- 1Department of Hepatobiliary Surgery, People’s Liberation Army of China (PLA) Navy Characteristic Medical Center, Shanghai, China
- 2Department of Organ Transplantation, Shanghai ChangZheng Hospital, Shanghai, China
- 3Department of Urology, Kidney Transplantation Center, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: We aim to analysis the impact of Human Leukocyte Antigen (HLA) mismatch between kidney transplant donors and recipients on the incidence of prostate cancer after kidney transplantation (KT). Meanwhile, understanding the use of T cell therapy is of great importance after kidney transplantation from the perspective of prostate cancer occurrence.
Methods: A retrospective study was conducted on kidney transplant recipients based on the United Network for Organ Sharing (UNOS) database from 2000 to 2019. General demographic data, socio-economic and educational data, personal medical history, immunosuppressive therapy regimens, and HLA typing of donors and recipients were collected to analyze the impact of: (1) baseline patient characteristics; (2) HLA mismatch; and (3) HLA subtype mismatch on the incidence of prostate cancer after transplantation.
Results: A total of 268–994 kidney transplant recipients were included, with 1–910 newly diagnosed prostate cancer patients after surgery. Both univariate and Cox multivariate analysis discovered that the use of T cell therapy could reduce the risk of prostate cancer after KT [0.89(0.86~0.91)]. We also found HLA mismatch ≥ 3 is a risk factor of prostate cancer after transplantation [1.07(1.02~1.11)]. Further subgroup analysis was conducted on HLA mismatch. The Cox multivariate analysis of HLA-A (0–2), HLA-B (0–2), and HLA-DR (0–2) mismatch showed that 2-mismatch in HLA-A and HLA-B was a risk factor of prostate cancer after KT [1.19(1.01~1.40)]; 2-mismatch and 1-mismatch were both risk factors of prostate cancer after KT in the HLA-DR group [1.32(1.13~1.54)], [1.20(1.03~1.39)].
Conclusions: From the perspective of prostate cancer occurrence after transplantation, the use of T cell therapy is of great significance. HLA mismatch ≥ 3 was a risk factor of prostate cancer after KT. HLA-A and HLA-B 2-mismatch were risk factors of prostate cancer after KT, while HLA-DR 1-mismatch and 2-mismatch were both risk factors of prostate cancer after KT. This research contributed to the focus on the relationship between induction therapy and cancer occurrence after KT, and also provide guidance for reasonable selections of HLA typing of prostate cancer before KT.
1 Introduction
Kidney transplantation commonly represents the optimal treatment for patients with end-stage renal disease (ESRD) (1–7). Besides the undisputable advantages of transplantation, an increased incidence of malignancies after transplantation was shown (8, 9). Malignant tumor is the second leading cause of death for kidney transplant recipients (10). Compared with non-transplant population, the incidence rate of malignant tumor in patients after kidney or liver transplantation is 2–4 times higher (11, 12). Common malignant tumors after KT include skin cancer, post-transplant lymphoproliferative disease, and urologic tumors (13). Worldwide, prostate cancer is the second most common form of solid tumor in men, surpassed only by nonmelanoma skin cancers such as basal and squamous cell carcinomas. In this research, we choose prostate cancer to analysis the impact of HLA mismatch after KT (14, 15).
Malignancies have been identified to be widely associated with multiple baseline patient characteristics, such as age, gender, race, smoking, body mass index (BMI), ABO blood types, education background, duration of dialysis, etc. (16–19). In addition, the polymorphism and expression of Human leukocyte antigen (HLA) molecules have also been demonstrated to be correlated with the occurrence and development of tumors (20). HLA is the expression product of human histocompatibility complex (MHC) (21). HLA is categorized into class I, II, and III (22–25). The HLA class I molecules include HLA-A, HLA-B, and HLA-C; the HLA class II antigens include HLA-DP, HLA-DQ, HLA-DR; and the HLA class III antigens encode complements, cytokines, and heat shock proteins (26–32). HLAs play an essential role in the cellular and humoral immune responses after KT, as well as in determining the outcome of the transplants (33). It is widely recognized that the fewer mismatches of HLA-A, HLA-B, and HLA-DR between donors and recipients in KT, the smaller the rejection reaction, and the higher survival rate of the transplanted kidney (21, 34, 35). However, finding a well-matched donor may not be possible for all patients and usually prolongs waiting time (36). Furthermore, the introduction of modern immunosuppressive regimens markedly declined the emphasis on HLA matching (37). Even though using immunosuppressants after transplantation has been proved to improve the survival rate of both the recipients and the transplanted kidneys (34, 38–42), it can still increase the risk of infection and malignancy, as well as other attendant side effects (36). Therefore, understanding the effects of HLA mismatching on recipients is of great importance for increasing graft survival and further reducing the risk of recipient mortality. Previous studies on HLA mismatch have mainly focused on acute rejection and kidney transplant prognosis after transplantation. However, how does HLA mismatch affect the incidence of tumor after KT remains unclear. To this regard, our study aims to bridge this gap by analyzing the impact of both HLA mismatch and HLA subtype mismatch on the incidence of prostate cancer after KT in the United States. In addition, we also explore the necessity of induction therapy after KT from the perspective of prostate cancer occurrence. In order to make this retrospective study more comprehensive, the role of multiple baseline characteristics of the transplant recipients in the incidence of prostate cancer after KT were also investigated. We expect this retrospective study can provide the reference for prostate cancer screening and treatment strategies after KT.
2 Materials and methods
2.1 Study population
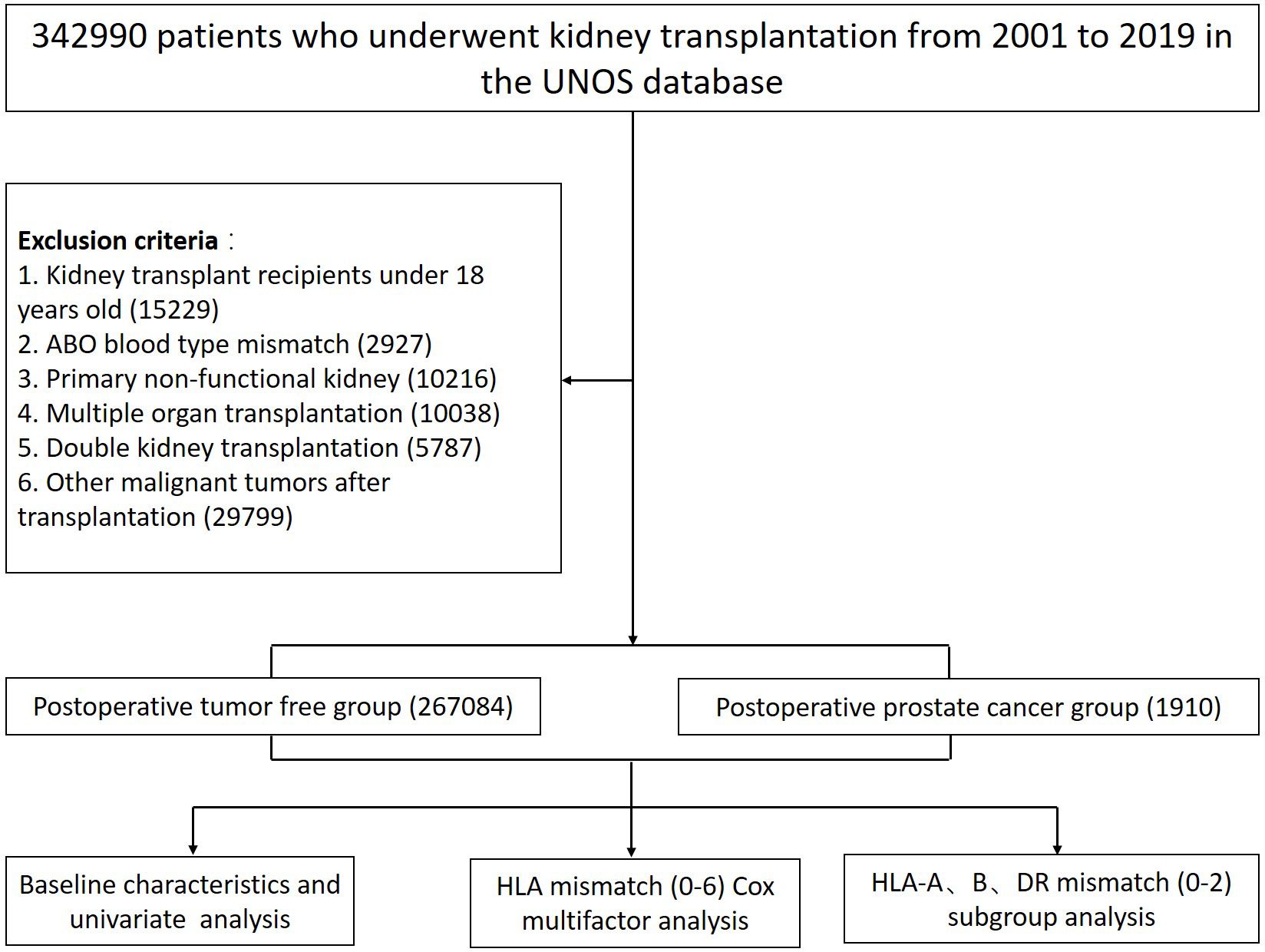
The present study retrospectively investigated all adult kidney transplant patients (i.e., age at transplantation ≥ 18 years) from 1 January 2000 to 31 December 2019 of the United Network for Organ Sharing (UNOS) database. Recipients with the following conditions including: (1) ABO blood type mismatch; (2) primary non-functional kidney; (3) multiple organ transplantation; (4) double KT; and (5) recipients developing other malignant cancers other than prostate cancer were excluded. 267–084 patients matched the inclusion criteria of whom 1–910 developed prostate cancer after KT were ultimately included (Figure 1).
2.2 Covariates and outcomes
The UNOS database records data regarding both recipient and allograft characteristics for all patients who received transplants. 14 variables were extracted from the UNOS database, including age, race, BMI, ABO blood type, cause of ESRD, commercial insurance, education level, history of malignant tumor, acute rejection, duration of dialysis, transplantation type, HLA mismatch number, whether to use interleukin-2 receptor subunit alpha (IL-2RA) or T cell therapy, and the type of immunosuppressive agents being used (i.e., cyclosporine (CSA), tacrolimus (TAC), mycophenolic acid (MPA), and mammalian target of rapamycin (mTOR) inhibitors). T cell therapy included ATGAM, Thymoglobulin, and Alemtuzumab.
The main observed outcomes of the study include 5-year and 10-year patient survival rates, as well as survival rate of transplanted kidneys. Patient survival refers to the time from transplantation to the death of the recipient. Graft survival refers to the time from transplantation to the failure of the transplanted kidney. When calculating survival rate, delete patients who have been lost to follow-up or died. Patients were divided into postoperative tumor free group and postoperative prostate cancer group.
2.3 Statistical analysis
The continuous variables were summarized using means and standard deviations, and were expressed as percentage as well. The age was expressed as the median (interquartile range (IQR)). Chi-square test and t-test were used to compare the baseline feature classification variables and continuous variables of patients, respectively. Kaplan Meier survival curves were presented after KT. The influencing factors of prostate cancer incidence were analyzed using Cox multiple regression, and HR and 95% confidence interval (CI) were calculated. A p-value of <0.05 was considered significant for all analyses. Inspection level α=0.05 (double tailed). Analyses were performed using R 3.6.2.
Patients were divided into postoperative tumor free group and postoperative prostate cancer group based on whether malignant tumors occur after surgery. Cox multivariate analysis was conducted on HLA (0–6), HLA-A (0–2), HLA-B (0–2), and HLA-DR (0–2) mismatch.
3 Results
3.1 Baseline characteristics and univariate analysis of prostate cancer incidence after KT
Univariate analysis showed that gender, age, race, cause of ESRD, education level, commercial insurance, history of malignant tumor, living transplantation, HLA mismatch, and use of immunosuppressants were the significant influencing factors of postoperative prostate cancer. As compared to the tumor free group, the prostate cancer group had an older average age (59 vs. 51), more white recipients (52.2% vs. 49.0%), shorter dialysis duration before transplantation (2 vs. 3), more likely to get college or graduate degree (51.0% vs. 46.3%), more likely to have private insurance (42.0% vs. 36.3%), more likely to have history of malignant tumor (1.0% vs. 0.2%), less likely to have acute rejection (2.4% vs. 9.7%), more likely to have HLA mismatch ≥3 (82.5% vs. 79.3%); more likely to use interleukin-2 receptor subunit alpha (31.5% vs. 25.7%), more likely to use CSA, TAC, MPA, MTOR (13.0% vs. 10.6%), (69.6% vs. 61.9%), (80.7% vs. 73.5%), (7.1% vs. 5.7%), less likely to use T cell therapy (51.7% vs. 58.8%). The leading cause of ESRD in the prostate cancer group was hypertensive nephrosclerosis (27.2%), while in the tumor free group was diabetes mellitus (25.2%) (all P < 0.05) (Table 1). In this research, recipient ABO blood types and living transplantation have no significant difference between the tumor free group and prostate cancer group.
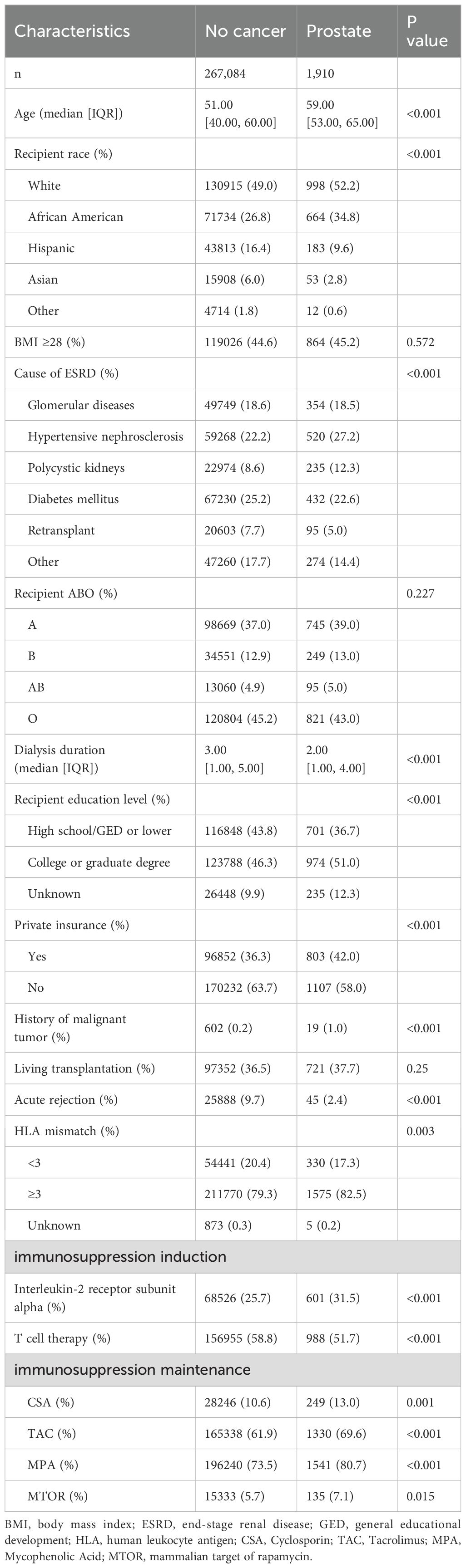
Table 1. Analysis of influencing factors for the incidence of prostate cancer in 268–994 recipients after KT.
3.2 Survival analysis between tumor free group and prostate cancer group
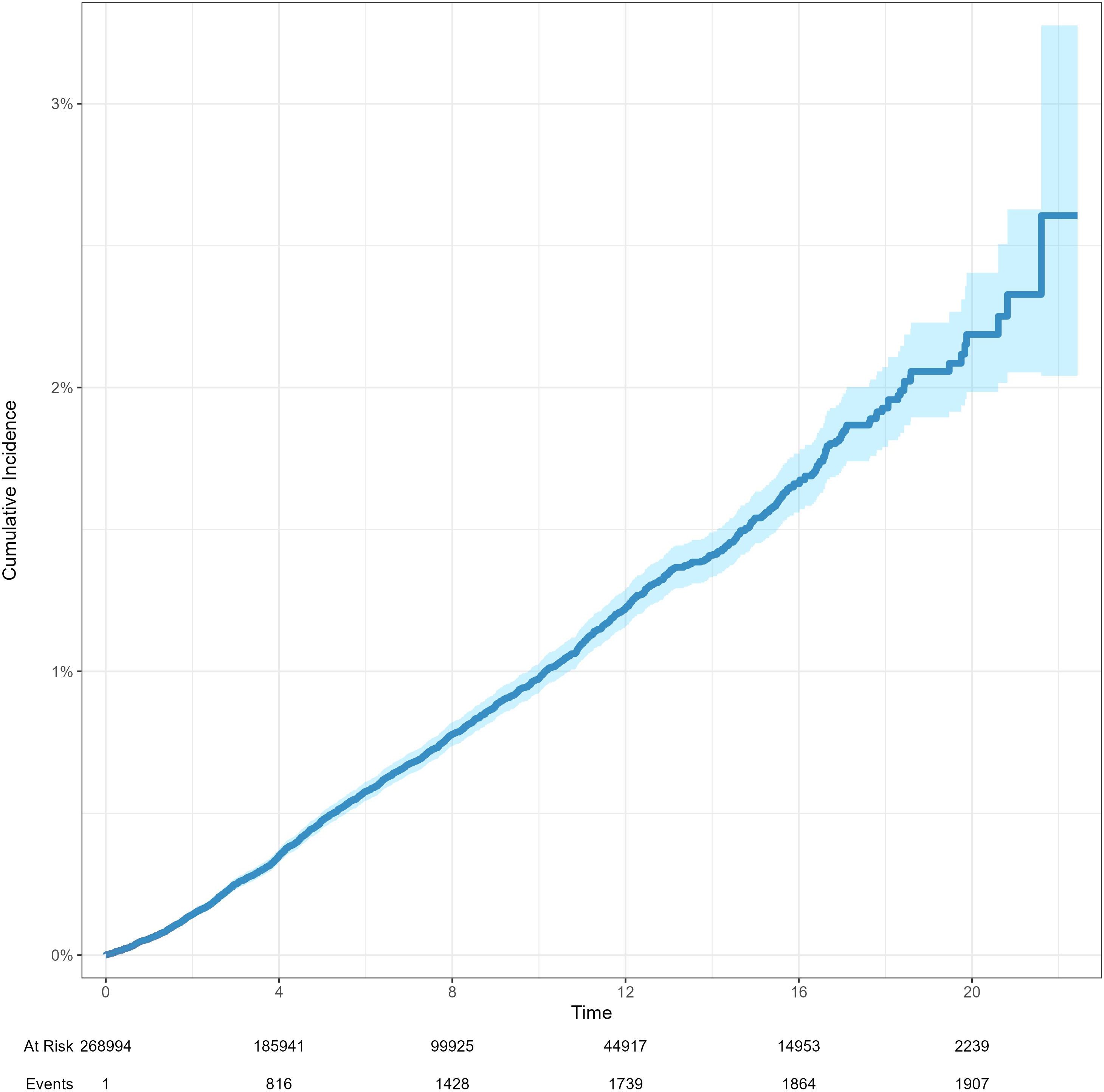
The 5-year and 10-year patient survival rates of postoperative tumor free group were 87.0% and 65.2%, respectively. The 5-year and 10-year patient survival rates of postoperative prostate cancer group were 93.4% and 74.4%, respectively. The 5-year and 10-year graft survival rates of postoperative tumor free group were 79.4% and 55.4%, respectively. The 5-year and 10-year graft survival rates of postoperative prostate cancer group were 91.9% and 70.4%, respectively. The 5-year and 10-year death censored graft survival rates of postoperative tumor free group were 88.0% and 73.1%, respectively. The 5-year and 10-year death censored graft survival rates of postoperative prostate cancer group were 96.8% and 88.5%, respectively (Figure 2). The cumulative incidence rates of prostate cancer and renal cancer after KT are presented in Figure 3 and Supplementary Figure 1, respectively.
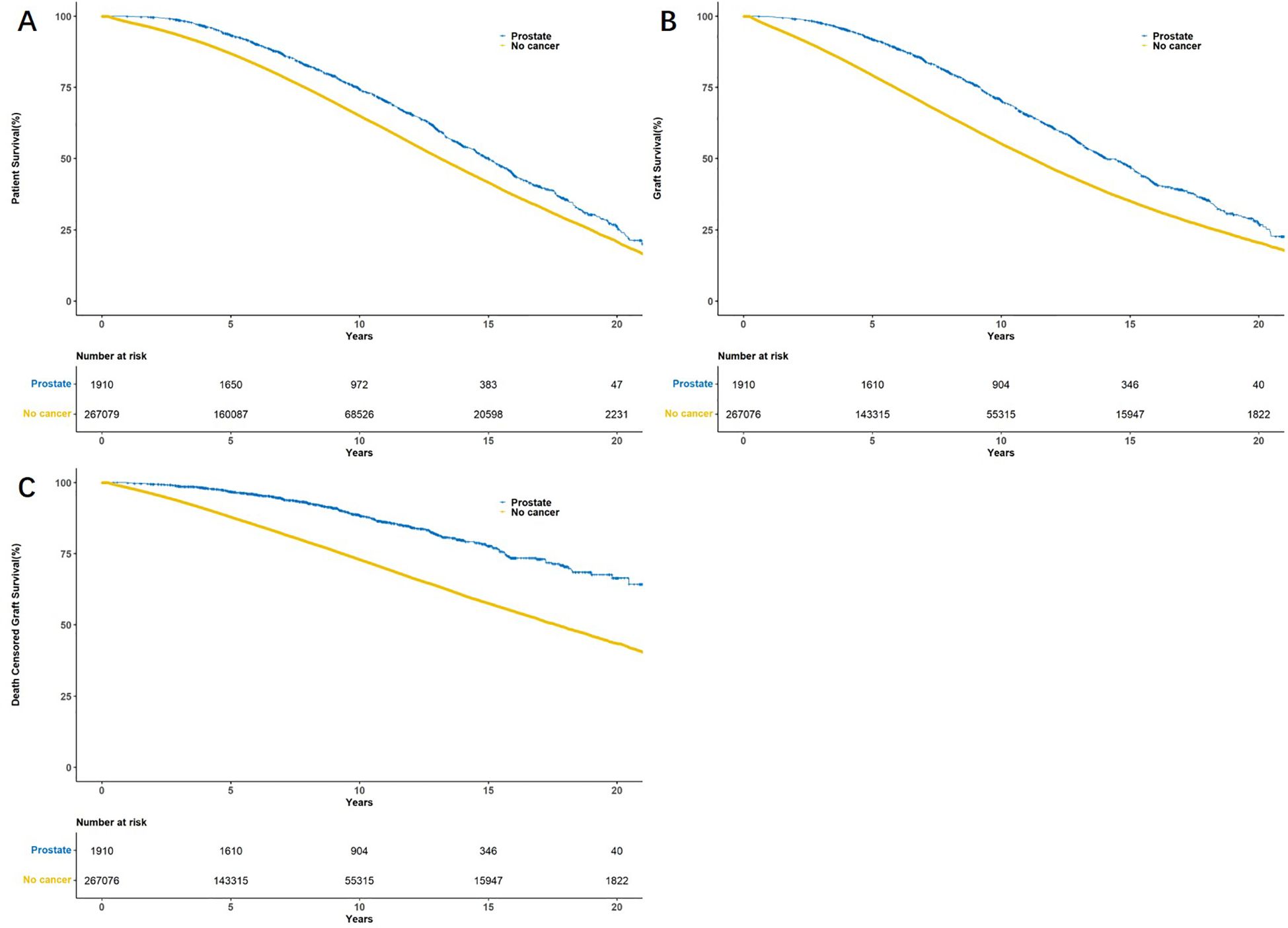
Figure 2. Kaplan–Meier survival curves showed patient survival (A), graft survival (B) and death censored graft survival (C) between tumor free group and prostate cancer group.
3.3 Cox multiple regression analysis of the influencing factors of prostate cancer incidence after KT
The adjusted Cox multivariate analysis showed that the elderly, white, history of malignant tumor, high education level, ESRD caused by polycystic kidney disease, nonprivate insurance, living transplantation, HLA mismatch ≥ 3, and the use of immunosuppressants were related to the incidence of prostate cancer after KT (Figure 4). HLA mismatch ≥ 3 is a risk factor of the occurrence of prostate cancer after KT [1.07(1.02~1.11)]. Multiple factor analysis was conducted on HLA match 0–6 and mismatch 0–6, while the results were not statistically significant (Figure 5).
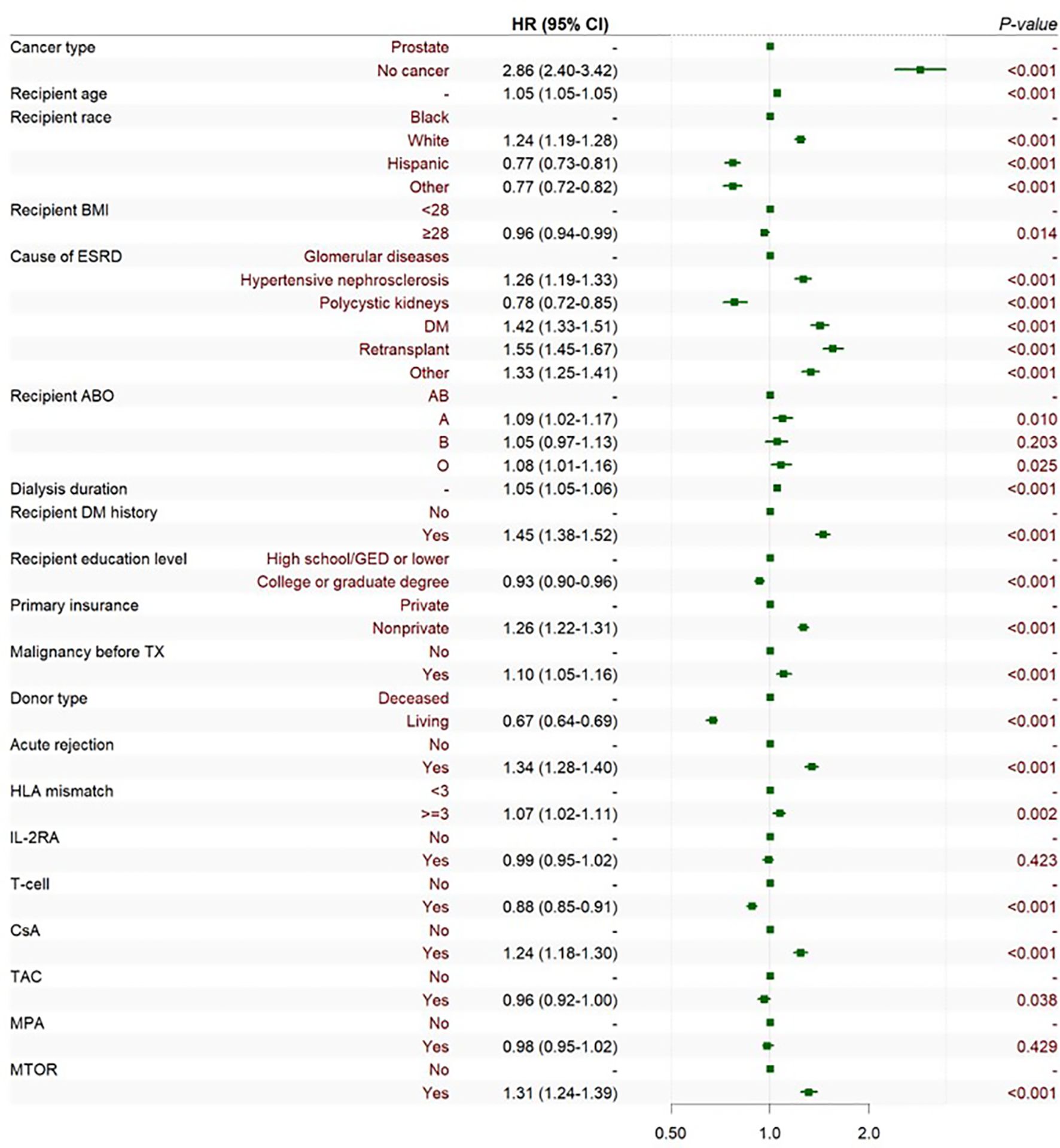
Figure 4. Cox multiple regression analysis of the influencing factors of prostate cancer incidence after KT. BMI is short for body mass index; ESRD is short for end-stage renal disease; DM is short for diabetes mellitus; GED is short for general educational development; TX is short for transplantation; HLA is short for human leukocyte antigen; CSA is short for Cyclosporin; TAC is short for Tacrolimus; MPA is short for Mycophenolic Acid; and MTOR is short for mammalian target of rapamycin.
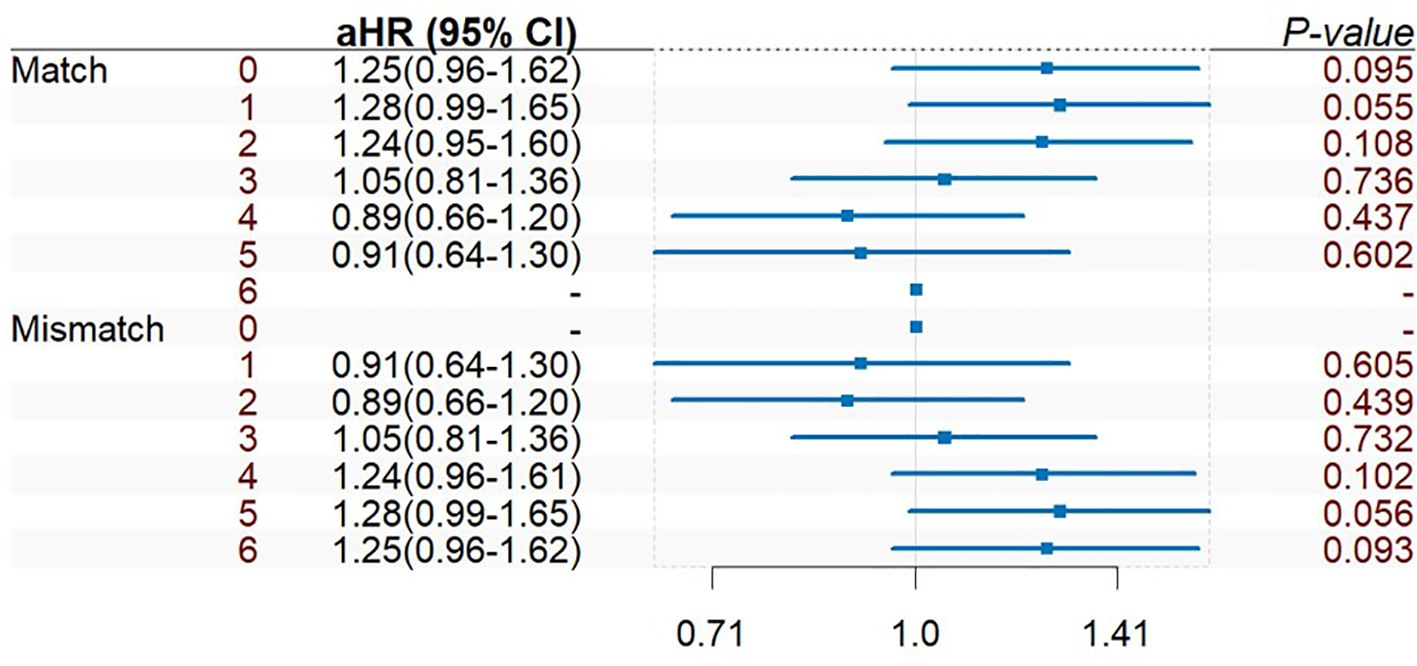
Figure 5. Multiple factor analysis of HLA match 0–6 and mismatch 0–6 of prostate cancer incidence after KT.
During induction therapy after KT, the use of T cell therapy could reduce the risk of prostate cancer [0.89(0.86~0.91)]. For maintenance therapy after KT, the use of TAC might be the protective factor of prostate cancer [0.92(0.96~1.00)], while CSA and mTOR could increase the risk of prostate cancer [1.24(1.18~1.31)], [1.31(1.24~1.39)]. Additional era-stratified analyses were performed to further validate our findings (Figure 6). During 2005–2009 and 2010-2014, T cell therapy was associated with reduced prostate cancer risk ([0.92(0.86~0.99)], [0.91(0.85~0.99)], respectively). This protective effect, however, did not reach statistical significance in the 2000–2004 and 2015–2019 cohorts.
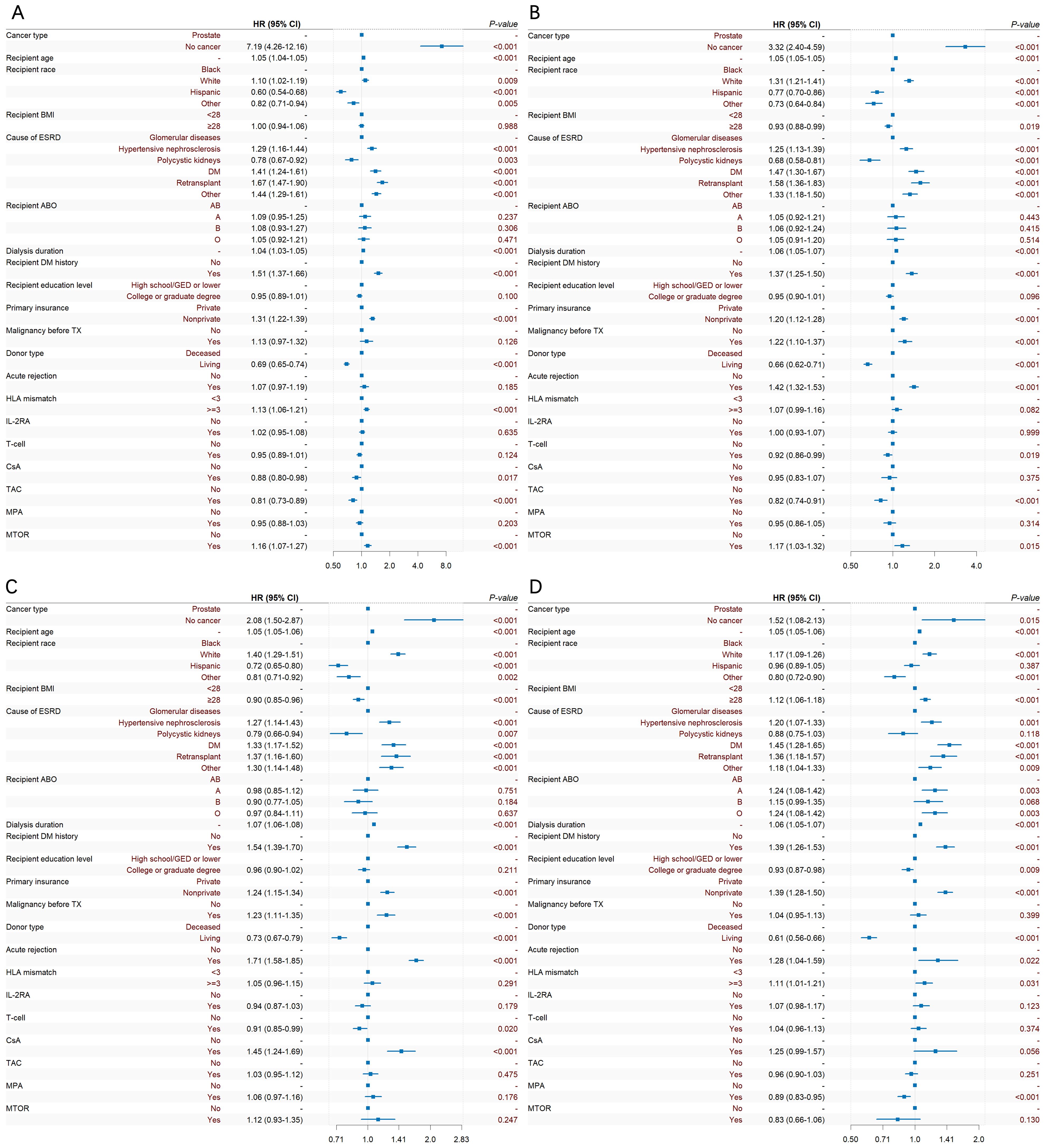
Figure 6. Temporal trends in prostate cancer risk factors after KT: Forest plots of Cox regression analyses stratified by era. (A) 2000-2004, (B) 2005-2009, (C) 2010-2014, (D) 2015-2019.
3.4 Subgroup analysis of HLA match and mismatch on the occurrence of prostate cancer after KT
Cox multiple regression analysis was also performed on HLA-A, HLA-B, HLA-DR mismatch 0–2 and match 0–2 (Figure 7). In the subgroup analysis results, it was found that HLA-A, HLA-B, and HLA-DR groups had consistent HLA 0-match and 2-mismatch results, while HLA 1-match and 1-mismatch results were consistent. 2-mismatch in HLA-A and HLA-B groups was a risk factor of prostate cancer after KT [1.19(1.01~1.40)]; 2-mismatch and 1-mismatch were both risk factors of prostate cancer after KT in the HLA-DR group [1.32(1.13~1.54)], [1.20(1.03~1.39)].
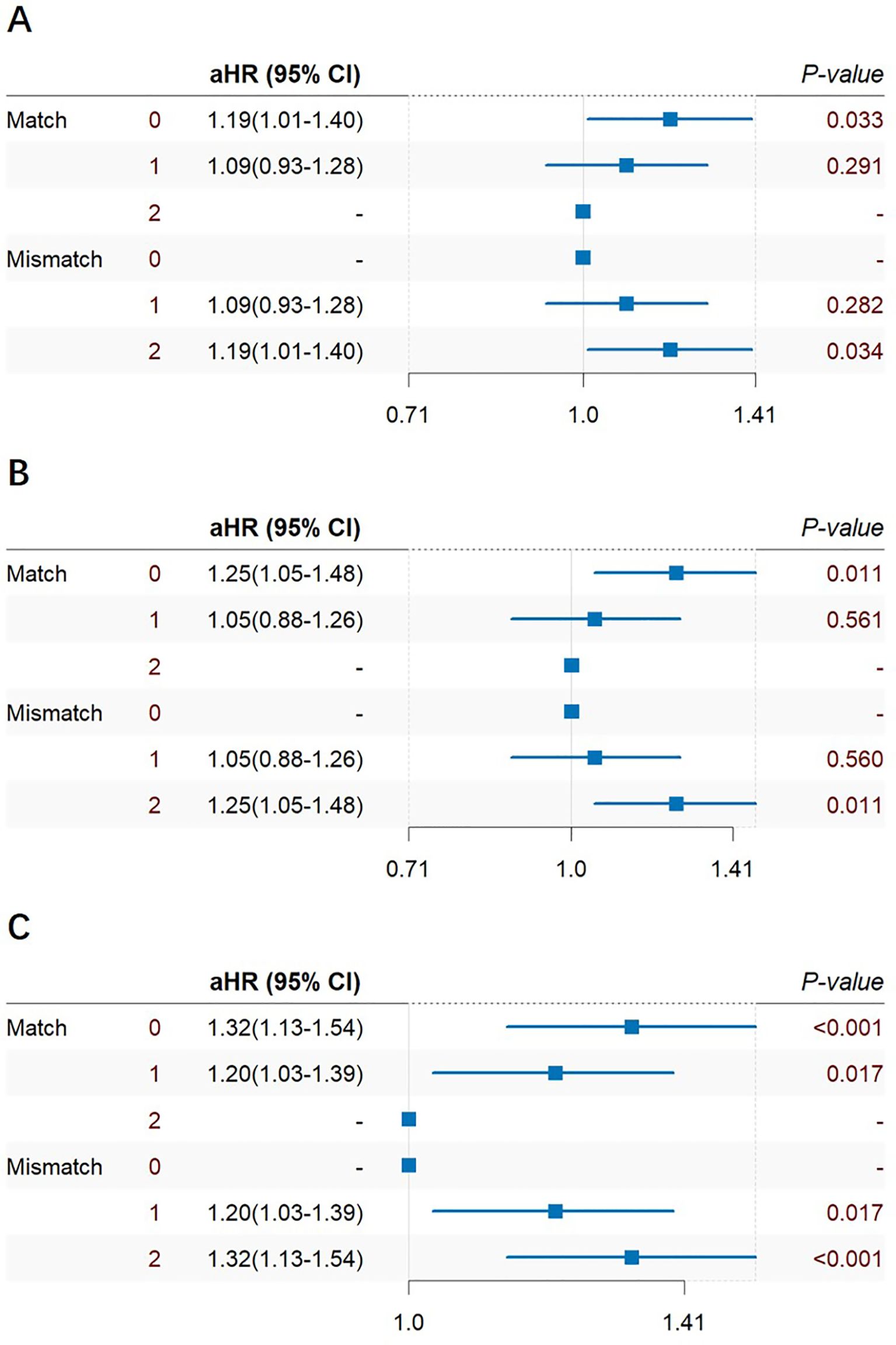
Figure 7. Cox multiple regression analysis of HLA-A (A), HLA-B (B), HLA-DR (C) match 0–2 and mismatch 0–2 of prostate cancer incidence after KT.
4 Discussion
The Cox multivariate analysis showed that the elderly, white, history of malignant tumor, nonprivate insurance, HLA mismatch ≥ 3, and the use of CSA could all significantly increase the risk of prostate cancer after KT, while higher education, ESRD caused by polycystic kidney, living transplantation, the use of T cell therapy and TAC could reduce the risk of prostate cancer after KT. Additional era-stratified analyses were performed to clarify the protective effect of T cell therapy. Collectively, these data suggest that the protective association of T cell therapy against prostate cancer has become increasingly evident with the evolution of immunosuppressive regimens. Notably, while Cox multivariate models demonstrated significant risk reduction during 2005-2014, the 2015–2019 cohort showed no statistically significant association. This non-significance may reflect the relatively short median follow-up in this recent era, warranting extended surveillance for definitive assessment.
Interestingly, in terms of survival analysis, the 5-year and 10-year survival rates of prostate cancer are better than the tumor free group after transplantation. The possible reasons are as follows: Patients with malignant tumors after KT have more regular follow-up, and prostate cancer have lower invasiveness and less distant metastasis. Regular physical examination and early treatment make the prognosis of patients with prostate cancer better than the tumor free group. Furthermore, prostate cancer diagnosis inherently requires sufficient post-transplant survival time to be detected. This immortal time bias may artificially inflate observed survival rates in the cancer cohort.
It is general acknowledged in tumor immunology that the occurrence and progression of tumors is due to the decrease in the body’s immune surveillance ability, which leads to the immune escape of abnormally growing potential tumor cells (43, 44). The decline in immune function implies natural immune tolerance, which may benefit patients in reducing transplant kidney rejection. In addition, transplant patients with HLA mismatch ≥ 3 would normally use stronger immunosuppressive regimens after surgery, and thus the decrease in the patient’s immune function may increase the risk of tumor development after transplantation.
The multivariate subgroup analysis of HLA mismatch 0–6 and match 0–6 showed no statistical significance. HLA-A, HLA-B 2-mismatch were risk factors of prostate cancer after KT, while HLA-DR both 1-mismatch and 2-mismatch were risk factors of prostate cancer after KT, which can be explained as follows: Firstly, HLA-A, HLA-B 2-mismatch increased the risk of prostate cancer after transplantation, while 1-mismatch results were not statistically significant, indicating that an increase in mismatches in HLA-A and HLA-B could add to the risk of prostate cancer after transplantation. HLA-A and HLA-B belong to MHC class I antigens, and former research have shown that abnormal MHC-I expression and function regulation may be hijacked by tumor cells to evade immune surveillance, thereby promoting tumor progression and impairing the efficacy of cancer immunotherapy (45–47). In addition, in the subgroup analysis of HLA-DR, 1-mismatch and 2-mismatch were both risk factors of prostate cancer after KT, indicating the special role of HLA-DR in HLA matching during transplantation. Previous studies on KT have found a significant correlation between the number of HLA-DR mismatches and the incidence of non-Hodgkin’s lymphoma and hip fractures (48). For example, the research based on hematopoietic stem cell transplantation (HSCT) by Biernacki has found out that most recurrent HSCT recipients have 3- to 12- fold reduction in HLA class II gene expression (49). IFN-γ exposure can reverse class II downregulation, while HLA class I expression is not downregulated, possibly due to the specific effect of CD4+T cells on HLA class II antigens during the immune response process after transplantation. In KT, the role of HLA-DR still requires more in-depth basic research.
In this study, we observed that HLA mismatch ≥ 3 also increases the risk of renal carcinoma after KT [1.05(1.03~1.09)] (Supplementary Table 1, Supplementary Figure 2). Further subgroup analysis showed that the results of HLA-A, HLA-B, and HLA-DR groups were not statistically significant (Supplementary Figure 3, Supplementary Figure 4), which indicate that there are differences in the impact of HLA mismatch in different types of tumors after KT, and further research is required to verify this conclusion.
Our study still has certain limitations. Firstly, the study could not explain the differences in HLA match and mismatch between kidney transplant donors and recipients. As shown in Table 2, there is no corresponding relationship between HLA match and mismatch due to the presence of NA. Specifically, an NA cannot be classified as either a match or a mismatch. This ambiguity can lead to analytical inconsistencies. In the case of HLA 1-mismatch, 3-match, 4-match, and 5-match can be present. However, the Cox multivariate analysis and subgroup analysis results of this study showed a one-to-one correspondence between match and mismatch, which is inconsistent with the expected results. This issue has been mentioned before, but still remained unclear (50). The reason may be attributed to the advancement of HLA detection technology, which in other words, the HLA loci that could not be detected before 2000 can now be detected, resulting in very few cases of HLA typing as NA. In this case, the Cox multivariate analysis results of HLA match and mismatch are consistent. Secondly, this retrospective study lacks control interventions for all confounding factors. Although the variables collected in UNOS are sufficiently rich, they still do not fully cover all the baseline characteristics of patients, which is also one of the drawbacks of retrospective studies. Thirdly, our study observed higher survival rates in kidney transplant recipients diagnosed with prostate cancer compared to non-cancer controls. Specifically, both 5-year and 10-year survival outcomes were significantly better in the prostate cancer group. This counterintuitive finding may be attributable to immortal time bias, as patients must survive long enough after transplantation to receive the cancer diagnosis. This methodological limitation could potentially inflate survival estimates in the cancer group. The persistent influence of this bias cannot be fully excluded. Further validation studies using validated methods and extended follow-up analyses are warranted to confirm these findings.
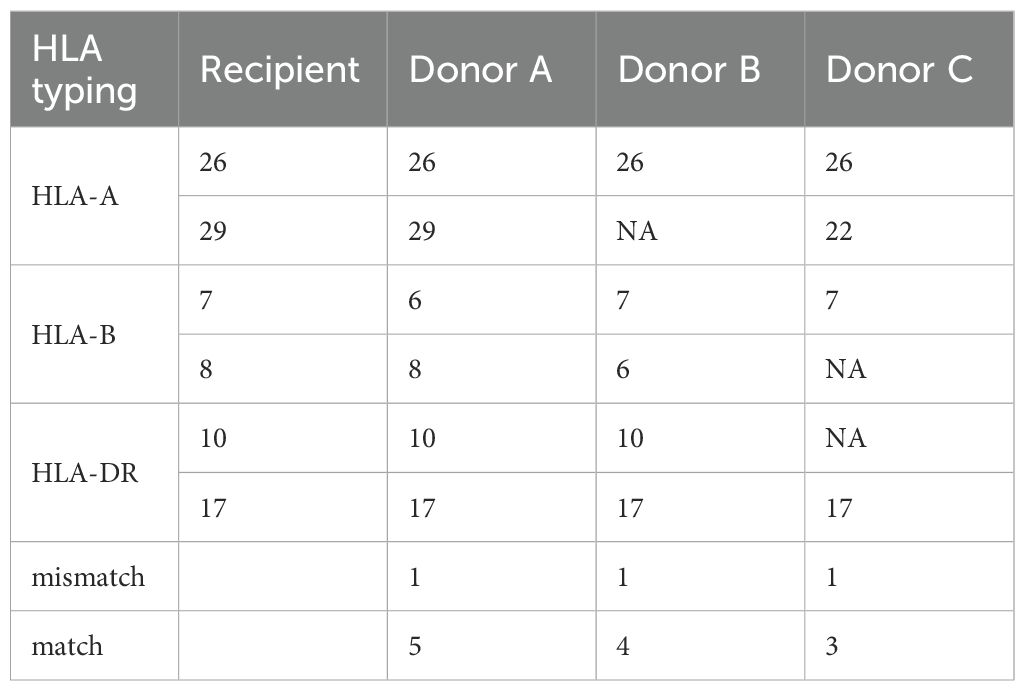
Table 2. In the case of HLA 1-mismatch between recipient and donors, 5-match, 4-match, and 3-match can be present with donor A, B, and C, respectively.
5 Conclusions
This retrospective study quantitatively analyzed the impact of: (1) baseline patient characteristics; (2) HLA mismatch; and (3) HLA subtype mismatch on the incidence of prostate cancer after KT. The use of T cell therapy could reduce the risk of prostate cancer after transplantation. HLA mismatch ≥ 3 is a risk factor of prostate cancer after KT. HLA-A and HLA-B 2-mismatch are risk factors of prostate cancer after KT, while HLA-DR 1-mismatch and 2-mismatch are both risk factors of prostate cancer after KT. The research contributes to emphasize advantages of T cell therapy from the perspective of prostate cancer occurrence, focus on HLA mismatch in KT and provide guidance for reasonable selection of HLA typing of prostate cancer.
Data availability statement
The data analyzed in this study were sourced from a publicly available dataset, available at: https://optn.transplant.hrsa.gov/data/request-data/.
Author contributions
YG: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. JL: Formal analysis, Investigation, Supervision, Writing – original draft. JM: Methodology, Validation, Writing – review & editing. AJ: Data curation, Investigation, Writing – original draft. WG: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. SF: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding: Our research was funded by the Chinese National Natural Science Funds (82371792).
Acknowledgments
We gratefully acknowledge the research guided by Dr. Qing Yuan at the Third Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1562869/full#supplementary-material
References
1. Moein M, Otero MT, Rood GJ, Hanlon M, and Saidi R. Kidney transplantation and obesity: are there any differences in outcomes? World J Surg. (2023) 47:510–8. doi: 10.1007/s00268-022-06806-4
2. Kostroa JZ, Bzoma B, Proczko-Stepaniak M, Hellmann AR, Hać S, Kaska Ł., et al. Kidney transplantation in patients after bariatric surgery: high-volume bariatric and transplant center experience. Transplant Proc. (2022) 54:955–9. doi: 10.1016/j.transproceed.2022.03.014
3. Mudiayi D, Shojai S, Okpechi I, Christie EA, Wen K, Kamaleldin M, et al. Global estimates of capacity for kidney transplantation in world countries and regions. Transplantation. (2022) 106:1113–22. doi: 10.1097/TP.0000000000003943
4. Karadag S, Eksi M, Ozdemir O, Kargi T, Haciislamoglu A, Evren I, et al. Comparison of open and robot-Assisted kidney transplantation in terms of perioperative and postoperative outcomes. Int J Clin Pract. (2022) 2022:2663108. doi: 10.1155/2022/2663108
5. Kanbay M, Ureche C, Copur S, Covic AM, Tanriover C, Sekmen M, et al. Kidney transplantation: is it a solution to endothelial dysfunction? Int Urol Nephrol. (2023) 55:1183–91. doi: 10.1007/s11255-022-03415-x
6. Gunawardena T and Ridgway D. Transplant nephrectomy: current concepts. Saudi J Kidney Dis Transpl. (2022) 33:716–25. doi: 10.4103/1319-2442.389431
7. Barmoussa O, Bentata Y, and Haddiya I. Is there gender discrimination in living-donor kidney transplantation? Saudi J Kidney Dis T. (2022) 33:168–71. doi: 10.4103/1319-2442.367810
8. Fröhlich FA, Halleck F, Lehner L, Schrezenmeier EV, Naik M, Schmidt D, et al. De-novo Malignancies after kidney transplantation: A long-term observational study. PloS One. (2020) 15:e0242805. doi: 10.1371/journal.pone.0242805
9. Vajdic CM and van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. (2009) 125:1747–54. doi: 10.1002/ijc.24439
10. Al-Adra D, Al-Qaoud T, Fowler K, and Wong G. De novo Malignancies after kidney transplantation. Clin J Am Soc Nephrol. (2022) 17:434–43. doi: 10.2215/CJN.14570920
11. Buxeda A, Redondo-Pachon D, Perez-Saez MJ, Crespo M, and Pascual J. Sex differences in cancer risk and outcomes after kidney transplantation. Transplant Rev (Orlando). (2021) 35:100625. doi: 10.1016/j.trre.2021.100625
12. Patel JA, Daoud D, and Jain A. Review of Standardized Incidence Ratios (SIR) of non-lymphoid de novo Malignancies after liver transplantation: Structured analysis of global differences. Transplant Rev (Orlando). (2022) 36:100670. doi: 10.1016/j.trre.2021.100670
13. Turshudzhyan A. Post-renal transplant Malignancies: Opportunities for prevention and early screening. Cancer Treat Res Commun. (2021) 26:100283. doi: 10.1016/j.ctarc.2020.100283
14. Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate cancer epidemiology and risk factors-A systematic review. Eur Urol. (2023) 84:191–206. doi: 10.1016/j.eururo.2023.04.021
15. Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. (2021) 4:877–92. doi: 10.1016/j.euo.2021.09.006
16. Ng MSY, Ullah S, Wilson G, McDonald S, Sypek M, and Mallett AJ. ABO blood group relationships to kidney transplant recipient and graft outcomes. PloS One. (2020) 15:e0236396. doi: 10.1371/journal.pone.0236396
17. Lin MY, Kuo MC, Hung CC, Wu WJ, Chen LT, Yu ML, et al. Association of dialysis with the risks of cancers. PloS One. (2015) 10:e0122856. doi: 10.1371/journal.pone.0122856
18. Franco-García JM, Castillo-Paredes A, Rodríguez-Redondo Y, Carlos-Vivas J, García-Carrillo RM, and Denche-Zamorano Á. Greater physical activity levels are associated with lower prevalence of tumors and risk of cancer in Spanish population: A cross-sectional study. Heliyon. (2024) 10:e29191. doi: 10.1016/j.heliyon.2024.e29191
19. Ulanja MB, Ntafam C, Beutler BD, Antwi-Amoabeng D, Rahman GA, Ulanja RN, et al. Race, age, and sex differences on the influence of obesity on colorectal cancer sidedness and mortality: A national cross-sectional study. J Surg Oncol. (2023) 127:109–18. doi: 10.1002/jso.27096
20. Liu DH, Mou FF, An M, and Xia P. Human leukocyte antigen and tumor immunotherapy (Review). Int J Oncol. (2023) 62:68. doi: 10.3892/ijo.2023.5516
21. Kim JJ, Fuggle SV, and Marks SD. Does HLA matching matter in the modern era of renal transplantation? Pediatr Nephrol. (2021) 36:31–40. doi: 10.1007/s00467-019-04393-6
22. Barbaro G, Inversetti A, Cristodoro M, Ticconi C, Scambia G, and Di Simone N. HLA-G and recurrent pregnancy loss. Int J Mol Sci. (2023) 24:2557. doi: 10.3390/ijms24032557
23. Scavuzzi BM, van Drongelen V, and Holoshitz J. HLA-G and the MHC cusp theory. Front Immunol. (2022) 13:814967. doi: 10.3389/fimmu.2022.814967
24. Bruijnesteijn J. HLA/MHC and KIR characterization in humans and non-human primates using Oxford Nanopore Technologies and Pacific Biosciences sequencing platforms. Hla. (2023) 101:205–21. doi: 10.1111/tan.14957
25. Naito T and Okada Y. HLA imputation and its application to genetic and molecular fine-mapping of the MHC region in autoimmune diseases. Semin Immunopathol. (2022) 44:15–28. doi: 10.1007/s00281-021-00901-9
26. Beretta-Piccoli BT, Mieli-Vergani G, and Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. (2022) 19:158–76. doi: 10.1038/s41423-021-00768-8
27. Wolday D, Fung CYJ, Morgan G, Casalino S, Frangione E, Taher J, et al. HLA variation and SARS-coV-2 specific antibody response. Viruses. (2023) 15:906. doi: 10.3390/v15040906
28. Admon A. The biogenesis of the immunopeptidome. Semin Immunol. (2023) 67:101766. doi: 10.1016/j.smim.2023.101766
29. Plasil M, Futas J, Jelinek A, Burger PA, and Horin P. Comparative genomics of the major histocompatibility complex (MHC) of felids. Front Genet. (2022) 13:829891. doi: 10.3389/fgene.2022.829891
30. Sandalova T, Sala BM, and Achour A. Structural aspects of chemical modifications in the MHC-restricted immunopeptidome; Implications for immune recognition. Front Chem. (2022) 10:861609. doi: 10.3389/fchem.2022.861609
31. Dressman D and Elyaman W. T cells: A growing universe of roles in neurodegenerative diseases. Neuroscientist. (2022) 28:335–48. doi: 10.1177/10738584211024907
32. Ziemann M, Suwelack B, Banas B, Budde K, Einecke G, Hauser I, et al. Determination of unacceptable HLA antigen mismatches in kidney transplant recipients. Hla. (2022) 100:3–17. doi: 10.1111/tan.14521
33. Van Loon E, Bernards J, Van Craenenbroeck AH, and Naesens M. The causes of kidney allograft failure: more than alloimmunity. A viewpoint article. Transplantation. (2020) 104:e46–56. doi: 10.1097/TP.0000000000003012
34. Callemeyn J, Lamarthée B, Koenig A, Koshy P, Thaunat O, and Naesens M. Allorecognition and the spectrum of kidney transplant rejection. Kidney Int. (2022) 101:692–710. doi: 10.1016/j.kint.2021.11.029
35. Ehlayel A, Simms KJA, and Ashoor IF. Emerging monitoring technologies in kidney transplantation. Pediatr Nephrol. (2021) 36:3077–87. doi: 10.1007/s00467-021-04929-9
36. Zachary AA and Leffell MS. HLA mismatching strategies for solid organ transplantation - A balancing act. Front Immunol. (2016) 7:575. doi: 10.3389/fimmu.2016.00575
37. Hafeez MS, Awais SB, Razvi M, Bangash MH, Hsiou DA, Malik TH, et al. HLA mismatch is important for 20-year graft survival in kidney transplant patients. Transpl Immunol. (2023) 80:101861. doi: 10.1016/j.trim.2023.101861
38. Kamal J and Doyle A. Immunosuppression and kidney transplantation. Handb Exp Pharmacol. (2022) 272:165–79. doi: 10.1007/164_2021_546
39. Aiyegbusi O, McGregor E, McManus SK, and Stevens KI. Immunosuppression therapy in kidney transplantation. Urol Clin North Am. (2022) 49:345–60. doi: 10.1016/j.ucl.2021.12.010
40. Cheung CY and Tang SCW. Personalized immunosuppression after kidney transplantation. Nephrol (Carlton). (2022) 27:475–83. doi: 10.1111/nep.14035
41. Qayyum S and Shahid K. Comparative safety and efficacy of immunosuppressive regimens post-kidney transplant: A systematic review. Cureus. (2023) 15:e43903. doi: 10.7759/cureus.43903
42. Wong G and Lim WH. Prior cancer history and suitability for kidney transplantation. Clin Kidney J. (2023) 16:1908–16. doi: 10.1093/ckj/sfad141
43. Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P, et al. NK cells and ILCs in tumor immunotherapy. Mol Aspects Med. (2021) 80:100870. doi: 10.1016/j.mam.2020.100870
44. Blass E and Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. (2021) 18:215–29. doi: 10.1038/s41571-020-00460-2
45. Wu XY, Li TH, Jiang R, Yang X, Guo HQ, and Yang R. Targeting MHC-I molecules for cancer: function, mechanism, and therapeutic prospects. Mol Cancer. (2023) 22:194. doi: 10.1186/s12943-023-01899-4
46. Rana PS, Ignatz-Hoover JJ, and Driscoll JJ. Targeting proteasomes and the MHC class I antigen presentation machinery to treat cancer, infections and age-related diseases. Cancers (Basel). (2023) 15:5632. doi: 10.3390/cancers15235632
47. Schenkel JM and Pauken KE. Localization, tissue biology and T cell state - implications for cancer immunotherapy. Nat Rev Immunol. (2023) 23:807–23. doi: 10.1038/s41577-023-00884-8
48. Opelz G and Döhler B. Ceppellini Lecture 2012: Collateral damage from HLA mismatching in kidney transplantation. Tissue Antigens. (2013) 82:235–42. doi: 10.1111/tan.12147
49. Biernacki MA, Sheth VS, and Bleakley M. T cell optimization for graft-versus-leukemia responses. JCI Insight. (2020) 5:e134939. doi: 10.1172/jci.insight.134939
Keywords: kidney transplantation (KT), HLA typing, prostate cancer, UNOS/OPTN, induction therapy
Citation: Gao Y, Li J, Mao J, Jiang A, Guo W and Fu S (2025) Analyzing the impact of human leukocyte antigen mismatch on the incidence of prostate cancer and the advantage of T cell therapy in patients after kidney transplantation based on the United Network for Organ Sharing database. Front. Oncol. 15:1562869. doi: 10.3389/fonc.2025.1562869
Received: 18 January 2025; Accepted: 21 August 2025;
Published: 10 September 2025.
Edited by:
Claude Lambert, Centre Hospitalier Universitaire (CHU) de Saint-Étienne, FranceReviewed by:
Yupeng Wu, First Affiliated Hospital of Fujian Medical University, ChinaDiaa Eldin Mohamed, Kafrelsheikh University, Egypt
Copyright © 2025 Gao, Li, Mao, Jiang, Guo and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shangxi Fu, ZnVzaGFuZ3hpQDEyNi5jb20=; Wenyuan Guo, Z3Vvd2VueXVhbkBzbW11LmVkdS5jbg==
 Yang Gao
Yang Gao Jingjing Li
Jingjing Li Jiaxi Mao
Jiaxi Mao Aijun Jiang1
Aijun Jiang1