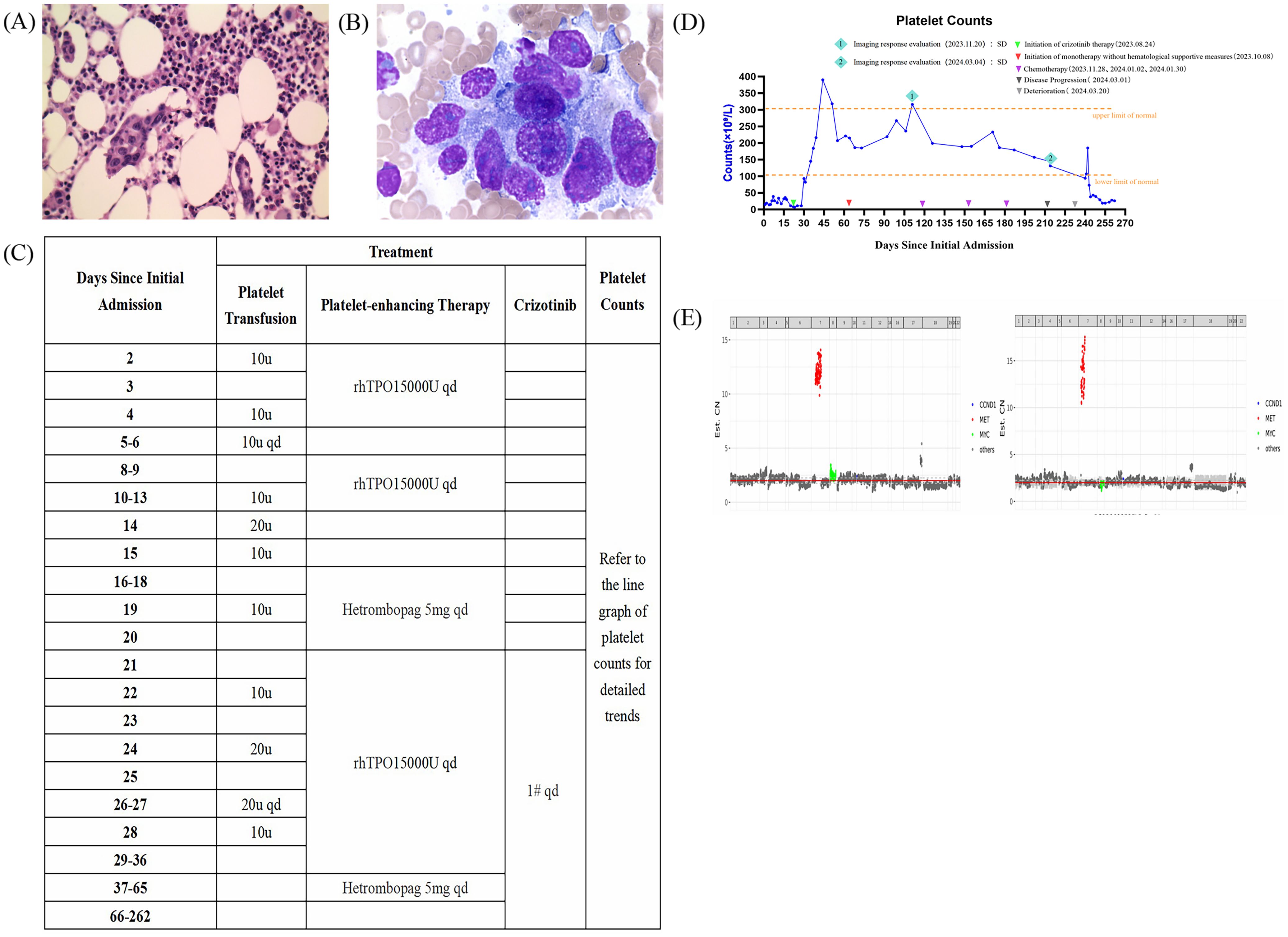
Figure 2. Bone marrow smear and biopsy of the patient after bone marrow infiltration and tendencies in hematopoietic function after the crizotinib treatments. Gene copy number distribution charts from the genetic reports. (A) The bone marrow smear and (B) the bone marrow biopsy showing metastatic poorly differentiated adenocarcinoma (hematoxylin-eosin, original magnification ×400). (C) Detailed supportive therapy and crizotinib administration timeline. (D) Dynamic monitoring of platelet counts suggested that the hematopoietic function was improved rapidly after the crizotinib treatments received in the hospital. E The gene copy number distribution charts obtained from the patient’s peripheral blood (left) and malignant bone marrow effusion (right).
2.4 Definitive diagnosis
On September 1, 2023, with the patient’s condition significantly improved, a gastroscopy was performed. The pathology results indicated poorly differentiated carcinoma at the angular incisure and low-adhesion carcinoma near the angular incisure in the gastric body, with areas of signet ring cell carcinoma. Lauren classification indicated a diffuse type. This led to the final diagnosis of gastric cancer.
2.5 Further treatment
After the treatment of crizotinib for three cycles, the patients’ subsequent imaging reports (Figures 3A-E) and laboratory test results showed marked improvement. According to RECIST 1.1 scoring standard, the therapeutic response was assessed as stable disease (SD), and the patient met the criteria for renewed chemotherapy eligibility. Consequently, the patient initiated a new anti-tumor regimen on November 28, 2023 for a total of three cycles, consisting of oxaliplatin combined with S-1 (Tegafur).
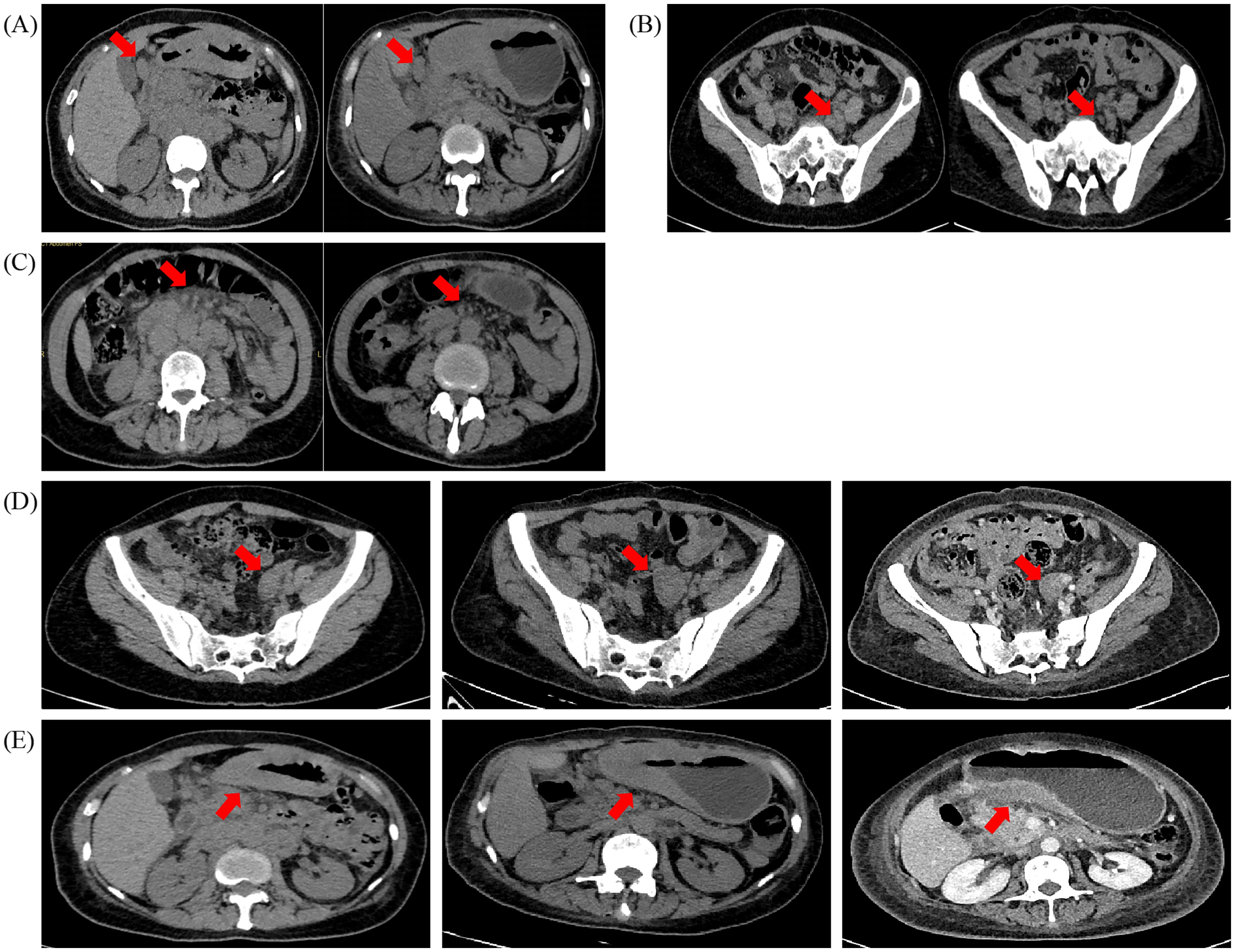
Figure 3. CT (Computed tomography) scans before (2023–08–03) and after (2023–11–20) (2024–03–04), the crizotinib treatments. (A) Metastatic lymph nodes around the gastric antrum and (B) pelvic metastatic lymph nodes were the two lesions that gradually shrunk significantly after the crizotinib treatments. (C) Omental metastatic lesions and (D) the pelvic adnexal mass shrunk significantly as well. E.The diffuse thickened gastric wall remained stable. All assessed lesions were indicated with red arrows.
2.6 Disease progression
On March 1, 2024, the patient developed symptoms of cough, sputum production, and fatigue. The follow-up CT scan demonstrated increased bilateral pleural effusions and progressive omental lesions, while notably revealing significant size reduction of the pelvic adnexal mass (Figure 3D). Changes in some non-target lesions may be associated with inflammation or hypoalbuminemia and do not meet the criteria for progressive disease. According to RECIST 1.1 criteria, the therapeutic response after three cycles of chemotherapy was assessed as SD. Immunohistochemical analysis confirmed that the atypical cells observed in the pleural fluid were likely metastatic in origin from gastric carcinoma. Following this, the patient received relevant symptomatic and supportive treatments.
2.7 Deterioration
On March 20, 2024, the patient developed jaundice and abnormal liver function. It was considered that the intrahepatic bile duct dilatation, caused by internal liver compression, led to obstructive jaundice. On March 28, 2024, the patient underwent percutaneous transhepatic biliary drainage in the interventional radiology department. The patient developed polymicrobial infection after the surgery.
2.8 Outcome
No alternative therapy initiated due to rapid clinical decline and family preference for palliative care, the patient was transferred back to a local hospital for treatment. On April 23, 2024, the patient was pronounced dead (Figure 4), with an overall survival of 8 months.
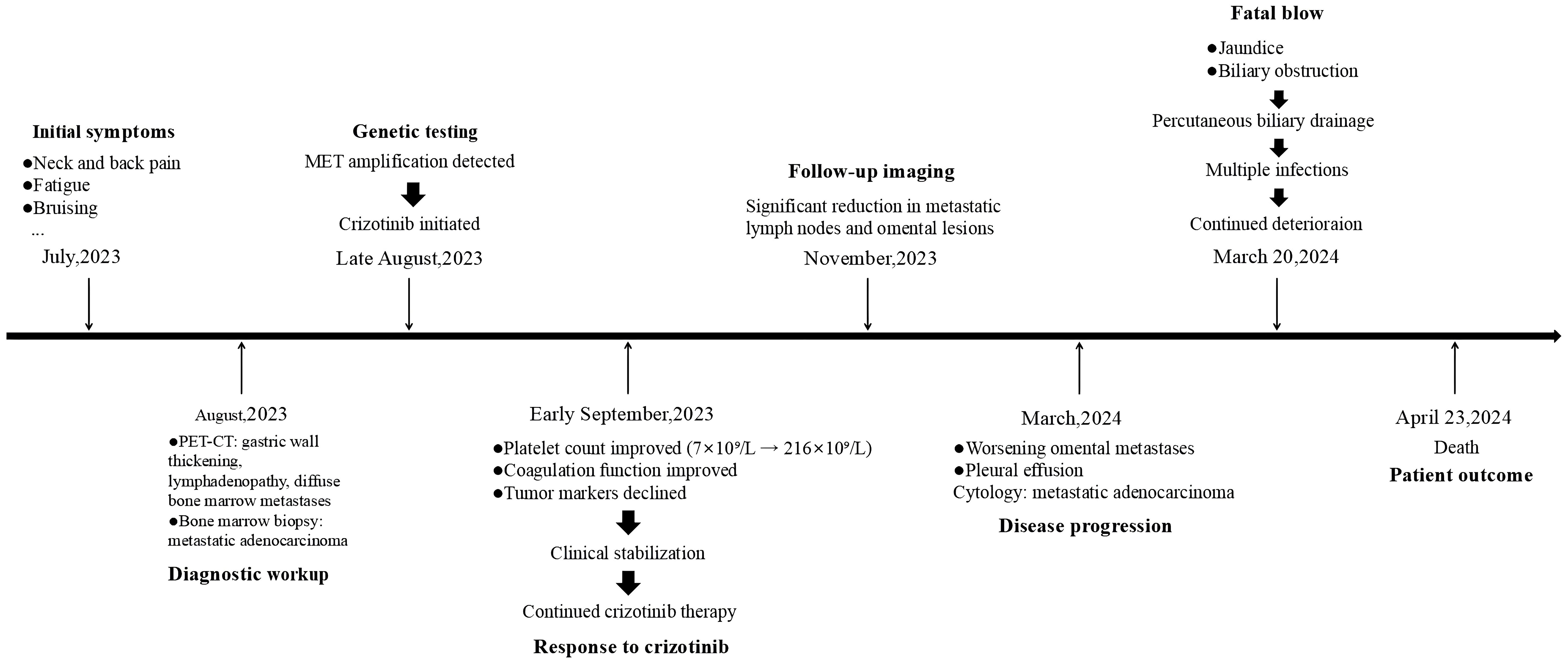
Figure 4. Timeline of the patient’s whole therapeutic process. Timeline for diagnosis and treatment form July 2023 to April 2024.
3 Discussion
Gastric adenocarcinoma accounts for approximately 95% of all gastric cancer cases and exhibits significant histological and molecular heterogeneity (9). The diffuse-type gastric cancer classified by the Lauren system, including signet ring cell carcinoma (SRCC), is characterized by poor differentiation, lack of cell adhesion, and an aggressive metastatic pattern, leading to a worse prognosis and limited treatment options (10) This patient’s disease, marked by systemic bone marrow metastasis, exemplifies the aggressive nature of this subtype, further compounded by a poor response to conventional therapies.
A significant challenge in this case was the development of severe thrombocytopenia and disseminated intravascular coagulation (DIC), conditions often associated with bone marrow metastasis. The concept of DCBM has been proposed in several case reports, referring to extensive bone marrow infiltration of solid tumors. DCBM is characterized by anemia, back pain, and bleeding tendencies, often associated with DIC, and can result in severe outcomes such as brain hemorrhage and death (11–20). Interestingly, in these reports, the most frequent primary tumors were either signet ring cell carcinoma or poorly cohesive carcinomas containing signet ring cell components (21). Infiltration of the bone marrow by highly aggressive tumor cells disrupts normal hematopoiesis and exacerbates pro-coagulant activity, leading to severe outcomes, as seen in this patient (22). This complex interaction between tumor burden and hematological complications underscores the need for early and aggressive management of the primary tumor in patients with widespread metastasis.
MET is involved in the metastatic progression of various cancers. In breast cancer, MET signaling activates the MAPK pathway and promotes the release of inflammatory cytokines such as IL-1β, IL-8, and CXCL1, thereby reshaping the brain microenvironment and enhancing brain metastasis (23). In lung cancer, METΔ14 leads to sustained MET activation, enhancing cell migration and resistance to apoptosis (24);TP53 - Induced Glycolysis and Apoptosis Regulator (TIGAR) has also been reported to promote lung cancer cell migration and metastasis via MET (25). In hepatocellular carcinoma, aberrant activation of the HGF/MET axis promotes angiogenesis and is closely associated with metastasis and drug resistance (26). Increased MET expression in metastatic cells of head and neck cancer is considered a marker of lymph node metastasis (27). In certain renal cancer stem cells, MET is overexpressed and contributes to bone metastasis (28). MET amplification is a well-established driver of tumor proliferation, invasion, and metastasis (29), particularly in poorly cohesive gastric carcinomas. A study has indicated that in gastric cancer, MET amplification drives epithelial–mesenchymal transition (EMT), extracellular matrix degradation via MMPs, and VEGF pathway activation, collectively promoting metastasis (30). Cross-tumor evidence supports MET as a key factor in the formation of metastatic niches. Its active role in the rare but fatal bone marrow metastasis observed in this case highlights the therapeutic potential of MET inhibitors, such as crizotinib, in metastatic gastric cancer. However, the heterogeneous response—where some lesions shrank while others progressed—highlights the challenges of treating a genetically diverse tumor.
A key challenge in MET-targeted therapy lies in the correlation between treatment efficacy and MET amplification levels. Current evidence suggests that patients with high-level MET amplification respond better to MET inhibitors (31–35), yet standardized thresholds remain elusive. The 2023 NCCN guidelines define high amplification as MET copy number >10 via NGS (36), while FISH categorizes amplification based on MET/Centromere of Chromosome 7 (CEP7) ratios (low: 1.8–2.2; intermediate: >2.2–<5; high: ≥5) (37). In our case, NGS revealed focal MET amplification (copy number >10), a subtype validated as a clinically significant driver. However, NGS has notable limitations (1): it cannot assess CEP7 status, and (2) its algorithms for copy number calculation (based on sequencing depth and variant allele frequency) require refinement (38). Studies indicate that even with high tumor content (≥10%) and deep sequencing (≥500×), concordance between NGS and FISH for MET amplification is only 62.5%, and NGS results poorly correlate with clinical outcomes, underscoring the need for multi-platform validation (39). Establishing robust biomarker-based screening methods could help identify patients most likely to benefit from MET-targeted therapies and optimize treatment strategies (40, 41). Furthermore, although in vitro and in vivo studies have shown that gastric cancer cell lines are highly sensitive to crizotinib (8, 41–43), large-scale clinical trials in gastric cancer patients are lacking. This limits the ability to draw definitive conclusions about its efficacy and broad applicability in treating MET-amplified gastric cancers. Additionally, baseline genetic testing was conducted at diagnosis. However, repeat testing was not performed in later treatment stages despite declining efficacy of targeted therapy. This gap hinders identification of secondary resistance mechanisms, such as clonal evolution and therapeutic selection pressure which may arise during treatment.
The heterogeneous treatment response observed in this patient may reflect clonal evolution within the tumor. Different metastatic sites may harbor distinct genetic mutations and resistance mechanisms (44–46), leading to variable drug sensitivity. Under selective pressure from targeted therapy, tumor cells with pre-existing or acquired resistance mutations may become the dominant clones, reducing treatment efficacy (47). Some studies have suggested that high-level MET amplification (≥5 gene copies per cell) correlates with greater sensitivity to MET inhibitors, whereas lower amplification levels or concurrent resistance mutations (e.g., RAS or PI3K pathway alterations) may limit benefit (35, 48). Although the initial pre-treatment genetic testing did not detect any relevant alterations, clonal evolution and therapeutic selection pressure may lead to divergent genomic profiles, including altered amplification status, emerging resistance mutations, or activation of bypass pathways. Unfortunately, follow-up testing was not conducted. Additionally, our initial gene panel did not include Programmed cell death ligand 1(PD-L1) expression status, limiting comprehensive assessment of its therapeutic benefit. Beyond intrinsic genetic factors, the tumor microenvironment (TME) plays a crucial role in modulating drug response, as certain sites could have been more conducive to tumor growth or more resistant to treatment (44). Differences in stromal support, angiogenesis, and immune cell infiltration, among other factors, may lead to varying sensitivity to MET inhibition across different metastatic sites (47). Studies in NSCLC suggest that MET-amplified tumors often exhibit immune exclusion, which could further diminish responses to targeted therapy (49).Given the heterogeneity and clonal evolution of MET-amplified tumors, monotherapy with MET inhibitors like crizotinib may be insufficient for durable disease control. Combination therapy strategies are being actively explored to overcome resistance and enhance efficacy: Combining MET inhibitors with chemotherapy (e.g., fluorouracil, leucovorin, and oxaliplatin in the mFOLFOX6 regimen) has shown synergistic effects in some studies, as chemotherapy may help target resistant tumor subclones while maintaining selective MET inhibition (50).MET amplification is often associated with an immunosuppressive tumor microenvironment, suggesting a potential role for immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 therapy) in combination with MET-targeted therapy. In NSCLC, early trials combining MET inhibitors with immune checkpoint blockade have yielded promising results, and similar strategies could be evaluated in gastric cancer (49). Other approaches, such as simultaneous inhibition of MET and alternative oncogenic pathways (e.g., PI3K, EGFR, or VEGF signaling), may further enhance treatment efficacy and delay resistance (51).
These therapeutic challenges were acutely evident in our patient’s case. Despite the potential of combination strategies, clinical urgency necessitated a prioritized intervention. This case report outlines the clinical trajectory of a single patient, and its observational design inherently precludes causal conclusions. The absence of a control cohort (e.g., MET-amplified patients managed with standard chemotherapy or best supportive care) limits direct comparisons between crizotinib and alternative therapies. Nevertheless, the patient’s rapid clinical decline—characterized by severe thrombocytopenia (7×109/L) and disseminated intravascular coagulation—posed an immediate threat, rendering conventional chemotherapy (e.g., fluorouracil-platinum combinations) exceptionally high-risk. We feared chemotherapy’s myelosuppressive effects might worsen blood toxicity, potentially triggering life-threatening bleeding or infections. In contrast, crizotinib, an oral MET inhibitor, demonstrated a favorable hematologic safety profile in prior studies, alongside preliminary evidence of antitumor activity in MET-amplified malignancies (8, 43). Following multidisciplinary consensus, crizotinib was selected as the initial therapy to swiftly reduce tumor burden, stabilize blood counts, and establish a foundation for subsequent chemotherapy. While these observations highlight the potential utility of MET inhibition in this critical context, the lack of controlled data underscores the urgent need for prospective trials or matched cohort analyses to validate the relative efficacy of MET-targeted strategies in similar high-risk populations.
This case highlights the critical role of molecular profiling in guiding targeted therapy. The identification of MET amplification facilitated the use of crizotinib, which led to rapid improvement in thrombocytopenia and coagulation abnormalities. However, the emergence of resistance and subsequent disease progression emphasize the necessity of (1): Comprehensive genomic profiling (e.g., NGS, FISH, and liquid biopsy) to enable real-time detection of resistant clones (2). Multi-region tumor sampling to account for intratumoral heterogeneity and identify potential resistance mechanisms (3). Longitudinal molecular monitoring to inform adaptive treatment strategies and facilitate timely therapeutic adjustments. Future clinical practice should integrate routine molecular profiling to enable truly personalized therapeutic interventions.
4 Conclusion
In summary, this case highlights the potential of crizotinib in MET-amplified gastric cancer while also illustrating the challenges posed by tumor heterogeneity and acquired resistance. The mixed response observed in this patient suggests that biomarker-driven selection and combination strategies may be necessary to optimize MET-targeted therapy. This case further reinforces the importance of molecular profiling in guiding treatment decisions and underscores the urgent need for expanded clinical research on MET inhibitors in gastric cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans as it does not involve experimental research. Informed consent was obtained from the patient before publication. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Conceptualization, Investigation, Writing – original draft. YX: Conceptualization, Investigation, Visualization, Writing – review & editing. JS: Writing – review & editing, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82072721), the Natural Science Foundation of Jiangsu Province (BK20201493) and the Nanjing Medical University-Qilu Clinical Research Fund Project(2024KF0270).
Acknowledgments
We are very grateful to the individual participants, research staff and students who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, and Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. (2009) 250:878–87. doi: 10.1097/SLA.0b013e3181b21c7b
3. Puccini A, Poorman K, Catalano F, Seeber A, Goldberg RM, Salem ME, et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene. (2022) 41:3455–60. doi: 10.1038/s41388-022-02350-6
4. Kwon CH, Kim YK, Lee S, Kim A, Park HJ, Choi Y, et al. Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes. Histopathology. (2018) 72:556–68. doi: 10.1111/his.13383
5. Zhang J, Guo L, Liu X, Li W, and Ying J. MET overexpression, gene amplification and relevant clinicopathological features in gastric adenocarcinoma. Oncotarget. (2017) 8:10264–73. doi: 10.18632/oncotarget.14382
6. Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. (1999) 85:1894–902. doi: 10.1002/(SICI)1097-0142(19990501)85:9<1894::AID-CNCR3>3.0.CO;2-J
7. Drilon A, Cappuzzo F, Ou SI, and Camidge DR. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol. (2017) 12:15–26. doi: 10.1016/j.jtho.2016.10.014
8. Ji J, Chen W, Lian W, Chen R, Yang J, Zhang Q, et al. (S)-crizotinib reduces gastric cancer growth through oxidative DNA damage and triggers pro-survival akt signal. Cell Death Dis. (2018) 9:660. doi: 10.1038/s41419-018-0667-x
9. Nakamura K, Sugano H, and Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. (1968) 59:251–8.
10. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. (1992) 52:6735–40.
11. Namikawa H, Takemoto Y, Makuuchi A, Kobayashi M, Kinuhata S, Morimura M, et al. Disseminated carcinomatosis of the bone marrow from pancreatic cancer: a case report. BMC Cancer. (2016) 16:801. doi: 10.1186/s12885-016-2849-1
12. Hong YM, Yoon KT, Cho M, Kang DH, Kim HW, Choi CW, et al. Bone marrow metastasis presenting as bicytopenia originating from hepatocellular carcinoma. Clin Mol Hepatol. (2016) 22:267–71. doi: 10.3350/cmh.2015.0017
13. Iguchi H. Recent aspects for disseminated carcinomatosis of the bone marrow associated with gastric cancer: What has been done for the past, and what will be needed in future? World J Gastroenterol. (2015) 21:12249–60. doi: 10.3748/wjg.v21.i43.12249
14. Fujita K, Okubo A, Nakamura T, and Takeuchi N. Disseminated carcinomatosis of the bone marrow caused by granulocyte colony-stimulating factor: A case report and review of literature. World J Gastrointest Oncol. (2022) 14:2077–84. doi: 10.4251/wjgo.v14.i10.2077
15. Koizumi K, Haseyama Y, Machino R, Sato Y, Sawada K, and Koike T. The hemophagocytic syndrome in prostate cancer revealed by disseminated carcinomatosis of the bone marrow. J Urol. (2002) 168:1101–2. doi: 10.1016/S0022-5347(05)64588-0
16. Kamiya T, Honda K, Kizaki M, Maruyama T, Masuda T, Suzuki O, et al. Clinicopathological studies on disseminated carcinomatosis of the bone marrow occurring through metastasis of gastric carcinoma. Gan No Rinsho. (1985) 31:819–26.
17. Suyama T, Yokoyama M, Nishimura T, Kobayashi K, and Katagiri K. Sweat gland carcinoma of the left axilla with disseminated carcinomatosis of the bone marrow. Int J Dermatol. (2020) 59:e281–e4. doi: 10.1111/ijd.14854
18. Higashino Y, Nakamura T, Kato M, Asano E, Akimoto R, Uchida H, et al. Effective postoperative chemotherapy of gastric cancer associated with disseminated carcinomatosis of the bone marrow. Gan No Rinsho. (1985) 31:1456–62.
19. Honda Y, Kawaoka T, Aikata H, Kan H, Fujino H, Kobayashi T, et al. Disseminated carcinomatosis of the bone marrow originating from hepatocellular carcinoma. A Case Rep Hepatol Res. (2015) 45:705–10. doi: 10.1111/hepr.12391
20. Kawakami Y, Ueki K, Chikama M, Shimamura Y, and Naito T. Intracranial hemorrhage associated with nontraumatic disseminated intravascular coagulation–report of four cases. Neurol Med Chir (Tokyo). (1990) 30:610–7. doi: 10.2176/nmc.30.610
21. Kusumoto H, Haraguchi M, Nozuka Y, Oda Y, Tsuneyoshi M, and Iguchi H. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep. (2006) 16:735–40. doi: 10.3892/or.16.4.735
22. Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res. (2018) 164 Suppl 1:S77–s81. doi: 10.1016/j.thromres.2018.01.016
23. Xing F, Liu Y, Sharma S, Wu K, Chan MD, Lo HW, et al. Activation of the c-met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer. Cancer Res. (2016) 76:4970–80. doi: 10.1158/0008-5472.CAN-15-3541
24. Cerqua M, Botti O, Arigoni M, Gioelli N, Serini G, Calogero R, et al. METΔ14 promotes a ligand-dependent, AKT-driven invasive growth. Life Sci Alliance. (2022) 5:e202201409. doi: 10.26508/lsa.202201409
25. Shen M, Zhao X, Zhao L, Shi L, An S, Huang G, et al. Met is involved in TIGAR-regulated metastasis of non-small-cell lung cancer. Mol Cancer. (2018) 17:88. doi: 10.1186/s12943-018-0839-4
26. Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, and Atabey N. Elevated hepatocyte growth factor expression as an autocrine c-Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci. (2016) 107:407–16. doi: 10.1111/cas.12891
27. Choe JY, Yun JY, Nam SJ, and Kim JE. Expression of c-Met Is Different along the Location and Associated with Lymph Node Metastasis of Head and Neck Carcinoma. Korean J Pathol. (2012) 46:515–22. doi: 10.4132/KoreanJPathol.2012.46.6.515
28. D’Amico L, Belisario D, Migliardi G, Grange C, Bussolati B, D’Amelio P, et al. C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget. (2016) 7:45525–37. doi: 10.18632/oncotarget.9997
29. Trusolino L, Bertotti A, and Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. (2010) 11:834–48. doi: 10.1038/nrm3012
30. Kato T. Biological roles of hepatocyte growth factor-Met signaling from genetically modified animals. BioMed Rep. (2017) 7:495–503. doi: 10.3892/br.2017.1001
31. Camidge DR, Otterson GA, Clark JW, Ignatius Ou SH, Weiss J, Ades S, et al. Crizotinib in patients with MET-amplified NSCLC. J Thorac Oncol. (2021) 16:1017–29. doi: 10.1016/j.jtho.2021.02.010
32. Raghav K, Bailey AM, Loree JM, Kopetz S, Holla V, Yap TA, et al. Untying the gordion knot of targeting MET in cancer. Cancer Treat Rev. (2018) 66:95–103. doi: 10.1016/j.ctrv.2018.04.008
33. Van Cutsem E, Karaszewska B, Kang YK, Chung HC, Shankaran V, Siena S, et al. A multicenter phase II study of AMG 337 in patients with MET-amplified gastric/gastroesophageal junction/esophageal adenocarcinoma and other MET-amplified solid tumors. Clin Cancer Res. (2019) 25:2414–23. doi: 10.1158/1078-0432.CCR-18-1337
34. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. (2020) 383:944–35. doi: 10.1056/NEJMoa2002787
35. Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, et al. MET 20 amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. (2011) 29:4803–10. doi: 10.1200/JCO.2011.35.4928
36. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version 2 (2023). Available online at: https://www.nccn.org (Accessed February 17, 2023).
37. Noonan SA, Berry L, Lu X, Gao D, Barón AE, Chesnut P, et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol. (2016) 11:1293–304. doi: 10.1016/j.jtho.2016.04.033
38. Sun B, Qiu T, Zeng X, Duan J, Bai H, Xu J, et al. Detection of MET polysomy by next-generation sequencing and its clinical relevance for MET inhibitors. Cancer Res Commun. (2023) 3:532–9. doi: 10.1158/2767-9764.CRC-22-0438
39. Peng LX, Jie GL, Li AN, Liu SY, Sun H, Zheng MM, et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors. Exp Hematol Oncol. (2021) 10:52. doi: 10.1186/s40164-021-00245-y
40. Moosavi F, Giovannetti E, Saso L, and Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. (2019) 56:533–66. doi: 10.1080/10408363.2019.1653821
41. Yang Y, Wu N, Shen J, Teixido C, Sun X, Lin Z, et al. MET overexpression and amplification define a distinct molecular subgroup for targeted therapies in gastric cancer. Gastric Cancer. (2016) 19:778–88. doi: 10.1007/s10120-015-0545-5
42. Sabree S, Berg D, and Sato M. Treatment of a pediatric patient with MET-amplified signet ring cell adenocarcinoma of the stomach with crizotinib. Pediatr Blood Cancer. (2018) 65:e26984. doi: 10.1002/pbc.26984
43. Okamoto W, Okamoto I, Arao T, Kuwata K, Hatashita E, Yamaguchi H, et al. Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther. (2012) 11:1557–64. doi: 10.1158/1535-7163.MCT-11-0934
44. Junttila MR and de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. (2013) 501:346–54. doi: 10.1038/nature12626
45. Lee YS and van Galen P. Mutation signatures reveal clonal evolution. Blood. (2023) 141:2292–3. doi: 10.1182/blood.2022019510
46. Liang Y, He H, Wang W, Wang H, Mo S, Fu R, et al. Malignant clonal evolution drives multiple myeloma cellular ecological diversity and microenvironment reprogramming. Mol Cancer. (2022) 21:182. doi: 10.1186/s12943-022-01648-z
47. Labrie M, Brugge JS, Mills GB, and Zervantonakis IK. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat Rev Cancer. (2022) 22:323–39. doi: 10.1038/s41568-022-00454-5
48. Janjigian YY, Tang LH, Coit DG, Kelsen DP, Francone TD, Weiser MR, et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev. (2011) 20:1021–7. doi: 10.1158/1055-9965.EPI-10-1080
49. Zhang Y, Yang Q, Zeng X, Wang M, Dong S, Yang B, et al. MET amplification attenuates lung tumor response to immunotherapy by inhibiting STING. Cancer Discov. (2021) 11:2726–37. doi: 10.1158/2159-8290.CD-20-1500
50. Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. (2017) 3:620–7. doi: 10.1001/jamaoncol.2016.5580
Keywords: targeted therapy, MET-amplified, precision medicine, crizotinib, gastric cancer
Citation: Shen Y, Xu Y and Sun J (2025) Case Report: A rare case of MET-amplified gastric cancer with systemic metastasis: remarkable efficacy of crizotinib and the role of precision medicine. Front. Oncol. 15:1555801. doi: 10.3389/fonc.2025.1555801
Received: 05 January 2025; Accepted: 16 July 2025;
Published: 08 August 2025.
Edited by:
Rohit Upadhyay, Tulane University, United StatesReviewed by:
Ina Valeria Zurlo, ASL Lecce, ItalyDongshi Chen, University of Southern California, United States
Copyright © 2025 Shen, Xu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Sun, c3VuakBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yan Shen
Yan Shen Yaxin Xu
Yaxin Xu Jing Sun
Jing Sun