- 1Center for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Key Laboratory of Chinese Internal Medicine of Ministry of Education, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3Institute for Excellence in Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 4School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Background: Breast cancer is one of the most prevalent tumors worldwide, significantly compromising the survival and quality of life of patients. This study aims to evaluate the global burden and the application of patient-reported outcomes (PROs) in clinical trials of breast cancer.
Methods: Data of breast cancer burden is extracted from the Global Burden of Disease Study (GBD) 2021 database. This study analyzes geographic patterns, temporal trends and age patterns of female breast cancer disease burden globally and explored the association between age standardised rates for disability adjusted life years (ASDR) of female breast cancer and sociodemographic index (SDI). The interventional clinical trials of breast cancer are selected in the WHO International Clinical Trial Register database from January 1, 2010, to December 31, 2022. The application of PROs is classified into three categories: 1) precisely listed PRO instruments as outcomes, 2) mentioned patient subjective feelings without clarifying specified PRO instruments, and 3) not mentioned any PROs as outcomes.
Results: Globally, in 2021 the age standardised rates for point prevalence of female breast cancer per 100000 population was 450.64 (427.02 to 475.96), the age standardised rates for incidence (ASIR) per 100000 population was 46.40 (43.26 to 49.56), and the ASDR per 100000 population was 455.56 (426.64 to 485.30). Compared with 1990, the ASIR of female breast cancer in 2021 had increased while the ASDR had decreased globally. Trials involving PROs only account for 37.87% (3968/10478). The Visual Analog Scale and Cancer Quality of Life Questionnaire-Core 30 are the most common instruments in these trials.
Conclusions: The disease burden of breast cancer is severe and varied worldwide while the application of PROs in clinical trials remains noteworthy. Increasing population awareness about policy for breast cancer care and the application of specific PRO instruments is warranted to reduce the future burden of disease.
1 Introduction
According to the International Agency for Research on Cancer (IARC), breast cancer is a significant global health challenge, ranking as the first leading cause of cancer-related deaths worldwide for the year 2020 (1, 2). Globally, the incidence of breast cancer is still increasing, with 4.4 million cases anticipated by 2070 (3). Female breast cancer led in most countries in terms of cancer incidence and death rates in 2020, accounting for approximately 24.5% of all cancer cases and 15.5% of cancer deaths in women (2). The burden of breast cancer has a significant impact on survival rates and quality of life, not limited to its high incidence.
Female breast cancer affects every country, but significant geographical variations exist worldwide (1). Despite the continuous improvement of medical services, female breast cancer is still diagnosed in the advanced stages in the countries without adequate medical resources and appropriate treatment. Studying the regional differences in spatial-temporal patterns (incidence, mortality, prevalence) of female breast cancer can be helpful to determine the underlying causes of these differences and offer guidance for public health policy interventions locally and regionally. Most previous studies concerning female breast cancer incidence and mortality in various regions were insufficient and the reports based on the Global Burden of Disease (GBD) 2021 lacked. All of the required information is connected with the local cancer epidemiology, which can be provided accurately by the GBD 2021. Given the substantial burden of female breast cancer, the incorporation of patient-reported outcomes (PROs) in clinical trials can offer distinct advantages, including improved patient-clinician communication, enhanced health-related quality of life, and financial savings from reduced healthcare utilization.
With the increasing burden of female breast cancer, the importance of utilizing PROs as one of the primary or secondary end points became more prominent (4). PROs play a significant role in informing patient-centered care, enhancing clinical decisions, and improving health policy (5–7), reported by patients themselves and not explained by clinicians or other individuals (8). Data collected using PROs is more comprehensive than conventional medical records and has better sensitivity. The present study extracted data on female breast cancer burden from the GBD 2021, while the registration information of clinical trials was retrieved from the WHO international clinical trial register database. This study aims to facilitate interpretation of female breast cancer burden and analyze the application of PROs in clinical trials for breast cancer, offering insights for future directions.
2 Methods
2.1 Data sources and collection
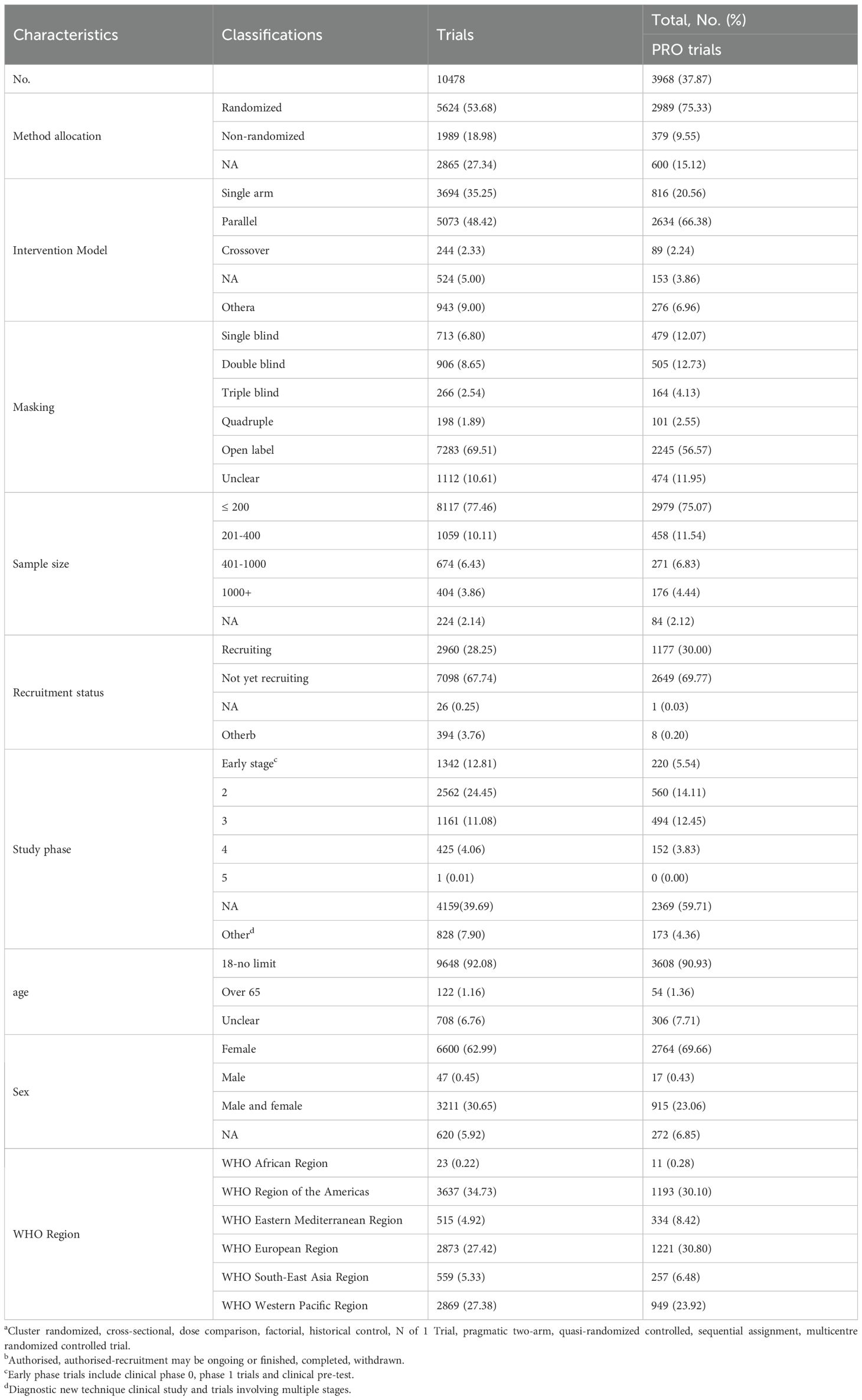
The disease burden data for female breast cancer is acquired from the GBD 2021 database. The global burden of disease is a resource that could comprehensively and comparably measure the epidemiological level and trends all over the world. Data on incidence, prevalence, death, disability adjusted life years (DALYs), and percentage change between 1990 and 2021 are retrieved in different age, region, and country groups. Geographical factors are used to identify the 21 GBD regions. The data covered 204 countries/territories globally. The study includes interventional clinical trials of breast cancer which recruited participants aged 18 and older. The clinical trials of breast cancer are selected in the WHO International Clinical Trial Register database from January 1, 2010, to December 31, 2022. The information used to evaluate the conditions and characteristics of the clinical experiments was as follows: 1) basic information, containing the number of registrations, registration date, formal title and country, 2) key information, including PRO instrument, target size, age and gender of participants, and 3) characteristic information, such as primary sponsor and study phase.
2.2 Data classification
To estimate the distribution of the disease across different age groups, the individuals are divided into different age groups. Sociodemographic index (SDI) is calculated based on the per capita national income, the total fertility rate of people under 25 and average educational attainment (age ≥ 15 years) (9). According to SDI values, countries and territories are divided into five ranges, named: low SDI, low-middle SDI, middle SDI, high- middle SDI and high SDI region. Countries with higher levels of SDI have higher levels of development (10). The US Food and Drug Administration defined PRO instruments in 2009 as any report of a patient’s health status obtained directly from the patient, without the involvement of a clinician or anyone else to interpret the patient’s response (11). In our study, trials were classified into three categories according to 1) explicitly specified PROs (trial registration information mentioned the use of specified PRO instruments), 2) implicitly specified PROs (trial registration information mentioned the application of PRO instruments, but PRO instruments were not specified), and 3) did not mention any PROs as outcomes.
2.3 Statistical analysis
The current study used Joinpoint regression models, smoothing splines models, and annual percent change (APC) to estimate female breast cancer burden. Data concerned with the characteristics of the included studies was extracted independently by two authors using pre-designed data extraction tables. The intervention model, masking method, study phase, participant age and sex, and sample size were summarized. Trials involving PROs were defined as those that used PRO instruments as primary or secondary outcomes (8). This study summarized the most frequently used PRO instruments of included trials, which are used in primary, secondary or co-primary outcomes. The study limited quantitative analysis to items that mentioned the names of PRO instruments to have a more comprehensive understanding of the most often utilized evaluation instruments.
Joinpoint regression models are applied to demonstrate the time trends in the age standardised incidence rate (ASIR) and age standardised mortality rate (ASMR) for female breast cancer in five SDI regions for the period 1990-2021. A maximum number of seven-line segments were used in these models. APC represents the annual percent change, which is calculated in this study. APC is greater than zero, which proves an increasing trend during this period. APC is less than zero, which proves a decreasing trend during this period (12). In order to ascertain the form of the relationship between the age standardised rates for disability adjusted life years (ASDR) and the SDI for 21 regions and 204 countries/territories, our research employed smoothing splines models. The GHDx query tool was utilized to obtain SDI data gathered for 21 regions and 204 countries/territories globally between 1990 and 2021. R software version 4.3.2 and Python 3.8 were used to map the result of female breast cancer burden.
3 Results
3.1 The burden of female breast cancer at the global level
Table 1 shows the prevalent cases of female breast cancer was 20.32 million (95% uncertainty interval 19.25 million to 21.45 million), with an age standardised rate prevalence per 100000 population of 450.64 (95% uncertainty interval 427.02 to 475.96), which changed from 1990 (0.11%, uncertainty interval 0.02% to 0.20% per 100000 population). Globally, the number of incident cases of female breast cancer was 2.08 million in 2021 (95% uncertainty interval 1.94 million to 2.23 million), with an age standardised point incidence per 100000 population of 46.40 (95% uncertainty interval 43.26 to 49.56), which changed from 1990 (0.16%, 95% uncertainty interval 0.09% to 0.24%) per 100000. Globally, the number of DALYs due to female breast cancer in 2021 was 20.25 million (95% uncertainty interval 18.96 million to 21.57 million), with an age standardised rate per 100000 population of 455.56 (95% uncertainty interval 426.64 to 485.30), which changed from 1990 to 2021 (-0.10%, 95% uncertainty interval -0.15% to -0.03% per 100000 population).
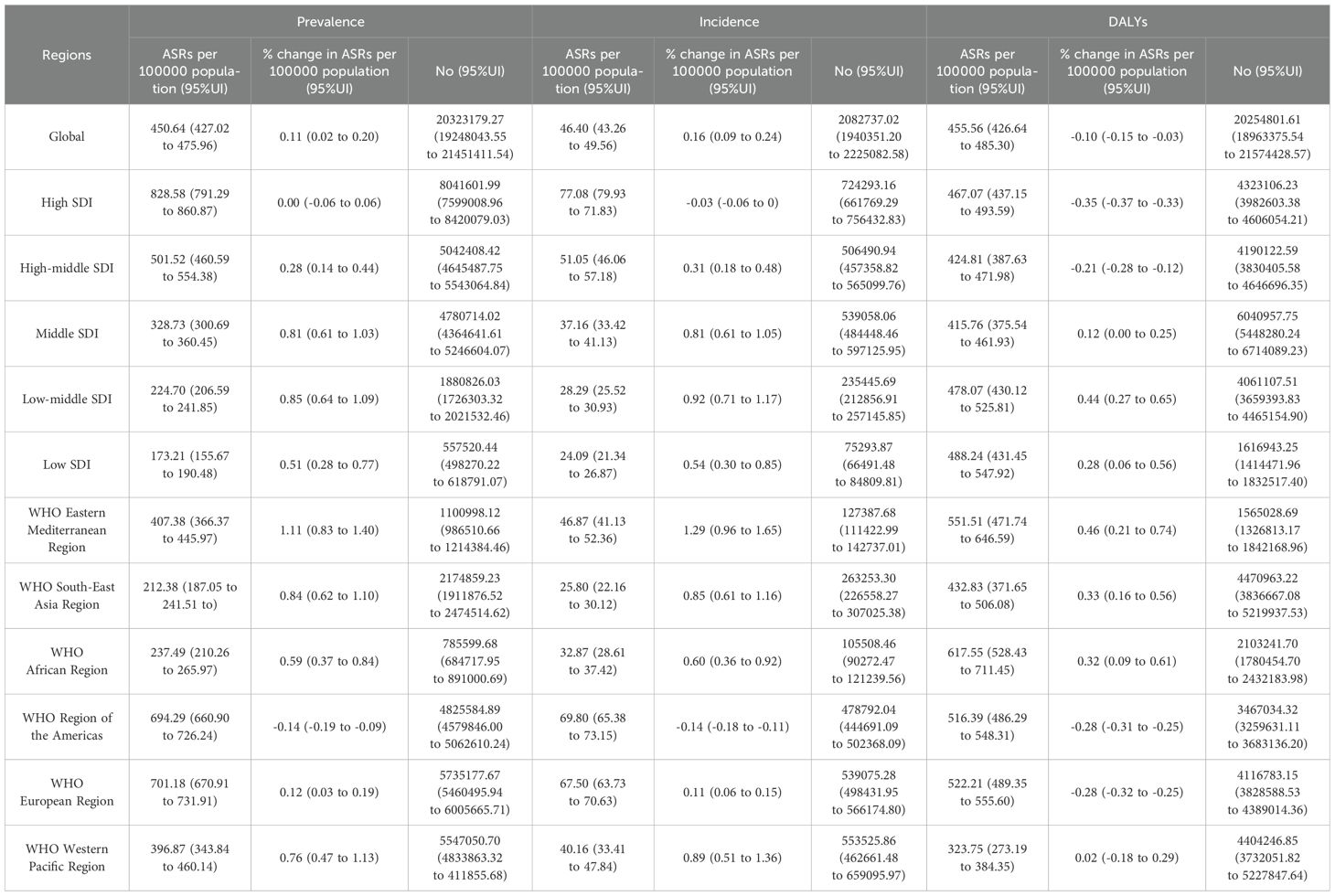
Table 1. Prevalence, incidence, and DALYs of female breast cancer in 2021, and percentage change of ASR per 100000 population between 1990 and 2021.
3.2 Burden of female breast cancer in five SDI regions
Table 1 presents that the highest age standardised point prevalence of female breast cancer per 100000 population in 2021 was in the high SDI region [828.58 (95% uncertainty interval 791.29 to 860.87)], while the lowest was in the low SDI region [173.21 (95% uncertainty interval 155.67 to 190.48)]. In 2021, the highest ASIR of female breast cancer per 100000 population was the high SDI region [77.08 (95% uncertainty interval 71.83 to 79.93)], while the lowest was the low SDI region [24.09 (21.34 to 26.87)]. In 2021, the highest ASDR of female breast cancer per 100000 population was the low SDI region [488.24 (95% uncertainty interval 431.45 to 547.92)], while the lowest was the middle SDI region [415.76 (375.54 to 461.93)].
3.3 Burden of female breast cancer in six WHO regions
Table 1 shows that the highest age standardised point prevalence of female breast cancer per 100000 population was in the WHO European Region [701.18 (95% uncertainty interval 670.91 to 731.91)], while the lowest was in the WHO South-East Asia Region [212.38 (95% uncertainty interval 187.05 to 241.51)] by 2021. Except for the WHO Region of the Americas, other regions were on the increase. The highest ASIR of female breast cancer per 100000 population was in the WHO Region of the Americas [69.80 (95% uncertainty interval 65.38 to 73.15)], while the lowest was in the WHO South-East Asia Region [26.0 (95% uncertainty interval 22.0 to 30.4)] by 2021. Except for the WHO Region of the Americas, other WHO regions were on the increase. The highest ASDR of female breast cancer per 100000 population was in the WHO African Region [617.55 (95% uncertainty interval 528.43 to 711.45)], while the lowest was in the WHO Western Pacific Region [323.75 (95% uncertainty interval 273.19 to 384.35)] by 2021. All regions, except for the WHO Region of the Americas and the WHO European Region, showed an upward trend.
3.4 The burden of female breast cancer at the national level
The countries with the highest ASIR estimated per 100000 population in 2021 were the Principality of Monaco [163.82 (120.64 to 221.94)], United Arab Emirates [131.81 (97.03 to 174.18)], Qatar [125.28 (92.03 to 174.18)], whereas Mongolia [11.37 (8.74 to 14.12)], Niger [13.02 (8.94 to 18.03)], The Gambia [15.07 (10.97 to 20.04)] had the lowest rates (Supplementary eFigure 1 in the (Supplementary Material). The countries with the highest age standardised point prevalence estimates per 100000 population in 2021 were Principality of Monaco [1689.45(1348.34 to 2153.73)], United Arab Emirates [1235.12 (979.40 to 1550.47)], Qatar [1145.79 (894.26 to 1457.43)], whereas Niger [91.26 (67.87 to 119.97)], Mongolia [99.65 (83.12 to 118.37)], The Gambia [(109.59 (84.85 to 140.94)] had the lowest rates.
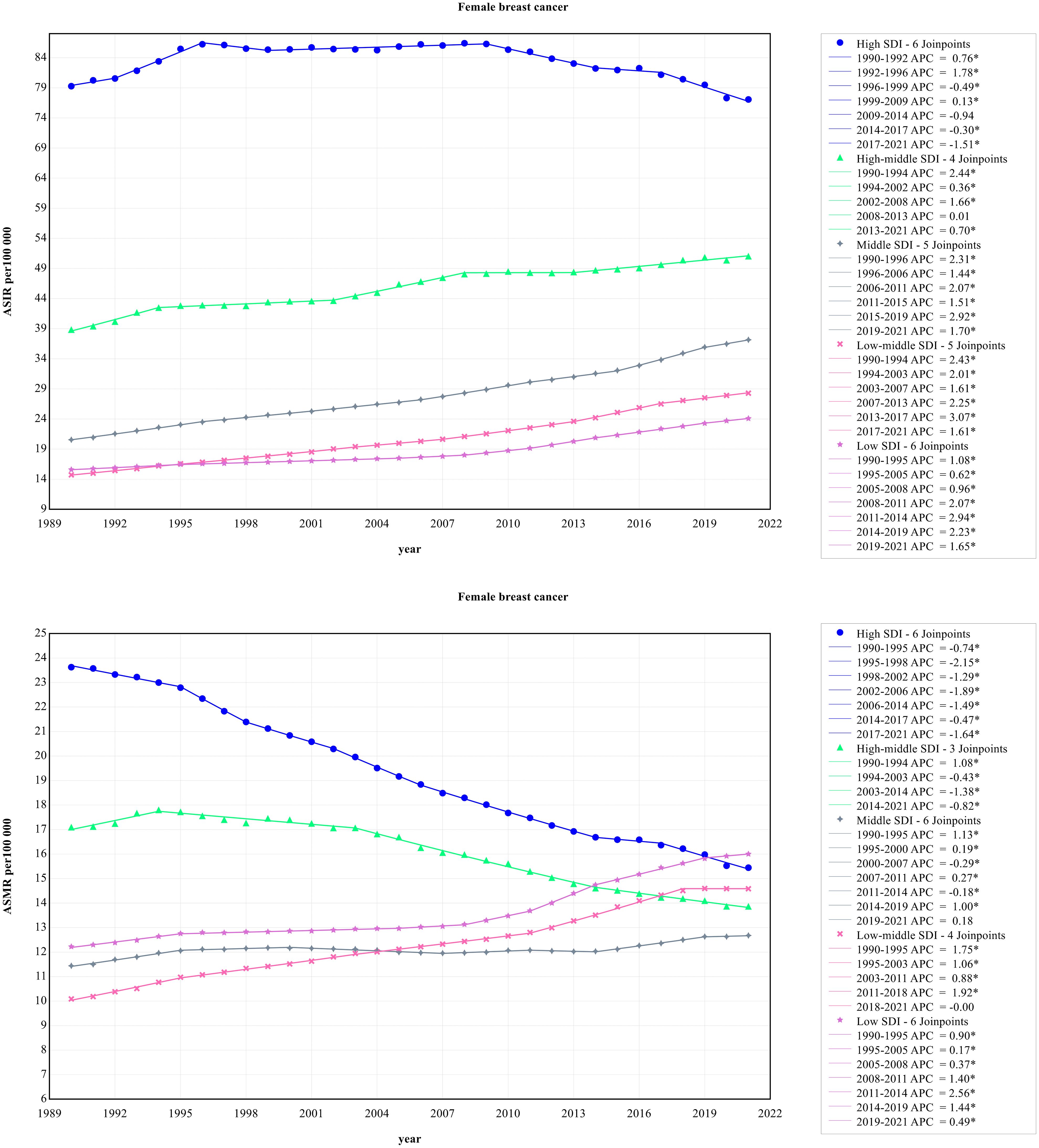
3.5 Time trends of female breast cancer incidence and mortality in five SDI regions
Figure 1 presents the time trends of female breast cancer incidence that were significantly different in five SDI regions. From the perspective of incidence, the ASIR of the high SDI region was obviously higher than other SDI regions. From 1990 to 2021, the ASIR of the high SDI region remained high, while the other four SDI regions showed a small growth trend. From 2009 to 2021, although the high SDI region had the highest ASIR, a steady downward trend was observed. The high SDI region had the highest ASMR of female breast cancer, meanwhile with a sharp decrease throughout the study period. The ASMR of female breast cancer in the middle SDI region slowly increased from 1990 to 1994 and decreased between 1995 and 2021. However, other SDI regions showed an andante upward trend.
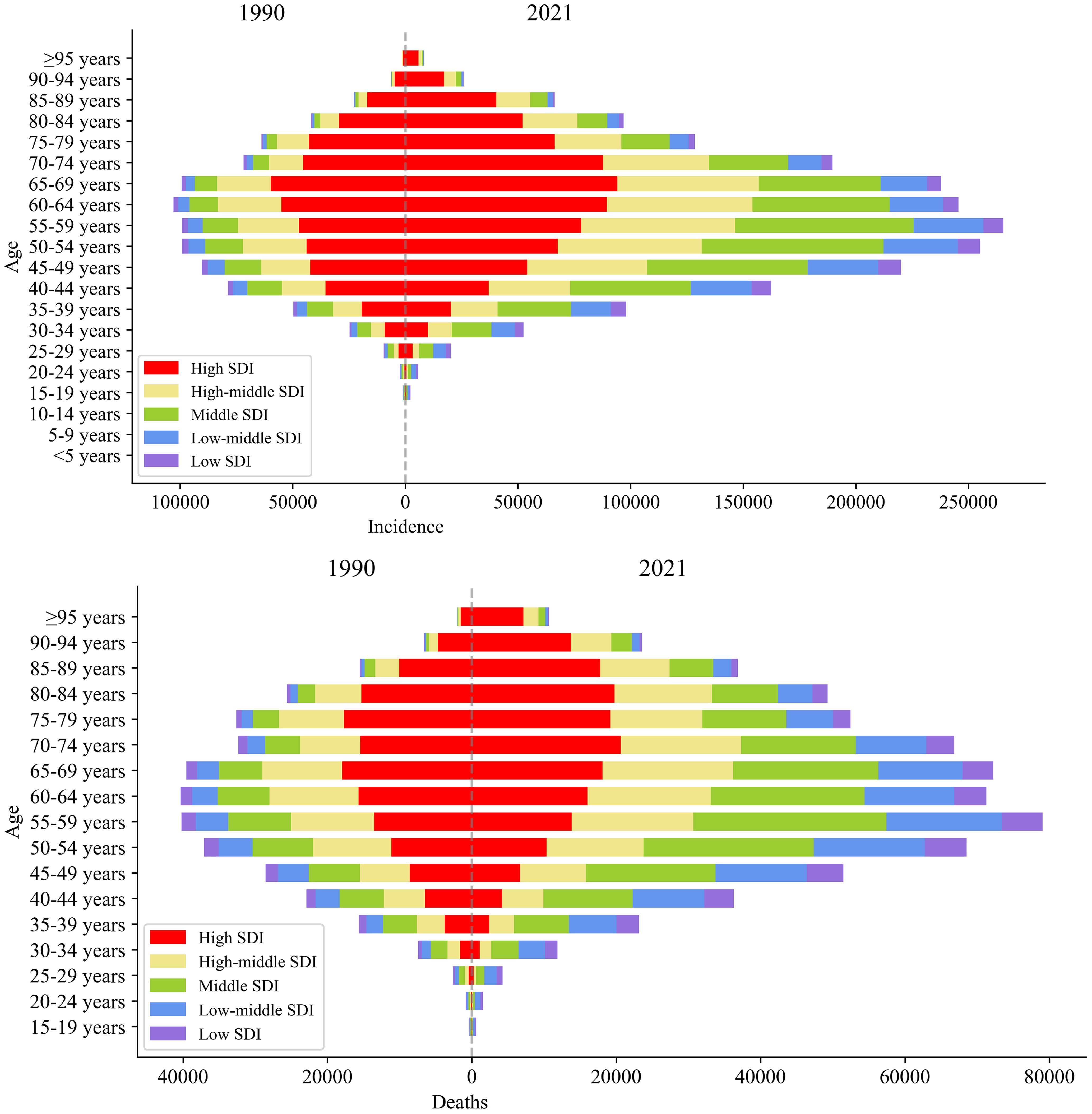
3.6 Age patterns of female breast cancer in five SDI regions
Figure 2 illustrates that age had a significant impact on the incidence and mortality of female breast cancer. In 1990 the number of incidence cases of female breast cancer increased with age up to 60-64 years, then decreased with older age. In 2021 the peak of female breast cancer incidence was found in the 55-59 age group, which was in a younger age group than that in 1990. Similarly, in 2021 the mortality increased with age and peaked at the 55-59 and 65-69 age groups for females, then decreased with older age. Compared with 1990, the mortality in 2021 declined generally with ages up to 65-69 years, especially in the high SDI regions.
3.7 Observed burden of female breast cancer compared with expected by SDI
Figure 3 demonstrates a time trend of the relationship for each of the countries and regions, with each colored line denoting a particular year. Based on data from all nations from 1990 to 2021, the average expected association between SDI and DALYs for breast cancer was represented by the black line with a 95% confidence range. DALYs were calculated by summing YLDs (years lived with disability) and YLLs (years of life lost). One DALY corresponded to one healthy year lost (13).
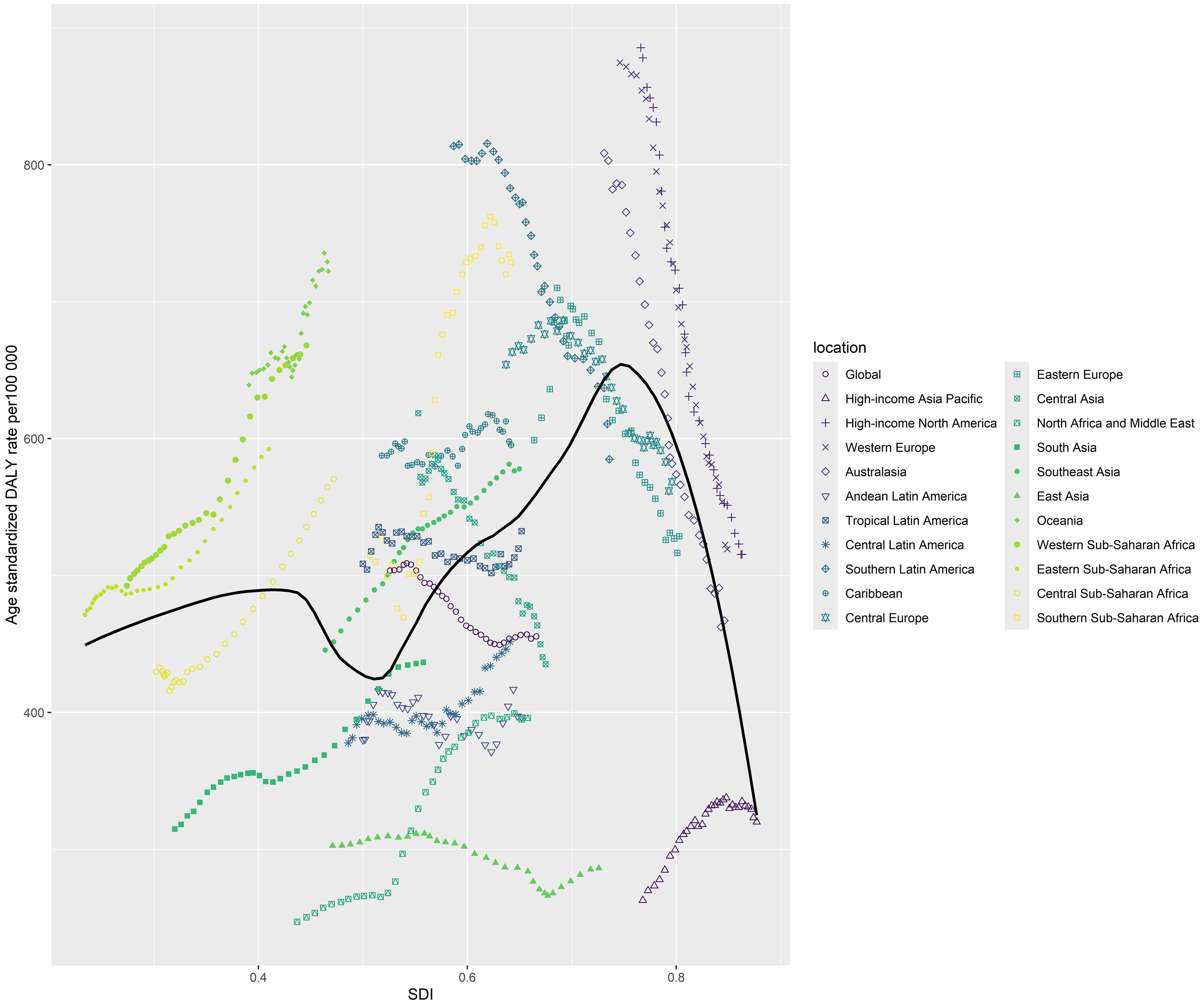
Figure 3. Age standardised DALY rate from female breast cancer per 100 000 population for 21 Global Burden of Disease regions by sociodemographic index, 1990-2021.
Overall, a positive association was observed between the ASDR of female breast cancer and SDI across the GBD regions of which SDI was under 0.75. At the regional level, the observed burden was higher than expected given the sociodemographic index for high-income North America, Western Europe, Western Sub-Saharan Africa, Eastern Sub-Saharan Africa and Southern Sub-Saharan Africa.
Figure 4 illustrates the high ASDR of female breast cancer was not limited to developed countries. In developing countries such as Malaysia, Nigeria and Jamaica, the ASDR was much higher than the expected level based on SDI. Whereas in developed countries such as Japan, Australia and South Korea, the ASDR was much lower than expected.
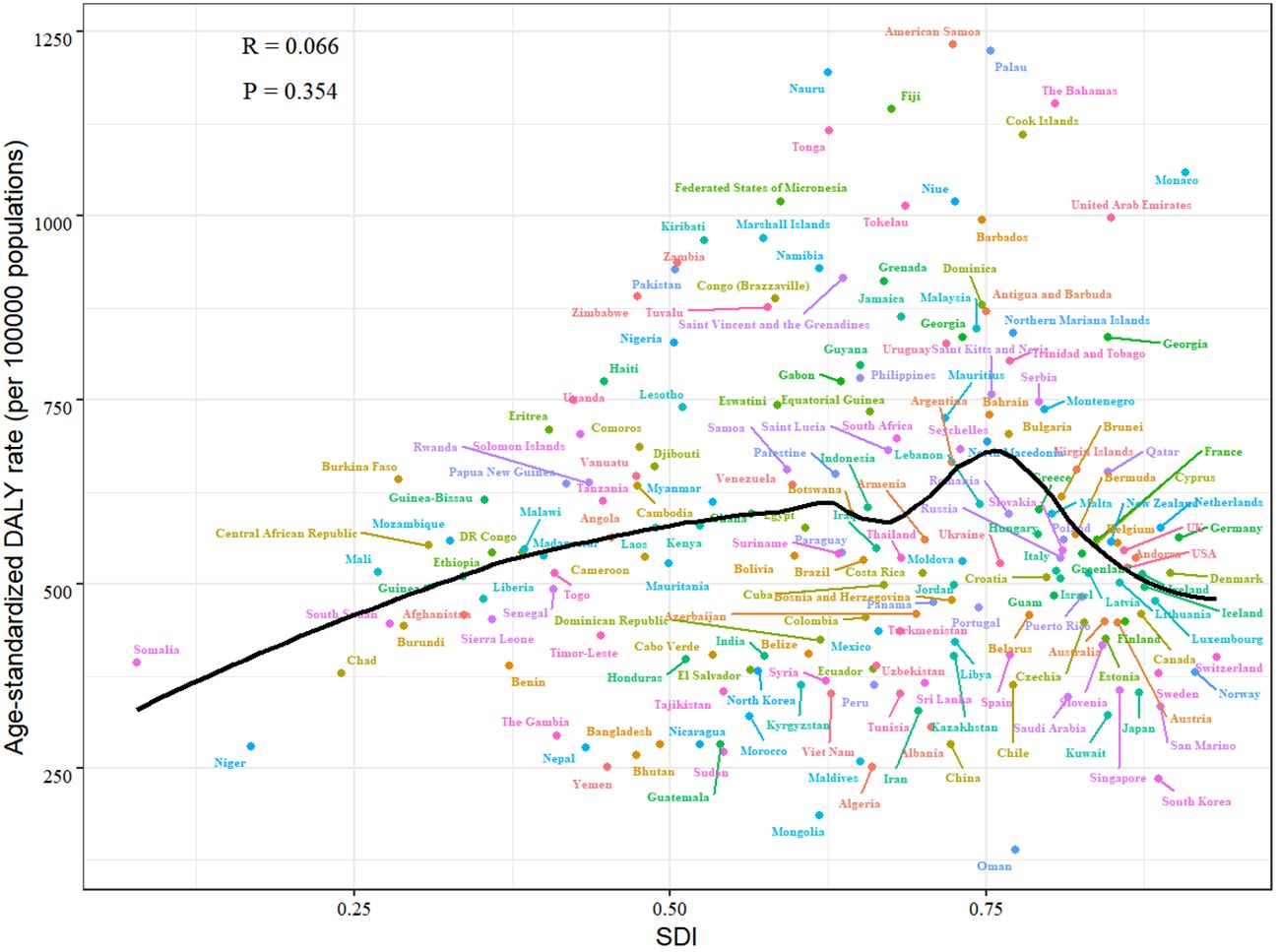
Figure 4. Age standardised DALY rate from female breast cancer per 100 000 population by countries and SDI, 2021.
3.8 Trial characteristics
The sex characteristics of the included trials are presented in Supplementary eFigure 2 in the Supplementary Material. Our study identified 11043 interventional studies conducted from 2010 to 2022 all over the world. Our study excludes 565 trials, including 110 duplicates, 428 clinical trials including children, and 27 outcomes not filled or reported. A total of 10478 eligible trials are identified for analysis. Among the 10478 included trials, 3968 trials mention the use of PROs. Among the 3968 trials involving PROs, 997 trials mention the use of PROs but instruments are not specified, and 2971 trials mentioned the use of PROs and instruments are specified.
A comprehensive overview of eligible trials is presented in Table 2. Among the included trials, phase-2 trials [2562 (24.45%)] are the most common, followed by phase-3 trials [(1161 (11.08%))]. Of the 3968 trials involving PROs, phase 2 trials [560 (14.11%)] were still the most common, followed by phase 3 [494 (12.45%)] trials (Table 1). Among the included trials, most of the experimental subjects were over 18 years old [9648 (92.08%)], and only a few of them were over 65 years old [122 (1.16%)]. Of the 3968 trials involving PROs, most of the trial participants are still over 18 years old [3608 (90.93%)], and only a few of them are over 65 years old [54 (1.36%)]. Among the included trials, more than 60.0% of the studies chose women as the research objects, while 30.65% had no limitation on sex, and only 0.45% of trials chose men as the research participants. Of the 3968 trials involving PROs, more than 65.00% of the trials chose women as the research participants, while 23.06% had no limitation on sex, and only 0.43% of trials chose men as the research participants. Most primary sponsors were located in the WHO Region of the Americas, followed by the WHO European Region and the WHO Western Pacific Region. The other WHO regions, including the WHO South-East Asia Region, the WHO Eastern Mediterranean Region and the WHO African Region accounted for less than 11% of the trial locations (Table 1). Similar findings were found when only PRO-related studies were considered, with more than 80% of primary sponsors coming from the WHO Region of the Americas, the WHO European Region and the WHO Western Pacific Region. Meanwhile fewer than 20% of sponsors came from the WHO Eastern Mediterranean Region, the WHO South-East Asia Region and the WHO African Region.
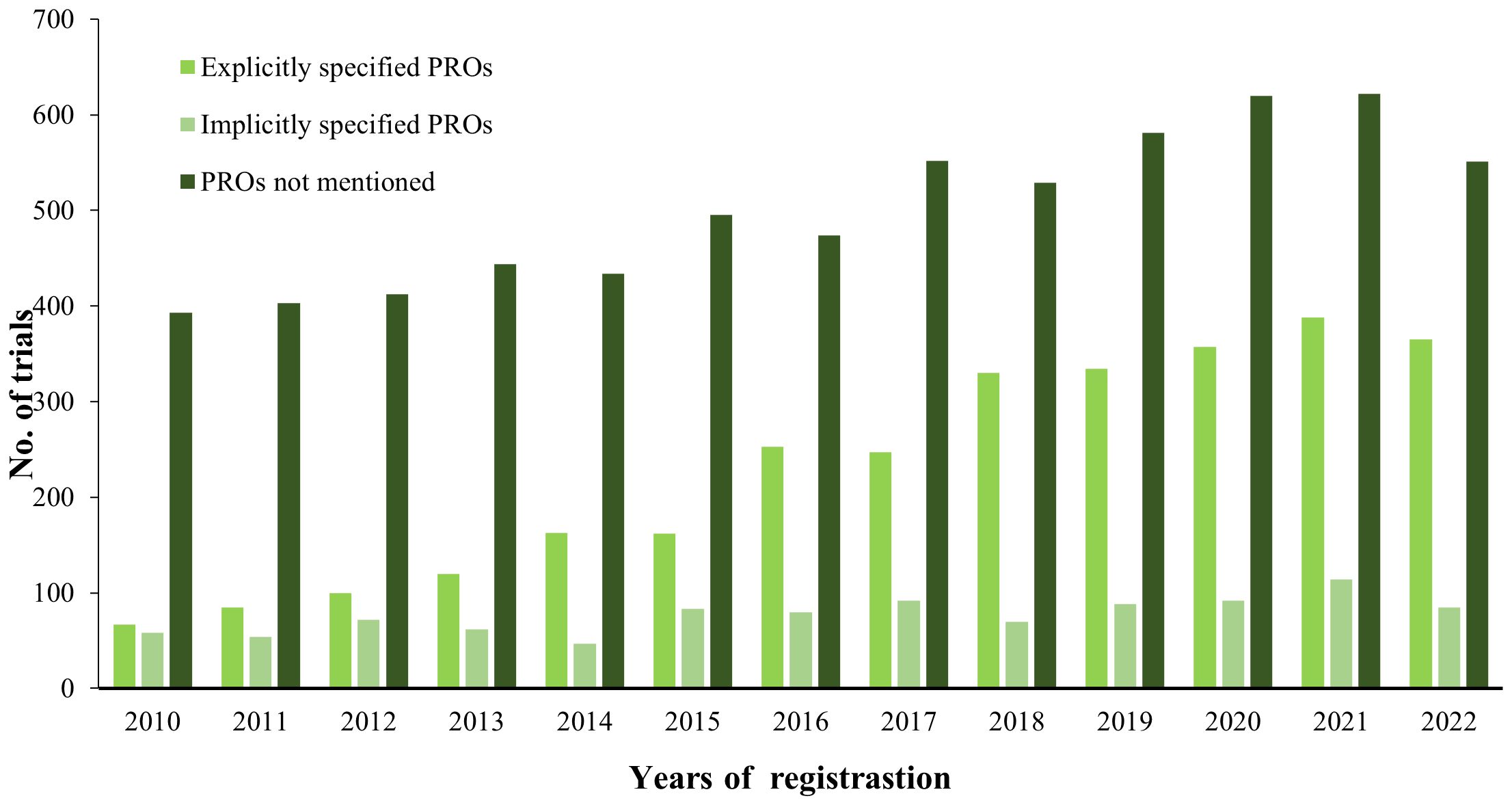
Figure 5 illustrates the increase in the number of breast cancer clinical registration trials from 2010 to 2022 and demonstrates the proportion of trials that listed PROs as the outcome among the breast cancer trials. Of the 10478 trials, 28.4% (n = 2971) report specific PRO instruments, 9.5% (n = 997) are trials that use a vague PRO description, and 62.1% (n=6510) are trials that not use PROs. PRO-related trials are also on the increase, particularly the trials with explicitly specified PROs.
3.9 Global distribution of trials involving PROs
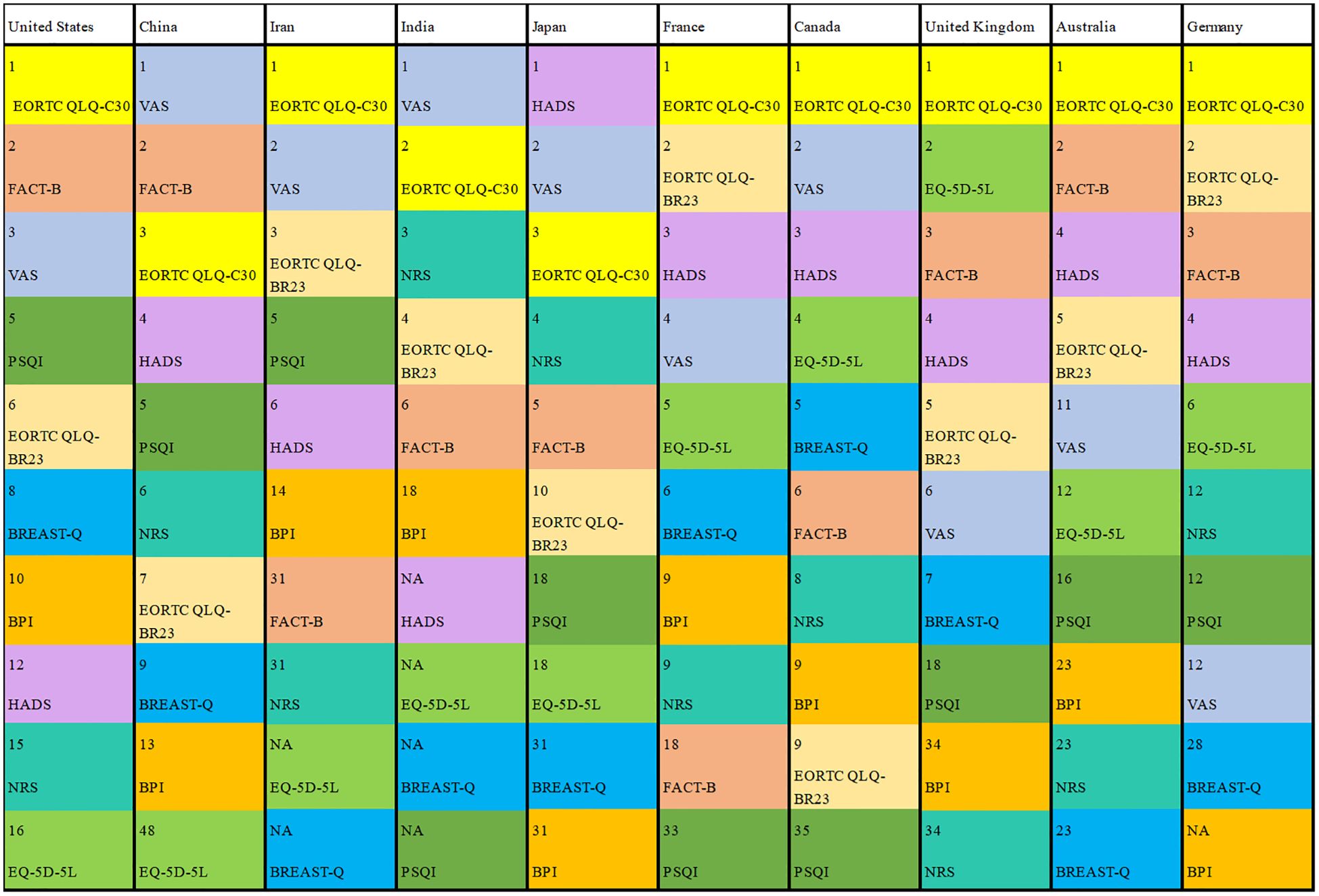
There are among different countries. The distribution of primary sponsors among the different countries of trials involving PROs is shown in Supplementary eFigure 3 in the Supplementary Material. Among the trials involving PROs, most primary sponsors are seated in Europe, followed by Asia, North America and Africa. The other regions, including South America, Oceania and Antarctica accounted for 0%–6.9% (Table 2). The top ten countries are the United States, China, Iran, India, Japan, France, Canada, United Kingdom, Australia and Germany. Meanwhile United States, Canada and Australia were also countries with high incidence and prevalence of female breast cancer.
3.10 PRO instruments used in clinical trials
To summarize the overall application of PROs, our study collected the specific PRO instruments applied in trials that explicitly mention the PRO instruments as primary and/or secondary outcomes (Table 3). In the trials that applied PRO instruments as primary outcomes, VAS was the most frequently used PRO instrument. Moreover, EORTC QLQ-C30 was the most frequently used PRO instrument in the trials that applied PRO instruments as secondary outcomes. The frequently utilized PRO instruments applied as co-primary outcomes were EORTC QLQ-C30, VAS, EORTC QLQ-BR23, FACT-B, HADS, NRS, EQ-5D-5L, BREAST-Q, PSQI and BPI. Although PRO instruments were used in different outcomes, VAS, EORTC QLQ-C30, FACT-B, HADS, NRS and EORTC QLQ-BR23 were still used frequently. EORTC QLQ-C30, EORTC QLQ-BR23 and EQ-5D-5L are scales to assess health-related quality of life. VAS, NRS and BPI are scales to evaluate pain. HADS and PSQI are scales to measure symptoms of mental health. FACT-G, FACIT-F and FACT-B are scales to analyze function.
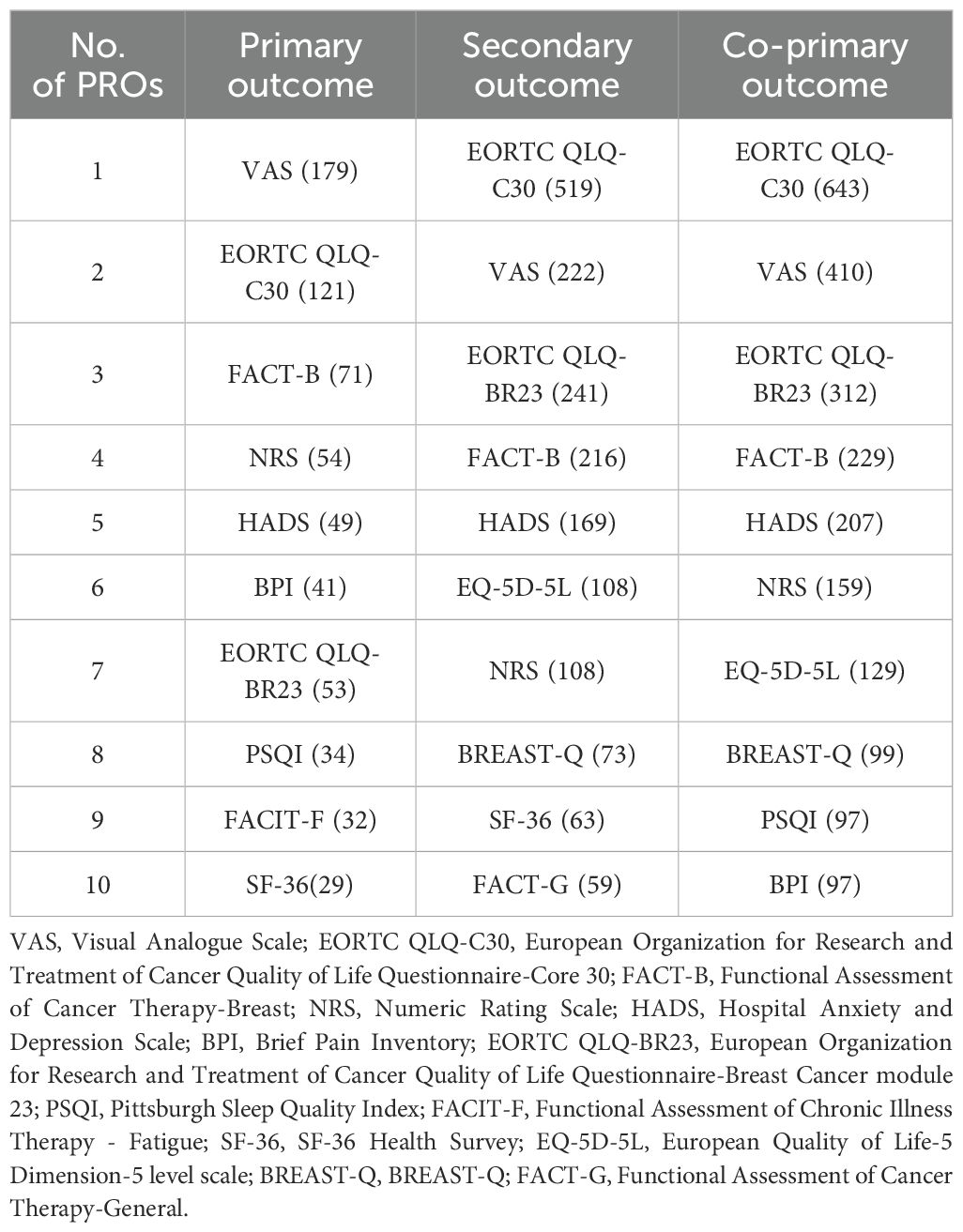
Table 3. Frequency of PRO instruments utilized as primary outcomes, secondary outcomes and co-primary outcomes.
Figure 6 illustrates the frequency of the top ten used PRO instruments of co-primary outcomes in the US, China, Iran, India, Japan, France, Canada, the UK, Australia and Germany. Different colors represented different instruments while the number represents the ranking of the frequency of use of the instruments in this country. EORTC QLQ-C30 was most frequently applied except for China, India, and Japan. In these countries, health-related quality of life was the main evaluation index of clinical trials involving PROs. VAS was the most frequently used instrument in China and India. HADS was the most frequently used instrument in Japan, which was used to evaluate patients’ anxiety and depression. It can be indicated that anxiety and depression of breast cancer patients are the main indices of clinical trials involving PROs of breast cancer in Japan. BREAST-Q was not used in India and Iran, which reflected less evaluation of satisfaction among patients undergoing breast surgery. HADS and PSQI were out of use in India, which reflected the lack of evaluation of mental health.
4 Discussion
To our knowledge, this study is among the first systematic researches to integrate the global burden of disease with the application of PROs in clinical trials for breast cancer. The study had two major findings. Firstly, the burden of female breast cancer is still not alleviated and varied across territories, ages, and SDI levels. Secondly, the study finds that over 60% of the clinical trials neglected subjective burden by patients. Meanwhile, the standard as well as widely used PRO instruments for breast cancer are not enough. It is significant to introduce PROs to the evaluation of breast cancer and assess the burden of breast cancer, which can improve the comprehensiveness, accuracy, and degree of individuation of the evaluation.
According to the GBD 2021, the number of prevalent cases of female breast cancer was 20.32 million with large geographical variations, which is in accordance with the previous studies (14, 15). At the regional level, our study found that the highest age standardised point prevalence estimated in 2021 was in High SDI regions followed by High-middle SDI regions globally, which proved that higher level of SDI was correlated with increased age standardised prevalence rates of female breast cancer. At the national level, Mongolia, Niger and The Gambia had the lowest incidence and prevalence in 2021. This may be related to the national development level, which approved public health policy concerning female breast cancer as fundamental in developing countries (16). It is also significant to develop and adopt the cost-effective screening and therapeutic regimen, which can mitigate the risk factors of female breast cancer in developing countries (17). Meanwhile improving health data of female breast cancer in all countries and regions is strongly suggested for a better evaluation of the Global Burden of Disease.
The incidence estimates of female breast cancer in our study peaks in the middle-aged group, which is also found in other studies (16, 18, 19). The highest incidence was aged 60-64 years in 1990 and aged 55-59 years in 2021. Compared with 1990, the peak of incidence appeared in the younger age groups. The variation may be due to the higher healthcare service level the earlier detection and the change in the population age structure (18, 20). Mammographic screening can reduce mortality of female breast cancer aged 50-74 years (21) and benefit women aged 45-49 years similarly (22). Therefore, it is important to improve the early detection and advanced treatment of women in the middle-aged group, which can increase the survival rate and life expectancy of female breast cancer patients. The development and adoption of cost-effective screening and therapeutic solutions, the mitigation of risk factors, and the establishment of a cancer infrastructure were essential.
Clarity and control of risk factors are two common methods in clinical prevention programs (23). The risk factors for female breast cancer involved different categories, such as various genetic, environmental, lifestyle, demographic and socioeconomic risk factors (24, 25). DALYs and related socioeconomic risk factors had an influence on the incidence and development of female breast cancer around the world. Our study found that the high ASDR of female breast cancer was not limited to regions and territories with high SDI, while the mortality among high SDI regions and high-middle SDI regions declined during 1990-2021. Since 1990, the correlation between socioeconomic development and discrepancy in the global female breast cancer incidence had continued to decline (15). This phenomenon proved the centralization of the disease burden had transformed from developed to undeveloped countries. This change in DALYs from female breast cancer can be attributed to the following factors. Firstly, alcohol consumption was the greatest distributor of breast cancer DALYs and mortality (19, 26). Studies which demonstrated the use of alcohol declined significantly in the past three decades especially in the high and the high middle SDI countries, while increased in most developing countries. Therefore, policy makers are recommended to reject commercial marketing for products especially alcohol, which can raise the risk of breast cancer. Secondly, raising awareness among the general public and improving treatment in high SDI regions can contribute to mortality rates reduction and survival rates improvement, especially Mammographic screening (27). Meanwhile, expanding the scope of early detection efforts for breast cancer in low-income and middle-income countries is recommended under the permission of economic conditions, rather than concentrating just on mammography screening. Equitable access to early diagnosis and treatment is a fundamental need for all individuals to improve their breast cancer survival rates and quality of life in different income regions. Moreover, factors of female breast cancer including but not limited to BMI, contraceptive use, family history and menopause could impact the DALYs in countries with different levels of SDI (28, 29). Our study stressed the importance of breast cancer risk factor education in preventing potential future cases of breast cancer. People should have a healthy lifestyle and address attributable risk factors. Non-genetic, modifiable risk factors should be emphasized to reduce the prevalence of female breast cancer in countries with lower SDI (30).
Our study emphasizes that, despite enormous advances in breast cancer research and treatment over the past three decades, making efforts to an over 40% reduction in breast cancer mortality in some high-income countries, the subjective emotions and suffering of breast cancer patients were still disregarded (1). Many patients with breast cancer suffered from mental health problems as well as none appropriate care even in high-income countries. However, the effects including but not restricted to physical, psychological, social and economic aspects on patients with breast cancer had an impact on patients themselves, their families and society without being fully evaluated. Therefore, enhanced patient decision-making and communication in breast cancer care are encouraged, which can lead to improvements in adherence to therapy, quality of life, and body image. Our research analyzed the application of PROs in clinical trials to improve patient communication and decision-making in breast cancer treatment and reduce the suffering sustained by patients. The findings revealed that among the 10478 eligible trials, only 3968 trial registrations mentioned the use of PROs, which accounted for 37.87%. Among the trials mentioning PROs, 778 applied PROs as primary outcomes, 1930 as secondary outcomes and 1260 as co-primary outcomes. Of the 3968 trials applied PROs, 28.4% (n = 2971) were involved in trials that reported specific PRO instruments. Considering that PROs could appropriately represent patients’ subjective feelings and opinions, they should be emphasized in the study of breast cancer (31). It is of great significance to prioritize patients’ benefits and value patient opinions in clinical trials (32). However, faced with the small proportion of breast cancer-related clinical trials involving PROs, more attention is encouraged to the enormous costs and suffering sustained by patients with breast cancer, which can reduce patients’ suffering and society’s burden of breast cancer.
The number of breast cancer clinical registration trials increased from 2010 to 2022, especially those applied specific PRO instruments. Trials applied PROs of breast cancer are more prevalent in the WHO Region of the Americas followed by the WHO European Region. Conversely, in other regions, especially in the WHO African Region and the WHO South-East Asia Region, the number of clinical trials applied to PROs was conspicuously lower. The reasons were attributed to the following points. Firstly, the economic development level and medical resources of different countries and regions played a significant role in this phenomenon (33). Among the trials involving PROs, most primary sponsors were seated in developed countries including but not limited to the United States, Japan, France, Canada, United Kingdom, Australia and Germany. It was disproportionally distributed with a prominent difference in density between developed and developing countries in health workforce reserve by 2020. It is predicted that the health workforce shortage would improve a lot globally, but the shortage in the WHO African region would continue to be severe. Therefore, developed countries had more medical resources to invest in clinical trials involving PROs of breast cancer (34). Secondly, there existed an intimate relationship between the adoption of PROs in clinical trials of breast cancer and the disease burden. The regions with a great deal of primary sponsors of clinical trials involving PROs, such as the WHO Region of the Americas and the WHO European Region, also had the highest age standardised point prevalence. Clinical Trials on breast cancer in these regions can provide valuable evidence to support collaborative decision-making, claim labeling, medicinal recommendations, and health policy (5). Considering PROs can’t be applied in health resource-constrained regions, it is necessary to apply more simplified instruments and indicators to reveal patients’ subjective pain, including physical, mental and psychological thus meeting the demand of the patients suffering from breast cancer (32). More concentration on treatment considering patients’ subjective pain should be given especially in low-income regions, which had high risk of death concerning breast cancer.
Analyzing the frequency of PRO instruments application in different types of trials, we found the most frequently used questionnaires for breast cancer patients are EORTC-QLQ C30 and VAS. EORTC QLQ-C30 is a proverbial and extensively used representative self-administered questionnaire of breast cancer, which covers multidimensional aspects of health-related quality of life. Meanwhile, breast cancer patients had poor quality of life generally (35, 36). VAS has precision, simplicity, and sensitivity. VAS is accurate, simple and sensitive (37). In addition, a considerable proportion of breast cancer patients suffered from breast cancer pain which was common and heavy but lacked objective evaluation indices (1). Therefore, VAS has become the first choice for pain evaluation in clinical research. There can be variations in the use of PRO instruments concerning breast cancer across different countries. Questionnaires frequently applied in clinical trials concerning breast cancer including BREAST-Q, and HADS scales were not used in India, reflecting the lack of evaluation of mental and patient satisfaction related to breast surgery (38, 39). Our study speculated the reason that the patients in India ignored the treatment-related side effects when customizing the treatment plan, such as the breast shape and psychological conditions of breast cancer patients after an operation because of financial hardship (40). HADS used to evaluate patients’ anxiety and depression is the most frequently used instrument in Japan, which is utilized to evaluate patients’ anxiety and depression (39). Japanese patients generally felt stressed and anxious suffering from breast cancer and even their parents’ mental conditions were affected as well (41). This had a guiding significance for future policy-making and health medical level assessment of breast cancer in Japan (42).
Relevant medical departments are recommended to make a patient-centered questionnaire with empathy and compassion according to different social environments and national medical levels (43). Meanwhile, we should take into account the difficulties of the elderly in answering questionnaires, which suggests simplifying the questionnaires or formulating different questionnaires for different age groups (44). When selecting which PRO instruments to use, balancing clinicians’ and patients’ preferences should be taken into consideration. Considering that clinicians lacked the knowledge of how to effectively use PRO data in clinical diagnosis and treatment, training concerning PROs should be encouraged to be included in regular medical education curricula (45). Real patient cases and problem-based learning with audio/video clips proved to be the most successful and efficient methods of instruction, enabling physicians to understand how to utilize the PRO instruments and consult the PRO data. Meanwhile, clinical researchers and clinicians should be encouraged to cooperate aiming to formulate patient-centered treatment and care (45).
5 Conclusions
The disease burden of female breast cancer is severe and varies greatly throughout countries and regions, while the application of PROs in clinical trials remained noteworthy. It is highly recommended to establish health data on female breast cancer across all countries and territories to raise more awareness about preventive measures and policy-making for female breast cancer to reduce future burdens. Collaboration between clinical researchers and clinicians is encouraged to better estimate the global health impact of female breast cancer, providing valuable evidence to improve health policies and reduce inequalities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JH: Data curation, Methodology, Visualization, Writing – original draft. YG: Writing – original draft, Software. XZ: Validation, Writing – original draft. MD: Data curation, Writing – original draft. HW: Data curation, Writing – original draft. GG: Data curation, Methodology, Writing – original draft. MZ: Data curation, Methodology, Writing – original draft. TT: Supervision, Validation, Writing – review & editing. HR: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (NO:82204963) and the Key project of “Jie Bang Gua Shuai” of Beijing University of Chinese Medicine Fundamental Research Funds in 2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1557080/full#supplementary-material
References
1. Coles CE, Earl H, Anderson BO, Barrios CH, Bienz M, Bliss JM, et al. The lancet breast cancer commission. Lancet. (2024) 403:1895–950. doi: 10.1016/s0140-6736(24)00747-5
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Soerjomataram I and Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. (2021) 18:663–72. doi: 10.1038/s41571-021-00514-z
4. Santosa KB, Qi J, Kim HM, Hamill JB, Wilkins EG, and Pusic AL. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. (2018) 153:891–99. doi: 10.1001/jamasurg.2018.1677
5. Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. Jama. (2018) 319:483–94. doi: 10.1001/jama.2017.21903
6. Aiyegbusi OL, Cruz Rivera S, Roydhouse J, Kamudoni P, Alder Y, Anderson N, et al. Recommendations to address respondent burden associated with patient-reported outcome assessment. Nat Med. (2024) 30:650–59. doi: 10.1038/s41591-024-02827-9
7. Snyder CF, Jensen RE, Segal JB, and Wu AW. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. (2013) 51:S73–9. doi: 10.1097/MLR.0b013e31829b1d84
8. Calvert M, Blazeby J, Altman D, Revicki D, Moher D, and Brundage M. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. (2013) 309:814–22. doi: 10.1001/jama.2013.879
9. Wang H, Abbas KM, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1160–203. doi: 10.1016/s0140-6736(20)30977-6
10. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
11. Wang W, Wang Y, Shao K, Lei Z, Cheng L, Wang F, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Out. (2006) 4:79. doi: 10.1186/1477-7525-4-79
12. Qiu H, Cao S, and Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). (2021) 41:1037–48. doi: 10.1002/cac2.12197
13. Wang W, Wang Y, Shao K, Lei Z, Cheng L, Wang F, et al. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiat. (2022) 9:137–50. doi: 10.1016/s2215-0366(21)00395-3
14. Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. (2017) 389:847–60. doi: 10.1016/s0140-6736(16)31392-7
15. Hu K, Ding P, Wu Y, Tian W, Pan T, and Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open. (2019) 9:e028461. doi: 10.1136/bmjopen-2018-028461
16. Linlin L, Binggong Z, Jie K, Shujing L, and Huijian W. Trend of disease burden and risk factors of breast cancer in developing countries and territories, from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front Public Health. (2023) 10:1078191. doi: 10.3389/fpubh.2022.1078191
17. Mishra GA, Pimple SA, Mittra I, and Badwe RA. Screening for breast cancer: Cost-effective solutions for low- & middle-income countries. Indian J Med Res. (2021) 154:229–36. doi: 10.4103/ijmr.IJMR_2635_20
18. Li Y, Zheng J, Deng Y, Deng X, Lou W, Wei B, et al. Global burden of female breast cancer: age-period-cohort analysis of incidence trends from 1990 to 2019 and forecasts for 2035. Front Oncol. (2022) 12:891824. doi: 10.3389/fonc.2022.891824
19. Li N, Deng Y, Zhou L, Tian T, Yang S, Wu Y, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. (2019) 12:140. doi: 10.1186/s13045-019-0828-0
20. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. (2019) 5:1749–68. doi: 10.1001/jamaoncol.2019.2996
21. Krager SC and Prochazka AV. ACP Journal Club. Review: In women 50 to 69 y of age at average risk, mammography screening reduces breast cancer mortality. Ann Intern Med. (2016) 164:Jc38. doi: 10.7326/acpjc-2016-164-8-038
22. Lauby-Secretan B, Loomis D, and Straif K. Breast-cancer screening–viewpoint of the IARC working group. N Engl J Med. (2015) 373:1479. doi: 10.1056/NEJMc1508733
23. Safiri S, Kolahi AA, Hoy D, Buchbinder R, Mansournia MA, Bettampadi D, et al. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the Global Burden of Disease Study 2017. Bmj. (2020) 368:m791. doi: 10.1136/bmj.m791
24. Mubarik S, Yu Y, Wang F, Malik SS, Liu X, Fawad M, et al. Epidemiological and sociodemographic transitions of female breast cancer incidence, death, case fatality and DALYs in 21 world regions and globally, from 1990 to 2017: An Age-Period-Cohort Analysis. J Adv Res. (2022) 37:185–96. doi: 10.1016/j.jare.2021.07.012
25. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. (2021) 17:350–63. doi: 10.1038/s41574-021-00487-0
26. Zhang J, Lu Y, Zhang N, Yu Z, Li H, He R, et al. Global burden of female breast cancer and its association with socioeconomic development status, 1990-2044. Cancer Rep (Hoboken). (2023) 6 Suppl 1:e1827. doi: 10.1002/cnr2.1827
27. Bhavika KP, Molly BC, Donald N, Karen A, Gina LM, Victor JP, et al. Prospective study of supplemental screening with contrast-enhanced mammography in women with elevated risk of breast cancer: results of the prevalence round. J Clin Oncol. (2024) 42:3826–36. doi: 10.1200/jco.22.02819
28. Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, et al. Global increase in breast cancer incidence: risk factors and preventive measures. BioMed Res Int. (2022) 2022:9605439. doi: 10.1155/2022/9605439
29. Nindrea RD, Aryandono T, and Lazuardi L. Breast cancer risk from modifiable and non-modifiable risk factors among women in southeast asia: A meta-analysis. Asian Pac J Cancer Prev. (2017) 18:3201–06. doi: 10.22034/apjcp.2017.18.12.3201
30. Winters S, Martin C, Murphy D, and Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. (2017) 151:1–32. doi: 10.1016/bs.pmbts.2017.07.002
31. Jia Y, Li Q, Zhang X, Yan Y, Yan S, Li S, et al. Application of patient-reported outcome measurements in adult tumor clinical trials in China: cross-sectional study. J Med Internet Res. (2024) 26:e45719. doi: 10.2196/45719
32. Zhou H, Yao M, Gu X, Liu M, Zeng R, Li Q, et al. Application of patient-reported outcome measurements in clinical trials in China. JAMA Netw Open. (2022) 5:e2211644. doi: 10.1001/jamanetworkopen.2022.11644
33. Liniker E, Harrison M, Weaver JM, Agrawal N, Chhabra A, Kingshott V, et al. Treatment costs associated with interventional cancer clinical trials conducted at a single UK institution over 2 years (2009-2010). Br J Cancer. (2013) 109:2051–7. doi: 10.1038/bjc.2013.495
34. Boniol M, Kunjumen T, Nair TS, Siyam A, Campbell J, and Diallo K. The global health workforce stock and distribution in 2020 and 2030: a threat to equity and ‘universal’ health coverage? BMJ Glob Health. (2022) 7:e009316. doi: 10.1136/bmjgh-2022-009316
35. Kim YB, Lee IJ, Byun HK, Choi YY, Hong B, and Lee J. Symptom network and quality of life of breast cancer patients receiving multimodal cancer treatment: Cross-sectional study. Eur J Oncol Nurs. (2024) 71:102661. doi: 10.1016/j.ejon.2024.102661
36. Alem T, Nigatu D, Birara A, Fetene T, and Giza M. Quality of life of breast cancer patients in Amhara region, Ethiopia: A cross-sectional study. PloS One. (2024) 19:e0305263. doi: 10.1371/journal.pone.0305263
37. Qu M, Zhao J, Zhang Y, Xu Z, Ma C, and Cui H. Utilizing the visual analogue scale (VAS) to monitor and manage pain in post-operative skin wounds after thoracic surgery. Int Wound J. (2023) 21:e14503. doi: 10.1111/iwj.14503
38. MeGhana GS, Jacqueline JC, Thais OP, Shen Y, Rosario CM, Monique CJ, et al. The impact of psychiatric diagnoses on patient-reported satisfaction and quality of life in postmastectomy breast reconstruction. Ann Surg. (2022) 277:e1313-e1323. doi: 10.1097/sla.0000000000005478
39. Annunziata MA, Muzzatti B, Bidoli E, Flaiban C, Bomben F, Piccinin M, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer. (2020) 28:3921–26. doi: 10.1007/s00520-019-05244-8
40. Patra A, deSouza R, Nag S, Pant HB, Agiwal V, YN A, et al. Burden of financial hardship among breast cancer survivors in maharashtra, India. Cureus. (2024) 16:e61625. doi: 10.7759/cureus.61625
41. Oshiro R, Soejima T, Tada K, Suzuki M, Ohno S, Yubune K, et al. Anxiety and related factors among parents of patients with breast cancer after surgery in Japan: A multi-informant and multilevel study. JPN J Nurs Sci. (2022) 19:e12452. doi: 10.1111/jjns.12452
42. Ohno S, Chen Y, Sakamaki H, Matsumaru N, and Tsukamoto K. A population-based study of the humanistic burden among cancer patients in Japan. J Med Econ. (2020) 23:429–41. doi: 10.1080/13696998.2019.1707213
43. David TE, Timothy JB, Philip TH, Michele YH, Victor MM, James MN, et al. Harmonizing and consolidating the measurement of patient-reported information at health care institutions: a position statement of the Mayo Clinic. Patient-Relat Outcom. (2014) 5:7–15. doi: 10.2147/prom.S55069
44. David R, Jens L, Maria R, Daniel D, Vincent G, Michael JF, et al. Usability of electronic patient-reported outcome measures for older patients with cancer: secondary analysis of data from an observational single center study. J Med Internet Res. (2023) 25:e49476. doi: 10.2196/49476
Keywords: breast cancer, global burden of disease, patient-reported outcomes, clinical trials, systematic analysis, quality of life
Citation: Hao J, Gong Y, Zhang X, Du M, Wang H, Guo G, Zhou M, Tian T and Rong H (2025) Global burden of breast cancer and application of patient-reported outcomes in clinical trials: a systematic analysis based on the global burden of disease study 2021 and WHO international clinical trial register database. Front. Oncol. 15:1557080. doi: 10.3389/fonc.2025.1557080
Received: 17 March 2025; Accepted: 28 May 2025;
Published: 12 June 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Janet L. Wale, HTAi Patient and Citizen Involvement in HTA Interest Group, CanadaHesong Wang, Fourth Hospital of Hebei Medical University, China
Copyright © 2025 Hao, Gong, Zhang, Du, Wang, Guo, Zhou, Tian and Rong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Tian, dHQ4MzI0QDE2My5jb20=; Hongguo Rong, aGdyb25nQGhzYy5wa3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jiaxin Hao
Jiaxin Hao Yijia Gong1,2†
Yijia Gong1,2† Hongguo Rong
Hongguo Rong