- 1NYU Grossman School of Medicine, New York, NY, United States
- 2NYU Langone Health, Perlmutter Cancer Center, New York, NY, United States
- 3Department of Neurosurgery, NYU Langone Health, New York, NY, United States
- 4Department of Neurology, Smilow Cancer Hospital, Yale Cancer Center, New Haven, CT, United States
- 5Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
Leptomeningeal carcinomatosis (LC) is a severe complication of metastatic breast cancer (mBC), with rising incidence. The prognosis for patients with LC has been poor, with a median overall survival of approximately four months. However, recent therapeutic advances, in particular the introduction of trastuzumab deruxtecan have dramatically changed the landscape of CNS metastases and improved outcomes. Here, we present the case of a 42-year-old woman with recurrent HER2+ breast cancer who developed extensive LC after multiple lines of treatment. Despite progressive disease, the patient exhibited a sustained response to trastuzumab deruxtecan, a novel antibody-drug conjugate (ADC), for 15 months, which was further extended by adding tucatinib. This case underscores the potential of ADCs, like trastuzumab deruxtecan, in controlling both brain metastases and leptomeningeal disease, offering hope for prolonged survival in patients with aggressive HER2+ mBC. Additionally, we highlight the evolving role of clinical trials, molecular profiling, and interdisciplinary care in managing this challenging condition. Ongoing trials continue to investigate new therapeutic options for HER2+ mBC with CNS involvement, promising to further improve outcomes and quality of life for patients facing this devastating disease.
1 Introduction
The incidence of leptomeningeal carcinomatosis (LC) has shown an upward trend, particularly in patients with metastatic breast cancer (mBC), likely due to improved survival outcomes from advancements in systemic therapies (1). For some women, progression in the central nervous system has emerged as the primary factor limiting survival (2). Among mBC subtypes, those with HER2-positive (HER2+) tumors are at a notably higher risk for central nervous system (CNS) involvement (3, 4); brain metastases typically retain HER-2 expression (5). In the adjuvant KATHERINE trial (comparing trastuzumab emtansine versus trastuzumab) enrolling patients who had residual disease after neoadjuvant HER2-directed therapy, the incidence of brain metastasis (BM) was approximately five percent in both arms (6). Current treatment guidelines for HER2-positive breast cancer with CNS involvement vary depending on extracranial disease (ECD) status and concordance between primary and brain (7).
The management of BM and/or LC is tailored based on individual patient characteristics, including performance status, age, control of the primary tumor, and the extent of extracranial disease. For patients with favorable prognostic factors – such as good performance status, age below 65, controlled primary tumor, and limited or absent extracranial metastases – intensive local treatments like surgery, whole-brain radiotherapy, or stereotactic radiosurgery are more frequently recommended (8, 9). Available systemic therapies include biologics such as HER2-directed antibodies (trastuzumab), HER2/3-directed antibodies (pertuzumab), small molecule HER2-directed tyrosine kinase inhibitors (lapatinib, neratinib, tucatinib), several chemotherapeutics (especially taxanes and capecitabine) as well as HER2-directed ADCs (trastuzumab emtansine and trastuzumab deruxtecan). The goals of systemic therapy in patients with intracranial disease, including LC, are to prevent further extracranial progression, enhance intracranial disease control, prevent or palliate neurological symptoms to maintain functional status, and prolong survival. An exploratory analysis of the HER2CLIMB study, which randomized patients to receive tucatinib versus placebo in combination with capecitabine and trastuzumab in patients with advanced HER-2 positive breast cancer, showed that systemic treatment with tucatinib in combination with trastuzumab and capecitabine improved intracranial response rates versus control (47.3% and 20.0%, respectively), in addition to extended overall survival (10). Over recent years, we have witnessed the flourishing of ADCs as a new class of anticancer therapy with enormous capability to improve survival outcomes in patients with metastatic breast cancer, even in heavily treated individuals (11–13). A pooled analysis conducted to evaluate the effectiveness of trastuzumab deruxtecan in the HER2-positive population with BM supported its intracranial activity with an overall response rate (ORR) of 68% in patients with active brain lesions (14). In the DESTINY-Breast03 study, confirmed intracranial ORR was 65.7% with trastuzumab deruxtecan versus 34.3% with trastuzumab emtansine (15). However, none of the studies included in the pooled analysis allowed patients with LC. Here, we present a case of recurrent HER2-positive breast cancer with extensive LC, demonstrating a sustained response to trastuzumab deruxtecan after multiple lines of anti-HER2 therapy (Figure 1).
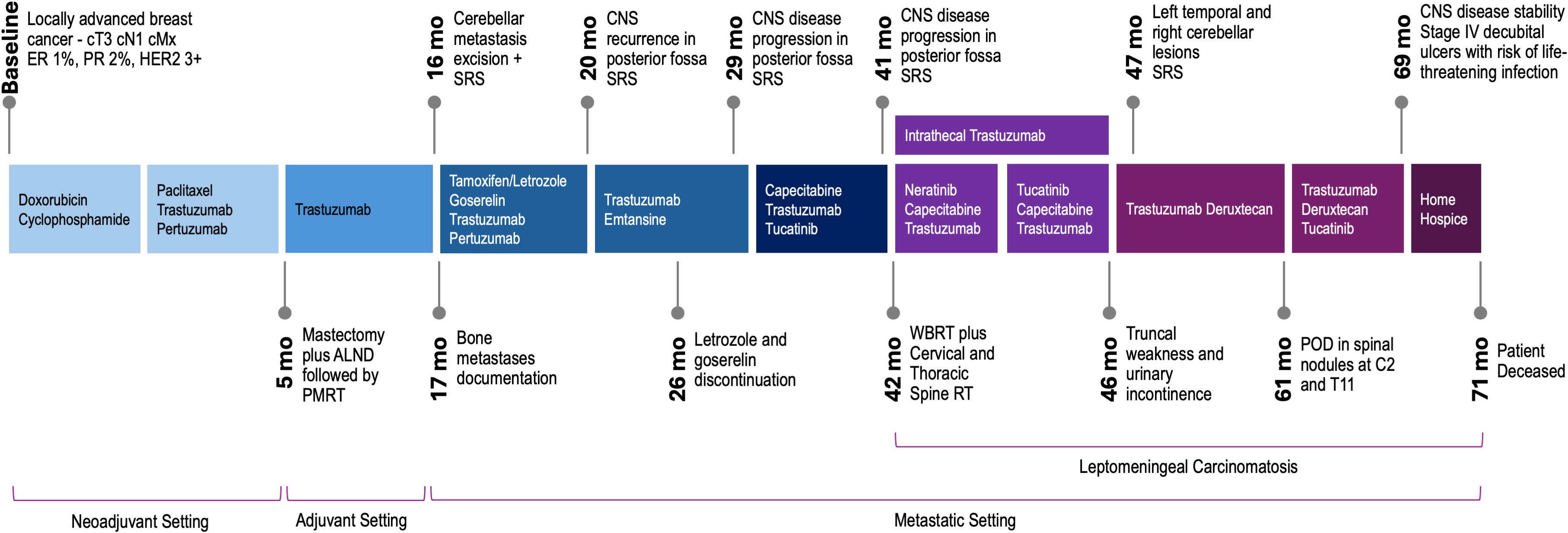
Figure 1. Clinical course and treatment approaches for recurrent HER2 breast cancer with leptomeningeal involvement. ER, estrogen receptor; PR, progesterone receptor; ALND, axillary lymph node dissection; PMRT, post-mastectomy radiation therapy; SRS, stereotactic radiosurgery; POD, progression of disease.
2 Case report
A 42-year-old premenopausal woman with no significant past medical history or family history of cancer initially presented with a palpable lump on the right breast. She had recently completed breast feeding for her second child, which was conceived after in vitro fertilization. After diagnostic workup, pathology revealed a poorly differentiated invasive ductal carcinoma with lymphovascular invasion, ER low positive (1%), PR low positive (2%), HER2-positive (3+), Ki67 65% and positive axillary lymph node. The patient underwent neoadjuvant chemotherapy with doxorubicin and cyclophosphamide followed by paclitaxel, trastuzumab, and pertuzumab, followed by mastectomy with axillary lymph node dissection which report revealed no residual in situ or invasive carcinoma in breast or axillary lymph nodes (ypT0 ypN0), consistent with pathologic complete response. She completed post-mastectomy radiation therapy and adjuvant trastuzumab but declined hormonal therapy due to low hormone receptor positivity. Germline genetic testing was negative for pathogenic variants.
Sixteen months after diagnosis, she presented in a follow up visit with headaches and paresthesia. A brain MRI revealed a 3-cm left cerebellar mass with edema, a craniotomy with lesion excision confirmed metastatic carcinoma consistent with breast origin (GATA3 positive, ER 11-50%, PR-negative, HER2-positive (3+)). Somatic tissue sequencing using a clinical grade panel detected TP53 D281E, ERBB3 V104M, CREBBP S807N, ERCC4 I729T, GALNT12 G272W, JAK1 Y41C, and ERBB2 amplification, with a tumor mutation burden of 6 mutations/Mb. She underwent stereotactic gamma knife radiosurgery (SRS) to the tumor bed and further staging workup revealed sustained low burden bone metastases. She started tamoxifen, goserelin, trastuzumab, and pertuzumab and a subsequent bone scan 3 months after first-line initiation showed interval improvement. At the same time, a brain MRI revealed two new dural-based lesions adjacent to the cerebellar resection cavity, CSF cytology was negative, this was followed by SRS to these lesions and initiation of trastuzumab emtansine. A nonspecific CNS lesion was retrospectively noted and subsequently grew to 8-9 mm in the left cerebellum after 8 months on trastuzumab emtansine, she was again treated with SRS.
The patient then received tucatinib (unblinded at disease progression), trastuzumab, and capecitabine in the HER2CLIMB trial for 10 months, when serial imaging showed slight worsening of posterior fossa lesions, and she was re-treated with SRS. Shortly after, a brain MRI revealed dural deposits at the left cranial nerve V root extending to the left temporal uncus and hippocampus, concerning for leptomeningeal disease. A spine MRI showed multifocal nodular leptomeningeal deposits and larger lesions at C6 and T2 (Figure 2). Whole-brain radiation therapy (WBRT) was initiated, along with targeted spinal radiation. An Ommaya reservoir was placed, and she began intrathecal trastuzumab, along with oral neratinib and capecitabine plus intravenous trastuzumab. Repeated CSF cytology tests were negative. Approximately 5 months after, the patient experienced lower extremity weakness and urinary incontinence, and intrathecal trastuzumab was discontinued due to progression, with new left temporal and right cerebellar lesions treated with SRS. She then started trastuzumab deruxtecan, noting gradual lower extremity strength improvement and increased muscle mass. Five months after trastuzumab deruxtecan initiation, a spine MRI revealed significant improvement of leptomeningeal deposits. Increased edema surrounding the cerebellar lesion resolved with low dose dexamethasone. A subsequently obtained FDG-PET demonstrated low uptake in this area, therefore attributed to prior radiation.
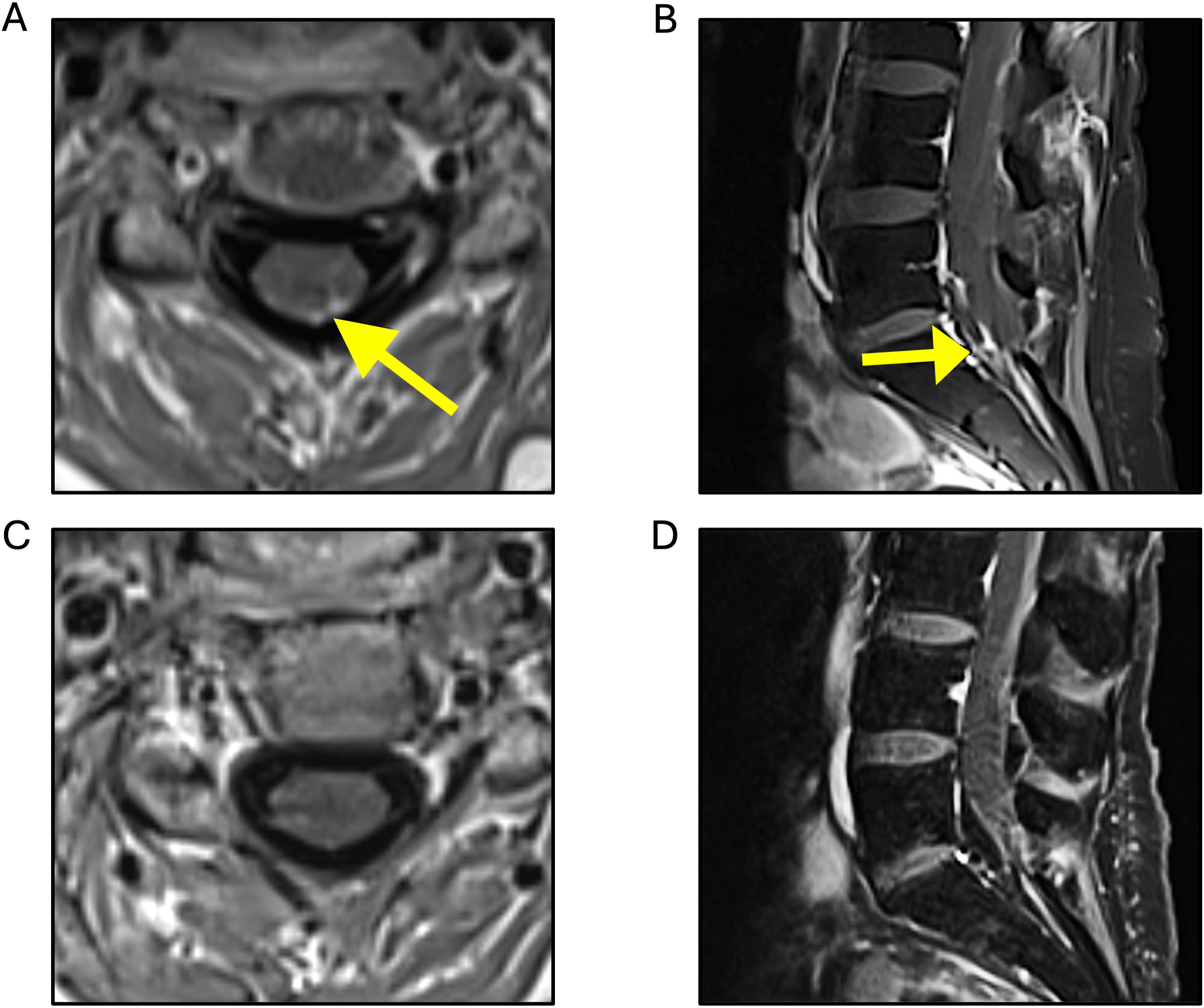
Figure 2. T1-weighted MRI sequences. Multiple areas of nodular enhancement detected, predominantly concentrated along the lower cervical (A) and the left-sided cauda equina nerve roots (B), with decreased enhancement observed 2 months after trastuzumab deruxtecan initiation (C, D, respectively).
After 12 months of trastuzumab deruxtecan therapy, the patient reported increased left lower extremity weakness. A brain MRI showed no significant changes, but the spinal imaging revealed multiple segmental abnormalities suggestive of a demyelinating process, along with possible new lesions at C2-C4 and T2. Immunofixation showed oligoclonal bands in both CSF and serum, consistent with an autoimmune phenomenon. Three months later, she reported worsening blurry vision, headaches and paresthesia. Brain MRI revealed stable findings, but spine MRI showed disease progression in spinal nodules at C2 and T11. Due to preliminary safety of the combination (personal communication, Dr. Lin), the patient restarted tucatinib, in combination with trastuzumab deruxtecan. After 8 months on this combined regimen, both brain and spine MRIs still showed disease stability, but given the patient’s declining functional status and stage IV decubital ulcers which posed significant risk of life-threatening infection with continued systemic therapy, she was offered comfort care and passed away a few weeks later.
3 Leptomeningeal carcinomatosis in HER2-positive mBC
Leptomeningeal carcinomatosis occurs as an advanced-stage complication in approximately 5% of patients with mBC, coinciding with BM in 14% of cases (16–18). While HER-2-positive disease has a higher likelihood of cerebral metastases, this association is not consistently observed for LC. In addition, survival for those with LM, has been poor regardless of subtype, with a median overall survival of approximately 4 months (19). Survival in LC directly correlates with younger age, better performance status, and the ability to receive appropriate treatment, including locoregional, intrathecal, and systemic therapies (20, 21). Furthermore, early access to novel therapies through participation in clinical trials and optimized care through integrated interdisciplinary management, both of which we highlight in our patient, can extend survival. Remarkably, despite bulky LM, and extensive prior systemic therapy, the patient had a sustained 15-month response to trastuzumab deruxtecan, which was then extended by 8 months with the addition of tucatinib.
The challenges of obtaining leptomeningeal tissue samples as well as the low cell count in CSF contribute to the paucity of studies addressing genomic analysis in breast cancer patients with LC. As an alternative, recent studies have shown that molecular profiling of LC can be achieved through circulating tumor DNA sequencing from CSF (22–24). This approach not only aids in diagnosing LC but can also serve as a quantitative marker for monitoring therapeutic responses. In the case presented, genomic analysis of the CSF was not feasible. However, sequencing of brain metastasis tissue obtained at recurrence identified a mutation profile typical of HER2-positive breast cancer.
DESTINY-Breast12 has demonstrated significant BM activity with trastuzumab deruxtecan; within the active BM subgroup, CNS ORR was reported in 19 of 23 patients (82.6%) and in 19 of 38 patients (50%) with untreated and previously treated/progressing BM, respectively (25). The effectiveness of trastuzumab deruxtecan for LC has been studied prospectively, and preliminary data from the LC cohort of the phase II DEBRAH trial (n=7) are promising, with a median overall survival of 13.3 months and 16-week and 24-week overall survival rates of 86% and 71%, respectively (26).
Several ongoing clinical trials expand treatment options for LM in HER2-positive mBC (Table 1), with promising early data from a radiotherapeutic trial with intrathecal Rhenium (186Re) obisbemeda (27). Importantly, two phase 3 clinical trials – CompassHER2 RD and DESTINY-Breast05 have been designed to explore the efficacy of newer agents for preventing relapse, including in the CNS, in high-risk HER2-positive breast cancer with residual disease after neoadjuvant HER2-directed therapy (Table 1).
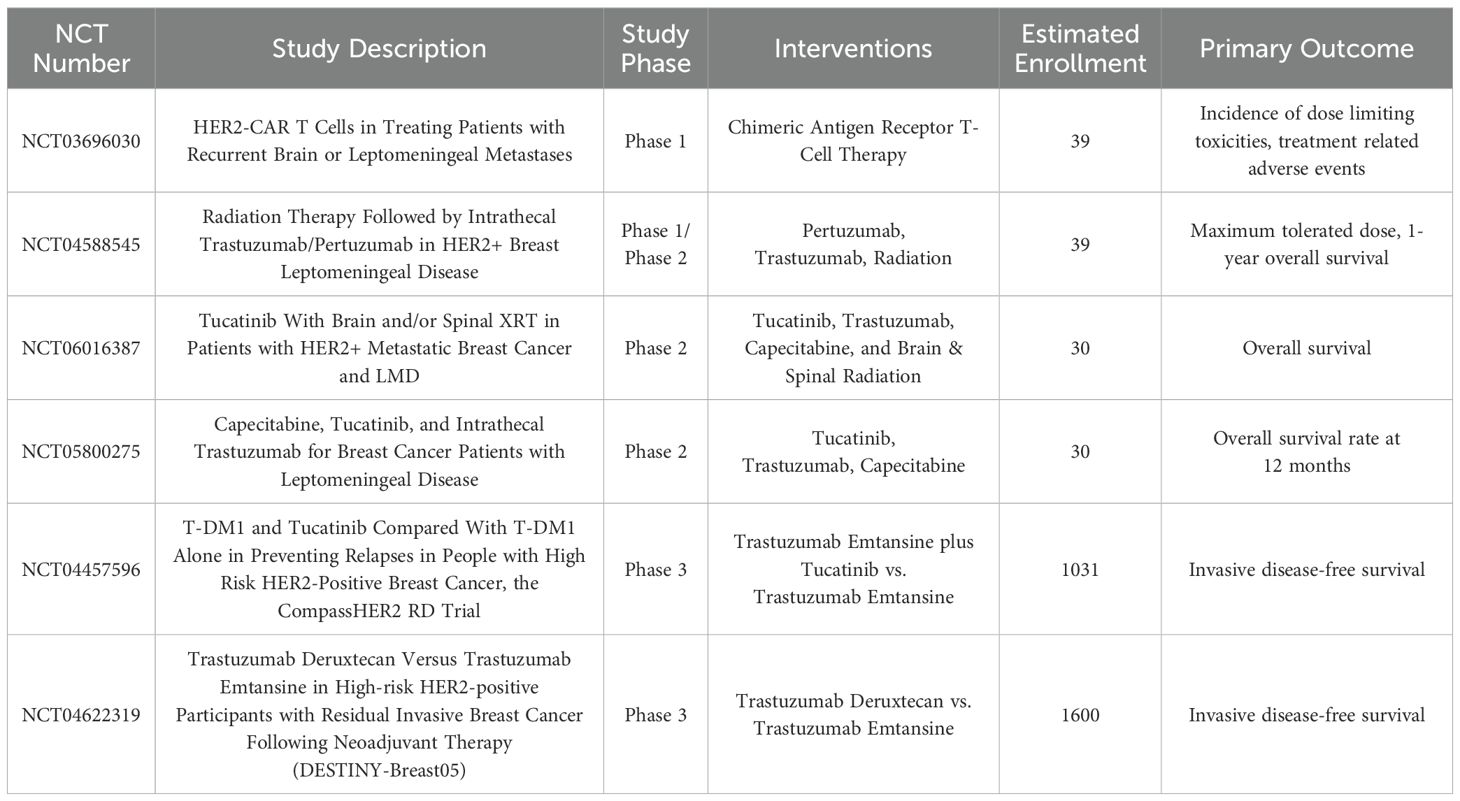
Table 1. Ongoing clinical trials for leptomeningeal carcinomatosis treatment (top) or CNS disease prevention (bottom) in HER2-positive breast cancer.
4 Final considerations
The management of LC in HER2-positive mBC remains a highly complex clinical challenge, however, the introduction of antibody-drug conjugates such as trastuzumab deruxtecan, with accumulating data showing prolonged intracranial and leptomeningeal disease control gives hope for extending survival and improving quality of life for patients with this aggressive disease. Furthermore, investigation of ADCs in patients with early, high-risk breast cancer will hopefully lessen recurrence risk and the burden metastatic disease puts on patients and their families.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
AL: Writing – original draft, Writing – review & editing. DK: Writing – review & editing. DP: Writing – review & editing. SAn: Writing – review & editing. SK: Writing – review & editing. NL: Writing – review & editing. SAd: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, and Komorowski AS. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. (2021) 23:894–904. doi: 10.1093/neuonc/noaa285
2. Lin NU, Gaspar LE, and Soffietti R. Breast cancer in the central nervous system: multidisciplinary considerations and management. Am Soc Clin Oncol Educ Book. (2017) 37:45–56. doi: 10.1200/EDBK_175338
3. Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. (2006) 17:935–44. doi: 10.1093/annonc/mdl064
4. Lamba N, Cagney DN, Catalano PJ, Elhalawani H, Haas-Kogan DA, Wen PY, et al. Incidence proportion and prognosis of leptomeningeal disease among patients with breast vs. non-breast primaries. Neuro Oncol. (2023) 25:973–83. doi: 10.1093/neuonc/noac249
5. Pereslete AM, Hughes ME, Martin AR, Files J, Nguyen K, Buckley L, et al. Analysis of HER2 expression changes from breast primary to brain metastases and the impact of HER2-low expression on overall survival. Neuro Oncol. (2024). doi: 10.1093/neuonc/noae163
6. von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. (2019) 380:617–28. doi: 10.1056/NEJMoa1814017
7. Giordano SH, Franzoi MAB, Temin S, Anders CK, Chandarlapaty S, and Crews JR. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J Clin Oncol. (2022) 40:2612–35. doi: 10.1200/JCO.22.00519
8. Narita Y, Sato S, and Kayama T. Review of the diagnosis and treatment of brain metastases. Jpn J Clin Oncol. (2022) 52:3–7. doi: 10.1093/jjco/hyab182
9. Mashiach E, Alzate JD, Vasconcellos FDN, Bernstein K, Donahue BR, and Schnurman Z. Long-term survival from breast cancer brain metastases in the era of modern systemic therapies. Neurosurgery. (2024) 94:154–64.
10. Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, and Bedard PL. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. (2023) 9:197–205. doi: 10.1001/jamaoncol.2022.5610
11. Andre F, Park YH, Kim S-B, Takano T, Im S-A, Borges G, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2023) 401:1773–85.
12. Hurvitz SA, Hegg R, Chung W-P, Im S-A, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. (2023) 401:105–17. doi: 10.1016/S0140-6736(22)02420-5
13. Cortes J, Hurvitz SA, Im S-A, Iwata H, Curigliano G, Kim S-B, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-Breast03 trial. Nat Med. (2024) 30:2208–15. doi: 10.1038/s41591-024-03021-7
14. Michelon I, Vilbert M, Marinho AD, Castro CER, Dacoregio MI, and Stecca C. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-positive breast cancer brain metastases: a systematic review and meta-analysis. ESMO Open. (2024) 9:102233. doi: 10.1016/j.esmoop.2024.102233
15. Hurvitz SA, Kim S-B, Chung W-P, Im S-A, Park YH, and Hegg R. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open. (2024) 9:102924. doi: 10.1016/j.esmoop.2024.102924
16. Corbin ZA and Nagpal S. Leptomeningeal metastases. JAMA Oncol. (2016) 2:839. doi: 10.1001/jamaoncol.2015.3502
17. Franzoi MA and Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. (2019) 135:85–94. doi: 10.1016/j.critrevonc.2019.01.020
18. de Azevedo CRAS, Cruz MRS, Chinen LTD, Peres SV, Peterlevitz MA, Pereira AEAP, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. (2011) 104:565–72. doi: 10.1007/s11060-010-0524-y
19. Abouharb S, Ensor J, Loghin ME, Katz R, Moulder SL, and Esteva FJ. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. (2014) 146:477–86. doi: 10.1007/s10549-014-3054-z
20. Niwinska A, Pogoda K, Michalski W, Kunkiel M, and Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. (2018) 138:191–8. doi: 10.1007/s11060-018-2790-z
21. Carausu M, Carton M, Darlix A, Pasquier D, Leheurteur M, and Debled M. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open. (2021) 6:100150. doi: 10.1016/j.esmoop.2021.100150
22. Fitzpatrick A, Iravani M, Mills A, Childs L, Alaguthurai T, and Clifford A. Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin Cancer Res. (2022) 28:1180–91. doi: 10.1158/1078-0432.CCR-21-3017
23. Siravegna G, Geuna E, Mussolin B, Crisafulli G, Bartolini A, and Galizia D. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open. (2017) 2:e000253. doi: 10.1136/esmoopen-2017-000253
24. Morganti S, Parsons HA, Lin NU, and Grinshpun A. Liquid biopsy for brain metastases and leptomeningeal disease in patients with breast cancer. NPJ Breast Cancer. (2023) 9:43. doi: 10.1038/s41523-023-00550-1
25. Harbeck N, Ciruelos E, Jerusalem G, Müller V, Niikura N, and Viale G. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial. Nat Med. (2024).
26. Batista MV, Pérez-García JM, Garrigós L, García-Sáenz JÁ, Cortez-Castedo P, and Racca F. Trastuzumab deruxtecan in patients with HER2[+] or HER2-low advanced breast cancer and pathologically confirmed leptomeningeal carcinomatosis: results from cohort 5 of the DEBBRAH study. Cancer Res. (2024) 84. doi: 10.1158/1538-7445.SABCS23-PS11-05
Keywords: metastatic breast cancer, leptomeningeal disease, HER2-targeted therapy, antibody-drug conjugate, trastuzumab deruxtecan
Citation: Leal A, Kondziolka D, Pacione D, Antwi S, Kurz S, Lin N and Adams S (2025) Case Report: Unlocking opportunities in HER2-targeted antibody-drug conjugates for bulky leptomeningeal metastatic breast cancer. Front. Oncol. 15:1559085. doi: 10.3389/fonc.2025.1559085
Received: 11 January 2025; Accepted: 07 March 2025;
Published: 13 August 2025.
Edited by:
Xinpei Deng, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2025 Leal, Kondziolka, Pacione, Antwi, Kurz, Lin and Adams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvia Adams, c3lsdmlhLmFkYW1zQG55dWxhbmdvbmUub3Jn
 Alessandro Leal
Alessandro Leal Douglas Kondziolka
Douglas Kondziolka Donato Pacione
Donato Pacione Stacy Antwi1,2
Stacy Antwi1,2 Sylvia Kurz
Sylvia Kurz