- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, Nanning, China
- 2Guangxi key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer, Nanning, China
- 3Guangxi Clinical Research Center for Enhanced Recovery after Surgery, Nanning, China
- 4Guangxi Zhuang Autonomous Region Engineering Research Center for Artificial Intelligence Analysis of Multimodal Tumor Images, Nanning, China
- 5Department of Surgery, University of Michigan Medical School, Ann Arbor, MI, United States
- 6Department of Urology, Xiangya Hospital, Central South University, Changsha, Hunan, China
Background: This umbrella review aims to critically appraise and synthesize epidemiological evidence from meta-analyses to identify and classify risk and protective factors associated with gastric cancer.
Methods: PubMed, Embase, Web of Science, and the Cochrane were used to search, including meta-analyses up to April 2024. Emphasis was placed on non-interventional studies, and the inclusion criteria focused on meta-analyses that involved diverse ethnic groups and genders from various countries and settings. Two reviewers independently evaluated the methodological quality using the AMSTAR tool and classified evidence strength based on established criteria.
Results: Of 245 meta-analyses meeting inclusion criteria, 117 unique risk factors were identified, including 77 significantly associated factors (42 adverse and 35 protective) and 40 non-significant factors. 17 (14.5%) risk factors were classified as class I or II evidence in this umbrella review. Protective factors included cruciferous vegetable intake, total cholesterol (TC), HDL cholesterol (HDL-C), NSAIDs, β-carotene, vitamins, and dietary polyphenols. Risk factors included depression, Helicobacter pylori (Hp) infection, dermatomyositis, and Graves’ disease. Class III evidence confirmed that aspirin, non-aspirin NSAIDs, soy food intake, non-fermented soy food intake, physical activity, vitamin A, ginseng, dietary fiber, tooth brushing frequency, folate, and green tea consumption were associated with reduced GC risk. Conversely, Epstein-Barr virus infection, red meat, processed meat, intestinal metaplasia, gastric atrophy, a western-style diet, dietary cholesterol, dietary salt, and proton pump inhibitors were linked to higher GC risk.
Conclusion: This umbrella review identified 77 risk factors significantly associated with gastric cancer (GC), the majority of which are linked to personal traits and lifestyle behaviors. These findings enhance our understanding of GC etiology and can inform strategies to reduce incidence, delay progression, and alleviate the global burden.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023447199.
Introduction
Gastric cancer (GC) is a prevalent digestive system malignancy with a poor prognosis. Ranking fifth in incidence among common cancers worldwide, its distribution varies considerably across geographic regions and ethnic groups (1, 2). Early-stage GC is often asymptomatic or presents with nonspecific symptoms, resulting in delayed diagnosis and treatment. Consequently, many cases are diagnosed at advanced stages, substantially worsening patient outcomes (3). Data show that the 5-year survival rate for early GC exceeds 90%, but it drops significantly to below 20% once the disease progresses to intermediate or late stages (4, 5). GC is a multifactorial disease influenced by both modifiable and non-modifiable risk factors. While genetic predispositions and aging are beyond control, environmental exposures, infections, dietary habits, drug use, and psychological factors represent modifiable contributors, highlighting the potential for prevention through targeted interventions. Numerous meta-analyses have identified various risk factors for GC (6–18). Among these, environmental factors are increasingly recognized as significant. Excessive smoking, alcohol consumption, high-salt and fried food intake, and the consumption of red and processed meats are strongly associated with an elevated GC risk in a dose- or time-dependent manner (1, 19, 20). Insufficient fruit intake, physical inactivity, and obesity also increase the risk of GC (21–23). Environmental pollutants, including poor drinking water quality, contaminated water sources, and soil pollution, have also been linked to higher GC incidence. Helicobacter pylori (Hp) infection remains a primary high-risk factor, with early eradication showing a favorable cost-effectiveness ratio for GC prevention (14). Emerging evidence indicates that the proton pump inhibitors (PPIs)’s long-term use may lead to GC risk, presenting new challenges in prevention and treatment strategies (12). Further research is needed to optimize drug use and inform clinical guidelines effectively. However, these studies suffer from issues such as methodological heterogeneity, differences in population characteristics, and inconsistent classification criteria for risk factors, resulting in significant contradictions among the results of different meta - analyses. This dispersion of evidence seriously hinders the formulation of clinical decisions and prevention strategies.
Although several meta-analyses of observational studies have investigated various risk factors for GC, differences in research design, exposure assessments, and outcomes have complicated the drawing of definitive conclusions. It is worth noting that although some systematic reviews have explored the association between specific risk factors and GC, there is currently a lack of research that uses the umbrella review method to hierarchically integrate existing evidence. Before effective prevention strategies can be formulated, it is crucial to systematically assess the quality, potential biases, and validity of the existing studies on GC risk factors. To address this gap, we conducted an umbrella review to consolidate the available evidence on the risk factors for GC.
Methods and analysis
Design and registration
Systematic literature searching, data extraction, and studies’ analysis focusing on GC risk factors were conducted. The process followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24). This study adhered to the methodological principles outlined in the Joanna Briggs Institute Manual for Evidence Synthesis of Umbrella Reviews (25), as well as the procedures detailed in the Cochrane Handbook (26). Additionally, the review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023447199 (https://www.crd.york.ac.uk/PROSPERO/).
Eligibility criteria
Meta-analyses assessing GC risk factors across any ethnicity, sex, country, or setting were eligible for inclusion. If a single meta-analysis reported multiple risk factors, data on each risk factor were extracted separately. In cases where several meta-analyses (published more than 24 months apart) evaluated the same risk factor, we included the most recent one for analysis. For studies published within 24 months, priority was given to the meta-analysis with the most prospective cohorts. If the number of cohorts was the same, preference was given to the study with the higher AMSTAR score (27, 28). Study quality was independently assessed by two reviewers using AMSTAR-2 criteria. Discrepancies in scoring were resolved through iterative re-examination of the original articles followed by consultation with a third senior researcher when necessary. Moreover, if the latest meta-analysis did not perform a dose-response analysis, while another did, both were included for data extraction. Meta-analyses focusing on therapeutic interventions for GC, as well as non-English studies, animal studies, and cell culture studies, were excluded.
Population
This study systematically examines meta-analyses assessing risk factors for GC. The primary focus of the included studies was to identify factors that influence the risk of GC, either by increasing or reducing it. Studies on the efficacy of treatments for GC, pathogenesis of GC, and factors related to GC exacerbation or recurrence were excluded.
Exposure
Meta-analyses that identified at least one risk factor for gastric cancer (GC) were included in this review. These factors covered a broad range, including environmental, lifestyle, disease-related, treatment-related, demographic, genetic, social, and psychophysiological aspects. These risk factors was evaluated through the use of odds ratios (OR), relative risks (RR), or hazard ratios (HR), accompanied by 95% confidence intervals (CIs).
Outcomes
The diagnosis of GC in the included studies must follow internationally accepted guidelines, such as the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines, which outline the diagnostic, treatment, and follow-up protocols for patients with GC (29).
Study designs
Only meta-analyses of studies assessing GC risk factors across various ethnicities, sexes, countries, or settings were eligible for inclusion. Studies focused on GC risk factors and provided comprehensive descriptions of their methods, including the search strategy, inclusion and exclusion criteria, quality assessment, result evaluation, analysis procedures, and criteria for interpreting the results. The original studies included in the reviews consisted of prospective or retrospective cohort studies, case-control studies, or cross-sectional studies.
Information sources
A systematic search was performed in PubMed, Embase, Web of Science, and the Cochrane from the establishment of the database to April 2024 to find relevant meta-analyses, including both interventional and non-interventional studies. Additionally, we reviewed the reference lists to locate other relevant articles.
Search strategy
These databases were searched using Medical Subject Headings (MeSH), keywords, and text terms associated with GC, in accordance with the Scottish Intercollegiate Guidelines Network (SIGN) recommendations for literature search methodology: (((risk) OR (incidence)) AND ((systematic review) OR (meta-analysis))) AND (((gastric cancer) OR (stomach cancer)) OR (stomach neoplasms)) (30).
Study selection
All studies identified were initially screened with EndNote X9. After eliminating duplicates, two authors independently reviewed the titles and abstracts to select meta-analyses that met the inclusion criteria, followed by a full-text review. Any discrepancies between the two authors were resolved by a third author. Furthermore, a manual search of reference lists was conducted to identify additional meta-analyses that may have been overlooked (Figure 1).
Assessment of methodological quality
Two reviewers independently assessed the methodological rigor, employing the AMSTAR instrument, which is a well-established and dependable method for evaluating the quality of meta-analyses (27, 31). The strength of epidemiological data supporting each risk factor was graded into one of four categories: Class I (indicating strong evidence), Class II (suggesting strong evidence), Class III (indicating evidence), Class IV (suggesting limited evidence), and NS (indicating no significant evidence), as detailed in Table 1 (32–34).
Data extraction
Two researchers separately assessed the eligible studies to collect relevant data, such as the names of the authors, publication years, risk factors, types of gastric cancer, sample sizes, number of cases, total number of participants, study designs (e.g., cross-sectional, case-control, cohort), durations of follow-up, and risk measures (including RR, OR, HR, etc.) along with their 95% confidence intervals. The methods used for meta-analysis (whether random or fixed effects), heterogeneity evaluations (I², Cochran’s Q), and assessments of publication bias (through Egger’s and Begg’s tests, as well as funnel plots) were also recorded. For studies involving dose-response relationships or subgroup analyses, we noted P-values concerning nonlinearity and subgroup results. Any discrepancies were addressed by consulting another author. All papers contain reported data from unrelated individuals.
Data summary
RR, OR, or HR along with their 95% CIs were re-estimated by applying either random or fixed-effects models. Additionally, we evaluated heterogeneity (using I² and Cochran’s Q-test) and the potential for small-study effects (via Egger’s or Begg’s tests) in meta-analyses, provided that the analysis involved more than 10 studies and sufficient data were available (35–37). Heterogeneity testing and publication bias detection were performed using Review Manager v5.4.1 (Cochrane Collaboration, Oxford, UK) and Stata v15.0, respectively. The risk factors were categorized into five distinct groups according to the health ecological model (38, 39): individual characteristics (such as age, gender, genetic factors, birth status, height, weight, BMI, pre-existing conditions, and prior treatments), lifestyle choices (including diet, physical activity, smoking, alcohol consumption, sleep habits, and working hours), social relationships (such as marital status, family dynamics, and social interactions), economic variables (like occupation, household income, and debt), and environmental influences (including urban vs. rural location, presence of pets, immigration status, and living conditions). When the OR, RR, or HR value of a factor is greater than 1 and there is a significant statistical difference, we consider this factor to be a risk factor; On the contrary, it is a protective factor for the stomach.
For risk factors classified as Class I or II evidence, a sensitivity analysis was performed when adequate data were available, in order to assess the individual studies’ impact on the overall evidence strength. We also conducted a dose-response analysis for GC risk factors based on the meta-analyses included in the review. Additionally, if the latest meta-analysis did not incorporate clinical studies from other reviews, we pooled data from these studies and carried out a new analysis. Heterogeneity tests were considered significant with a P-value of < 0.10, while other statistical tests were deemed significant at a P-value of < 0.05. Evidence synthesis was conducted using Review Manager v5.4.1 (Cochrane Collaboration, Oxford, UK), while sensitivity analysis and the Egger and Begg tests were performed using Stata v15.0.
Major outcomes
Meta-analyses characteristics
Literature searching and selection was depicted in Figure 1. 6,230 unique articles were identified. 245 meta-analyses (7, 9–17, 21–23, 40–271) met the inclusion criteria. We extracted 117 distinct risk factors, consisting of 77 significantly associated and 40 non-significantly associated factors (Supplementary Table S1). Our analysis identified 42 adverse and 35 favorable associations with statistical significance. After a comprehensive quality assessment using established guidelines, most outcomes were categorized as Class IV (low quality) or NS (non-significant) evidence. Notably, only 17 risk factors (14.5%) were classified as Class I or II evidence. Among the identified factors, innate personal traits and behavioral lifestyles emerged as the primary contributors to GC risk.
Class I evidence
Cruciferous vegetable
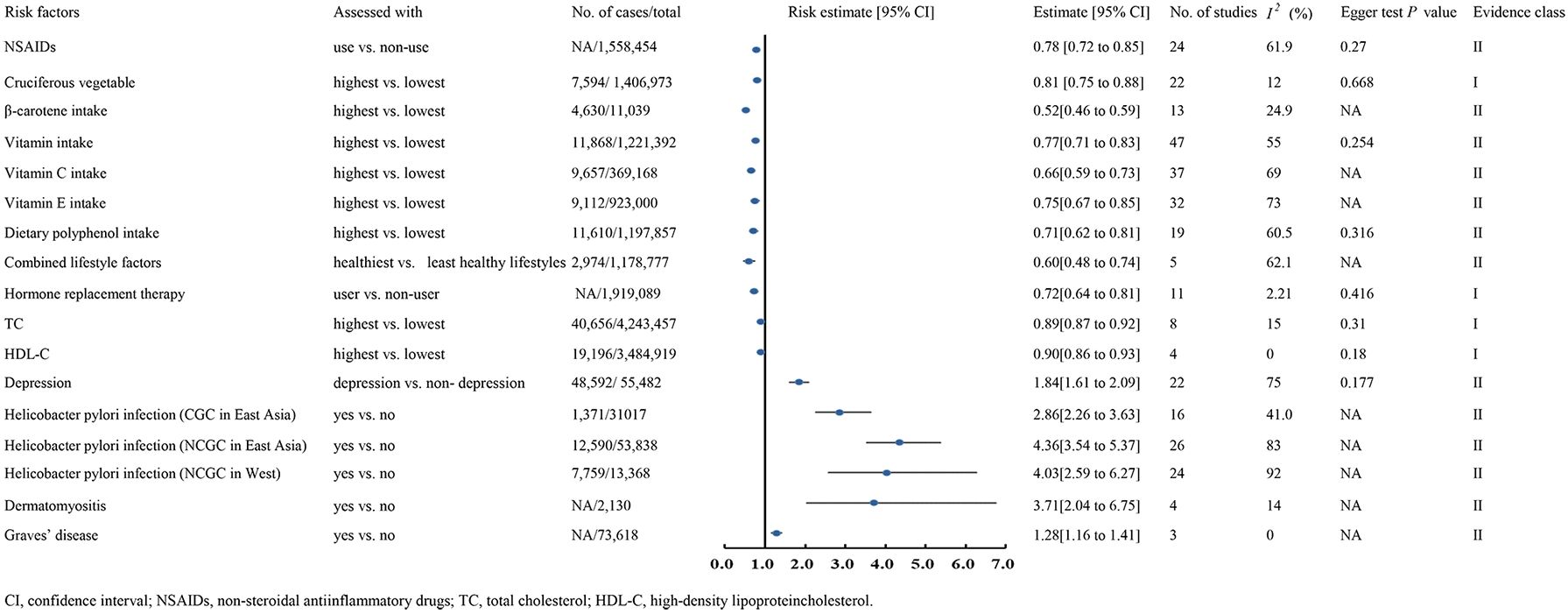
A meta-analysis including 6 cohorts and 16 case-control studies, with 1,406,973 patients and 7,594 cases, showed that the cruciferous vegetables consumption can significantly decrease GC risk(RR 0.81, 95% CI 0.75-0.88) (21) (Figure 2).
High-density lipoprotein cholesterol
A meta-analysis involving 4 cohorts, comprising 3,484,919 participants and 19,196 cases, demonstrated that higher HDL-C were linked to lower GC risk (HR 0.90, 95% CI 0.86-0.93) (9) (Figure 2).
Total cholesterol
A meta-analysis encompassing 8 cohorts, with 4,243,457 participants and 40,656 cases, reported that elevated TC levels were linked to a lower GC risk (HR 0.89, 95% CI 0.87-0.92) (9) (Figure 2).
Hormone replacement therapy
A meta-analysis with 7 cohorts and 4 case-control studies, comprising 1,919,089 participants, found a significant association between estrogen-based hormone replacement therapy and a reduced GC risk (RR 0.72, 95% CI 0.64-0.81) (245) (Figure 2).
Class II evidence
Innate personal trait
A meta-analysis of 22 studies, including 2 cohorts and 22 case-control studies, reported that depression increases the GC risk (OR 1.84, 95% CI 1.61-2.09) (259). Similarly, a meta-analysis of 4 case-control studies with 2,130 participants revealed a significant link between dermatomyositis and elevated GC risk (SIR 3.71, 95% CI 2.04-6.75) (236). In comparison, a meta-analysis of three studies involving 73,618 participants showed that individuals with Graves’ disease had a higher GC risk than healthy populations (SIR 1.28, 95% CI 1.16-1.41) (236). Conversely, a meta-analysis of 24 studies with 1,558,454 patients found NSAID use significantly reduced GC risk (RR 0.78, 95% CI 0.72-0.85) (169). Moreover, a meta-analysis of 26 studies demonstrated that Hp infection significantly raised GC risk (RR 4.36, 95% CI 3.54-5.37) (14) (Figure 2).
Behavioural lifestyles
A meta-analysis of 47 studies, involving 11 randomized controlled trial, 7 cohorts and 29 case-control studies, comprising 1,221,392 participants and 11,868 cases, reported that higher vitamin intake was associated with a lower risk of GC compared to lower intake (RR 0.77, 95% CI 0.71-0.83) (112). The analysis further demonstrated that increased intake of vitamin C (RR 0.66, 95% CI 0.59-0.73) and vitamin E (RR 0.75, 95% CI 0.67-0.85) reduced GC risk (112). Regarding dietary polyphenol intake, a meta-analysis of 7 cohort and 12 case-control studies involving 1,197,857 participants revealed that individuals with higher polyphenol consumption had a significantly lower GC risk (RR 0.71, 95% CI 0.62-0.81) (240). Similarly, a large-scale meta-analysis of 13 case-control studies found that higher β-carotene intake significantly reduced GC risk (OR 0.52, 95% CI 0.46-0.59) (162). Moreover, a meta-analysis of 5 cohort studies concluded that healthier combined lifestyle factors significantly decreased GC risk (OR 0.60, 95% CI 0.48-0.74) (220) (Figure 2).
Class III evidence
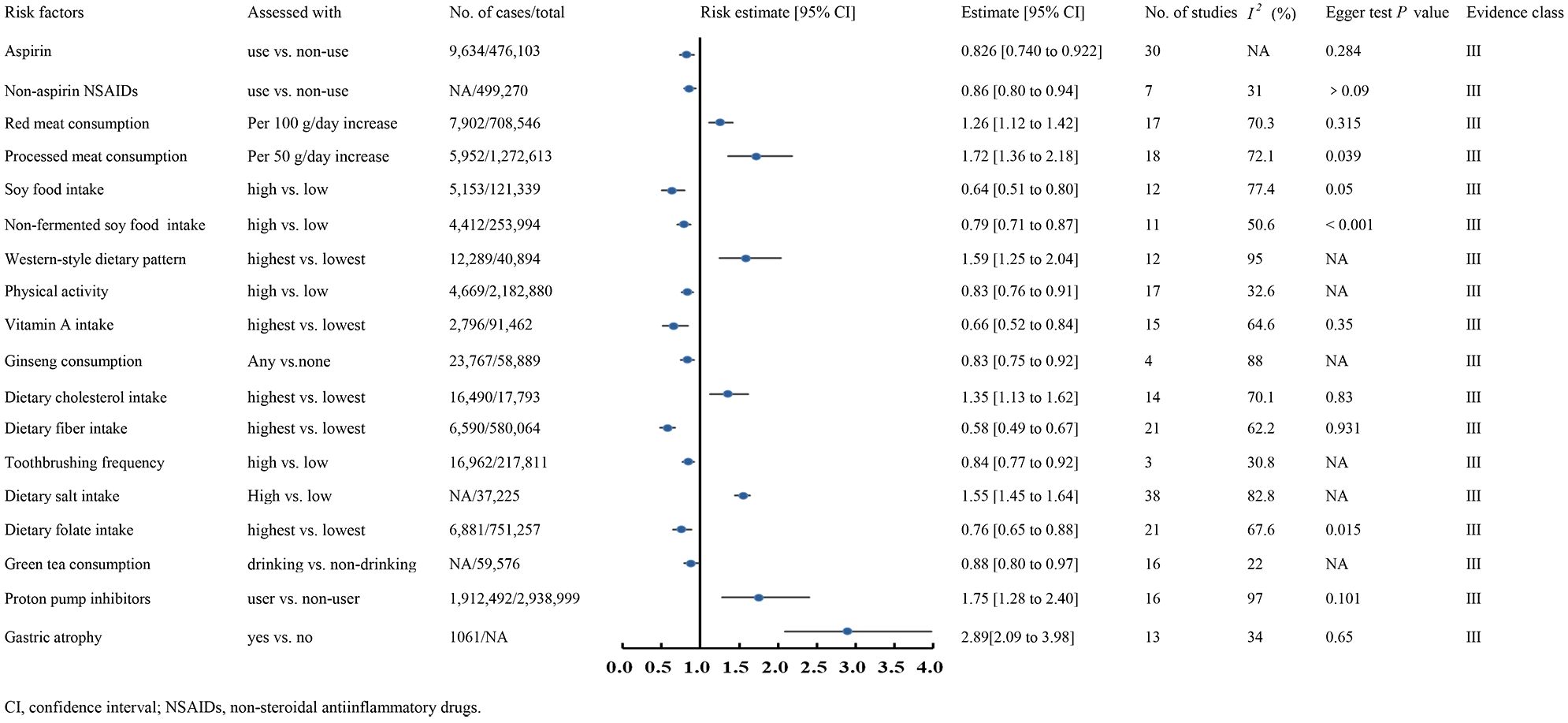
Class III evidence demonstrated that aspirin use (RR 0.826, 95% CI 0.740-0.922) (210), non-aspirin NSAID use (RR 0.86, 95% CI 0.80-0.94) (169), higher soy food intake (RR 0.64, 95% CI 0.51-0.80) (233), non-fermented soy food intake (RR 0.79, 95% CI 0.71-0.87) (233), increased physical activity (RR 0.83, 95% CI 0.76-0.91) (22), higher vitamin A intake (RR 0.66, 95% CI 0.52-0.84) (144), ginseng consumption (RR 0.83, 95% CI 0.75-0.92) (152), higher dietary fiber intake (OR 0.58, 95% CI 0.49-0.67) (107), frequent toothbrushing (OR 0.84, 95% CI 0.77-0.92) (235), higher folate intake (OR 0.76, 95% CI 0.65-0.88) (171), and increased green tea consumption (OR 0.88, 95% CI 0.80-0.97) (212) were associated with reduced GC risk (Figure 3).
Conversely, EB virus infection (OR 18.57, 95% CI 15.69-21.98) (215), higher red meat consumption (RR 1.26, 95% CI 1.12-1.42) (193), processed meat consumption (RR 1.72, 95% CI 1.36-2.18) (193), intestinal metaplasia (RR 5.16, 95% CI 3.28-5.16) (224), gastric atrophy (RR 2.89, 95% CI 2.09-3.98) (261), a western-style dietary pattern (OR 1.59, 95% CI 1.25-2.04) (97), higher dietary cholesterol intake (OR 1.35, 95% CI 1.13-1.62) (229), increased dietary salt intake (OR 1.55, 95% CI 1.45-1.64) (256), and proton pump inhibitor use (OR 1.75, 95% CI 1.28-2.40) (12) were linked to elevated GC risk (Figure 3). Dose-response analysis revealed that every 100g increase in daily red meat intake raised GC risk by 26%, while each 50g increase in processed meat intake per day elevated the risk by 72%.
Class IV and NS evidence
This umbrella review identified 40 class IV evidence-based risk factors, including 13 protective and 27 risk factors. The top ten protective factors were refrigerator use (OR 0.70, 95% CI 0.56-0.88) (265), higher allium vegetable intake (RR 0.78, 95% CI 0.67-0.91) (141), greater garlic consumption (OR 0.65, 95% CI 0.49-0.87) (244), adherence to a healthy dietary pattern (OR 0.69, 95% CI 0.53-0.89) (97), the Mediterranean dietary score (MDS) (OR 0.69, 95% CI 0.53-0.90) (209), the dietary inflammatory index (DII) (RR 0.63, 95% CI 0.45-0.88) (209), metformin use (RR 0.84, 95% CI 0.73-0.96) (253), statin use (OR 0.74, 95% CI 0.67-0.80) (16), higher tomato product consumption (OR 0.73, 95% CI 0.60-0.90) (104), and increased selenium levels (OR 0.87, 95% CI 0.78-0.97) (151).
The top ten risk factors with the highest effect values were MAFLD (RR 1.49, 95% CI 1.17-1.91) (7), pernicious anemia (OR 6.8, 95% CI 2.6-18.1) (102), frequent consumption of refined grains (≥3 times/week vs. <3 times/week) (OR 1.63, 95% CI 1.49-1.79) (216), papillomavirus infection (OR 5.80, 95% CI 3.27-10.31) (221), higher educational attainment (OR 2.97, 95% CI 1.93-4.58) (101), combined socioeconomic position (OR 2.64, 95% CI 1.05-6.63) (101), current cigarette smoking (OR 1.61, 95% CI 1.49-1.75) (212), insulin use (RR 1.65, 95% CI 1.02-2.68) (95), inflammatory myopathies (SIR 2.68, 95% CI 1.40-5.12) (236), and higher chili consumption (OR 1.51, 95% CI 1.02-2.00) (228).
Additionally, this umbrella review identified 40 non-significant risk factors, with detailed data provided in Supplementary Table S1.
Heterogeneity
In this study, 59% of risk factors were reanalyzed using random- or fixed-effects models, revealing significant heterogeneity in approximately 63% (I² > 50% or Cochran’s Q-test P < 0.1). Heterogeneity in most outcomes was likely influenced by factors such as study setting, geographical region, ethnicity, gender, age, sample size, study design, follow-up duration, and adjustments for confounding variables. Among the remaining 41% of risk factors, 56% exhibited considerable heterogeneity, while 2.6% did not report heterogeneity results.
Assessment of risk of bias
In the reassessment, publication bias was evaluated for 56.2% of the identified risk factors using Egger’s test, which detected bias in nine factors. For outcomes not reanalyzed, statistical tests or funnel plots revealed publication bias in 5.1% of the risk factors. The remaining outcomes showed no significant evidence of publication bias or lacked bias assessment.
AMSTAR score
The median AMSTAR score for all identified risk factors was 8 (range 6-10). Detailed AMSTAR scores for each outcome are listed in Supplementary Table S2.
Discussion
Principal findings and possible explanations
Gastric cancer remains a common malignancy worldwide, ranking third in cancer-related mortality. In China, the burden of GC is particularly severe, with an incidence rate of 34.6 per 100,000 and a mortality rate of 30.2 per 100,000 (267). The etiology of GC is multifaceted, involving intricate interactions among genetic, environmental, and lifestyle factors, many of which remain poorly understood. Despite extensive research, several risk factors contributing to GC have not received adequate attention (268). Current studies categorize GC risk factors into demographic, socioeconomic, environmental, infectious, genetic, drug-related, and psychological factors. A subset of these risk factors is modifiable, prompting some researchers to classify GC as “one of the preventable cancers” (269). Over the past decades, clinical and evidence-based studies have explored these risk factors extensively, using systematic reviews and meta-analyses to synthesize findings from diverse populations. This umbrella review aims to evaluate the strengths and limitations of existing evidence from systematic reviews and meta-analyses on GC risk factors, providing a comprehensive understanding of the potential contributors to its development and progression. By synthesizing insights from systematic reviews and meta-analyses, this review offers a robust theoretical foundation for the design of more effective prevention and control strategies and highlights priorities for future research. This umbrella review identified 117 unique risk factors for GC, including 77 factors significantly associated with GC and 40 with non-significantly associations. Among the significant risk factors, 42 were adverse while 35 were favorable. Following a rigorous quality assessment using established classification criteria, most outcomes were classified as Class IV or NS evidence. Only 17 risk factors (14.5%) met the criteria for Class I or II evidence. Notably, personal traits and behavioral lifestyles were the primary contributors to the risk of GC.
Dietary factors play an important role in the occurrence and development of gastric cancer
This study observed a significant negative correlation between cruciferous vegetable intake and the risk of GC (Class I evidence). Supporting this finding, Fang et al. (270) analyzed 76 cohort studies and reported an inverse relationship between fruit and white vegetable consumption and gastric cancer risk. However, a Japanese cohort study found no significant association between fresh fruit and vegetable intake and gastric cancer risk (271). A hypothesis suggests that a protective threshold exists for vegetables and fruits consumption, beyond which no additional benefit occurs (272). This discrepancy may also arise from contextual factors: the Japanese diet traditionally includes high salt intake (e.g., pickled vegetables and soy sauce), which could counteract the benefits of fresh vegetables. Additionally, variations in Helicobacter pylori infection rates, genetic susceptibility, or differences in vegetable preparation methods (e.g., fermentation vs. raw consumption) might contribute to conflicting results. Future studies should account for dietary patterns holistically and adjust for salt intake as a potential confounder to clarify these associations. Notably, the studies reviewed here did not establish a significant dose-response relationship between vegetable intake and gastric cancer risk, highlighting the need for further investigation. Additionally, a meta-analysis by Ren et al. (265, 273) among Korean and Chinese populations found that consuming pickled vegetables increased the risk of gastric cancer by 50%, while refrigerator use reduced cancer risk. This implies that processed vegetables, with their high salt and sugar content, may counteract the protective effects of fresh vegetables.
Vitamin Effects
This study indicates that the intake of vitamins, including vitamin C (Class II evidence), vitamin E (Class II evidence), and vitamin A (Class III evidence), is inversely correlated with GC risk. Vitamin C, a water-soluble antioxidant and enzyme cofactor, is present in various animal and plant sources. It exists in two forms: reduced (ascorbic acid, AA) and oxidized (dehydroascorbic acid, DHA), with AA being the predominant form in the human body, essential for normal physiological functions in many organisms (274). As an antioxidant and free radical scavenger, vitamin C plays a key role in collagen synthesis. Moderate intake may help reduce tissue or DNA damage (275). Vitamin C has been shown to disrupt the microenvironment created by bacteria, enhance the diffusion of antibiotics into the gastric mucosa, inhibit Hp colonization and growth, and thereby reduce the risk of progression of precancerous lesions, modifying Hp’s effect on gastric cancer (270, 276). In 2007, experts from the American Cancer Institute suggested that high intake of vitamin C-rich foods may enhance anti-cancer effects in GC patients (277). An ecological study in Poland found a significant negative correlation between high intake of vegetables, fruits, and vitamin C and GC incidence. Experiments indicated that increasing vitamin C-rich food intake may help prevent GC development (278). A meta-analysis found that daily Vitamin C intake reduced GC risk by 26% (279). However, controversies remain regarding its protective role, as some studies report limited or nonsignificant effects in specific subpopulations (280). The precise role of vitamin C in GC and its potential as a diagnostic marker, including the significance of serum vitamin C levels, remain uncertain.
Vitamin E, a fat-soluble antioxidant, protects against oxidative stress, lipid peroxidation, and tumor cell proliferation. It exerts a protective effect on the gastric mucosa. Vitamin E also neutralizes nitroso ions, inhibits nitrosamine production, activates cellular immunity, suppresses humoral immunity, prevents tumor cell proliferation, and induces tumor cell apoptosis (281, 282). Studies have shown that vitamin E can protect DNA from oxidative damage and prevent potential gene mutations, thereby playing an anti-cancer role (283). In a prospective cohort study by Egnell et al. (284), follow-up of the trial population revealed that increasing total vitamin E intake in adults reduces the risk of gastrointestinal tumors. Although higher vitamin E intake reduces the risk of GC, its effects vary by anatomical site and histological subtype. Specifically, increased vitamin E intake lowers the risk of non-cardia gastric cancer but shows no significant association with diffuse gastric cancer. A meta-analysis of dietary vitamin E intake, involving 24 studies with 7,095 participants, examined the relationship between dietary vitamin E and GC risk. Comparing the highest and lowest doses of vitamin E intake, the study found significant heterogeneity and indicated that vitamin E’s effects differ across GC subtypes. However, it did not address the relationship between serum vitamin E levels and GC risk (285). Therefore, while increasing vitamin E intake may help reduce GC risk, the diagnostic significance and clinical value of serum vitamin E levels for GC remain uncertain and warrant further investigation.
Similarly, β-carotene intake was associated with a significant reduction in gastric cancer risk (Class II). Consistent with these findings, a Chinese meta-analysis also supports the inverse association between tomato consumption and gastric cancer risk (162). In a Finnish cohort study, carotenoids did not affect the risk of cardia cancer but significantly reduced the risk of non-cardia gastric cancer. They inhibit carcinogen synthesis, reduce carcinogen activity, and exhibit anti-mutagenic and anti-teratogenic effects (286). In vitro and in vitro studies have also confirmed the anti-cancer and cancer-preventive properties of carotenoids and their extracts, with therapeutic efficacy positively correlated with dose (287). A study by Silvia et al. (288) in northern Italy found that higher carotenoid intake was associated with a greater inhibition of GC cells.
The impact of underlying diseases on the risk of gastric cancer cannot be ignored
Psychological factors
This study highlights the significant influence of depression and psychological distress on GC risk. Depression increases the risk of GC by 84% compared to non-depressed individuals (Class II). Under the modern bio-psycho-social model, the influence of psychological factors on cancer incidence has gained increasing attention. Research has shown that depression raises the risk of gastric adenoma or GC by 4.54 times (OR: 4.54; 95% CI: 2.42-8.55) (289). In a Japanese cross-sectional survey involving 29,926 GC patients, only 36% of individuals with severe psychological stress participated in cancer screening, underscoring the negative impact of psychological stress on screening uptake (290). Chronic psychological distress and stress disrupts the hypothalamus-pituitary-adrenal axis, leading to endocrine disruptions that impair immune cells activity, including natural killer(NK) cells, T cell subsets, and B cells, thereby increasing GC susceptibility (291). Psychological distress also increases the production of reactive oxygen species (ROS). Excessive ROS production due to psychological distress can activate the ABL proto-oncogene 1 (ABL1), promoting inflammatory pathways and GC progression (292). Psychological distress is common among GC patients at all stages and is associated with poor prognosis (293). These findings emphasize the need for integrating mental health interventions in GC prevention strategies.
Helicobacter pylori infection
This study corroborates previous findings, confirming that Helicobacter pylori infection significantly increases the risk of GC (Class II evidence). A recent 10-year prospective study showed that gastric mucosal atrophy and intestinal metaplasia are reversible after Hp eradication. In this study, 65 participants were Hp-negative, and 533 were Hp-positive (442 in the eradication group and 91 in the non-eradication group). After 1 year of follow-up, the Hp-positive eradication group exhibited significant improvement in gastric mucosal atrophy. The differences in gastric antral intestinal metaplasia were eliminated after 5 years, and those in gastric body intestinal metaplasia were eliminated after 3 years (294). Studies have shown that Hp eradication is especially beneficial for asymptomatic patients and those after endoscopic early cancer resection, reducing the gastric cancer risk by 34% (295). In first-degree relatives with a family history of gastric cancer and Hp infection, the risk of gastric cancer was reduced by 55% following Hp eradication (296). Hp is a key controllable risk factor for gastric cancer, particularly in patients with a family history. Early eradication significantly reduces gastric cancer risk. Widespread Hp screening offers promising prospects for gastric cancer prevention and treatment.
The use of certain drugs may reduce the risk of gastric cancer
This study found that long-term NSAID use significantly reduces GC risk (Class II evidence), with both aspirin and non-aspirin NSAIDs demonstrating protective effects (Class III evidence). NSAIDs primarily exert anti-tumor effects by inhibiting COX, a key enzyme in PG synthesis. This inhibition reduces COX-derived PGE2 production, which is closely linked to GC development. Possible mechanisms include: 1) Echizen et al. (297) identified that the interaction between the TLR/MyD88 and COX-2/PGE2 pathways plays a key role in tumor microenvironment formation. NSAIDs inhibit COX-2, reducing PGE2 levels and disrupting the signal transduction of both pathways, thereby exerting anti-tumor effects. 2) PGE2 suppresses macrophage and natural killer cell activity, decreases the production of lymphokines such as tumor necrosis factor-α, interferon-γ. 3) Gene mutations such as those in APC, ras, and p53 lead to increased COX-2 expression, enhanced PGE2 synthesis, and promote tumor cell proliferation. In contrast, NSAIDs reduce PGE2 synthesis and inhibit tumor cell growth. However, NSAIDs do not solely rely on the COX-2 pathway to suppress tumor cell growth. β-catenin, a key regulator of cell adhesion, is influenced by the APC gene. As a tumor suppressor, APC regulates β-catenin’s adhesion function. When APC is mutated, β-catenin dissociates from APC, activating the expression of oncogenes like c-myc and cyclin D1, thereby promoting tumor cell growth. Liggett et al. (298) demonstrated that NSAIDs downregulate β-catenin and reduce Smad2/3 transfer protein complex activity, inhibiting tumor cell proliferation, adhesion, and growth. Akrami et al. (299) investigated the anti-tumor effects of NSAIDs on human gastric cancer cells (AGS) and found that NSAIDs upregulate p53 expression, induce tumor cell apoptosis, and inhibit tumor onset and progression.
This study demonstrates that estrogen replacement therapy (ERT) significantly reduces the risk of GC (Class I evidence). While gastric cancer is typically regarded as an estrogen-independent tumor, in contrast to breast and endometrial cancers, emerging evidence suggests that estrogen may play a protective role in its progression (300, 301). The incidence of gastric cancer is higher in men compared to premenopausal women (2-3:1), but it increases in postmenopausal women (1, 2). An analysis of the relationship between estrogen exposure and GC risk indicates that ERT decreases the incidence of GC, while anti-estrogen therapies may increase the risk (302). Estrogen also exhibits potential immunomodulatory effects within the tumor microenvironment (303). Reyes-Ramos et al. (304) reported that ERα is expressed on both GC cells and macrophages within the tissue microenvironment. Estrogen interacts with these macrophages, modulating their activation, recruitment, and chemokine secretion, which collectively influencing tumor progression. However, since ERT is primarily used in postmenopausal women, its precise role in reducing GC risk requires confirmation through further prospective studies.
Blood lipid-related indicators may be significantly associated with the risk of gastric cancer
TC Factors
This study found that higher levels of TC (Class I evidence) and HDL-C (Class I evidence) are associated with a reduced risk of GC. Serum TC levels are crucial for cell structure and function, playing a key role in maintaining the structure and activity of biological membranes (305). Changes in TC levels can affect various cell functions, including enzyme activity, endocytosis, and receptor function (306). In some malignancies, elevated serum TC levels promote tumor progression, such as in testicular, prostate, and colorectal cancers (307). In contrast, reduced serum TC levels can accelerate tumor progression in other malignancies, including GC, hepatocellular carcinoma, intrahepatic bile duct carcinoma, and pancreatic cancer (308). The findings suggest that serum TC levels play a complex role in the occurrence and progression of malignant tumors. TC serves as a precursor for several biochemical pathways involved in the synthesis of key signaling molecules, such as vitamin D and steroid hormones, which are implicated in the etiology of certain malignancies (309). Studies have shown that long-term serum TC deficiency can activate nuclear factor κB, a key transcription factor involved in regulating immunity, inflammation, apoptosis, carcinogenesis, and other processes (310) Additionally, low serum TC levels may deplete CD8+ T cells in the tumor microenvironment, weakening the immune system’s protective function (311, 312). These factors may explain the negative correlation between serum TC levels and gastric cancer, although the underlying mechanisms require further investigation.
Blood lipid components
The mechanisms underlying the correlation between blood lipid components and GC include both anti-tumor and carcinogenic pathways. HDL-C exhibits anti-tumor effects through its reverse cholesterol transport function, antioxidant, and anti-inflammatory properties, while LDL-C may contribute to carcinogenesis by impairing immune system function. A key function of HDL-C is reverse cholesterol transport. HDL-C binds to cell surface receptors, promoting the efflux of intracellular cholesterol to the liver and steroidogenic cells for metabolism, thereby maintaining normal intracellular cholesterol balance (313). Elevated HDL-C levels may lower peripheral cholesterol, thereby inhibiting tumor development and lymph node metastasis (314). Furthermore, the antioxidant properties of HDL-C contribute to its anti-tumor role. HDL-C can neutralize harmful oxidants, preventing oxidative stress-induced DNA damage, which otherwise promotes tumorigenesis and tumor transformation (315).
This study proposes a tiered prevention strategy for gastric cancer based on evidence levels: prioritizing the promotion of measures supported by Class I-II evidence, including increasing intake of cruciferous vegetables and vitamin C/E, improving high-density lipoprotein cholesterol, and standardizing Helicobacter pylori eradication treatment; Strictly control the main risk factors, such as implementing PHQ-9 scale screening and initiating cognitive-behavioral interventions for patients with depression, and regular gastroscopy monitoring for patients with autoimmune diseases. For protective factors such as dietary fiber and green tea, as well as risk factors such as red meat and high salt diet, it is recommended to develop a limit control plan based on individual metabolic characteristics. At the same time, it is called for to carry out multi center RCTs to verify potential protective effects such as beta carotene, and to construct a collaborative prevention and control system of gastroenterology psychiatry nutrition to optimize the prevention and treatment of gastric cancer.
Research gaps and future directions
While this umbrella review consolidates extensive evidence, several gaps remain. First, most studies focused on Asian and Western populations, with limited data from Africa, South America, and indigenous communities, where genetic and environmental risk profiles may differ. Second, emerging exposures (e.g., microplastics, antibiotic overuse, and alterations in the gut microbiome) remain underexplored. Third, biomarker-based risk assessments (e.g., genetic polymorphisms, epigenetic markers, or metabolic signatures) are rarely integrated with traditional epidemiological factors, limiting personalized prevention strategies. Additionally, interactions between psychological stress and biological pathways (e.g., immune dysfunction or oxidative stress) warrant deeper investigation. Future research should prioritize longitudinal studies in underrepresented populations, incorporate multi-omics approaches, and evaluate the cost-effectiveness of targeted interventions (e.g., H. pylori eradication in high-risk subgroups). Addressing these gaps will enhance our ability to mitigate the global burden of gastric cancer through precision prevention.
Limitations and strengths
This study has several limitations. Firstly, we only searched English-language databases, which may have introduced publication bias and language bias by excluding studies published in other languages. Secondly, we included only published data, excluding unpublished or forthcoming evidence. Third, data for this study were extracted and analyzed directly from systematic reviews and meta-analyses, excluding original studies not included in these reviews. In addition, this study included more retrospective studies than RCTs, with limited evidence levels. Despite these limitations, this umbrella review provides the first comprehensive summary of existing evidence from previous meta-analyses on gastric cancer risk factors. This umbrella review evaluated the strengths and weaknesses of the current evidence from systematic reviews and meta-analyses on risk factors for GC. It presents a thorough understanding of potential factors influencing the onset and progression of GC, lays the groundwork for the development of more effective prevention and control strategies, and proposes avenues for future clinical research. The study adhered to stringent systematic procedures, with two independent authors performing literature searches, selecting relevant studies, and extracting data. When adequate data were available, we reanalyzed the RR, OR, or HR using 95% CIs and applying either random or fixed-effects models. We evaluated heterogeneity and assessed publication bias for each included meta-analysis. Furthermore, we utilized two established approaches-AMSTAR and evidence classification criteria-to assess methodological quality and categorize the evidence for each risk factor. They reduce subjective bias and improve the accuracy and reliability of evidence assessment by providing standardized and objective frameworks. This helps to make informed decisions in clinical practice and policy-making. It also promotes global research exchange and cooperation, and can guide future research directions by highlighting areas with insufficient evidence. However, these standards are not without flaws. Their applications may be subjective, and different standards may lead to different classifications. They may have overlooked the diversity of evidence and not fully considered various research backgrounds and non-traditional sources of evidence. In addition, they often lag behind the rapid development of medical research and run the risk of over reliance on high-level evidence, which may overlook valuable information from low-level research.
Conclusion
This umbrella review identified 77 risk factors significantly associated with GC, including 42 risk factors and 35 protective factors. Most were linked to innate traits and behavioral lifestyles. After assessing evidence quality, only 17 risk factors were classified as Class I or II. Protective factors included cruciferous vegetable intake, TC, HDL-C, NSAIDs, β-carotene, vitamin intake, and dietary polyphenol intake. Key risk factors comprised depression, Helicobacter pylori infection, dermatomyositis, and Graves’ disease. These findings provide a foundation for developing improved prevention strategies and treatments to reduce GC incidence, slow progression, and alleviate the global burden of GC-related diseases. However, current research has the drawback that the analysis of traditional epidemiological factors has not yet been systematically integrated with genetic and molecular markers. Future overall reviews should focus on combining genetic and molecular risk markers with traditional epidemiological factors. Future umbrella reviews should focus on integrating genetic and molecular risk markers alongside traditional epidemiological factors.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: PubMed, Embase, Web of Science, and the Cochrane.
Author contributions
JL: Data curation, Formal analysis, Visualization, Writing – original draft. HY: Data curation, Investigation, Writing – original draft. CQ: Data curation, Validation, Writing – review & editing. JC: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the colleagues from department of gastrointestinal surgery in the First Affiliated Hospital of Guangxi Medical University and Guangxi Key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer for their kind assistance during the preparation and revision of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1564575/full#supplementary-material
Abbreviations
GC, gastric cancer; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; NSAIDs, non-steroidal anti-inflammatory drugs; Hp, Helicobacter pylori; PPIs, proton pump inhibitors; HR, hazard ratios; OR, odds ratios; RR, relative risks; HR, hazard ratios; BMI, body mass index; SIR, standardized incidence rate; MDS, mediterranean diet score; DII, dietary inflammatory index; MAFLD, metabolic-associated fatty liver disease; LDL-C, low-density lipoprotein cholesterol.
References
1. Inoue M. Epidemiology of gastric cancer-changing trends and global disparities. Cancers. (2024) 16:2948. doi: 10.3390/cancers16172948
2. Leja M. Where are we with gastric cancer screening in Europe in 2024? Gut. (2024) 73:2074–82. doi: 10.1136/gutjnl-2024-332705
3. Khalili-Tanha G, Khalili-Tanha N, Rouzbahani AK, Mahdieh R, Jasemi K, Ghaderi R, et al. Diagnostic, prognostic, and predictive biomarkers in gastric cancer: from conventional to novel biomarkers. Trans Res. (2024) 274:35–48. doi: 10.1016/j.trsl.2024.09.001
4. Chong X, Madeti Y, Cai J, Li W, Cong L, Lu J, et al. Recent developments in immunotherapy for gastrointestinal tract cancers. J Hematol Oncol. (2024) 17:65. doi: 10.1186/s13045-024-01578-x
5. Svrcek M, Voron T, André T, Smyth EC, and de la Fouchardière C. Improving individualised therapies in localised gastro-oesophageal adenocarcinoma. Lancet Oncol. (2024) 25:e452–63. doi: 10.1016/S1470-2045(24)00180-3
6. Chen W, Xia C, Zheng R, Zhou M, Lin C, Zeng H, et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Global Health. (2019) 7:e257–69. doi: 10.1016/S2214-109X(18)30488-1
7. Zou Q, Tan H-Y, Li J-C, Li Y-D, and Yang K. Metabolic-associated fatty liver disease and risk of esophagogastric cancer: a systematic review and meta-analysis. Japanese J Clin Oncol. (2023) 53:680–90. doi: 10.1093/jjco/hyad038
8. Yao P, Kartsonaki C, Butt J, Jeske R, de Martel C, Plummer M, et al. Helicobacter pylori multiplex serology and risk of non-cardia and cardia gastric cancer: a case-cohort study and meta-analysis. Int J Epidemiol. (2023) 52:1197–208. doi: 10.1093/ije/dyad007
9. Xu S, Fan Y, Tan Y, Zhang L, and Li X. Association between blood lipid levels and risk of gastric cancer: A systematic review and meta-analysis. PLoS One. (2023) 18:e0288111–e0288111. doi: 10.1371/journal.pone.0288111
10. Seyyedsalehi MS, Mohebbi E, Tourang F, Sasanfar B, Boffetta P, and Zendehdel K. Association of dietary nitrate, nitrite, and N-nitroso compounds intake and gastrointestinal cancers: A systematic review and meta-analysis. Toxics. (2023) 11:190. doi: 10.3390/toxics11020190
11. Piovani D, Tsantes AG, Schunemann HJ, and Bonovas S. Meta-analysis: Use of proton pump inhibitors and risk of gastric cancer in patients requiring gastric acid suppression. Alimentary Pharmacol Ther. (2023) 57:653–65. doi: 10.1111/apt.17360
12. Peng TR, Wu TW, and Li CH. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review and meta-analysis. Int J Clin Oncol. (2023) 28:99–109. doi: 10.1007/s10147-022-02253-2
13. Naemi Kermanshahi M, Safaei E, Tutunchi H, Naghshi S, Mobarak S, Asadi M, et al. Fruit and vegetable intake in relation to gastric cancer risk: A comprehensive and updated systematic review and dose-response meta-analysis of cohort studies. Front Nutr. (2023) 10:973171. doi: 10.3389/fnut.2023.973171
14. Han Z, Liu J, Zhang W, Kong Q, Wan M, Lin M, et al. Cardia and non-cardia gastric cancer risk associated with Helicobacter pylori in East Asia and the West: A systematic review, meta-analysis, and estimation of population attributable fraction. Helicobacter. (2023) 28:e12950. doi: 10.1111/hel.12950
15. Guo H, Zhang R, Zhang P, Chen Z, Hua Y, Huang X, et al. Association of proton pump inhibitors with gastric and colorectal cancer risk: A systematic review and meta-analysis. Front Pharmacol. (2023) 14:1129948. doi: 10.3389/fphar.2023.1129948
16. Chen Y, Zhang J, Zhang Y, and Zhu L. Effect of statin use on risk and mortality of gastric cancer: a meta-analysis. Anti-cancer Drugs. (2023) 34:901–9. doi: 10.1097/CAD.0000000000001524
17. Cao C, Gan X, He Y, Nong S, Su Y, Liu Z, et al. Association between nut consumption and cancer risk: a meta-analysis. Nutr Cancer. (2023) 75:82–94. doi: 10.1080/01635581.2022.2104880
18. Ba DM, Ssentongo P, Pelucchi C, Negri E, Palli D, Ferraroni M, et al. Mushroom consumption and risk of gastric cancer: a pooled analysis within the stomach cancer pooling project and a combined meta-analysis with other observational studies. Eur J Cancer Prev. (2023) 32:222–8. doi: 10.1097/CEJ.0000000000000754
19. Reytor-González C, Zambrano AK, Montalvan M, Frias-Toral E, Simancas-Racines A, and Simancas-Racines D. Adherence to the Mediterranean Diet and its association with gastric cancer: health benefits from a Planeterranean perspective. J Trans Med. (2024) 22:483. doi: 10.1186/s12967-024-05176-w
20. Zeng R, Gou H, Lau HCH, and Yu J. Stomach microbiota in gastric cancer development and clinical implications. Gut. (2024) 73:2062–73. doi: 10.1136/gutjnl-2024-332815
21. Wu Q-J, Yang Y, Wang J, Han L-H, and Xiang Y-B. Cruciferous vegetable consumption and gastric cancer risk: A meta-analysis of epidemiological studies. Cancer Sci. (2013) 104:1067–73. doi: 10.1111/cas.2013.104.issue-8
22. Xie F, You Y, Huang J, Guan C, Chen Z, Fang M, et al. Association between physical activity and digestive system cancer: An updated systematic review and meta-analysis. J sport Health Sci. (2021) 10:4–13. doi: 10.1016/j.jshs.2020.09.009
23. Azizi N, Zangiabadian M, Seifi G, Davari A, Yekekhani E, Safavi-Naini SAA, et al. Gastric cancer risk in association with underweight, overweight, and obesity: A systematic review and meta-analysis. Cancers. (2023) 15:2778. doi: 10.3390/cancers15102778
24. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
25. Cavaleiro-Pinto M, Peleteiro B, Lunet N, and Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. ancer Causes Control. (2011) 22:375–87. doi: 10.1007/s10552-010-9707-2
26. Ge Z, Ben Q, Qian J, Wang Y, and Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. (2011) 23:1127–35. doi: 10.1097/MEG.0b013e32834b8d73
27. Kim J, Kang M, Lee JS, Inoue M, Sasazuki S, and Tsugane S. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. (2011) 102:231–44. doi: 10.1111/j.1349-7006.2010.01770.x
28. Li Y, Yang H, and Cao J. Association between alcohol consumption and cancers in the Chinese population-A systematic review and meta-analysis. PloS One. (2011) 6:e18776. doi: 10.1371/journal.pone.0018776
29. Shitara K, Fleitas T, Kawakami H, Curigliano G, Narita Y, Wang F, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with gastric cancer. ESMO Open. (2024) 9:102226. doi: 10.1016/j.esmoop.2023.102226
30. SIGN. Scottish intercollegiate guidelines network search filters (2020). Available online at: https://www.sign.ac.uk/what-we-do/methodology/search-filters/ (Accessed April 15 2021).
31. Wu S, Liang J, Zhang L, Zhu X, Liu X, and Miao D. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. (2011) 11:26. doi: 10.1186/1471-2407-11-26
32. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. (2009) 181:488–93. doi: 10.1503/cmaj.081086
33. Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. (2018) 107:436–44. doi: 10.1093/ajcn/nqx082
34. Wallace TC, Bailey RL, Blumberg JB, Burton-Freeman B, Chen CO, Crowe-White KM, et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr. (2020) 60:2174–211. doi: 10.1080/10408398.2019.1632258
35. Theodoratou E, Tzoulaki I, Zgaga L, and Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ (Clinical Res ed.). (2014) 348:g2035. doi: 10.1136/bmj.g2035
36. Huang Y, Cao D, Chen Z, Chen B, Li J, Guo J, et al. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. (2021) 356:129697–7. doi: 10.1016/j.foodchem.2021.129697
37. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed.). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
38. Kennedy W, Fruin R, Lue A, and Logan SW. Using ecological models of health behavior to promote health care access and physical activity engagement for persons with disabilities. J patient Exp. (2021) 8:23743735211034031. doi: 10.1177/23743735211034031
39. Lu J, Wang Y, Hou L, Zuo Z, Zhang N, and Wei A. Multimorbidity patterns in old adults and their associated multi-layered factors: a cross-sectional study. BMC geriatrics. (2021) 21:372. doi: 10.1186/s12877-021-02292-w
40. Partanen T and Boffetta P. Cancer risk in asphalt workers and roofers: review and meta-analysis of epidemiologic studies. Am J Ind Med. (1994) 26:721–40. doi: 10.1002/ajim.4700260602
41. Tredaniel J, Boffetta P, Buiatti E, Saracci R, and Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. (1997) 72:565–73. doi: 10.1002/(SICI)1097-0215(19970807)72:4<565::AID-IJC3>3.0.CO;2-O
42. Jacobs DR Jr., Marquart L, Slavin J, and Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. (1998) 30:85–96. doi: 10.1080/01635589809514647
43. Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Alimentary Pharmacol Ther. (1999) 13:851–6. doi: 10.1046/j.1365-2036.1999.00546.x
44. Eslick GD, Lim LL, Byles JE, Xia HH, and Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. (1999) 94:2373–9. doi: 10.1111/j.1572-0241.1999.01360.x
45. Fleischauer AT, Poole C, and Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. (2000) 72:1047–52. doi: 10.1093/ajcn/72.4.1047
46. Wu AH, Yang D, and Pike MC. A meta-analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer epidemiology Biomarkers Prev. (2000) 9:1051–8.
47. Bagnardi V, Blangiardo M, La Vecchia C, and Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health. (2001) 25:263–70.
48. Bagnardi V, Blangiardo M, La Vecchia C, and Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. (2001) 85:1700–5. doi: 10.1054/bjoc.2001.2140
49. Xue FB, Xu YY, Wan Y, Pan BR, Ren J, and Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. (2001) 7:801–4. doi: 10.3748/wjg.v7.i6.801
50. Norat T and Riboli E. Fruit and vegetable consumption and risk of cancer of the digestive tract: meta-analysis of published case-control and cohort studies. IARC Sci publications. (2002) 156:123–5.
51. González-Pérez A, García Rodríguez LA, and López-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. (2003) 3:28. doi: 10.1186/1471-2407-3-28
52. Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, and Wong BC. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Institute. (2003) 95:1784–91. doi: 10.1093/jnci/djg106
53. Bjelakovic G, Nikolova D, Simonetti RG, and Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet (London England). (2004) 364:1219–28. doi: 10.1016/S0140-6736(04)17138-9
54. Lunet N, Lacerda-Vieira A, and Barros H. Fruit and vegetables consumption and gastric cancer: A systematic review and meta-analysis of cohort studies. Nutr Cancer-an Int J. (2005) 53:1–10. doi: 10.1207/s15327914nc5301_1
55. Botelho F, Lunet N, and Barros H. Coffee and gastric cancer: systematic review and meta-analysis. Cadernos saude publica. (2006) 22:889–900. doi: 10.1590/S0102-311X2006000500002
56. Kubo A and Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2006) 15:872–8. doi: 10.1158/1055-9965.EPI-05-0860
57. Larsson SC, Orsini N, and Wolk A. Processed meat consumption and stomach cancer risk: A meta-analysis. J Natl Cancer Institute. (2006) 98:1078–87. doi: 10.1093/jnci/djj301
58. Nishino Y, Inoue M, Tsuji I, Wakai K, Nagata C, Mizoue T, et al. Tobacco smoking and gastric cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Japanese J Clin Oncol. (2006) 36:800–7. doi: 10.1093/jjco/hyl112
59. Browning DRL and Martin RM. Statins and risk of cancer: A systematic review and metaanalysis. Int J Cancer. (2007) 120:833–43. doi: 10.1002/ijc.v120:4
60. Kubo A and Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. (2007) 102:2323–30. doi: 10.1111/j.1572-0241.2007.01374.x
61. Mahjub H and Sadri G. Association between alcohol consumption and gastric cancer: A meta-analysis. J Res Health Sci. (2007) 7:63–72.
62. Kuoppala J, Lamminpää A, and Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer (Oxford England: 1990). (2008) 44:2122–32. doi: 10.1016/j.ejca.2008.06.025
63. Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. (2008) 19:689–701. doi: 10.1007/s10552-008-9132-y
64. Zhou Y, Li N, Zhuang W, Liu G, Wu T, Yao X, et al. Green tea and gastric cancer risk: meta-analysis of epidemiologic studies. Asia Pacific J Clin Nutr. (2008) 17:159–65.
65. Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, and Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. (2009) 100:551–7. doi: 10.1038/sj.bjc.6604880
66. Huang Y-x, Qin L-q, and Wang P-y. Meta-analysis of the relationship between dairy product consumption and gastric cancer. Zhonghua yu fang yi xue za zhi [Chinese J Prev medicine]. (2009) 43:193–6.
67. La Torre G, Chiaradia G, Gianfagna F, De lauretis A, Boccia S, Mannocci A, et al. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori. (2009) 95:13–22. doi: 10.1177/030089160909500103
68. Myung SK, Bae WK, Oh SM, Kim Y, Ju W, Sung J, et al. Green tea consumption and risk of stomach cancer: A meta-analysis of epidemiologic studies. Int J Cancer. (2009) 124:670–7. doi: 10.1002/ijc.v124:3
69. Cavaleiro-Pinto M, Peleteiro B, Lunet N, and Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. ancer Causes Control. (2011) 22:375–87. doi: 10.1007/s10552-010-9707-2
70. Yang P, Zhou Y, Chen B, Wan H-W, Jia G-Q, Bai H-L, et al. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur J Cancer. (2009) 45:2867–73. doi: 10.1016/j.ejca.2009.04.019
71. Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. (2010) 127:172–84. doi: 10.1002/ijc.25008
72. Gatto NM, Kelsh MA, Mai DH, Suh M, and Proctor DM. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer Epidemiol. (2010) 34:388–99. doi: 10.1016/j.canep.2010.03.013
73. Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, et al. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. (2010) 101:508–16. doi: 10.1111/j.1349-7006.2009.01374.x
74. Kim S. 박은환, 정현재, 황인홍, ginseng intake and gastric cancer risk: A meta-analysis of observational epidemiological studies. Korean J Family Med. (2010) 31:930–6. doi: 10.4082/kjfm.2010.31.12.930
75. Tian W, Zhao Y, Liu S, and Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur J Cancer Prev. (2010) 19:288–98. doi: 10.1097/CEJ.0b013e328339648c
76. Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Aspirin use and the risk of gastric cancer: a meta-analysis. Digestive Dis Sci. (2010) 55:1533–9. doi: 10.1007/s10620-009-0915-0
77. Tio M, Andrici J, Cox MR, and Eslick GD. Folate intake and the risk of upper gastrointestinal cancer: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2011) 4:80–1. doi: 10.1111/jgh.12446
78. Tramacere I, La Vecchia C, and Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology. (2011) 22:344–9. doi: 10.1097/EDE.0b013e31821092cd
79. Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, and Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. (2011) 141:80–9. doi: 10.1053/j.gastro.2011.03.057
80. Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, and Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev. (2012) 21:20–38. doi: 10.1158/1055-9965.EPI-11-0834
81. D’Elia L, Rossi G, Ippolito R, Cappuccio FP, and Strazzullo P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin Nutr. (2012) 31:489–98. doi: 10.1016/j.clnu.2012.01.003
82. Ge S, Feng X, Shen L, Wei Z, Zhu Q, and Sun J. Association between habitual dietary salt intake and risk of gastric cancer: A systematic review of observational studies. Gastroenterol Res Pract. (2012) 2012:808120. doi: 10.1155/2012/808120
83. Goto Y, Camargo MC, Zabaleta J, Morgan DR, Correa P, and Rabkin CS. Hormone replacement therapy and gastric cancer risk: A meta-analysis. Gastroenterology. (2012) 142:S402–3. doi: 10.1016/S0016-5085(12)61525-X
84. Green J, Czanner G, Reeves G, Watson J, Wise L, Roddam A, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. (2012) 130:2387–96. doi: 10.1002/ijc.v130.10
85. Oh YH, Yoon C, and Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: Meta-analysis of observational studies. World J Gastroenterol. (2012) 18:5779–88. doi: 10.3748/wjg.v18.i40.5779
86. Ren JS, Kamangar F, Forman D, and Islami F. Pickled food and risk of gastric cancer - A systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev. (2012) 21:905–15. doi: 10.1158/1055-9965.EPI-12-0202
87. Tian T, Zhang LQ, Ma XH, Zhou JN, and Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: A meta-analysis. Exp Clin Endocrinol Diabetes. (2012) 120:217–23. doi: 10.1055/s-0031-1297969
88. Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. (2012) 23:28–36. doi: 10.1093/annonc/mdr135
89. Tramacere I, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, et al. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol. (2012) 23:287–97. doi: 10.1093/annonc/mdr136
90. Yu XF, Wang YQ, Zou J, and Dong J. A meta-analysis of the effects of energy intake on risk of digestive cancers. World J Gastroenterol. (2012) 18:7362–70. doi: 10.3748/wjg.v18.i48.7362
91. Ahn JS, Eom CS, Jeon CY, and Park SM. Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol. (2013) 19:2560–8. doi: 10.3748/wjg.v19.i16.2560
92. Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, et al. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol. (2013) 24:1450–8. doi: 10.1093/annonc/mdt108
93. Bonequi P, Meneses-Gonzalez F, Correa P, Rabkin CS, and Camargo MC. Risk factors for gastric cancer in Latin America: A meta-analysis. Cancer Causes Control. (2013) 24:217–31. doi: 10.1007/s10552-012-0110-z
94. Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: A meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. (2013) 22:1395–408. doi: 10.1158/1055-9965.EPI-13-0042
95. Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, et al. Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf. (2013) 8:333–48. doi: 10.2174/15680266113136660067
96. Shimoyama S. Diabetes mellitus carries a risk of gastric cancer: A metaanalysis. World J Gastroenterol. (2013) 19:6902–10. doi: 10.3748/wjg.v19.i40.6902
97. Shu L, Wang X-Q, Wang S-F, Wang S, Mu M, Zhao Y, et al. Dietary patterns and stomach cancer: A meta-analysis. Nutr Cancer-an Int J. (2013) 65:1105–15. doi: 10.1080/01635581.2013.828086
98. Singh PP and Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. (2013) 24:1721–30. doi: 10.1093/annonc/mdt150
99. Singh S, Varayil JE, Devanna S, Murad M, and Iyer P. Physical activity is associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Am J Gastroenterol. (2013) 1:S38–9. doi: 10.14309/00000434-201310001-00119
100. Turati F, Tramacere I, La Vecchia C, and Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. (2013) 24:609–17. doi: 10.1093/annonc/mds244
101. Uthman OA, Jadidi E, and Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health. (2013) 67:854–60. doi: 10.1136/jech-2012-201108
102. Vannella L, Lahner E, Osborn J, and Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Alimentary Pharmacol Ther. (2013) 37:375–82. doi: 10.1111/apt.2013.37.issue-4
103. Wu XD, Zeng K, Xue FQ, Chen JH, and Chen YQ. Statins are associated with reduced risk of gastric cancer: a meta-analysis. Eur J Clin Pharmacol. (2013) 69:1855–60. doi: 10.1007/s00228-013-1547-z
104. Yang T, Yang X, Wang X, Wang Y, and Song Z. The role of tomato products and lycopene in the prevention of gastric cancer: A meta-analysis of epidemiologic studies. Med Hypotheses. (2013) 80:383–8. doi: 10.1016/j.mehy.2013.01.005
105. Ye X, Fu J, Yang Y, Gao Y, Liu L, and Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: A systematic review and meta-analysis. PLoS One. (2013) 8:e71522. doi: 10.1371/journal.pone.0071522
106. Yoon JM, Son KY, Eom CS, Durrance D, and Park SM. Pre-existing diabetes mellitus increases the risk of gastric cancer: A meta-analysis. World J Gastroenterol. (2013) 19:936–45. doi: 10.3748/wjg.v19.i6.936
107. Zhang Z, Xu G, Ma M, Yang J, and Liu X. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology. (2013) 145:113–20.e3. doi: 10.1053/j.gastro.2013.04.001
108. Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, et al. Red and processed meat intake is associated with higher gastric cancer risk: a meta-analysis of epidemiological observational studies. PloS One. (2013) 8:e70955. doi: 10.1371/journal.pone.0070955
109. Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, and Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. (2014) 29:151–70. doi: 10.1007/s10654-014-9895-2
110. Chen Y, Yu C, and Li Y. Physical activity and risks of esophageal and gastric cancers: a meta-analysis. PLoS One. (2014) 9:e88082. doi: 10.1371/journal.pone.0088082
111. Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. (2014) 57:2261–9. doi: 10.1007/s00125-014-3361-2
112. Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One. (2014) 9:e116060. doi: 10.1371/journal.pone.0116060
113. Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, et al. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin Nutr. (2014) 33:415–20. doi: 10.1016/j.clnu.2013.10.001
114. Lin XJ, Wang CP, Liu XD, Yan KK, Li S, Bao HH, et al. Body mass index and risk of gastric cancer: a meta-analysis. Japanese J Clin Oncol. (2014) 44:783–91. doi: 10.1093/jjco/hyu082
115. Ma Z, Wang W, Jin G, Chu P, and Li H. Effect of statins on gastric cancer incidence: A meta-Analysis of case control studies. J Cancer Res Ther. (2014) 10:859–65. doi: 10.4103/0973-1482.138218
116. Pabalan N, Jarjanazi H, and Ozcelik H. The impact of capsaicin intake on risk of developing gastric cancers: a meta-analysis. J gastrointestinal Cancer. (2014) 45:334–41. doi: 10.1007/s12029-014-9610-2
117. Shimazu T, Wakai K, Tamakoshi A, Tsuji I, Tanaka K, Matsuo K, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. (2015) 25:1228–33. doi: 10.1093/annonc/mdu115
118. Song P, Lu M, Yin Q, Wu L, Zhang D, Fu B, et al. Red meat consumption and stomach cancer risk: a meta-analysis. J Cancer Res Clin Oncol. (2014) 140:979–92. doi: 10.1007/s00432-014-1637-z
119. Sun Y, Lin LJ, Sang LX, Dai C, Jiang M, and Zheng CQ. Dairy product consumption and gastric cancer risk: a meta-analysis. World J Gastroenterol. (2014) 20:15879–98. doi: 10.3748/wjg.v20.i42.15879
120. Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, and Cao ZJ. Association between dairy intake and gastric cancer: a meta-analysis of observational studies. PloS One. (2014) 9:e101728. doi: 10.1371/journal.pone.0101728
121. Tio M, Andrici J, Cox MR, and Eslick GD. Folate intake and the risk of upper gastrointestinal cancers: A systematic review and meta-analysis. J Gastroenterol Hepatol. (2014) 29:250–8. doi: 10.1111/jgh.2014.29.issue-2
122. Tong G-X, Liang H, Chai J, Cheng J, Feng R, Chen P-L, et al. Association of risk of gastric cancer and consumption of tobacco, alcohol and tea in the Chinese population. Asian Pacific J Cancer Prev. (2014) 15:8765–74. doi: 10.7314/APJCP.2014.15.20.8765
123. Wang Q, Chen Y, Wang X, Gong G, Li G, and Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: Results from a meta-analysis of cohort studies. Eur J Cancer. (2014) 50:1498–509. doi: 10.1016/j.ejca.2014.02.009
124. Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, et al. Diet and cancer risk in the Korean population: a meta- analysis. Asian Pacific J Cancer prevention: APJCP. (2014) 15:8509–19. doi: 10.7314/APJCP.2014.15.19.8509
125. Xie F, Wang D, Huang Z, and Guo Y. Coffee consumption and risk of gastric cancer: A large updated meta-analysis of prospective studies. Nutrients. (2014) 6:3734–46. doi: 10.3390/nu6093734
126. Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, et al. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. (2014) 14:197. doi: 10.1186/1471-2407-14-197
127. Yu X-F, Zou J, and Dong J. Fish consumption and risk of gastrointestinal cancers: A meta-analysis of cohort studies. World J Gastroenterol. (2014) 20:15398–412. doi: 10.3748/wjg.v20.i41.15398
128. Fallahzadeh H, Jalali A, Momayyezi M, and Bazm S. Effect of carrot intake in the prevention of gastric cancer: A meta-analysis. J gastric Cancer. (2015) 15:256–61. doi: 10.5230/jgc.2015.15.4.256
129. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. (2015) 51:2820–32. doi: 10.1016/j.ejca.2015.09.010
130. Fortunato L and Rushton L. Stomach cancer and occupational exposure to asbestos: a meta-analysis of occupational cohort studies. Br J Cancer. (2015) 112:1805–15. doi: 10.1038/bjc.2014.599
131. Goodman JE, Loftus CT, and Zu K. 2,4-Dichlorophenoxyacetic acid and non-Hodgkin’s lymphoma, gastric cancer, and prostate cancer: meta-analyses of the published literature. Ann Epidemiol. (2015) 25:626–36. doi: 10.1016/j.annepidem.2015.04.002
132. Guo Y, Shan Z, Ren H, and Chen W. Dairy consumption and gastric cancer risk: A meta-analysis of epidemiological studies. Nutr Cancer-an Int J. (2015) 67:555–68. doi: 10.1080/01635581.2015.1019634
133. Han J, Jiang Y, Liu X, Meng Q, Xi Q, Zhuang Q, et al. Dietary fat intake and risk of gastric cancer: A meta-analysis of observational studies. PloS One. (2015) 10:e0138580. doi: 10.1371/journal.pone.0138580
134. Khayatzadeh S, Feizi A, Saneei P, and Esmaillzadeh A. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: A systematic review and meta-analysis. J Res Med Sci. (2015) 20:790–6. doi: 10.4103/1735-1995.168404
135. Kodali RT and Eslick GD. Meta-analysis: Does garlic intake reduce risk of gastric cancer? Nutr Cancer. (2015) 67:1–11. doi: 10.1080/01635581.2015.967873
136. Li L, Gan Y, Wu C, Qu X, Sun G, and Lu Z. Coffee consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. BMC Cancer. (2015) 15:733. doi: 10.1186/s12885-015-1758-z
137. Liu H, Hua Y, Zheng X, Shen Z, Luo H, Tao X, et al. Effect of coffee consumption on the risk of gastric cancer: A systematic review and meta-analysis of prospective cohort studies. PLoS One. (2015) 10:e0128501. doi: 10.1371/journal.pone.0128501
138. Odesanya MO, Abioye AI, and Ibrahim NA. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J sports Med. (2015) 49:224–9. doi: 10.1136/bjsports-2013-092778
139. Shen Z, Liu H, and Cao H. Coffee consumption and risk of gastric cancer: An updated meta-analysis. Clinics Res Hepatol Gastroenterol. (2015) 39:245–53. doi: 10.1016/j.clinre.2014.09.005
140. Song P, Wu L, and Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients. (2015) 7:9872–95. doi: 10.3390/nu7125505
141. Turati F, Pelucchi C, Guercio V, La Vecchia C, and Galeone C. Allium vegetable intake and gastric cancer: A case-control study and meta-analysis. Mol Nutr Food Res. (2015) 59:171–9. doi: 10.1002/mnfr.201400496
142. Welling R, Beaumont JJ, Petersen SJ, Alexeeff GV, and Steinmaus C. Chromium VI and stomach cancer: a meta-analysis of the current epidemiological evidence. Occup Environ Med. (2015) 72:151–9. doi: 10.1136/oemed-2014-102178
143. Wright E, Schofield PT, and Molokhia M. Bisphosphonates and evidence for association with esophageal and gastric cancer: A systematic review and metaanalysis. BMJ Open. (2015) 5:e007133. doi: 10.1136/bmjopen-2014-007133
144. Wu Y, Ye Y, Shi Y, Li P, Xu J, Chen K, et al. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: A meta-analysis. Clin Nutr. (2015) 34:620–6. doi: 10.1016/j.clnu.2014.06.007
145. Zeng SB, Weng H, Zhou M, Duan XL, Shen XF, and Zeng X-T. Long-term coffee consumption and risk of gastric cancer A PRISMA-compliant dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore). (2015) 94:e1640. doi: 10.1097/MD.0000000000001640
146. Zhang Y-F, Xu Q, Lu J, Wang P, Zhang H-W, Zhou L, et al. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Cancer Prev. (2015) 24:353–62. doi: 10.1097/CEJ.0000000000000094
147. Bae JM and Kim EH. Epstein-barr virus and gastric cancer risk: A meta-analysis with meta-regression of case-control studies. J Prev Med Public Health = Yebang Uihakhoe chi. (2016) 49:97–107. doi: 10.3961/jpmph.15.068
148. Bae JM and Kim EH. Dietary intakes of citrus fruit and risk of gastric cancer incidence: an adaptive meta-analysis of cohort studies. Epidemiol Health. (2016) 38:e2016034. doi: 10.4178/epih.e2016034
149. Bo Y, Sun J, Wang M, Ding J, Lu Q, and Yuan L. Dietary flavonoid intake and the risk of digestive tract cancers: a systematic review and meta-analysis. Sci Rep. (2016) 6:24836. doi: 10.1038/srep24836
150. Deng W, Yang H, Wang J, Cai J, Bai Z, Song J, et al. Coffee consumption and the risk of incident gastric cancer–A meta-analysis of prospective cohort studies. Nutr Cancer. (2016) 68:40–7. doi: 10.1080/01635581.2016.1115093
151. Gong H-Y, He J-G, and Li B-S. Meta-analysis of the association between selenium and gastric cancer risk. Oncotarget. (2016) 7:15600–5. doi: 10.18632/oncotarget.v7i13
152. Jin X, Che D-b, Zhang Z-h, Yan H-m, Jia Z-y, and Jia X-b. Ginseng consumption and risk of cancer: A meta-analysis. J ginseng Res. (2016) 40:269–77. doi: 10.1016/j.jgr.2015.08.007
153. Kong P, Wu R, Liu X, Liu J, Chen S, Ye M, et al. The effects of anti-inflammatory drug treatment in gastric cancer prevention: an update of a meta-analysis. J Cancer. (2016) 7:2247–57. doi: 10.7150/jca.16524
154. Lee W, Ahn Y-S, Lee S, Song BM, Hong S, and Yoon J-H. Occupational exposure to crystalline silica and gastric cancer: a systematic review and meta-analysis. Occup Environ Med. (2016) 73:794–801. doi: 10.1136/oemed-2016-103552
155. Lippi G, Mattiuzzi C, and Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol. (2016) 97:1–14. doi: 10.1016/j.critrevonc.2015.11.008
156. Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, and Sergentanis TN. Physical activity and gastric cancer risk: A systematic review and meta-analysis. Clin J sport Med. (2016) 26:445–64. doi: 10.1097/JSM.0000000000000316
157. Tse G and Eslick GD. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr. (2016) 55:63–73. doi: 10.1007/s00394-014-0824-7
158. Xie L, Mo M, Jia H-X, Liang F, Yuan J, and Zhu J. Association between dietary nitrate and nitrite intake and site-specific cancer risk: evidence from observational studies. Oncotarget. (2016) 7:56915–32. doi: 10.18632/oncotarget.10917
159. Xie Y, Huang S, He T, and Su Y. Coffee consumption and risk of gastric cancer: an updated meta-analysis. Asia Pac J Clin Nutr. (2016) 25:578–88. doi: 10.6133/apjcn.092015.07
160. Xie Y, Huang S, and Su Y. Dietary flavonols intake and risk of esophageal and gastric cancer: A meta-analysis of epidemiological studies. Nutrients. (2016) 8:91. doi: 10.3390/nu8020091
161. Yin XH, Wang YD, Luo H, Zhao K, Huang GL, Luo SY, et al. Association between tooth loss and gastric cancer: A meta-analysis of observational studies. PLoS One. (2016) 11:e0149653. doi: 10.1371/journal.pone.0149653
162. Zhou Y, Wang T, Meng Q, and Zhai S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin Nutr. (2016) 35:109–16. doi: 10.1016/j.clnu.2015.02.003
163. Boniol M, Koechlin A, and Boyle P. Meta-analysis of occupational exposures in the rubber manufacturing industry and risk of cancer. Int J Epidemiol. (2017) 46:1940–7. doi: 10.1093/ije/dyx146
164. Cai D, Qin J, Chen G, Feng W, and Liu J. Bisphosphonates use and risk of gastric cancer: an updated meta-analysis of cohort and case-control studies. Minerva Med. (2017) 108:464–72. doi: 10.23736/S0026-4806.17.05055-8
165. Chen YH, Zou XN, Zheng TZ, Zhou Q, Qiu H, Chen YL, et al. High spicy food intake and risk of cancer: A meta-analysis of case-control studies. Chin Med J. (2017) 130:2241–50. doi: 10.4103/0366-6999.213968
166. Grosso G, Godos J, Lamuela-Raventos R, Ray S, Micek A, Pajak A, et al. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol Nutr Food Res. (2017) 61. doi: 10.1002/mnfr.201600930
167. Han X, Xiao L, Yu Y, Chen Y, and Shu HH. Alcohol consumption and gastric cancer risk: a meta-analysis of prospective cohort studies. Oncotarget. (2017) 8:83237–45. doi: 10.18632/oncotarget.19177
168. He Z, Zhao TT, Xu HM, Wang ZN, Xu YY, Song YX, et al. Association between alcohol consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. Oncotarget. (2017) 8:84459–72. doi: 10.18632/oncotarget.20880
169. Huang XZ, Chen Y, Wu J, Zhang X, Wu CC, Zhang CY, et al. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget. (2017) 8:4781–95. doi: 10.18632/oncotarget.13591
170. Huang Y, Chen H, Zhou L, Li G, Zhang Y, Wu Y, et al. Association between green tea intake and risk of gastric cancer: a systematic review and dose-response meta-analysis of observational studies. Public Health Nutr. (2017) 20:3183–92. doi: 10.1017/S1368980017002208
171. Liu W, Zhou H, Zhu Y, and Tie C. Associations between dietary folate intake and risks of esophageal, gastric and pancreatic cancers: an overall and dose-response meta-analysis. Oncotarget. (2017) 8:86828–42. doi: 10.18632/oncotarget.18775
172. Lu D, Pan C, Ye C, Duan H, Xu F, Yin L, et al. Meta-analysis of soy consumption and gastrointestinal cancer risk. Sci Rep. (2017) 7:4048. doi: 10.1038/s41598-017-03692-y
173. Ma K, Baloch Z, He T-T, and Xia X. Alcohol consumption and gastric cancer risk: A meta-analysis. Med Sci Monitor. (2017) 23:238–46. doi: 10.12659/MSM.899423
174. Mamtani R, Cheema S, Sheikh J, Al Mulla A, Lowenfels A, and Maisonneuve P. Cancer risk in waterpipe smokers: a meta-analysis. Int J Public Health. (2017) 62:73–83. doi: 10.1007/s00038-016-0856-2
175. Miao ZF, Xu H, Xu YY, Wang ZN, Zhao TT, Song YX, et al. Diabetes mellitus and the risk of gastric cancer: a meta-analysis of cohort studies. Oncotarget. (2017) 8:44881–92. doi: 10.18632/oncotarget.16487
176. Schwingshackl L, Schwedhelm C, Galbete C, and Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. (2017) 9:1063. doi: 10.3390/nu9101063
177. Wang PL, Xiao FT, Gong BC, and Liu FN. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget. (2017) 8:99013–23. doi: 10.18632/oncotarget.20918
178. Waziry R, Jawad M, Ballout RA, Al Akel M, and Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol. (2017) 46:32–43. doi: 10.1093/ije/dyw021
179. Weng KG and Yuan YL. Soy food intake and risk of gastric cancer: A dose-response meta-analysis of prospective studies. Medicine (Baltimore). (2017) 96:e7802. doi: 10.1097/MD.0000000000007802
180. Ye Y, Wu Y, Xu J, Ding K, Shan X, and Xia D. Association between dietary carbohydrate intake, glycemic index and glycemic load, and risk of gastric cancer. Eur J Nutr. (2017) 56:1169–77. doi: 10.1007/s00394-016-1166-4
181. Zhao Z, Yin Z, and Zhao Q. Red and processed meat consumption and gastric cancer risk: a systematic review and meta-analysis. Oncotarget. (2017) 8:30563–75. doi: 10.18632/oncotarget.15699
182. Zhou XL, Xue WH, Ding XF, Li LF, Dou MM, Zhang WJ, et al. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget. (2017) 8:55622–31. doi: 10.18632/oncotarget.16973
183. Fang HJ, Shan SB, Zhou YH, and Zhong LY. Diabetes mellitus and the risk of gastrointestinal cancer in women compared with men: a meta-analysis of cohort studies. BMC Cancer. (2018) 18:422. doi: 10.1186/s12885-018-4351-4
184. Ferro A, Morais S, Rota M, Pelucchi C, Bertuccio P, Bonzi R, et al. Tobacco smoking and gastric cancer: meta-analyses of published data versus pooled analyses of individual participant data (StoP Project). Eur J Cancer Prev. (2018) 27:197–204. doi: 10.1097/CEJ.0000000000000401
185. Li Z, Ying X, Shan F, and Ji J. The association of garlic with Helicobacter pylori infection and gastric cancer risk: A systematic review and meta-analysis. Helicobacter. (2018) 23:e12532. doi: 10.1111/hel.2018.23.issue-5
186. Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, et al. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer. (2018) 18:288. doi: 10.1186/s12885-018-4156-5
187. Shi J, Leng W, Zhao L, Deng C, Xu C, Wang J, et al. Tooth loss and cancer risk: a dose-response meta analysis of prospective cohort studies. Oncotarget. (2018) 9:15090–100. doi: 10.18632/oncotarget.23850
188. Sona MF, Myung SK, Park K, and Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer: a meta-analysis of observational studies. Japanese J Clin Oncol. (2018) 48:426–33. doi: 10.1093/jjco/hyy047
189. You J, Sun Y, Bo Y, Zhu Y, Duan D, Cui H, et al. The association between dietary isoflavones intake and gastric cancer risk: a meta-analysis of epidemiological studies. BMC Public Health. (2018) 18:510. doi: 10.1186/s12889-018-5424-7
190. You S, Sun G, Yao Q, Wan Z, and Huang X. Statin use and risk of gastrointestinal cancer: a meta-analysis of cohort studies. Int J Clin Exp Med. (2018) 11:1437–47.
191. Bae JM. Body mass index and risk of gastric cancer in Asian adults: A meta-epidemiological meta-analysis of population-based cohort studies. Cancer Res Treat. (2020) 52:369–73. doi: 10.4143/crt.2019.241
192. Du S, Li Y, Su Z, Shi X, Johnson NL, Li P, et al. Index-based dietary patterns in relation to gastric cancer risk: a systematic review and meta-analysis. Br J Nutr. (2019) 123:964–74. doi: 10.1017/S0007114519002976
193. Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose⁻Response meta-analysis. Nutrients. (2019) 11:826. doi: 10.3390/nu11040826
194. Li WY, Han Y, Xu HM, Wang ZN, Xu YY, Song YX, et al. Smoking status and subsequent gastric cancer risk in men compared with women: a meta-analysis of prospective observational studies. BMC Cancer. (2019) 19:377. doi: 10.1186/s12885-019-5601-9
195. Li Z, Han H, and Chang Y. Association between metabolic syndrome and the incidence of gastric cancer: a meta-analysis of cohort studies. Diabetol Metab syndrome. (2019) 11:83. doi: 10.1186/s13098-019-0478-y
196. Pormohammad A, Mohtavinejad N, Gholizadeh P, Dabiri H, Chirani AS, Hashemi A, et al. Global estimate of gastric cancer in Helicobacter pylori-infected population: A systematic review and meta-analysis. J Cell Physiol. (2019) 234:1208–18. doi: 10.1002/jcp.v234.2
197. Song M, Latorre G, Ivanovic-Zuvic D, Camargo MC, and Rabkin CS. Autoimmune diseases and gastric cancer risk: A systematic review and meta-analysis. Cancer Res Treat. (2019) 51:841–50. doi: 10.4143/crt.2019.151
198. Suh M, Wikoff D, Lipworth L, Goodman M, Fitch S, Mittal L, et al. Hexavalent chromium and stomach cancer: a systematic review and meta-analysis. Crit Rev Toxicol. (2019) 49:140–59. doi: 10.1080/10408444.2019.1578730
199. Turati F, Galeone C, Augustin LSA, and La Vecchia C. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients. (2019) 11:2342. doi: 10.3390/nu11102342
200. Wan QY, Wu XT, Li N, Du L, and Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926–386 participants. Gut. (2019) 68:762–4. doi: 10.1136/gutjnl-2018-316416
201. Xu Y, Yang J, Du L, Li K, and Zhou Y. Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: A meta-analysis of observational studies. Food Sci Nutr. (2019) 7:256–65. doi: 10.1002/fsn3.2019.7.issue-1
202. Yang DY, Wang X, Yuan WJ, and Chen ZH. Intake of anthocyanins and gastric cancer risk: A comprehensive meta-analysis on cohort and case-control studies. J Nutr Sci vitaminology. (2019) 65:72–81. doi: 10.3177/jnsv.65.72
203. Yoo JY, Cho HJ, Moon S, et al. Abstract 636: Salted food intake and risk of gastric cancer: A pooled analysis of Korean cohorts and a global meta-analysis [J]. Cancer Res. (2019) 79(13-Sup):1. doi: 10.1158/1538-7445.AM2019-636
204. Zhang F-X, Miao Y, Ruan J-G, Meng S-P, Dong J-D, Yin H, et al. Association between nitrite and nitrate intake and risk of gastric cancer: A systematic review and meta-analysis. Med Sci Monitor. (2019) 25:1788–99. doi: 10.12659/MSM.914621
205. Chang C-J, Tu Y-K, Chen P-C, and Yang H-Y. Talc exposure and risk of stomach cancer: Systematic review and meta-analysis of occupational cohort studies. J Formosan Med Assoc. (2020) 119:781–92. doi: 10.1016/j.jfma.2018.07.015
206. Gaesser GA. Whole grains, refined grains, and cancer risk: A systematic review of meta-analyses of observational studies. Nutrients. (2020) 12:3756. doi: 10.3390/nu12123756
207. Liang Y, Jiao H, Qu L, and Liu H. Positive association between dietary inflammatory index and gastric cancer risk: A systematic review and meta-analysis. Nutr Cancer-an Int J. (2020) 72:1290–6. doi: 10.1080/01635581.2019.1679197
208. Mariani M, Sassano M, and Boccia S. Metabolic syndrome and gastric cancer risk: a systematic review and meta-analysis. Eur J Cancer Prev. (2021) 30:239–50. doi: 10.1097/CEJ.0000000000000618
209. Moazzen S, van der Sloot KWJ, Vonk RJ, de Bock GH, and Alizadeh BZ. Diet quality and upper gastrointestinal cancers risk: A meta-analysis and critical assessment of evidence quality. Nutrients. (2020) 12:1863. doi: 10.3390/nu12061863
210. Niikura R, Hirata Y, Hayakawa Y, Kawahara T, Yamada A, and Koike K. Effect of aspirin use on gastric cancer incidence and survival: A systematic review and meta-analysis. JGH Open. (2020) 4:117–25. doi: 10.1002/jgh3.12226
211. Park Y and Ki M. Population attributable fraction of helicobacter pylori infection-related gastric cancer in Korea: A meta-analysis. Cancer Res Treat. (2021) 53:744–53. doi: 10.4143/crt.2020.610
212. Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, and Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. (2020) 42:e2020004. doi: 10.4178/epih.e2020004
213. Segna D, Brusselaers N, Glaus D, Krupka N, and Misselwitz B. Association between long-term use of proton pump inhibitors and the risk of gastric cancer: a systematic review and meta-analysis. Therap Adv Gastroenterol. (2020) 14:17562848211051463. doi: 10.1177/17562848211051463
214. Song HJ, Jeon N, and Squires P. The association between acid-suppressive agent use and the risk of cancer: a systematic review and meta-analysis. Eur J Clin Pharmacol. (2020) 76:1437–56. doi: 10.1007/s00228-020-02927-8
215. Tavakoli A, Monavari SH, Solaymani Mohammadi F, Kiani SJ, Armat S, and Farahmand M. Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer. (2020) 20:493. doi: 10.1186/s12885-020-07013-x
216. Wang T, Zhan R, Lu J, Zhong L, Peng X, Wang M, et al. Grain consumption and risk of gastric cancer: a meta-analysis. Int J Food Sci Nutr. (2020) 71:164–75. doi: 10.1080/09637486.2019.1631264
217. Win TT, Aye SN, Fern JLC, and Fei CO. Aspirin and reducing risk of gastric cancer: systematic review and meta-analysis of the observational studies. J Gastrointestinal Liver Dis. (2020) 29:191–8. doi: 10.15403/jgld-818
218. Yoo JY, Cho HJ, Moon S, Choi J, Lee S, Ahn C, et al. Pickled vegetable and salted fish intake and the risk of gastric cancer: two prospective cohort studies and a meta-analysis. Cancers. (2020) 12:996. doi: 10.3390/cancers12040996
219. Zhang X-F, Wang X-K, Tang Y-J, Guan X-X, Guo Y, Fan J-M, et al. Association of whole grains intake and the risk of digestive tract cancer: a systematic review and meta-analysis. Nutr J. (2020) 19:52. doi: 10.1186/s12937-020-00556-6
220. Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, et al. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. (2020) 122:1085–93. doi: 10.1038/s41416-020-0741-x
221. Bae J-M. Human papillomavirus infection and gastric cancer risk: A meta-epidemiological review. World J Virol. (2021) 10:209–16. doi: 10.5501/wjv.v10.i5.209
222. Bae JM. Green tea consumption and stomach cancer risk in women: A meta-analysis of population-based cohort studies. Cancer Res Treat. (2021) 53:289–90. doi: 10.4143/crt.2020.624
223. Deng W, Jin L, Zhuo H, Vasiliou V, and Zhang Y. Alcohol consumption and risk of stomach cancer: A meta-analysis. Chemico-biological Interact. (2021) 336:109365. doi: 10.1016/j.cbi.2021.109365
224. Du S, Yang Y, Fang S, Guo S, Xu C, Zhang P, et al. Gastric cancer risk of intestinal metaplasia subtypes: A systematic review and meta-analysis of cohort studies. Clin Trans Gastroenterol. (2021) 12:e00402. doi: 10.14309/ctg.0000000000000432
225. Du Y, Lv Y, Zha W, Hong X, and Luo Q. Chili consumption and risk of gastric cancer: A meta-analysis. Nutr Cancer-an Int J. (2021) 73:45–54. doi: 10.1080/01635581.2020.1733625
226. Duan F, Song C, Shi J, Wang P, Ye H, Dai L, et al. Identification and epidemiological evaluation of gastric cancer risk factors: based on a field synopsis and meta-analysis in Chinese population. Aging-Us. (2021) 13:21451–69. doi: 10.18632/aging.203484
227. He F, Sha Y, and Wang B. Relationship between alcohol consumption and the risks of liver cancer, esophageal cancer, and gastric cancer in China: Meta-analysis based on case-control studies. Medicine (Baltimore). (2021) 100(33):e26982. doi: 10.1097/MD.0000000000026982
228. Luo L, Yan J, Wang X, and Sun Z. The correlation between chili pepper consumption and gastric cancer risk: A meta-analysis. Asia Pacific J Clin Nutr. (2021) 30(1):130–9. doi: 10.6133/apjcn.202103_30(1).0016
229. Miao P and Guan L. Association of dietary cholesterol intake with risk of gastric cancer: A systematic review and meta-analysis of observational studies. Front Nutr. (2021) 8:722450. doi: 10.3389/fnut.2021.722450
230. Segna D, Brusselaers N, Glaus D, Krupka N, and Misselwitz B. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Ther Adv Gastroenterol. (2021) 14:17562848211051463. doi: 10.1177/17562848211051463
231. Sun C, Chen Y, Ismail MR, Tuason JPW, Cheng X, Hu L, et al. Is acid suppression therapy associated with increased risk of cardia gastric cancer? A meta-analysis. Am J Gastroenterol. (2021) 116:S655. doi: 10.14309/01.ajg.0000779240.78130.d8
232. Wan Q, Zhao R, Xia L, Wu Y, Zhou Y, Wang Y, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol. (2021) 147:1077–87. doi: 10.1007/s00432-020-03496-0
233. Wang Y, Guo J, Yu F, Tian Y, Wu Y, Cui L, et al. The association between soy-based food and soy isoflavone intake and the risk of gastric cancer: a systematic review and meta-analysis. J Sci Food Agric. (2021) 101:5314–24. doi: 10.1002/jsfa.v101.13
234. Wu B, Yang D, Yang S, and Zhang G. Dietary salt intake and gastric cancer risk: A systematic review and meta-analysis. Front Nutr. (2021) 8:801228. doi: 10.3389/fnut.2021.801228
235. Wu H, Zhang J, and Zhou B. Toothbrushing frequency and gastric and upper aerodigestive tract cancer risk: A meta-analysis. Eur J Clin Invest. (2021) 51:e13478. doi: 10.1111/eci.13478
236. Zadori N, Szako L, Vancsa S, Voerhendi N, Ostarijas E, Kiss S, et al. Six autoimmune disorders are associated with increased incidence of gastric cancer: A systematic review and meta-analysis of half a million patients. Front Immunol. (2021) 12:750533. doi: 10.3389/fimmu.2021.750533
237. Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, et al. Tea drinking and risk of cancer incidence: A meta-analysis of prospective cohort studies and evidence evaluation. Adv Nutr. (2021) 12:402–12. doi: 10.1093/advances/nmaa117
238. Chen C, Zhang M, Zheng X, and Lang H. Association between chili pepper consumption and risk of gastrointestinal-tract cancers: A meta-analysis. Front Nutr. (2022) 9:935865. doi: 10.3389/fnut.2022.935865
239. Chen X, Li L, Liang Y, Huang T, Zhang H, Fan S, et al. Relationship of vitamin D intake, serum 25(OH) D, and solar ultraviolet-B radiation with the risk of gastric cancer: A meta-analysis. J Cancer Res Ther. (2022) 18:1417–24. doi: 10.4103/jcrt.jcrt_527_21
240. Fagundes M, Silva ARC, Fernandes GA, and Curado MP. Dietary polyphenol intake and gastric cancer: A systematic review and meta-analysis. Cancers. (2022) 14:5878. doi: 10.3390/cancers14235878
241. Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, et al. Intake of soy, soy isoflavones and soy protein and risk of cancer incidence and mortality. Front Nutr. (2022) 9:847421. doi: 10.3389/fnut.2022.847421
242. Guo J, Liu C, Pan J, and Yang J. Relationship between diabetes and risk of gastric cancer: A systematic review and meta-analysis of cohort studies. Diabetes Res Clin Pract. (2022) 187:109866. doi: 10.1016/j.diabres.2022.109866
243. He J, Fu H, Li C, Deng Z, and Chang H. Association between vitamin B-12 and risk of gastric cancer: A systematic review and meta-analysis of epidemiological studies. Nutr Cancer-an Int J. (2022) 74:3263–73. doi: 10.1080/01635581.2022.2074062
244. Wang Y, Huang P, Wu Y, Liu D, Ji M, and Li H. Association and mechanism of garlic consumption with gastrointestinal cancer risk: A systematic review and meta-analysis. Oncol Lett. (2022) 23:125. doi: 10.3892/ol.2022.13245
245. Jang YC, Leung CY, and Huang HL. Association of hormone replacement therapy with risk of gastric cancer: a systematic review and meta-analysis. Sci Rep. (2022) 12:12997. doi: 10.1038/s41598-022-17345-2
246. Liu X, Zhou Y, and Zou X. Correlation between serum 25-hydroxyvitamin D levels and gastric cancer: A systematic review and meta-analysis. Curr Oncol. (2022) 29:8390–400. doi: 10.3390/curroncol29110661
247. Lou D, Fu R, Gu L, Su H, and Guan L. Association between statins’ exposure with incidence and prognosis of gastric cancer: an updated meta-analysis. Expert Rev Clin Pharmacol. (2022) 15:1127–38. doi: 10.1080/17512433.2022.2112178
248. Pan S, Akhdar G, Thrift A, and El-Serag H. The risk of gastric cancer (Overall and cardia only) with the use of proton pump inhibitors in observational studies: A systematic review and meta-analysis. Am J Gastroenterol. (2022) 117:S1149–50. doi: 10.14309/01.ajg.0000863072.72710.b4
249. Picetti R, Deeney M, Pastorino S, Miller MR, Shah A, Leon DA, et al. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ Res. (2022) 210:112988. doi: 10.1016/j.envres.2022.112988
250. Poly TN, Lin MC, Syed-Abdul S, Huang CW, Yang HC, and Li YJ. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers. (2022) 14:3052. doi: 10.3390/cancers14133052
251. Reng Q, Zhu LL, Feng L, Li YJ, Zhu YX, Wang TT, et al. Dietary meat mutagens intake and cancer risk: A systematic review and meta-analysis. Front Nutr. (2022) 9:962688. doi: 10.3389/fnut.2022.962688
252. Said Abasse K, Essien EE, Abbas M, Yu X, Xie W, Sun J, et al. Association between dietary nitrate, nitrite intake, and site-specific cancer risk: A systematic review and meta-analysis. Nutrients. (2022) 14:666. doi: 10.3390/nu14030666
253. Seo SI, Park CH, Kim TJ, Bang CS, Kim JY, Lee KJ, et al. Aspirin, metformin, and statin use on the risk of gastric cancer: A nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med. (2022) 11:1217–31. doi: 10.1002/cam4.v11.4
254. Song H, Shen X, Chu Q, and Zheng X. Coffee consumption is not associated with the risk of gastric cancer: An updated systematic review and meta-analysis of prospective cohort studies. Nutr Res. (2022) 102:35–44. doi: 10.1016/j.nutres.2022.03.002
255. Su C-H, Islam MM, Jia G, and Wu C-C. Statins and the risk of gastric cancer: A systematic review and meta-analysis. J Clin Med. (2022) 11:7180. doi: 10.3390/jcm11237180
256. Wu X, Chen L, Cheng J, Qian J, Fang Z, and Wu J. Effect of dietary salt intake on risk of gastric cancer: A systematic review and meta-analysis of case-control studies. Nutrients. (2022) 14 4260. doi: 10.3390/nu14204260
257. Yang Y, Liu M-H, and Li Y. Association between cholecystectomy and gastric cancer risk: A systematic review and meta-analysis. Front Oncol. (2022) 12:667736. doi: 10.3389/fonc.2022.667736
258. Zhang ML, Fan YX, Meng R, Cai WK, Yin SJ, Zhou T, et al. Proton pump inhibitors and cancer risk: an umbrella review and meta-analysis of observational studies. Am J Clin Oncol. (2022) 45:475–85. doi: 10.1097/COC.0000000000000949
259. Zhang Y, Ma N, Duan F, Yin J, He G, Wang L, et al. Depression and the occurrence of gastric cancer: a meta-analysis based on their relationship and epidemiological evaluation. J Public Health (Germany). (2022) 30:1533–43. doi: 10.1007/s10389-020-01469-8
260. Zheng J, Gao Y, Xie S-H, Santoni G, and Lagergren J. Haemoglobin A1c and serum glucose levels and risk of gastric cancer: a systematic review and meta-analysis. Br J Cancer. (2022) 126:1100–7. doi: 10.1038/s41416-021-01693-3
261. Islami F, Sheikhattari P, Ren JS, and Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma–a systematic review and meta-analysis. Ann Oncol. (2011) 22:754–60. doi: 10.1093/annonc/mdq411
262. Chen J, Gong TT, and Wu QJ. Parity and gastric cancer risk: a systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. (2016) 6:18766. doi: 10.1038/srep18766
263. Mundt KA, Dell LD, Crawford L, Sax SN, and Boffetta P. Cancer risk associated with exposure to bitumen and bitumen fumes: an updated systematic review and meta-analysis. J Occup Environ Med. (2018) 60:e6–e54. doi: 10.1097/JOM.0000000000001202
264. Shao L, Li P, Ye J, Chen J, Han Y, Cai J, et al. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int J Cancer. (2018) 143:1671–7. doi: 10.1002/ijc.v143.7
265. Yan S, Gan Y, Song X, Chen Y, Liao N, Chen S, et al. Association between refrigerator use and the risk of gastric cancer: A systematic review and meta-analysis of observational studies. PloS One. (2018) 13:e0203120. doi: 10.1371/journal.pone.0203120
266. Cohen SS, Sadoff MM, Jiang X, Fryzek JP, and Garabrant DH. A review and meta-analysis of cancer risks in relation to Portland cement exposure. Occup Environ Med. (2014) 71:796–802. doi: 10.1136/oemed-2014-102193
267. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.v144.8
268. Liu Q, Zeng X, Wang W, Huang R-l, Huang Y-j, Liu S, et al. Awareness of risk factors and warning symptoms and attitude towards gastric cancer screening among the general public in China: a cross-sectional study. BMJ Open. (2019) 9:e029638. doi: 10.1136/bmjopen-2019-029638
269. Wiseman MJ. Nutrition and cancer: prevention and survival. Br J Nutr. (2019) 122:481–7. doi: 10.1017/S0007114518002222
270. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. (2015) 51:2820–32. doi: 10.1016/j.ejca.2015.09.010
271. Shimazu T, Wakai K, Tamakoshi A, Tsuji I, Tanaka K, Matsuo K, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. (2014) 25:1228–33. doi: 10.1093/annonc/mdu115
272. Persson C, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Miura T, et al. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: a nested case–control study. Carcinogenesis. (2008) 29:1042–8. doi: 10.1093/carcin/bgn072
273. Ren J-S, Kamangar F, Forman D, and Islami F. Pickled food and risk of gastric cancer—a systematic review and meta-analysis of English and Chinese literature. Cancer epidemiology Biomarkers Prev. (2012) 21:905–15. doi: 10.1158/1055-9965.EPI-12-0202
274. Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, and Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer. (2011) 63:479–94. doi: 10.1080/01635581.2011.539315
275. Jain A, Tiwari A, Verma A, and Jain SK. Vitamins for cancer prevention and treatment: an insight. Curr Mol Med. (2017) 17:321–40. doi: 10.2174/1566524018666171205113329
276. Sezikli M, Çetinkaya ZA, Güzelbulut F, Çimen B, Özcan Ö, Özkara S, et al. Effects of alpha tocopherol and ascorbic acid on Helicobacter pylori colonization and the severity of gastric inflammation. Helicobacter. (2012) 17:127–32. doi: 10.1111/j.1523-5378.2011.00925.x
277. Vingeliene S, Chan DSM, Aune D, Vieira AR, Polemiti E, Stevens C, et al. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control. (2016) 27:837–51. doi: 10.1007/s10552-016-0755-0
278. Jarosz M, Sekuła W, Rychlik E, and Figurska K. Impact of diet on long-term decline in gastric cancer incidence in Poland. World J Gastroenterology: WJG. (2011) 17:89. doi: 10.3748/wjg.v17.i1.89
279. Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One. (2014) 9:e116060. doi: 10.1371/journal.pone.0116060
280. González CA and López-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scandinavian J Gastroenterol. (2010) 45:6–14. doi: 10.3109/00365520903401959
281. Fesharaki M, Nasimi A, Mokhtari S, Mokhtari R, Moradian R, and Amirpoor N. Reactive oxygen metabolites and anti-oxidative defenses in aspirin-induced gastric damage in rats: Gastroprotection by Vitamin E. Pathophysiology. (2006) 13:237–43. doi: 10.1016/j.pathophys.2006.08.003
282. Sun YQ, Girgensone I, Leanderson P, Petersson F, and Borch K. Effects of antioxidant vitamin supplements on Helicobacter pylori-induced gastritis in Mongolian gerbils. Helicobacter. (2005) 10:33–42. doi: 10.1111/j.1523-5378.2005.00289.x
283. Zhao Y, Li R, Xia W, Neuzil J, Lu Y, Zhang H, et al. Bid integrates intrinsic and extrinsic signaling in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. Cancer Lett. (2010) 288:42–9. doi: 10.1016/j.canlet.2009.06.021
284. Egnell M, Fassier P, Lécuyer L, Gonzalez R, Zelek L, Vasson M-P, et al. Antioxidant intake from diet and supplements and risk of digestive cancers in middle-aged adults: results from the prospective NutriNet-Santé cohort. Br J Nutr. (2017) 118:541–9. doi: 10.1017/S0007114517002392
285. Li P, Zhang H, Chen J, Shi Y, Cai J, Yang J, et al. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int J Cancer. (2014) 135:1444–53. doi: 10.1002/ijc.v135.6
286. De Stefani E, Boffetta P, Brennan P, Deneo-Pellegrini H, Carzoglio JC, Ronco A, et al. Dietary carotenoids and risk of gastric cancer: a case-control study in Uruguay. Eur J Cancer Prev. (2000) 9:329–34. doi: 10.1097/00008469-200010000-00007
287. Gomathinayagam S, Srinivasan R, Gomathi A, Jayaraj R, Vasconcelos V, Sudhakaran R, et al. Oral Administration of Carotenoid-Rich Dunaliella salina Powder Inhibits Colon Carcinogenesis via Modulation of Wnt/β-catenin Signaling Cascades in a Rat Model. Appl Biochem Biotechnol. (2024) 297:159–8. doi: 10.1007/s12010-024-05024-z
288. Kim MJ and Kim H. Anticancer effect of lycopene in gastric carcinogenesis. J Cancer Prev. (2015) 20:92. doi: 10.15430/JCP.2015.20.2.92
289. Lee SP, Sung I-K, Kim JH, Lee S-Y, Park HS, and Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. (2015) 21:273. doi: 10.5056/jnm14116
290. Fujiwara M, Inagaki M, Nakaya N, Fujimori M, Higuchi Y, Kakeda K, et al. Association between serious psychological distress and nonparticipation in cancer screening and the modifying effect of socioeconomic status: analysis of anonymized data from a national cross-sectional survey in Japan. Cancer. (2018) 124:555–62. doi: 10.1002/cncr.v124.3
291. Lutgendorf SK and Andersen BL. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am Psychol. (2015) 70:186. doi: 10.1037/a0035730
292. Huang T, Zhou F, Yuan X, Yang T, Liang X, Wang Y, et al. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through ABL1-mediated inflammation signaling pathway. Oxid Med Cell Longevity. (2019) 2019:5813985. doi: 10.1155/2019/5813985
293. Kim GM, Kim SJ, Song SK, Kim HR, Kang BD, Noh SH, et al. Prevalence and prognostic implications of psychological distress in patients with gastric cancer. BMC Cancer. (2017) 17:1–8. doi: 10.1186/s12885-017-3260-2
294. Hwang YJ, Kim N, Lee HS, Lee JB, Choi YJ, Yoon H, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication-a prospective study for up to 10 years. Alimentary Pharmacol Ther. (2018) 47:380–90. doi: 10.1111/apt.2018.47.issue-3
295. Lee YC, Chiang tH, Chou CK, tu YK, Liao WC, Wu MS, et al. Association Between Helicobacter pylori eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. (2016) 150:1113–24. doi: 10.1053/j.gastro.2016.01.028
296. Choi IJ, Kim CG, Lee JY, Kim Y-I, Kook M-C, Park B, et al. Family history of gastric cancer and Helicobacter pylori treatment. New Engl J Med. (2020) 382:427–36. doi: 10.1056/NEJMoa1909666
297. Echizen K, Hirose O, Maeda Y, and Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. (2016) 107:391–7. doi: 10.1111/cas.2016.107.issue-4
298. Liggett JL, Zhang X, Eling TE, and Baek SJ. Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets. Cancer Lett. (2014) 346:217–24. doi: 10.1016/j.canlet.2014.01.021
299. Akrami H, Aminzadeh S, and Fallahi H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumor Biol. (2015) 36:3237–43. doi: 10.1007/s13277-014-2952-3
300. Wang X, Xia X, Xu E, Yang Z, Shen X, Du S, et al. Estrogen Receptor Beta Prevents Signet Ring Cell Gastric Carcinoma Progression in Young Patients by Inhibiting Pseudopodia Formation via the mTOR-Arpc1b/EVL Signaling Pathway. Front Cell Dev Biol. (2020) 8:592919. doi: 10.3389/fcell.2020.592919
301. Okamoto M, Miura A, Ito R, Kamada T, Mizukami Y, and Kawamoto K. G-protein-coupled estrogen receptor prevents nuclear factor-kappa B promoter activation by Helicobacter pylori cytotoxin-associated gene A in gastric cancer cells. J veterinary Med Sci. (2023) 85:1348–54. doi: 10.1292/jvms.23-0054
302. Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One. (2014) 9:e116060. doi: 10.1371/journal.pone.0116060
303. Wang T, Jin J, Qian C, Lou J, Lin J, Xu A, et al. Estrogen/ER in anti-tumor immunity regulation to tumor cell and tumor microenvironment. Cancer Cell Int. (2021) 21:1–13. doi: 10.1186/s12935-021-02003-w
304. Reyes-Ramos AM, Álvarez-García YR, Solodin N, Almodovar J, Alarid ET, Torres-Garcia W, et al. Collagen I fibrous substrates modulate the proliferation and secretome of estrogen receptor-positive breast tumor cells in a hormone-restricted microenvironment. ACS biomaterials Sci Eng. (2021) 7:2430–43. doi: 10.1021/acsbiomaterials.0c01803
305. Yamauchi Y and Rogers MA. Sterol metabolism and transport in atherosclerosis and cancer. Front Endocrinol. (2018) 9:509. doi: 10.3389/fendo.2018.00509
306. Calleros L, Lasa M, Toro MJ, and Chiloeches A. Low cell cholesterol levels increase NFκB activity through a p38 MAPK-dependent mechanism. Cell signalling. (2006) 18:2292–301. doi: 10.1016/j.cellsig.2006.05.012
307. Radišauskas R, Kuzmickienė I, Milinavičienė E, and Everatt R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina. (2016) 52:89–98. doi: 10.1016/j.medici.2016.03.002
308. Strohmaier S, Edlinger M, Manjer J, Stocks T, Bjørge T, Borena W, et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PloS One. (2013) 8:e54242. doi: 10.1371/journal.pone.0054242
309. Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. (2011) 29:1592–8. doi: 10.1200/JCO.2010.31.5200
310. Asano K, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. Int J Cancer. (2008) 122:909–14. doi: 10.1002/ijc.v122:4
311. Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. (2019) 30:143–56. doi: 10.1016/j.cmet.2019.04.002
312. King RJ, Singh PK, and Mehla K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. (2022) 43:78–92. doi: 10.1016/j.it.2021.11.007
313. Ganjali S, Ricciuti B, Pirro M, Butler AE, Atkin SL, Banach M, et al. High-density lipoprotein components and functionality in cancer: state-of-the-art. Trends Endocrinol Metab. (2019) 30:12–24. doi: 10.1016/j.tem.2018.10.004
314. Shen JG, Jin LD, Dong MJ, Wang LB, Zhao WH, and Shen J. Low level of serum high-density lipoprotein cholesterol in gastric cancer correlates with cancer progression but not survival. Trans Cancer Res. (2020) 9:6206. doi: 10.21037/tcr-20-1220
Keywords: gastric cancer, risk factor, umbrella review, meta-analysis, system review
Citation: Liang JL, Yuan HM, Quan C and Chen JQ (2025) Risk factors for gastric cancer: an umbrella review of systematic reviews and meta-analyses. Front. Oncol. 15:1564575. doi: 10.3389/fonc.2025.1564575
Received: 21 January 2025; Accepted: 22 May 2025;
Published: 26 June 2025.
Edited by:
Hussain Gadelkarim Ahmed, Prof. Medical Research Consultancy Center -MRCC, SudanReviewed by:
Rocio Gomez, Center for Research and Advanced Studies (CINVESTAV), MexicoMichela Giulii Capponi, santo spirito in sassia hospital, taly
Danish Jamil, University of Santiago de Compostela, Spain
Copyright © 2025 Liang, Yuan, Quan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Qiang Chen, Y2hlbmp1bnFpYW5nQGd4bXUuZWR1LmNu
 Jin Long Liang
Jin Long Liang Hui Ming Yuan
Hui Ming Yuan Chao Quan
Chao Quan Jun Qiang Chen
Jun Qiang Chen