- 1The First Affiliated Hospital of Guangxi University of Science and Technology, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
- 2School of Economics and Management, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
- 3Guangxi College Key Laboratory of Innovation Research on Medical and Engineering Integration, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Introduction: This meta-analysis was designed to compare the outcomes of preoperative exercise training versus no preoperative exercise for lung cancer patients scheduled for lung resection.
Materials and methods: Four databases (Medline, Embase, Web of Science, and CENTRAL) were searched for randomized controlled trials (RCTs) comparing preoperative exercise training versus no preoperative exercise for lung cancer patients scheduled for lung resection. The primary outcomes were postoperative complications and postoperative length of hospital stay. The secondary outcomes included post-intervention pulmonary function, severe postoperative complications, postoperative 30-day mortality, postoperative duration of chest tube drainage, post-intervention dyspnea, and post-intervention health-related quality of life (HRQoL).
Results: A total of 16 RCTs with 1,022 individuals were included in this meta-analysis. Compared with no preoperative exercise, preoperative exercise training significantly reduced the postoperative complications (OR = 0.33, 95%CI: 0.24 to 0.46, P < 0.0001) and postoperative length of hospital stay (95%CI: −3.11 to −1.40, P < 0.0001). In addition, preoperative exercise training significantly improved forced expiratory volume in 1 s (FEV1%) of predicted norm values (95%CI: 5.30 to 8.10, P < 0.0001), forced vital capacity (FVC%) of predicted norm values (95%CI: 1.90 to 4.23, P < 0.0001), peak expiratory flow (PEF) (95%CI: 12.44 to 60.93, P = 0.003), and peak oxygen uptake (VO2peak) (95%CI: 2.41 to 4.17, P < 0.0001), while reducing severe postoperative complications (OR = 0.35, 95%CI: 0.21 to 0.56, P < 0.0001) and post-intervention dyspnea (95%CI: −0.61 to 0.04, P = 0.02). There was no significant difference between the two groups regarding FEV1, FVC, carbon monoxide diffusing capacity (DLCO), six-minute walk distance (6MWD), postoperative 30-day mortality, postoperative chest tube drainage time, and post-intervention HRQoL.
Conclusions: This meta-analysis indicated that preoperative exercise training was effective for lung cancer patients scheduled for lung resection, potentially reducing postoperative complications and hospital stay duration, while improving post-intervention pulmonary function and exercise capacity.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024607156.
1 Introduction
Lung cancer is the leading cause of cancer-related morbidity and mortality, responsible for around 2.5 million new cases and over 1.8 million fatalities globally. It accounts for approximately one in eight (12.4%) cancer diagnoses worldwide and one in five (18.7%) cancer-related deaths. The disease ranks first in incidence and mortality among men and second among women (1, 2). Surgery often leads to postoperative complications, which prolong hospitalization, increase the probability of admission to the critical care unit, and elevate mortality rates during the perioperative period (3). Postoperative outcomes are affected by multiple factors, including the type of surgical procedure, cancer stage, gender, and neoadjuvant medications; however, emerging evidence suggests that patients’ physical functions are pivotal. Pulmonary function and cardiorespiratory fitness prior to surgery have been recognized as predicting factors for postoperative complications and overall survival in lung cancer patients (4).
Exercise training is a systematic, organized, and repetitive kind of physical activity designed to enhance or sustain physical fitness as a primary or secondary objective (5). Studies demonstrated that exercise training enhanced functional and cardiorespiratory fitness (CRF) in persons with chronic obstructive pulmonary disease (6–8). This comprehension has led to the integration of exercise training into the preoperative therapy of patients scheduled for lung resection owing to lung cancer, with the objective of improving physical fitness to overcome the physiological stress caused by surgery, hence reducing postoperative morbidity and mortality. Multiple worldwide guidelines have been established to mandate specific perioperative cardiopulmonary exercise testing prior to the initiation of preoperative exercise training (9), in order to improve patients’ physical function and better manage the homeostatic disruption and stress response associated with surgery (10). Preoperative exercise training in patients slated for lung resection aims to improve health, namely, aerobic fitness, during the period between diagnosis and surgery, thereby reducing the risk of complications and decreasing hospital length of stay (LoS) (11). Preoperative exercise training has shown a decrease in hospitalizations and postoperative complications in patients following lobectomies or lung resections (12).
A previous meta-analysis indicated that higher preoperative cardiorespiratory fitness was associated with a reduction in postoperative pulmonary complications (13). Preoperative sarcopenia, characterized by diminished skeletal muscle mass and strength, might negatively impact postoperative outcomes, including complications and overall survival in colorectal, esophageal, pancreatic, and bladder cancers (14). Sarcopenia can develop into frailty, characterized by diminished reserve and resistance to stressors due to cumulative losses in numerous organ systems, resulting in an increased prevalence of unfavorable consequences, which is an independent risk factor for surgical complications, extended hospital stay, and fatality (15–18). Therefore, it is clear that enhancing the functional and physiological capacities of individuals is crucial for their ability to withstand stressful events like surgery and to promote recovery afterward (19). Postoperative complications are common in elderly individuals with low physical fitness, physical inactivity, malnutrition, and tobacco-related comorbidities (2, 20–22).
The available data for preoperative exercise training for patients with lung cancer are somewhat restricted. The previous systematic review on this subject demonstrated that preoperative exercise training decreased the incidence of postoperative complications, decreased LoS, and enhanced postoperative exercise capacity (23). Yet, this conclusion was derived from a mere 10 studies. More recently, another meta-analysis has yielded comparable findings (24). Regrettably, the conclusions were constrained by methodological limitations: four of the 16 studies included were not RCTs, which might have resulted in bias. Therefore, we conducted an updated meta-analysis that exclusively included RCTs, with the aim of providing clearer insights into the outcomes of patients with lung cancer who received preoperative exercise training and informing clinical decision-making.
2 Materials and methods
2.1 Search strategy
The present meta-analysis carefully followed the guidelines established by the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines. The study has been formally registered at PROSPERO with the designation number CRD42024607156. A systematic search was conducted in four databases, namely, PubMed, Embase, Web of Science, and the Cochrane Library, to identify literature items published up to July 22, 2024. The search strategy used a combination of MeSH and free-text words following the PICOS principle. The search keywords were “lung cancer” AND “preoperative exercise” AND “randomized controlled trial”. Supplementary Tables provided a comprehensive listing of the search results.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Patients diagnosed with lung cancer who were about to undergo lung resection. (2) Patients in the intervention group received preoperative exercise training. The exercise sessions may be supervised, unsupervised, or a combination of both and can encompass aerobic, resistance, high-intensity interval or respiratory muscle training, or a combination thereof. (3) Patients in the control group received no preoperative exercise. (4) At least one of the following outcomes were reported: postoperative complications, postoperative length of hospital stay, post-intervention pulmonary function by FEV1, FVC and FEV1% of predicted norm values, FVC% of predicted norm values, PEF, DLCO, post-intervention exercise capacity measured by 6MWD and VO2peak, severe postoperative complications, postoperative 30-day mortality, postoperative chest tube drainage time, post-intervention dyspnea, and post-intervention HRQoL. (5) Study design: RCTs.
The exclusion criteria were as follows: (1) Other types of articles, such as case reports, publications, letters, reviews, editorials, pharmacological intervention, animal trials, and protocols. (2) Not relevant. (3) Full text not available. (4) Duplicate patient cohort. (5) Failed to obtain data.
2.3 Selection of studies
Selection of studies, including elimination of duplicates, was undertaken using EndNote (Version 20; Clarivate Analytics). An initial search was undertaken by two reviewers who independently deleted duplicate entries, assessed the titles and abstracts for relevance, and classified each study as either included or excluded. The settlement was arrived at through the attainment of consensus. A third author of the review would take on the role of an arbitrator if lacking a consensus.
2.4 Data extraction
Two separate reviewers conducted a thorough examination of the title and abstract, subsequently engaging in an exhaustive review of the entire text. A third reviewer was consulted to resolve the inconsistencies. Publication year, country, first author, sample size (preoperative exercise training group and no preoperative exercise group), study design, age, sex, current smoker, Non-Small Cell Lung Cancer (NSCLC) stage, American Society of Anesthesiologists (ASA) status, postoperative complications, postoperative length of hospital stay, post-intervention pulmonary function by FEV1 (25), FVC and FEV1% of predicted norm values (26, 27), FVC% of predicted norm values, PEF (28), DLCO (29), post-intervention exercise capacity measured by 6MWD (30) and VO2peak (31, 32), severe postoperative complications, postoperative 30-day mortality, postoperative chest tube drainage time, post-intervention dyspnea, and post-intervention HRQoL were all extracted. The postoperative complications assessed with the Clavien-Dindo classification (33) score ≥2 were classified as severe postoperative complications. HRQoL was evaluated using EORTC-QLQ-C30, a disease-specific health-related quality of life (QOL) scale ranging from 0 to 100, wherein a higher score reflects either better function or worse symptomatic effect (34, 35).
2.5 Risk of bias assessment
Two independent reviewers assessed the risk of bias using the Cochrane Risk of Bias tool, which has seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Group discussions were employed to resolve disputed results and correct discrepancies.
2.6 Data analysis and statistical methods
EndNote (Version 20; Clarivate Analytics) was used for article selection and duplication removal. The Cochrane Collaboration in Oxford, UK’s Review Manager 5.3 was used to analyze all study results. With a 95% confidence interval (CI), odds ratios (OR) were used to compare binary variables. A 95% CI was used to compare continuous variables. The medians and interquartile ranges of the continuous data were converted into corresponding means and standard deviations. The Cochrane Q p value and I2 statistic were used to evaluate the heterogeneity of each meta-analysis. A fixed-effect model (FEM) was used for low heterogeneity (I2 < 50%), and a random-effect model (REM) was used for high heterogeneity (I2 ≥ 50%) when analyzing pooled data. Using a traditional chi-square test, the statistical heterogeneity was assessed and shown to be statistically significant at a significance level of P < 0.05. The funnel plots’ visual evaluation was used to determine whether publication bias was present.
3 Results
3.1 Literature search
Figure 1 illustrates the procedure of selecting and integrating literature. The initial search approach facilitated the identification of 499 potential research studies. A total of 23 papers fulfilled the criteria and were evaluated for potential inclusion following the examination of titles and abstracts. Finally, 16 RCTs were included in this meta-analysis following a comprehensive review of the full text (12, 28, 29, 36–48).
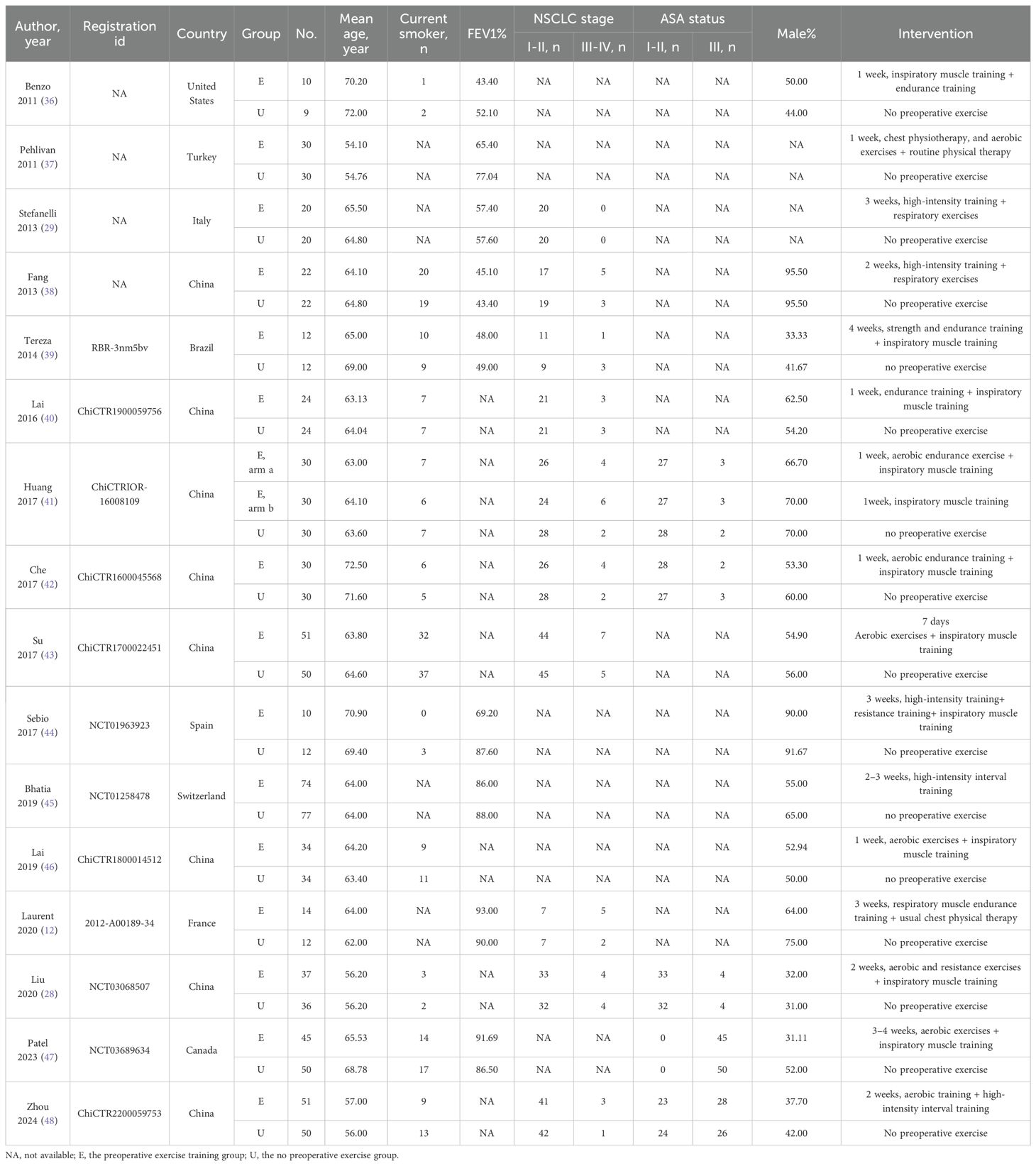
3.2 Characteristics of the included studies and quality assessment
The meta-analysis comprised 16 trials including 1,022 individuals, with 524 allocated to the preoperative exercise training group and 498 to the no preoperative exercise group. The registration ID, country, number, age, mean age, smoker, FEV1% of predicted norm values, NSCLC stage, ASA status, gender, and intervention are presented in Table 1.
3.3 Risk of bias
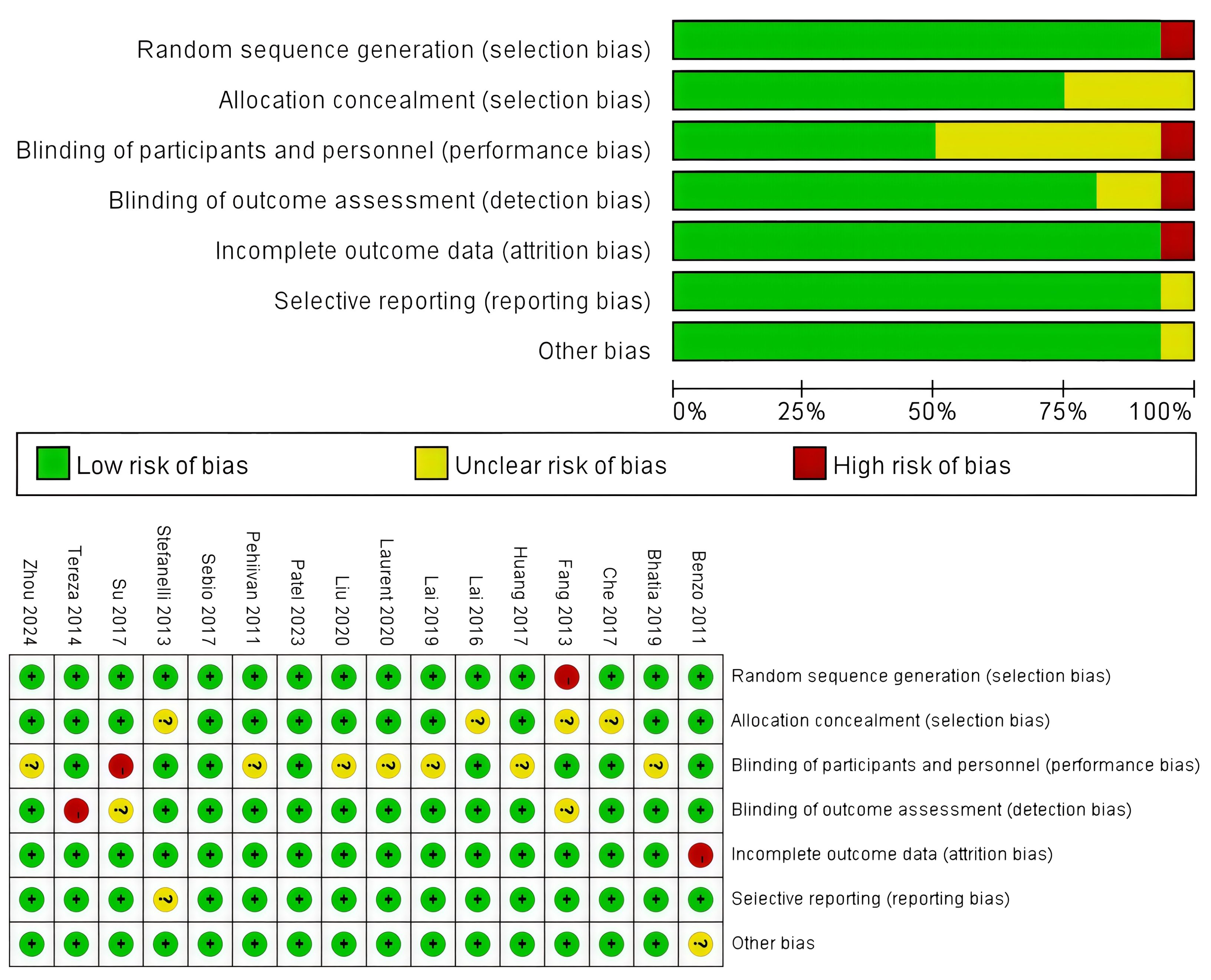
The assessment of the risk of bias is summarized in Figure 2, and all these 16 RCTs were of high quality. To be more specific, an adequate randomized sequence was reported in 11 RCTs, appropriate allocation concealment was generated in 8 RCTs, the blinding of participants was clear in 12 RCTs, the blinding of outcome assessors was generated in 15 RCTs, outcome data were complete in 15 RCTs, 15 RCTs had no selective reporting, and 15 RCTs had no other bias.
3.4 Clinical outcomes
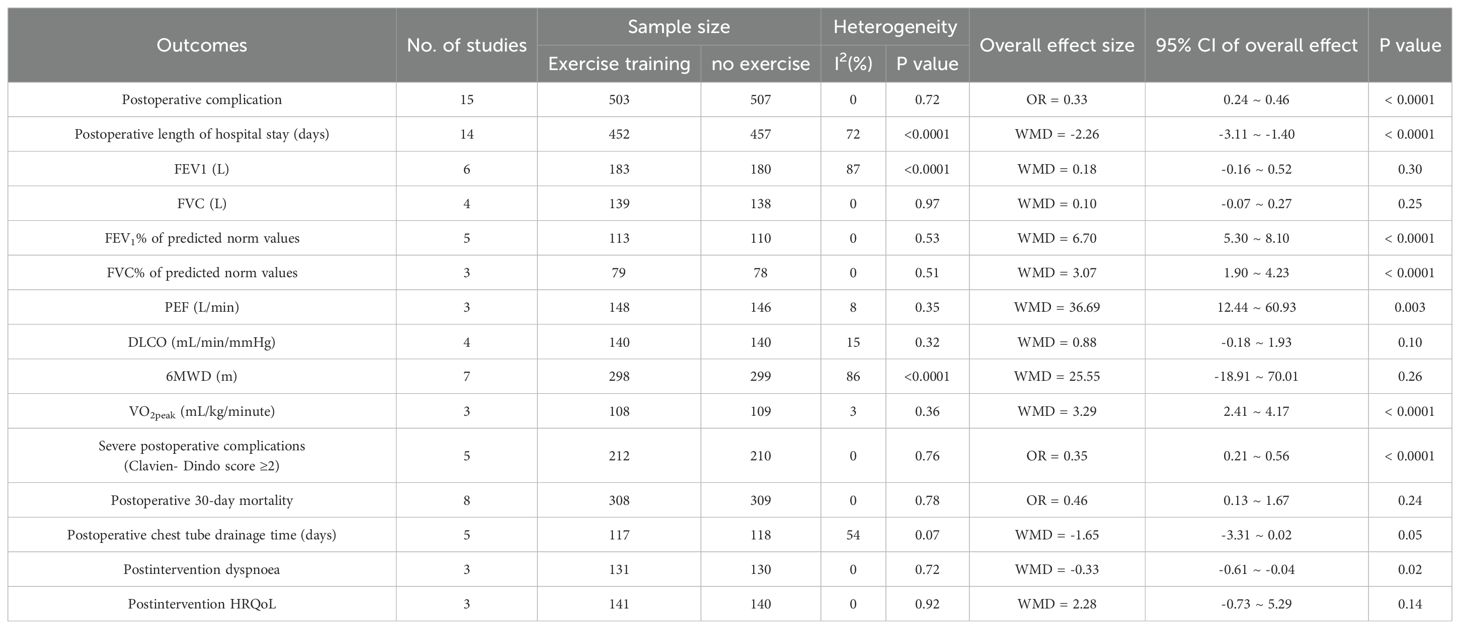
Table 2 presents the findings of the meta-analysis for all clinical outcomes.
3.4.1 Primary outcomes
3.4.1.1 Postoperative complication
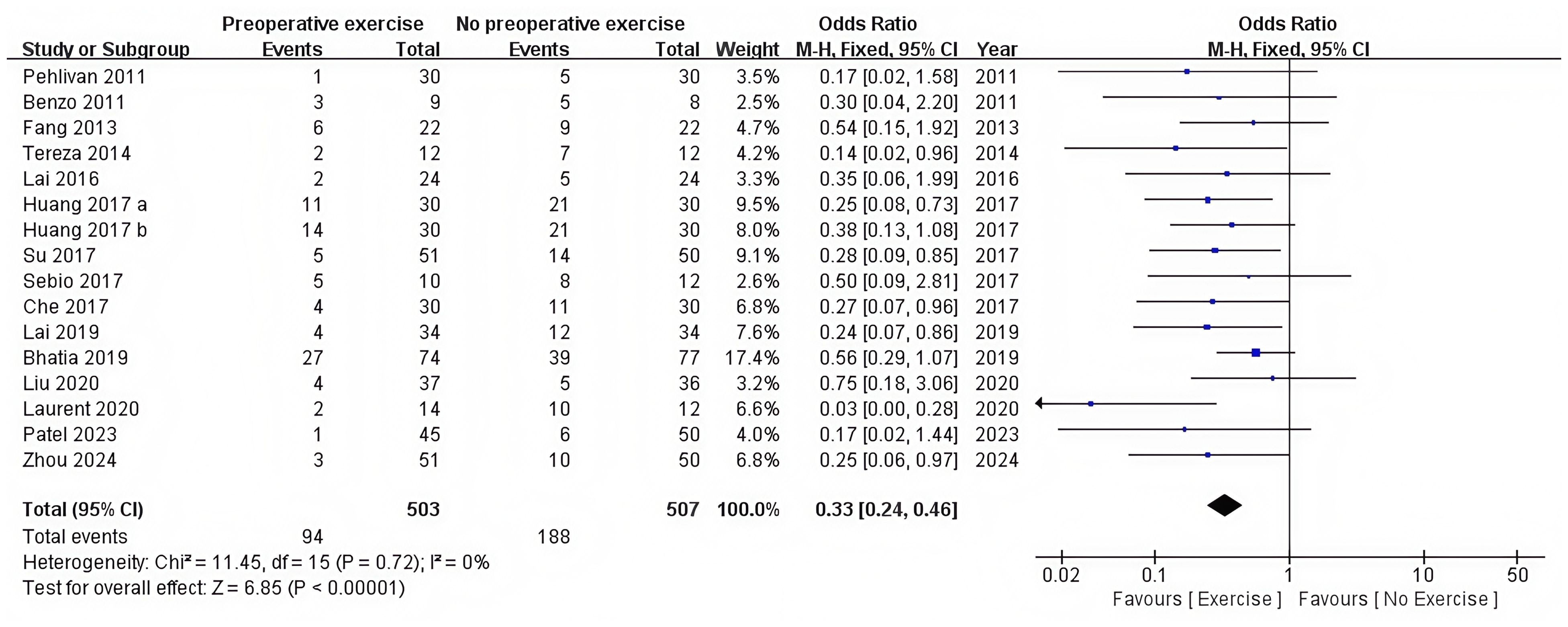
There were 15 RCTs who reported postoperative complications (12, 28, 36–48). Preoperative exercise training significantly reduced the postoperative complications compared with no preoperative exercise (OR = 0.33, 95%CI: 0.24 to 0.46, P < 0.0001) (Table 2, Figure 3).
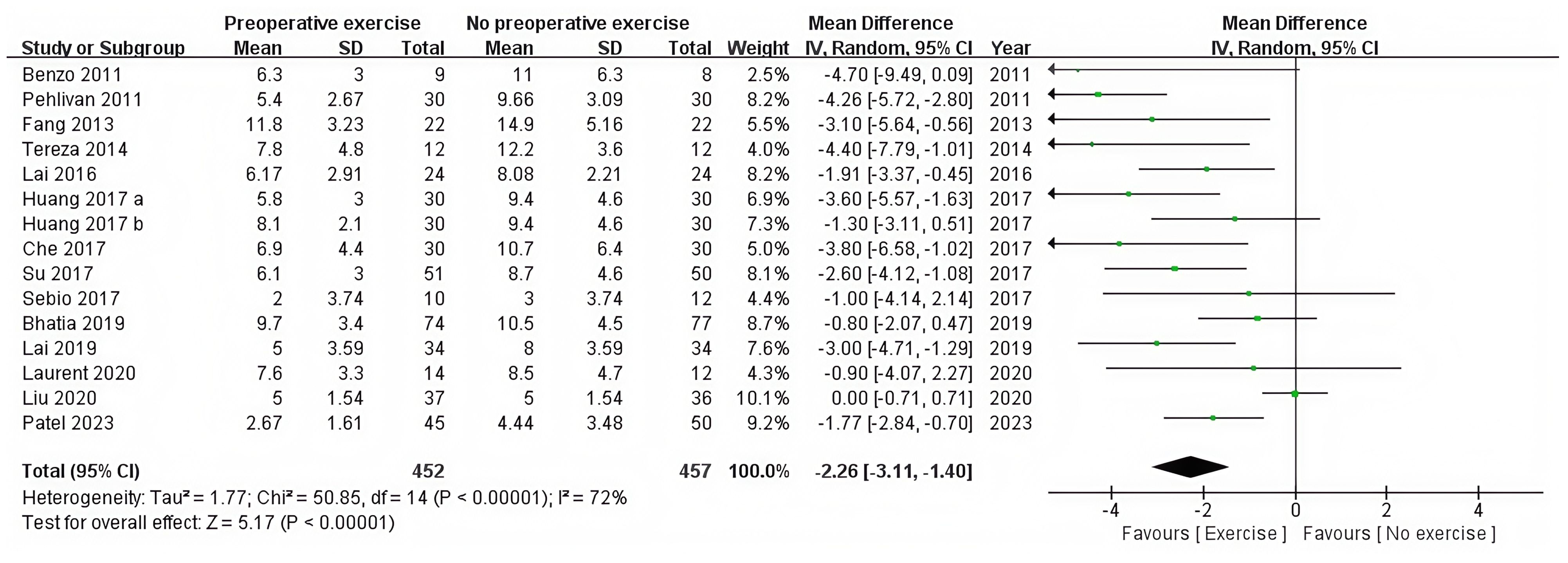
3.4.1.2 Postoperative length of hospital stay (days)
The postoperative hospital stay was recorded in 14 RCTs (12, 28, 36–47). The statistical analysis revealed that preoperative exercise training resulted in a significantly shorter hospital stay compared with usual care (95%CI: −3.11 to −1.40, P < 0.0001) (Table 2, Figure 4).
3.4.2 Secondary outcomes
3.4.2.1 Post-intervention pulmonary function
Six RCTs compared post-intervention FEV1 between preoperative exercise training and no preoperative exercise (12, 28, 37, 39, 41, 42). Preoperative exercise training and no preoperative exercise did not show any statistically significant change (95%CI: −0.16 to 0.52, P = 0.30) (Table 2, Supplementary Figure 1). Four RCTs investigated the difference in FVC between preoperative exercise training and no preoperative exercise (28, 39, 41, 42). There was no statistically significant change between preoperative exercise training and no preoperative exercise (95%CI: −0.07 to 0.27, P = 0.25) (Table 2, Supplementary Figure 2). Five RCTs were conducted to compare the FEV1% of predicted norm values between preoperative exercise training and no preoperative exercise (12, 28, 29, 37, 39). Preoperative exercise training produced a significantly greater improvement in FEV1% of predicted norm values compared with no preoperative exercise (95%CI: 5.30 to 8.10, P < 0.0001) (Table 2, Supplementary Figure 3). Three RCTs reported the FVC% of predicted norm values for preoperative exercise training and no preoperative exercise (28, 37, 39). Preoperative exercise training significantly enhanced the FVC% of predicted norm values compared with no preoperative exercise (95%CI: 1.90 to 4.23, P < 0.0001) (Table 2, Supplementary Figure 4). Three RCTs compared PEF between preoperative exercise training and no preoperative exercise (28, 41, 43). Significant disparities existed between preoperative exercise training and no preoperative exercise. The preoperative exercise training significantly enhanced the PEF compared with no preoperative exercise (95%CI: 12.44 to 60.93, P = 0.003) (Table 2, Supplementary Figure 5). A total of four RCTs documented differences in DLCO between preoperative exercise training and no preoperative exercise (29, 37, 41, 42), and no statistically significant difference was seen between the two groups (95%CI: −0.18 to 1.93, P = 0.10) (Table 2, Supplementary Figure 6).
3.4.2.2 Post-intervention exercise capacity
Seven RCTs examined the impact of the preoperative exercise training on exercise capacity using the 6MWD text compared with no preoperative exercise (28, 39, 41–43, 45, 46). Preoperative exercise training and no preoperative exercise did not show any statistically significant difference (95%CI: −18.91 to 70.01, P = 0.26) (Table 2, Supplementary Figure 7). Three RCTs reported post-intervention VO2peak as their measure of exercise capacity (12, 29, 45). Significant disparities existed between preoperative exercise training and no preoperative exercise. Preoperative exercise training increased post-intervention exercise capacity measured by VO2peak (95%CI: 2.41 to 4.17, P < 0.0001) (Table 2, Supplementary Figure 8).
3.4.2.3 Severe postoperative complications
Five RCTs reported severe postoperative complications (28, 41–43, 46). Preoperative exercise training substantially decreased severe postoperative complications (OR = 0.35, 95%CI: 0.21 to 0.56, P < 0.0001) (Table 2, Supplementary Figure 9).
3.4.2.4 Postoperative 30-day mortality
Eight RCTs evaluated postoperative 30-day mortality (28, 37, 38, 41, 42, 45, 46, 48). Preoperative exercise training and no preoperative exercise exhibited no statistically significant difference (OR = 0.46, 95%CI: 0.13 to 1.67, P = 0.24) (Table 2, Supplementary Figure 10).
3.4.2.5 Postoperative chest tube drainage time (days)
Postoperative chest tube drainage time was reported in five RCTs (12, 28, 36, 39, 47). No statistically significant difference was seen between preoperative exercise training and no preoperative exercise (95%CI: −3.31 to 0.02, P = 0.05) (Table 2, Supplementary Figure 11).
3.4.2.6 Post-intervention dyspnea
Three RCTs documented post-intervention dyspnea on exertion as judged by the BORG scale (29, 41, 43). Preoperative exercise training significantly reduced post-intervention dyspnea compared with no preoperative exercise (95%CI: -0.61 to 0.04, P = 0.02) (Table 2, Supplementary Figure 12).
3.4.2.7 Post-intervention HRQoL
Three RCTs evaluated post-intervention HRQoL (41–43). There was no statistically significant difference between preoperative exercise training and no preoperative exercise (95%CI: −0.73 to 5.29, P = 0.14) (Table 2, Supplementary Figure 13).
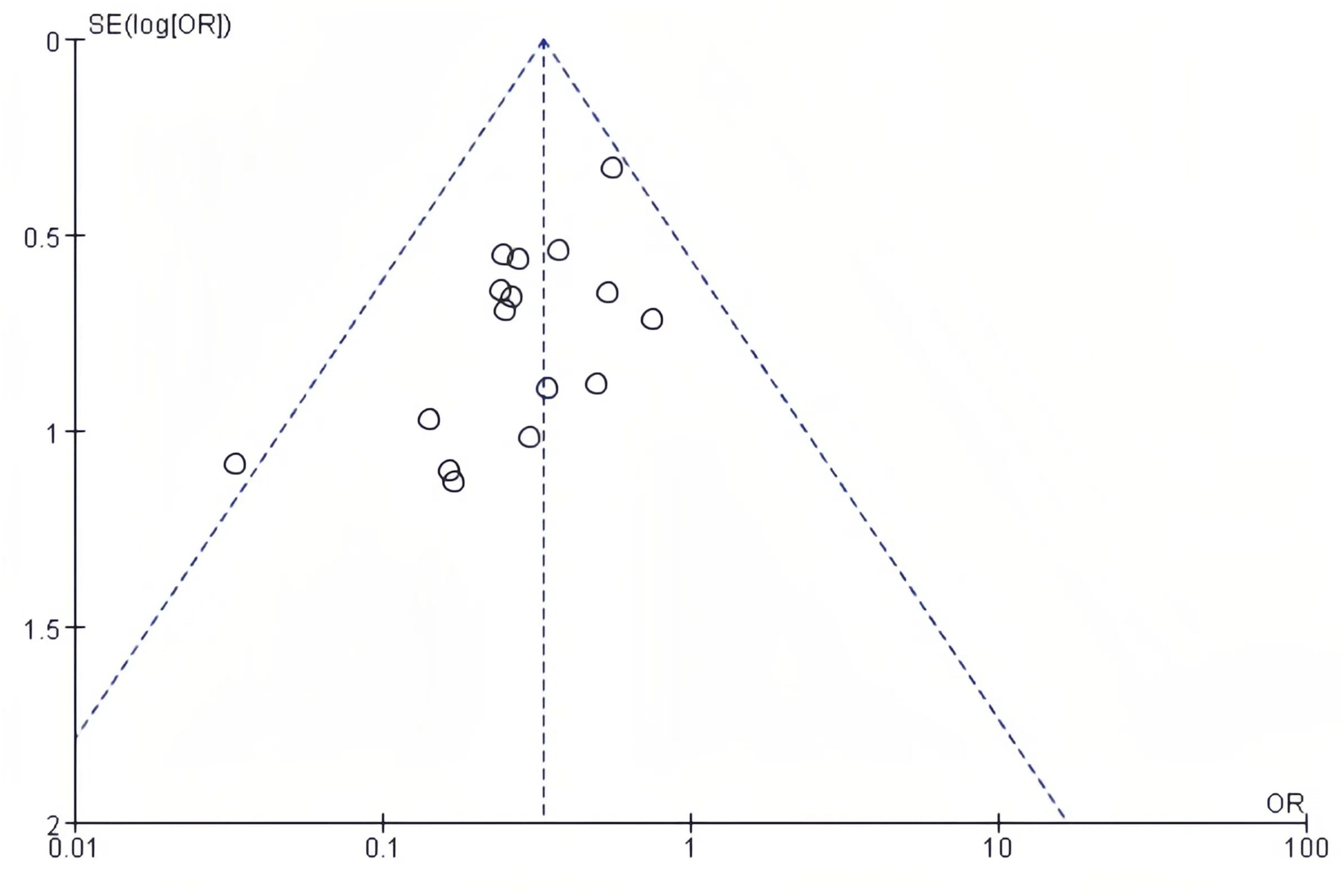
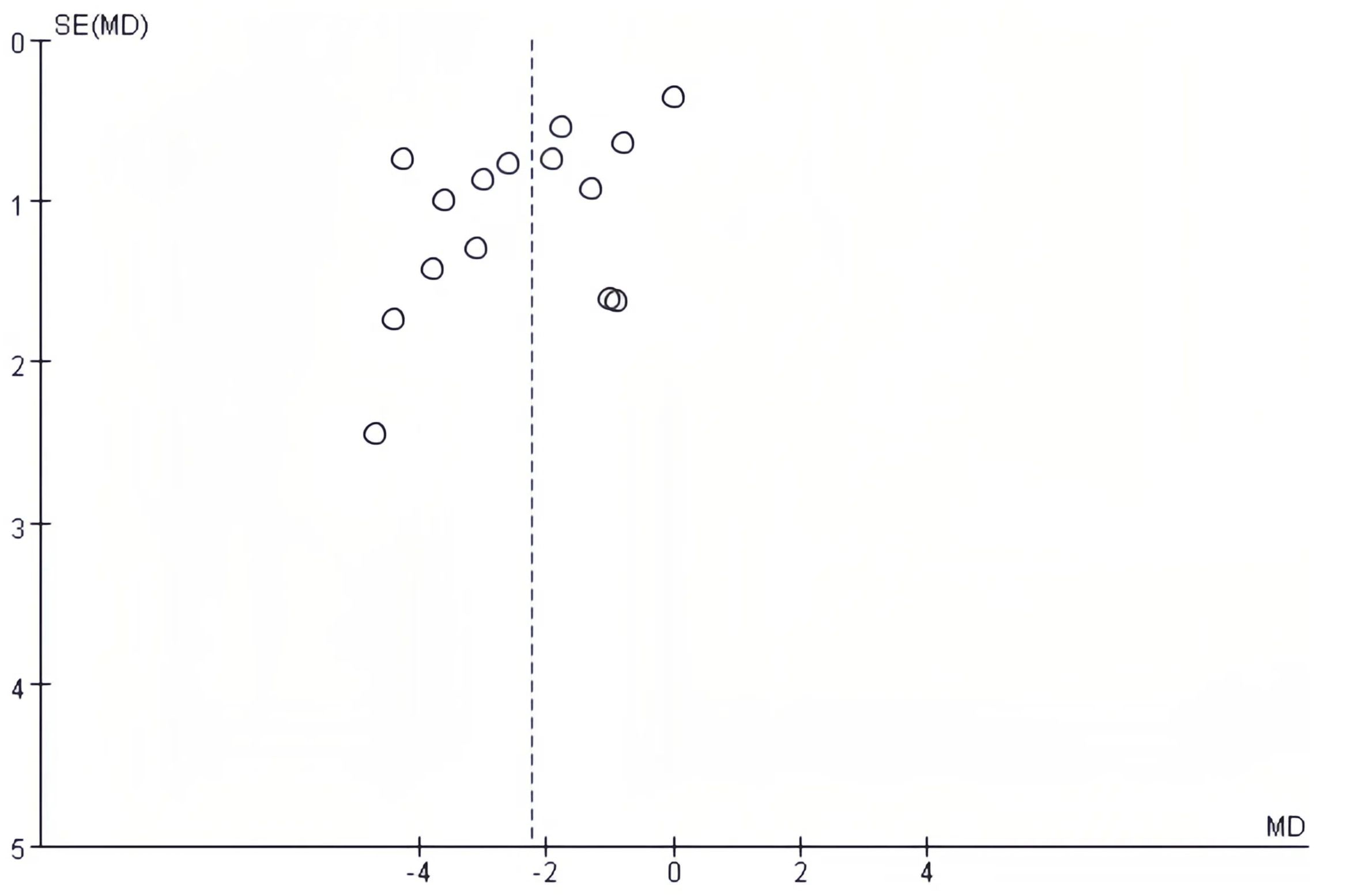
3.5 Publication bias
The publication bias on postoperative complications and postoperative length of hospital stay was evaluated using funnel plots. There was no notable publication bias detected in the bilaterally symmetrical funnel plots regarding postoperative complications (Figure 5) or postoperative length of hospital stay (Figure 6).
4 Discussion
This updated meta-analysis included 16 RCTs of high quality and assessed the clinical outcomes of patients with lung cancer who received preoperative exercise training. The results of this meta-analysis revealed that preoperative exercise training reduced postoperative complications and decreased postoperative length of hospital stay, which was similar to prior studies (23, 24, 49–52). Preoperative exercise training reduces hypermetabolic, stress, and inflammatory symptoms (53). Practicing deep breathing, coughing, and incentive spirometry before surgery improved lung function and reduced postoperative pneumonia and atelectasis patients (54). Preoperative exercise training can improve inspiratory muscle endurance, exercise capabilities, cardiac output, and muscle oxygen extraction, lowering postoperative complications in the exercise training group. This may boost exercise resistance and aerobic capacity, improving health before surgery and recuperation thereafter (43, 46). Breathing training increases respiratory muscle function, coughing, expectoration, and sputum excretion post-surgery, lowering lung infections and atelectasis (55, 56).
Several pathways have been proposed, which include the modulation of metabolic and sex-steroid hormone levels, enhancement of immune surveillance, reduction of systemic inflammation, and attenuation of oxidative damage through the induction of antioxidant responses to exercise-induced transient oxidative stress, although little evidence substantiates these hypotheses (56–60). The decrease in postoperative complications due to preoperative exercise training likely led to the reduction in postoperative length of hospital stay.
Our findings indicated that preoperative exercise training enhanced post-intervention exercise capacity measured by VO2peak more effectively than no preoperative exercise. Physical deconditioning significantly increases the risk for surgical patients, with low VO2peak serving as an indicator of perioperative mortality and cardiopulmonary complications (61, 62). The VO2peak indicates the comprehensive capacity of the pulmonary, circulatory, and autonomic nervous systems to optimally supply oxygen to the active skeletal muscles (63). Recent data have underscored the power of exercise training to elicit a protective cardiovascular phenotype while improving oxygen extraction in skeletal muscle through increased capillary density and mitochondrial oxidative capacity (64, 65). During maximal activity, the elevated cardiac output, along with enhanced oxygen extraction by the working muscles, leads to an increased VO2peak (66).
Our results demonstrated that preoperative exercise training enhanced preoperative pulmonary function regarding FEV1% of predicted norm values, FVC% of predicted norm values, PEF, and decreased preoperative dyspnea. The evidence regarding the impact of preoperative exercise training on lung function was highly equivocal due to the limited number of RCTs that have documented preoperative lung function metrics. The increased occurrence of postoperative complications in the elderly may not be solely attributable to age, but rather to more advanced chronic obstructive pulmonary disease, despite all patients exhibiting similar FEV1 levels (67). It was suggested that personalized preoperative exercise training should be obligatory for patients with chronic obstructive pulmonary disease, especially for symptomatic individuals with an FEV1 below 50% of the predicted value, and recommended for symptomatic or exercise-limited patients with an FEV1 exceeding 50% of the predicted value (68–70). The augmentation of the PEF signifies an improved clearance capacity of endotracheal hypersecretion in the intervention group, suggesting a potential reduction in the postoperative pulmonary complications (PPCs) rate (42). In recent years, several studies have linked PEF to surgical complications, mortality, and the ability to cough and expectorate, which can be used as an index to predict surgery prognosis (40). Preoperative exercise may play a potential role in rendering physiologically inoperable patients operable. Numerous resectable malignancies manifest in patients with impaired lung function, typically attributable to tobacco use, COPD, and/or atherosclerotic vascular disease as underlying comorbidities. This cohort of patients has an elevated risk of surgical complications and may be deemed inoperable (71, 72). The results of this meta-analysis indicated that preoperative exercise training could enhance preoperative pulmonary function. Therefore, it seems reasonable to assume that the patients in high risk of complications and mortality, considered inoperable due to lung function impairment, might be operated after preoperative exercise training. Preliminary findings suggested that preoperative exercise training markedly enhanced cardiopulmonary fitness in low-fit older persons undergoing lobectomy (29). Further evaluation in bigger cohorts and among individuals with highest postoperative risk is necessary.
To our knowledge, this updated meta-analysis included the largest number of RCTs comparing outcomes of preoperative exercise training versus no preoperative exercise for patients with lung cancer who were about to undergo lung resection, which could result in relatively robust conclusions. Nonetheless, we recognize the potential limitations of our study. First of all, the sample size of the included trials were relatively small, and only 16 RCTs were included due to our strict inclusion and exclusion criteria. The statistical results of partial clinical outcomes were difficult to reflect the difference between the two groups due to the relatively small sample size. Second, since the short follow-up periods of the included RCTs, we were unable to analyze long-term outcomes, such as 1-year postoperative mortality. Third, we were unable to manage confounding variables, including varying inclusion criteria, population disparities, and differing intervention of preoperative exercise training. These variables, particularly regarding the variability in exercise interventions and patient populations, may lead to significant heterogeneity. Heterogeneity in exercise interventions, made direct comparisons across studies challenging. Methodological weaknesses or conflicts of interest in included studies might lead to potential selection bias. Fourth, the absence of a gray literature search may contribute to publication bias. Furthermore, certain effects might be overestimated, particularly improvements in VO2peak over short-term interventions. VO2peak thresholds were limited by not using different thresholds for men and women. We failed to resolve this issue because the original literature did not provide data on gender subgroups. In addition, a subject that could provide very interesting information is whether preoperative exercise training improves final outcomes in patients undergoing minimally invasive surgery approaches. However, most of these RCTs did not disaggregate outcome data for patients categorized by type of surgery, which prevented further subgroup analysis regarding minimally invasive procedures. Therefore, more clinical outcomes reported by well-designed RCTs with longer follow-up periods are necessary to further confirm the advantage of preoperative exercise training.
In summary, this meta-analysis indicated that preoperative exercise training was advantageous for lung cancer patients undergoing lung resection, as it could reduce postoperative complications and length of hospital stay, while enhancing post-intervention pulmonary function and exercise capacity.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
CL: Conceptualization, Data curation, Formal Analysis, Project administration, Writing – original draft. HM: Conceptualization, Data curation, Formal Analysis, Writing – original draft. YW: Investigation, Methodology, Writing – original draft. YL: Investigation, Methodology, Writing – original draft. YX: Software, Supervision, Writing – original draft. XH: Data curation, Methodology, Writing – original draft. WL: Investigation, Methodology, Software, Writing – original draft. JQ: Conceptualization, Supervision, Writing – original draft. SW: Investigation, Methodology, Supervision, Writing – original draft. XW: Funding acquisition, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007) and the Scientific Research Foundation of Guangxi Health Commission (Z20210794).
Acknowledgments
All individuals who made substantial contributions to this work have been enumerated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1563478/full#supplementary-material
References
1. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Sadler SJ, Torio EF, and Golby AJ. Global cancer surgery in low-resource settings: A strengths, weaknesses, opportunities, and threats analysis. Cancer. (2023) 129:671–84. doi: 10.1002/cncr.34630
4. Avancini A, Cavallo A, Trestini I, Tregnago D, Belluomini L, Crisafulli E, et al. Exercise prehabilitation in lung cancer: getting stronger to recover faster. Eur J Surg Oncol. (2021) 47:1847–55. doi: 10.1016/j.ejso.2021.03.231
5. Piggin J. What is physical activity? A holistic definition for teachers, researchers and policy makers. Front sports active living. (2020) 2:72. doi: 10.3389/fspor.2020.00072
6. Wouters EF, Posthuma R, Koopman M, Liu WY, Sillen MJ, Hajian B, et al. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. (2020) 14:149–61. doi: 10.1080/17476348.2020.1700796
7. Ward TJC, Plumptre CD, Dolmage TE, Jones AV, Trethewey R, Divall P, et al. Change in V˙O(2peak) in response to aerobic exercise training and the relationship with exercise prescription in people with copd: A systematic review and meta-analysis. Chest. (2020) 158:131–44. doi: 10.1016/j.chest.2020.01.053
8. Troosters T, Janssens W, Demeyer H, and Rabinovich RA. Pulmonary rehabilitation and physical interventions. Eur Respir Rev. (2023) 32:1–14. doi: 10.1183/16000617.0222-2022
9. Ohde Y, Ueda K, Okami J, Saito H, Sato T, Yatsuyanagi E, et al. Guidelines for preoperative pulmonary function assessment in patients with lung cancer who will undergo surgery (the Japanese association for chest surgery). Gen Thorac Cardiovasc Surg. (2025) 73(6):385–404. doi: 10.1007/s11748-025-02120-7
10. Cohen JB, Smith BB, and Teeter EG. Update on guidelines and recommendations for enhanced recovery after thoracic surgery. Curr Opin anaesthesiology. (2024) 37:58–63. doi: 10.1097/aco.0000000000001328
11. Cruz Mosquera FE, Murillo SR, Naranjo Rojas A, Perlaza CL, Castro Osorio D, and Liscano Y. Effect of exercise and pulmonary rehabilitation in pre- and post-surgical patients with lung cancer: systematic review and meta-analysis. Medicina (Kaunas Lithuania). (2024) 60:1–25. doi: 10.3390/medicina60111725
12. Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, et al. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med. (2020) 56:73–81. doi: 10.23736/s1973-9087.19.05781-2
13. Steffens D, Ismail H, Denehy L, Beckenkamp PR, Solomon M, Koh C, et al. Preoperative cardiopulmonary exercise test associated with postoperative outcomes in patients undergoing cancer surgery: A systematic review and meta-analyses. Ann Surg Oncol. (2021) 28:7120–46. doi: 10.1245/s10434-021-10251-3
14. Dikova TS, Zatsepina AY, Fedorinov DS, and Lyadov VK. The impact of sarcopenic obesity on treatment outcomes in gastrointestinal cancer: A systematic review. Clin Nutr ESPEN. (2022) 47:135–46. doi: 10.1016/j.clnesp.2021.11.004
15. Allison R 2nd, Assadzandi S, and Adelman M. Frailty: evaluation and management. Am Fam Physician. (2021) 103:219–26.
16. Panayi AC, Orkaby AR, Sakthivel D, Endo Y, Varon D, Roh D, et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am J Surg. (2019) 218:393–400. doi: 10.1016/j.amjsurg.2018.11.020
17. Subramaniam A, Tiruvoipati R, Lodge M, Moran C, and Srikanth V. Frailty in the older person undergoing elective surgery: A trigger for enhanced multidisciplinary management - a narrative review. ANZ J Surg. (2020) 90:222–9. doi: 10.1111/ans.15633
18. Becerra-Bolaños Á, Hernández-Aguiar Y, and Rodríguez-Pérez A. Preoperative frailty and postoperative complications after non-cardiac surgery: A systematic review. J Int Med Res. (2024) 52:3000605241274553. doi: 10.1177/03000605241274553
19. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for Enhanced Recovery after Lung Surgery: Recommendations of the Enhanced Recovery after Surgery (Eras®) Society and the European Society of Thoracic Surgeons (Ests). Eur J cardio-thoracic Surg. (2019) 55:91–115. doi: 10.1093/ejcts/ezy301
20. Fukui M, Matsunaga T, Hattori A, Takamochi K, Oh S, Nojiri S, et al. Exercise oxygen desaturation is a predictor of cardiopulmonary complications after lung resection. BMJ Open Respir Res. (2022) 9:1–8. doi: 10.1136/bmjresp-2022-001397
21. Svoboda M, Cundrle I Jr., Plutinsky M, Homolka P, Mitas L, Chovanec Z, et al. New models for prediction of postoperative pulmonary complications in lung resection candidates. ERJ Open Res. (2024) 10:1–11. doi: 10.1183/23120541.00978-2023
22. Himbert C, Klossner N, Coletta AM, Barnes CA, Wiskemann J, LaStayo PC, et al. Exercise and lung cancer surgery: A systematic review of randomized-controlled trials. Crit Rev Oncol Hematol. (2020) 156:103086. doi: 10.1016/j.critrevonc.2020.103086
23. Granger C and Cavalheri V. Preoperative exercise training for people with non-small cell lung cancer. Cochrane Database Systematic Rev. (2022) 2022:1–57. doi: 10.1002/14651858.CD012020.pub3
24. Voorn M, van Kampen-van den Boogaart VEM, Bootsma G, Bongers B, Franssen R, and Hoogeboom T. Evidence base for exercise prehabilitation suggests favourable outcomes for patients undergoing surgery for non-small cell lung cancer despite being of low therapeutic quality: A systematic review and meta-analysis. J Thorac Oncol. (2023) 18:S99. doi: 10.1016/S1556-0864(23)00356-8
25. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
26. ATS. Ats/ers statement on respiratory muscle testing. Am J Respir Crit Care Med. (2002) 166:518–624. doi: 10.1164/rccm.166.4.518
27. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. (2005) 26:511–22. doi: 10.1183/09031936.05.00035005
28. Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: A randomized controlled trial. Anesth analgesia. (2020) 131:840–9. doi: 10.1213/ANE.0000000000004342
29. Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardio-Thoracic Surg. (2013) 44:e260–e5. doi: 10.1093/ejcts/ezt375
30. Statement A. Ats statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
31. Marjanski T, Badocha M, Wnuk D, Dziedzic R, Ostrowski M, Sawicka W, et al. Result of the 6-min walk test is an independent prognostic factor of surgically treated non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. (2019) 28:368–74. doi: 10.1093/icvts/ivy258
32. Statement AA. Ats/accp statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. (2003) 167:211–77. doi: 10.1164/rccm.167.2.211
33. Dindo D, Demartines N, and Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
34. Maringwa JT, Quinten C, King M, Ringash J, Osoba D, Coens C, et al. Minimal important differences for interpreting health-related quality of life scores from the eortc qlq-C30 in lung cancer patients participating in randomized controlled trials. Supportive Care Cancer. (2011) 19:1753–60. doi: 10.1007/s00520-010-1016-5
35. Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, et al. Replication and validation of higher order models demonstrated that a summary score for the eortc qlq-C30 is robust. J Clin Epidemiol. (2016) 69:79–88. doi: 10.1016/j.jclinepi.2015.08.007
36. Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer (Amsterdam Netherlands). (2011) 74:441–5. doi: 10.1016/j.lungcan.2011.05.011
37. Pehlivan E, Turna A, Gurses A, and Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients:A randomized controlled trial. Ann Thorac Cardiovasc Surg. (2011) 17:461–8. doi: 10.5761/atcs.oa.11.01663
38. Fang YZQ, Huang D, Guan S, and Wang J. The impact of exercise training on surgery tolerability in lung cancer patients with impaired pulmonary function. Chinese J Rehab Med. (2013) 28(7):619–23. doi: 10.3969/j.issn.1001-1242.2013.07.006
39. Tereza MMR, Silva Da FPG, Araújo Souza A, Pinto Sousa DMJ, Neto Gomes A, Viana Sampaio MC, et al. Comparison of the effects of pulmonary rehabilitation with chest physical therapy on the levels of fibrinogen and albumin in patients with lung cancer awaiting lung resection: A randomized clinical trial. BMC Pulmonary Med. (2014) 14:121. doi: 10.1186/1471-2466-14-121
40. Lai YYM, Zhou K, Che G, and Su J. Impact and effect of preoperative short-term pulmonary rehabilitation training on lung cancer patients with mild to moderate chronic obstructive pulmonary disease: A randomized trial. Chinese J Lung Cancer. (2016) 19(11):746–53. doi: 10.3779/j.issn.1009-3419.2016.11.05
41. Huang J, Lai Y, Zhou X, Li S, Su J, Yang M, et al. Short-term high-intensity rehabilitation in radically treated lung cancer: A three-armed randomized controlled trial. J Thorac Dis. (2017) 9:1919–29. doi: 10.21037/jtd.2017.06.15
42. Lai Y, Huang J, Yang M, Su J, Liu J, and Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: A randomized controlled trial. J Surg Res. (2017) 209:30–6. doi: 10.1016/j.jss.2016.09.033
43. Lai Y, Su J, Qiu P, Wang M, Zhou K, Tang Y, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: A randomized trial. Interactive Cardiovasc Thorac Surg. (2017) 25:476–83. doi: 10.1093/icvts/ivx141
44. Sebio García R, Yáñez-Brage MI, Giménez Moolhuyzen E, Salorio Riobo M, Lista Paz A, and Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: A randomized, single-blind controlled trial. Clin Rehabil. (2017) 31:1057–67. doi: 10.1177/0269215516684179
45. Bhatia C and Kayser B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: A randomized clinical trial. J Rehabil Med. (2019) 51:712–8. doi: 10.2340/16501977-2592
46. Lai Y, Wang X, Zhou K, Su J, and Che G. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: A randomized trial. Ann Transl Med. (2019) 7:544. doi: 10.21037/atm.2019.09.151
47. Patel YS, Sullivan KA, Churchill IF, Beauchamp MK, Wald J, Mbuagbaw L, et al. Preconditioning program reduces the incidence of prolonged hospital stay after lung cancer surgery: results from the move for surgery randomized clinical trial. Br J Surg. (2023) 110:1467–72. doi: 10.1093/bjs/znad252
48. Zhou N, Ripley-Gonzalez JW, Zhang W, Xie K, You B, Shen Y, et al. Preoperative exercise training decreases complications of minimally invasive lung cancer surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. (2024) 169(2):516–28. doi: 10.1016/j.jtcvs.2024.04.009
49. Gravier F-E, Smondack P, Prieur G, Medrinal C, Combret Y, Muir J-F, et al. Effects of exercise training in people with non-small cell lung cancer before lung resection: A systematic review and meta-analysis. Thorax. (2022) 77:486–96. doi: 10.1136/thoraxjnl-2021-217242
50. Rosero ID, Ramírez-Vélez R, Lucia A, Martínez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers. (2019) 11:1–19. doi: 10.3390/cancers11070944
51. Ni H-J, Pudasaini B, Yuan X-T, Li H-F, Shi L, and Yuan P. Exercise training for patients pre- and postsurgically treated for non-small cell lung cancer: A systematic review and meta-analysis. Integr Cancer Therapies. (2017) 16:63–73. doi: 10.1177/1534735416645180
52. Cavalheri V and Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Systematic Rev. (2017) 2017:1–33. doi: 10.1002/14651858.CD012020.pub2
53. Scheffer DDL and Latini A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165823. doi: 10.1016/j.bbadis.2020.165823
54. Takaoka ST and Weinacker AB. The value of preoperative pulmonary rehabilitation. Thorac Surg Clin. (2005) 15:203–11. doi: 10.1016/j.thorsurg.2005.02.001
55. Van Hollebeke M, Poddighe D, Gojevic T, Clerckx B, Muller J, Hermans G, et al. Measurement validity of an electronic training device to assess breathing characteristics during inspiratory muscle training in patients with weaning difficulties. PLoS One. (2021) 16:e0255431. doi: 10.1371/journal.pone.0255431
56. Wang Q and Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J sport Health Sci. (2021) 10:201–10. doi: 10.1016/j.jshs.2020.07.008
57. Hong BS and Lee KP. A systematic review of the biological mechanisms linking physical activity and breast cancer. Phys activity Nutr. (2020) 24:25–31. doi: 10.20463/pan.2020.0018
58. Sessa F, Messina G, Russo R, Salerno M, Castruccio Castracani C, Distefano A, et al. Consequences on aging process and human wellness of generation of nitrogen and oxygen species during strenuous exercise. Aging male. (2020) 23:14–22. doi: 10.1080/13685538.2018.1482866
59. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, and Hyatt H. Exercise-induced oxidative stress: friend or foe? J sport Health Sci. (2020) 9:415–25. doi: 10.1016/j.jshs.2020.04.001
60. Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP, and Louzada RA. Exercise-stimulated ros sensitive signaling pathways in skeletal muscle. Antioxidants (Basel Switzerland). (2021) 10:1–21. doi: 10.3390/antiox10040537
61. Chouinard G, Roy P, Blais MC, Lippens A, Pelletier É, Roy E, et al. Exercise testing and postoperative complications after minimally invasive lung resection: A cohort study. Front Physiol. (2022) 13:951460. doi: 10.3389/fphys.2022.951460
62. Orlandi R, Rinaldo RF, Mazzucco A, Baccelli A, Mondoni M, Marchetti F, et al. Early outcomes of "Low-risk" Patients undergoing lung resection assessed by cardiopulmonary exercise testing: single-institution experience. Front Surg. (2023) 10:1130919. doi: 10.3389/fsurg.2023.1130919
63. Sadaqat S, Khan AW, Ashfaq AD, and Rehman SU. Role of cardiopulmonary exercise testing in predicting perioperative outcomes in cancer patients undergoing thoracoabdominal surgeries; an observational cohort study. J Cancer Allied specialties. (2021) 7:e313. doi: 10.37029/jcas.v7i1.313
64. Penna C, Alloatti G, and Crisafulli A. Mechanisms involved in cardioprotection induced by physical exercise. Antioxidants Redox Signaling. (2020) 32:1115–34. doi: 10.1089/ars.2019.8009
65. Chen H, Chen C, Spanos M, Li G, Lu R, Bei Y, et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduct Target Ther. (2022) 7:306. doi: 10.1038/s41392-022-01153-1
66. Mølmen KS and Rønnestad BR. A narrative review exploring advances in interval training for endurance athletes. Appl physiology nutrition Metab = Physiologie appliquee Nutr metabolisme. (2024) 49:1008–13. doi: 10.1139/apnm-2023-0603
67. Boushy SF, Billig DM, North LB, and Helgason AH. Clinical course related to preoperative and postoperative pulmonary function in patients with bronchogenic carcinoma. Chest. (1971) 59:383–91. doi: 10.1378/chest.59.4.383
68. Martinez FJ, Han MK, Lopez C, Murray S, Mannino D, Anderson S, et al. Discriminative accuracy of the capture tool for identifying chronic obstructive pulmonary disease in us primary care settings. Jama. (2023) 329:490–501. doi: 10.1001/jama.2023.0128
69. Rochester CL, Alison JA, Carlin B, Jenkins AR, Cox NS, Bauldoff G, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. (2023) 208:e7–e26. doi: 10.1164/rccm.202306-1066ST
70. Xiong T, Bai X, Wei X, Wang L, Li F, Shi H, et al. Exercise rehabilitation and chronic respiratory diseases: effects, mechanisms, and therapeutic benefits. Int J chronic obstructive pulmonary Dis. (2023) 18:1251–66. doi: 10.2147/copd.S408325
71. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (Nsclc): esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
72. Brunelli A, Cassivi SD, Fibla J, Halgren LA, Wigle DA, Allen MS, et al. External validation of the recalibrated thoracic revised cardiac risk index for predicting the risk of major cardiac complications after lung resection. Ann Thorac Surg. (2011) 92:445–8. doi: 10.1016/j.athoracsur.2011.03.095
Keywords: lung cancer, lung resection, exercise training, complication, pulmonary function, exercise capacity, meta-analysis
Citation: Li C, Meng H, Wei Y, Liang Y, Xu Y, Huang X, Liang W, Quan J, Wu S and Wei X (2025) Postoperative outcomes of preoperative exercise training in patients with operable non-small cell lung cancer: a systematic review and meta-analysis. Front. Oncol. 15:1563478. doi: 10.3389/fonc.2025.1563478
Received: 20 January 2025; Accepted: 26 August 2025;
Published: 12 September 2025.
Edited by:
Melanie Keats, Dalhousie University, CanadaReviewed by:
Serghei Covantsev, National Medical Research Treatment and Rehabilitation Centre of the Ministry of Health of Russia, RussiaAkif Turna, Istanbul University-Cerrahpasa, Türkiye
Morten Quist, University of Copenhagen, Denmark
Copyright © 2025 Li, Meng, Wei, Liang, Xu, Huang, Liang, Quan, Wu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueyan Wei, MTM2Njc3MjY1MDhAMTYzLmNvbQ==
†These authors share first authorship
 Cuifang Li
Cuifang Li Haidan Meng1†
Haidan Meng1† Weiming Liang
Weiming Liang Xueyan Wei
Xueyan Wei