- 1Department of Neurosurgery, Brain Research Institute, Niigata University, Niigata, Japan
- 2Advanced Treatment of Neurological Diseases Branch, Brain Research Institute, Niigata University, Niigata, Japan
- 3Department of Neurosurgery, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan
- 4Department of Pathology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan
- 5Department of Otolaryngology, Head and Neck Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 6Department of Pathology, Brain Research Institute, Niigata University, Niigata, Japan
Introduction: Brain stem gliomas harboring IDH mutations can be sensitive to temozolomide (TMZ) treatment, unlike their H3K27-altered counterparts, so distinguishing the two is essential.
Case presentation: Here, we report an adult brainstem glioma patient whose hearing loss normalized after treatment. He presented with gradual left hearing loss from two years before, and magnetic resonance (MR) images showed a diffuse mass lesion involving the pons to left middle cerebral peduncle, including the vestibular and cochlear nuclei. On MR spectroscopy (MRS), 2-hydroxyglutarate (2HG) was elevated to 3.602 mM, suggesting an IDH-mutant glioma. Subsequently, an open biopsy was performed via the lateral suboccipital approach, and the pathological diagnosis was astrocytoma, IDH-mutant, CNS WHO grade 3. Molecular analysis revealed a non-canonical IDH2 R172S mutation. Left hearing improved from 87.5 dB to 8.3dB by 6-frequency pure tone audiogram (PTA) and 90% speech discrimination at 35 dB after concomitant TMZ and radiation treatment, followed by 12 cycles of adjuvant TMZ treatment. 2HG also decreased to 0.186 mM on MRS after treatment determining treatment strategy.
Discussion: Studies have shown that as high as 31% of adult brainstem gliomas are IDH mutant, with most of these mutations being non-canonical IDH1/2 mutations. Approximately 70% of IDH-mutant astrocytomas are known to harbor a methylated O6-methylguanine-DNA-methyltransferase (MGMT) promoter and respond to TMZ treatment, whereas almost all H3K27M-mutant diffuse midline gliomas have unmethylated MGMT promoters and generally are not sensitive to TMZ treatment. Detection of 2HG by MRS and molecular analysis, including non-canonical IDH1/2 mutations, were helpful in determining treatment response in this adult brainstem glioma case. Notably, hearing loss normalized after TMZ treatment.
Conclusion: The diagnosis of IDH-mutant brainstem gliomas by MRS and integrated analysis of surgically obtained specimens is essential to determine the proper treatment of these rare cases.
Introduction
Adult brainstem gliomas are rare, accounting for less than 2% of all adult gliomas (1). Large-scale studies reporting the genetic profile of adult brainstem gliomas are scarce (2). Their pediatric counterparts, which are known to harbor recurrent alterations of H3F3A K27M (3–5), are now classified along with diffuse gliomas arising from the thalamus (6) and spine (7) as diffuse midline gliomas, H3K27-altered (8). These gliomas almost universally have unmethylated O6-methylguanine-DNA-methyltransferase (MGMT) promoter (9, 10) and generally do not respond to the oral alkylating agent temozolomide (TMZ) (11, 12), with a dismal prognosis (13–17). A couple of studies have shown that mutations in IDH1 and IDH2 genes can be found in 18-31% of adult brainstem gliomas (18–20) and that a majority of these mutations are non-canonical IDH1/2-mutations (21, 22), compared to a high frequency of IDH1 R132H mutations in IDH-mutant supratentorial diffuse gliomas (23, 24). Determining the molecular profile of adult brainstem gliomas is very important clinically because IDH-mutant diffuse gliomas frequently harbor a methylated MGMT promoter and can be expected to respond to TMZ treatment (11, 25).
Here, we present a case of adult brainstem glioma with non-canonical IDH2 mutation and treatment response to radiation and TMZ. Pre-operatively, 2-hydroxyglutarate (2HG) was detected by magnetic resonance spectroscopy (MRS). This rare case report provides rationale that biopsy and genetic testing should be performed in adult brainstem gliomas when feasible to obtain valuable information about treatment response.
Case presentation
A 33-year-old Japanese male with no significant past medical history was referred to Niigata University Medical and Dental Hospital with progressive left hearing loss and tinnitus, which had been worsening for two years. A 6-frequency pure tone audiogram (PTA) was 87.5 dB in the left ear compared to 7.5 dB in the right, and only wave I could be identified on the left auditory brainstem response (ABR) (Supplementary Figure 1A).
Magnetic resonance (MR) images showed a hyperintense lesion extending from the pons to the left middle cerebellar peduncle on T2-weighted and fluid attenuation inverted recovery (FLAIR) images, including the vestibular and cochlear nuclei at the rhomboid fossa (Figures 1A). Post-contrast images showed no enhancement (Figure 1). Magnetic resonance spectroscopy (MRS) showed an increased choline (Cho)-to-creatine (Cr) ratio and decreased N-acetyl aspartate (NAA), suggestive of a malignant brain tumor (Figure 1). In addition, on single voxel MRS with a TE at 97 msec to optimize the detection of 2HG (26), 2HG was detected (3.602 mM at TE =97 msec; S/N =7, Cramer-Rao lower bounds [CRLB] = 32%), suggesting the presence of IDH mutation (Figure 1). 2HG was also detected at a TE of 35 msec (5.692 mM; S/N = 6, CRLB = 42%), although the CRLB was high, suggesting the possible contamination of macromolecules.
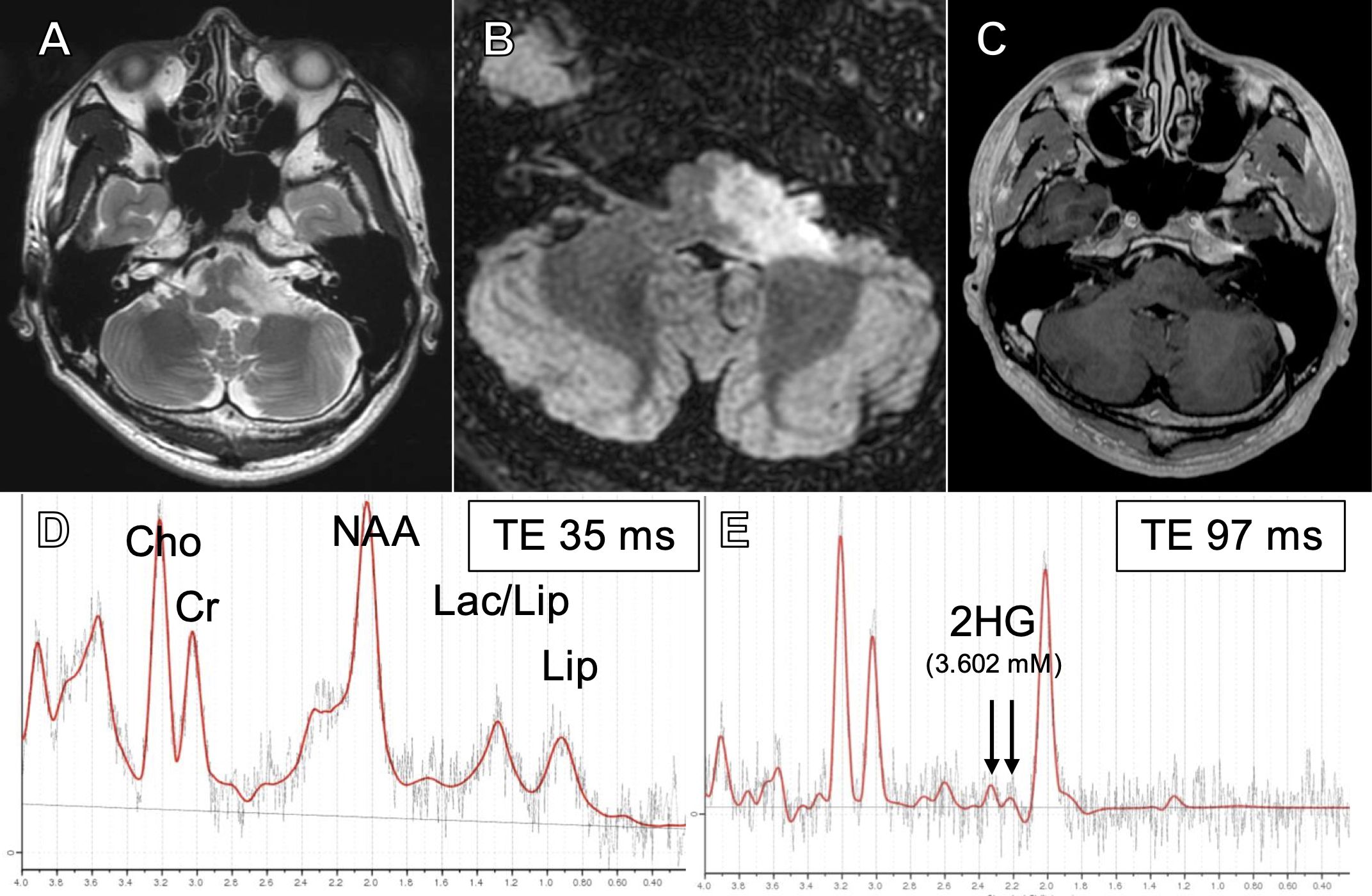
Figure 1. T2-weighted (A), fluid-attenuated inversion recovery (FLAIR) (B), and post-contrast (C) magnetic resonance (MR) images at presentation. Short TE (35 msec) single voxel MR spectroscopy (MRS) showing elevated Cho/Cr and decreased NAA (D) and intermediate TE (97 msec) SVMRS showing elevated 2-hydroxyglutarate (E).
A typical DIPG displays more than 50% T2/FLAIR hyperintensity of the ventral pons on MR images (27). In the present case, the tumor progressed to the left middle cerebellar peduncle. Furthermore, considering the results of the MRS and the relatively slow progression of symptoms, the clinicoradiographical presentation was atypical for H3K27-altered DMG of the brainstem. Therefore, we decided that a biopsy was necessary.
We performed a biopsy via the left lateral suboccipital approach, and small pieces of edematous tumor between the root exit zones of the V and VII/VIII complex were sampled. Hematoxylin and eosin (HE) sections showed diffuse astrocytic tumor with nuclear atypia (Figure 2). Immunohistochemically, the tumor cells were negative for H3K27M and positive for H3K27me3 (Figures 2B). Also, IDH1 R132H was negative, but ATRX staining was diminished and P53 was strongly positive, suggestive of an IDH-mutant astrocytoma (Figures 2D). The MIB-1 labeling index was 8% (Figure 2). MGMT was positive in only 5% of tumor cells (Figure 2), below the cutoff of 30% (28), suggesting that the MGMT promoter is methylated. Sanger sequencing (IRB approval #G2022-0012) revealed IDH1 R132 wildtype and IDH2 R172S mutation (Figures 2I). A bimodal DNA and RNA next-generation sequencing panel for integrative diagnosis of glioma (7, 29) was performed (IRB approval #C2023-0039), and IDH2 R172S mutation (variant allele frequency [VAF] 48.1%), as well as TP53 C275F (VAF 91.2%) and ATRX c.3809 + 1G>C (VAF 92.1%), were detected. There was no loss of CDKN2A/B. The integrated diagnosis was astrocytoma, IDH-mutant, CNS WHO grade 3.
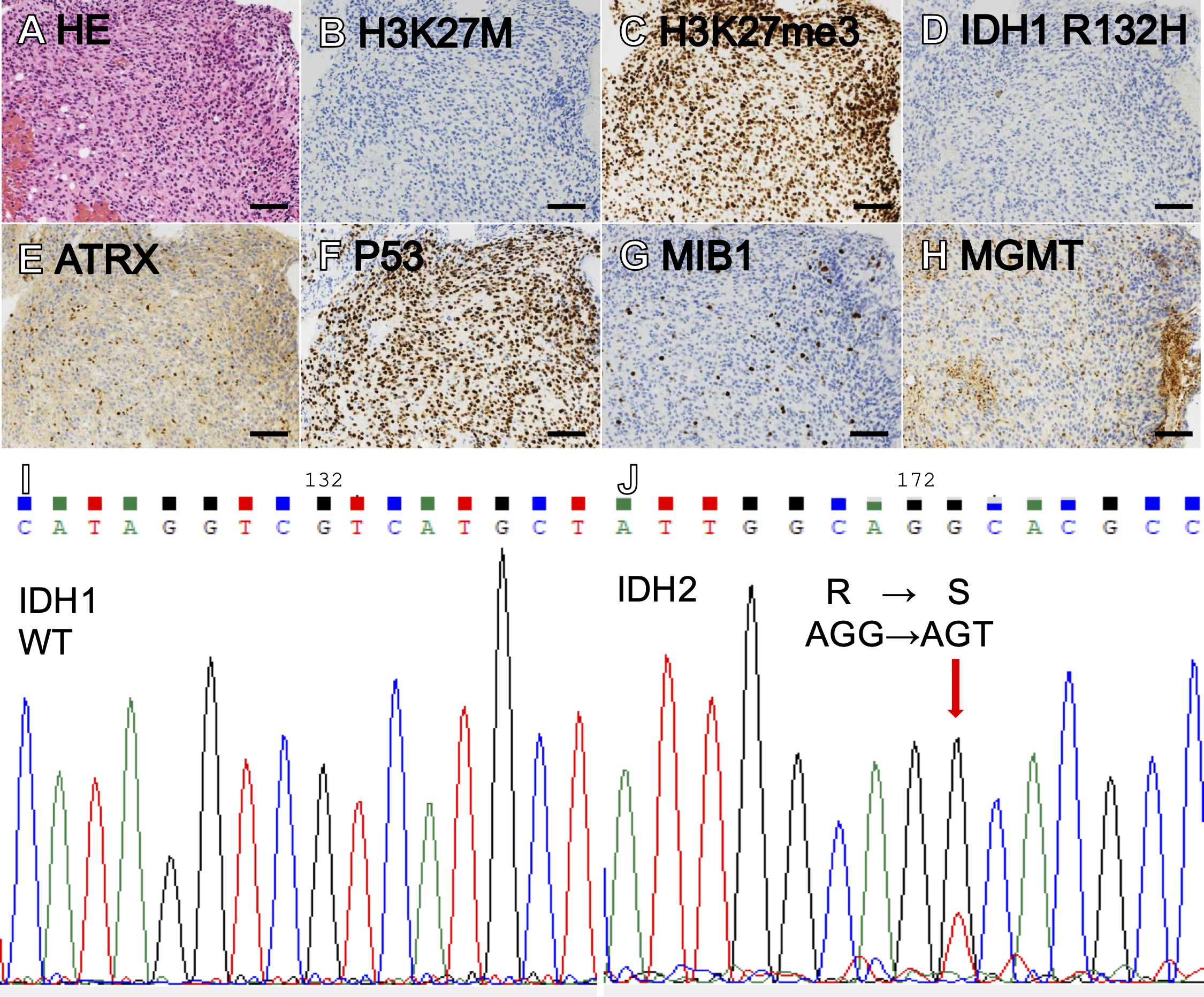
Figure 2. Hematoxylin-eosin (HE) staining showed a diffuse, astrocytic tumor with nuclear atypia and moderately increased cellularity (A). The tumor showed negative H3K27M (B), intact H3K27me3 (C), negative IDH1 R132H (D), loss of ATRX (E), and marked staining for P53 (F). MIB labeling index was 8% (G) and MGMT was positive in 5% of tumor cells (H). Sanger sequencing showing IDH1 wildtype (I) and IDH2 R172S (J). (Scale bars = 100 µm).
After the surgery, the patient experienced minimal hypesthesia of the left perioral area. The patient underwent concomitant TMZ and 54 Gy (30 fractions) of intensity-modulated radiation therapy (IMRT), followed by 12 courses of maintenance TMZ. Three months after the completion of treatment, the area of T2/FLAIR hyperintensity on MR images decreased by 51% by volumetric analysis (30) (Figure 3), and 2HG on MRS had drastically decreased to 0.186 mM, suggesting a treatment effect (Figure 3). After finishing TMZ treatment, the patient noticed a partial improvement in his left hearing. His left hearing loss had normalized from 87.5 dB on 6-frequency PTA (Figures 3) to 8.3 dB (Figure 3) and 90% speech discrimination at 35 dB (Figure 3). However, ABR findings remained unchanged, with only wave I identified on the left ABR (Supplementary Figure 1). The patient’s partial improvement of left hearing has persisted, and he is recurrence-free eight months after completion of treatment and 22 months from diagnosis.
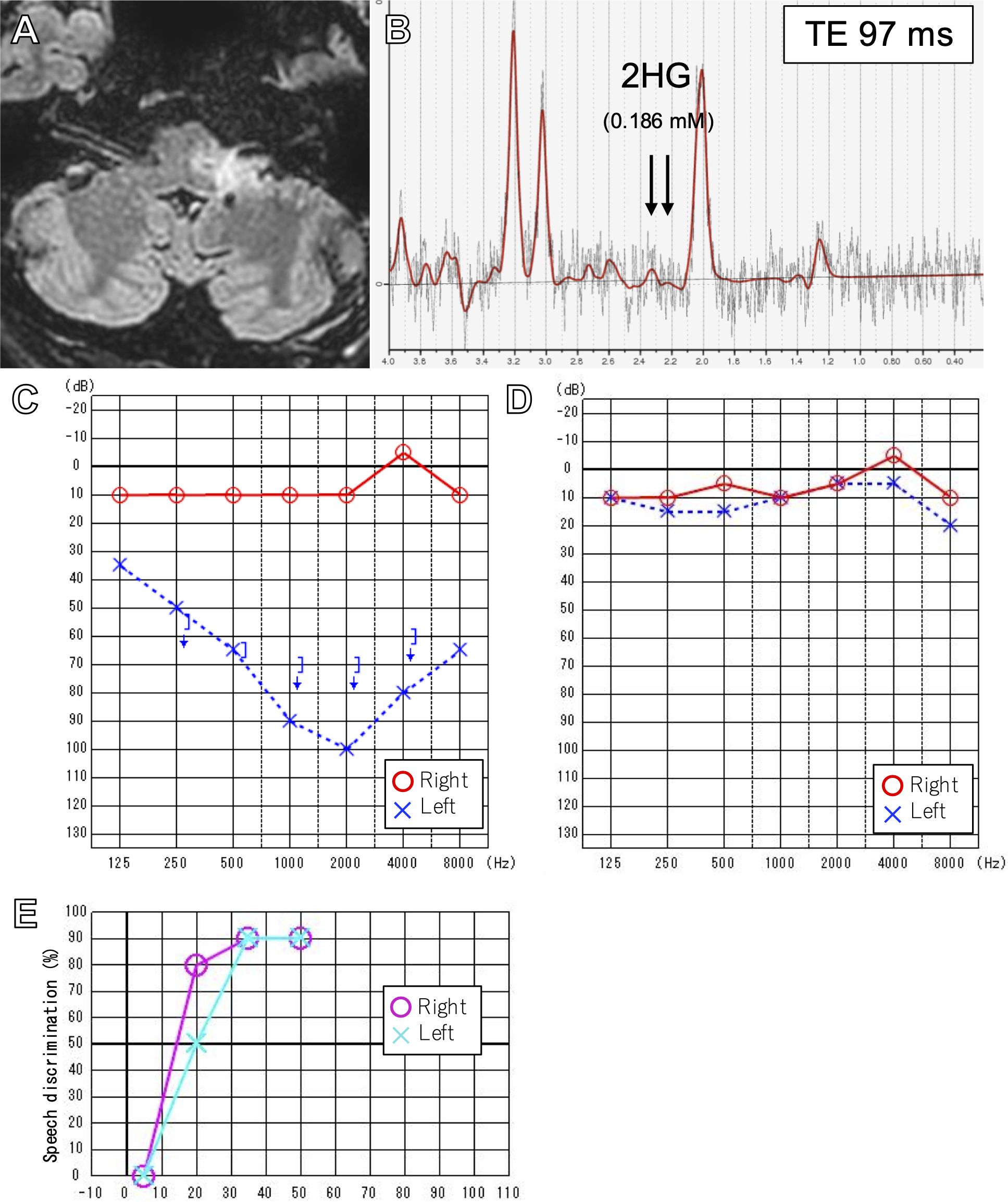
Figure 3. FLAIR image after 12 cycles of adjuvant temozolomide (A). Intermediate TE (97msec) SVMRS after treatment showing decreased 2HG (B). Pure tone audiometry before (C) and after treatment (D) showing normalization of left hearing. Speech discrimination after treatment was also normal (E).
Discussion
In the present case, a dramatic response to radiation and temozolomide treatment and subsequent improved hearing were observed in a rare IDH2 R172S-mutant brainstem glioma patient who presented with left hearing loss. Detection of 2HG by MRS served as a less-invasive adjunct to screen for potential IDH mutation, which was confirmed by integrated diagnosis of surgically obtained tissue.
A multicenter review by Shumacher et al. deemed that biopsies are rarely needed to diagnose pediatric brainstem lesions, and MR imaging would suffice in most cases (31). With the introduction of molecular analysis to brain tumor diagnosis, stereotactic and robot-assisted techniques have been implicated in the biopsy of brainstem lesions, however, with the potential for significant morbidity (32).
Up to 90% of pediatric DIPGs are known to harbor H3F3A K27M or HIST1H3B K27M mutations (3–5, 33). Almost all of these H3K27-altered DMGs have unmethylated MGMT promoters (9, 10) and are resistant to TMZ treatment (11, 12, 34). However, a significant percentage of IDH-mutant astrocytomas have been implicated in adult brainstem gliomas, with rates ranging from 18-31% in relatively large series (18–20). It is important to consider that most IDH-mutant astrocytomas arise after the second decade of life (35), likely contributing to the scarcity of IDH-mutant pediatric brainstem gliomas. Almost 70% of IDH-mutant astrocytomas are known to harbor methylated MGMT promoters (23), making them more likely to respond to TMZ treatment (11). In the present case, the diagnosis of IDH mutant astrocytoma was vital in determining treatment with TMZ.
Remarkably, hearing loss dramatically improved after radiation and TMZ treatment. This is especially surprising as hearing loss had been noted for over two years before the presentation. To date, dramatic improvement of cranial nerve signs after treatment has not been extensively reported in gliomas. Visual acuity markedly improved after bevacizumab treatment in 4 cases of pediatric optic pathway gliomas previously treated with chemotherapy or proton-beam radiation (36). Objective hearing improvement was observed in 8 out of 13 (61%) patients with hearing loss in neurofibromatosis type 2-related vestibular schwannomas after receiving bevacizumab (37). We speculate that in the present case, a reduction in compression of the auditory tract by the tumor due to treatment effects led to improved hearing. As seen in both pre- and post-treatment ABR, wave I, which originates from the cochlea, is preserved, suggesting that the cochlear periphery was not affected by the tumor. Different from insults to cochlear peripheries, central auditory pathways may have the potential to recover from injuries via mechanisms such as neural redundancy. Therefore, normal cochlea and reduction of auditory tract compression due to tumor shrinkage could bring about the improvement of hearing on PTA. However, the patient continues to subjectively claim that his left hearing is worse than the right, suggesting that the patient did not attain usable conversational hearing even after the TMZ treatment. Although the post-treatment speech discrimination test seems to be good even in the affected ear, it may be due to the usage of relatively simple syllables in the speech discrimination test in Japan.
In the present case, MRS was performed at presentation, and a high Cho/Cr ratio, decreased NAA, and accumulation of 2HG were noted, suggesting a malignant tumor with possible IDH mutation. We (38, 39) and others (26, 40–42) have succeeded in the detection of 2HG by MRS and also have reported the usefulness of 2HG detection by MRS in non-canonical IDH-mutant gliomas (41, 43). A report by Iwahashi et al. nicely illustrates the diagnosis of non-canonical IDH mutations in brainstem gliomas by MRS (44). 2HG detection by MRS is an important adjunct in diagnosing IDH-mutant brainstem astrocytomas because 72% of infratentorial IDH-mutant gliomas (21) and 59% of IDH-mutant brainstem gliomas (22) have been reported to harbor non-canonical IDH mutations. The IDH2 R172S mutation found in the present case is extremely rare in gliomas. This mutation was not reported in a large series of 170 IDH-mutant gliomas from the US (24) and 286 from Japan (23). Banan et al. report 2 out of 42 (5%) infratentorial and 0 out of 50 (0%) supratentorial IDH-mutant gliomas to be IDH2 R172S-mutant (21).
In addition to the less-invasive detection of 2HG by MRS, surgical biopsy of the lesion and integrated diagnosis of the specimen is strongly recommended when feasible. We have previously reported a specificity of 72.2-81.3% of 2HG accumulation by MRS to detect IDH mutation in gliomas (38, 43). Therefore, false-positive cases can exist. Also, the CRLB of 2HG detection in the present case was 32%, higher than the optimal <20% (26), further necessitating histological confirmation. In the present case, an open biopsy was safely performed, enabling pathological and molecular confirmation.
Conclusion
Marked improvement of hearing was observed after TMZ and radiation treatment in a rare IDH2 R172S-mutant, adult brainstem glioma case. Detection of 2HG by MRS is important for the less-invasive screening of IDH mutation, but a surgical biopsy is strongly recommended when feasible to determine the proper treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Niigata University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TO: Conceptualization, Investigation, Methodology, Writing – original draft. MN: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. HF: Investigation, Writing – review & editing. NH: Data curation, Investigation, Methodology, Writing – review & editing. YW: Investigation, Writing – review & editing. KT: Investigation, Writing – review & editing. KN: Investigation, Writing – review & editing. TA: Data curation, Investigation, Methodology, Writing – review & editing. YT: Investigation, Writing – review & editing, Funding acquisition. SO: Investigation, Methodology, Writing – review & editing. AH: Investigation, Methodology, Supervision, Writing – review & editing. AT: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. RH: Investigation, Methodology, Supervision, Writing – review & editing. HS: Investigation, Writing – original draft, Writing – review & editing. AK: Resources, Supervision, Writing – review & editing. MO: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported in part by the Japan Society for Promotion of Science KAKENHI grants 24K12238, 21KK0156 (MN), and 22K16679 (YT). MN has received research support from the New Sustainable Growth (NSG) group.
Acknowledgments
The authors would like to thank Dr. Shuji Izumi for helpful insight on the mechanism of hearing improvement, Shingo Nigorikawa, Akiko Yoshii, and Tamami Murakami for their technical assistance, and Hiroaki Saito for MRS analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1555986/full#supplementary-material
Supplementary Figure 1 | Auditory brainstem response before (A) and after (B) treatment.
References
1. Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre JY, and Laigle-Donadey F. Adult brainstem gliomas. Oncologist. (2012) 17:388–97. doi: 10.1634/theoncologist.2011-0335
2. Eisele SC and Reardon DA. Adult brainstem gliomas. Cancer. (2016) 122:2799–809. doi: 10.1002/cncr.29920
3. Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. (2014) 46:444–50. doi: 10.1038/ng.2938
4. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. (2012) 124:439–47. doi: 10.1007/s00401-012-0998-0
5. Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. (2017) 32:520–37. doi: 10.1016/j.ccell.2017.08.017
6. Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. (2014) 16:140–6. doi: 10.1093/neuonc/not144
7. Tanaka Y, Natsumeda M, Ohashi M, Saito R, Higa N, Akahane T, et al. Primary spinal cord gliomas: Pathologic features associated with prognosis. J Neuropathol Exp Neurol. (2024) 83:1010–19. doi: 10.1093/jnen/nlae084
8. Louis DN. World Health Organization classification of tumours of the central nervous system. 5th ed edn. Lyon: International Agency for Research on Cancer (2021).
9. Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. (2015) 129:669–78. doi: 10.1007/s00401-015-1405-4
10. Banan R, Christians A, Bartels S, Lehmann U, and Hartmann C. Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun. (2017) 5:98. doi: 10.1186/s40478-017-0500-2
11. Abe H, Natsumeda M, Kanemaru Y, Watanabe J, Tsukamoto Y, Okada M, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas and MGMT silencing to temozolomide sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo). (2018) 58:290–5. doi: 10.2176/nmc.ra.2018-0044
12. Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. (2011) 13:410–6. doi: 10.1093/neuonc/noq205
13. Vuong HG, Ngo TNM, Le HT, and Dunn IF. The prognostic significance of HIST1H3B/C and H3F3A K27M mutations in diffuse midline gliomas is influenced by patient age. J Neurooncol. (2022) 158:405–12. doi: 10.1007/s11060-022-04027-2
14. Ostrom QT, Shoaf ML, Cioffi G, Waite K, Kruchko C, Wen PY, et al. National-level overall survival patterns for molecularly-defined diffuse glioma types in the United States. Neuro Oncol. (2023) 25:799–807. doi: 10.1093/neuonc/noac198
15. Kleinschmidt-DeMasters BK and Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. (2018) 37:53–63. doi: 10.5414/NP301085
16. Schulte JD, Buerki RA, Lapointe S, Molinaro AM, Zhang Y, Villanueva-Meyer JE, et al. Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neurooncol Adv. (2020) 2:vdaa142. doi: 10.1093/noajnl/vdaa142
17. Zheng L, Gong J, Yu T, Zou Y, Zhang M, Nie L, et al. Diffuse midline gliomas with histone H3 K27M mutation in adults and children- A retrospective series of 164 cases. Am J Surg Pathol. (2022) 46:863–71. doi: 10.1097/PAS.0000000000001897
18. Reyes-Botero G, Giry M, Mokhtari K, Labussière M, Idbaih A, Delattre JY, et al. Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J Neurooncol. (2014) 116:405–11. doi: 10.1007/s11060-013-1312-2
19. Zhang L, Chen LH, Wan H, Yang R, Wang Z, Feng J, et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat Genet. (2014) 46:726–30. doi: 10.1038/ng.2995
20. Wang Y, Pan C, Xie M, Zuo P, Li X, Gu G, et al. Adult diffuse intrinsic pontine glioma: clinical, radiological, pathological, molecular features, and treatments of 96 patients. J Neurosurg. (2022) 137:1628–38. doi: 10.3171/2022.2.JNS211920
21. Banan R, Stichel D, Bleck A, Hong B, Lehmann U, Suwala A, et al. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol. (2020) 140:569–81. doi: 10.1007/s00401-020-02194-y
22. Pan C, Zhang M, Xiao X, Li T, Liu Z, Wang Y, et al. Brainstem gliomas with isocitrate dehydrogenase mutation: natural history, clinical-radiological features, management strategy, and long-term outcome. Neurosurgery. (2024) 95:1407–17. doi: 10.1227/neu.0000000000003020
23. Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. (2016) 4:79. doi: 10.1186/s40478-016-0351-2
24. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH 1 and IDH 2 mutations in gliomas. N Engl J Med. (2009) 360:765–73. doi: 10.1056/NEJMoa0808710
25. SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. (2012) 103:269–73. doi: 10.1111/j.1349-7006.2011.02134.x
26. Choi C, Ganji S, Hulsey K, Madan A, Kovacs Z, Dimitrov I, et al. A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR BioMed. (2013) 26:1242–50. doi: 10.1002/nbm.2943
27. Vitanza NA and Monje M. Diffuse intrinsic pontine glioma: from diagnosis to next-generation clinical trials. Curr Treat Options Neurol. (2019) 21:37. doi: 10.1007/s11940-019-0577-y
28. Ogura R, Tsukamoto Y, Natsumeda M, Isogawa M, Aoki H, Kobayashi T, et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology. (2015) 35:324–35. doi: 10.1111/neup.12196
29. Higa N, Akahane T, Kirishima M, Yonezawa H, Makino R, Uchida H, et al. All-in-one bimodal DNA and RNA next-generation sequencing panel for integrative diagnosis of glioma. Pathol Res Pract. (2024) 263:155598. doi: 10.1016/j.prp.2024.155598
30. Takahashi H, Natsumeda M, On J, Watanabe J, Tada M, Shimizu H, et al. Administration of glucocorticoids prior to liquid biopsy dramatically reduces the detection rate of MYD88 L265P mutation in cerebrospinal fluid of primary CNS lymphoma patients. Leuk Lymphoma. (2023) 64:1219–22. doi: 10.1080/10428194.2023.2199895
31. Schumacher M, Shulte-Monting J, Stoeter P, Warmuth-Metz M, and Solymosi L. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood- a multicenter review. J Neurosurg. (2007) 106:111–9. doi: 10.3171/ped.2007.106.2.111
32. Chaturvedi A, Sadashiva N, Kalahasti S, Konar S, Krishna U, Ar P, et al. Safety and efficacy of biopsy in patients with diffuse intrinsic pontine gliomas. World Neurosurg. (2024) 187:e870–e82. doi: 10.1016/j.wneu.2024.05.003
33. Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. (2015) 130:815–27. doi: 10.1007/s00401-015-1478-0
34. Abe H, Natsumeda M, Okada M, Watanabe J, Tsukamoto Y, Kanemaru Y, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas. Front Oncol. (2019) 9:1568. doi: 10.3389/fonc.2019.01568
35. Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. (2012) 22:425–37. doi: 10.1016/j.ccr.2012.08.024
36. Avery RA, Hwang EI, Jakacki RI, and Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. (2014) 132:111–4. doi: 10.1001/jamaophthalmol.2013.5819
37. Renzi S, Michaeli O, Salvador H, Alderete D, Ponce NF, Zapotocky M, et al. Bevacizumab for NF2-associated vestibular schwannomas of childhood and adolescence. Pediatr Blood Cancer. (2020) 67:e28228. doi: 10.1002/pbc.28228
38. Natsumeda M, Igarashi H, Nomura T, Ogura R, Tsukamoto Y, Kobayashi T, et al. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: a study by 3.0-tesla magnetic resonance spectroscopy. Acta Neuropathol Commun. (2014) 2:158. doi: 10.1186/s40478-014-0158-y
39. Natsumeda M, Motohashi K, Igarashi H, Nozawa T, Abe H, Tsukamoto Y, et al. Reliable diagnosis of IDH-mutant glioblastoma by 2-hydroxyglutarate detection: a study by 3-T magnetic resonance spectroscopy. Neurosurg Rev. (2018) 41:641–7. doi: 10.1007/s10143-017-0908-y
40. Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. (2012) 4:116ra4. doi: 10.1126/scitranslmed.3002693
41. Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. (2012) 18:624–9. doi: 10.1038/nm.2682
42. Nagashima H, Tanaka K, Sasayama T, Irino Y, Sato N, Takeuchi Y, et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol. (2016) 18:1559–68.
43. Natsumeda M, Igarashi H, Gabdulkhaev R, Takahashi H, Motohashi K, Ogura R, et al. Detection of 2-hydroxyglutarate by 3.0-tesla magnetic resonance spectroscopy in gliomas with rare IDH mutations: making sense of “False-positive” Cases. Diagnostics (Basel). (2021) 11:2129. doi: 10.3390/diagnostics11112129
Keywords: adult diffuse intrinsic pontine glioma, non-canonical IDH mutation, 2-hydroxyglutarate, magnetic resonance spectroscopy, temozolomide sensitivity
Citation: Okada T, Natsumeda M, Fujiwara H, Higa N, Akahane T, Watabe Y, Tomikawa K, Nishita K, Tsukamoto Y, Ohshima S, Horii A, Tanimoto A, Hanaya R, Shimizu H, Kakita A and Oishi M (2025) Case Report: Improved hearing in a rare, adult IDH2-mutant brainstem astrocytoma successfully treated with radiation and temozolomide. Front. Oncol. 15:1555986. doi: 10.3389/fonc.2025.1555986
Received: 06 January 2025; Accepted: 15 June 2025;
Published: 08 July 2025.
Edited by:
David D. Eisenstat, Royal Children’s Hospital, AustraliaCopyright © 2025 Okada, Natsumeda, Fujiwara, Higa, Akahane, Watabe, Tomikawa, Nishita, Tsukamoto, Ohshima, Horii, Tanimoto, Hanaya, Shimizu, Kakita and Oishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manabu Natsumeda, bmF0c3VtZWRhQGJyaS5uaWlnYXRhLXUuYWMuanA=
 Takuya Okada
Takuya Okada Manabu Natsumeda
Manabu Natsumeda Hidemoto Fujiwara1
Hidemoto Fujiwara1 Arata Horii
Arata Horii Akihide Tanimoto
Akihide Tanimoto Ryosuke Hanaya
Ryosuke Hanaya Makoto Oishi
Makoto Oishi