- 1Department of Anesthesiology, University-Town Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
- 2Department of Gynecology and Obstetrics, University-Town Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
Purpose of review: The incidence of pheochromocytoma and paraganglioma (PPGL) in pregnancy is extremely low, yet it poses a significant threat to maternal and fetal safety. While studies are published annually, progress in this field remains slow. A key question is how researchers can better utilize limited case data to gain valuable insights. This bibliometric review summarizes the current research landscape, highlights recent findings, and suggests areas for future investigations.
Methods: A comprehensive search was conducted in the Web of Science Core Collection (WoSCC), PubMed, and Embase databases for PPGL in pregnancy from 1990 to 2024. Data analysis was performed using Excel 2022 and CiteSpace.
Current state: A total of 391 articles were included in the analysis. The United States was the most prolific country, and the Mayo Clinic was the most productive institution. Lenders JWM was identified as both the most published and most co-cited author. Journal of the Endocrine Society was the most frequently targeted journal for publication, while the Journal of Clinical Endocrinology & Metabolism had the highest number of co-citations. Evidence-based practice in this field primarily depends on case reports, case series, case-control studies, and systematic reviews. The primary focus in this research area is on clinical management and pregnancy complications.
Recent findings: Maternal and infant mortality in pheochromocytoma during pregnancy has significantly decreased due to improved awareness and advances in diagnosis and treatment. Antepartum diagnosis is the most vital element in reducing mortality; hypertension at admission and history of PPGL were independent factors of antepartum diagnosis. Abdominal/pelvic tumor location and catecholamine levels ≥10 times the upper limit of the reference range were associated with adverse outcomes. Women with hereditary disease and risk of developing PPGL should be screened before becoming pregnant for occult PPGL and should be treated adequately.
Conclusion: Enhanced collaboration between countries and institutions is needed to advance the field. Diagnostic and therapeutic strategies, as well as complications associated with PPGL during pregnancy, have consistently been core areas of research. Future studies should prioritize the clarification of detailed clinical management protocols and the underlying pathophysiological mechanisms, with the goal of generating high-quality evidence to guide the care of this high-risk population.
1 Introduction
Pheochromocytoma and paraganglioma (PPGL) are rare neuroendocrine tumors. Pheochromocytomas originate in the adrenal medulla, while paragangliomas arise from extra-adrenal chromaffin tissue. Due to their similar histological features and clinical behavior, they are collectively referred to as PPGL (1). PPGL is a rare cause of secondary hypertension in pregnancy, with an extremely low incidence but it poses significant risks to both maternal and fetal health. The reported mortality rates for mothers and infants have reached as high as 48% and 54%, respectively (2). Although international perioperative guidelines for PPGL have been published, there are currently no specialized guidelines for cases involving pregnancy. Clinically, the symptoms of pregnancy-associated PPGL closely resemble those of pregnancy-induced hypertension, which often leads to misdiagnosis and delayed treatment. Diagnosis after delivery is widely recognized as a critical risk factor that increases mortality and complications for both mothers and fetuses (3–7). In fact, the diagnosis of this condition itself is not particularly difficult; the primary challenge is considering PPGL as a potential diagnosis when treating a pregnant patient (8). Therefore, increased awareness of PPGL in pregnancy is essential.
Almost every year, case reports of PPGL in pregnancy are published in medical databases, underscoring its rarity. The earliest report dates back to 1949, and to date, case studies remain an essential source of evidence-based knowledge for managing pregnant patients with PPGL. Significant advancements have been made in many areas of endocrine research; however, progress in studying PPGL in pregnancy remains slow. The scarcity of cases makes it challenging to obtain high-quality evidence-based data for this condition (8). After searching the Cochrane Library, we found no relevant randomized controlled trials. The current evidence in evidence-based medicine comes mainly from case reports, systematic reviews. A key issue in the current context is how to better utilize sporadic clinical cases to obtain more valuable insights. Herein, we summarize existing literature on PPGL in pregnancy to create an up-to-date knowledge map and identify future research directions.
Bibliometrics is a quantitative method used to analyze publication trends and research output within a specific field. CiteSpace, one of the most widely used tools for bibliometric analysis, was developed by Professor Chaomei Chen (9). It enables researchers to identify influential authors, institutions, countries, references and keywords, as well as to analyze citation patterns and emerging trends. Bibliometric methods have been widely applied in oncology research to explore the current landscape and evolving directions of the field. However, to date, no bibliometric analysis has focused specifically on PPGL in pregnancy. The present study aims to perform a bibliometric analysis of research on PPGL during pregnancy, with the goal of summarizing recent developments and identifying major research hotspots in the field.
2 Materials and methods
2.1 Data source
Three researchers independently searched the Web of Science Core Collection (WoSCC), PubMed, and Embase databases for articles related to pregnancy complicated by PPGL, covering the period from 1990 to 2024.
2.2 Search strategy
The search strategies for PubMed and Embase were based on a combination of Medical Subject Headings (MeSH) or Emtree terms and free-text keywords. Controlled vocabulary terms included “Pregnancy,” “Pheochromocytoma,” and “Paraganglioma,” while the free-text terms consisted of synonyms and variations for each concept. The search strategy for WoSCC involved the use of keywords appearing in the title and abstract of the articles. Details of the search strategy can be found in the Supplementary Material.
2.3 Data collection
The inclusion criteria were as follows: (1) the content of the article, or specific sections thereof, pertains to pregnant women with PPGL, regardless of whether the diagnosis was made antepartum or postpartum; patients with PPGL during pregnancy who have other comorbidities were also included; (2) article types limited to original research articles and reviews; (3) publications written in English; and (4) publication period spanning from 1990 to 2024.
The exclusion criteria were as follows: (1) studies unrelated to the topic, such as those involving non-pregnant patients with PPGL or pregnancy-related hypertension not caused by PPGL; (2) non-journal publications, such as books, conference abstracts, proceedings, and letters; and (3) studies involving non-human subjects.
2.4 Data analysis
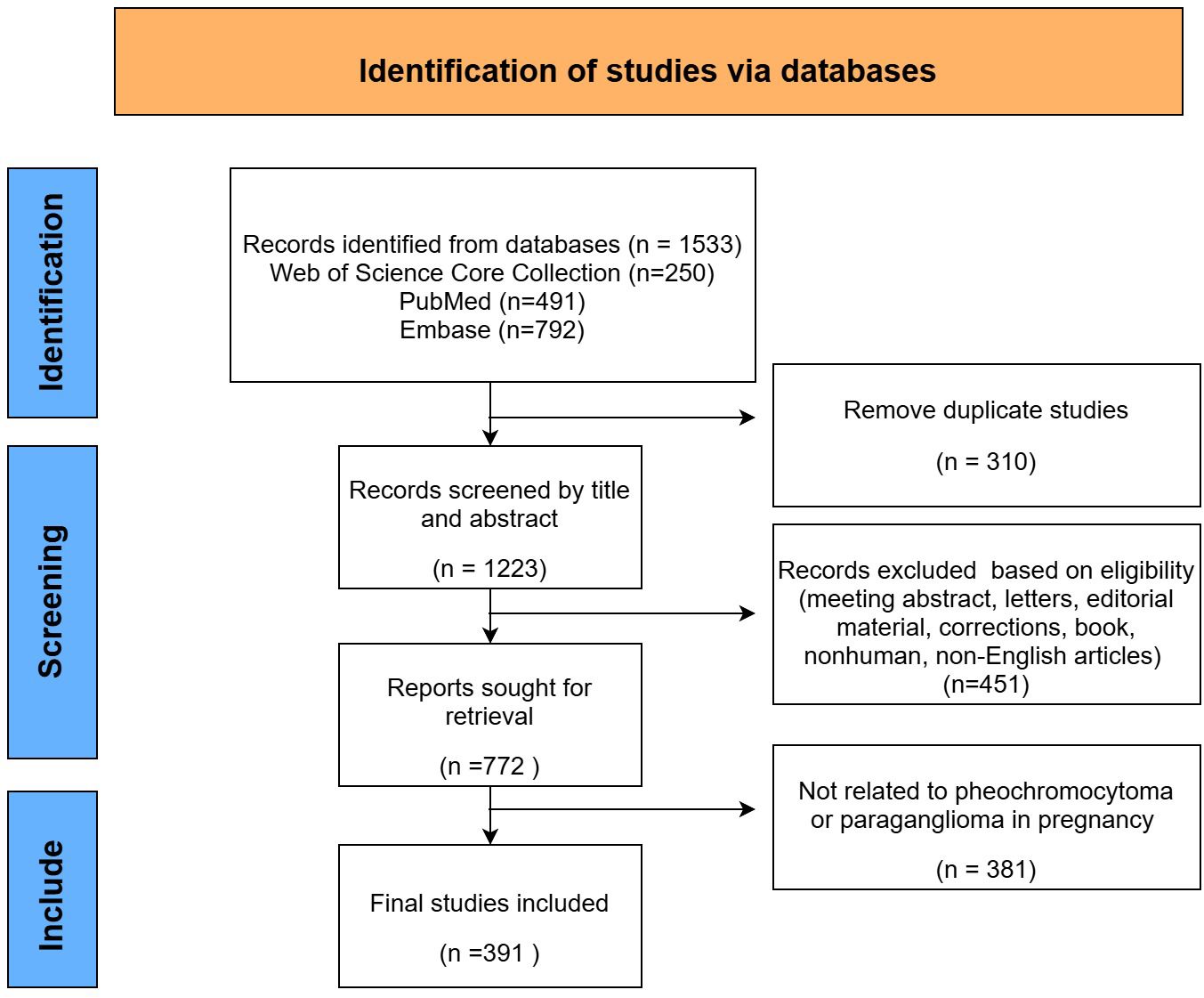
After data collection and filtering (Figure 1), 391 articles were imported into Microsoft Office Excel 2022, and a publication volume chart was created.
Epidemiological characteristics of pregnancies complicated by PPGL were extracted from the included literature, including prevalence, maternal and fetal mortality rates, as well as the rates of antepartum and postpartum diagnosis.
Bibliometric analysis was performed using CiteSpace. Key indicators analyzed included publishing countries, institutions, authors, co-cited authors, co-cited journals, references, and keywords. The evaluation metrics included publication count, co-citation frequency, betweenness centrality, burst strength, and JCR journal rankings. Co-citation frequency reflects how often two documents or authors are cited together in subsequent publications. A higher co-citation frequency indicates a stronger intellectual linkage or shared relevance between the cited entities within the field. Betweenness centrality quantifies the extent to which a node lies on the shortest paths between other nodes, indicating its role as a bridge or connector within the network. A betweenness centrality score greater than 0.1 is typically considered indicative of a node with significant bridging influence in the network. Citation burst strength reflects the intensity of a sharp increase in citations of a particular reference over a specific period. A higher burst strength indicates that the reference received considerable attention in a short time, suggesting its impact on emerging research frontiers. The clustering analysis of major keywords employed the Log-Likelihood Ratio (LLR) algorithm. The modularity Q and weighted average silhouette S were calculated to assess clustering effectiveness. The Q value ranges from 0 to 1, reflecting the network structure’s quality. A Q value greater than 0.3 indicates a significant clustering structure. The S value ranges from -1 to 1, positively correlating with the clustering network’s rationality. An S value greater than 0.5 indicates a reasonably structured network, while an S value greater than 0.7 suggests reliable clustering results. JCR classifications were obtained from Journal Citation Reports 2023.
3 Results
3.1 Global trend of publications and epidemiological overview
3.1.1 Annual number of publications
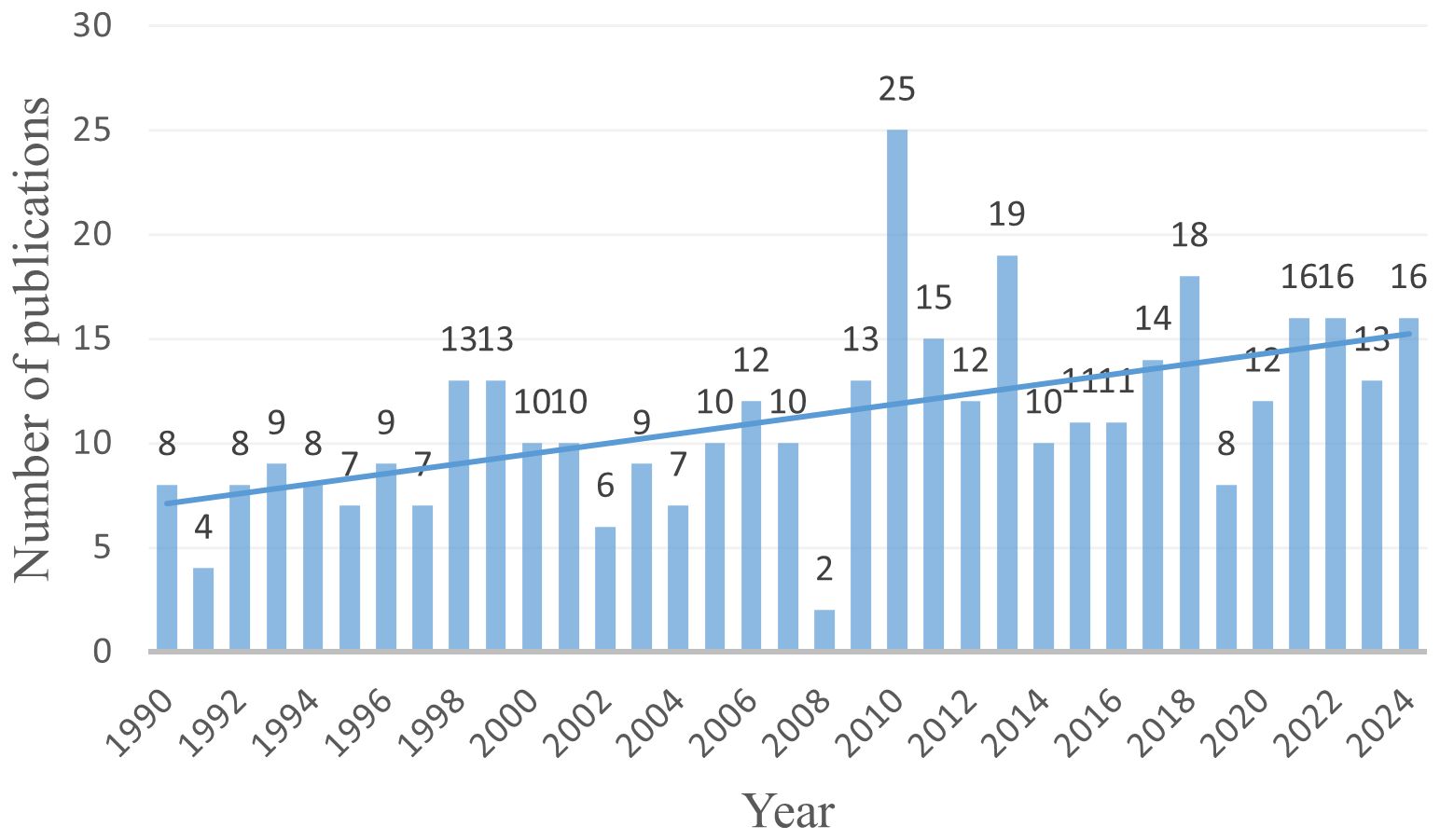
Based on the search strategy, a total of 391 articles related to pregnancy complicated by PPGL were identified from January 1, 1990, to December 30, 2024. As shown in Figure 2, the annual number of publications has shown a steady upward trend, reflecting growing academic interest in PPGL during pregnancy.
3.1.2 Country and institution contributions analysis
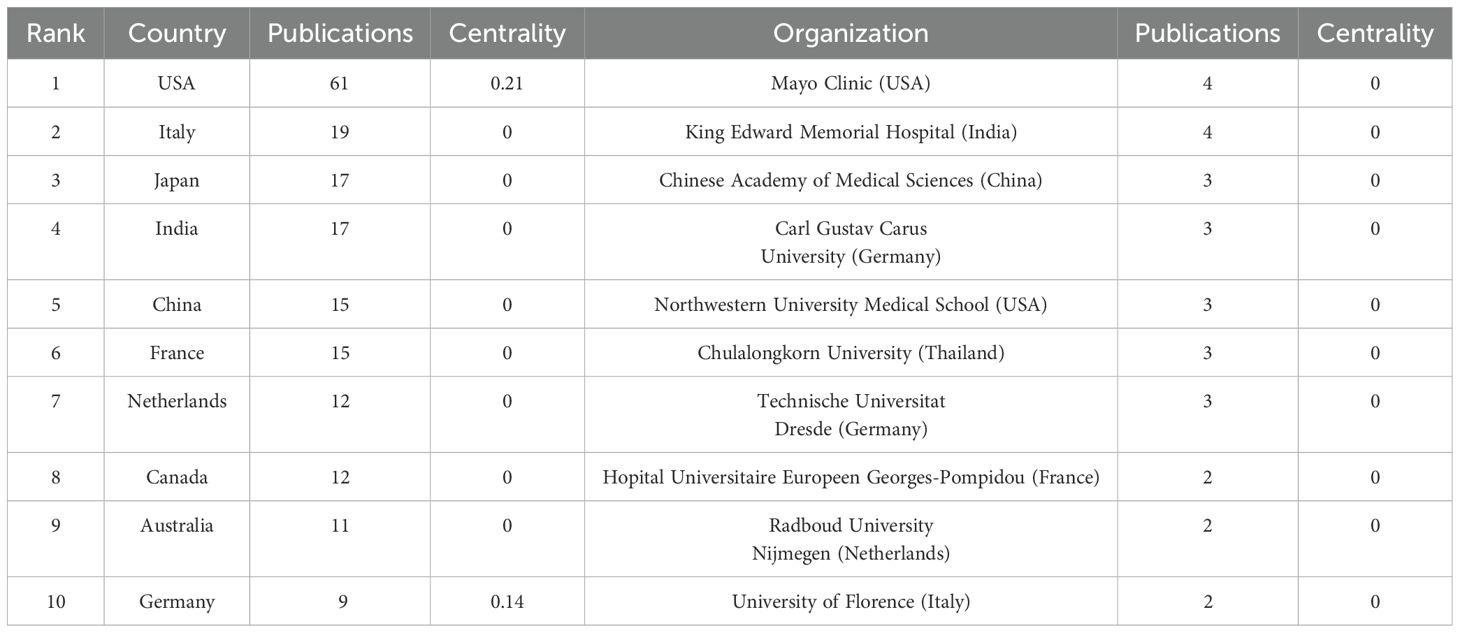
Table 1 presents the top 10 countries and institutions ranked by the number of publications, along with their corresponding publication counts and betweenness centrality scores. The countries with the highest number of publications were the United States (n = 61, 15.6%), Italy (n = 19, 4.8%), Japan (n = 17, 4.3%), and India (n = 17, 4.3%). The countries with the highest centrality were the United States (0.21) and Germany (0.14), while all other countries had a centrality score of 0. The leading institutions in terms of publication volume were the Mayo Clinic (USA, n = 4), King Edward Memorial Hospital (India, n = 4), and the Chinese Academy of Medical Sciences (China, n = 3). All institutions had a centrality score of 0. A centrality score of zero indicates that the country or institution did not function as a bridge within the collaboration network, suggesting limited connectivity or a relatively isolated research position.
3.1.3 Authors and co-cited authors analysis
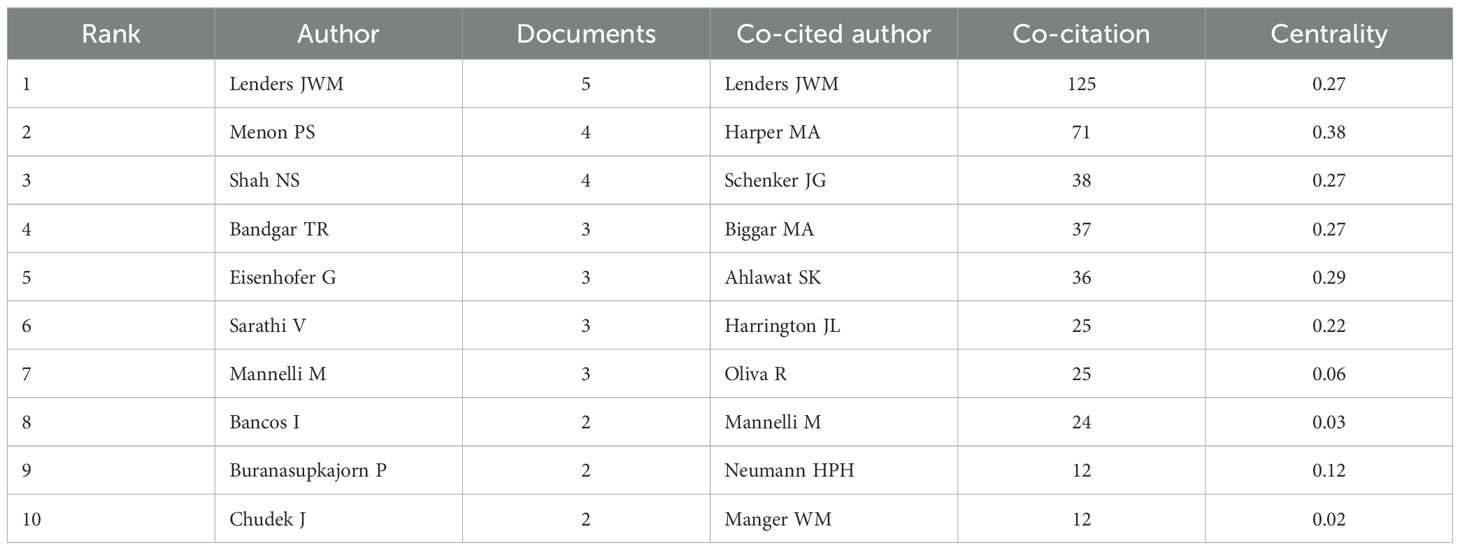
The top 10 authors by publication count and co-citation frequency are listed in Table 2. The most prolific authors were Lenders JWM (n = 5), Menon PS (n = 4), and Shah NS (n = 5). The top three co-cited authors were Lenders JWM (co-citation count = 125, centrality = 0.27), Harper MA (co-citation count = 71, centrality = 0.38), and Schenker JG (co-citation count = 38, centrality = 0.27). Notably, Lenders JWM from Radboud University Nijmegen in the Netherlands was both the most prolific and most co-cited author, with a centrality score of 0.27, indicating his pivotal position within the co-citation network.
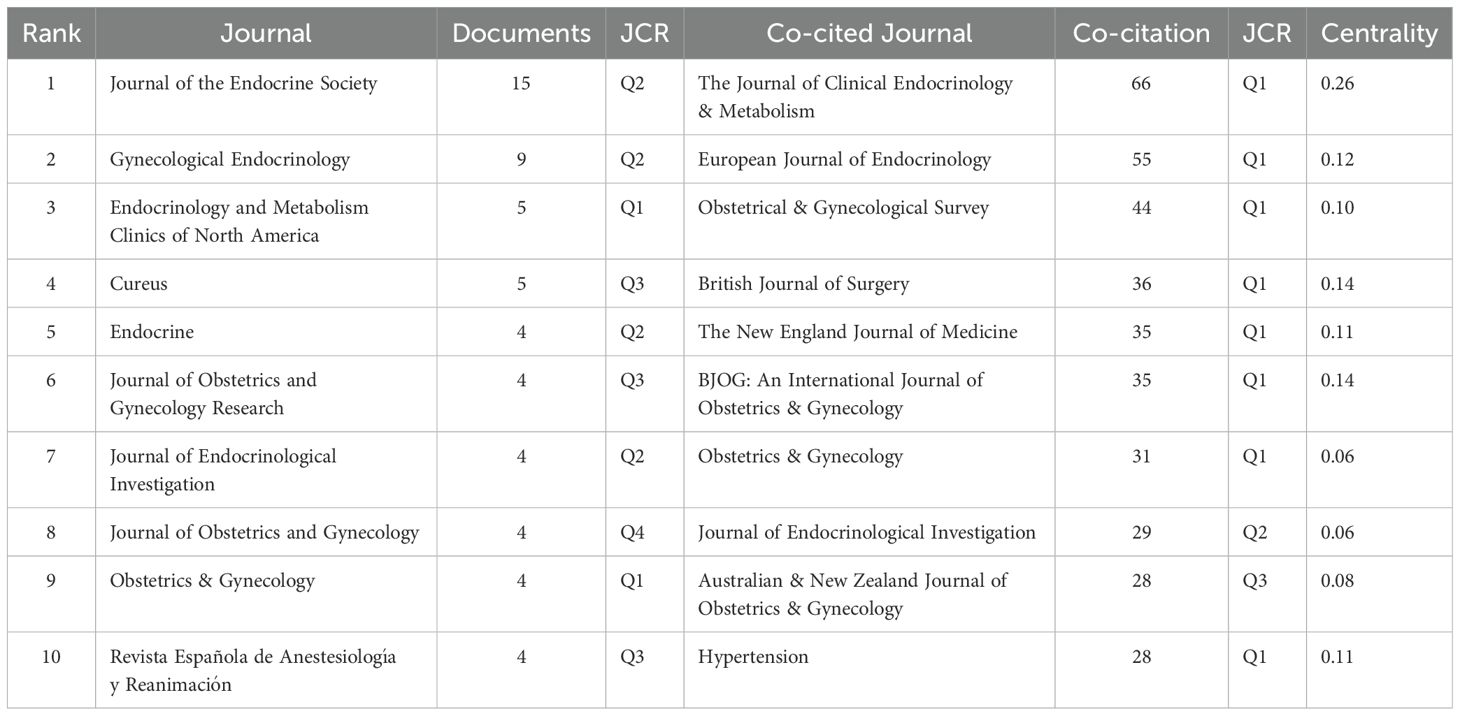
3.1.4 Journals and co-cited journals analysis
Journal publication counts were analyzed using EndNote, and Table 3 displays the top 10 journals ranked by publication volume and co-citation frequency. The top three journals by publication volume were Journal of the Endocrine Society (n = 15, Q2), Gynecological Endocrinology (n = 9, Q2), and Endocrinology and Metabolism Clinics of North America (n = 5, Q1). Among the top 10 journals publishing on this topic, five were classified as endocrinology journals, four as obstetrics and gynecology journals, and one was indexed in both categories. Co-cited journals were analyzed using CiteSpace, and eight of the top 10 co-cited journals were classified as Q1 journals according to the JCR. The three most co-cited journals were The Journal of Clinical Endocrinology & Metabolism (co-citation count = 66, Q1, centrality = 0.26), European Journal of Endocrinology (co-citation count = 55, Q1, centrality = 0.12), and Obstetrical & Gynecological Survey (co-citation count = 44, Q1, centrality = 0.10).
3.1.5 Epidemiological trends
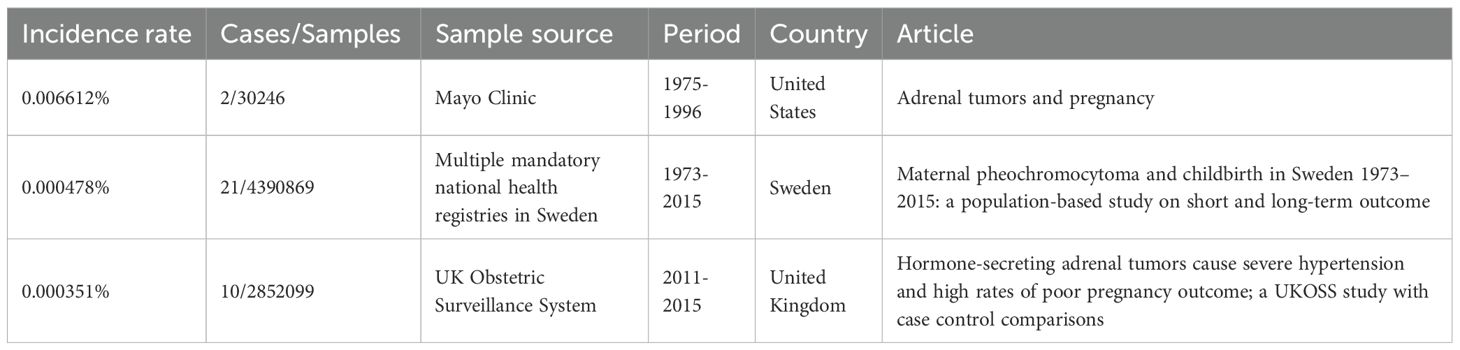
The incidence rate of pregnancy-associated PPGL is generally low, estimated to be 0.000351% to 0.006612% (10–12), as shown in Table 4.
According to retrospective studies and systematic reviews, maternal and fetal mortality rates have markedly improved in recent years compared to earlier periods (3). Antepartum diagnosis remains the most critical factor in reducing both maternal and fetal mortality (3–6, 13), with hypertension at admission and a history of PPGL identified as independent predictors of timely diagnosis (6). Initiation of alpha-adrenergic blockade after diagnosis effectively controls symptoms caused by catecholamine excess and reduces the risk of adverse outcomes (3, 14). Tumors located in the abdomen or pelvis, and plasma catecholamine levels exceeding 10 times the upper limit of the reference range, are both associated with poorer outcomes (3). Maternal mortality is also higher among patients who experience hypertensive crises compared to those who do not (7).
Six studies reported outcomes for pregnant women and fetuses, as detailed in Table 5. Among them, Bancos I et al. (3), published in 2021 in The Lancet Diabetes & Endocrinology, conducted the longest retrospective study spanning from 1980 to 2019, including 232 cases and a total of 249 pregnancies, showed a maternal mortality rate of 1% and a fetal mortality rate of 9% for pregnancies associated with PPGL. Although the mortality rate has significantly decreased, 6.5% of mothers experienced severe cardiac complications related to catecholamine excess. Other systematic reviews covered different periods: 1988-1997 (15); 1998-2008 (14); 1980-2019 (3); 1988-2019 (6); 2000-2011 (7); and 2010-2017 (13), reporting maternal and fetal mortality rates respectively, as shown in Table 5. These rates are significantly reduced compared to the 48% maternal mortality rate reported before 1979 (2).
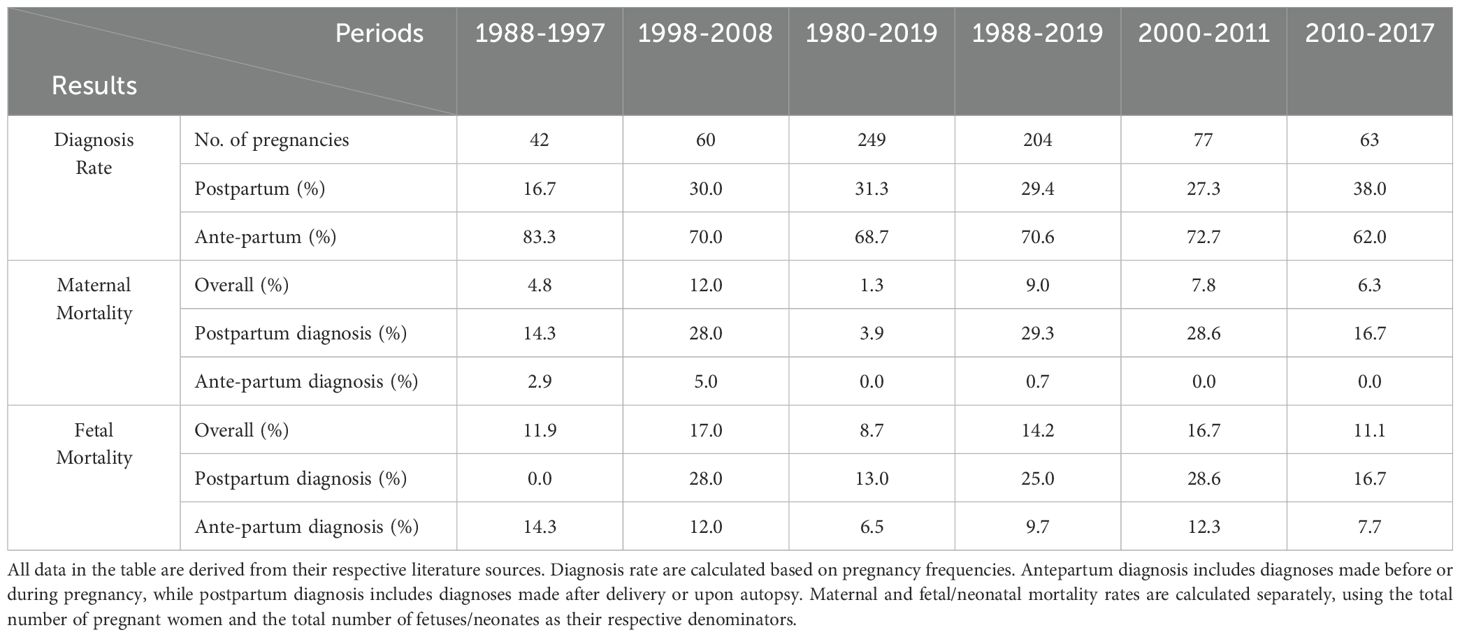
Table 5. Diagnosis rate, maternal and fetal mortality according to the time of diagnosis, as reported in the literature for different periods.
3.2 Effects of hormones on mothers and fetuses
Figure 3 summarizes the impact of excessive catecholamine secretion on both the mother and the fetus as reported in case reports.
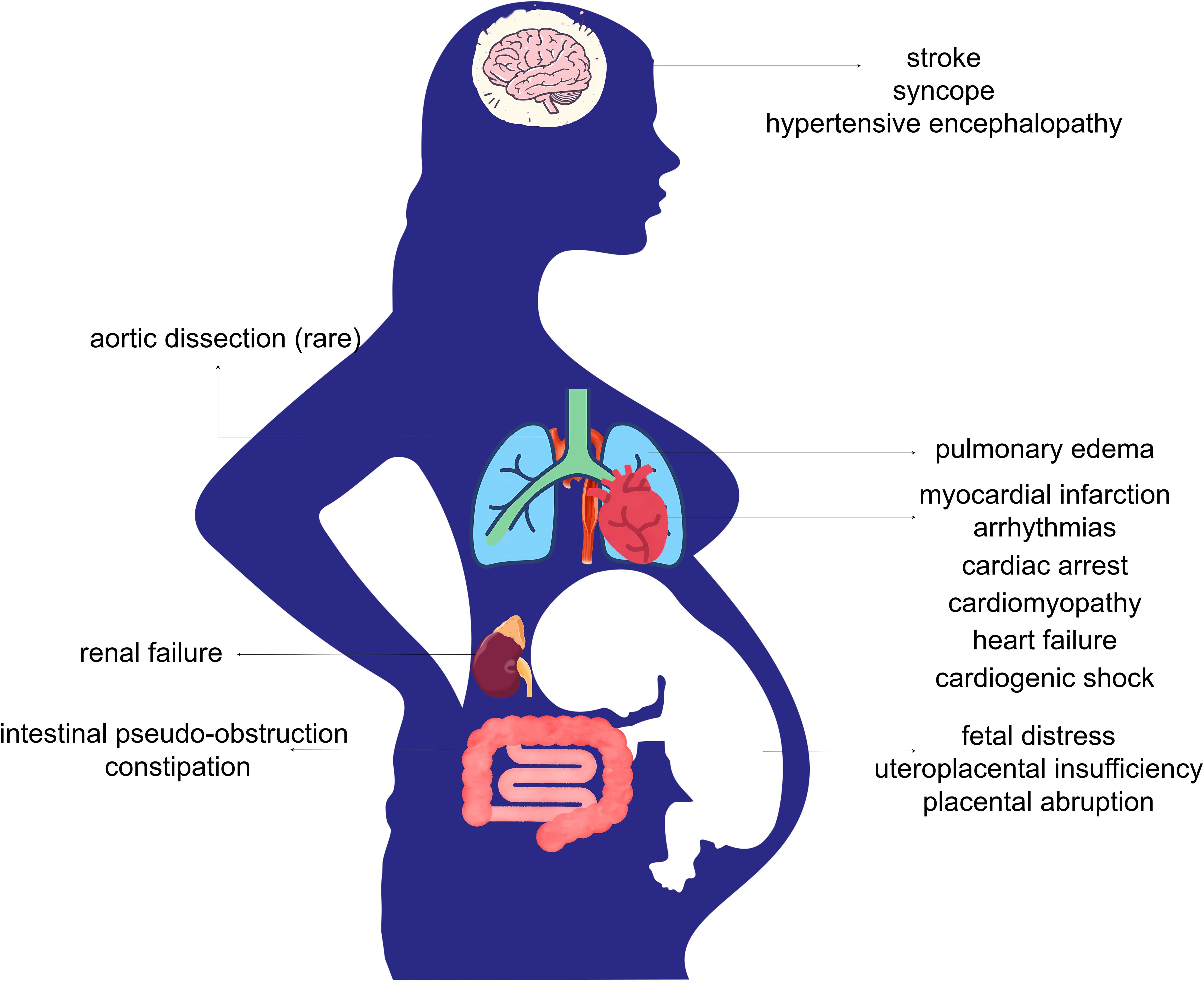
Figure 3. Complications of functional PPGL in pregnancy. Excessive secretion of catecholamines in functional PPGL leads to systemic vasoconstriction, heightened sympathetic activity, hemodynamic instability, and increased cardiac workload. These pathophysiological changes may result in serious maternal complications, including cardiovascular and cerebrovascular events, pulmonary edema, renal failure, and gastrointestinal dysfunction. Fetal complications may arise from impaired placental perfusion, leading to intrauterine fetal distress, uteroplacental insufficiency, and placental abruption.
Excessive catecholamines exert differing effects on mothers and fetuses. In the mother, elevated catecholamine levels cause widespread vascular spasm, intense myocardial contraction, and a hypermetabolic state, leading to potential damage across multiple organ systems that are directly exposed. However, for the fetus, the risks associated with PPGL are primarily linked to maternal placental vasoconstriction (13). The placental barrier protects the fetus from direct exposure to high maternal catecholamine levels. Research evidence shows that the concentrations of norepinephrine, epinephrine, and metanephrine in umbilical cord blood are lower than those in maternal plasma (16, 17). Therefore, placental vasospasm-induced hypoxia, placental insufficiency, and placental abruption represent the primary threats to the fetus (18). The uteroplacental circulation lacks autoregulation it is directly affected by changes in maternal blood flow, indicating that effective control of maternal blood pressure is beneficial for positive fetal health outcomes (13, 16).
Following the diagnosis of PPGL, 3% of pregnant women opted for elective termination, while 4% experienced miscarriage or intrauterine fetal loss between 8 and 37 weeks of gestation (3). Preterm delivery occurred in 26%–30% of cases (3, 11), with some reports indicating rates as high as 45% (6). However, the incidence of small for gestational age (SGA) infants did not significantly differ from that in the general obstetric population, possibly due to timely termination or appropriate perinatal management. The incidence of infant respiratory distress syndrome (IRDS) was 13% among neonates born to mothers with PPGL, substantially higher than the 1.8% observed in the general population (11). In the long term, no significant difference in hospitalization rates was observed between children born to mothers with PPGL and those born to healthy mothers, based on a median follow-up period of 12 years (11).
3.3 Citation analysis and keyword analysis
3.3.1 Co-citation analysis and citation burst analysis
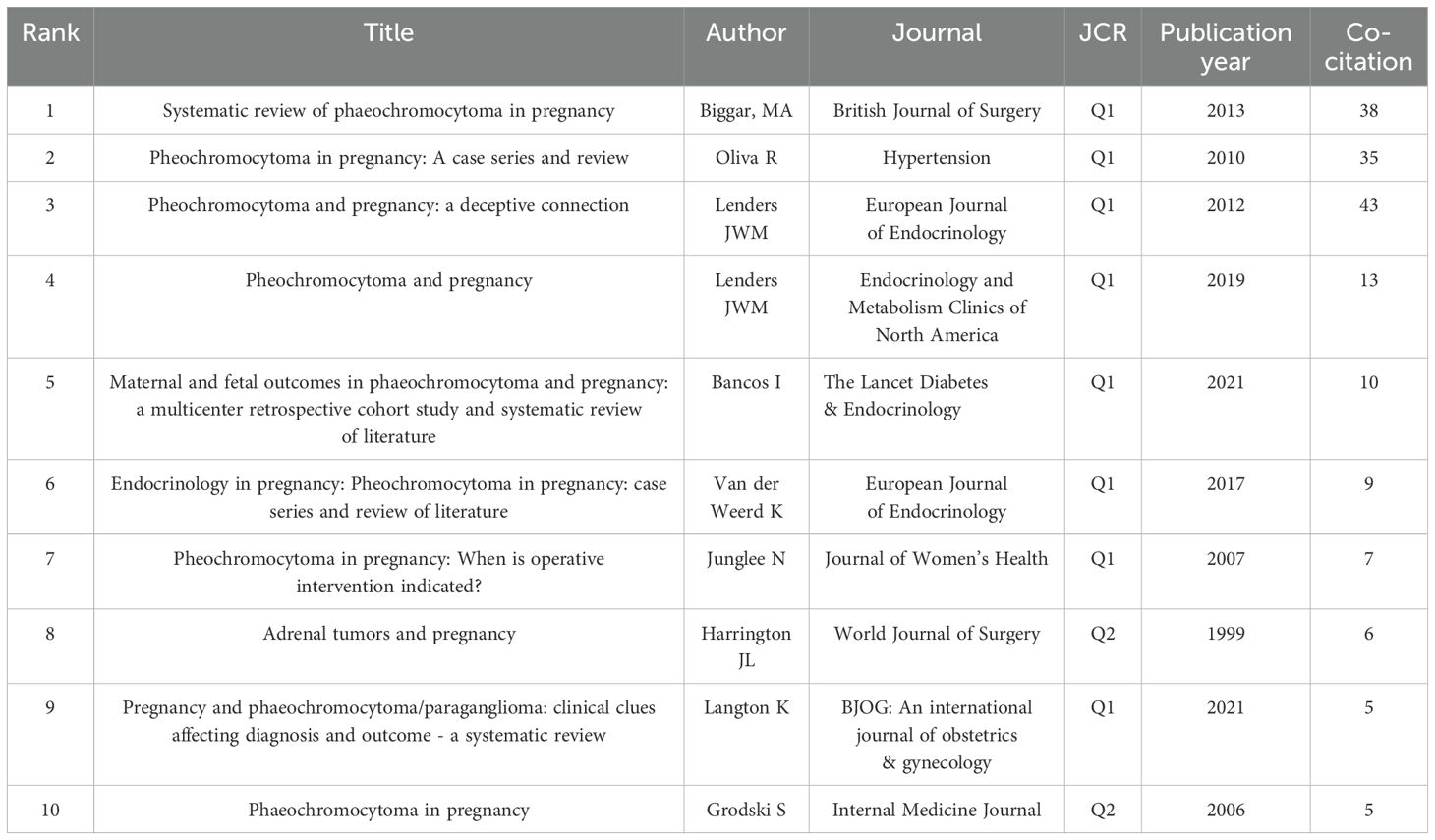
The top 10 co-cited references and their bibliographic details are listed in Table 6. Highly co-cited references represent foundational literature that has shaped the development of this research field. The top three citations are as follows: in 2013, Biggar MA et al. published a paper titled “Systematic review of pheochromocytoma in pregnancy” in the British Journal of Surgery. In 2010, Oliva R et al. published a paper titled “Pheochromocytoma in Pregnancy: A Case Series and Review” in Hypertension. In 2012, Lenders JWM et al. published a paper titled “Pheochromocytoma and pregnancy: a deceptive connection” in the European Journal of Endocrinology.
Figure 4A displays the co-citation reference network generated by CiteSpace. Each node represents a cited reference, with larger nodes indicating higher co-citation frequency. The color of the nodes reflects the publication year, showing the temporal distribution of influential literature. Purple outer rings highlight references with high betweenness centrality, signifying their bridging role across different thematic areas. Edges between nodes indicate co-citation links, where thicker lines reflect stronger intellectual connections between references. Figure 4B illustrates the top 8 references with the strongest citation bursts identified by CiteSpace. The red segments indicate the period during which each reference experienced a significant surge in citations, while the blue lines represent the overall timespan from 1995 to 2024. The burst strength reflects the intensity of the citation increase within a specific period. Among these, the article by Biggar MA (2013) in the British Journal of Surgery exhibited the highest burst strength (strength=11.42, from 2014 to 2018), suggesting that it received a rapid and concentrated increase in scholarly attention during that time. Since 2021, citation bursts have been observed for the articles by Lenders JWM (2019), titled “Pheochromocytoma and Pregnancy” in Endocrinology and Metabolism Clinics of North America, and Bancos I (2021), “Maternal and fetal outcomes in phaeochromocytoma and pregnancy: a multicenter retrospective cohort study and systematic review of literature,” published in The Lancet Diabetes & Endocrinology, indicating a recent surge of academic interest in clinical management strategies and outcome-based research on PPGL in pregnancy.
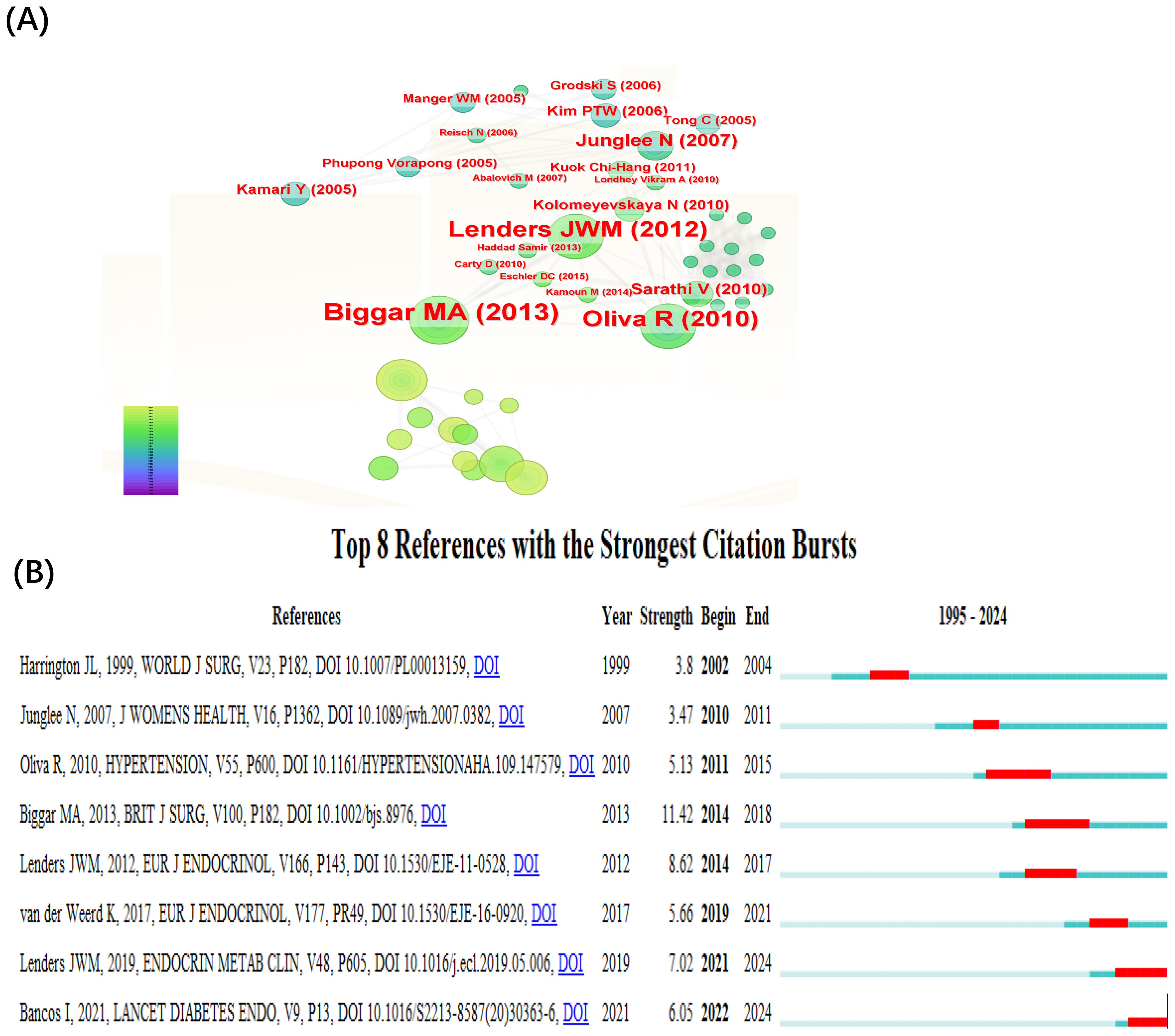
Figure 4. Co-citation reference visualization and citation burst. (A) Co-cited literature interaction. (B) Reference burst analysis.
3.3.2 Keyword analysis
A keyword co-occurrence network was visualized using CiteSpace, with the g-index set to k=25 to enhance the clarity of keyword distribution. The top 10 keywords include pregnancy complications (n = 128, centrality = 0.21), cesarean section (n = 60, centrality = 0.59), management (n = 34, centrality = 0.15), case report (n = 34, centrality = 0.08), pheochromocytoma (n = 33, centrality = 0.18), diagnosis (n = 26, centrality = 0.35), young adult (n=23, centrality = 0.20), nuclear magnetic resonance imaging (n=19, centrality = 0.02), clinical article (n=19, centrality = 0.08), and pregnancy (n=15, centrality = 0.07). Figure 5 presents the visualized keyword network for studies on pregnancy complicated by PPGL from 1995 to 2024. Figure 5A illustrates the keyword co-occurrence network, with node colors ranging from purple (1995) to red (2024), representing temporal evolution. The size of each node reflects the frequency of keyword occurrences, the width of the connecting lines indicates the strength of co-occurrence, and the color of the lines represents the time of co-appearance. Nodes with a purple outer ring represent high burst strength. Figure 5B shows the results of keyword clustering analysis, with representative cluster labels including “ 0 humans”, “ 1 pregnancy complications”, “ 2 management”, “ 3 coronary angiography”, “ 4 cesarean section”, and “ 5 beta blockers”. These cluster labels classify keywords into distinct research themes; clusters with smaller label numbers contain a larger number of keywords. The degree of overlap among cluster labels reflects the thematic relevance and conceptual overlap between different research areas. The modularity Q score of 0.5 and silhouette S score of 0.84 indicate a stable and reasonable clustering structure. Burst analysis of keywords, as shown in Figure 5C, identified ten keywords with significant citation bursts. Among them, the most recent keywords with notable burst strengths were “clinical article” (burst strength = 4.99) and “case series” (burst strength = 4.58). The keyword with the strongest burst strength was “pregnancy complications” (burst strength = 18.49).
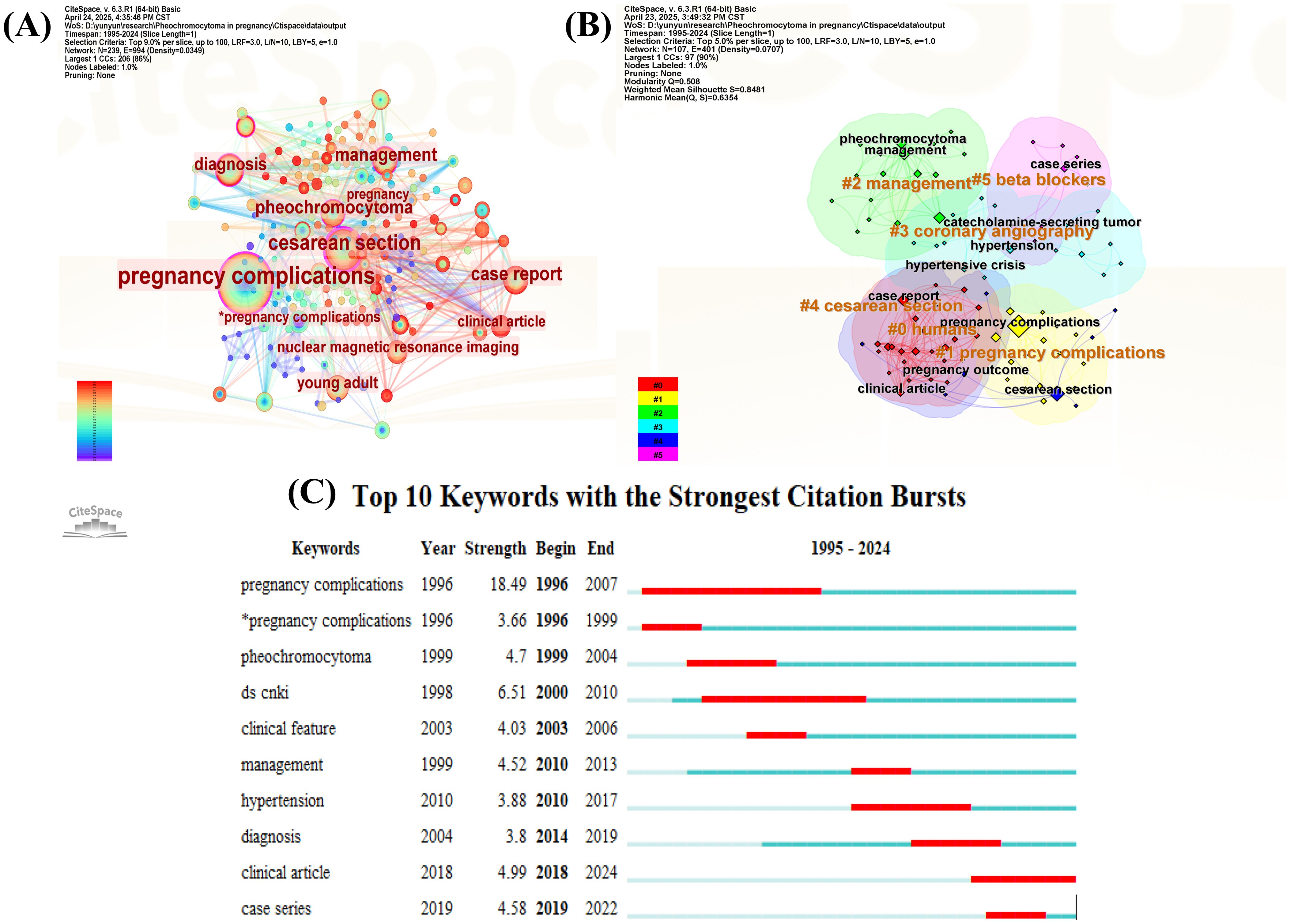
Figure 5. Visual network of keywords in pregnancy complicated by PPGL. (A) Keywords visualization map of the CiteSpace network. (B) Cluster diagram of keywords co-occurrence analysis network. (C) Keywords burst analysis.
4 Discussion
4.1 General information
Research on pregnancy complicated by PPGL remains limited in quantity, with case reports being the predominant type of publication, followed by review articles. There is a lack of strong collaborative networks among publishing countries and institutions, and greater international and inter-institutional collaboration is encouraged in future research. Lenders JWM is a highly influential author in this field, with a primary research focus on the clinical management of pregnancy complicated by PPGL. Endocrinology and obstetrics and gynecology journals are the most preferred publication venues in this area of research.
4.2 Key considerations in the management of pregnancy complicated by PPGL
Highly co-cited references and citation burst analyses across different time periods have revealed the foundational literature and evolving research hotspots in this field. Keyword co-occurrence and clustering analyses further illustrate the major themes and directions of research. Diagnosis and treatment remain central topics in the study of PPGL during pregnancy. As illustrated in Figure 6, prior studies have established a general consensus regarding multidisciplinary care, diagnostic strategies, and antihypertensive management. However, there are still many details in the clinical management strategies that need to be further investigated. These include the sensitivity and specificity of diagnostic tools, additional tests following diagnosis, target blood pressure levels, the necessity of fluid resuscitation, the role of the anesthesiologist, timing and mode of delivery, timing of tumor resection, medication-related precautions, and considerations for breastfeeding in the postpartum period. These key aspects will be discussed in detail in the following subsections.
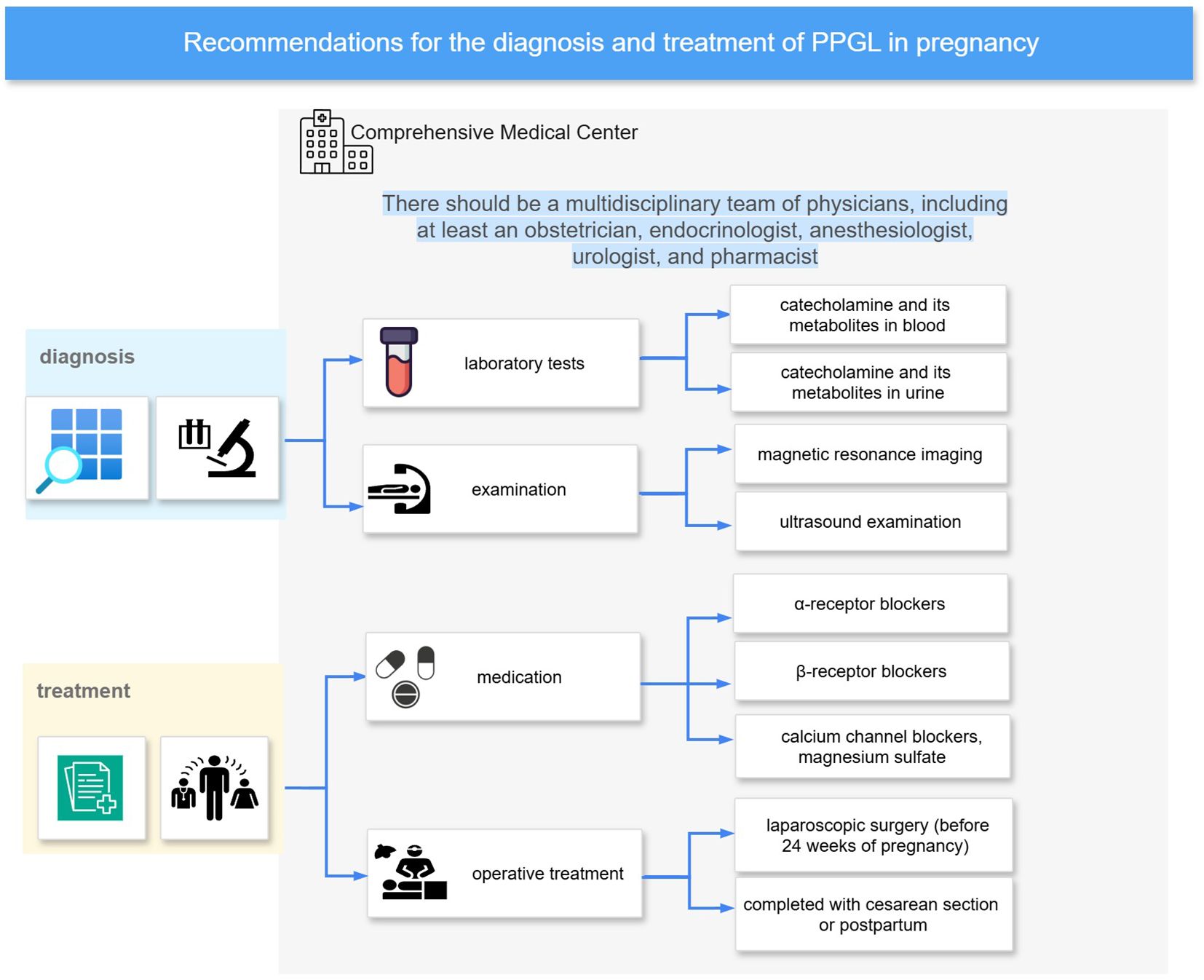
Figure 6. Diagnosis and treatment recommendations. It summarizes a recommended management pathway for PPGL in pregnancy, based on bibliometric analysis and key literature. The approach emphasizes care in Comprehensive Medical Center with multidisciplinary teams. Diagnostic evaluation includes catecholamine testing (blood and urine), MRI, and ultrasonography. Initial pharmacologic management typically involves α-blockers, with β-blockers and calcium channel blockers or magnesium sulfate added as needed.
4.2.1 What is the sensitivity and specificity of the diagnostic methods?
Recommended diagnostic methods for pregnant patients suspected of having PPGL include hormone assays, ultrasound, and magnetic resonance imaging (MRI). MRI has a sensitivity of 90–100% for detecting adrenal tumors in late pregnancy, compared to 54% for ultrasound (14, 19). There is a lack of data regarding the specificity of biochemical testing in pregnant patients with PPGL. Generally, biochemical testing demonstrates relatively high specificity in diagnosing PPGL during pregnancy, as catecholamine and metabolite levels in plasma and urine are typically low in normal pregnancies (15), resulting in a low rate of false positives. When false positives do occur, they are commonly attributed to interference from medications such as methyldopa, labetalol, or tricyclic antidepressants, increased sympathetic activity, or improper sample collection. To minimize the risk of false-positive results, it is recommended that blood samples be obtained after the patient has fasted and remained in a supine resting position for at least 20 minutes (18, 20). In terms of diagnostic sensitivity, urine tests are superior to plasma tests: urinary catecholamines demonstrate a sensitivity of 93%, metanephrines 100%, plasma catecholamines 79%, and plasma metanephrines 100% (14).
Computed tomography (CT) offers the highest diagnostic sensitivity for PPGL; however, due to concerns about fetal radiation exposure, it is often used with caution in pregnant patients (21). In fact, studies have shown that fetal exposure to CT radiation in late pregnancy is well below the threshold considered to be harmful (22–25). At the same time, the potential risk of breast radiation exposure should also be considered when performing CT examinations on pregnant patients. Ionizing radiation is a known risk factor for breast cancer, and this risk is particularly relevant in younger women (26). Abdominal CT does not directly expose the breast to radiation; only a minimal amount of scattered radiation reaches the breast, resulting in a negligibly low dose (27). In contrast, chest CT examinations expose the breast to relatively high doses of radiation, with an average effective dose of approximately 7 mSv, depending on the scanning protocol and technical parameters (26). Therefore, radiologists should optimize scanning protocols to minimize breast radiation exposure while ensuring diagnostic quality. A retrospective cohort study based on health databases from Ontario, Canada (1995–2014), showed that thoracic CT or V/Q scan exposure during pregnancy or the early postpartum period was not associated with an increased short-term risk of breast cancer over a median follow-up of at least 5 years. (28). This result is consistent with the conclusion observed in the general population of women (26). However, evidence regarding long-term effects of chest CT exposure during pregnancy remains limited, and further studies are warranted to confirm these findings. Maternal mortality has been shown to increase significantly in the presence of hypertensive crises (7). Therefore, in cases of unexplained hypertensive emergencies or acute cardiovascular events during pregnancy, CT imaging may be appropriately considered by the attending physician to enable timely diagnosis and intervention (29).
4.2.2 What are the additional tests after diagnosis?
Pregnant patients diagnosed with PPGL may have coexisting endocrine disorders, such as diabetes mellitus and excessive secretion of adrenal corticosteroids (30, 31). It is recommended to evaluate fasting blood glucose and serum cortisol levels following diagnosis. Fasting glucose testing is simple, widely available, and routinely performed during antenatal care. If the initial serum cortisol level is within the normal range, repeated testing is generally unnecessary; however, clinicians should remain alert to any signs or symptoms suggestive of hypercortisolism during pregnancy.
PPGL is well known for its highly variable clinical presentations. In severe cases, excessive catecholamine secretion can cause extensive cardiovascular and multi-organ damage. Reported complications include Takotsubo cardiomyopathy, myocardial infarction, pulmonary edema, cerebrovascular stroke, ischemic bowel obstruction, and acute renal failure (32). In pregnant patients with marked blood pressure fluctuations accompanied by symptoms such as palpitations, chest pain, nausea, vomiting, or dyspnea, clinical evaluation should include cardiac injury biomarkers, B-type natriuretic peptide (BNP), echocardiography, serum creatinine, and urinary microalbumin or albumin levels. Oliva R et al. pointed out in their review that proteinuria may serve as a useful marker to distinguish hypertension induced by pheochromocytoma from that of other pregnancy-related causes (33). However, a case series reported in China found that 3 out of 6 pregnant women with pheochromocytoma exhibited positive urinary albumin results (34). This may be attributed to sustained hypertension damaging the glomerular capillary walls, resulting in increased permeability and subsequent protein leakage into the urine. Therefore, proteinuria may also occur in pregnant patients with PPGL when blood pressure is poorly controlled.
4.2.3 What is the goal of antihypertensive medication?
For functional PPGL, once the diagnosis is confirmed, α-blocker antihypertensive therapy is recommended. β-blockers are introduced only after the initiation of alpha-adrenergic blockade to counteract catecholamine-induced tachyarrhythmias and reflex tachycardia caused by alpha-adrenergic receptor inhibition. The most commonly used α-blockers are phenoxybenzamine and doxazosin (5, 35), and the β-blocker of choice is metoprolol (36). While drug selection and application have been extensively studied, fewer studies have addressed specific antihypertensive targets. ESC/ESH Guidelines for the management of arterial hypertension recommend a target of 140/90 mmHg for antihypertensive treatment during pregnancy (37). Van der Weerd et al. suggest in their literature review that for chronic hypertension without signs of end-organ damage, the target blood pressure during pregnancy should be < 150/100 mmHg with a diastolic pressure > 80 mmHg. If signs of end-organ damage are present, the target should be lowered to < 140/90 mmHg (5). Blood Pressure (BP) control strategies were last discussed in 2022, when Lucinda et al. proposed lower systolic BP targets of 90-110 mmHg and the heart rate should average less than 90 beats per minute (36). There is no evidence to support that less tight or tight BP control is better for pregnant women and fetuses. Keeping blood pressure at a stable value in pregnant patients with PPGL is challenging due to the enlarging uterus and fetal movements potentially compressing the tumor. Moreover, PPGLs express luteinizing hormone/chorionic gonadotropin (LHCG) receptors, and pregnancy may trigger catecholamine surges and hypertensive crises through hCG-mediated epinephrine stimulation (38). Although there are differences in antihypertensive goals, if BP is stabilized at the current dose and an increase in dose would result in orthostatic hypotension, it is recommended that the current dose be maintained (36, 39).
4.2.4 Is rehydration therapy warranted?
Blood volume expansion through rehydration is a common protocol for perioperative preparation in patients with PPGL. However, during pregnancy, maternal blood volume increases by at least 30% compared to pre-pregnancy levels, peaking at 32–34 weeks. This may raise controversy regarding the necessity of rehydration therapy in managing PPGL during pregnancy. In the systematic review by Lucinda et al., they emphasize increasing fluid and sodium intake when starting an alpha-adrenergic blocker (36). Although pregnancy increases maternal blood volume, relative hypovolemia in PPGL is well recognized. Vasodilation and hypotension after alpha-blocker administration can further exacerbate volume deprivation; therefore, careful and balanced fluid management is advisable.
4.2.5 What can anesthesiologists do to help?
Cesarean section is currently the primary mode of delivery (3, 6), which can be performed under intrathecal anesthesia, general anesthesia, or combined anesthesia techniques. In general, the preferred form of anesthesia for cesarean delivery is intrathecal anesthesia, which avoids the effects of general anesthesia drugs on the fetus. In uncomplicated parturients, general anesthesia is typically reserved for emergency situations or when neuraxial anesthesia is contraindicated (40). It is recommended that women with catecholamine levels exceeding 10 times the upper limit of normal and tumors located in the pelvic-abdominal cavity be administered general anesthesia during cesarean section. For these patients, general anesthesia can provide better circulatory control than intrathecal anesthesia and allows for a timely response to malignant hypertension and refractory hypotension. The standard general anesthesia regimen is propofol, rocuronium, and remifentanil for induction, with inhalation anesthetics for maintenance. The concentration of inhaled agents is kept low due to their uterine relaxation effects, which may compromise uterine tone. General anesthesia combined with intrathecal anesthesia can combine the advantages of both, guaranteeing good analgesia and reducing the dose of general anesthetic drugs, and is recommended for high-risk women with PPGL who are undergoing non-emergency surgery.
There are also cases where vaginal delivery has been safely performed with the involvement of an anesthesiologist (41–44). For women with indications for vaginal delivery, it is recommended that vaginal delivery be attempted with the participation of an anesthesiologist. Anesthesiologists can provide analgesia during labor, including intrathecal analgesia and intravenous analgesia, and effective analgesia can reduce the occurrence of malignant hypertension. Additionally, anesthesiologist can monitor and manage hypertensive crises with short-acting antihypertensive drugs.
4.2.6 When should labor be induced?
In non-emergency situations—excluding conditions such as fetal distress or maternal life-threatening events—it is advisable to prolong pregnancy beyond 34 weeks if fetal growth and development are deemed normal, as neonatal survival improves with advancing gestational age. In China, the average survival rate for infants born before 28 weeks is 62.3% (45), increasing to 79.1% at 30 weeks and 85.8% at 31 weeks (46). Among the general obstetric population, antenatal corticosteroid administration has been shown to improve outcomes in preterm neonates (47). However, the use of corticosteroids for fetal lung maturation in pregnant patients with PPGL remains controversial. Some experts argue that corticosteroids may stimulate tumor secretion of dopamine, potentially triggering a pheochromocytoma crisis; therefore, their use is generally avoided in this population. Nevertheless, several case reports have documented successful corticosteroid administration in PPGL patients with threatened preterm labor (48–51). Moreover, since PPGL may coexist with Cushing’s syndrome (30, 52), maternal hypercortisolism should be excluded prior to corticosteroid initiation. Therefore, in non-emergency situations, antenatal corticosteroids may be cautiously administered in pregnant patients with PPGL at risk of preterm delivery before 34 weeks, provided that maternal blood pressure is closely monitored throughout the course of treatment.
4.2.7 Cesarean section or vaginal delivery?
A Swedish population-based survey conducted between 1973 and 2015 reported that 57% of mothers with PPGL delivered vaginally (11). In contrast, most previous studies have indicated that the rate of cesarean section in patients with PPGL during pregnancy is generally higher than that of vaginal delivery (3–6, 14). This pattern likely reflects clinical concerns about the elevated risk of adverse outcomes associated with PPGL, which may influence decisions regarding the mode of delivery. However, current evidence suggests that the choice of delivery mode is not independently associated with maternal or neonatal outcomes (3, 11). In a systematic review by Langton et al., the maternal mortality rate was higher in vaginal deliveries (1 in 3) than in cesarean sections (1 in 12) (14); however, this conclusion was based on a limited sample size and requires confirmation through studies with larger cohorts.
4.2.8 When to perform a tumor resection?
Surgical resection at the time of delivery or postpartum is the most commonly adopted strategy, accounting for approximately 70% of cases (3, 6, 11, 12). For patients diagnosed before 24 weeks of gestation with hormonally active PPGLs that are poorly controlled with medical therapy, antenatal tumor resection may be considered, typically between 14 and 23 weeks (5). Data suggest that patients who undergo antenatal tumor removal are more likely to achieve full-term gestation, and their neonates experience a lower incidence of low neonatal Apgar scores (defined by an Apgar score of <7) (6). Adverse maternal or fetal outcomes have been reported in approximately 3.8% (3/78) to 4.5% (4/88) of antenatal surgical cases, although it remains unclear whether antenatal surgery is conclusively associated with improved overall outcomes (3, 6). If surgical resection of the tumor is required, preoperative preparation with α-adrenergic blockade is essential (3, 18, 21).
4.2.9 What are the precautions for drug use?
The use of beta-adrenergic blockers without prior alpha-adrenergic blockade is strictly contraindicated, as blocking peripheral beta-mediated vasodilation while unopposed alpha-adrenergic stimulation persists can result in a paradoxical elevation of blood pressure (53). Labetalol, a first-line agent for pregnancy-induced hypertension with an alpha-to-beta blockade ratio of approximately 1:3, is not recommended for use as monotherapy in pregnant patients with PPGL (53, 54). Phenoxybenzamine, a frequently used non-selective alpha-blocker, can cross the placenta and may cause neonatal hypotension and respiratory depression, with a reported fetal–maternal plasma accumulation ratio of approximately 1.6:1 (51, 55, 56). Doxazosin also crosses the placenta, but fetal plasma concentrations are lower than maternal levels, with a fetal-to-maternal concentration ratio of 0.8, indicating a relatively low risk of neonatal hypotension (17). To date, no cases of neonatal hypotension associated with doxazosin have been reported, and it is considered a safe alternative for use in pregnant patients with PPGL (5, 17, 36).
Medications that may stimulate catecholamine release from the tumor, trigger histamine release, or produce sympathomimetic effects should be avoided. These include opioid analgesics such as morphine and meperidine, antiemetics like metoclopramide, anesthetic agents such as thiopental and ketamine, neuromuscular blockers including mivacurium and succinylcholine, as well as sympathomimetic drugs such as ephedrine (54, 57, 58).
4.2.10 Can postpartum mothers breastfeed?
Following tumor resection, plasma catecholamine and metabolite levels decrease rapidly (59). Patients who undergo tumor removal prior to delivery may breastfeed without restriction. In cases where cesarean section is combined with tumor resection, breastfeeding can be initiated once the final dose of medication has been sufficiently metabolized. For mothers requiring continued postpartum antihypertensive therapy without prior tumor removal, certain medications may be excreted into breast milk. Therefore, breastfeeding is generally not recommended or should only proceed under close clinical supervision. Limited evidence suggests that only 0.1% or less of doxazosin is transferred into breast milk (60), which is well below the commonly accepted 10% threshold for infant relative dose. However, this finding is based on isolated case reports and requires further validation. A case report by Aplin SC et al. (56) documented a mother–infant pair in which oral phenoxybenzamine was continued during breastfeeding without the occurrence of neonatal hypotension, suggesting a potential safety profile of phenoxybenzamine in lactating patients. Nevertheless, this conclusion is drawn from a single case and requires confirmation in larger cohorts.
4.3 Genetic studies on PPGL in pregnant patients
Patients carrying certain susceptibility genes have an increased risk of developing PPGL. Statistics show that approximately 40% of patients with PPGL have genetic mutations (61). The identified susceptibility genes for PPGL include NF1, RET, VHL, succinate dehydrogenase subunits (SDHA, SDHB, SDHC, SDHD), succinate dehydrogenase complex assembly factor (SDHAF2), TMEM127, MAX, EGLN1/PHD2, EGLN2/PHD1, KIF1β, IDH1, HIF2α, FH, and ATRX (35, 39, 62, 63). Mutations detected in pregnant patients include NF1 (64), RET (65–67), VHL, succinate dehydrogenase subunits (SDHA, SDHB, SDHC, SDHD) (3, 42, 68–70), MAX, FH, CDKN2B (3), and HIF2α (71). Associated endocrine disorders include multiple endocrine neoplasia type 2A (MEN2A) (65–67), von Hippel–Lindau (VHL) disease (72, 73), neurofibromatosis type 1 (NF1) (11, 64, 74–77), and Carney Triad (3).
Individuals with a past history of PPGL and/or heritable PPGL syndrome are considered high-risk and should undergo genetic counseling and tumor screening prior to conception (4, 14, 78), a process typically overseen by endocrinologists. Metastatic or invasive forms of PPGL are generally recognized as high-risk conditions, and maternal deaths have been reported in such cases (79). However, a large retrospective study by Bancos I et al. (3), which included 249 pregnancies complicated by PPGL, found that neither metastatic disease nor the presence of pathogenic germline mutations was associated with an increased risk of maternal or fetal complications or mortality. This investigation represents the largest cohort study to date on PPGL in pregnancy, and its findings challenge previous assumptions—possibly due to the exclusion of case reports and small case series with fewer than five patients, which may have introduced selection bias into earlier literature.
5 Limitations
Due to the rarity of PPGL in pregnancy, there is a lack of randomized controlled trials in this field. Large-scale cohort studies and case-control studies are also limited. Currently, the main body of evidence is derived from case reports, case series, systematic reviews, and a small number of observational studies. This concentration on lower levels of evidence may introduce publication bias and limit the overall strength and generalizability of the findings.
This study performed a bibliometric analysis of literature published between 1990 and 2024. Publications from 2025 were excluded due to insufficient time for citation accumulation. Additionally, non-English articles were excluded from the analysis, which may have led to the omission of relevant studies published in other languages.
6 Conclusion
This study represents the first bibliometric analysis specifically focusing on pregnancy complicated by PPGL. Through bibliometric and visual analytic methods, it highlights publication trends, current research landscapes, major hotspots, and emerging directions in this field. Future research should prioritize international collaboration, refine diagnostic and therapeutic strategies, and investigate the factors contributing to adverse maternal and fetal outcomes to provide more robust evidence-based support for clinical decision-making in this high-risk population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SD: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. FL: Data curation, Formal analysis, Visualization, Validation, Writing – review & editing. JT: Data curation, Supervision, Writing – review & editing. CS: Data curation, Supervision, Writing – review & editing. YZ: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Senior Java Engineer Yunsen Yang, for his meticulous data correction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1557376/full#supplementary-material
References
1. Neumann HPH, Young WF Jr, and Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. (2019) 381:552–65. doi: 10.1056/NEJMra1806651
2. Schenker JG and Chowers I. Pheochromocytoma and pregnancy. Rev 89 cases Obstet Gynecol Surv. (1971) 26:739–47. doi: 10.1097/00006254-197111000-00001
3. Bancos I, Atkinson E, Eng C, Young WF, Neumann HPH, and Int Pheochromocytoma Pregnancy S. Maternal and fetal outcomes in phaeochromocytoma and pregnancy: a multicentre retrospective cohort study and systematic review of literature. Lancet Diabetes Endocrinol. (2021) 9:13–21. doi: 10.1016/s2213-8587(20)30363-6
4. Clifton-Bligh RJ. The diagnosis and management of pheochromocytoma and paraganglioma during pregnancy. Rev Endocr Metab Disord. (2023) 24:49–56. doi: 10.1007/s11154-022-09773-2
5. Van der Weerd K, van Noord C, Loeve M, Knapen M, Visser W, De Herder WW, et al. Endocrinology in pregnancy: pheochromocytoma in pregnancy: case series and review of literature. Eur J Endocrinol. (2017) 177:R49–58. doi: 10.1530/eje-16-0920
6. Langton K, Tufton N, Akker S, Deinum J, Eisenhofer G, Timmers H, et al. Pregnancy and phaeochromocytoma/paraganglioma: clinical clues affecting diagnosis and outcome - a systematic review. Bjog. (2021) 128:1264–72. doi: 10.1111/1471-0528.16635
7. Biggar MA and Lennard TWJ. Systematic review of phaeochromocytoma in pregnancy. Br J Surg. (2013) 100:182–90. doi: 10.1002/bjs.8976
8. Turcu AF and Lacroix A. Adrenal disorders in pregnancy. Rev Endocr Metab Disord. (2023) 24:1–3. doi: 10.1007/s11154-022-09779-w
9. Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
10. Harrington JL, Farley DR, van Heerden JA, and Ramin KD. Adrenal tumors and pregnancy. World J Surg. (1999) 23:182–86. doi: 10.1007/pl00013159
11. Gunnesson L, Ragnarsson O, Nilsson M, Sengpiel V, Elfvin A, Elias E, et al. Maternal pheochromocytoma and childbirth in Sweden 1973–2015: a population-based study on short and long-term outcome. Endocrine. (2024) 84:720–26. doi: 10.1007/s12020-024-03749-9
12. Quartermaine G, Lambert K, Rees K, Seed PT, Dhanjal MK, Knight M, et al. Hormone-secreting adrenal tumours cause severe hypertension and high rates of poor pregnancy outcome; a UK Obstetric Surveillance System study with case control comparisons. Bjog. (2018) 125:719–27. doi: 10.1111/1471-0528.14918
13. Iijima S. Impact of maternal pheochromocytoma on the fetus and neonate. Gynecol Endocrinol. (2019) 35:280–86. doi: 10.1080/09513590.2018.1540568
14. Sarathi V, Lila AR, Bandgar TR, Menon PS, and Shah NS. Pheochromocytoma and pregnancy: a rare but dangerous combination. Endocr Pract. (2010) 16:300–9. doi: 10.4158/EP09191.RA
15. Ahlawat SK, Jain S, Kumari S, Varma S, and Sharma BK. Pheochromocytoma associated with pregnancy: case report and review of the literature. Obstet Gynecol Surv. (1999) 54:728–37. doi: 10.1097/00006254-199911000-00025
16. Dahia PLM, Hayashida CY, Strunz C, Abelin N, and Toledo SPA. Low cord blood levels of catecholamine from a newborn of a pheochromocytoma patient. Eur J Endocrinol. (1994) 130:217–19. doi: 10.1530/eje.0.1300217
17. Versmissen J, Koch BC, Roofthooft DW, Ten Bosch-Dijksman W, Van den Meiracker AH, Hanff LM, et al. Doxazosin treatment of phaeochromocytoma during pregnancy: placental transfer and disposition in breast milk. Br J Clin Pharmacol. (2016) 82:568–9. doi: 10.1111/bcp.12981
18. Lenders JWM. Pheochromocytoma and pregnancy: a deceptive connection. Eur J Endocrinol. (2012) 166:143–50. doi: 10.1530/eje-11-0528
19. Mechcatie. In pregnancy, tumor is often mistaken for preeclampsia. Clin Endocrinol News. (2006) 1:21. doi: 10.1016/s1558-0164(06)70021-9
20. Eisenhofer G and Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem. (2014) 60:1486–99. doi: 10.1373/clinchem.2014.224832
21. Mannelli M and Bemporad D. Diagnosis and management of pheochromocytoma during pregnancy. J Endocrinol Invest. (2002) 25:567–71. doi: 10.1007/bf03345503
22. Sensakovic WF, Royall I, Hough M, Potrebko P, Grekoski V, and Vicenti R. Fetal dosimetry at CT: a primer. Radiographics. (2020) 40:1061–70. doi: 10.1148/rg.2020190166
23. Burton CS, Frey K, Fahey F, Kaminski MS, Brown RKJ, Pohlen JM, et al. Fetal dose from PET and CT in pregnant patients. J Nucl Med. (2023) 64:312–19. doi: 10.2967/jnumed.122.263959
24. Xie T, Poletti PA, Platon A, Becker CD, and Zaidi H. Assessment of CT dose to the fetus and pregnant female patient using patient-specific computational models. Eur Radiol. (2018) 28:1054–65. doi: 10.1007/s00330-017-5000-z
25. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee opinion No. 656: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. (2016) 127:e75–80. doi: 10.1097/AOG.0000000000001316
26. Lahham A, AL H, and Kameel S. Estimation of female radiation doses and breast cancer risk from chest CT examinations. Radiat Prot Dosimetry. (2018) 179:303–09. doi: 10.1093/rpd/ncx283
27. Huda W, Atherton JV, Ware DE, and Cumming WA. An approach for the estimation of effective radiation dose at CT in pediatric patients. Radiology. (1997) 203:417–22. doi: 10.1148/radiology.203.2.9114097
28. Burton KR, Park AL, Fralick M, and Ray JG. Risk of early-onset breast cancer among women exposed to thoracic computed tomography in pregnancy or early postpartum. J Thromb Haemost. (2018) 16:876–85. doi: 10.1111/jth.13980
29. Roseland ME, Zhang M, and Caoili EM. Imaging of pregnant and lactating patients with suspected adrenal disorders. Rev Endocr Metab Disord. (2023) 24:97–106. doi: 10.1007/s11154-022-09733-w
30. Finkenstedt G, Gasser RW, Höfle G, Lhotta K, Kölle D, Gschwendtner A, et al. Pheochromocytoma and sub-clinical Cushing’s syndrome during pregnancy: diagnosis, medical pre-treatment and cure by laparoscopic unilateral adrenalectomy. J Endocrinol Invest. (1999) 22:551–57. doi: 10.1007/bf03343608
31. Sherer DM, Dalloul M, Salame G, Kalidas P, Zinn HL, and Abulafia O. Gestational diabetes leading to diagnosis and management of multiple endocrine neoplasia type 2a. Obstet Gynecol. (2010) 115:455–57. doi: 10.1097/AOG.0b013e3181c3cace
32. Riester A, Weismann D, Quinkler M, Lichtenauer UD, Sommerey S, Halbritter R, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. (2015) 173:757–64. doi: 10.1530/eje-15-0483
33. Oliva R, Angelos P, Kaplan E, and Bakris G. Pheochromocytoma in pregnancy a case series and review. Hypertension. (2010) 55:600–06. doi: 10.1161/hypertensionaha.109.147579
34. Song Y, Liu J, Li H, Zeng Z, Bian X, and Wang S. Outcomes of concurrent caesarean delivery and pheochromocytoma resection in late pregnancy. Intern Med J. (2013) 43:588–91. doi: 10.1111/imj.12118
35. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2014) 99:1915–42. doi: 10.1210/jc.2014-1498
36. Gruber LM, Young WF, and Bancos I. Pheochromocytoma and paraganglioma in pregnancy: a new era. Curr Cardiol Rep. (2021) 23:8. doi: 10.1007/s11886-021-01485-4
37. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041. doi: 10.1097/HJH.0000000000001940
38. Lopez AG, Duparc C, Renouf S, Machevin E, Le Guillou V, Sabourin JC, et al. Expression of LHCGR in pheochromocytomas unveils an endocrine mechanism connecting pregnancy and epinephrine overproduction. Hypertension. (2022) 79:1006–16. doi: 10.1161/hypertensionaha.121.18864
39. Prete A, Paragliola RM, Salvatori R, and Corsello SM. Management of catecholamine-secreting tumors in pregnancy: a review. Endocr Pract. (2016) 22:357–70. doi: 10.4158/ep151009.Ra
40. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin No. 209: obstetric analgesia and anesthesia. Obstet Gynecol. (2019) 133:e208–e25. doi: 10.1097/AOG.0000000000003132
41. Wing LA, Conaglen JV, Meyer-Rochow GY, and Elston MS. Paraganglioma in pregnancy: a case series and review of the literature. J Clin Endocrinol Metab. (2015) 100:3202–09. doi: 10.1210/jc.2015-2122
42. Junglee N, Harries SE, Davies N, Scott-Coombes D, Scanlon MF, and Rees DA. Pheochromocytoma in pregnancy: when is operative intervention indicated? J Womens Health. (2007) 16:1362–65. doi: 10.1089/jwh.2007.0382
43. Strachan AN, Claydon P, and Caunt JA. Phaeochromocytoma diagnosed during labour. Br J Anaesth. (2001) 86:288–89. doi: 10.1093/bja/85.4.635
44. Kariya N, Nishi S, Hosono Y, Hamaoka N, Nishikawa K, and Asada A. Cesarean section at 28 weeks' gestation with resection of pheochromocytoma: perioperative antihypertensive management. J Clin Anesth. (2005) 17(4):296–9. doi: 10.1016/j.jclinane.2004.07.006
45. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Netw Open. (2021) 4:e219382. doi: 10.1001/jamanetworkopen.2021.9382
46. Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. (2016) 16:174. doi: 10.1186/s12887-016-0716-5
47. Roberts D, Brown J, Medley N, and Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2017) 3:CD004454. doi: 10.1002/14651858.CD004454.pub3
48. Duquesnay C, Espiard S, Cardot-Bauters C, Carnaille B, Gonzalez M, Jourdain M, et al. Pheochromocytomas and paragangliomas in pregnancy: about four cases and key messages on management. Gynecol Obstet Fertil Senol. (2021) 49:881–88. doi: 10.1016/j.gofs.2021.04.012
49. Giampaolino P, Della Corte L, Formisano C, Cuomo L, Maurea S, Romeo V, et al. Successful management of a third-trimester pregnancy complicated by pheochromocytoma: case report. Gynecol Endocrinol. (2018) 34:1016–18. doi: 10.1080/09513590.2018.1480712
50. Malinowski AK, Maxwell C, Sermer M, Rubin B, Gandhi S, and Silversides CK. Pheochromocytoma in a pregnant woman with prior traumatic aortic injury. Obstet Gynecol. (2015) 126:1089–94. doi: 10.1097/aog.0000000000000909
51. Kothari A, Bethune M, Manwaring J, Astley N, and Wallace E. Massive bilateral phaeochromocytomas in association with Von Hippel Lindau syndrome in pregnancy. Aust N Z J Obstet Gynaecol. (1999) 39:381–4. doi: 10.1111/j.1479-828x.1999.tb03425.x
52. Langton K, Gruber M, Masjkur J, Steenblock C, Peitzsch M, Meinel J, et al. Hypertensive crisis in pregnancy due to a metamorphosing pheochromocytoma with postdelivery Cushing’s syndrome. Gynecol Endocrinol. (2018) 34:20–4. doi: 10.1080/09513590.2017.1379497
53. Mazza A, Armigliato M, Marzola MC, Schiavon L, Montemurro D, Vescovo G, et al. Anti-hypertensive treatment in pheochromocytoma and paraganglioma: current management and therapeutic features. Endocrine. (2014) 45:469–78. doi: 10.1007/s12020-013-0007-y
54. Negro A, Verzicco I, Tedeschi S, Santi R, Palladini B, Calvi A, et al. Unrecognised pheochromocytoma in pregnancy discovered through metoclopramide-triggered hypertensive emergency. Blood Pressure. (2021) 30:322–26. doi: 10.1080/08037051.2021.1945428
55. Santeiro ML, Stromquist C, and Wyble L. Phenoxybenzamine placental transfer during the third trimester. Ann Pharmacother. (1996) 30:1249–51. doi: 10.1177/106002809603001108
56. Aplin SC, Yee KF, and Cole MJ. Neonatal effects of long-term maternal phenoxybenzamine therapy. Anesthesiology. (2004) 100:1608–10. doi: 10.1097/00000542-200406000-00039
57. Pacu I, Zygouropoulos N, Furau CG, Navolan D, Tit DM, Ionescu CA, et al. Pheochromocytoma as a rare hypertensive complication rarely associated with pregnancy: diagnostic difficulties (review). Exp Ther Med. (2021) 22:7. doi: 10.3892/etm.2021.10780
58. Takai Y, Seki H, and Kinoshita K. Pheochromocytoma in pregnancy manifesting hypertensive crisis induced by metoclopramide. Int J Gynaecol Obstet. (1997) 59:133–37. doi: 10.1016/S0020-7292(97)00203-8
59. Wolf A, Goretzki PE, Röhrborn A, Feldkamp J, Simon D, Scherbaum WA, et al. Pheochromocytoma during pregnancy: laparoscopic and conventional surgical treatment of two cases. Exp Clin Endocrinol Diabet. (2004) 112:98–101. doi: 10.1055/s-2004-815764
60. Jensen BP, Dalrymple JM, and Begg EJ. Transfer of doxazosin into breast milk. J Hum Lact. (2013) 29:150–3. doi: 10.1177/0890334412473203
61. Fishbein L, Merrill S, Fraker DL, Cohen DL, and Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. (2013) 20:1444–50. doi: 10.1245/s10434-013-2942-5
62. Favier J, Amar L, and Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. (2015) 11:101–11. doi: 10.1038/nrendo.2014.188
63. Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, et al. Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncol. (2017) 3:1204–12. doi: 10.1001/jamaoncol.2017.0223
64. Inkollu S, Faizi N, and Kohlenberg J. Pheochromocytoma discovery during pregnancy leads to neurofibromatosis diagnosis. JCEM Case Rep. (2024) 2(4):luae032. doi: 10.1210/jcemcr/luae032
65. Sarathi V, Bandgar TR, Menon PS, and Shah NS. Pheochromocytoma and medullary thyroid carcinoma in a pregnant Multiple endocrine neoplasia-2A patient. Gynecol Endocrinol. (2011) 27:533–35. doi: 10.3109/09513590.2010.507285
66. Wattanachanya L, Bunworasate U, Plengpanich W, Houngngam N, Buranasupkajorn P, Sunthornyothin S, et al. Bilateral pheochromocytoma during the postpartum period. Arch Gynecol Obstet. (2009) 280:1055–58. doi: 10.1007/s00404-009-1057-5
67. Frayssinet C, Vezzosi D, Huyghe E, Lorenzini F, Bennet A, and Caron P. Retroperitoneal laparoscopic adrenalectomy in a pregnant woman presenting MEN2a with a pheochromocytoma: case report and review of the literature. Ann Endocrinol. (2008) 69:53–7. doi: 10.1016/j.ando.2007.10.005
68. Kolomeyevskaya N, Blazo M, van den Veyver I, Strehlow S, and Aagaard-Tillery KM. Pheochromocytoma and von hippel-lindau in pregnancy. Am J Perinatol. (2010) 27:257–63. doi: 10.1055/s-0029-1239489
69. Ganguly S, LeBeau S, Pierce K, Ramanathan R, and Salata R. Multiple paragangliomas in a pregnant patient with a succinate dehydrogenase B mutation. Postgrad Med. (2010) 122:46–50. doi: 10.3810/pgm.2010.11.2222
70. Salazar-Vega JL, Levin G, Sansó G, Vieites A, Gómez R, and Barontini M. Pheochromocytoma associated with pregnancy: unexpected favourable outcome in patients diagnosed after delivery. J Hypertens. (2014) 32:1458–63. doi: 10.1097/hjh.0000000000000215
71. Abdullah AE, Guerin C, Annebarlier AI, Barlier A, Battini S, Pertuit M, et al. Paraganglioma of the organ of Zuckerkandl associated with a somatic HIF2α mutation: a case report. Oncol Lett. (2017) 13:1083–86. doi: 10.3892/ol.2017.5599
72. Frantzen C, Kruizinga RC, van Asselt SJ, Zonnenberg BA, Lenders JWM, de Herder WW, et al. Pregnancy-related hemangioblastoma progression and complications in Von Hippel-Lindau disease. Neurology. (2012) 79:793–96. doi: 10.1212/WNL.0b013e3182661f3c
73. Donatini G, Kraimps JL, Caillard C, Mirallie E, Pierre F, De Calan L, et al. Pheochromocytoma diagnosed during pregnancy: lessons learned from a series of ten patients. Surg Endosc. (2018) 32:3890–900. doi: 10.1007/s00464-018-6128-x
74. Cecchi R, Frati P, Capri O, and Cipolloni L. A rare case of sudden death due to hypotension during cesarean section in a woman suffering from pheochromocytoma and neurofibromatosis. J Forensic Sci. (2013) 58:1636–39. doi: 10.1111/1556-4029.12279
75. Remón-Ruiz P, Aliaga-Verdugo A, and Guerrero-Vázquez R. Pheochromocytoma in neurofibromatosis type 1 during pregnancy. Gynecol Endocrinol. (2017) 33:93–5. doi: 10.1080/09513590.2016.1254181
76. Strauss S, Pansky M, and Lewinsohn G. Hemorrhagic pheochromocytoma in a pregnant patient with neurofibromatosis. Sonographic appearance J Ultrasound Med. (1990) 9:165–67. doi: 10.7863/jum.1990.9.3.165
77. Takahashi N, Nishijima K, Orisakama M, Tsuyoshi H, Kurokawa T, Kato K, et al. Amniotic fluid embolism triggered by hypertensive crisis due to undiagnosed pheochromocytoma in a pregnant subject with neurofibromatosis type 1. AACE Clin Case Rep. (2015) 1:178–81. doi: 10.4158/EP14108.CR
78. Araujo-Castro M, Chavez LN, Lorca AM, Molina-Cerrillo J, Alonso-Gordoa T, and Pascual-Corrales E. Special situations in pheochromocytomas and paragangliomas: pregnancy, metastatic disease, and cyanotic congenital heart diseases. Clin Exper Med. (2022) 22:359–70. doi: 10.1007/s10238-021-00763-3
Keywords: pheochromocytoma, pregnancy, bibliometric, CiteSpace, paraganglioma
Citation: Deng S, Li F, Tian J, Sun C and Zhang Y (2025) What should we focus on in pregnancy complicated by pheochromocytoma? a bibliometric analysis (1990-2024). Front. Oncol. 15:1557376. doi: 10.3389/fonc.2025.1557376
Received: 08 January 2025; Accepted: 24 June 2025;
Published: 17 July 2025.
Edited by:
Karin Windsperger, Medical University of Vienna, AustriaReviewed by:
Andrea Goldmann, Cantonal Hospital Winterthur, SwitzerlandEva Karner, Medical University of Vienna, Austria
Copyright © 2025 Deng, Li, Tian, Sun and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqing Zhang, ODAwMzg1QGhvc3BpdGFsLmNxbXUuZWR1LmNu
 Shiyun Deng1
Shiyun Deng1 Fan Li1
Fan Li1