- 1Department of Gastrointestinal Surgery, Cancer Hospital Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 2Department of Colorectal Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Gastrointestinal surgery, Hebei General Hospital, Shijiazhuang, Hebei, China
Background: The occurrence of isolated splenic metastasis in association with colorectal cancer is exceedingly rare. This paper presents a case of isolated splenic metastasis identified one year after radical resection of rectal cancer. The patient underwent laparoscopic splenectomy, achieving favorable therapeutic outcomes. Furthermore, we conduct a literature review of analogous cases and examine the clinical diagnosis and therapeutic approaches for this uncommon condition.
Case Review: A 76-year-old male patient underwent laparoscopic radical resection for rectal cancer, accompanied by ileostomy, at our institution. Postoperatively, the patient received two cycles of XELOX chemotherapy but continued with oral capecitabine for six cycles due to oxaliplatin intolerance. Follow-up enhanced CT scans of the chest, abdomen, and pelvis revealed an isolated metastatic tumor in the spleen. Subsequently, the patient underwent laparoscopic splenectomy. Histopathological examination post-surgery confirmed the metastasis of rectal cancer to the spleen.
Conclusion: Isolated splenic metastasis originating from colorectal cancer is exceedingly rare, and laparoscopic splenectomy constitutes an effective treatment strategy.
Background
Colorectal cancer is currently the third most common malignancy worldwide. Despite its prevalence, splenic metastasis from colorectal cancer is rare.
Splenic metastasis is an exceedingly rare condition, largely attributable to the spleen’s unique anatomical structure and immunological features (1, 2). Anatomically, the splenic artery branches at an acute angle from the celiac artery, the splenic sinuses undergo rhythmic contraction, and the spleen lacks afferent lymphatic vessels. Immunologically, the spleen’s microenvironment, abundant in macrophages and monocytes, further impedes the development of metastatic tumors.
Based on the timing of splenic metastasis relative to the primary tumor, splenic metastases are classified as either synchronous or metachronous. Synchronous splenic metastases are defined in the literature as those identified concurrently with the primary tumor on imaging studies. Metachronous splenic metastases, in contrast, are those primarily detected during postoperative follow-up of the primary tumor lesion (3). However,the classification of splenic metastases found shortly after primary tumor resection—whether as synchronous or metachronous—remains controversial and lacks a definitive consensus. Drawing a parallel from the definition applied to liver metastases, metastases detected within 6 months following primary tumor resection are still classified as synchronous in the hepatic context. Critically, synchronous and metachronous hepatic metastases exhibit distinctly different patterns of incidence over time and divergence in survival outcomes. Therefore, a precise definition of synchronous splenic metastasis will likely necessitate future studies analyzing the clinical outcomes and long-term prognosis of a larger cohort of patients developing splenic metastases after resection of the primary tumor (4). In this patient, the splenic metastasis was detected at 1 year following resection of the primary tumor, consistent with a metachronous presentation.
When splenic metastasis does occur, it is typically associated with metastasis to other sites, rendering the incidence of isolated splenic metastasis even more uncommon. At present, there is a paucity of large-scale randomized controlled trials providing evidence for the treatment of splenic metastases, with current knowledge primarily derived from case reports.
This article presents a rare case of isolated splenic metastasis and reviews pertinent literature on isolated splenic metastasis associated with colorectal cancer, sourced from the PubMed database. The objective is to elucidate the mechanisms underlying the occurrence, diagnosis, and treatment of splenic metastasis, thereby offering additional references for future clinical management.
Case description
Splenic metastasis detected following curative resection of rectal cancer
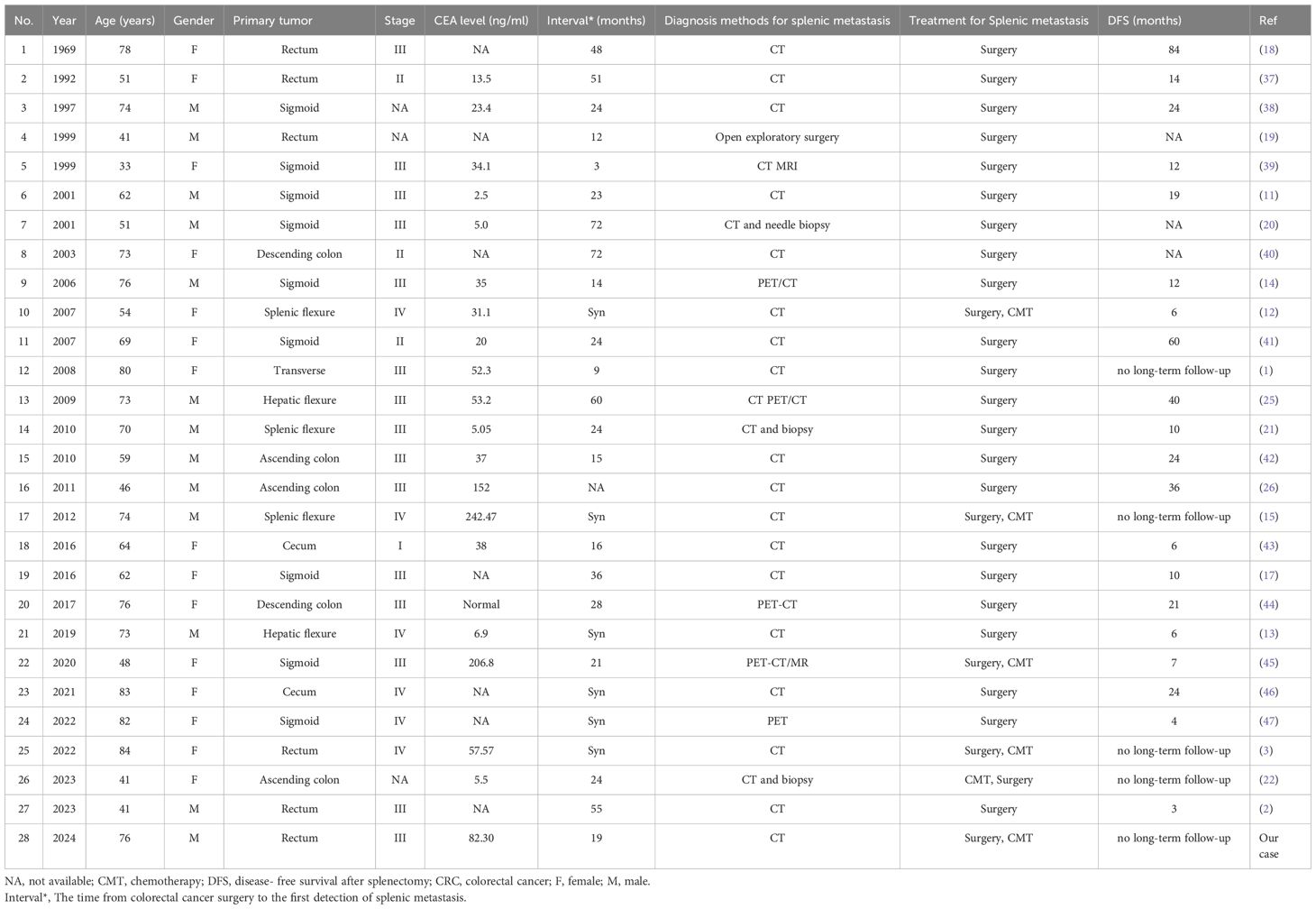
A 76-year-old male patient, with a medical history significant for hypertension, atrial fibrillation, and cerebral infarction and no familial predisposition to tumors, underwent laparoscopic radical resection for rectal cancer and a prophylactic ileostomy on January 6, 2023. Postoperative pathological analysis revealed a classification of pT4aN1b, accompanied by tumor budding and poorly differentiated tumor cell clusters, with evidence of vascular and neural invasion. Immunohistochemical analysis demonstrated the following results: BRAF-V600E negative, C-MET 1+, HER2 1+, MLH1 positive, MSH2 positive, MSH6 positive, and PMS2 positive, with a microsatellite status of stable (MSS). The patient was administered two cycles of adjuvant chemotherapy according to the XELOX regimen; however, due to oxaliplatin intolerance, treatment was continued with oral capecitabine for an additional six cycles. On September 19, 2023, the patient underwent ileostomy reversal. Routine follow-up assessments, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 242 (CA242), and enhanced computed tomography (CT) scans of the chest, abdomen, and pelvis, indicated normal parameters with no signs of tumor recurrence. However, on August 8, 2024, laboratory tests revealed elevated levels of CEA, CA19-9, and CA242. Subsequent CT imaging on August 14, 2024, identified a new lesion in the spleen, measuring approximately 4.0 × 3.7 cm (Figure 1). Mildly irregular, suggesting a metastatic tumor. However, there was no evidence of metastasis to the liver, lungs, or peritoneum. Given the potential for isolated metachronous splenic metastasis and the patient’s intolerance to oxaliplatin, a multidisciplinary team (MDT) discussion resulted in the decision to perform a laparoscopic splenectomy under general anesthesia on August 26, 2024.
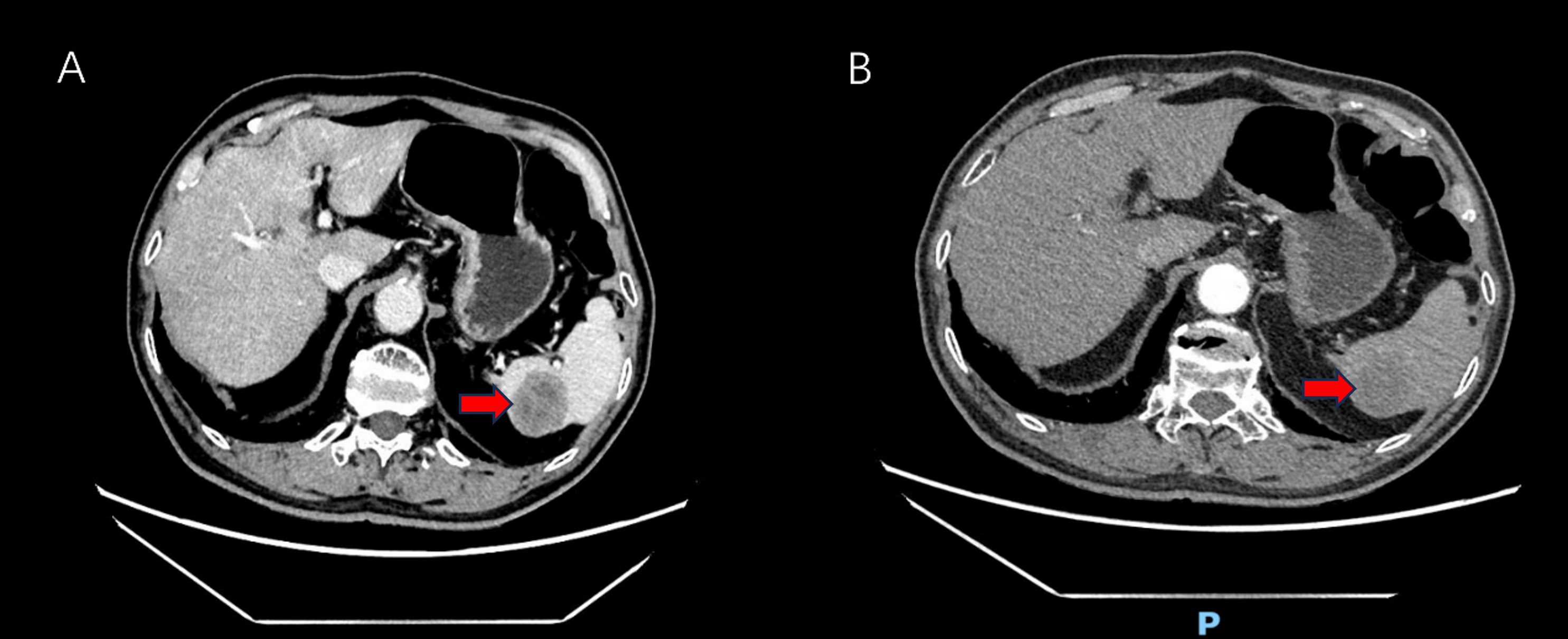
Figure 1. Abdomen enhanced CT showing low-density shadows in the spleen (red arrow). (A) Venous-phase splenic metastasis shows a hypodense lesion (red arrow). (B) Arterial-phase CT reveals a hypodense lesion with peripheral enhancement (red arrow).
Surgical treatment
After consent was obtained from the patient and his family, the procedure was performed. We conducted a five-port laparoscopic procedure.Intraoperative exploration revealed the absence of metastatic nodules in the liver, peritoneum, or pelvis, and no ascites was present. However, a firm, white nodule was observed at the lower pole of the spleen (Figure 2). So we performed a laparoscopic total splenectomy and placed a drainage tube in the splenic fossa.The surgery went smoothly without any intraoperative complications.
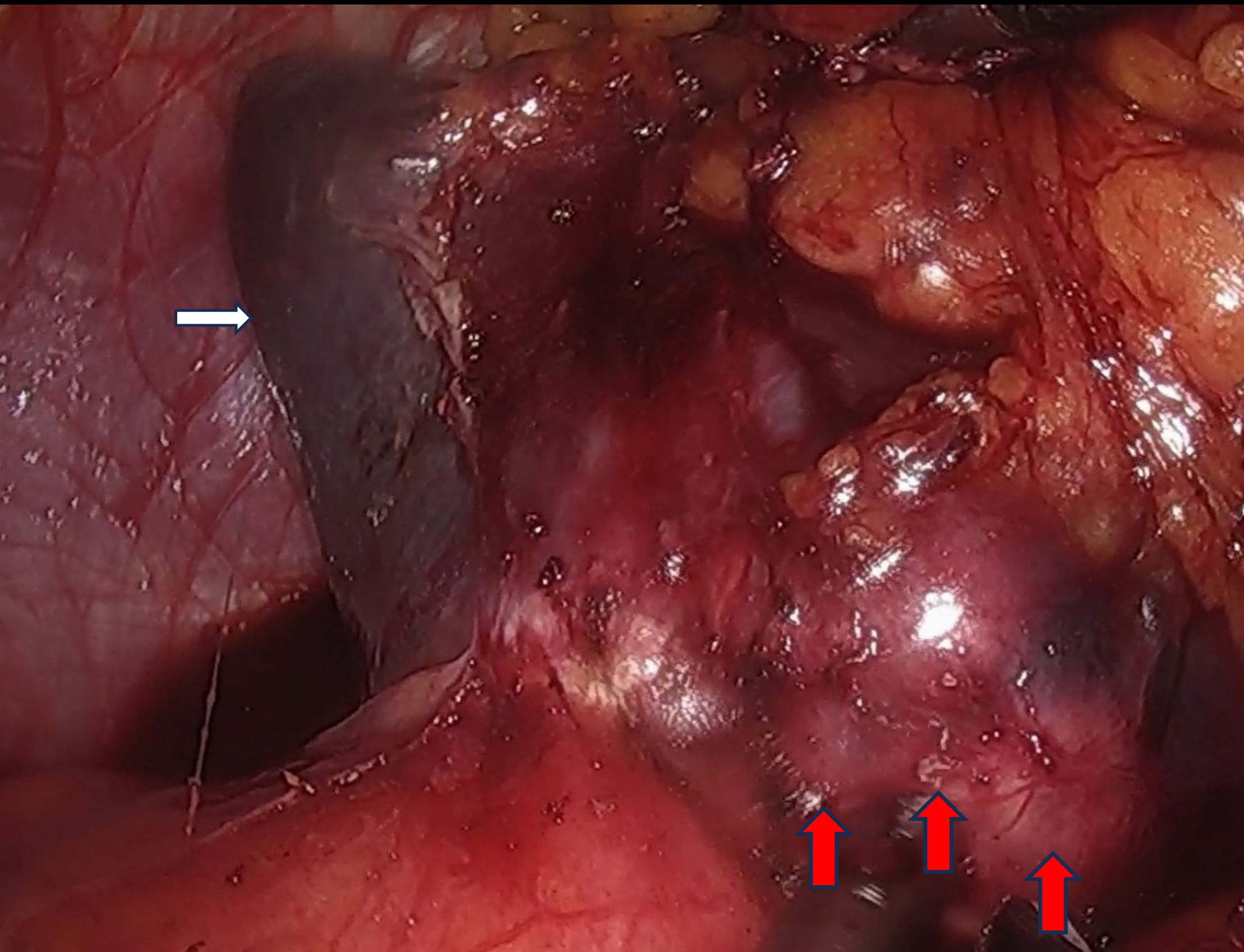
Figure 2. Laparoscopic exploration of a hard tumor in the spleen (The white arrow indicates the spleen, the red arrow denotes the metastatic lesion within the spleen).
Postoperative pathology
The postoperative pathological findings from the previous radical resection for rectal cancer demonstrated poorly differentiated adenocarcinoma (Figure 3A). Postoperative pathological analysis identified the nodule as moderately to poorly differentiated intestinal-type adenocarcinoma infiltrating the splenic parenchyma, with extensive necrosis and focal mucin secretion (Figure 3B). Correlating these findings with the patient’s medical history, morphological characteristics, and immunohistochemical results, the diagnosis was consistent with splenic metastasis originating from colorectal adenocarcinoma. The tumor involved the splenic capsule but did not extend into the splenic hilum or omentum. No definitive vascular or neural invasion was detected. The surrounding splenic tissue appeared unremarkable. The pathological staging was pTNM: pM1, to be interpreted in conjunction with clinical data. Immunohistochemical analysis demonstrated the following results: CDX-2 (2+), CD10 (3+), SATB2 (3+), Villin (3+), CK7 (-), and CK20 (-) (refer to Figures 3C, D). Postoperatively, the patient exhibited no fever and no elevation in platelet count (PLT). And the patient was discharged one week after the procedure. It was advised that the patient commence oral capecitabine therapy three weeks following surgery. At present, the patient is maintaining a favorable follow-up status.
Figure 3. Histological results of the patient we reported. (A) The primary rectal cancer: Poorly differentiated adenocarcinoma (HE, ×400); (B) Splenic metastasis from rectal cancer (HE, ×400); (C) CDX-2 positively stained in splenic metastasis (CDX2, ×400); (D) SATB2 positively stained in splenic metastasis (SATB2, ×400). HE: hematoxylin and eosin, CDX-2: Homeobox Transcription Factor 2; SATB2: Special AT-rich sequence-binding protein 2.
Discussion
Colorectal cancer ranks as the third most prevalent cancer globally and is associated with the fourth highest mortality rate (5). Metastasis accounts for approximately 90% of colorectal cancer-related deaths (6). The liver is the most frequent site of distant metastasis, followed by lymph nodes, lungs, and peritoneum, while metastases to the brain and bones are uncommon (7). The incidence of splenic metastasis is relatively low. A comprehensive autopsy study conducted by Professor Berge reported a 7.1% incidence of splenic metastases among cancer patients, with colorectal cancer exhibiting a 4.4% likelihood of splenic metastasis, which is lower than that observed in skin, breast, and ovarian cancers. However, the study did not address the incidence of isolated splenic metastasis (6). Professor Berge’s research identified that among cases exhibiting metastasis in five or more organs, 50% presented with microscopic splenic metastases. In comparison to subjects lacking splenic metastasis, the incidence of lymph node and other organ metastases was significantly lower (8). Consequently, splenic metastasis is often indicative of advanced disease. However, unlike splenic metastases associated with widespread metastases throughout the body, solitary splenic metastases do not signify the terminal stages of metastatic cancer. Instead, they reflect the proliferation of cancer cells in the spleen during the latent phase of early hematogenous dissemination (9). Indeed, the likelihood of isolated splenic metastasis is exceedingly low. Another study reported that among 29,364 cancer patients, 20.9% had metastases, yet only 59 patients exhibited splenic metastases (0.2% of the total cohort), and merely three patients had isolated splenic metastases (10). As previously mentioned, there is currently no definitive explanation for the relatively low incidence of splenic metastasis, although several hypotheses have been proposed in the academic community (1, 8, 11–16). First, the sharp angulation of the splenic artery at its origin from the celiac trunk impedes tumor emboli migration toward the spleen (8, 15). Second, rhythmic splenic contractions prevent colonization of tumor emboli (17). Third, the absence of afferent lymphatic vessels reduces tumor trafficking to the spleen (15). Finally, the immunologically active splenic microenvironment—abundant in macrophages and monocytes—inhibits metastatic development (16).
Through systematic literature searches conducted in PubMed, Embase, Web of Science, and the Cochrane Library using the keywords”colon cancer,” “rectal cancer,” “colorectal cancer,” and “splenic metastasis,” we identified only 27 documented case reports of isolated splenic metastases secondary to colorectal carcinoma (Table 1). The earliest documented case was reported by Professor Dunbar in 1969 (18). Among the cases examined, 6 involved synchronous splenic metastasis, while 21 involved metachronous splenic metastasis. The statement indicates that metachronous splenic metastasis is more prevalent than synchronous splenic metastasis. The cohort comprised 15 female and 12 male patients, with ages ranging from 33 to 84 years. The primary tumor sites included the cecum (2 case), ascending colon (3 cases), hepatic flexure (2 cases), transverse colon (1 case), splenic flexure (3 cases), descending colon (2 case), sigmoid colon (9 cases), and rectum (5 cases) (Figure 4). The sigmoid colon was the most frequent site of involvement (33.3%), followed by the rectum (18.5%). Splenic metastasis demonstrates a pronounced predilection for malignancies arising from the left colon and rectum, a clinicopathologic pattern attributable to vascular anatomic determinants. Tumor cells undergo retrograde venous transmission via the inferior mesenteric vein, directly accounting for both the predominantly intraparenchymal localization of metastatic deposits and their increased frequency in left-sided versus right-sided carcinomas (12). Notably, the splenic parenchyma lacks afferent lymphatics, structurally reinforcing hematogenous dissemination as the principal pathway (19). Contemporary consensus affirms this mechanistic dominance despite coexisting lymphatic routes (8, 19, 20).
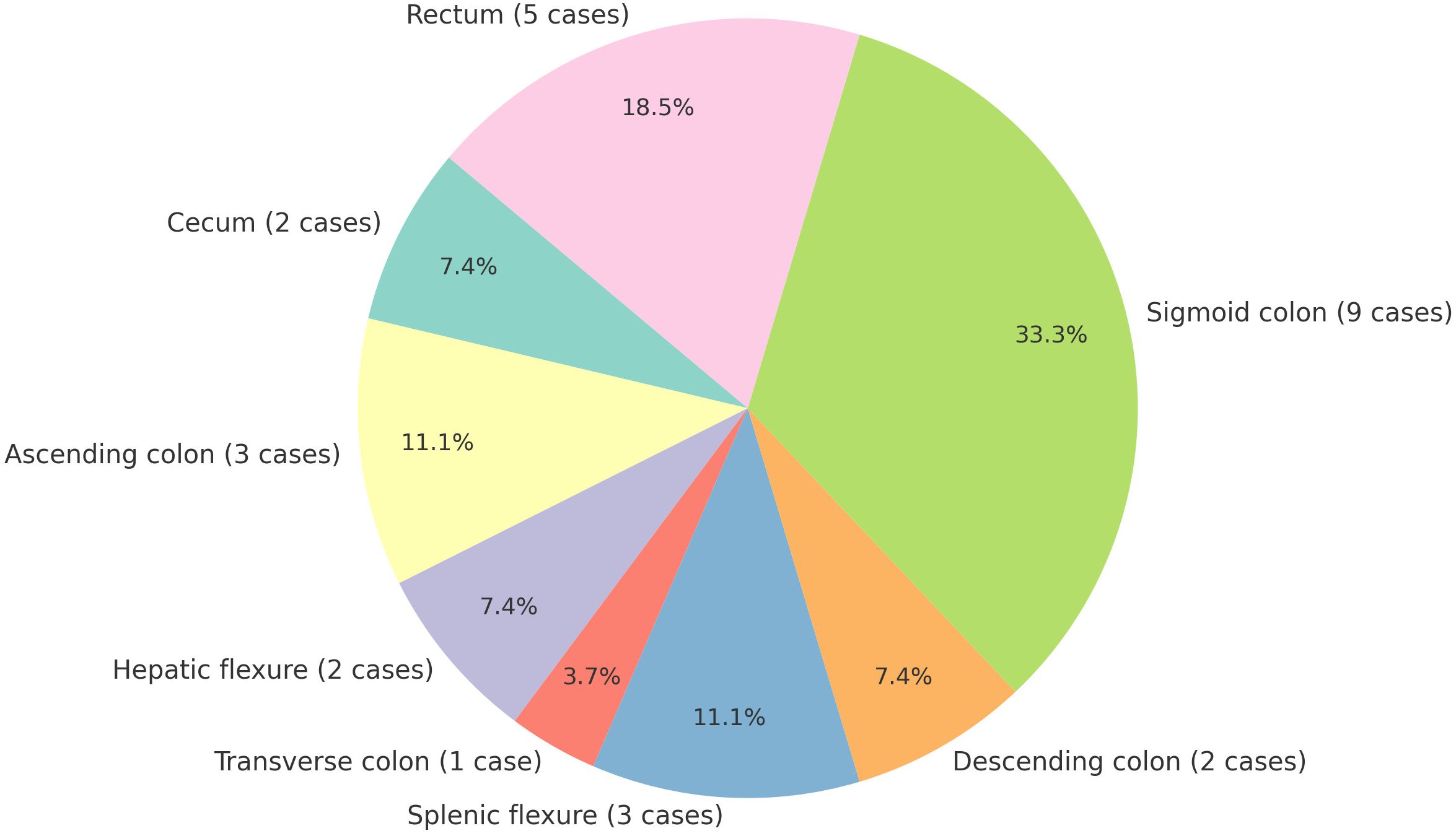
Figure 4. Distribution of primary tumor sites in metastatic spleen cases [Sigmoid colon was the most frequent site of involvement (33.3%, 9/27)].
Our case exemplifies this paradigm: A histologically confirmed solitary intraparenchymal splenic metastasis developed 19 months post-resection of an upper rectal adenocarcinoma, without hilar nodal involvement,Critical pathologic correlates(including absence of vascular tumor emboli and perineural invasion)further corroborate hematogenous spread. This spatiotemporal profile (primary tumor venous drainage via inferior mesenteric vasculature + latency interval) aligns precisely with established hemodynamic models of splenic seeding.
Notably, most patients with splenic metastasis are asymptomatic; however, one case was identified due to abdominal pain resulting from splenic rupture (19). The primary method of detection involves regular postoperative follow-up, which may reveal elevated carcinoembryonic antigen (CEA) levels or abnormalities on abdominal computed tomography (CT) scans. Among the reported patients, 80% exhibited elevated CEA levels, with the exception of five cases where CEA levels were not specified. In our case, the patient’s CEA level was 82.30 ng/ml, and elevated levels of CA19–9 and CA242 were also observed (Figure 5). Considering the initial preoperative elevation of CEA, its normalization following surgery, and subsequent elevation during splenic metastasis, we propose that dynamic monitoring of CEA levels can serve as a valuable tool for recurrence surveillance. Additionally, we identified several diagnostic modalities for detecting splenic metastasis, including abdominal ultrasound, fine-needle aspiration biopsy (20, 21), abdominal CT, abdominal magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT). Percutaneous biopsy enables pathological diagnosis that clarifies the nature of splenic lesions and informs subsequent therapeutic planning, albeit with inherent risks of hemorrhage and splenic abscess formation (20–22).PET/CT, due to its ability to rule out distant metastasis, plays a significant role in surgical decision-making, thereby increasing its utility in the diagnosis of splenic metastasis.
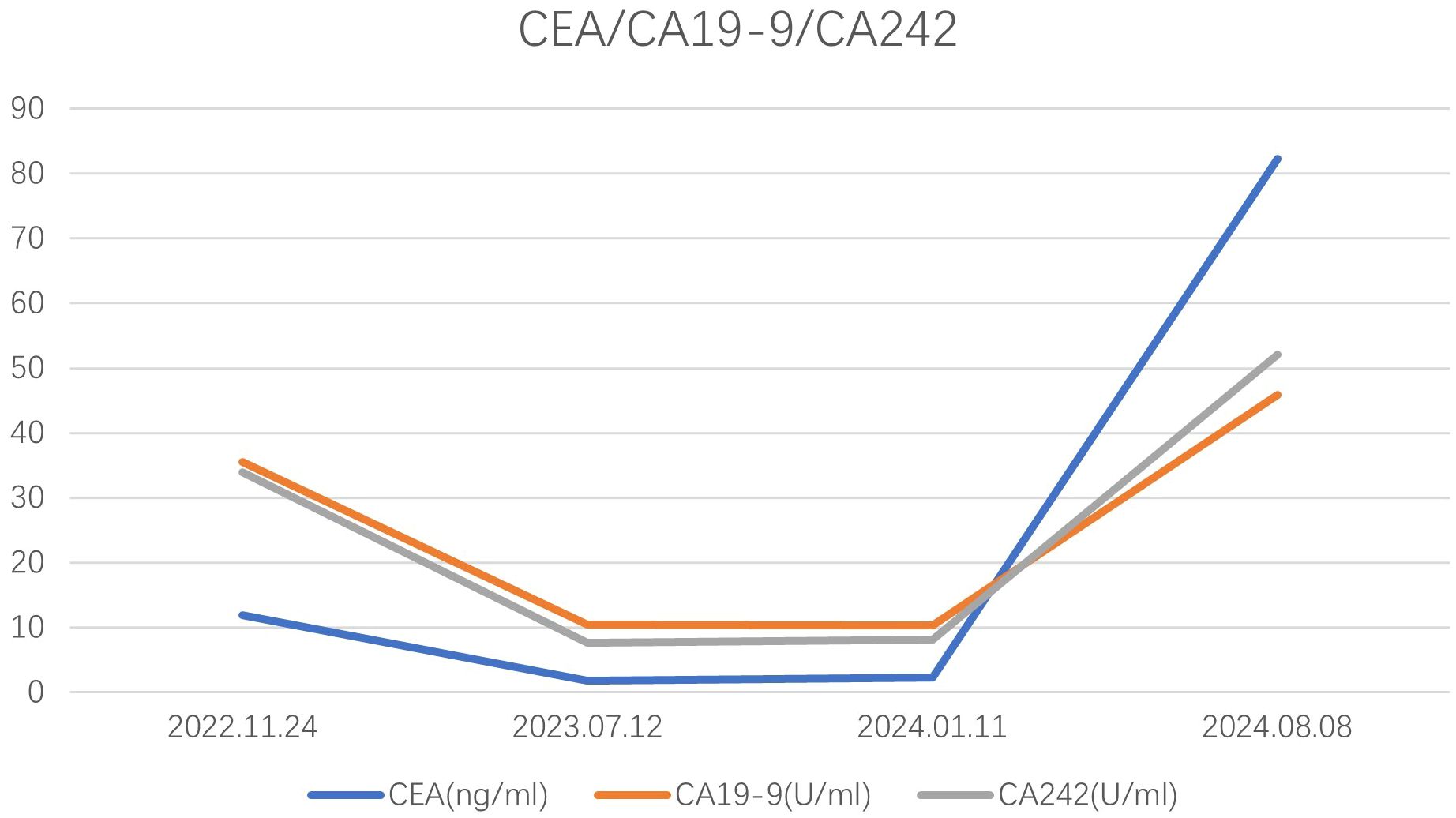
Figure 5. The concentration levels of CEA, CA19-9, and CA242 for the patient we reported at different time.
For metastatic colorectal cancer, treatment strategies encompass surgery, chemotherapy, targeted therapy, and radiotherapy. In the context of synchronous metastatic colorectal cancer, current consensus suggests that achieving an R0 resection should aim for a comprehensive tumor-free state, addressing both primary and metastatic lesions to enhance survival outcomes (23). For metachronous metastatic colorectal cancer, if the metastatic lesions are amenable to resection, the primary strategy involves excising these metastases initially. In cases where the lesions are not resectable, systemic therapy should be prioritized. Patients with initially unresectable disease should undergo follow-up evaluations every two months to reassess resectability. If resectability is achieved, surgical intervention is advised, followed by either observation or systemic therapy (3, 23, 24). Presently, there is limited evidence-based data regarding splenic metastases. However, for solitary liver or lung metastases from colorectal cancer, surgical resection has been shown to extend survival (23). As previously noted, solitary splenic metastasis does not necessarily indicate terminal metastatic disease. Case reports have suggested that surgical resection of solitary splenic metastasis can significantly prolong disease-free survival (25, 26). Furthermore, untreated splenic metastases may result in splenic rupture (19, 27). In the context of colorectal cancer splenic metastases, the adoption of laparoscopic surgery remains a subject of debate, primarily due to concerns regarding the potential risk of peritoneal dissemination. Nonetheless, certain studies suggest that laparoscopic surgery for colorectal cancer does not elevate the risk of intra-abdominal dissemination of cancer cells compared to conventional surgery (28). Moreover, laparoscopic splenectomy, in contrast to open surgery, is associated with reduced trauma, shorter hospitalization, expedited recovery, and lower rates of postoperative complications (29, 30). Laparoscopy also provides an opportunity to assess for peritoneal metastases. In our case, the patient presented with isolated splenic metastasis following laparoscopic radical resection of rectal cancer. Following a multidisciplinary team (MDT) discussion and consideration of the patient’s preferences, a laparoscopic splenectomy was performed. Intraoperatively, no hepatic or peritoneal metastases were detected, and the metastatic tumor was localized at the lower pole of the spleen. We removed the splenic metastatic tumor through the ileostomy closure incision to minimize wound size and recommended the patient continue oral capecitabine treatment. The patient is currently in good condition. Surgical treatment for isolated splenic metastases has shown promising results, with some patients achieving up to 7 years of disease-free survival (18).
For splenic metastases, treatment options extend beyond surgery and may include systemic chemotherapy, targeted therapy, immunotherapy, and ablation therapies. Currently, the evidence base supporting systemic treatment specifically for splenic metastases is limited, largely confined to case reports (31–34). The principles guiding systemic therapy for splenic metastases align with the overall treatment strategy for metastatic gastrointestinal (GI) cancers, with the primary objective being control of systemic disease.Available case reports also suggest the feasibility of medical therapy, indicating that chemotherapy can be safely administered in patients with pancytopenia secondary to hypersplenism, provided there is vigilant monitoring and supportive care (32).However, the emergence of anemia and thrombocytopenia during immunotherapy necessitates careful differentiation to determine their etiology: whether they are disease-related (due to metastases), treatment-related (attributable to chemotherapy/targeted agents), or immune-related toxicities.For severe bone marrow suppression or refractory hypersplenism, multidisciplinary team (MDT) consultation is strongly recommended to evaluate the need for splenectomy or interventional ablation.Ablation techniques for splenic metastases can serve as a curative treatment modality. The literature reports that radiofrequency ablation (RFA) and microwave ablation (MWA) display a safety profile that preserves splenic function while significantly reducing the risk of post-splenectomy infections and other associated complications (33). Nevertheless, splenectomy also remains an option, although it carries the risk of positive surgical margins, potentially necessitating subsequent radical splenectomy (35). Splenic metastases present a particular challenge for radiotherapy due to their subdiaphragmatic location and respiratory motion, hence its limited clinical adoption. Reports describe the placement of three fiducial markers (FMs) via a transradial intravascular approach around the lesion to guide radiotherapy (36). Nevertheless, this technique is likely reserved as an alternative option for patients unsuitable for surgery or ablation.
Due to the rarity of isolated splenic metastasis in colorectal cancer, we report this case to contribute to the existing data and offer insights for future treatment. Splenic metastasis in colorectal cancer typically manifests as a terminal-stage disease. Its management centers on a multidisciplinary approach (MDT-guided therapy), integrating systemic treatment with potentially curative-intent local interventions.
For patients presenting with solitary splenic metastasis, curative therapies (radical surgery or ablation therapy) are associated with significant survival benefits.
Looking ahead, we propose the establishment of international multicenter collaborative registries to systematically aggregate clinical treatment data and outcomes, thereby generating large-scale, high-quality evidence. Concurrently, leveraging artificial intelligence and radiomics holds promise for refining prognostic models and predicting therapeutic responses, ultimately contributing to personalized, optimized treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PR: Writing – original draft, Writing – review & editing. YiS: Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. LZ: Resources, Writing – review & editing. YuS: Data curation, Writing – review & editing, Resources. ZY: Writing – review & editing, Data curation. HL: Writing – review & editing, Supervision. SW: Writing – review & editing, Data curation. YC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Shenzhen High-level Hospital Construction Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Montemurro S, Maselli E, Ruggieri E, Caliandro C, Rucci A, Zito AF, et al. Isolated splenic metastasis from colon cancer. Report of a case. Tumori. (2008) 94:422–5. doi: 10.1177/030089160809400322
2. Xu J, He Y, Cai Y, Huang Y, and He Y. Isolated splenic metastases from rectal carcinoma 5 years after surgery: Case report. Med (Baltimore). (2023) 102:e32493. doi: 10.1097/MD.0000000000032493
3. Zhao L, Sui M, Li J, and Zhang K. Case report of isolated synchronous multiple splenic metastases from rectal cancer: A case report and brief review of the literature. Med (Baltimore). (2022) 101:e29613. doi: 10.1097/MD.0000000000029613
4. Reboux N, Jooste V, Goungounga J, Robaszkiewicz M, Nousbaum J-B, and Bouvier A-M. Incidence and survival in synchronous and metachronous liver metastases from colorectal cancer. JAMA Netw Open. (2022) 5:e2236666. doi: 10.1001/jamanetworkopen.2022.36666
5. Weitz J, Koch M, Debus J, Höhler T, Galle PR, and Büchler MW. Colorectal cancer. Lancet. (2005) 365:153–65. doi: 10.1016/S0140-6736(05)17706-X
6. Gupta GP and Massagué J. Cancer metastasis: building a framework. Cell. (2006) 127:679–95. doi: 10.1016/j.cell.2006.11.001
7. Haraldsdottir S, Einarsdottir HM, Smaradottir A, Gunnlaugsson A, and Halfdanarson TR. Colorectal cancer - review. Laeknabladid. (2014) 100:75–82. doi: 10.17992/lbl.2014.02.531
8. Berge T. Splenic metastases. Frequencies and patterns. Acta Pathol Microbiol Scandinavica Section A Pathol. (1974) 82:499–506. doi: 10.1111/j.1699-0463.1974.tb00379.x
9. Compérat E, Bardier-Dupas A, Camparo P, Capron F, and Charlotte F. Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med. (2007) 131:965–9. doi: 10.5858/2007-131-965-SMCPDD
10. Sauer J, Sobolewski K, and Dommisch K. Splenic metastases–not a frequent problem, but an underestimate location of metastases: epidemiology and course. J Cancer Res Clin Oncol. (2009) 135:667–71. doi: 10.1007/s00432-008-0502-3
11. Okuyama T, Oya M, and Ishikawa H. Isolated splenic metastasis of sigmoid colon cancer: a case report. Jpn J Clin Oncol. (2001) 31:341–5. doi: 10.1093/jjco/hye065
12. Pisanu A, Ravarino A, Nieddu R, and Uccheddu A. Synchronous isolated splenic metastasis from colon carcinoma and concomitant splenic abscess: a case report and review of the literature. World J Gastroenterol. (2007) 13:5516–20. doi: 10.3748/wjg.v13.i41.5516
13. Zhao H, Zhong W, Chen D, and Cheng X. Synchronous isolated splenic metastasis from cancer of hepatic flexure of colon. Medicine. (2019) 98(14):e15016. doi: 10.1097/MD.0000000000015016
14. Gencosmanoglu R, Aker F, Kir G, and Tozun N. Isolated metachronous splenic metastasis from synchronous colon cancer. World J Surg Oncol. (2006) 4:42. doi: 10.1186/1477-7819-4-42
15. Rosa N, Martins S, and Lamelas J. Isolated splenic metastasis of colon cancer: A case report and literature review. J Coloproctol (Rio Janeiro). (2012) 32:88–93. doi: 10.1590/S2237-93632012000100014
16. Andryukhova ES, Tashireva LA, Isaeva AV, Vtorushin SV, Zavyalova MV, and Perelmuter VM. The rarity of metastasis to the spleen — a phenomenon with an unknown mechanism. Bull Exp Biol Med. (2022) 173:575–82. doi: 10.1007/s10517-022-05591-3
17. Efared B, Mazti A, Atsame-Ebang G, Tahiri L, El Bouhaddouti H, Hammas N, et al. An unusual site of metastasis: splenic metastastasis from a colon cancer. J Surg Case Rep. (2016) 2016(11):rjw175. doi: 10.1093/jscr/rjw175
18. Dunbar WH, Beahrs OH, and Morlock CG. Solitary splenic metastasis incidental to rectal carcinoma: report of a case. Mayo Clin Proc. (1969) 44:40–5.
19. Achuthan R, Joseph A, and Haray PN. Splenic metastasis from a rectal tumour: an unusual presentation. Ann R Coll Surg Engl. (1999) 81:139.
20. Place RJ. Isolated colon cancer metastasis to the spleen. Am Surg. (2001) 67:454–7. doi: 10.1177/000313480106700515
21. Busić Z, Cupurdija K, Kolovrat M, Cavka V, Cavka M, Patrlj L, et al. Isolated splenic metastasis from colon cancer–case report and literature review. Collegium Antropol. (2010) 34 Suppl 1:287–90.
22. Ramos C, Santos V, Lopes A, Ferreira C, Viana P, and Quirino FJCS. Isolated splenic metastasis from colon cancer: case report. Clin Surg. (2023) 8:3618. doi: 10.25107/2474-1647.3618
23. Hernandez Dominguez O, Yilmaz S, and Steele SR. Stage IV colorectal cancer management and treatment. J Clin Med. (2023) 12(5):2072. doi: 10.3390/jcm12052072
24. Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, et al. Garrido-Laguna I et al: Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw: JNCCN. (2024) 22(2 D):e240029. doi: 10.6004/jnccn.2024.0029
25. Sileri P, D’Ugo S, Benavoli D, Stolfi VM, Palmieri G, Mele A, et al. Metachronous splenic metastasis from colonic carcinoma five years after surgery: a case report and literature review. South Med J. (2009) 102:733–5. doi: 10.1097/SMJ.0b013e3181a93c39
26. El M’rabet FZ, Brahmi SA, Rachidi S, Tizniti S, Amaarti A, Ait Taleb K, et al. Splenic metastasis from colonic adenocarcinoma - about a case and literature review. Pan Afr Med J. (2011) 10:44.
27. Krapohl BD, Kömürcü F, and Deutinger M. Spleen rupture due to metastasis of thin melanoma (Breslow thickness of 0.75 mm). Melanoma Res. (2005) 15:135. doi: 10.1097/00008390-200504000-00009
28. Jingli C, Rong C, and Rubai X. Influence of colorectal laparoscopic surgery on dissemination and seeding of tumor cells. Surg Endosc. (2006) 20:1759–61. doi: 10.1007/s00464-005-0694-4
29. Winslow ER and Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery. (2003) 134:647–53. doi: 10.1016/S0039-6060(03)00312-X
30. He LH, Yang B, Su XQ, Zhou Y, and Zhang Z. Comparison of clinical efficacy and postoperative inflammatory response between laparoscopic and open radical resection of colorectal cancer. World J Clin cases. (2022) 10:4042–9. doi: 10.12998/wjcc.v10.i13.4042
31. Güngör AUK, Aykut A, Sahin B, and Rüzgar HA. Successful chemotherapy administration despite hypersplenism and pancytopenia: A case of metastatic rectal adenocarcinoma. Hematol Transfusion Cell Ther. (2024) 46:S68–9. doi: 10.1016/j.htct.2024.11.064
32. Fujii J, Sugimura K, Masuzawa T, Yukawa Y, Katsuyama S, Arita A, et al. A case of stage IV Gastric cancer with distant metastasis that underwent curative surgery and achieved complete response in primary lesion after systemic chemotherapy with nivolumab. Gan Kagaku Ryoho Cancer Chemother. (2025) 52:279–81.
33. Lardière-Deguelte S, de Mestier L, Amroun KL, Volet J, Cart P, Bouche O, et al. Laparoscopic thermal ablation of splenic metastases initial experience and present aspects. J visceral Surg. (2013) 150:355–8. doi: 10.1016/j.jviscsurg.2013.07.003
34. Ogawa H, Komatsu S, Matsubara D, Hamada S, Nishiko M, Konishi T, et al. Patient with epstein-Barr virus-Positive esophagogastric junctional cancer with splenic metastasis and underwent metastasectomy following immune checkpoint therapy. Gan Kagaku Ryoho Cancer Chemother. (2022) 49:1163–5.
35. Marangio A, Prati U, Luinetti O, Brunetti E, and Fìlice C. Radiofrequency ablation of colorectal splenic metastasis. AJR Am J Roentgenol. (2002) 178:1481–2. doi: 10.2214/ajr.178.6.1781481
36. Timmers AC, Nuyttens J, and de Wolf MA. Endovascular fiducial placement in splenic metastatic disease as a novel option for radiotherapy: A case report. Cureus. (2025) 17:e77186. doi: 10.7759/cureus.77186
37. Capizzi PJ, Allen KB, Amerson JR, and Skandalakis JE. Isolated splenic metastasis from rectal carcinoma. South Med J. (1992) 85:1003–5. doi: 10.1097/00007611-199210000-00017
38. Indudhara R, Vogt D, Levin HS, and Church J. Isolated splenic metastases from colon cancer. South Med J. (1997) 90:633–6. doi: 10.1097/00007611-199706000-00010
39. Weathers BK, Modesto VL, and Gordon D. Isolated splenic metastasis from colorectal carcinoma: report of a case and review of the literature. Dis Colon Rectum. (1999) 42:1345–8. doi: 10.1007/BF02234228
40. Genna M, Leopardi F, Valloncini E, Molfetta M, De Manzoni G, and Castelli A. Metachronus splenic metastasis of colon cancer. A case report. Minerva Chirurgica. (2003) 58:811–4.
41. Bigot P, Goodman C, Hamy A, Teyssedou C, and Arnaud JP. Isolated splenic metastasis from colorectal cancer: report of a case. J Gastrointestinal Surg: Off J Soc Surg Alimentary Tract. (2008) 12:981–2. doi: 10.1007/s11605-007-0326-5
42. Genç V, Akbari M, Karaca AS, Çakmak A, Ekıncı C, and Gürel M. Why is isolated spleen metastasis a rare entity? Turkish J Gastroenterol: Off J Turkish Soc Gastroenterol. (2010) 21:452–3. doi: 10.4318/tjg.2010.0136
43. Abdou J. Isolated splenic metastasis from colon cancer: Case report. World J Gastroenterol. (2016) 22(18):4610–4. doi: 10.3748/wjg.v22.i18.4610
44. Rizzo F, Calamia S, Mingoia G, Fulfaro F, Grassi N, and Cipolla C. Isolated metachronous splenic metastasis from colon cancer: possible explanations for this rare entity. J Gastrointestinal Cancer. (2017) 50:143–6. doi: 10.1007/s12029-017-9992-z
45. Hu L, Zhu J-Y, Fang L, Yu X-C, and Yan Z-L. Isolated metachronous splenic multiple metastases after colon cancer surgery: A case report and literature review. World J Clin cases. (2020) 8:3320–8. doi: 10.12998/wjcc.v8.i15.3320
46. Yokokawa H, Imaizumi R, Ito Y, Kono T, Koike T, Miyano Y, et al. An octogenarian case of sequential laparoscopic surgery for synchronous isolated splenic metastasis from cancer of the cecum. Int Surg. (2021) 105:619–22. doi: 10.9738/INTSURG-D-20-00031.1
Keywords: colorectal cancer, isolated splenic metastasis, metachronous, laparoscopic splenectomy, literature review
Citation: Ren P, Shi Y, Zhang L, Sun Y, Ye Z, Liu H, Wang S and Chen Y (2025) Isolated metachronous splenic metastasis after rectal cancer surgery: a case report and literature review. Front. Oncol. 15:1563632. doi: 10.3389/fonc.2025.1563632
Received: 20 January 2025; Accepted: 17 July 2025;
Published: 07 August 2025.
Edited by:
Masaichi Ohira, Osaka City University, JapanReviewed by:
Fulin Zhou, Guizhou Medical University, ChinaTümay Ak, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2025 Ren, Shi, Zhang, Sun, Ye, Liu, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinggang Chen, Y2h5Z2FuZzc3N0AxNjMuY29t
 Peide Ren
Peide Ren Yihang Shi2
Yihang Shi2 Hengchang Liu
Hengchang Liu Shunda Wang
Shunda Wang Yinggang Chen
Yinggang Chen