- 1Department of Pathology, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Pathology, The Yaan People’s Hospital (Yaan Hospital of West China Hospital, Sichuan University), Yaan, Sichuan, China
- 3Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
Background: Numerous studies have reported considerable heterogeneity in breast ductal carcinoma in situ with microinvasion (DCIS-MI) regarding clinical presentation, progression potential, and treatment strategies.
Case presentation: In this work, we report a case of rapidly progressing multiple metastases in a patient with DCIS-MI following conventional treatment. Subsequent fluorescence in situ hybridization (FISH) testing revealed HER2 gene overexpression. After receiving 10 cycles of trastuzumab-targeted therapy, the patient achieved a favorable prognosis and remains stable to date.
Conclusion: This case suggests that even patients with microinvasive lesions who are HER2-positive may exhibit unexpected stability under targeted therapy, warranting close surveillance in such cases.
Introduction
Breast ductal carcinoma in situ with microinvasion (DCIS-MI) is classified as a T1mi tumor according to the American Joint Committee on Cancer (AJCC) Staging, 8th edition, defined by stromal invasion extending beyond the basement membrane with foci measuring ≤1 mm. Epidemiological studies have indicated that DCIS-MI occurs in approximately 5%–10% of DCIS cases (1–3). Its incidence has risen over recent decades due to widespread mammographic screening, color Doppler ultrasound, and increased clinical awareness. Numerous studies have reported considerable heterogeneity in DCIS-MI regarding clinical presentation and progression potential. Some scholars have considered DCIS-MI an early form of invasive breast carcinoma (IBC), suggesting comparable mortality and 5-year overall survival rates to IBC (4–6). However, recent studies have distinguished DCIS, DCIS-MI, and IBC (7, 8), indicating that DCIS-MI patients exhibit worse survival than DCIS patients but better outcomes than IBC patients (9). Significant controversy persists regarding the pathological characteristics, diagnosis, and treatment of DCIS-MI, necessitating further research.
In this study, we report the progression and prognosis of a DCIS-MI patient with multi-organ metastases following surgery at the Affiliated Hospital of Southwest Medical University. We also discuss current consensus and controversies in DCIS-MI management based on the latest literature. The patient provided written informed consent for the publication of this manuscript and any identifying images or data. This report details one of the longest prospective follow-ups of HER2+ DCIS-MI, addressing a gap in long-term outcome data.
Case presentation
In March 2012, a 58-year-old female patient presented to the Department of Breast Surgery at our hospital with a left-sided breast mass. Physical examination revealed a 2.0-cm firm, fixed mass in the upper outer quadrant of the left breast. Mammography identified an irregular 2.0-cm mass with pleomorphic calcifications [BI-RADS (Breast Imaging Reporting and Data System) category 5]. Ultrasound demonstrated a hypoechoic lesion with angular margins. An ultrasound-guided core needle biopsy (CNB) subsequently confirmed high-grade ductal carcinoma in situ (DCIS) with focal microinvasion. On March 8, 2012, the patient received one course of TAC (docetaxel + doxorubicin + cyclophosphamide) induction chemotherapy. A left modified radical mastectomy was performed on April 3, 2012. Postoperative pathological examination identified two discrete microinvasive foci (each <1 mm) within a 1.5 cm area of DCIS. The radiological size (2.0 cm on mammography) correlated with the pathological DCIS extent (1.5 cm) (Figures 1A, B). Examination of 19 left axillary lymph nodes showed no metastases. The entire lesion was serially sectioned at 3-mm intervals. All suspicious foci were embedded in paraffin, and ≥15 additional deeper sections per block were evaluated to exclude occult IBC. Exhaustive sectioning of the tumor bed and surrounding parenchyma revealed no further invasive foci. Immunohistochemistry of the microinvasive areas showed ER (estrogen receptor, −), PR(progesterone receptor, −), Ki-67 (+20%), and HER2 (3+) (Figures 2A–D). Immunohistochemical staining for p63 and SMMHC (smooth muscle myosin heavy chain) revealed a continuous layer of positive cells in DCIS lesions, while no positive staining was observed around the two discrete microinvasive lesions, confirming that these nests represent invasive breast cancer foci (micrometastasis). Following surgery, the patient completed five courses of TAC chemotherapy and 4 weeks of conventional radiotherapy. Post-chemotherapy re-examination revealed no breast mass recurrence [the patient declined HER2 fluorescence in situ hybridization (FISH) testing].
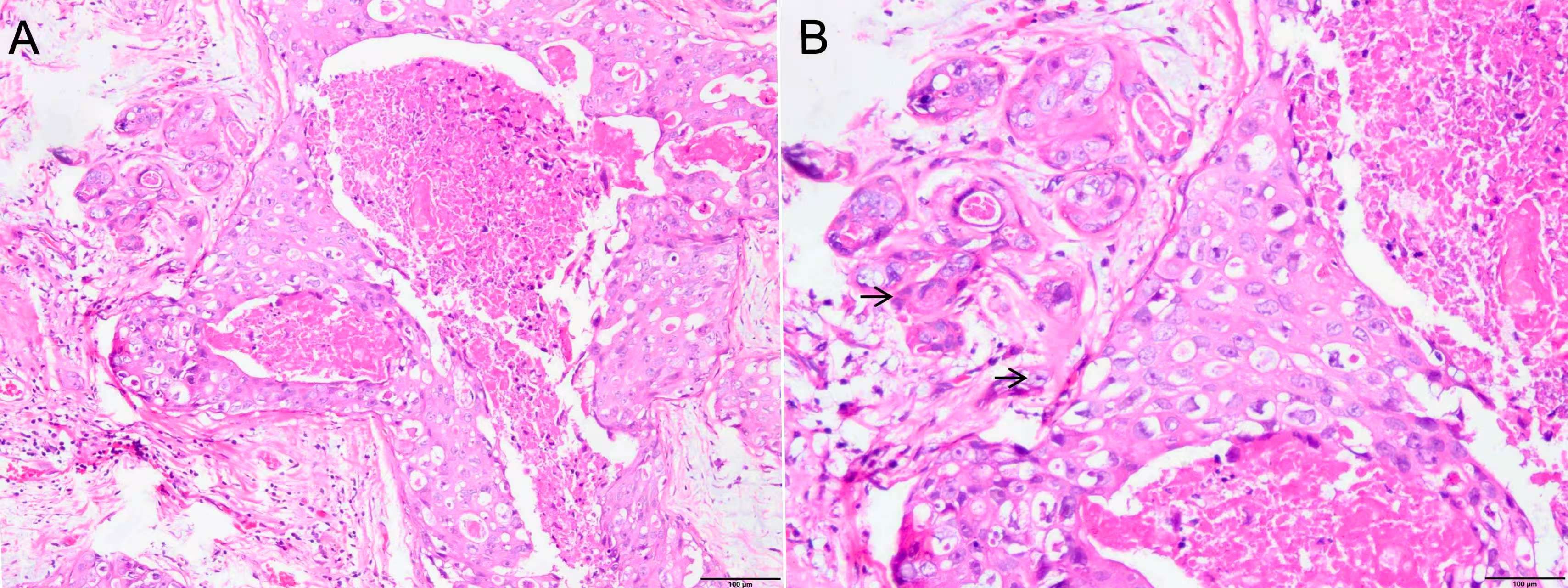
Figure 1. (A) Ductal carcinoma in situ of the left breast tissue with microinvasion (×100). (B) Ductal carcinoma in situ of the left breast tissue with two discrete microinvasive foci (each <1 mm) (black arrow, ×200).
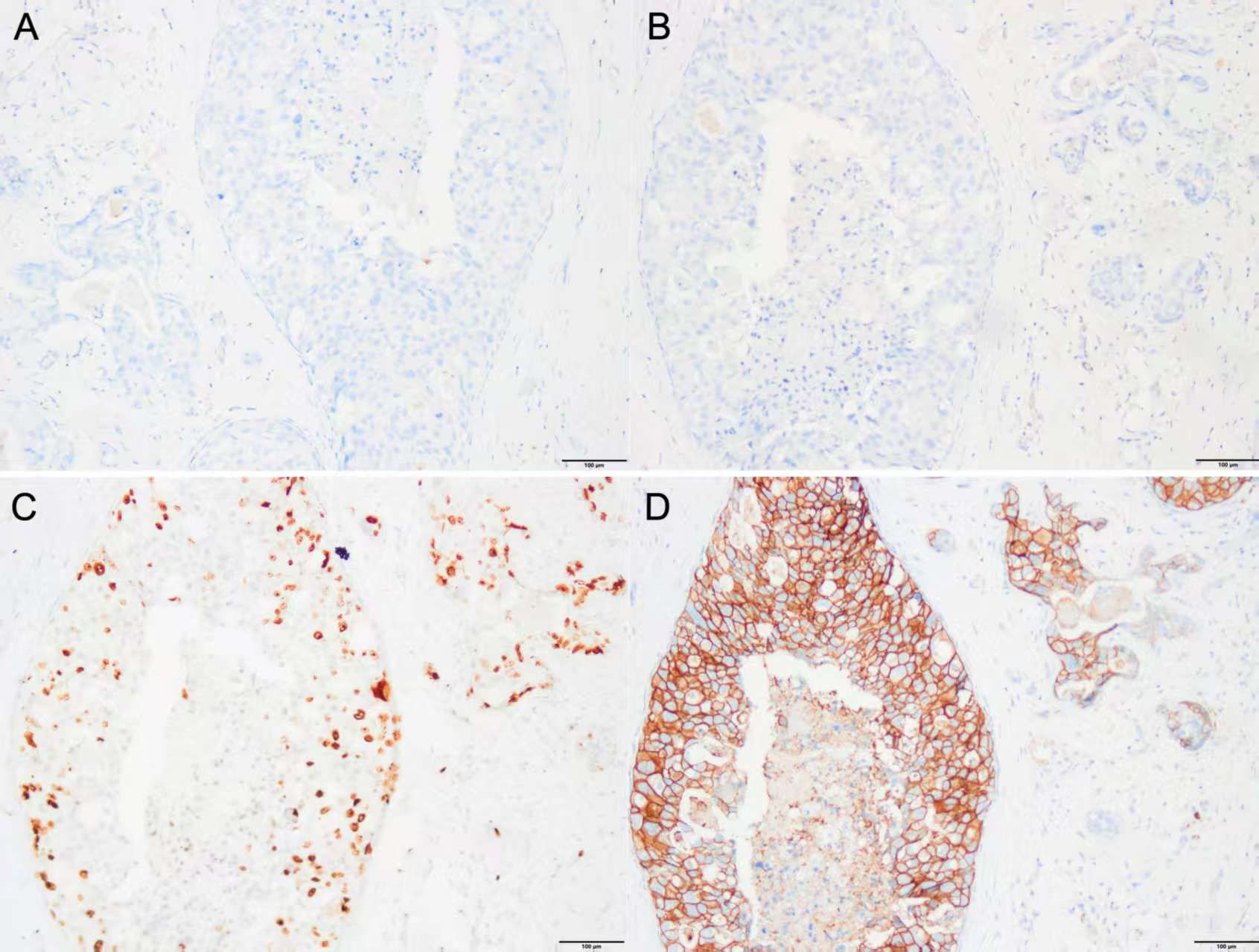
Figure 2. (A–D) IHC of the tissue of excisional biopsy of the left breast in 2012 (×200). (A) IHC for ER (−), (B) IHC for PR (−), (C) IHC for Ki-67 (+, 20%), and (D) IHC for HER2 (3+). IHC, immunohistochemistry; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
On August 14, 2013, the patient presented to the Department of Hepatobiliary Surgery at our hospital with upper abdominal pain. Ultrasound revealed substantial intrahepatic space-occupying lesions and multiple enlarged retroperitoneal lymph nodes. PET/CT showed (Figures 3A, C, E) the following: 1) multiple variably sized patchy and flaky low-density lesions in the liver with heterogeneously increased glucose metabolism, suggestive of breast cancer metastasis given the patient’s history; 2) multiple enlarged lymph nodes with increased glucose metabolism in the right cardiophrenic angle, porta hepatis, and retroperitoneal space, consistent with lymph node metastases from breast cancer; and 3) focally increased glucose metabolism in the sacrum, raising suspicion of breast cancer metastasis. On August 16, 2013, with the patient’s consent, the FISH test confirmed HER2 overexpression (cluster amplification of HER signals in microinvasive cancer cells) in both the pretreatment CNB and surgical specimen, per the American Society of Clinical Oncology / College of American Pathologists (ASCO / CAP) 2008 (Figure 4). Liver biopsy was deferred per institutional guidelines due to the patient’s rapid clinical deterioration and strong clinical/radiological evidence supporting a primary breast origin. Based on the National Comprehensive Cancer Network (NCCN) guidelines for HER2+ disease, treatment with trastuzumab (6 mg/kg every 3 weeks) plus capecitabine (1,250 mg/m2 twice daily on days 1–14 of a 21-day cycle) was initiated. The patient received eight courses of this regimen from August 17, 2013, to February 6, 2014. PET/CT re-evaluation after three courses showed a significant reduction in the size and metabolic activity of the intrahepatic lesions compared to those in August 2013. Post-treatment PET/CT on March 15, 2014 (Figures 3B, D, F) revealed the following: 1) hepatic cyst and calcification in the left lateral liver lobe, 2) partial mediastinal lymph node calcification, and 3) normal bone scan and CT findings. From March 14, 2014, to December 9, 2014, she received an additional 10 courses of trastuzumab plus capecitabine. Maintenance therapy with oral capecitabine (2.5 g twice daily) was followed from January 14, 2015, to January 12, 2016 (10 courses). Quarterly PET/CT surveillance during this period showed no abnormalities. In February 2016, the patient developed grade 2 hand–foot syndrome. Capecitabine dosing was reduced to 2.0 g twice daily, administered from February 5, 2016, to March 15, 2017 (16 courses). A PET/CT scan in March 2017 showed no new lesions or metastases. The patient continued single-agent capecitabine chemotherapy from April 2017 to January 2021. Semiannual PET/CT scans during this period showed no significant changes compared to the 2017 baseline. Since February 2021, all chemotherapy treatments have been discontinued. The patient has been undergoing annual CT surveillance and physical assessment, with no abnormalities detected to date. This case was identified through retrospective review of our institutional breast cancer registry (IRB#12732). Data collection adhered to STROBE (strengthening the reporting of observational studies in epidemiology) guidelines for case reports.
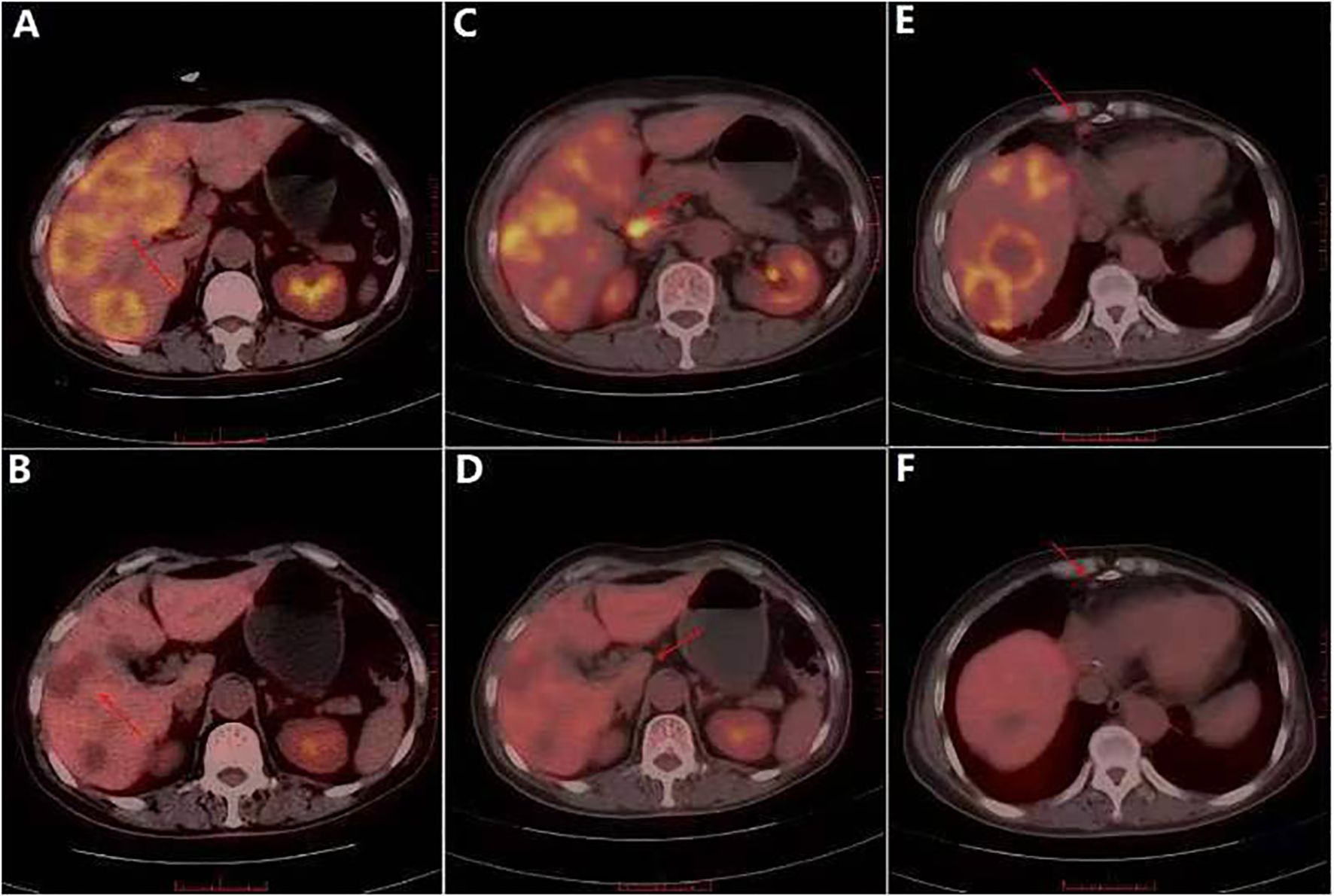
Figure 3. In August 2013, PET/CT showed liver and multiple lymph node metastases. (A) PET/CT showed multiple patchy and flaky low-density shadows of different sizes in the liver. (B) PET/CT showed the disappearance of patchy low-density shadows in the liver. (C) Multiple lymph node enlargement and increased glucose metabolism in right septal angle. (D) No swelling or increased glucose metabolism was observed in the right septal horn lymph node. (E) Local glucose metabolism of the sacrum was increased. (F) Local glucose metabolism of the sacrum was reduced.
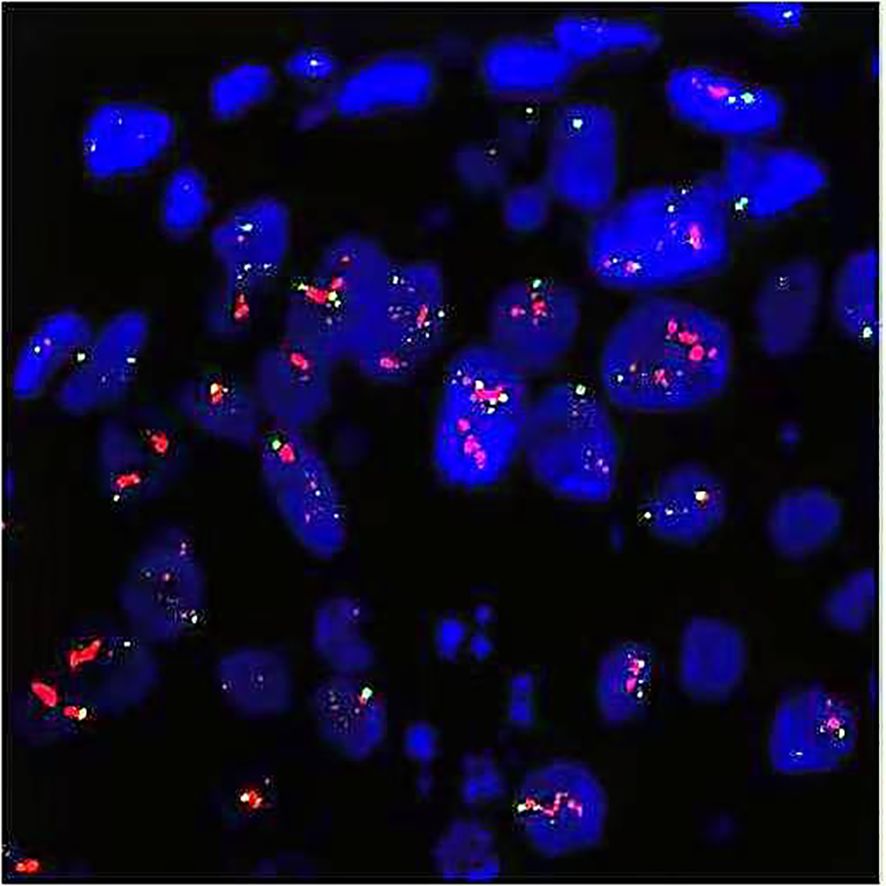
Figure 4. FISH test indicated HER2 overexpression (cluster amplification of HER2 signals in microinvasive carcinoma cells). Red for HER2 gene and green for CEP17. FISH, fluorescence in situ hybridization; CEP, centromere encoded probe.
Discussion
The patient was diagnosed with DCIS-MI based on biopsy and subsequent surgical excision. Histopathological examination of H&E-stained sections revealed two discrete microinvasive foci, each measuring <1 mm in the greatest dimension. Negative immunohistochemical staining for p63 and SMMHC confirmed that these foci represented invasive breast cancer (micrometastasis). However, due to prolonged storage and significant fading of the original slides, images from that time could not be retrieved. Additionally, re-staining the corresponding paraffin blocks failed to produce images of diagnostic quality. The absence of a myoepithelial marker immunohistochemistry image represents a limitation in this case. Notably, the patient developed widespread systemic metastases within a short timeframe. This case presents a paradoxical clinical course: while the majority of lesions represented DCIS with only minimal invasive components (<1 mm), the disease exhibited aggressive metastatic behavior atypical of pure DCIS. We elucidate the clinical significance of this case through a comparative analysis of the patient’s age, tumor dimensions, histopathological characteristics, biomarker profile, axillary lymph node status, and targeted therapy regimen.
First, regarding age, studies have indicated that DCIS-MI patients are typically older than those with pure DCIS (10, 11). In this case, the patient was 58 years old at diagnosis (postmenopausal), consistent with literature suggesting age >50 years as a potential independent risk factor for microinvasion (12, 13). We thus speculate that prolonged clonal evolution may drive DCIS microinvasion: extended lesion duration could permit the emergence of aggressive subclones through stochastic genetic alterations, increasing invasive potential. Second, concerning tumor size, Jia et al. (14) reported that 61.6% of DCIS-MI lesions exceed 3 cm in diameter, while Mori et al. (10) documented a median tumor diameter of 3.5 cm. These data suggest that larger tumors (particularly >2 cm) correlate with microinvasion risk. Notably, this patient’s tumor measured only 1.5 cm yet developed multiple metastases. We therefore postulate that while DCIS lesion size contributes to invasive potential, the presence of microinvasion itself constitutes a critical risk factor for disease progression.
Histopathologically, DCIS-MI typically presents as higher-grade lesions across histological subtypes compared to pure DCIS (15–18). Studies have indicated that 85.0% of DCIS-MI cases exhibit high-grade lesions, whereas only 63.5% of pure DCIS cases show high-grade histology. This suggests that high-grade DCIS may be a necessary precursor to most invasive carcinomas, with low-grade DCIS potentially progressing to high-grade disease before invasion. In the present case, the histological features align with reported high-grade DCIS (Figure 1). However, the clinical outcome diverged significantly from pure DCIS: the patient developed extensive systemic metastases rapidly, far exceeding the 15-year distant metastases rate of 0.9% observed in the NSABP B-17/24 cohort (19). This implies that DCIS-MI and pure DCIS may represent fundamentally distinct entities. We hypothesize that DCIS-MI exhibits inherent aggressiveness, progressing to invasive carcinoma more rapidly. This could explain the patient’s swift disease progression and early metastases. Biological markers further differentiate DCIS grades (20–23). Well-differentiated/low-grade DCIS typically expresses ER/PR (90%–100%), rarely shows HER2 amplification (~10%), and has low Ki-67 indices (5%–6%). In contrast, our patient’s immunohistochemistry revealed ER/PR negativity, HER2 amplification, and elevated Ki-67 (Figure 2). This HER2-enriched molecular subtype correlates with higher proliferation indices and microinvasion risk, potentially explaining early metastasis. Such aggressive DCIS-MI subtypes align with rare reports (24) and suggest that molecular biology may supersede conventional risk stratification. Collectively, the loss of ER/PR, HER2 overexpression, and elevated Ki-67 may serve as predictors of poor prognosis in DCIS-MI.
Published data have indicated metastatic axillary lymph node involvement in DCIS patients at frequencies of 0%–10% (25, 26), with an overall sentinel lymph node (SLN) positivity rate of 7.4% (27). Consequently, current guidelines from the NCCN and ASCO suggest that sentinel lymph node biopsy (SLNB) may be omitted in DCIS patients undergoing breast-conserving therapy (BCT) (28, 29). Conversely, the European Society for Medical Oncology (ESMO) recommends SLNB for high-grade DCIS with tumors exceeding 4 cm (30). In this case, the 1.5-cm lesion did not undergo SLNB, and axillary lymph node dissection revealed no metastases. However, the patient developed multi-organ metastases shortly after surgery. This implies that early sentinel lymph node metastasis cannot be excluded, underscoring SLNB’s diagnostic value. Our findings indicate that SLNB remains clinically significant for DCIS-MI patients, even when lesions are small (<4 cm).In addition, trastuzumab-targeted therapy is standard for HER2-overexpressing IBC. However, its efficacy in HER2-positive DCIS-MI remains unconfirmed by clinical trials. Studies have indicated that ~40% of DCIS cases are HER2-positive (31), yet insufficient evidence exists for trastuzumab’s ability to prevent DCIS progression to IBC; it currently serves only as a risk stratification reference. Consequently, trastuzumab is not standard care for HER2+ DCIS. The NSABP B-43 trial—the first prospective phase III study evaluating trastuzumab’s impact on local recurrence in high-risk HER2+ DCIS (32)—found no significant histological, antiproliferative, or pro-apoptotic effects from trastuzumab monotherapy. Although trastuzumab mediates NK cell-dependent ADCC (antibody-dependent cell-mediated cytotoxicity) and induces T-cell humoral immunity, no substantial toxicity was observed in DCIS patients, and the trial remains ongoing. Cobleigh et al. (33) investigated trastuzumab combined with radiotherapy for HER2+ DCIS but found that prognostic benefits lacked statistical significance. In this case, trastuzumab targeting HER2 (10 cycles) achieved complete remission after metastasis (Figure 3), suggesting clinical utility in metastatic DCIS-MI. However, as this represents a single case, therapeutic efficacy requires validation through larger trials. Notably, biomarker discordance between primary and metastatic sites occurs in 10%–20% of cases (34). We acknowledge this limitation and recommend metastatic lesion biopsy to reassess receptor status when feasible.
Conclusion
In this case, multiple metastases of the patient display the malignant nature of DCIS-MI, and while DCIS lacks metastatic potential, microinvasion represents the transition to IBC. This case underscores that even minimal invasive components in DCIS-MI can drive distant metastases, warranting aggressive surveillance. For DCIS-MI patients, HER2 testing should be considered in DCIS-MI given potential therapeutic implications, although broader adoption requires cohort validation. In addition, a comprehensive evaluation is required for tumor size, ER and PR expression, lymph node metastases, etc. The treatment for DCIS-MI patients is not invariable; clinicians need to know more about the commonness and individuality of patients to manage more individualized treatment plans for different DCIS-MI patients. At the same time, a more reasonable and accurate method is needed to predict the possibility of different patients developing IBC and give different treatments to different subtypes of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Southwest Medical University Affiliated Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
SR: Data curation, Writing – original draft, Writing – review & editing. JZ: Data curation, Formal Analysis, Writing – original draft. RT: Data curation, Formal Analysis, Writing – original draft. TW: Formal Analysis, Investigation, Project administration, Software, Writing – original draft. XG: Investigation, Writing – original draft. LM: Investigation, Writing – original draft. MT: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:290–303. doi: 10.3322/caac.21393
2. Song G and Zhang Y. Clinicopathological characteristics, treatments, and prognosis of breast ductal carcinoma in situ with microinvasion: A narrative review. Chronic Dis Transl Med. (2022) 9:5–13. doi: 10.1002/cdt3.53
3. Phantana-Angkool A, Voci AE, Warren YE, Livasy CA, Beasley LM, Robinson MM, et al. Ductal carcinoma in situ with microinvasion on core biopsy: Evaluating tumor upstaging rate, lymph node metastasis rate, and associated predictive variables. Ann Surg Oncol. (2019) 26:3874–82. doi: 10.1245/s10434-019-07604-4
4. Shi J, Yang S, Niu Q, Zhao L, Jia C, Du L, et al. Correlation of sonographic features with prognostic factors in ductal carcinoma in situ of the breast: an exploratory study using ultrasound and shear wave elastography. BMC Med Imaging. (2024) 24:327. doi: 10.1186/s12880-024-01494-z
5. Canas-Marques R and Schnitt SJ. Ductal carcinoma in situ with and without microinvasion: is there a clinically meaningful difference in outcome? Br J Cancer. (2023) 128:713–4. doi: 10.1038/s41416-023-02152-x
6. Champion CD, Ren Y, Thomas SM, Fayanju OM, Rosenberger LH, Greenup RA, et al. DCIS with microinvasion: Is it in situ or invasive disease. Ann Surg Oncol. (2019) 26:3124–32. doi: 10.1245/s10434-019-07556-9
7. Shiino S, Quinn C, Ball G, Syed BM, Kurozumi S, Tsuda H, et al. Prognostic significance of microinvasion with ductal carcinoma in situ of the breast: a meta-analysis. Breast Cancer Res Treat. (2023) 197:245–54. doi: 10.1007/s10549-022-06800-3
8. Magnoni F, Bianchi B, Corso G, Alloggio EA, Di Silvestre S, Abruzzese G, et al. Ductal carcinoma in situ (DCIS) and microinvasive DCIS: role of surgery in early diagnosis of breast cancer. Healthcare (Basel). (2023) 11:1324. doi: 10.3390/healthcare11091324
9. Li C, Yang Y, Wang J, Jin K, Yang Z, Yu X, et al. Characteristics, diagnosis, risk factors, and management of recently diagnosed ductal carcinoma in situ with microinvasion. Cancer Med. (2021) 10:7203–12. doi: 10.1002/cam4.4263
10. Mori M, Tsugawa K, Yamauchi H, Yagata H, Suzuki K, Ohde S, et al. Pathological assessment of microinvasive carcinoma of the breast. Breast Cancer. (2013) 20:331–5. doi: 10.1007/s12282-012-0339-0
11. Orzalesi L, Casella D, Criscenti V, Gjondedaj U, Bianchi S, Vezzosi V, et al. Microinvasive breast cancer: pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single- institution series. Breast Cancer. (2016) 23:640–8. doi: 10.1007/s12282-015-0616-9
12. van Leeuwen MM, Doyle S, van den Belt-Dusebout AW, van der Mierden S, Loo CE, Mann RM, et al. Clinicopathological and prognostic value of calcification morphology descriptors in ductal carcinoma in situ of the breast: a systematic review and meta-analysis. Insights Imaging. (2023) 14:213. doi: 10.1186/s13244-023-01529-z
13. Ambrosini-Spaltro A, Di Donato F, Saragoni L, Cserni G, Rakha E, and Foschini MP. Prognostic markers of microinvasive breast carcinoma: A systematic review and meta-analysis. Cancers (Basel). (2023) 15:3007. doi: 10.3390/cancers15113007
14. Jia H, Zhao P, Chen Z, Wang G, Dong X, Xing X, et al. Clinicopathological characteristics and prognostic analysis of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ (DCIS) and DCIS with microinvasion (DCIS-Mi) of the breast. Breast Cancer Res Treat. (2022) 193:111–20. doi: 10.1007/s10549-022-06553-z
15. Vieira CC, Mercado CL, Cangiarella JF, Moy L, Toth HK, and Guth AA. Microinvasive ductal carcinoma in situ: clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol. (2010) 73:102–7. doi: 10.1016/j.ejrad.2008.09.037
16. De Mascarel I, MacGrogan G, Mathoulin-Pélissier S, Soubeyran I, Picot V, and Coindre JM. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer. (2002) 94:2134–42. doi: 10.1002/cncr.10451
17. Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Viale G, Renne G, et al. Minimal and small size invasive breast cancer with no axillary lymph node involvement: the need for tailored adjuvant therapies. Ann Oncol. (2004) 15:1633–9. doi: 10.1093/annonc/mdh434
18. Margalit DN, Sreedhara M, Chen YH, Catalano PJ, Nguyen PL, Golshan M, et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast- conserving therapy or mastectomy. Ann Surg Oncol. (2013) 20:811–8. doi: 10.1245/s10434-012-2640-8
19. Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. (2011) 103:478–88. doi: 10.1093/jnci/djr027
20. Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, and Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. (2010) 57:171–92. doi: 10.1111/j.1365-2559.2010.03568.x
21. Bai M, Agnantis NJ, Kamina S, Demou A, Zagorianakou P, Katsaraki A, et al. In vivo cell kinetics in breast carcinogenesis. Breast Cancer Res. (2001) 3:276–83. doi: 10.1186/bcr306
22. Harn HJ, Shen KL, Yueh KC, Ho LI, Yu JC, Chiu SC, et al. Apoptosis occurs more frequently in intraductal carcinoma than in infiltrating duct carcinoma of human breast cancer and correlates with altered p53 expression: detected by terminal-deoxynucleotidyl-transferase-mediated dUTP-FITC nick end labelling (TUNEL). Histopathology. (1997) 31:534–9. doi: 10.1046/j.1365-2559.1997.3270906.x
23. Bodis S, Siziopikou KP, Schnitt SJ, Harris JR, and Fisher DE. Extensive apoptosis in ductal carcinoma in situ of the breast. Cancer. (1996) 77:1831–5. doi: 10.1002/(sici)1097-0142(19960501)77:9<1831::aid-cncr11>3.3.co;2-n
24. Solek J, Chrzanowski J, Cieslak A, Zielinska A, Piasecka D, Braun M, et al. Subtype-specific tumour immune microenvironment in risk of recurrence of ductal carcinoma in situ: prognostic value of HER2. Biomedicines. (2022) 10:1061. doi: 10.3390/biomedicines10051061
25. Francis AM, Haugen CE, Grimes LM, Crow JR, Yi M, Mittendorf EA, et al. Is sentinel lymph node dissection warranted for patients with a diagnosis of ductal carcinoma in situ? Ann Surg Oncol. (2015) 22:4270–9. doi: 10.1245/s10434-015-4547-7
26. Meretoja TJ, Heikkilä PS, Salmenkivi K, and Leidenius MH. Outcome of patients with ductal carcinoma in situ and sentinel node biopsy. Ann Surg Oncol. (2012) 19:2345–51. doi: 10.1245/s10434-012-2287-5
27. Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, and Thompson AM. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. (2008) 95:547–54. doi: 10.1002/bjs.6396
28. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl ComprCanc Netw. (2018) 16:310–20. doi: 10.6004/jnccn.2018.0012
29. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, and Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. (2014) 32:1365–83. doi: 10.1200/jco.2013.54.1177
30. Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. (2017) 35:203–17. doi: 10.1016/j.breast.2017.07.017
31. Lari SA and Kuerer HM. Biological markers in DCIS and risk of breast recurrence: A systematic review. J Cancer. (2011) 2:232–61. doi: 10.7150/jca.2.232
32. Siziopikou KP, Anderson SJ, Cobleigh MA, Julian TB, Arthur DW, Zheng P, et al. Preliminary results of centralized HER2 testing in ductal carcinoma in situ (DCIS): NSABP B- 43. Breast Cancer Res Treat. (2013) 142:415–21. doi: 10.1007/s10549-013-2755-z
33. Cobleigh MA, Anderson SJ, Siziopikou KP, Arthur DW, Rabinovitch R, Julian TB, et al. Comparison of radiation with or without concurrent trastuzumab for HER2-positive ductal carcinoma in situ resected by lumpectomy: A phase III clinical trial. J Clin Oncol. (2021) 39:2367–74. doi: 10.1200/jco.20.02824
Keywords: breast cancer, microinvasion, metastasis, therapy, HER2 gene
Citation: Ruan S, Zhou J, Tang R, Wang T, Gao X, Mou L and Tang M (2025) Case Report: Breast ductal carcinoma in situ with microinvasion: a case tracked over 13 years. Front. Oncol. 15:1560366. doi: 10.3389/fonc.2025.1560366
Received: 14 January 2025; Accepted: 15 July 2025;
Published: 12 August 2025.
Edited by:
Hossein Schandiz, University of Oslo, NorwayReviewed by:
Dharmendra Kumar Yadav, Gachon University, Republic of KoreaDesh Deepak Singh, Amity University Jaipur, India
Torill Sauer, University of Oslo, Norway
Copyright © 2025 Ruan, Zhou, Tang, Wang, Gao, Mou and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sibei Ruan, cnNpYmVpODdAMTYzLmNvbQ==; Mingxi Tang, bXh0YW5nNjlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Sibei Ruan
Sibei Ruan Jian Zhou1†
Jian Zhou1† Mingxi Tang
Mingxi Tang